Abstract
We report that the lymphocytic choriomeningitis virus (LCMV) matrix protein, which drives viral budding, is phosphorylated at serine 41 (S41). A recombinant (r)LCMV bearing a phosphomimetic mutation (S41D) was impaired in infectious and defective interfering (DI) particle release, while a non-phosphorylatable mutant (S41A) was not. The S41D mutant was disproportionately impaired in its ability to release DI particles relative to infectious particles. Thus, DI particle production by LCMV may be dynamically regulated via phosphorylation of S41.
Keywords: Arenavirus, LCMV, matrix protein Z, phosphorylation, budding, defective interfering particle
Arenaviruses are enveloped RNA viruses that establish lifelong, asymptomatic infections in reservoir rodents, but can cause severe disease in humans (Buchmeier et al., 2007). The prototypic arenavirus, lymphocytic choriomeningitis virus (LCMV), produces high levels of defective interfering (DI) particles during acute and persistent infection (Popescu & Lehmann-Grube, 1977; Staneck et al., 1972; Welsh & Pfau, 1972). DI particles, which are replication-deficient virus particles that can interfere with the propagation of standard infectious virus (Huang & Baltimore, 1970; Welsh et al., 1972), are thought to be critical for the establishment of LCMV persistence (Burns & Buchmeier, 1993; Huang & Baltimore, 1970; Oldstone, 1998), possibly by reducing virus-induced cytopathology in reservoir rodents. The arenavirus matrix protein Z is a multifunctional protein that drives the assembly and release of standard infectious virus (Fehling et al., 2012; Perez et al., 2003; Strecker et al., 2003) and DI particles (Ziegler et al., 2016). For many viruses, budding is controlled by one or more late domain(s) encoded in the matrix protein that recruit the cellular endosomal sorting complex required for transport (ESCRT) pathway, which drives the final membrane scission step (Votteler & Sundquist, 2013). The LCMV matrix protein encodes a single late domain, PPXY, which has been shown to be important for the formation of infectious virus-like particles (VLPs) (Perez et al., 2003). In the context of fully infectious virus, we recently demonstrated that the LCMV matrix protein uses different cellular pathways for the production of standard versus DI particles. In particular, DI particle formation absolutely requires LCMV’s PPXY late domain and the cellular ESCRT pathway, while the production of standard infectious particles does not (Ziegler et al., 2016). Further, phosphorylation of the terminal tyrosine in the PPXY late domain may be important for regulating this late domain-driven pathway of DI particle formation. In the current study, we discovered an additional phosphorylation site in LCMV Z at serine 41 (S41). Furthermore, using site-specific mutant viruses we found that S41 plays an important regulatory role in the production of LCMV infectious virions and DI particles.
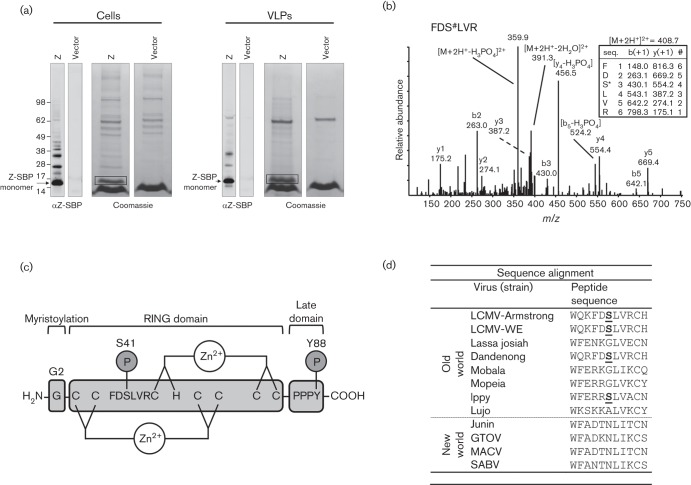
To screen for phosphorylation sites in LCMV Z, we transfected HEK-293T cells with a plasmid encoding the LCMV strain Armstrong Z protein fused to a C-terminal streptavidin binding peptide (SBP) and subsequently used magnetic streptavidin beads [as described by Ziegler et al. (2016)] to affinity purify SBP-tagged Z from the transfected cells as well as from VLPs that had been released into the tissue culture media. Purified Z-SBP was separated by SDS-PAGE (Fig. 1a), prepared for mass spectrometry analysis by in-gel tryptic digestion, and analysed by liquid chromatography-mass spectrometry essentially as described previously (Ziegler et al., 2016). This analysis revealed a novel serine phosphorylation site at S41 in Z-transfected cells (Fig. 1b). S41, a site conserved in the Old World arenaviruses Dandenong and Ippy (Fig. 1d), is located in the central really interesting new gene (RING) domain of Z (Fig. 1c) and is outside any motif known to be important for arenavirus budding and release (Fehling et al., 2012).
Fig. 1.
The LCMV matrix protein Z is phosphorylated at S41. (a) HEK-293T cells were transfected with a plasmid encoding SBP-tagged LCMV strain Armstrong Z or an empty vector. Two days later cells and virus-like particle (VLP)-containing supernatant were lysed and Z-SBP was affinity purified using streptavidin-coated magnetic beads. The purified Z-SBP was subjected to SDS-PAGE and detected by Western blotting using an anti-SBP tag antibody or Coomassie stain. The presumptive monomeric Z bands from cells or VLPs were excised from the Coomassie stained gels (as indicated by the boxes) and subjected to reduction, alkylation and in-gel tryptic digestion prior to mass spectrometry analysis of extracted peptides. (b) A representative low energy collision-induced dissociation tandem mass spectrum with its corresponding fragment ion table from low energy collision-induced dissociation of a Z-derived tryptic peptide (FDS#LVR) containing the phosphorylated S41 where # denotes phosphorylation. The fragment ion table lists the predicted m/z values of the singly charged b and y ions. Major measured b and y ions, as well as dominant losses of phosphoric acid are labelled. Phosphoric acid loss is a major signature in tandem mass spectra of phosphoserine/threonine-containing peptides. (c) Cartoon of the LCMV Z protein depicting the G2 myristoylation site, the central zinc-binding RING domain and the C-terminal PPPY late domain. The S41 phosphorylation site and its flanking amino acids as well as the previously described Y88 phosphorylation site (Ziegler et al., 2016) are indicated. (d) Alignment of Old and New World arenavirus Z protein sequences shows that S41 (bold and underlined for LCMV strains Armstrong and WE) is conserved with the Old World arenaviruses Dandenong and Ippy virus.
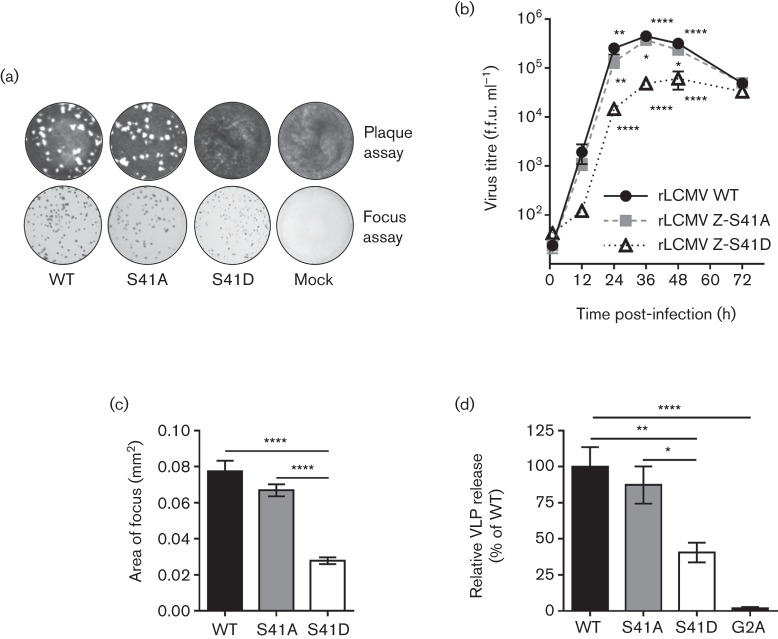
To determine the importance of the S41 residue for viral fitness, recombinant (r)LCMV containing a non-phosphorylatable alanine (S41A) or a phosphomimetic aspartic acid (S41D) substitution at position 41 were recovered using reverse genetics as previously described (Emonet et al., 2009; Flatz et al., 2006; Ziegler et al., 2016). Initially, it appeared that the S41D phosphomimetic mutant could not be recovered as it did not produce plaques in a standard plaque assay (Fig. 2a). However, staining for viral nucleoprotein (NP) (mouse anti-LCMV NP, 1.1.3, kindly provided by M. J. Buchmeier, University of California, Irvine) via immunofocus assay (Battegay et al., 1991) revealed that the S41D mutant was recoverable despite its inability to form plaques (Fig. 2a). Growth curve analysis revealed that the phosphomimetic S41D virus was attenuated in its growth kinetics, while the non-phosphorylatable S41A mutant grew to similar levels as wild-type (Fig. 2b). The attenuation of the S41D mutant was also apparent in the smaller foci it formed, which were less than 50 % of WT size (Fig. 2a, c). To determine whether the reduction in infectious titre of the phosphomimetic S41D virus was due to decreased virus budding and release, we employed a Z VLP release assay as previously described (Ziegler et al., 2016). As a control, we also included the LCMV Z G2A mutant, which exhibits a pronounced defect in VLP formation due to its inability to be myristoylated at this glycine residue (Perez et al., 2004; Strecker et al., 2006). This experiment demonstrated that the budding efficiency of the phosphomimetic Z-S41D was reduced ~60 %, while the budding activity of the non- phosphorylatable S41A was not different from WT (Fig. 2d). Collectively, these findings demonstrate that the S41 residue possesses a previously unappreciated capacity to drive virus budding and that this function may be regulated by phosphorylation.
Fig. 2.
Phosphomimetic mutation of S41 significantly reduces the efficiency of infectious virus release and the ability of Z to form VLPs. (a) Reverse genetics were used to generate rLCMV containing a non-phosphorylatable S41A mutation or a phosphomimetic S41D mutation. To determine whether infectious virus was recovered, both a standard plaque assay and an immunofocus assay [using an anti-nucleoprotein antibody (1.1.3)] were performed on Vero E6 cells. (b) The kinetics of infectious virus production was examined by growth curve analysis on Vero E6 cells [a multiplicity of infection (MOI) of 0.01 was used for each virus]. Data represent the mean±sem from three independent experiments. For statistical analysis, the data were first log-transformed then a two-way ANOVA with Holm–Sidak’s test for multiple comparisons was performed. (c) The area of foci obtained from the immunofocus assay wells shown in (a) for each rLCMV strain was measured using Image J. Data represent the mean±sem of foci from eight wells for each virus. The Kruskal–Wallis non-parametric test with Dunn’s multiple comparisons test was used to compare mean values. (d) The budding activity of WT or S41-mutant LCMV Z proteins was measured by a VLP release assay. The LCMV Z G2A mutant, which has a budding defect due to its inability to be myristoylated, was included as a control (Perez et al., 2004). The results shown represent the mean±sem from three independent experiments. A one-way ANOVA with the Holm–Sidak’s test for multiple comparisons was used to compare the mean values. For the indicated statistical tests in (b–d): *, P<0.05; **; P<0.01; ****; P<0.0001.
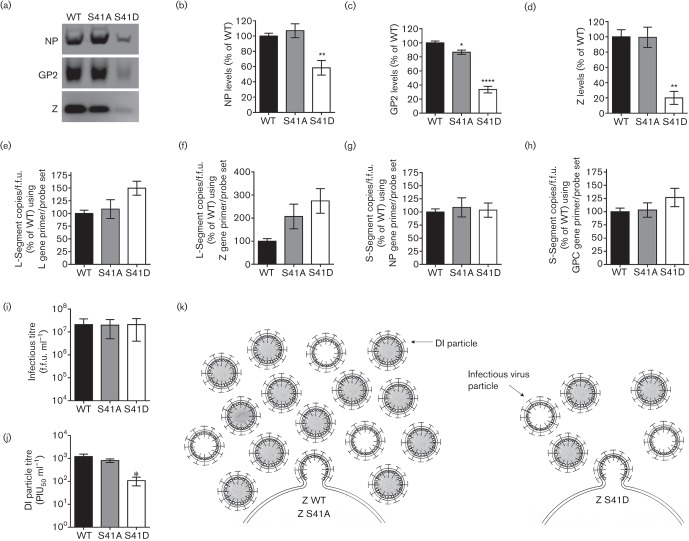
As the reduction in VLP release by Z-S41D (Fig. 2d) did not appear to fully explain the greater than 10-fold reduction in virus titre observed in Fig. 2(b), we probed rLCMV WT or S41 mutant virion preparations (that were generated in Vero E6 cells infected at a multiplicity of infection (MOI) of 0.0001 and collected 72 h after infection) for structural protein and/or genome deficits. To determine the composition of each virus particle preparation, an equal number of focus forming units (f.f.u.) of cell-free rLCMV WT, S41A or S41D viruses were concentrated through a 20 % sucrose cushion by ultracentrifugation as described by Ziegler et al. (2016) and virion protein quantity was analysed by quantitative Western blotting (Fig. 3a–d). The S41D phosphomimetic virus preparation, despite containing equivalent infectious units as the WT and S41A preparations, had markedly reduced levels of NP, glycoprotein (GP2) and Z (Fig. 3a–d). Interestingly, the quantities of viral genomic S- and L-segment RNAs, measured by quantitative PCR as described by Haist et al. (2015) and Ziegler et al. (2016), did not differ between the preparations of WT, S41A or S41D viruses (Fig. 3e–h). The loss in viral structural protein content without a corresponding loss in infectious titre led us to hypothesize that the S41D phosphomimetic virus may be defective in its ability to generate DI particles, which could explain the reduced levels of viral protein observed relative to infectious units. To test whether S41 can indeed act as a regulatory motif to control DI particle production, we next measured the infectious virus levels and DI particle activity of the same virus preparations used above in Fig. 3(a–h) using the immunofocus assay and the plaque interference assay, respectively, as described by Welsh & Pfau (1972) and Ziegler et al. (2016). All three viruses had approximately equivalent titres of infectious virus (Fig. 3i). The DI particle titre of the S41D phosphomimetic virus, however, was reduced greater than 10-fold compared to WT virus, while the DI particle titre of the S41A virus was not different from WT (Fig. 3j). These results indicate that the loss of viral structural protein content observed in the phosphomimetic S41D virus preparation (Fig. 3a–d) was likely due to the reduced production of DI particles, not infectious virus particles.
Fig. 3.
The S41 phosphomotif regulates DI particle production. (a–h) Comparison of viral structural protein and genome content in preparations of rLCMV WT, S41A or S41D virus. Vero E6 cells were infected with WT or S41-mutant rLCMV at a MOI of 0.0001 and clarified supernatants were collected 72 h later. Equivalent f.f.u.s of each rLCMV were then concentrated through a 20 % sucrose cushion by ultracentrifugation. Viral protein content in these concentrated virus preparations was analysed by quantitative Western blotting. Representative Western blots (a) as well as the quantity (mean±sem) of NP (b), GP (c) or Z (d) contained in each rLCMV virus preparation are shown. The copies of LCMV genomic L-segment (e–f) or S-segment (g–h) were determined by quantitative real-time PCR (qRT-PCR) (Haist et al., 2015) and then normalized to the infectious titre (f.f.u.). To enumerate copies of L-segment vRNA, RT was performed using the RT primer 5906−, followed by qPCR using the primer probe sets located in either the L gene (primers L5517+ and L5645− and probe L5582−P) (e) or Z gene (primers L212+ and L276− and probe L251−P) (f). To determine the copies of S-segment vRNA, RT was performed using the RT primer 2865−, followed by qPCR using the primer probe sets located in either the NP gene (primers S2275+ and S2338− and probe S2295+P) (g) or GPC gene (primers S929+ and S988− and probe S952+P) (h). (i–j) Measurement of standard infectious virus and DI particles produced by rLCMV WT, S41A or S41D. The infectious titre of each of the clarified supernatants used in (a–h) was determined by focus forming assay (i) and the DI particle titre was assessed by plaque interference assay (j). PIU50 ml−1, plaque interfering units50 ml−1. For (b–j), values represent the mean±sem of protein (b–d), viral genome (e–h), f.f.u. ml−1 (i) or DI particle titre (j) from four independent experiments and statistical analyses were performed by one-way ANOVA with Holm–Sidak’s test for multiple comparisons for which *, P<0.05; **, P<0.01; ****, P<0.0001. (k) Model of S41’s impact on infectious virus and DI particle formation. WT virus containing the native S41 (Z WT) produces high levels of infectious and DI particles. Mutation of S41 to alanine (Z S41A) to prevent phosphorylation has little effect on infectious or DI particle production. Mutation of S41 to aspartic acid (Z S41D) to mimic the negative charge associated with phosphorylation of S41 results in decreased infectious virus and DI particle release. The S41D mutation disproportionately impacts DI formation over standard infectious virus.
The S41 phosphomotif represents a novel regulatory site within the LCMV Z protein (see Fig. 3k for our proposed model). We recently demonstrated that the PPXY late domain in LCMV Z is not absolutely required for the production of infectious LCMV virions (Ziegler et al., 2016). Provided that the only other motif in Z with a known role in budding activity is the myristoylation site at the glycine at position 2 (Figs 1c and 2d) (Perez et al., 2004; Strecker et al., 2006), our finding here expands the functional repertoire of motifs in LCMV Z that regulate the efficiency of infectious virus release. Further, we have built upon our previous findings regarding the PPXY late domain (Ziegler et al., 2016) by showing that S41 also serves as a key regulator of DI particle formation. To our knowledge, these are the only two motifs known to specifically regulate DI particle formation over standard particles for any virus family. While we have shown that DI particle formation requires a functional ESCRT pathway (Ziegler et al., 2016), it will be important to determine whether the PPXY late domain and/or the S41 phosphomotif directly engage ESCRT machinery and, if so, the mechanistic basis for this interaction as it relates to DI particle production. Further, our findings support the hypothesis that phosphorylation of Z is an important mechanism by which the virus can adjust its rate of DI particle formation in response to the dynamic environment of the host cell (e.g. phosphorylation at Y88 appears to increase DI particle production) (Ziegler et al., 2016), whereas phosphorylation of S41 represses it (Fig. 3j). In this scenario, the S41 site could function as an important rheostat for regulating the potency of the PPXY-driven DI production pathway. Other arenaviruses, if they produce fewer DI particles than LCMV (e.g. Junin virus Candid #1) (Ziegler et al., 2016), may not require a secondary regulatory motif such as S41, which could explain why the S41 site is only found in LCMV-like viruses and the closely related Old World arenavirus Ippy (Fig. 1d). Identifying the key kinases and phosphatases that target S41 and Y88 and determining how their activity is regulated in response to environmental stimuli will be important for understanding how DI particle formation is regulated during infection. It will also be important to determine whether phosphorylation of matrix proteins can play a similar role in the regulation of DI particle production for other relevant virus families.
Acknowledgements
We thank the UVM Immunobiology Group for insightful discussions and Drs Alan Howard and Cory Teuscher for statistical support and advice. We are grateful to Drs Michael Buchmeier and Juan Carlos de la Torre for providing critical reagents. The authors gratefully acknowledge NIH grants T32 AI055402 (C. M. Z.), T32 HL076122 (B. R. K.), R21 AI088059 (J. B.), AI065359 (J. B.) and P20RR021905 (Immunobiology and Infectious Disease COBRE) (J. B.). The mass spectrometry analysis was supported by the Vermont Genetics Network through NIH grant 8P20GM103449 from the INBRE program and of the NIGMS. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
References
- Battegay M., Cooper S., Althage A., Bänziger J., Hengartner H., Zinkernagel R. M.(1991). Quantification of lymphocytic choriomeningitis virus with an immunological focus assay in 24- or 96-well plates. J Virol Methods 33191–198. 10.1016/0166-0934(91)90018-U [DOI] [PubMed] [Google Scholar]
- Buchmeier M. J., de la Torre J. C., Peters C. J.(2007). Arenaviridae: the viruses and their replication. Fields Virology, 5th edn, 1791–1827. Edited by Knipe D. M., Howley P. M., Griffin D. E., Lamb R. A., Martin M. A., Roizman B., Straus S. E.Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins. [Google Scholar]
- Burns J. W., Buchmeier M. J.(1993). Glycoproteins of the Arenaviruses. The Arenaviridae, 17–35. Edited by Salvato M. S.New York: Plenum Press. [Google Scholar]
- Emonet S. F., Garidou L., McGavern D. B., de la Torre J. C.(2009). Generation of recombinant lymphocytic choriomeningitis viruses with trisegmented genomes stably expressing two additional genes of interest. Proc Natl Acad Sci U S A 1063473–3478. 10.1073/pnas.0900088106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehling S. K., Lennartz F., Strecker T.(2012). Multifunctional nature of the arenavirus RING finger protein Z. Viruses 42973–3011. 10.3390/v4112973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatz L., Bergthaler A., de la Torre J. C., Pinschewer D. D.(2006). Recovery of an arenavirus entirely from RNA polymerase I/II-driven cDNA. Proc Natl Acad Sci U S A 1034663–4668. 10.1073/pnas.0600652103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haist K., Ziegler C., Botten J.(2015). Strand-specific quantitative reverse transcription-polymerase chain reaction assay for measurement of arenavirus genomic and antigenomic RNAs. PLoS One 10e0120043. 10.1371/journal.pone.0120043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. S., Baltimore D.(1970). Defective viral particles and viral disease processes. Nature 226325–327. 10.1038/226325a0 [DOI] [PubMed] [Google Scholar]
- Oldstone M. B.(1998). Viral persistence: mechanisms and consequences. Curr Opin Microbiol 1436–441. 10.1016/S1369-5274(98)80062-3 [DOI] [PubMed] [Google Scholar]
- Perez M., Craven R. C., de la Torre J. C.(2003). The small RING finger protein Z drives arenavirus budding: implications for antiviral strategies. Proc Natl Acad Sci U S A 10012978–12983. 10.1073/pnas.2133782100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez M., Greenwald D. L., de la Torre J. C.(2004). Myristoylation of the RING finger Z protein is essential for arenavirus budding. J Virol 7811443–11448. 10.1128/JVI.78.20.11443-11448.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popescu M., Lehmann-Grube F.(1977). Defective interfering particles in mice infected with lymphocytic choriomeningitis virus. Virology 7778–83. 10.1016/0042-6822(77)90407-X [DOI] [PubMed] [Google Scholar]
- Staneck L. D., Trowbridge R. S., Welsh R. M., Wright E. A., Pfau C. J.(1972). Arenaviruses: cellular response to long-term in vitro infection with parana and lymphocytic choriomeningitis viruses. Infect Immun 6444–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strecker T., Eichler R., Meulen J., Weissenhorn W., Dieter Klenk H., Garten W., Lenz O.(2003). Lassa virus Z protein is a matrix protein and sufficient for the release of virus-like particles [corrected]. J Virol 7710700–10705. 10.1128/JVI.77.19.10700-10705.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strecker T., Maisa A., Daffis S., Eichler R., Lenz O., Garten W.(2006). The role of myristoylation in the membrane association of the Lassa virus matrix protein Z. Virol J 393. 10.1186/1743-422X-3-93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Votteler J., Sundquist W. I., Wesley I.(2013). Virus budding and the ESCRT pathway. Cell Host Microbe 14232–241. 10.1016/j.chom.2013.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh R. M., Pfau C. J.(1972). Determinants of lymphocytic choriomeningitis interference. J Gen Virol 14177–187. 10.1099/0022-1317-14-2-177 [DOI] [PubMed] [Google Scholar]
- Welsh R. M., O'Connell C. M., Pfau C. J.(1972). Properties of defective lymphocytic choriomeningitis virus. J Gen Virol 17355–359. 10.1099/0022-1317-17-3-355 [DOI] [PubMed] [Google Scholar]
- Ziegler C. M., Eisenhauer P., Bruce E. A., Weir M. E., King B. R., Klaus J. P., Krementsov D. N., Shirley D. J., Ballif B. A., Botten J.(2016). The lymphocytic choriomeningitis virus matrix protein PPXY late domain drives the production of defective interfering particles. PLoS Pathog 12e1005501. 10.1371/journal.ppat.1005501 [DOI] [PMC free article] [PubMed] [Google Scholar]