Abstract
As the largest endocrine organ, adipose tissue secretes many bioactive molecules that circulate in blood, collectively termed adipokines. Efforts to identify such metabolic regulators have led to the discovery of a family of secreted proteins, designated as C1q tumor necrosis factor (TNF)-related proteins (CTRPs). The CTRP proteins, adiponectin, TNF-alpha, as well as other proteins with the distinct C1q domain are collectively grouped together as the C1q/TNF superfamily. Reflecting profound biological potency, the initial characterization of these adipose tissue-derived CTRP factors finds wide-ranging effects upon metabolism, inflammation, and survival-signaling in multiple tissue types. CTRP3 (also known as CORS26, cartducin, or cartonectin) is a unique member of this adipokine family. In this review we provide a comprehensive overview of the research concerning the expression, regulation, and physiological function of CTRP3.
Introduction
Since the discovery of Leptin in 1994 and then later adiponectin there has been a fundamental shift in how adipose tissue is viewed within the medical and research community, as an active endocrine organ which effects human health and physiology (57,87). In 2004, Wong et al. characterized a novel family of adipose tissue-derived cytokines, collectively called adipokines, referred to as Complement C1q Tumor necrosis factor-Related Proteins (CTRPs) (76), like adiponectin and tumor necrosis factor (TNF) these CTRPs all contain a C1q globular domain and are characterized together as the C1q/TNF superfamily (62). To date, this superfamily has been documented to have a wide range and opposing effects on metabolism, food intake, inflammation, tumor metastasis, apoptosis, vascular disorders, ischemic injury, and even sexual reproduction (7, 8, 25, 29, 30, 49, 51, 57, 62, 64, 68, 69, 74-76, 81, 84, 89). The purpose of this review is to carefully summarize the research that has been accomplished on one of these proteins, CTRP3. A list of abbreviations used in this article is found in Table 1.
Table 1.
Abbreviations
| SP-1: specificity protein 1 |
| CTRP3: C1q TNF-related protein 3 |
| LPS: Lipopolysaccharide |
| TLR: Toll-like receptor |
| MCP-1: Monocyte chemotactic protein 1 |
| PPAR: Peroxisome proliferator-activated receptor |
| pIgA: Polymeric Immunoglobulin A |
| RANKL: Receptor activator of nuclear factor kappa-B ligand |
| TNF: Tumor necrosis factor |
| IL-6: Interleukin-6 |
| AP-1: Activator protein 1 |
| c-FOS: Fos proto-oncogene |
| Pit-1a: POU domain, class 1, transcription factor 1 |
| C/EBP-α/β: CCAAT-enhancer-binding proteins-alpha/beta |
| MyoD: Myogenic differentiation |
| c-JUN: Jun proto-oncogene, AP-1 transcription factor subunit |
| TY-IID: Transcription factor II D—“TATAA” |
| CREB: cAMP response element-binding protein |
| GATA-1: GATA-binding factor 1 |
| SRY: Sex-determining region Y |
| Sox-5: SRY-related HMG-box 5 |
| c-Myc: similar to myelocytomatosis viral oncogene |
| RXR: Retinoid X receptor |
| PGC-1α: Peroxisome proliferators activated receptor-γ co-activator-1α |
| NRF-1/NRF-2: Nuclear respiratory factor 1/2 |
| TFAM: Mitochondrial transcription factor A |
| TBXAS1: Thromboxane A synthase 1 |
| HUVEC: Human umbilical vascular endothelial cells |
| ROS: Reactive oxygen species |
| TGF-β: Transforming growth factor beta |
History of CTRP3
Initial discovery
CTRP3 was first discovered in 2001 (43) in C3H10T1/2 mouse mesenchymal stem cells treated to induce chondrogenic differentiation. Because of its size and 23 Gly-X-Y repeats in the N-terminal collagen domain it was originally named CORS26 (Collagenous repeat-containing sequence 26 kDa protein). Later Wong et al. (2004) identified CTRP3 as a member of a family of highly conserved adiponectin paralogs designated as CTRPs and CORS26 was renamed CTRP3 (76). Alternative names that have been used for CTRP3 are cartducin (1, 44) and cartonectin (56, 73), both due to the detection of CTRP3 expression in developing cartilage.
Structure
Analysis of the primary structure predicts that CTRP3 is a highly hydrophilic secreted protein, with an N-terminal hydrophobic signal peptide, and no transmembrane domains (43). Experimental work confirms that CTRP3 is a secreted protein and circulates in the blood, which indicates that the physiological function of CTRP3 occurs through endocrine mechanisms (51, 75). Additionally, CTRP3 has a series of N-terminal Collagenous repeats (Gly-X-Y), and a highly conserved C-terminal globular domain (76), thus placing CTRP3 within the expanding C1q TNF Superfamily (62) (Fig. 1). CTRP3 shares sequence homology with adiponectin (38% in mouse and 36% in human), and is highly conserved (95.9% identity between human and mouse proteins) (36). Additionally, there are two splice variants of CTRP3 that have been identified. The longer splice variant, designated as CTRP3B, encodes an extra 73 N-terminal amino acids due to the retention of intron 1. CTRP3B contains a highly conserved N-linked glycosylation site that is not present on the original splice variant, CTRP3A (51). At this time the functional significance of the splice variants of CTRP3 are unknown as research has focused almost exclusively on CTRP3A. Regardless, both CTRP3A and CTRP3B are secreted proteins which are detectable in human serum. Although only CTRP3A has been detected in mouse serum, both variants are expressed in mouse adipose tissue (51, 75). One of the reasons for the difficulty in detecting the CTRP3B variant is that unlike CTRP3A, CTRP3B degrades rapidly unless it forms a higher order oligomer with CTRP3A (51).
Figure 1.
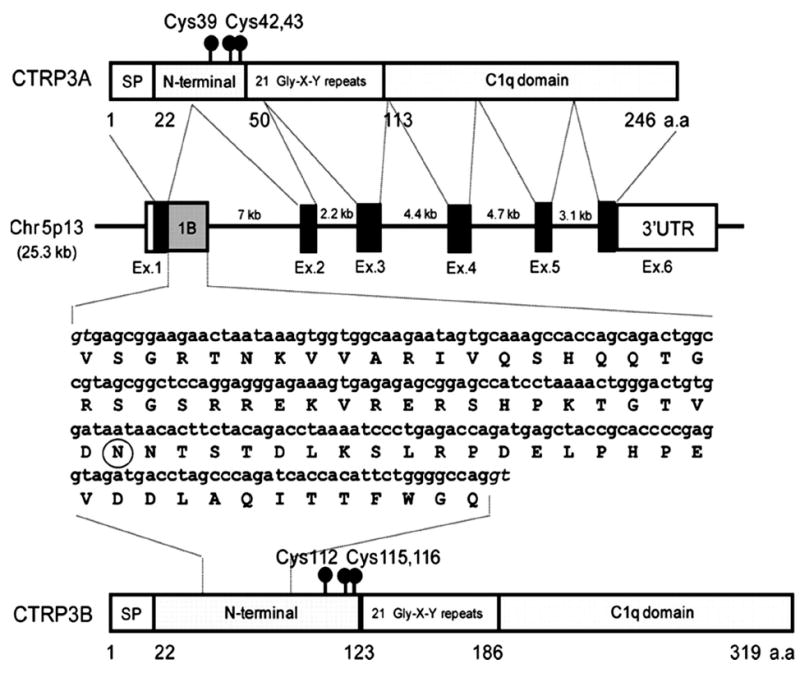
Structural overview of CTRP3. (A) Two splice variants were identified and designated as CTRP3A and CTRP3B. (B) Organization of the CTRP3A and CTRP3B genes and proteins. The human CTRP3A gene is 25.3 kb in size, consists of six exons and five introns, and is located on chromosome 5p13. Exons 1, 2, 3, 4, 5, and 6 of the CTRP3A gene are 171, 112, 155, 130, 100, and 2885 bp in size, respectively. The size of each intron is also indicated. Exon 1B (gray square) contains a 219-nucleotide sequence found in CTRP3B cDNA, coding for an extra 73 amino acid residues. A potential Nlinked glycosylation site is circled. The consensus splice donors are shown in italic type. This figure was originally published in The Journal of Biological Chemistry (51) and image reproduced according to the copyright policy of the ASBMB. © the American Society for Biochemistry and Molecular Biology.
CTRP3 is endogenously co-expressed in many tissues with other proteins within the C1q TNF superfamily. However, CTRP3 is unique as it does not form hetero-oligomeric complexes with adiponectin or any other CTRP protein (76). Most CTRP proteins will form hetero-oligomers with adiponectin or at least 1 other CTRP protein when coexpressed (75, 76). However, higher-order oligomers are the primary form by which CTRP3 is found circulating in either human or mouse serum, indicating that CTRP3 high-order oligomeric complexes occur solely between its two splice variants, CTRP3A and CTRP3B (51). While the significance of these posttranslational modifications and higherorder structure formations have yet to be explored, it is likely that these modifications influence the function of CTRP3. Therefore, it is important to differentiate between functional studies and experimental findings gleaned from bacterialproduced compared with other types of recombinant CTRP3 protein. Bacterial-produced recombinant CTRP3 protein does not possess the potentially physiologically relevant posttranslational modifications or multimeric structures, and may explain any observed lack of biological activity (19, 61).
Recently using the novel ligand-receptor capture method, and mammalian-cell expressed CTRP3, Li et al. (2016) (41) identified Lysosomal-associated membrane protein 1 (LAMP-1) and Lysosome membrane protein 2 (LIMP II) as potential receptors for CTRP3. Although it remains to be determined whether either of these proteins directly mediate the intercellular effects of CTRP3 or act as coreceptors for a yet unidentified protein, both LAMP1 and LIMPII are widely expressed in a number of different tissues corresponding to the variety of functions attributed to CTRP3. A comprehensive list of in vitro functions documented for CTRP3 is listed in Table 2. CTRP3 may also act without directly initiating intracellular action but rather through inhibiting the binding of other ligands. For example, lipopolysaccharide (LPS) is a potent endotoxin that binds to Toll-like receptor 4 (TLR4) and promotes a cellular inflammatory response. However, even though CTRP3 does not bind directly to either LPS or TLR4, CTRP3 prevents their interaction through an unestablished mechanism (30).
Table 2.
Complete Summary of the In Vitro Functions of CTRP3
| Cell line | Citation | Type of treatment | Effect |
|---|---|---|---|
| N1511 (mouse chondrogenic progenitor) | Akiyama et al. 2006 (1) | 10 μg/ml [E. coli produced His6-tagged signal peptide removed] | Increased ERK 1/2, and Akt phosphorylation from 5 min-1 hour, but not JNK or p38 MAPK |
| MSS31 (mouse endothelial cells) | Akiyama et al. 2007 (2) | 1-5 μg/mL [E. coli produced His6-tagged signal peptide removed] | ↑ Proliferation and migration, ↑ ERK1/2 and MAPK p38 within 15 min |
| LM8 (mouse osteosarcoma cell line) | Akiyama et al. 2009 (3) | 2-10 μg/mL [E. coli produced His6-tagged signal peptide removed] | CTRP3 treatment ↑ proliferation and ↑ ERK1/2 phosphorylation with no effect on migration or p38, JNK1/2, or Akt phosporylation |
| NOHS (mouse osteosarcoma cell line) | Akiyama et al. 2009 (3) | 2-10 μg/mL [E. coli produced His6-tagged signal peptide removed] | CTRP3 treatment ↑ proliferation and ↑ ERK1/2 phosphorylation with no effect on migration or p38, JNK1/2, or Akt |
| Rat vascular smooth muscle cells (VSMCs) | Feng et al. 2016 (15) | Human globular CTRP3 (Aviscera Bioscience) Catalog # not reported | CTRP3 promotes ATP synthesis, oxidative phosphorylation complex proteins, and ROS levels; whereas siRNA knockdown reduces ATP and oxidative phosphorylation complex proteins levels |
| Human primary colonic lamina propria fibroblasts (CLPF) | Hofmann et al. 2011 (21) | Recombinant CTRP-3 (High Five insect cells) | Significantly and dose-dependently reduced LPS-induced IL-8 secretion in CLPF within 8 hours after LPS exposure, whereas LPS-induced IL-6 and TNF release was not affected. CTRP-3 inhibited TGF-β production and the expression of CTGF and collagen I in CLPF, whereas collagen III expression remained unchanged |
| RWPE-1 prostate cells (ATCC Number CRL-11609) | Hou et al. 2015 (23) | Mammalian expressed CTRP3 (3, 10, or 30 μg/mL) | Increased cell number in a dose-dependent manner at 24-72 h, and 10 μg/mL prevented apoptosis. Cytokeratin-19, GLRX3, and DDAH1 were upregulated and cytokeratin-17 and 14-3-3 sigma were downregulated proteins in CTRP3-treated cells |
| Bone marrow macrophages (BMM) | Kim et al. 2015 (27) | Recombinant human CTRP3 was purchased from AdipoGen (catalog number not reported) | Decreased osteoclast differentiation from BMM treated with IL-1, 1,25(OH2)D3, receptor activator of nuclear factor kappa-B ligand (RANKL) (27), further CTRP3 prevented mature osteoclasts from reabsorbing bone |
| Adipocyte (3T3-L1) | Kopp et al. 2010 (30) | 1-10 μg/mL recombinant full length CTRP3 (H5 insect cells) | Suppressed LPS, lauric acid, TLR1/2, or TLR3 ligand-induced MCP-1 release. CTRP3 had no effect on TLR2/6 ligand-induced MCP-1 release. Further, siRNA knockdown of CTRP3 increased MCP-1 release and decreased adiponectin secretion and reduced lipid accumulation |
| Primary human monocytes | Kopp et al. 2010 (30) | 1 μg/mL recombinant full length CTRP3 (H5 insect cells) | Suppressed LPS-induced MIF and CCL4 release in lean subjects, and MCP-1 in monocytes from both diabetic and lean subjects. Prevents Lauric acid induced release of IL-6 and TNF |
| Primary human monocytes | Kopp et al. 2010 (31) | 1 μg/mL recombinant full length CTRP3 (H5 insect cells) | ↓ LPS-induced secretion of IL-6 in monocytes from control but not T2D (31). CTRP3 did not significantly affect TNF secretion |
| H4IIE (rat hepatoma cells) | Li et al. 2016 (41) | Mammalian expressed recombinant CTRP3 (2 μg/mL) | Increased lipid oxidation. Also, LAMP1 and LIMPII were identified as potential receptors |
| Adipocyte (3T3-L1) | Li et al. 2014 (38) | Recombinant CTRP3 (uncited origin) | Increased adipocyte glucose uptake and decreased TNF and IL6 secretion in insulin resistant 3T3-L1A |
| Adipocyte (3T3-L1) | Li et al. 2014 (39) | Mammalian expressed recombinant CTRP3 (250 ng/mL) | ↑ the secretion of adiponectin, leptin, visfatin, and apelin with peak detectable effect at 12 h (effects prevented by the addition of an AMPK inhibitor) |
| Adventitial fibroblasts | Lin et al. 2014 (42) | CTRP3 protein (10 μg/mL) (source/type of CTRP3 was not reported) | CTRP3 prevents TGF-β1 induced fibroblasts phenotypic conversion, proliferation, migration, collagen I expression, and connective tissue growth factor (all indicators of inappropriate of vascular remodeling and neointima formation) |
| HSC-2/8 (human chondrocytes) | Maeda et al. 2006 (44) | 10 μg/mL [E. coli produced His6-tagged signal peptide removed] | Increase proliferation |
| N1511 (mouse chondrogenic progenitor) | Maeda et al. 2006 (44) | 2-10 μg/mL [E. coli produced His6-tagged signal peptide removed] | Increase proliferation |
| TM3 mouse Leydig cells | Otani et al. 2012 (47) | Mammalian expressed full length (3-30 ug/mL) | Dose responsive ↑ testosterone production, ↑ steroidogenic acute regulatory protein mRNA and protein levels |
| L6 Myotubes (Rat) | Peterson et al. 2010 (51) | Mammalian expressed recombinant CTRP3 (2 μg/mL) | No effect on glucose uptake |
| 3T3-L1A (preadipocyte) | Peterson et al. 2010 (51) | Mammalian expressed recombinant CTRP3 (2 μg/mL) | No effect on glucose uptake |
| H4IIE (rat hepatoma cells) | Peterson et al. 2013 (50) | Mammalian expressed recombinant CTRP3 (2 μg/mL) | Reduced intracellular intra-lipid accumulation and decreased de novo fatty acid synthesis. No effect on lipid uptake |
| H4IIE (rat hepatoma cells) | Peterson et al. 2010 (51) | Mammalian expressed recombinant CTRP3 (2 μg/mL) | Suppressed glucose production and gluconeogenic genes (PEPCK and G6Pase). Increased Akt phosphorylation with no effect on AMPK phosphorylation |
| Adipocyte (3T3-L1) | Schmid et al. 2012 (59) | siRNA knockdown of CTRP3 | Reduced resistin secretion and lipolysis |
| Adipocyte (3T3-L1) | Schmid et al. 2012 (59) | siRNA knockdown of CTRP3 | siRNA knockdown of CTRP3 reduced resistin secretion and lipolysis |
| Adipocyte (3T3-L1) | Schmid et al. 2013 (58) | siRNA-mediated cellular knockdown of CTRP3 | siRNA-mediated cellular knockdown of CTRP-3 in adipocytes resulted in an upregulation of CTRP-5 expression |
| THP-1 (human—acute monocytic leukemia) | Weigert et al. 2005 (70) | 1 μg/mL recombinant full length CTRP3 (H5 insect cells) | Suppressed LPS induced: IL-6 and TNF mRNA and release |
| Adipocyte (3T3-L1) | Wolfing et al. 2008 (73) | 10 ng/mL recombinant full length CTRP3 (H5 insect cells) | Increased adiponectin and resistin secretion but not promoter activity with no effect on IL-6 (no effect on human-derived adipocytes) |
| Primary cultured adult rat cardiac fibroblast (CF) | Wu et al. 2015 (77) | 2 μg/mL recombinant human gCTRP3 (Aviscera Bioscience; 00082-01-100; E. Coli expressed); also used siRNA knockdown of CTRP3 | Inhibited TGF-β1 α-induced α-SMA, III, and connective tissue growth factor (CTGF) expression [markers for myofibroblast differentiation]. Whereas, SiRNA knockdown of CTRP3 increased TGF-β1-induced α-smooth muscle actin (α-SMA) and smooth muscle 22α (SM22α) collagen I, collagen III, and CTGF expression. Further CTRP3 attenuated TGF-β1 induced-elevations in phosphor-Smad3 (Ser204) |
| Cardiomyocytes (adult mouse) | Yi et al. 2012 (81) | Bacterial expressed globular domain of mouse CTRP3 | CTRP3 upregulated levels of phosphorylated Akt, HIF1α, and VEGF. Further cardiomyocyte hypoxia-induced apoptosis was attenuated with condition media from 3t3-L1A adipocytes; however, this effect was abolished when the conditioned media came from 3T3-L1a cells treated with CTRP3 siRNA |
| human umbilical vascular endothelial cells (HUVECs) | Yi et al. 2012 (81) | Bacterial expressed globular domain of mouse CTRP3 | CTRP3 had no effect on tube formation, Akt phosphorylation or HIF1α or VEGF expression. These results suggest that the in vivo proangiogenic effect of CTRP3 is no through direct mechanisms on endothelial cells. Further, treatment of HUVECs cell with condition media from primary cardiomyocytes treated with CTRP3 significantly enhanced HUVEC tube formation |
| Primary meckel’s cartilage | Yokohama-Tamaki et al. 2011 (82) | Knock-down of CTRP3 (antisense ogliodeoxynucleotide) | Caused severe curvature deformation and suppressed growth |
| Neonatal rat ventricular myocytes | Zhang et al. 2016 (85) | 0.5-4 μg/mL human recombinant globular and full-length CTRP3 (Aviscera Bioscience) Catalog # not reported | Both increased the expression of PGC-1α, nuclear respiratory factors 1 and 2, and markers of mitochondrial biogenesis |
| human mesangial cells (HMCs) | Zhang et al. 2016 (86) | Full length human CTRP3 adenovirus-mediated overexpression | ↓ pIgA-induce IL-6 and TGF-beta secretion from HMC cells |
| Human mesangial cells (HMCs) | Zhang et al. 2016 (86) | Treated with serum polymeric IgA (pIgA) from control or IgAN patients | pIgA markedly increased IL-6 and TGF-β in HMCs, which were suppressed after Ad-CTRP3 transfection |
| Rat cultured carotid arterial rings | Zhou et al. 2014 (90) | (1, 2, and 4 μg/mL) Recombinant human globular domain CTRP3 Aviscera Bioscience (00082-01-100) | CTRP3 promotes vascular calcification in high-phosphate cultured carotid arterial rings |
| Rat vascular smooth muscle cells (VSMCs) | Zhou et al. 2014 (90) | (1, 2, and 4 μg/mL) Recombinant human globular domain CTRP3 Aviscera Bioscience (00082-01-100) | CTRP3 promotes β-glycerophosphate-induced VSMC calcification and promoted the phenotypic transition from contractile to osteogenic phenotype. Increase mitochondrial ROS levels in VSMC |
Regulation
Analysis of the upstream untranslated region for CTRP3 identified a number of putative consensus sequences for transcription factor regulation of CTRP3 expression. The proximal region of the CTRP3 promoter is highly conserved between rodent and human (28), indicating that there are conserved functional regulation sites. These predicted regulatory sites include loci for the following transcription factors: specificity protein 1 (SP-1); Activator protein 1 (AP-1); Peroxisome proliferator-activated receptor (PPAR); Fos proto-oncogene (c-FOS); POU domain, class 1, transcription factor 1 (Pit-1a); CCAAT-enhancer-binding proteins-alpha/beta (C/EBP-α/β); myogenic differentiation (MyoD); c-JUN; transcription factor II D – “TATAA” box (TY-IID); cAMP response element-binding protein (CREB); GATA-binding factor 1 (GATA-1); sex-determining region Y (SRY); SRY-related HMG-box 5 (Sox-5); similar tomyelocytomatosis viral oncogene (c-Myc); and Retinoid X receptor (RXR) (53-55). However, to date, only a few transcription factors have been demonstrated to regulate CTRP3 expression experimentally: c-FOS, SP-1, c-JUN, and PPAR-gamma.
Although CTRP3, SP-1 and PPAR-gamma are induced during adipocyte differentiation, promoter activity assays demonstrate that PPAR-gamma, SP-1, and c-FOS are all negative regulators of CTRP3 expression (53, 56). Electrophoretic mobility shift assays confirmed that both SP-1 and PPAR-gamma (but not SRY, c-FOS, C/EBPβ, or PPAR-alpha) bind to the promoter region for CTRP3. To date only the transcription factor c-Jun has been shown to be an unequivocal positive regulator for CTRP3 transcription (28). The transcription factor c-Jun is one of three Jun family proteins which make up the activator protein-1 (AP-1) transcription factor group. Chromatin immunoprecipitation assay confirmed that c-Jun binds to the AP-1 region (-184/-177) of CTRP3 (28), whereas, other Jun and Fos members JunB, JunD, FosB, Fra-1, and Fra-2 were tested by a reporter gene assay and had no effect on CTRP3 promoter activity (28). Further, treatment of adipocytes, in vitro, or diet-induced obese rats, in vivo, with the glucagon-likepeptide-1 (GLP-1) receptor agonist, Exendin-4 (Ex-4), increased CTRP3 expression and circulating levels through activation of the Protein kinase A (PKA) pathway (37,40). Briefly, the activation ofGLP-1 receptor and PKA pathway activates HOB1 motif within the A1 activation domain of c-JUN and promotes c-JUN’s binding to the AP-1 region (6). However, the regulation of CTRP3 under physiological conditions in vivo has not yet been established. Unlike most adipokines, circulating CTRP3 levels are increased with fasting (51), indicating that CTRP3 levels may be suppressed by either insulin or activated by glucagon signaling pathways. Further, CTRP3 levels are negatively associated with insulin and leptin levels in high fat fed mice (51). Taken together, these data show a potential reciprocal relationship between food intake and CTRP3. However, the clinical implications have yet to be explored, especially regarding the significance of the by-phasic regulation of CTRP3 and lipid metabolism.
Tissues expressed
A summary of cell lines, which express CTRP3, in vitro, are listed in Table 3. CTRP3 is not detectable in undifferentiated adipocytes, but can be detected at 4 days of differentiation (55, 56). These data match in vivo data which shows that CTPR3 is highly expressed in adipose tissue. In addition, CTRP3 is also detected during development, starting at mouse embryonic day 15 (43,75,76), in developing chondrocytes (43) and cartilage (44). These data have led to speculation that CTRP3 is essential for appropriate bone growth and development. This is supported by experimental evidence that demonstrates that CTRP3 stimulates the proliferation and differentiation of chondrogenic and osteogenic precursors, inhibits osteoclast activity, and is essential for appropriate bone formation in vitro (1, 27, 44, 82). However, a clinical association between abnormal development and deregulation of CTRP3 protein has yet to be documented. On the other hand, CTRP3 is highly expressed in both osteoscarcoma and chandroblastoma cell lines but not in the MC3T3-E1 mouse osteoblast-like non-cancer cell line (3). These data have led to speculation that elevated CTRP3 level in adults may be a risk factor and/or biomarker for certain types of cancer, specifically osteoscarcoma, but again this hypothesis remains to be tested either in vivo or in a clinical population.
Table 3.
CTRP3 Expression In Vitro
| Cell line | Reference | Finding |
|---|---|---|
| Adipose tissue collected from control (n = 3) or diabetic (n = 3) female donors) | Schmid et al. 2012 (59) | CTRP3 protein levels were higher in subcutaneous adipocytes from an obese compared with lean donor, no differences were observed from visceral adipocytes. (Note: small sample size, n = 3) |
| N1511 (mouse chondrogenic progenitor) | Maeda et al. 2006 (44) | CTRP3 is induced w/differentiation by TGF-beta |
| 3T3-L1a (mouse preadipocyte) | Schaffler et al. 2007 (56) | Produce CTRP3 at day 4 differentiation and CTRP3 promoter activity was suppressed when treated with PPAR ligands: troglitazone (α/γ), fenofibrate (α), but not 15-Deoxy-Delta-12,14-prostaglandin J2 |
| 3T3-L1a (mouse preadipocyte) | Li et al. 2015 (37) | Exendin-fragment 4 (Ex-4), a specific GLP-1 receptor agonist, specifically used to treat T2D, increased CTRP3 mRNA and protein whereas Ex-9 (and GLP-1 antagonist) or blocking activation of PKA (thr197) phosphorylation by H89, a selective antagonist of PKA, blocked Ex-4 induced increases in CTRP3. Neither H89 nor Ex-9 had any effect on CTRP3 alone |
| 3T3-L1a (mouse preadipocyte) | Schmid et al. 2012 (59) | CTRP3 is positively regulated by insulin, and inhibited by chronic LPS-exposure or Intracellular infection of adipocytes by S. aureus. Further, siRNA knockdown of CTRP3 reduced resistin secretion and lipolysis |
| MC3T3-E1 (mouse osteoblast-like cell line; noncancer) | Akiyama et al. 2009 (3) | Neither CTRP3 mRNA or protein was detected |
| Human mesangial cells (HMCs) | Zhang et al. 2016 (86) | Polymeric IgA (pIgA) suppressed CTRP3 mRNA and protein whereas the IgA1 fractions (mIgA and pIgA) had no effect on CTRP3. Further, galactose-deficient IgA (gd-IgA; the type of IgA found in IgAN patients) suppressed CTRP3 mRNA levels even more than pIgA at identical concentrations (~60% compared with 30%) |
| LM8 (mouse osteosarcoma cell line) | Akiyama et al. 2009 (3) | CTRP3 mRNA and protein detected |
| NOHS (mouse osteosarcoma cell line) | Akiyama et al. 2009 (3) | CTRP3 mRNA and protein detected |
| Human omental adipose tissue explants | Tan et al. 2013 (65) | Metformin increased CTRP3 secretion |
| TM3 mouse Leydig cells | Otani et al. 2012 (47) | Express CTRP3 |
In adult mice CTRP3 is considered an adipokine, as it is predominantly expressed in adipose tissue (55, 56, 75), indicating that CTRP3 may have a functional role in energy storage and metabolism. However, CTRP3 is also expressed in the lung, kidneys, spleen, testis, and macrophages with moderate expression in heart, bone, small intestine, liver, kidney, skeletal muscle, and vascular smooth muscle cells (1, 43, 44, 47, 51, 54, 59, 75, 76, 90). Combined these data indicate that CTRP3 has a variety of factors which could contribute to its regulation and function. In the following sections we will detail the experimental evidence regarding the functional activity of CTRP3 in regards to metabolism, cardiovascular health, inflammation, and growth and development.
Metabolism, Metabolic Disease and CTRP3
Overview
CTRP3 has been documented to have a variety of effects on metabolism. The complete overview of in vitro effects is listed in Table 2. Briefly, CTRP3 increases adipokine secretion, attenuates inflammatory signaling, promotes proliferation, increases cellular differentiation, and increases hepatic lipid oxidation (39, 41, 50, 51, 58). Whereas, the in vivo the effects of CTRP3 are less well examined. The summary of all animal experiments and CTRP3 are listed in Table 4. For example, in rodents an acute injection with recombinant CTRP3 protein decreases serum glucose levels for up to 8-hours with no change to insulin levels (51). The chronic transgenic overexpression of CTRP3 had no effect upon glucose levels, which demonstrates the development of a potential compensatory mechanism (51). On the other hand, both transgenic overexpression and daily administration of CTRP3 were effective in attenuating high fat diet-induced hepatic insulin resistance and hepatic steatosis (50). Conversely, hepatic triglycerides were elevated in high fat-fed CTRP3 knockout mice when compared to high fat-fed wild-type mice (71).Considered together, these data suggest that the metabolic effects of CTRP3 are specific to the liver, as no changes to metabolism were observed in skeletal muscle in any experimental (in vivo or in vitro) model examined. Interestingly, neither transgenic overexpression nor genetic deletion of CTRP3 resulted in a measurable metabolic effect in mice fed a low fat diet (50, 71), which indicates that CTRP3 may function specifically to help regulate metabolism in response to elevated lipid consumption.
Table 4.
Metabolic Effects of CTRP3 In Vivo
| Model | Reference | Results |
|---|---|---|
| Male rats HFD+low dose Streptozotocin | Li et al. 2014 (40) | CTRP3 mRNA increased between birth and 10 weeks of age. Insulin resistance or T2D decreased CTRP3 (protein and mRNA) and treatment with Ex-4 slightly increased CTRP3 |
| CTRP3 knockout and collagen-induced arthritis model | Murayama et al. 2014 (46) | CTRP3 knockout results in severe histopathological changes |
| Transgenic CTRP3 overexpression with low or high-fat diet | Petersen et al. 2016 (48) | LF-diet; ↑ the circulating levels of CCL11, CXCl9, CXCL10, CCL17, CX3CL1, CCL22, AND Scd30. HF-Diet: ↓ circulating levels of IL-5, TNF-α, sVEGF2, and sVEGFR3, and ↑ circulating levels soluble gp130 |
| Transgenic CTRP3 overexpression with low- or high-fat diet | Peterson et al. 2012 (50) | No metabolic effect LF-diet; on HF-diet: mild shift to lipid oxidation, ↓ hepatic triglyceride synthesis, ↓ hepatic stenosis, ↑ insulin sensitivity. ↓ circulating TNF, IL5 and cholesterol levels and more than doubled soluble gp130 (sgp130) levels (an inhibitor of IL-6) |
| Acute injection of 2 μg/g body weight of (mammalian expressed recombinant CTRP3) | Peterson et al. 2010 (51) | ↓glucose, ↑pAkt and hepatic gluconeogenesis (specifically phosphoenolpyruvate carboxykinase and glucose 6-phosphatase) |
| Viral suspension consisting of 1×109 genomic copies of the lentivirus-CTRP3 (Lenti-CTRP3) | Wang et al. 2016 (67) | Attenuates brain injury after intracerebral hemorrhage via AMPK-dependent pathway in rat |
| CTRP3 knockout mice fed a high-fat diet | Wolf et al. 2016 (71) | No differences in metabolic factors between KO and WT; however the liver was smaller with a higher triglyceride content |
| Mouse model of myocardial infarction (MI); Adenovirus-mediated overexpression of human full-length CTRP3 | Yi et al. 2012 (81) | MI-reduced adipocyte CTRP3 mRNA and plasma protein levels. Whereas, replenishment of CTRP3 after MI improves survival, cardiac function, promotes angiogenesis |
Regardless, the clinical implications of these effects have yet to be explored, as the reported associations between obesity and/or type 2 diabetes (T2D) with CTRP3 levels are contradictory in the literature. The summary finding of all cross sectional human studies, which examine CTRP3 levels, are listed in Table 5. Briefly, CTRP3 levels are reported to be elevated (12), not different (16, 66), or reduced (5,14,52,65,72,83) with obesity and/or T2D. In most reports circulating CTRP3 levels are higher in women than in men (10,14,72,83), with one exception (52). Further complicating the relationship between CTRP3 levels and human health, Wager et al. (2016) reported that CTRP3 levels are elevated with obesity in male but are reciprocally reduced with obesity in female subjects (66). This contradictory gender dependent association of obesity and circulating CTRP3 levels provides some explanation regarding the conflicting data in the literature, as almost all the reported studies combined varying proportions of male and female subjects within each experimental group. Nevertheless, these data demonstrate that there is a gender specific regulation and function of CTRP3 that needs to be explored in more detail. Further, all of these studies have examined the total amount of CTRP3 with no attention being given to the different splice variants or in the multimeric structures of the circulating CTRP3. This is worth noting as high molecular weight adiponectin is thought to be the active form (20, 32), and similarly the different splice variants and multimeric structure may also contribute to the function of CTRP3. Lastly, the associations between circulating CTRP3 levels (splice variants and multimeric structures) and hepatic steatosis in human subjects have not been explored even though animal experiments have shown a specific hepatic effect of both CTRP3A and CTRP3B (51).
Table 5.
Cross-Sectional Studies Regarding CTRP3 Levels
| Population (Male:Female) | Reference | Result |
|---|---|---|
| Newly diagnosed T2D (25:22) vs. control (35:28) | Ban et al. 2014(5) | CTRP3 levels were lower in T2D (150 ng/mL) vs. control (249 ng/mL). CTRP3 was negatively associated with C-reactive protein levels and positively associated with insulin levels in control subjects. No associations were observed in T2D |
| 126 singleton live births (67:59) | Chen et al. 2016 (9) | CTRP3 was positively correlated with Ponderal index (a measurement of thinness) and birth weight |
| Control (40:79), prediabetic (40:71), or T2D (54:65) | Choi et al. 2012 (12) | Overall: CTRP3 levels were positively correlated with total cholesterol, fasting blood glucose, AST and ALT, creatinine, and C-reactive protein levels, and negatively correlated with estimated glomerular filtration rate. CTRP3 increased significantly with T2D (Normal 273, prediabetes 482, and T2D 516 ng/mL) |
| 453 nondiabetic Korean adults (137:316) | Choi et al. 2013 (10) | CTRP3 levels were higher in women than men. Independently associated with age, sex, and triglyceride, LDL cholesterol, adiponectin, and retinol-binding protein 4 (RBP4) levels |
| 362 Korean adults: with acute coronary syndrome (49:20), stable angina pectoris (58:27), or control subjects (137:71) | Choi et al. 2014 (11) | CTRP-3 levels were lower in patients with acute coronary syndrome or stable angina pectoris compared to control subjects. CTRP-3 levels negatively associated with glucose and C-reactive protein positively associated with HDL-cholesterol and adiponectin. |
| Newly diagnosed obese and hypertensive patients (124:83) | Deng et al. 2015 (14) | Regardless of groups women had higher CTRP3 levels than men. Both obesity and High blood pressure were associated with lower CTRP3 levels, there was no further reduction in obesity combined with high BP |
| Obese (33:39) and T2D (34:35) | Flehmig et al. 2014 (16) | No difference in CTRP3 levels with obesity or T2D. Of the 69 T2D, 46 were taking metformin and CTRP3 levels were increased with metformin (267 vs. 343 ng/mL) |
| COPD patients (50:23) and healthy controls (29:25) | Li et al. 2015 (34) | CTRP3 levels (COPD 950 ng/mL; Control 820 ng/mL) had no correlation to lung function. No association between CTRP-3 and CRP, TNF-α, or adiponectin |
| Lean (25:20), obese (19:24), T2D (17:24), and obese+T2D (22:23) | Qu et al. 2015 (52) | No difference in CTRP3 between men and women (397.51 ± 122.67 vs. 416.17 ± 131.24 ng/mL,). CTRP3 levels were reduced in obese and T2D and further reduced in obese+T2D. CTRP3 levels were negatively associated with IL-6 levels, HOMA-IR, and HbA1c |
| Women with Polycystic ovary syndrome (PCOS) | Tan et al. 2013 (65) | CTRP3 levels were lower in women with PCOS (200 ng/mL) compared with control (330 ng/mL); Metformin intervention increased CTRP3 levels to (272 ng/mL). BMI, insulin, LDL, triglycerides, CRP and carotid intima-media thickness were all negatively associated with CTRP3 levels. TNF levels were not associated with CTRP3 |
| Lean (20:40) and obese (6:44) | Wolf et al. 2015 (72) | CTRP3 was inversely associated with BMI and triglyceride levels. Men had significantly lower CTRP3 levels compared to women (397.7 vs. 432 ng/mL, P < 0.01) |
| Control (61:22) vs. metabolic syndrome (32:12) | Yoo et al. 2013 (83) | CTRP3 concentrations exhibit a significant negative association with cardiometabolic risk factors and positive association with adiponectin |
| 16 patients diagnosed with IgA nephropathy (IgAN) compared to 12 controls | Zhang et al. 2016 (86) | CTRP3 levels were lower in patients with IgAN (TGF-β and IL-6 levels were elevated in these patients) |
| Patients with symptoms requiring heart catheterization to diagnosis obstructive CAD (52:48) | Wagner et al. 2016 (66) | No association between obstructive coronary artery disease and CTRP3 levels. CTRP3 levels were higher in lean female than males. CTRP3 levels increased with obesity in males, but decreased with obesity in female patients |
Human studies
To date very few experimental intervention studies have been performed with human subjects. The first, Wurm et al. (2007) (78) examined circulating CTRP3 levels before and 2 hours after a glucose load (n = 20, 14 males and 6 females) and observed no change. However, this study occurred before there were reliable ELISA’s developed for CTRP3 and examined CTRP3 levels solely though immunoblot analysis. Further complicating the results from this study is that although they reported the supplier (R&D Systems) they did not include the catalog number for the antibody used and they reported band migration of 50 kDa for CTRP3 on a denaturing SDS-PAGE. CTRP3 has a predicted and gel migration ~30 kDa and although the posttranslational modifications for CTRP3 could result in a higher than predicted migration pattern on a SDS gel, this has not been observed by other researchers (1, 43, 51, 70, 75). Indeed, the antibody for human CTRP3 from R&D Systems (R and D Systems Cat#AF7925, RRID:AB_2619735) also reports detecting a band at ~30 kDa. Moreover, using an ELISA based method Ban et al. (2014) observed that in type 2 diabetic patients CTRP3 levels decreased from ~150 to 50 ng/ml in response to a 2-hour oral glucose load (5). In light of these data and the progress within the past 9 years in CTRP3 antibody and ELISA development, the effects of glucose on CTRP3 levels in healthy human subject population should be reexamined. In addition, due to the potential role of CTRP3 in lipid metabolism, experiments should also examine the effects of acute lipid loading on circulating CTRP3 levels.
Only one study has examined the effects of exercise on circulating CTRP3 levels in human subjects. Briefly, Choi et al. (2013) examine changes to circulating CTRP3 levels after a 3-month exercise program (45 min cardio/20 min resistance 5×/week) in 76 obese Korean females (10). They found that CTRP3 levels decreased ~15% (444-374 ng/mL) after exercise intervention. The exercise training program also resulted in ~9% loss in body weight, so it is unclear if the change in CTRP3 levels are due to exercise or the reduction in body fat. The effects of acute exercise or exercise training on CTRP3 levels in a healthy human population or in the absence of weight loss have not been investigated.
Lastly, Tan et al. (2013) (65) reported that women with polycystic ovary syndrome had lower levels of CTRP3 than control subjects. Polycystic ovary syndrome is an endocrine system disorder associated with obesity, diabetes, dyslipidemia, and cardiovascular complications and Metformin is a common medication used in the treatment of insulin resistance, obesity and type 2 diabetes. CTRP3 levelswere restored with Metformin treatment along with a general improvement in insulin sensitivity.
CTRP3 and Cardiovascular Disease
Cardiovascular disease (CVD) is the leading cause of death in the world, accounting for 30% of all deaths (17). The identification of novel biomarkers indicating the progression, or signaling pathways which can be exploited as a treatment for CVD is a subject of ongoing interest for combating this disease. It is well established that obesity is a leading risk factor for CVD (63), however very little attention has been given to the endocrine function of adipose tissue and its role in CVD. In both cell culture and animal models CTRP3 has been shown to be protective following heart attack (myocardial infarction, MI) or stroke (intracerebral hemorrhage, ICH) (11,42,67,77,80,81,83,89). Table 6 contains the complete summary of the treatment effects of CTRP3 treatment as it related to CVD. Specifically, CTRP3 stimulates mitochondrial biogenesis in cardiac tissue and promotes vascular relaxation (85,89), whereas immediately following a MI there is a significant decrease of adipose tissue CTRP3 mRNA and circulating CTRP3 protein levels (77, 81). In animals models of MI exogenous CTRP3 pretreatment (adenovirus-delivered or recombinant CTRP3) increases survival, improves postevent cardiac function, and prevents pathological remodeling (77, 81). Progressive remodeling after myocardial infarction (MI) is a leading cause of morbidity and mortality associated with MI. Specifically Transforming growth factor beta (TGF-β) has been reported to be involved in ventricular remodeling by promoting myocardial fibrosis (88). However, CTRP3 attenuates TGF-β1-induced signaling and pathogenic remodeling post-MI both in vivo and in vitro (77). Regarding stroke recovery, preconditioning with CTRP3 reduced cerebral edema, reduced blood-brain barrier damage, improved neurological function, and reduced oxidative stress following ICH (67,80). Collectively, these data illustrate the potential of CTRP3 and CTRP3-mediated signaling pathways as a prospective post-MI/ICH treatment targets.
Table 6.
Summary of Cardiovascular Effects of CTRP3 Treatment
| Model | Reference | Treatment/Measurement | Effect |
|---|---|---|---|
| MSS31 (mouse endothelial cells (79)) | Akiyama (2) et al. (2007) | 1-5 μg/mL [E. coli produced His6-tagged signal peptide removed] | ↑ Proliferation and migration, ↑ ERK1/2 and MAPK p38 within 15 min (2) |
| Rat vascular smooth muscle cells (VSMCs) | Feng et al. 2016 (15) | Recombinant human globular CTRP3 (Aviscera Bioscience, no catalog # provided), or siRNA knockdown of CTRP3 | CTRP3 increase ATP synthesis and oxidative phosphorylation complex proteins, reversed with knockdown. CTRP3 increases mitochondrial ROS |
| Rat vascular smooth muscle cells (VSMCs) | Feng et al. 2016 (15) | Recombinant human globular domain CTRP3 (Aviscera Bioscience, no catalog # provided) | CTRP3 increases mitochondrial Reactive oxygen species levels in VSMC |
| Adventitial fibroblasts | Lin et al. 2014 (42) | CTRP3 protein (10 μg/mL) (source/type of CTRP3 was not reported) | CTRP3 prevents TGF-β1 induced fibroblasts phenotypic conversion, proliferation, migration, and collagen I expression. |
| Primary cultured adult rat cardiac fibroblast (CF) | Wu et al. 2015 (77) | 2 μg/mL recombinant human gCTRP3 (Aviscera Bioscience; 00082-01-100; E. coli expressed); also used siRNA knockdown of CTRP3 | CTRP3 inhibited TGF-β1 induced markers for myofibroblast differentiation and TGF-β1 induced- phosphorylation of Smad3. Whereas, CTRP3 knockdown increased TGF-β1-induced markers for myofibroblast differentiation |
| Rat intracerebral hemorrhage (ICH) | Yang et al. 2016 (80) | +/− lentivirus or recombinant CTRP3 (80 μg/kg, Chimerigen, USA) | Reduced cerebral edema and blood-brain barrier damage and improved neurological functions and reduced oxidative stress via PKA signaling pathway |
| Cardiomyocytes (adult mouse) | Yi et al. 2012 (81) | Bacterial expressed globular domain of Mouse CTRP3, and condition media from 3T3-L1a cell +/− CTRP3 siRNA | CTRP3 upregulated levels of phosphorylated Akt, HIF1α, and VEGF. Cardiomyocyte hypoxia-induced apoptosis was attenuated with condition media from 3t3-L1A adipocytes, effect abolished with CTRP3 siRNA |
| HUVEC | Yi et al. 2012 (81) | Bacterial expressed globular domain of mouse CTRP3 | Condition media from primary cardiomyocytes treated with CTRP3 significantly enhanced HUVEC tube formation. No direct effect of CTRP3 |
| Mouse-myocardial infarction (MI) | Yi et al. 2012 (81) | Adenovirus or bacterial expressed globular domain of mouse CTRP3 | MI reduced CTRP3 levels, CTRP3 pre-treatment improved survival rate, restored cardiac function, increased revascularization, and reduced fibrosis |
| Neonatal rat ventricular myocytes | Zhang et al. 2016 (85) | 0.5–4 μg/mL Human recombinant globular and full-length CTRP3 (Aviscera Bioscience, no catalog #) | CTRP3 increased AMPK activated transcription factors and restored hypoxia-reoxygenation injury induced reduction in sirtuin1, PGC-1α, NRF-1, complex III, and ATP content |
| Aortic rings from C57BL/6 mice | Zheng et al. 2011 (89) | Mammalian expressed CTRP3 (1, 3, and 10 μg/mL) | 3 and 10 (but not 1) μg/mL of mammalian expressed CTRP3 caused significant vasorelaxation |
| Rat cultured carotid arterial rings | Zhou et al. 2014 (90) | (1-4 μg/mL) human globular CTRP3 (Aviscera Bioscience, 00082-01-100) | CTRP3 promotes vascular calcification in high-phosphate cultured carotid arterial rings |
| Rat vascular smooth muscle cells (VSMCs) | Zhou et al. 2014 (90) | (1-4 μg/mL) human globular CTRP3 (Aviscera Bioscience, 00082-01-100) | CTRP3 Promotes β-Glycerophosphate-Induced VSMC Calcification and mitochondrial Reactive oxygen species levels in VSMC |
| Mouse: Adenine-induced chronic renal failure (CRF) | Zhou et al. 2014 (90) | CTRP3 levels measured by ELISA and RT-qPCR or Adenovirus encoding full-length human CTRP3 | Protein CTRP3 levels increased but adipose mRNA expression was reduced with CRF. CTRP3 increased vascular calcification |
CTRP3 is also demonstrated to play a large role in angiogenesis and endothelial cell proliferation (2, 23, 45, 81). For example, during the recovery period following rat carotid artery balloon-injury model CTRP3 expression increases dramatically (35). Interestingly CTRP3 may not act directly on the endothelial cells, but through indirect pathways mediated by cardiac or smooth muscles cells. Direct treatment of Human Umbilical Vein Endothelial Cells (HUVEC) with CTRP3 had no direct effect on capillary-like structures formation (tube formation), Akt phosphorylation or hypoxiainducible factor 1, alpha subunit (HIF1α) orVascular endothelial growth factor (VEGF) expression (81). However, conditioned media from primary cardiomyocytes treated with CTRP3 induced HUVEC tube formation, indicating the presence of CTRP3-induced cardiomyocyte-secreted paracrine factors (81). However, the direct effect of CTRP3 on HUVEC cells cannot be completely ruled out as Yi et al. (2012) (81) used bacterial expressed CTRP3 directly on HUVEC cells and used mammalian expressed CTRP3 in vivo. This discrepancy may indicate a post-translational modification is required for the CTRP3-induced effects on vascular endothelium.
As shown in Table 6, very few studies have examined the association between CVD and circulating CTRP3 levels in a human patient population. Specifically, Wagner et al. 2016 (66) observed no association between the presence of CVD and CTRP3 levels among patients with symptoms requiring catherization for detecting the presence of coronary artery blockage. Whereas, Deng et al. 2015 (14), observed that both obesity and high blood pressure were associated with lower CTRP3 levels and Choi et al. 2014 (11) reported that CTRP3 concentrations in patients with acute coronary syndrome or stable angina pectoris were significantly decreased compared to control subjects. Further, CTRP3 concentrations have been shown to exhibit a significant negative association with many cardiometabolic risk factors (11, 83). As CTRP3 levels decrease with obesity, at least in men (66), the lower levels of CTRP3 may contribute to the impaired mitochondrial biogenesis in cardiac cells and increase the susceptibility/severity of an MI event. On the other hand, excessive sustained elevation of CTRP3 may also be detrimental to cardiovascular health as CTRP3 as promotes vascular and aortic ring calcification (90). Increase vascular calcification promotes arterial hardening and the development of arteriosclerosis. Regardless the associations between CTRP3-mediated signaling and CVD appear promising and need to be investigated in more detail.
Inflammation
Metabolic syndrome is defined as a cluster of risk factors which directly increase the risk of CVD, T2D, and allcause mortality (4, 26). One of the major defining risk factors for metabolic syndrome is a chronic state of low-grade inflammation marked by elevated circulating proinflammatory cytokines (TNF, IL-6, and C-reactive protein). The adipose tissue immune response plays amajor role in the development of the chronic proinflammatory state (22, 26). Whereas, CTRP3 is a potential anti-inflammatory mediator, and is negatively associated with the proinflammatory cytokines TNF, IL-6, and C-reactive protein (5, 11, 12, 14, 48, 50, 52, 65, 83). In animal models, diet-induced obesity results in decreased CTRP3 levels concurrent with an elevation in TNF and IL-6 (50). Similarly, diet-induced obese Ctrp3-knockout mice have an enhanced elevation of IL-6 and TNF levels (71). Further, transgenic overexpression of CTRP3 attenuates the high-fat diet induced rise in cytokine levels and more than doubles circulating soluble gp130 (sgp130) levels (50). Circulating sgp130 is a potent inhibitor of inflammatory cytokines such as IL-6 (24).On another note, although essential for regulating platelet aggregation, excessive levels of the cytokine thromboxane A2 are associated with the development of insulin resistance and arteriosclerosis (18), and are elevated in both human and mouse models of obesity (33). However, loss of Thromboxane synthase (TBXAS), the enzyme necessary to produce thromboxane A2, markedly enhances insulin sensitivity concurrent with the up-regulation of CTRP3, as well as other adipokines (CTRP9, CTRP12) (33). CTRP3 has also demonstrated potential as a treatment for a specific inflammatory disorder, IgA nephropathy (IgAN). IgAN is a kidney disorder caused by deposits of the protein immunoglobulin A (IgA) inside the glomeruli of kidney and excessive mesangial cell activation. In patients with IgAN CTRP3 levels are significantly reduced and the cytokines IL-6 and TGF-β are elevated (86). Treatment of isolated adult human mesangial cells (HMCs) with CTRP3 reduced IL-6 and TGF-β production and inhibited HMC activation (86). Combined these data demonstrate the potential of CTRP3 in the treatment of inflammatory disorders. The summary finding of all studies examining the connection between inflammation and CTRP3 are listed in Table 7.
Table 7.
CTRP3 and Inflammation
| Model | Reference | Type of treatment | Effect |
|---|---|---|---|
| Human primary colonic lamina propria fibroblasts (CLPF) | Hofmann et al. 2011 (21) | Recombinant CTRP-3 (high-five insect cells) | Significantly and dose-dependently reduced LPS-induced IL-8 secretion in CLPF within 8 hours after LPS exposure, whereas LPS-induced IL-6 and TNF release was not affected. CTRP-3 inhibited TGF-β production and the expression of CTGF and collagen I in CLPF, whereas collagen III expression remained unchanged (21) |
| Primary human monocytes | Kopp et al. 2010 (30) | 1 μg/mL recombinant full length CTRP3 (H5 insect cells) | Suppressed LPS-induced MIF & CCL4 release in lean subjects, and MCP-1 in monocytes from both diabetic and leans subjects. Prevents Lauric acid induced release of IL-6 and TNF |
| Primary human monocytes | Kopp et al. 2010 (31) | 1 μg/mL recombinant full length CTRP3 (H5 insect cells) | ↓ LPS-induced secretion of IL-6 in monocytes from control but not T2D. CTRP3 did not significantly affect TNF secretion |
| Adipocyte (3T3-L1) | Li et al. 2014 (38) | Recombinant CTRP3 (uncited origin) | Decreased TNF and IL6 secretion in insulin resistant 3T3-L1A |
| Transgenic CTRP3 overexpression and CTRP3 knockout | Petersen et al. 2016 (48) | Response to sublethal dose of LPS challenge | No effect on IL-1β, IL-6, TNF-α, or MIP-2 induction |
| LPS (1 μg i.p.) into male mice +/− CTRP3 | Schmid et al. 2014 (60) | Recombinant CTRP-3 (H5 insect cells) | Intraperitoneal injection (but not IV) injection of CTRP-3 significantly reduced LPS-induced IL-6 and levels MIP-2 |
| 16 patients diagnosed with IgA nephropathy (IgAN) compared to 12 controls | Zhang et al. 2016 (86) | CTRP3 levels were lower in patients with IgAN (TGF-B and IL-6 levels were elevated in the patients) | |
| THP-1 (human—acute monocytic leukemia cell line) | Weigert et al. 2005 (70) | 1 μg/mL recombinant full length CTRP3 (H5 insect cells) | Suppressed LPS induced: IL-6 and TNF expression and secretion |
LPS is a well-established endotoxin used as a model of systemic immune response, in vivo. In cell culture models LPS inhibits adipose tissue differentiation, induces insulin resistance, and prevents the expression of CTRP3 (59). Whereas, CTRP3 has been shown to specifically block the binding of LPS to its receptor TLR4 (13,30) and inhibit the inflammatory response (30). Further, in animalmodels intraperitoneal injection (IP) injection of CTRP3 (0.4ug/g body weight) significantly reduced the LPS-induced (IP) inflammatory response (65). Although intravenous administration of CTRP3 was unable to prevent LPS-induced inflammation, this is thought to be a dose related limitation and future studies are ongoing to determine the clinically relevant levels of CTRP3 administration needed to inhibit a systematic immune response. Further, CTRP3 potently inhibited LPS-induced IL-6 in monocytes isolated from healthy subjects but not in monocytes derived from subject with T2D (31, 70). Similarly, the anti-inflammatory effects of CTRP3 have also been observed in isolated primary human colonic fibroblasts (21). Combined, these data show promise for the use of CTRP3 and CTRP3-mediated pathways as a potential anti-inflammatory mediator. However, neither chronic CTRP3 deficiency nor overexpression altered the inflammatory response to a sublethal challenge to LPS (48), indicating that CTRP3 levels need to be regulated transiently to demonstrate an anti-inflammatory effect. Regardless, the potential use of CTRP3 as an inhibitor of inflammation needs to be examined in more detail.
Growth, Reproduction, and Tumorigenesis
CTRP3 has been linked to normal and pathogenic cellular proliferation, growth and development. CTRP3 has been consistently shown to stimulate the proliferation of chondrogenic precursors, chondrocytes, and osteocytes, in vitro, through activation of ERK1/2 and PI3K pathways (1, 3, 27, 44, 82). During early development CTRP3 is detected in developing chondrocytes (43) and cartilage (44) which has led to the hypothesis that CTRP3 Is essential for normal cartilage and bone development. In support of this CTRP3 is appears to be essential for proper mandible formation through perichondrium maintenance and new cartilage formation (82). However, the role of CTRP in development may be dispensatory as CTRP3-knockout animals do not demonstrate a growth phenotype indicating a possible compensatory mechanism during development. However, CTRP3-knockout mice do exhibit increased susceptibility to collagen-induced arthritis, indicating CTRP3 may be important for the maintenance and repair of cartilage tissue (46). However, elevated CTRP3 may also be detrimental as CTRP3 is highly secreted from and increases growth rates of osteosarcoma and chondroblastoma tumor cell lines indicating that CTRP3 may be linked to these types of cancers (54).However, no cancers have been reported in CTRP3 transgenic overexpressing mice, although this has not been specifically examined. Lastly, CTRP3 is specifically expressed in the testosterone producing interstitial Leydig cells and treatment of TM3 mouse Leydig cells with CTRP3 increased testosterone production (47). Although these findings may shed some light on the discrepancy between male and female in circulating CTRP3 levels, the overall clinical significance has yet to be explored.
Summary and Conclusion
Adipose tissue secretes numerous adipokines that contribute to a wide array of biological processes. CTRP3, is a novel and unique member of the adipokine superfamily which appears to have a unique roles in a variety of tissue: hepatic lipid metabolism, cardiovascular response to ischemia, and chondrocyte proliferation and differentiation. CTRP3 can function in an autocrine, paracrine, and endocrinemanner and there are many aspects of CTRP3’s regulation and function that have yet to be explored. In addition, CTRP3 has been consistently linked to activation of the PKA signaling pathway, regardless of the tissue/treatment paradigm examined (37, 47, 80). This review should serve as a basis for the design of future experimental studies specifically examining: (i) the regulation of CTRP3 in response to food intake or exercise, (ii) associations between circulating CTRP3 levels, including the two different splice variants (CTRP3A and CTRP3B), and hepatic steatosis or osteoscarcoma in clinical population, and (iii) the associations between the different CTRP3 isoforms (CTRP3A and CTRP3B) and multimeric structures in clinical populations, (iv) the potential use of CTRP3 as an inhibitor of inflammation, and (v) whether the putative receptors, LAMP1 and LIMPII are directly responsible for the intracellular effects of CTRP3 or at as coreceptors for a yet unidentified protein. Finally, the gender-specific regulation of CTRP3 needs to be explored in more detail (splice variants and multimeric structures) especially in regards to the association between CTRP3 and human diseases such as non-alcoholic fatty liver disease, T2D, and metabolic syndrome.
Didactic Synopsis.
This article summarizes the current research on CTRP3 at the graduate level.
Because of its size and repeating amino acid sequence in the collagen domain CTRP3 was originally named CORS26 (Collagenous repeat-containing sequence 26 kDa protein), but later renamed as CTRP3 as it is one of many members of a protein family designated as C1q TNF Related Proteins. CTRP3 is a secreted protein and circulates in the blood.
Peroxisome proliferator-activated receptor-gamma (PPAR-γ), specificity protein 1 (SP-1), and c-FOS decreased CTRP3 expression and the transcription factor c-Jun is the only known transcription factor which increases CTRP3 expression.
CTRP3 increases liver lipid metabolism, reduces the amount of damage and improves recovery following a heart attack, and inhibits inflammation.
Although research indicates that CTRP3 appears to prevent arthritis and repair cartilage, CTRP3 may also increase the development of certain types of bone cancers osteosarcoma and chondroblastoma.
The function of CTRP3 is still being actively investigated as well as its role in human health especially in regards to Fatty liver and metabolic syndrome.
References
- 1.Akiyama H, Furukawa S, Wakisaka S, Maeda T. Cartducin stimulates mesenchymal chondroprogenitor cell proliferation through both extracellular signal-regulated kinase and phosphatidylinositol 3-kinase/Akt pathways. FEBS J. 2006;273:2257–2263. doi: 10.1111/j.1742-4658.2006.05240.x. [DOI] [PubMed] [Google Scholar]
- 2.Akiyama H, Furukawa S, Wakisaka S, Maeda T. CTRP3/cartducin promotes proliferation and migration of endothelial cells. Mol Cell Biochem. 2007;304:243–248. doi: 10.1007/s11010-007-9506-6. [DOI] [PubMed] [Google Scholar]
- 3.Akiyama H, Furukawa S, Wakisaka S, Maeda T. Elevated expression of CTRP3/cartducin contributes to promotion of osteosarcoma cell proliferation. Oncol Rep. 2009;21:1477–1481. doi: 10.3892/or_00000377. [DOI] [PubMed] [Google Scholar]
- 4.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 5.Ban B, Bai B, Zhang M, Hu J, Ramanjaneya M, Tan BK, Chen J. Low serum cartonectin/CTRP3 concentrations in newly diagnosed type 2 diabetes mellitus: In vivo regulation of cartonectin by glucose. PloS One. 2014;9:e112931. doi: 10.1371/journal.pone.0112931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bannister AJ, Brown HJ, Sutherland JA, Kouzarides T. Phosphorylation of the c-Fos and c-Jun HOB1 motif stimulates its activation capacity. Nucleic Acids Res. 1994;22:5173–5176. doi: 10.1093/nar/22.24.5173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Byerly MS, Petersen PS, Ramamurthy S, Seldin MM, Lei X, Provost E, Wei Z, Ronnett GV, Wong GW. C1q/TNF-related protein 4 (CTRP4) is a unique secreted protein with two tandem C1q domains that functions in the hypothalamus to modulate food intake and body weight. J Biol Chem. 2014;289:4055–4069. doi: 10.1074/jbc.M113.506956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Byerly MS, Swanson R, Wei Z, Seldin MM, McCulloh PS, Wong GW. A central role for C1q/TNF-related protein 13 (CTRP13) in modulating food intake and body weight. PLoS One. 2013;8:e62862. doi: 10.1371/journal.pone.0062862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen NN, He JR, Li WD, Kuang YS, Yuan MY, Liu XD, Zhang HZ, Hu SP, Xia HM, Qiu X. C1q and tumor necrosis factor-related protein 3 is present in human cord blood and is associated with fetal growth. Clin Chim Acta. 2016;453:67–70. doi: 10.1016/j.cca.2015.11.031. [DOI] [PubMed] [Google Scholar]
- 10.Choi HY, Park JW, Lee N, Hwang SY, Cho GJ, Hong HC, Yoo HJ, Hwang TG, Kim SM, Baik SH, Park KS, Youn BS, Choi KM. Effects of a combined aerobic and resistance exercise program on C1q/TNF-related protein-3 (CTRP-3) and CTRP-5 levels. Diabetes Care. 2013;36:3321–3327. doi: 10.2337/dc13-0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi KM, Hwang SY, Hong HC, Choi HY, Yoo HJ, Youn BS, Baik SH, Seo HS. Implications of C1q/TNF-related protein-3 (CTRP-3) and progranulin in patients with acute coronary syndrome and stable angina pectoris. Cardiovasc Diabetol. 2014;13:14. doi: 10.1186/1475-2840-13-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi KM, Hwang SY, Hong HC, Yang SJ, Choi HY, Yoo HJ, Lee KW, Nam MS, Park YS, Woo JT, Kim YS, Choi DS, Youn BS, Baik SH. C1q/TNF-related protein-3 (CTRP-3) and pigment epitheliumderived factor (PEDF) concentrations in patients with type 2 diabetes and metabolic syndrome. Diabetes. 2012;61:2932–2936. doi: 10.2337/db12-0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Compton SA, Cheatham B. CTRP-3: Blocking a toll booth to obesityrelated inflammation. Endocrinology. 2010;151:5095–5097. doi: 10.1210/en.2010-0916. [DOI] [PubMed] [Google Scholar]
- 14.Deng W, Li C, Zhang Y, Zhao J, Yang M, Tian M, Li L, Zheng Y, Chen B, Yang G. Serum C1q/TNF-related protein-3 (CTRP3) levels are decreased in obesity and hypertension and are negatively correlated with parameters of insulin resistance. Diabetol Metab Syndr. 2015;7:33. doi: 10.1186/s13098-015-0029-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng H, Wang JY, Zheng M, Zhang CL, An YM, Li L, Wu LL. CTRP3 promotes energy production by inducing mitochondrial ROS and upexpression of PGC-1alpha in vascular smooth muscle cells. Exp Cell Res. 2016;341:177–186. doi: 10.1016/j.yexcr.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Flehmig G, Scholz M, Kloting N, Fasshauer M, Tonjes A, Stumvoll M, Youn BS, Bluher M. Identification of adipokine clusters related to parameters of fat mass, insulin sensitivity and inflammation. PLoS One. 2014;9:e99785. doi: 10.1371/journal.pone.0099785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaziano T, Reddy KS, Paccaud F, Horton S, Chaturvedi V. Cardiovascular disease. In: Jamison DT, Breman JG, Measham AR, Alleyne G, Claeson M, Evans DB, Jha P, Mills A, Musgrove P, editors. Disease Control Priorities in Developing Countries. 2. Washington (DC): World Bank; 2006. [Google Scholar]
- 18.Graziani F, Biasucci LM, Cialdella P, Liuzzo G, Giubilato S, Della Bona R, Pulcinelli FM, Iaconelli A, Mingrone G, Crea F. Thromboxane production in morbidly obese subjects. Am J Cardiol. 2011;107:1656–1661. doi: 10.1016/j.amjcard.2011.01.053. [DOI] [PubMed] [Google Scholar]
- 19.Hada Y, Yamauchi T, Waki H, Tsuchida A, Hara K, Yago H, Miyazaki O, Ebinuma H, Kadowaki T. Selective purification and characterization of adiponectin multimer species from human plasma. Biochem Biophys Res Commun. 2007;356:487–493. doi: 10.1016/j.bbrc.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 20.Hirose H, Yamamoto Y, Seino-Yoshihara Y, Kawabe H, Saito I. Serum high-molecular-weight adiponectin as a marker for the evaluation and care of subjects with metabolic syndrome and related disorders. J Atheroscler Thromb. 2010;17:1201–1211. doi: 10.5551/jat.6106. [DOI] [PubMed] [Google Scholar]
- 21.Hofmann C, Chen N, Obermeier F, Paul G, Buchler C, Kopp A, Falk W, Schaffler A. C1q/TNF-related protein-3 (CTRP-3) is secreted by visceral adipose tissue and exerts antiinflammatory and antifibrotic effects in primary human colonic fibroblasts. Inflamm Bowel Dis. 2011;17:2462–2471. doi: 10.1002/ibd.21647. [DOI] [PubMed] [Google Scholar]
- 22.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: Direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 23.Hou Q, Lin J, Huang W, Li M, Feng J, Mao X. CTRP3 stimulates proliferation and anti-apoptosis of prostate cells through PKC signaling pathways. PLoS One. 2015;10:e0134006. doi: 10.1371/journal.pone.0134006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jostock T, Mullberg J, Ozbek S, Atreya R, Blinn G, Voltz N, Fischer M, Neurath MF, Rose-John S. Soluble gp130 is the natural inhibitor of soluble interleukin-6 receptor transsignaling responses. Eur J Biochem. 2001;268:160–167. doi: 10.1046/j.1432-1327.2001.01867.x. [DOI] [PubMed] [Google Scholar]
- 25.Kambara T, Ohashi K, Shibata R, Ogura Y, Maruyama S, Enomoto T, Uemura Y, Shimizu Y, Yuasa D, Matsuo K, Miyabe M, Kataoka Y, Murohara T, Ouchi N. CTRP9 protein protects against myocardial injury following ischemia-reperfusion through AMP-activated protein kinase (AMPK)-dependent mechanism. J Biol Chem. 2012;287:18965–18973. doi: 10.1074/jbc.M112.357939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaur JA. comprehensive review on metabolic syndrome. Cardiol Res Pract. 2014;2014:943162. doi: 10.1155/2014/943162. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27.Kim JY, Min JY, Baek JM, Ahn SJ, Jun HY, Yoon KH, Choi MK, Lee MS, Oh J. CTRP3 acts as a negative regulator of osteoclastogenesis through AMPK-c-Fos-NFATc1 signaling in vitro and RANKL-induced calvarial bone destruction in vivo. Bone. 2015;79:242–251. doi: 10.1016/j.bone.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 28.Kim MJ, Park EJ, Lee W, Kim JE, Park SY. Regulation of the transcriptional activation of CTRP3 in chondrocytes by c-Jun. Mol Cell Biochem. 2012;368:111–117. doi: 10.1007/s11010-012-1349-0. [DOI] [PubMed] [Google Scholar]
- 29.Klonisch T, Glogowska A, Thanasupawat T, Burg M, Krcek J, Pitz M, Jaggupilli A, Chelikani P, Wong GW, Hombach-Klonisch S. Structural commonality of C1q Tumor Necrosis Factor-related proteins and their potential to activate RXFP1 signaling pathways in cancer cells. Br J Pharmacol. 2016 doi: 10.1111/bph.13559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kopp A, Bala M, Buechler C, Falk W, Gross P, Neumeier M, Scholmerich J, Schaffler A. C1q/TNF-related protein-3 represents a novel and endogenous lipopolysaccharide antagonist of the adipose tissue. Endocrinology. 2010;151:5267–5278. doi: 10.1210/en.2010-0571. [DOI] [PubMed] [Google Scholar]
- 31.Kopp A, Bala M, Weigert J, Buchler C, Neumeier M, Aslanidis C, Scholmerich J, Schaffler A. Effects of the new adiponectin paralogous protein CTRP-3 and of LPS on cytokine release from monocytes of patients with type 2 diabetes mellitus. Cytokine. 2010;49:51–57. doi: 10.1016/j.cyto.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 32.Lara-Castro C, Luo N, Wallace P, Klein RL, Garvey WT. Adiponectin multimeric complexes and themetabolic syndrome trait cluster. Diabetes. 2006;55:249–259. [PubMed] [Google Scholar]
- 33.Lei X, Li Q, Rodriguez S, Tan SY, Seldin MM, McLenithan JC, Jia W, Wong GW. Thromboxane synthase deficiency improves insulin action and attenuates adipose tissue fibrosis. Am J Physiol Endocrinol Metab. 2015;308:E792–E804. doi: 10.1152/ajpendo.00383.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li D, Wu Y, Tian P, Zhang X, Wang H, Wang T, Ying B, Wang L, Shen Y, Wen F. Adipokine CTRP-5 as a potential novel inflammatory biomarker in chronic obstructive pulmonary disease. Medicine (Baltimore) 2015;94:e1503. doi: 10.1097/MD.0000000000001503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li JM, Zhang X, Nelson PR, Odgren PR, Nelson JD, Vasiliu C, Park J, Morris M, Lian J, Cutler BS, Newburger PE. Temporal evolution of gene expression in rat carotid artery following balloon angioplasty. J Cell Biochem. 2007;101:399–410. doi: 10.1002/jcb.21190. [DOI] [PubMed] [Google Scholar]
- 36.Li W, Cowley A, Uludag M, Gur T, McWilliam H, Squizzato S, Park YM, Buso N, Lopez R. The EMBL-EBI bioinformatics web and programmatic tools framework. Nucleic Acids Res. 2015;43:W580–W584. doi: 10.1093/nar/gkv279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li X, Jiang L, Yang M, Wu Y, Sun S, Sun J. GLP-1 receptor agonist increases the expression of CTRP3, a novel adipokine, in 3T3-L1 adipocytes through PKA signal pathway. J Endocrinol Invest. 2015;38:73–79. doi: 10.1007/s40618-014-0156-8. [DOI] [PubMed] [Google Scholar]
- 38.Li X, Jiang L, Yang M, Wu YW, Sun JZ, Sun SX. CTRP3 improves the insulin sensitivity of 3T3-L1 adipocytes by inhibiting inflammation and ameliorating insulin signalling transduction. Endokrynol Pol. 2014;65:252–258. doi: 10.5603/EP.2014.0034. [DOI] [PubMed] [Google Scholar]
- 39.Li X, Jiang L, Yang M, Wu YW, Sun SX, Sun JZ. CTRP3 modulates the expression and secretion of adipokines in 3T3-L1 adipocytes. Endocr J. 2014;61:1153–1162. doi: 10.1507/endocrj.EJ14-0161. [DOI] [PubMed] [Google Scholar]
- 40.Li X, Jiang L, Yang M, Wu YW, Sun SX, Sun JZ. Expression of CTRP3, a novel adipokine, in rats at different pathogenic stages of type 2 diabetes mellitus and the impacts of GLP-1 receptor agonist on it. J Diabetes Res. 2014;2014 doi: 10.1155/2014/398518. 398518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li Y, Ozment T, Wright GL, Peterson JM. Identification of putative receptors for the novel adipokine CTRP3 using ligand-receptor capture technology. Plos One. 2016;11:e0164593. doi: 10.1371/journal.pone.0164593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin S, Ma S, Lu P, Cai W, Chen Y, Sheng J. Effect of CTRP3 on activation of adventitial fibroblasts induced by TGF-beta1 from rat aorta in vitro. Int J Clin Exp Pathol. 2014;7:2199–2208. [PMC free article] [PubMed] [Google Scholar]
- 43.Maeda T, Abe M, Kurisu K, Jikko A, Furukawa S. Molecular cloning and characterization of a novel gene, CORS26, encoding a putative secretory protein and its possible involvement in skeletal development. J Biol Chem. 2001;276:3628–3634. doi: 10.1074/jbc.M007898200. [DOI] [PubMed] [Google Scholar]
- 44.Maeda T, Jikko A, Abe M, Yokohama-Tamaki T, Akiyama H, Furukawa S, Takigawa M, Wakisaka S. Cartducin,a paralog ofAcrp30/adiponectin, is induced during chondrogenic differentiation and promotes proliferation of chondrogenic precursors and chondrocytes. J Cell Physiol. 2006;206:537–544. doi: 10.1002/jcp.20493. [DOI] [PubMed] [Google Scholar]
- 45.Maeda T, Wakisaka S. CTRP3/cartducin is induced by transforming growth factor-beta1 and promotes vascular smooth muscle cell proliferation. Cell Biol Int. 2010;34:261–266. doi: 10.1042/CBI20090043. [DOI] [PubMed] [Google Scholar]
- 46.Murayama MA, Kakuta S, Maruhashi T, Shimizu K, Seno A, Kubo S, Sato N, Saijo S, Hattori M, Iwakura Y. CTRP3 plays an important role in the development of collagen-induced arthritis in mice. Biochem Biophys Res Commun. 2014;443:42–48. doi: 10.1016/j.bbrc.2013.11.040. [DOI] [PubMed] [Google Scholar]
- 47.Otani M, Kogo M, Furukawa S, Wakisaka S, Maeda T. The adiponectin paralog C1q/TNF-related protein 3 (CTRP3) stimulates testosterone production through the cAMP/PKA signaling pathway. Cytokine. 2012;58:238–244. doi: 10.1016/j.cyto.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 48.Petersen PS, Wolf RM, Lei X, Peterson JM, Wong GW. Immunomodulatory roles of CTRP3 in endotoxemia and metabolic stress. Physiol Rep. 2016;4 doi: 10.14814/phy2.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peterson JM, Aja S, Wei Z, Wong GW. CTRP1 protein enhances fatty acid oxidation via AMP-activated protein kinase (AMPK) activation and acetyl-CoA carboxylase (ACC) inhibition. J Biol Chem. 2012;287:1576–1587. doi: 10.1074/jbc.M111.278333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peterson JM, Seldin MM, Wei Z, Aja S, Wong GW. CTRP3 attenuates diet-induced hepatic steatosis by regulating triglyceride metabolism. Am J Physiol Gastrointest Liver Physiol. 2013;305:G214–G224. doi: 10.1152/ajpgi.00102.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Peterson JM, Wei Z, Wong GW. C1q/TNF-related protein-3 (CTRP3), a novel adipokine that regulates hepatic glucose output. J Biol Chem. 2010;285:39691–39701. doi: 10.1074/jbc.M110.180695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Qu H, Deng M, Wang H, Wei H, Liu F, Wu J, Deng H. Plasma CTRP-3 concentrations in Chinese patients with obesity and type II diabetes negatively correlate with insulin resistance. J Clin Lipidol. 2015;9:289–294. doi: 10.1016/j.jacl.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 53.Schaffler A, Ehling A, Neumann E, Herfarth H, Paul G, Tarner I, Gay S, Buechler C, Scholmerich J, Muller-Ladner U. Role of specificity protein-1, PPARgamma, and pituitary protein transcription factor-1 in transcriptional regulation of the murine CORS-26 promoter. Biochim Biophys Acta. 2004;1678:150–156. doi: 10.1016/j.bbaexp.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 54.Schaffler A, Ehling A, Neumann E, Herfarth H, Paul G, Tarner I, Gay S, Scholmerich J, Muller-Ladner U. Genomic organization, promoter, amino acid sequence, chromosomal localization, and expression of the human gene for CORS-26 (collagenous repeat-containing sequence of 26-kDa protein) Biochim Biophys Acta. 2003;1630:123–129. doi: 10.1016/j.bbaexp.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 55.Schaffler A, Ehling A, Neumann E, Herfarth H, Tarner I, Gay S, Scholmerich J, Muller-Ladner U. Genomic organization, chromosomal localization and adipocytic expression of the murine gene for CORS-26 (collagenous repeat-containing sequence of 26 kDa protein) Biochim Biophys Acta. 2003;1628:64–70. doi: 10.1016/s0167-4781(03)00114-3. [DOI] [PubMed] [Google Scholar]
- 56.Schaffler A, Weigert J, Neumeier M, Scholmerich J, Buechler C. Regulation and function of collagenous repeat containing sequence of 26-kDa protein gene product “cartonectin”. Obesity (Silver Spring) 2007;15:303–313. doi: 10.1038/oby.2007.566. [DOI] [PubMed] [Google Scholar]
- 57.Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. Journal of Biological chemistry. 1995;270:26746–26749. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 58.Schmid A, Kopp A, Aslanidis C, Wabitsch M, Muller M, Schaffler A. Regulation and function of C1Q/TNF-related protein-5 (CTRP-5) in the context of adipocyte biology. Exp Clin Endocrinol Diabetes. 2013;121:310–317. doi: 10.1055/s-0032-1333299. [DOI] [PubMed] [Google Scholar]
- 59.Schmid A, Kopp A, Hanses F, Bala M, Muller M, Schaffler A. The novel adipokine C1q/TNF-related protein-3 is expressed in human adipocytes and regulated by metabolic and infection-related parameters. Exp Clin Endocrinol Diabetes. 2012;120:611–617. doi: 10.1055/s-0032-1323803. [DOI] [PubMed] [Google Scholar]
- 60.Schmid A, Kopp A, Hanses F, Karrasch T, Schaffler A. C1q/TNF-related protein-3 (CTRP-3) attenuates lipopolysaccharide (LPS)-induced systemic inflammation and adipose tissue Erk-1/-2 phosphorylation in mice in vivo. Biochem Biophys Res Commun. 2014;452:8–13. doi: 10.1016/j.bbrc.2014.06.054. [DOI] [PubMed] [Google Scholar]
- 61.Seldin MM, Tan SY, Wong GW. Metabolic function of the CTRP family of hormones. Rev Endocr Metab Disord. 2014;15:111–123. doi: 10.1007/s11154-013-9255-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shapiro L, Scherer PE. The crystal structure of a complement-1q family protein suggests an evolutionary link to tumor necrosis factor. Current Biology. 1998;8:335–340. doi: 10.1016/s0960-9822(98)70133-2. [DOI] [PubMed] [Google Scholar]
- 63.Smith SC., Jr Multiple risk factors for cardiovascular disease and diabetes mellitus. Am J Med. 2007;120:S3–S11. doi: 10.1016/j.amjmed.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 64.Su H, Yuan Y, Wang XM, Lau WB, Wang Y, Wang X, Gao E, Koch WJ, Ma XL. Inhibition of CTRP9, a novel and cardiac-abundantly expressed cell survival molecule, by TNFalpha-initiated oxidative signaling contributes to exacerbated cardiac injury in diabetic mice. Basic Res Cardiol. 2013;108:315. doi: 10.1007/s00395-012-0315-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.TanB K, Chen J, Hu J, Amar O, Mattu HS, Adya R, Patel V, Ramanjaneya M, Lehnert H, Randeva HS. Metformin increases the novel adipokine cartonectin/CTRP3 in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2013;98:E1891–E1900. doi: 10.1210/jc.2013-2227. [DOI] [PubMed] [Google Scholar]
- 66.Wagner RM, Sivagnanam K, Clark WA, Peterson JM. Divergent relationship of circulating CTRP3 levels between obesity and gender: a cross-sectional study. PeerJ. 2016;4:e2573. doi: 10.7717/peerj.2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang S, Zhou Y, Yang B, Li L, Yu S, Chen Y, Zhu J. C1q/tumor necrosis factor-related protein-3 attenuates brain injury after intracerebral hemorrhage via AMPK-dependent pathway in rat. Front Cell Neurosci. 2016;10:237. doi: 10.3389/fncel.2016.00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wei Z, Peterson JM, Lei X, Cebotaru L, Wolfgang MJ, Baldeviano GC, Wong GW. C1q/TNF-related protein-12 (CTRP12), a novel adipokine that improves insulin sensitivity and glycemic control in mouse models of obesity and diabetes. J Biol Chem. 2012;287:10301–10315. doi: 10.1074/jbc.M111.303651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wei Z, Peterson JM, Wong GW. Metabolic regulation by C1q/TNFrelated protein-13 (CTRP13): activation OF AMP-activated protein kinase and suppression of fatty acid-induced JNK signaling. J Biol Chem. 2011;286:15652–15665. doi: 10.1074/jbc.M110.201087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Weigert J, Neumeier M, Schaffler A, Fleck M, Scholmerich J, Schutz C, Buechler C. The adiponectin paralog CORS-26 has anti-inflammatory properties and is produced by human monocytic cells. FEBS Lett. 2005;579:5565–5570. doi: 10.1016/j.febslet.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 71.Wolf RM, Lei X, Yang ZC, Nyandjo M, Tan SY, Wong GW. CTRP3 deficiency reduces liver size and alters IL-6 and TGFbeta levels in obese mice. Am J Physiol Endocrinol Metab. 2016;310:E332–E345. doi: 10.1152/ajpendo.00248.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wolf RM, Steele KE, Peterson LA, Magnuson TH, Schweitzer MA, Wong GW. Lower circulating C1q/TNF-related protein-3 (CTRP3) levels are associated with obesity: A cross-sectional study. PLoS One. 2015;10:e0133955. doi: 10.1371/journal.pone.0133955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wolfing B, Buechler C, Weigert J, Neumeier M, Aslanidis C, Schoelmerich J, Schaffler A. Effects of the new C1q/TNF-related protein (CTRP-3) “cartonectin” on the adipocytic secretion of adipokines. Obesity (Silver Spring) 2008;16:1481–1486. doi: 10.1038/oby.2008.206. [DOI] [PubMed] [Google Scholar]
- 74.Wong GW, Krawczyk SA, Kitidis-Mitrokostas C, Ge G, Spooner E, Hug C, Gimeno R, Lodish HF. Identification and characterization of CTRP9, a novel secreted glycoprotein, from adipose tissue that reduces serum glucose in mice and forms heterotrimers with adiponectin. FASEB J. 2009;23:241–258. doi: 10.1096/fj.08-114991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wong GW, Krawczyk SA, Kitidis-Mitrokostas C, Revett T, Gimeno R, Lodish HF. Molecular, biochemical and functional characterizations of C1q/TNF family members: adipose-tissue-selective expression patterns, regulation by PPAR-gamma agonist, cysteine-mediated oligomerizations, combinatorial associations and metabolic functions. Biochem J. 2008;416:161–177. doi: 10.1042/BJ20081240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wong GW, Wang J, Hug C, Tsao TS, Lodish HF. A family of Acrp30/adiponectin structural and functional paralogs. Proc Natl Acad Sci U S A. 2004;101:10302–10307. doi: 10.1073/pnas.0403760101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wu D, Lei H, Wang JY, Zhang CL, Feng H, Fu FY, Li L, Wu LL. CTRP3 attenuates post-infarct cardiac fibrosis by targeting Smad3 activation and inhibiting myofibroblast differentiation. J MolMed (Berl) 2015;93:1311–1325. doi: 10.1007/s00109-015-1309-8. [DOI] [PubMed] [Google Scholar]
- 78.Wurm S, Neumeier M, Weigert J, Schaffler A, Buechler C. Plasma levels of leptin, omentin, collagenous repeat-containing sequence of 26-kDa protein (CORS-26) and adiponectin before and after oral glucose uptake in slim adults. Cardiovasc Diabetol. 2007;6:7. doi: 10.1186/1475-2840-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yanai N, Satoh T, Obinata M. Endothelial cells create a hematopoietic inductive microenvironment preferential to erythropoiesis in the mouse spleen. Cell Struct Funct. 1991;16:87–93. doi: 10.1247/csf.16.87. [DOI] [PubMed] [Google Scholar]
- 80.Yang B, Wang S, Yu S, Chen Y, Li L, Zhang H, Zhao Y. C1q/tumor necrosis factor-related protein 3 inhibits oxidative stress during intracerebral hemorrhage via PKA signaling. Brain Res. 2017;1657:176–184. doi: 10.1016/j.brainres.2016.11.016. [DOI] [PubMed] [Google Scholar]
- 81.Yi W, Sun Y, Yuan Y, Lau WB, Zheng Q, Wang X, Wang Y, Shang X, Gao E, Koch WJ, Ma XL. C1q/tumor necrosis factor-related protein-3, a newly identified adipokine, is a novel antiapoptotic, proangiogenic, and cardioprotective molecule in the ischemic mouse heart. Circulation. 2012;125:3159–3169. doi: 10.1161/CIRCULATIONAHA.112.099937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yokohama-Tamaki T, Maeda T, Tanaka TS, Shibata S. Functional analysis of CTRP3/cartducin in Meckel’s cartilage and developing condylar cartilage in the fetal mouse mandible. J Anat. 2011;218:517–533. doi: 10.1111/j.1469-7580.2011.01354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yoo HJ, Hwang SY, Hong HC, Choi HY, Yang SJ, Choi DS, Baik SH, Bluher M, Youn BS, Choi KM. Implication of progranulin and C1q/TNF-related protein-3 (CTRP3) on inflammation and atherosclerosis in subjects with or without metabolic syndrome. PLoS One. 2013;8:e55744. doi: 10.1371/journal.pone.0055744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yuasa D, Ohashi K, Shibata R, Mizutani N, Kataoka Y, Kambara T, Uemura Y, Matsuo K, Kanemura N, Hayakawa S, Hiramatsu-Ito M, Ito M, Ogawa H, Murate T, Murohara T, Ouchi N. C1q/TNF-related protein-1 functions to protect against acute ischemic injury in the heart. FASEB J. 2016;30:1065–1075. doi: 10.1096/fj.15-279885. [DOI] [PubMed] [Google Scholar]
- 85.Zhang CL, Feng H, Li L, Wang JY, Wu D, Hao YT, Wang Z, Zhang Y, Wu LL. Globular CTRP3 promotes mitochondrial biogenesis in cardiomyocytes through AMPK/PGC-1alpha pathway. Biochim Biophys Acta. 2016;1861:3085–3094. doi: 10.1016/j.bbagen.2016.10.022. [DOI] [PubMed] [Google Scholar]
- 86.Zhang R, Zhong L, Zhou J, Peng Y. Complement-C1q TNFrelated protein 3 alleviates mesangial cell activation and inflammatory response stimulated by secretory IgA. Am J Nephrol. 2016;43:460–468. doi: 10.1159/000446353. [DOI] [PubMed] [Google Scholar]
- 87.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 88.Zhao M, Zheng S, Yang J, Wu Y, Ren Y, Kong X, Li W, Xuan J. Suppression of TGF-beta1/Smad signaling pathway by sesamin contributes to the attenuation of myocardial fibrosis in spontaneously hypertensive rats. PLoS One. 2015;10:e0121312. doi: 10.1371/journal.pone.0121312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zheng Q, Yuan Y, Yi W, LauW B, Wang Y, Wang X, Sun Y, Lopez BL, Christopher TA, Peterson JM, Wong GW, Yu S, Yi D, Ma XL. C1q/TNFrelated proteins, a family of novel adipokines, induce vascular relaxation through the adiponectin receptor-1/AMPK/eNOS/nitric oxide signaling pathway. Arterioscler Thromb Vasc Biol. 2011;31:2616–2623. doi: 10.1161/ATVBAHA.111.231050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhou Y, Wang JY, Feng H, Wang C, Li L, Wu D, Lei H, Li H, Wu LL. Overexpression of c1q/tumor necrosis factor-related protein-3 promotes phosphate-induced vascular smoothmuscle cell calcification both in vivo and in vitro. Arterioscler Thromb Vasc Biol. 2014;34:1002–1010. doi: 10.1161/ATVBAHA.114.303301. [DOI] [PubMed] [Google Scholar]


