Abstract
Surgical site infection (SSI) remains one of the most important causes of healthcare-associated infections, accounting for ~17 % of all hospital-acquired infections. Although short-term perioperative treatment with high fraction of inspired oxygen (FiO2) has shown clinical benefits in reducing SSI in colorectal resection surgeries, the true clinical benefits of FiO2 therapy in reducing SSI remain unclear because randomized controlled trials on this topic have yielded disparate results and inconsistent conclusions. To date, no animal study has been conducted to determine the efficacy of short-term perioperative treatments with high (FiO2>60 %) versus low (FiO2<40 %) oxygen in reducing SSI. In this report, we designed a rat model for muscle surgery to compare the effectiveness of short-term perioperative treatments with high (FiO2=80 %) versus a standard low (FiO2=30 %) oxygen in reducing SSI with Pseudomonas aeruginosa – one of the most prevalent Gram-negative pathogens, responsible for nosocomial SSIs. Our data demonstrate that 5 h perioperative treatment with 80 % FiO2 is significantly more effective in reducing SSI with P. aeruginosa compared to 30 % FiO2 treatment. We further show that whilst 80 % FiO2 treatment does not affect neutrophil infiltration into P. aeruginosa-infected muscles, neutrophils in the 80 % FiO2-treated and infected animal group are significantly more activated than neutrophils in the 30 % FiO2-treated and infected animal group, suggesting that high oxygen perioperative treatment reduces SSI with P. aeruginosa by enhancing neutrophil activation in infected wounds.
Keywords: P. aeruginosa, Surgical site infection, High fraction of inspired oxygen
Introduction
Surgical site infection (SSI) remains one of the most common and important healthcare-associated infections, accounting for ~17 % of all hospital-acquired infections (Klevens et al., 2007; Magill et al., 2012). Despite many advances in infection control practices – including improved operating room ventilation, barriers, sterilization methods, improved surgical techniques and administration of appropriate antimicrobial prophylaxis – SSI remains a significant cause of morbidity, prolonged hospitalization and death with a mortality rate of ~3 % (Awad, 2012). According to the Centers for Disease Control and Prevention (CDC), SSIs are estimated to cost between $3.5 billion and $10 billion annually in healthcare expenditures in the USA alone (Scott, 2009). It is not surprising that the US Department of Health and Human Services has identified combatting SSI as a top national priority.
Neutrophils are the first inflammatory leukocytes infiltrating into the wound where they play a crucial role defending wound tissue from invading pathogens (Martin, 1997; Nauseef & Borregaard, 2014). One of the most important mechanisms by which neutrophils destroy invading pathogens is through generation of antimicrobial oxidant species, such as HOCl, which is dependent on the availability of oxygen (Brinkmann et al., 2004; Dovi et al., 2004; Arsalan et al., 2014). In vitro studies have demonstrated that neutrophil oxygen consumption and antimicrobial oxidant production are substantially impaired at low oxygen tension, which is often the condition found in wounds, suggesting that reduced oxygen availability may play a pivotal role in attenuating neutrophils’ bacterial killing at surgical sites, leading to SSI (Allen et al., 1997; Greif et al., 2000; Anderson, 2011).
High fraction of inspired oxygen (FiO2=80 %) therapy given perioperatively for 5 h has been shown to be effective in reducing the incidence of SSI after colorectal surgery compared to the standard low 30 % FiO2 (Greif et al., 2000). However, the true clinical benefits of FiO2 therapy in reducing SSI remain uncertain because (i) randomized controlled trials on this topic have yielded disparate results with inconsistent conclusions – presumably owing to differences in protocols, surgical sites and/or insufficient power (Chura et al., 2007; Al-Niaimi & Safdar, 2009; Qadan et al., 2009; Brar et al., 2011; Togioka et al., 2012; Hovaguimian et al., 2013) – and (ii) since all surgical patients also receive prophylactic antibiotics, the real efficacy of FiO2 therapy in reducing SSI remains unknown. Moreover, no study has directly examined the impact of high inspired oxygen therapy on neutrophil influx and/or its activation at infected surgical sites, although it has been postulated that FiO2 therapy-induced reduction in SSI may be due to increased tissue oxygen tension within surgical wounds – leading to increased oxidative capacity of neutrophils and enhanced neutrophil killing capacity in surgical sites (Knighton et al., 1984, 1986; Allen et al., 1997). Since infection studies cannot be performed in patients, animal modelling could help address these gaps in our understanding of the impact of FiO2 therapy in reducing SSI.
The beneficial effects of oxygen therapy on SSI control have been demonstrated in animal models in three relatively old studies, which demonstrated that long-term (2–3 days) exposure to moderate oxygen levels (FiO2=45 %) reduced SSI compared to low oxygen levels (21 % or 12 %) (Hunt et al., 1975; Knighton et al., 1984, 1986). Although these studies have provided strong evidence to support the notion that oxygen therapy may be effective in reducing SSI in animals, it remains unclear whether they truly recapitulate the clinical SSI situations – given that 2–3 day long exposure to FiO2, used in these animal studies, is neither practical to apply to human patients in clinical settings nor advisable. Moreover, these studies did not examine the impact of high inspired oxygen (FiO2=80 %) on SSI, to match the landmark clinical study by Greif et al. (2000), which demonstrated that 80 % FiO2 is significantly more effective in reducing SSI than the standard low 30 % FiO2. Finally, these animal studies also did not provide any insights into possible mechanism(s) responsible for enhanced antimicrobial defences imparted by FiO2 treatments, although the authors again postulated that elevated oxygen levels may enhance neutrophils’ bactericidal activity through the increased production of oxygen free-radical intermediates (Hunt et al., 1975; Knighton et al., 1984, 1986). A recent letter on the benefits and risks of high inspired oxygen stated that ‘we can only tentatively conclude that applying high FiO2 is very likely to reduce SSI’, but ‘further research is still needed if we are to clarify the specific effects of perioperative high FiO2’ (Belda et al., 2014).
In this report, we examined the effects of a short-term (5 h) 80 % versus 30 % FiO2 exposure on reducing SSI with Pseudomonas aeruginosa, using a rat thigh muscle SSI model that we described previously (Kroin et al., 2015). We further evaluated the impact of 80 % versus 30 % FiO2 treatments on neutrophil influx and its activation status at surgical sites in response to P. aeruginosa infection.
Methods
Surgical infection model
All experimental procedures in this study were approved by the Animal Care and Use Committee of Rush University Medical Center and conformed to the Guide for the Care and Use of Laboratory Animals (National Research Council). The bacterial species chosen to induce a muscle surgical infection was P. aeruginosa, which is the most prevalent Gram-negative pathogen in all wounds, and it represents 25 % of surgical wound infections (Giacometti et al., 2000; Greif et al., 2000). In line with the importance of P. aeruginosa infection in SSI, we and others have demonstrated that P. aeruginosa uses a variety of virulence mechanisms to inhibit wound healing both in vivo and in vitro in order to propagate its favourite niche ‘the wound’ (Garrity-Ryan et al., 2004; Shafikhani & Engel, 2006; Zhao et al., 2010; Goldufsky et al., 2015b; Wood et al., 2015a, b). The strain of P. aeruginosa used in this study was PA103, which we and others have described previously (Shafikhani & Engel, 2006; Wood et al., 2013; Goldufsky et al., 2015a, b). Cultures were propagated in tryptic soy broth. The day before surgery and bacterial injection, frozen stock from the initial propagation of bacteria was grown overnight (37 °C incubator). Bacterial titres were determined as colony-forming units (CFU) by serial dilution and plating, as previously described (Shafikhani & Engel, 2006; Shafikhani et al., 2008), to provide a 2.5 × 107 CFU ml−1 concentration (based initially on OD and confirmed by serial dilution and plating) on the morning of surgery.
To induce infection, male Sprague–Dawley rats (300 g, Sasco; Charles River Laboratories) were anaesthetized with 1.5 % isoflurane in oxygen provided via a nose cone. Surgery was performed with sterile instruments, sterile surgical gloves and aseptic techniques as follows (Kroin et al., 2015). The left thigh was shaved and disinfected with alcohol swabs (three times), followed by a topical antiseptic solution (chlorhexidine gluconate 4 %) applied to the skin. A 2 cm long skin incision was made with a #15 scalpel blade to expose the biceps femoris muscle. The skin margins were retracted, and a 7–0 polypropylene suture was placed on the surface of the muscle for later identification of the injection site. Using a 25G needle and 1 ml syringe, 5×106 CFU of PA103 P. aeruginosa was slowly injected into the biceps femoris muscle in 0.2 ml volume (Lin et al., 2005) adjacent to the 7–0 suture. A plastic tubing sheath over most of the needle limited the injection depth to 2 mm. At the end of surgery, all skin margins were closed with 4–0 nylon sutures. The topical antiseptic solution was again applied to the skin, and the animal recovered from anaesthesia. In the experiments to determine muscle neutrophil activity, control mock rats were injected with 0.2 ml sterile saline (no bacteria).
Oxygen exposure
Immediately after surgery, animals were placed in a closed clear plastic chamber (four rats per chamber) with a ventilated lid and a high-flow oxygen–air mixture (Knighton et al., 1984, 1986), with either high FiO2 (80 % oxygen) or standard FiO2 (30 % oxygen) for 5 h. The 5 h perioperative exposure time was based on the study by Greif et al. (2000), in which patients received perioperative exposure to a total of 5 h of FiO2=80 % or 30 % oxygen. Oxygen levels were measured with a Datex Capnomac Ultima gas monitor. The volume of the chamber was 20 l, and the flow of the oxygen–air mixture was 7 l min−1 so that the gases turned over every 3 min, assuring that there were normal carbon dioxide and water vapor levels (Knighton et al., 1984, 1986). Preliminary experiments verified that the body temperature of four control rats maintained in the chamber at 80 % or 30 % FiO2 was normal over the 5 h. At the end of the 5 h inspired oxygen exposure, rats were returned to the vivarium and normal room air (21 % oxygen).
Postoperative outcome measures
Bacterial muscle burden determination.
At 24 h after surgery and P. aeruginosa muscle injection, rats were euthanized with carbon dioxide in a closed chamber. Under aseptic conditions, the left biceps femoris muscle was exposed, and a section was selected around the previously implanted 7–0 suture. A 7×7 mm area of infected muscle, 4 mm thick, was then removed from the body and used for determination of bacterial loads as follows (Kroin et al., 2015). The muscle was weighed (typical value=0.20 g), minced with a razor blade and homogenized (PowerGen 125; Fisher Scientific; 7 mm probe, at full speed for 10 s three times) in 1 ml of sterile PBS. The bacterial loads were determined by serial dilution and plating, as previously described (Shafikhani & Engel, 2006; Shafikhani et al., 2008). Briefly, the homogenized muscle mixture underwent 10-fold serial dilution in PBS to produce 10−1–10−5 dilutions in 1 ml volume, plus an undiluted sample 100; 100 µl aliquots of each muscle solution (10−5–100) were plated on tryptic soy agar plates and incubated for 24 h at 37 °C. The next day, muscle tissue bacterial counts (CFU) were determined from plates with 30–300 colonies. The final bacterial burden is expressed as CFU per tissue wet weight.
Neutrophil count determination and activity assessment.
Haematoxylin and eosin (H&E) histological analysis was performed as described (Wood et al., 2014; Goldufsky et al., 2015b). Briefly, at 24 h after surgery and P. aeruginosa or saline muscle injection, rats were euthanized, and the biceps femoris muscle was removed as described (Kroin et al., 2015). The muscle was placed on a glass plate over ice and bisected at the injection site (previously implanted suture). One piece of muscle per rat was placed in a tissue cassette and dropped into 10 % buffered formalin for H&E histology. The other piece of muscle per rat (0.10 g) was minced with a razor blade and homogenized (PowerGen 125; Fisher Sci; 7 mm probe, at full speed for 5 s five times) in 1 ml ice-cold lysis buffer [PBS with 0.2 % Triton X-100, plus protease inhibitor cocktail (COMPLETE Mini; Roche)]. The homogenate was centrifuged (5000 g, 10 min, 4 °C), and the supernatant was frozen at −80 °C for later myeloperoxidase (MPO) Western blot analysis. After Western blotting, MPO levels were determined by densitometer using Image J and normalized to GAPDH loading control, as we described (Wood et al., 2015a, b).
For H&E histological studies, muscle tissues were harvested by resecting the injected area of muscle before cross-sectioning the muscle at the injection site. Once collected, muscle samples were fixed in 10 % formalin for 48 h and embedded in paraffin. Muscle sections were transversely cut into 6 µm thick sections from the edge of the injection site and stained with H&E, and slides were visualized on a Nikon Eclipse Ti microscope using NIS-Elements AR software.
Statistical analysis
Difference in bacterial muscle burden between the 80 % FiO2 and 30 % FiO2 rats after surgery and bacteria muscle injection was compared with the two-tailed Student t-test. Differences in MPO activity from Western blot data were compared amongst four groups with ANOVA and the lsd post hoc test.
Results
Treatment with 80 % FiO2 is significantly more effective in reducing SSI with P. aeruginosa than 30 % FiO2
In order to evaluate the impact of high (80 %) and low/standard (30 %) FiO2 therapies on SSI, we injected either saline (mock) or 5×106 CFU of PA103 bacteria – [a wild-type P. aeruginosa strain described previously (Ohman et al., 1980; Wood et al., 2013; Goldufsky et al., 2015a, b)] – into biceps femoris muscle, using a rat muscle surgical model for infection that we described previously (Kroin et al., 2015) (for more detailed protocol, see Methods). Immediately after surgery and infection, animals were exposed to either high 80 % FiO2 or 30 % low (standard) FiO2 for 5 h, as described in Methods. The 5 h oxygen exposure time was chosen to match the landmark study by Greif et al. (2000), which demonstrated that 5 h perioperative exposure to 80 % FiO2 was more effective in reducing SSI in patients than the 30 % FiO2. P. aeruginosa was chosen because of its clinical importance to all wound infections including SSI. P. aeruginosa accounts for ~25 % of SSIs, and its presence in wound correlates with a poor prognosis for healing (Halbert et al., 1992; Madsen et al., 1996; Giacometti et al., 2000; Winstanley et al., 2005; Gjødsbøl et al., 2006; Ramakant et al., 2011; Malik et al., 2013).
Twenty-four hours after surgery and infection, infected muscles were then harvested, and the level of infections in the muscles was determined by determining the P. aeruginosa bacterial CFU counts per gram of infected muscles, as we described (Goldufsky et al., 2015b; Kroin et al., 2015). Our data indicated that treatment with 80 % FiO2 was more effective than 30 % FIO2 in reducing SSI in muscle by ~1.4 log order (Fig. 1) (mean CFU count for 80 % FiO2=5.1×104±3.4×103; mean CFU count for 30 % FiO2=7.1×105±2.1×105; P=0.017; n=8 animals/group). These results confirmed the clinical findings which demonstrated the more beneficial effect of 80 % FiO2 in reducing SSI in comparison to 30 % FiO2 (Greif et al., 2000).
Fig. 1.
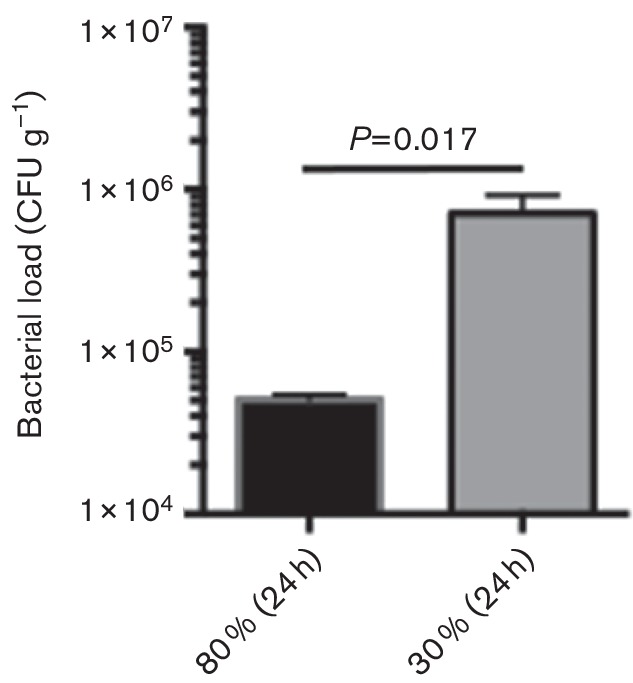
Treatment with 80 % FiO2 is significantly more effective in reducing SSI with P. aeruginosa than 30 % FiO2. After surgery, 5×106 bacteria were injected into biceps femoris muscles. Infected rats then received either 80 % or 30 % FiO2 for 5 h. After FiO2 treatments, rats were placed in normoxia conditions (21 % O2). Twenty-four hours later, infected muscles were removed, and their bacterial load was determined by serial dilution and CFU counts. Data are presented as mean±sem (P=0.017; n=8 rats/group, Student t-test).
Treatment with 80 % FiO2 does not affect neutrophil influx into infected muscle but increases neutrophil activation in P. aeruginosa-infected muscle
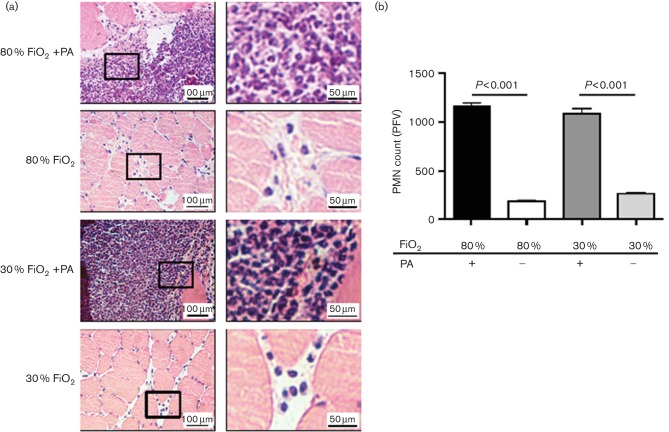
Given that neutrophils (a.k.a. PMNs) are the primary leukocytes in innate immune defences against P. aeruginosa and, without their function, tissues are completely vulnerable to P. aeruginosa infection (Tsai et al., 2000), we sought to evaluate the impact of 80 % and 30 % FiO2 treatments on the influx of neutrophils at surgical sites in the presence and absence of P. aeruginosa SSI. To this end, we harvested muscle tissues 24 h after saline treatment or P. aeruginosa infection and analysed their neutrophil contents by H&E staining, as we described (Wood et al., 2014) (Methods). As expected, P. aeruginosa infection significantly increased neutrophil infiltration in the infected muscles in both 80 % and 30 % FiO2-treated animals, compared to their uninfected counterpart animal groups (Fig. 2; P<0.001; n=4 animals/group, 10 random fields/animal). However, there were no differences in the neutrophil levels between the 80 % and 30 % FiO2-treated animal groups regardless of whether they were infected with P. aeruginosa or treated with PBS.
Fig. 2.
Treatment with 80 % FiO2 does not affect neutrophil migration into infected muscle. Rats treated with 80 % or 30 % FiO2 were either injected with saline or infected with 5×106 P. aeruginosa (PA) into biceps femoris muscle after surgery. (a) Twenty-four hours later, muscle sections were fixed and stained with H&E. Enlarged areas of the section are indicated by a box inset. (b) The corresponding tabulated number of polymorphoneuculear (PMN) cells per field of view is shown as mean±sem. P-values are indicated (n=4 rats/group, 6 random fields/rat, ANOVA with the lsd post hoc test).
We wondered if increased antimicrobial defences against P. aeruginosa in the 80 % FiO2-treated animal group, compared to 30 % FiO2-treated animal group (Fig. 1), may be due to enhanced neutrophil activation in these infected muscles. The heme enzyme MPO is a marker for activated neutrophils, and this enzyme is required for the production of antimicrobial oxidants in activated neutrophils (Klebanoff et al., 2013; Björnsdottir et al., 2015; Winterbourn et al., 2016). Twenty-four hours after surgery and P. aeruginosa infection, we evaluated the MPO protein levels of infected muscles or saline-treated uninfected muscles in animals treated with either 80 % or 30 % FiO2 by Western immunobloting. A representative Western blot gel is shown in Fig. 3(a), and the MPO levels, as determined by densitometer and normalized to GAPDH loading control, are shown in Fig. 3(b). As shown in Fig. 3, there was no difference in MPO levels between 80 % FiO2+saline (2.23±0.46) and 30 % FiO2+saline (2.11±0.60), indicating that additional oxygen exposure in the 80 % FiO2 group does not result in increased neutrophil activation in these animals in the absence of infection (P=0.112; n=6 rats/group). In contrast, MPO levels were significantly higher in the 80 % FiO2+P. aeruginosa (8.36±0.50) as compared to the 30 FiO2+P. aeruginosa (5.90±0.15) (P=0.001; n=6 rats/group), indicating that additional oxygen exposure results in enhanced neutrophil activation in infected wounds. Of note, the MPO levels in Pseudomonas-infected wounds in both the 80 % and the 30 % FiO2-treated animals were substantially higher than their uninfected saline-treated counterparts (P<0.001).
Fig. 3.
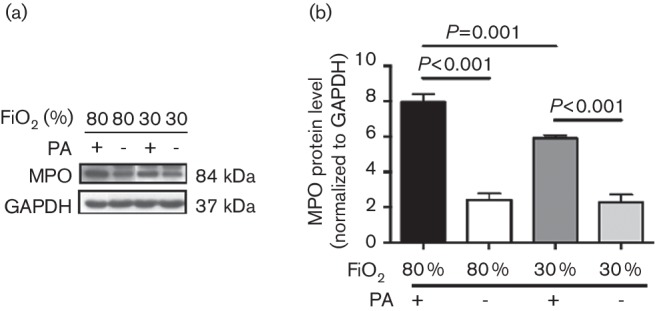
80 % FiO2 increases neutrophil activation in P. aeruginosa-infected muscle. Rats treated with 80 % or 30 % FiO2 were either injected with saline or infected with 5×106 P. aeruginosa (PA) into biceps femoris muscle after surgery. (a) Muscle tissue lysates were collected from the different treatment groups 24 h following surgery and probed for myeloperoxidase (MPO) by Western blotting. The experiment was performed in duplicate and repeated over four trials. (b) For each sample measured, the MPO levels were normalized to GAPDH. Data are shown as mean±sem, ANOVA with the lsd post hoc test.
Discussion
Despite many advances in infection control practices, SSIs are common complications with potentially devastating morbidity and mortality rates (Scott, 2009; Awad, 2012). A landmark clinical study, involving 500 patients undergoing colorectal resection, demonstrated that 5 h perioperative treatment with 80 % (high) FiO2 was significantly more effective in reducing SSI than the standard low 30 % FiO2 (Greif et al., 2000). These findings were subsequently confirmed by another clinical study involving 300 patients undergoing colorectal resection, which again showed that patients receiving perioperative 80 % FiO2 had a significant reduction in the risk of wound infection when compared to the 30 % FiO2 group (Belda et al., 2005). However, since all patients also had received prophylactic antibiotic therapy, there remained the possibility that the beneficial impact of high FiO2 on SSI reduction may be indirect through enhancement of the prophylactic antibiotic activity.
We designed a rat muscle surgical site infection model to address whether 80 % high FiO2 therapy is also more effective in reducing SSI than 30 % standard FiO2 therapy in the absence of prophylactic antibiotic treatment. Our data support the clinical findings by Greif et al. (2000) and demonstrate that 5 h perioperative treatment with 80 % FiO2 is also significantly more effective than 30 % FiO2 in reducing SSI with P. aeruginosa, even in the absence of prophylactic antibiotic treatment (Fig. 1). It would be interesting to use this rat model to study the effect of antibiotic treatment, with and without 80 % perioperative FiO2 on SSI.
FiO2 treatment at normobaric conditions is reported to have only modest effect on PO2 in the blood (Sjoberg & Singer, 2013). In contrast, FiO2 treatment has been shown to result in significant increases in tissue PO2 and shown to be predictive of the risk of wound infection in surgical patients (Hopf et al., 1997). These findings have led to the general acceptance in the field that the beneficial impact of FiO2 therapy on SSI reduction is due to its enhancement of neutrophil oxidative killing (Hunt et al., 1975; Knighton et al., 1984, 1986). To date, however, there are no published data to support this hypothesis. To our knowledge, for the first time, we provide strong evidence in support of this hypothesis. Our data demonstrate that high FiO2 perioperative treatment results in substantial increases in MPO levels in vivo (Fig. 3), without affecting the neutrophil influx into infected surgical site (Fig. 2). MPO is a critical enzyme that is required for the production of reactive antimicrobial oxidants in neutrophils (Klebanoff et al., 2013; Björnsdottir et al., 2015; Winterbourn et al., 2016).
How safe is it to use high FiO2 treatement in patients? Although future clinical studies are needed to evaluate all potential adverse impacts of high FiO2 therapy in patients, there are encouraging signs to suggest that this therapeutic approach may be safe in patients. A recent meta-analysis of randomized controlled trials found no evidence of atelectasis (lung collapse) or any other detrimental effect on postoperative gas exchange with high (80–100 %) FiO2 treatments (Hovaguimian et al., 2013). In fact, high FiO2 therapy has been shown to reduce the incidence of postoperative nausea and vomiting (Greif et al., 1999; Hovaguimian et al., 2013). Our data also support the notion that 80 % FiO2 therapy may be safe. We found that enhanced activation of neutrophils at surgical site in response to 80 % FiO2 only occured in infected muscles and not when the muscle wound was sterile (Fig. 3), indicating that 80 % FiO2 therapy only primes neutrophils to exhibit heightened response to infection and in itself is insufficient to activate neutrophils, which could have undesirable consequences.
In summary, our data suggest that short-term perioperative treatment with 80 % FiO2 should be adopted in clinical settings, as it may be more beneficial in reducing SSI than low 30 % FiO2.
Acknowledgements
This work was supported by National Institutes of Health grant R56DK107713-01 to S. H. S. and by the University Anesthesiologists Support Fund to J. S. K.
Abbreviations:
- CDC
Centers for Disease Control and Prevention
- CFU
colony-forming unit
- FiO2
fraction of inspired oxygen
- H&E
haematoxylin and eosin
- MPO
myeloperoxidase
- SSI
surgical site infection
References
- Al-Niaimi A., Safdar N.(2009). Supplemental perioperative oxygen for reducing surgical site infection: a meta-analysis. J Eval Clin Pract 15360–365. 10.1111/j.1365-2753.2008.01016.x [DOI] [PubMed] [Google Scholar]
- Allen D. B., Maguire J. J., Mahdavian M., Wicke C., Marcocci L., Scheuenstuhl H., Chang M., Le A. X., Hopf H. W., Hunt T. K.(1997). Wound hypoxia and acidosis limit neutrophil bacterial killing mechanisms. Arch Surg 132991–996. 10.1001/archsurg.1997.01430330057009 [DOI] [PubMed] [Google Scholar]
- Anderson D. J.(2011). Surgical site infections. Infect Dis Clin North Am 25135–153. 10.1016/j.idc.2010.11.004 [DOI] [PubMed] [Google Scholar]
- Arsalan A., Alam M., Naqvi S. B. S., Ahmad I., Anwar Z.(2014). Oxygen as a facilitator in the reduction of surgical site infections. Sri Lanka J Surg 31 10.4038/sljs.v31i3.6412 [DOI] [Google Scholar]
- Awad S. S.(2012). Adherence to surgical care improvement project measures and post-operative surgical site infections. Surg Infect 13234–237. 10.1089/sur.2012.131 [DOI] [PubMed] [Google Scholar]
- Belda F. J., Aguilera L., García de la Asunción J., Alberti J., Vicente R., Ferrándiz L., Rodríguez R., Company R., Sessler D. I., et al. (2005). Supplemental perioperative oxygen and the risk of surgical wound infection: a randomized controlled trial. JAMA 2942035–2042. 10.1001/jama.294.16.2035 [DOI] [PubMed] [Google Scholar]
- Belda F. J., Catalá-López F., Greif R., Canet J.(2014). Benefits and risks of intraoperative high inspired oxygen therapy: firm conclusions are still far off. Anesthesiology 1201051–1052. 10.1097/ALN.0000000000000156 [DOI] [PubMed] [Google Scholar]
- Björnsdottir H., Welin A., Michaëlsson E., Osla V., Berg S., Christenson K., Sundqvist M., Dahlgren C., Karlsson A., Bylund J.(2015). Neutrophil NET formation is regulated from the inside by myeloperoxidase-processed reactive oxygen species. Free Radic Biol Med 891024–1035. 10.1016/j.freeradbiomed.2015.10.398 [DOI] [PubMed] [Google Scholar]
- Brar M. S., Brar S. S., Dixon E.(2011). Perioperative supplemental oxygen in colorectal patients: a meta-analysis. J Surg Res 166227–235. 10.1016/j.jss.2009.06.007 [DOI] [PubMed] [Google Scholar]
- Brinkmann V., Reichard U., Goosmann C., Fauler B., Uhlemann Y., Weiss D. S., Weinrauch Y., Zychlinsky A.(2004). Neutrophil extracellular traps kill bacteria. Science 3031532–1535. 10.1126/science.1092385 [DOI] [PubMed] [Google Scholar]
- Chura J. C., Boyd A., Argenta P. A.(2007). Surgical site infections and supplemental perioperative oxygen in colorectal surgery patients: a systematic review. Surg Infect 8455–461. 10.1089/sur.2006.034 [DOI] [PubMed] [Google Scholar]
- Dovi J., Szpaderska A. M., DiPietro L. A.(2004). Neutrophil function in the healing wound: adding insult to injury? Thromb Haemost 92275–280. 10.1160/TH03-11-0720 [DOI] [PubMed] [Google Scholar]
- Garrity-Ryan L., Shafikhani S., Balachandran P., Nguyen L., Oza J., Jakobsen T., Sargent J., Fang X., Cordwell S., et al. (2004). The ADP ribosyltransferase domain of Pseudomonas aeruginosa ExoT contributes to its biological activities. Infect Immun 72546–558. 10.1128/IAI.72.1.546-558.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacometti A., Cirioni O., Schimizzi A. M., Del Prete M. S., Barchiesi F., D'Errico M. M., Petrelli E., Scalise G.(2000). Epidemiology and microbiology of surgical wound infections. J Clin Microbiol 38918–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjødsbøl K., Christensen J. J., Karlsmark T., Jørgensen B., Klein B. M., Krogfelt K. A.(2006). Multiple bacterial species reside in chronic wounds: a longitudinal study. Int Wound J 3225–231. 10.1111/j.1742-481X.2006.00159.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldufsky J., Wood S., Hajihossainlou B., Rehman T., Majdobeh O., Kaufman H. L., Ruby C. E., Shafikhani S. H.(2015a). Pseudomonas aeruginosa exotoxin T induces potent cytotoxicity against a variety of murine and human cancer cell lines. J Med Microbiol 64164–173. 10.1099/jmm.0.000003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldufsky J., Wood S. J., Jayaraman V., Majdobeh O., Chen L., Qin S., Zhang C., DiPietro L. A., Shafikhani S. H.(2015b). Pseudomonas aeruginosa uses T3SS to inhibit diabetic wound healing. Wound Repair Regen 23557–564. 10.1111/wrr.12310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greif R., Laciny S., Rapf B., Hickle R. S., Sessler D. I.(1999). Supplemental oxygen reduces the incidence of postoperative nausea and vomiting. Anesthesiology 911246–1252. 10.1097/00000542-199911000-00014 [DOI] [PubMed] [Google Scholar]
- Greif R., Akça O., Horn E. P., Kurz A., Sessler D. I., Outcomes Research Group (2000). Supplemental perioperative oxygen to reduce the incidence of surgical-wound infection. N Engl J Med 342161–167. 10.1056/NEJM200001203420303 [DOI] [PubMed] [Google Scholar]
- Halbert A. R., Stacey M. C., Rohr J. B., Jopp-McKay A.(1992). The effect of bacterial colonization on venous ulcer healing. Australas J Dermatol 3375–80. [DOI] [PubMed] [Google Scholar]
- Hopf H. W., Hunt T. K., West J. M., Blomquist P., Goodson W. H., Jensen J. A., Jonsson K., Paty P. B., Rabkin J. M., et al. (1997). Wound tissue oxygen tension predicts the risk of wound infection in surgical patients. Arch Surg 132997–1004. discussion 1005. 10.1001/archsurg.1997.01430330063010 [DOI] [PubMed] [Google Scholar]
- Hovaguimian F., Lysakowski C., Elia N., Tramèr M. R.(2013). Effect of intraoperative high inspired oxygen fraction on surgical site infection, postoperative nausea and vomiting, and pulmonary function: systematic review and meta-analysis of randomized controlled trials. Anesthesiology 119303–316. 10.1097/ALN.0b013e31829aaff4 [DOI] [PubMed] [Google Scholar]
- Hunt T. K., Linsey M., Grislis H., Sonne M., Jawetz E.(1975). The effect of differing ambient oxygen tensions on wound infection. Ann Surg 18135–39. 10.1097/00000658-197501000-00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J., Kettle A. J., Rosen H., Winterbourn C. C., Nauseef W. M.(2013). Myeloperoxidase: a front-line defender against phagocytosed microorganisms. J Leukoc Biol 93185–198. 10.1189/jlb.0712349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klevens R. M., Edwards J. R., Richards C. L., Horan T. C., Gaynes R. P., Pollock D. A., Cardo D. M.(2007). Estimating health care-associated infections and deaths in U.S. hospitals, 2002. Public Health Rep 122160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knighton D. R., Halliday B., Hunt T. K.(1984). Oxygen as an antibiotic. Arch Surg 119199–204. 10.1001/archsurg.1984.01390140057010 [DOI] [PubMed] [Google Scholar]
- Knighton D. R., Halliday B., Hunt T. K.(1986). Oxygen as an antibiotic. Arch Surg 121191–195. 10.1001/archsurg.1986.01400020077009 [DOI] [PubMed] [Google Scholar]
- Kroin J. S., Buvanendran A., Li J., Moric M., Im H. J., Tuman K. J., Shafikhani S. H., Moric H. J., Im K. J. T.(2015). Short-term glycemic control is effective in reducing surgical site infection in diabetic rats. Anesth Analg 1201289–1296. 10.1213/ANE.0000000000000650 [DOI] [PubMed] [Google Scholar]
- Lin W. Y., Tsai S. C., Hung G. U., Kwan P. C., Lin C. F., Yuan C. S., Lin Y. C.(2005). Comparison of animal models with soft tissue infection by different bacilli. J Vet Med Sci 6743–49. 10.1292/jvms.67.43 [DOI] [PubMed] [Google Scholar]
- Madsen S. M., Westh H., Danielsen L., Rosdahl V. T.(1996). Bacterial colonization and healing of venous leg ulcers. APMIS 104895–899. 10.1111/j.1699-0463.1996.tb04955.x [DOI] [PubMed] [Google Scholar]
- Magill S. S., Hellinger W., Cohen J., Kay R., Bailey C., Boland B., Carey D., de Guzman J., Dominguez K., et al. (2012). Prevalence of healthcare-associated infections in acute care hospitals in Jacksonville, Florida. Infect Control Hosp Epidemiol 33283–291. 10.1086/664048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik A., Mohammad Z., Ahmad J.(2013). The diabetic foot infections: biofilms and antimicrobial resistance. Diabetes Metab Syndr 7101–107. 10.1016/j.dsx.2013.02.006 [DOI] [PubMed] [Google Scholar]
- Martin P.(1997). Wound healing – aiming for perfect skin regeneration. Science 27675–81. 10.1126/science.276.5309.75 [DOI] [PubMed] [Google Scholar]
- Nauseef W. M., Borregaard N.(2014). Neutrophils at work. Nat Immunol 15602–611. 10.1038/ni.2921 [DOI] [PubMed] [Google Scholar]
- Ohman D. E., Sadoff J. C., Iglewski B. H.(1980). Toxin A-deficient mutants of Pseudomonas aeruginosa PA103: isolation and characterization. Infect Immun 28899–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qadan M., Akça O., Mahid S. S., Polk H. C.(2009). Perioperative supplemental oxygen therapy and surgical site infection: a meta-analysis of randomized controlled trials. Arch Surg 144359–366. discussion 366–357. 10.1001/archsurg.2009.1 [DOI] [PubMed] [Google Scholar]
- Ramakant P., Verma A. K., Misra R., Prasad K. N., Chand G., Mishra A., Agarwal G., Agarwal A., Mishra S. K.(2011). Changing microbiological profile of pathogenic bacteria in diabetic foot infections: time for a rethink on which empirical therapy to choose? Diabetologia 5458–64. 10.1007/s00125-010-1893-7 [DOI] [PubMed] [Google Scholar]
- Scott R.(2009). The Direct Medical Costs of Healthcare-Associated Infections in US Hospitals and the Benefits of Prevention. Atlanta, GA: Centers for Disease Control and Prevention. [Google Scholar]
- Shafikhani S. H., Engel J.(2006). Pseudomonas aeruginosa type III-secreted toxin ExoT inhibits host-cell division by targeting cytokinesis at multiple steps. Proc Natl Acad Sci U S A 10315605–15610. 10.1073/pnas.0605949103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafikhani S. H., Morales C., Engel J.(2008). The Pseudomonas aeruginosa type III secreted toxin ExoT is necessary and sufficient to induce apoptosis in epithelial cells. Cell Microbiol 10994–1007. 10.1111/j.1462-5822.2007.01102.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöberg F., Singer M.(2013). The medical use of oxygen: a time for critical reappraisal. J Intern Med 274505–528. 10.1111/joim.12139 [DOI] [PubMed] [Google Scholar]
- Togioka B., Galvagno S., Sumida S., Murphy J., Ouanes J. P., Wu C.(2012). The role of perioperative high inspired oxygen therapy in reducing surgical site infection: a meta-analysis. Anesth Analg 114334–342. 10.1213/ANE.0b013e31823fada8 [DOI] [PubMed] [Google Scholar]
- Tsai W. C., Strieter R. M., Mehrad B., Newstead M. W., Zeng X., Standiford T. J.(2000). CXC chemokine receptor CXCR2 is essential for protective innate host response in murine Pseudomonas aeruginosa pneumonia. Infect Immun 684289–4296. 10.1128/IAI.68.7.4289-4296.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley C., Kaye S. B., Neal T. J., Chilton H. J., Miksch S., Hart C. A., Microbiology Ophthalmic Group (2005). Genotypic and phenotypic characteristics of Pseudomonas aeruginosa isolates associated with ulcerative keratitis. J Med Microbiol 54519–526. 10.1099/jmm.0.46005-0 [DOI] [PubMed] [Google Scholar]
- Winterbourn C. C., Kettle A. J., Hampton M. B.(2016). Reactive oxygen species and neutrophil function. Annu Rev Biochem 85765–792. 10.1146/annurev-biochem-060815-014442 [DOI] [PubMed] [Google Scholar]
- Wood S., Pithadia R., Rehman T., Zhang L., Plichta J., Radek K. A., Forsyth C., Keshavarzian A., Shafikhani S. H.(2013). Chronic alcohol exposure renders epithelial cells vulnerable to bacterial infection. PLoS One 8e54646. 10.1371/journal.pone.0054646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood S., Jayaraman V., Huelsmann E. J., Bonish B., Burgad D., Sivaramakrishnan G., Qin S., DiPietro L. A., Zloza A., et al. (2014). Pro-inflammatory chemokine CCL2 (MCP-1) promotes healing in diabetic wounds by restoring the macrophage response. PLoS One 9e91574. 10.1371/journal.pone.0091574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood S., Goldufsky J., Shafikhani S. H.(2015a). Pseudomonas aeruginosa ExoT induces atypical anoikis apoptosis in target host cells by transforming crk adaptor protein into a cytotoxin. PLoS Pathog 11e1004934 10.1371/journal.ppat.1004934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood S. J., Goldufsky J. W., Bello D., Masood S., Shafikhani S. H.(2015b). Pseudomonas aeruginosa ExoT induces mitochondrial apoptosis in target host cells in a manner that depends on its GAP domain activity. J Biol Chem 2729063–29073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao G., Hochwalt P. C., Usui M. L., Underwood R. A., Singh P. K., James G. A., Stewart P. S., Fleckman P., Olerud J. E.(2010). Delayed wound healing in diabetic (db/db) mice with Pseudomonas aeruginosa biofilm challenge: a model for the study of chronic wounds. Wound Repair Regen 18467–477. 10.1111/j.1524-475X.2010.00608.x [DOI] [PMC free article] [PubMed] [Google Scholar]