Abstract
Data on cutaneous human papillomavirus (HPV) seroprevalence are primarily derived from skin cancer case–control studies. Few studies have reported the seroprevalence of cutaneous HPV among healthy men. This study investigated the seroprevalence of cutaneous HPV types and associated risk factors among men residing in Brazil, Mexico and the USA. Six hundred men were randomly selected from the HPV Infection in Men study. Archived serum specimens were tested for antibodies against 14 cutaneous HPV genotypes, β-HPV types (5/8/12/14/17/22/23/24/38/48), α-HPV 27, γ-HPV 4, µ-HPV1 and ν-HPV 41 using a glutathione S-transferase L1-based multiplex serology assay. Risk factor data were collected by a questionnaire. Binomial proportions were used to estimate seroprevalence, and logistic regression to examine factors associated with seropositivity. Overall, 65.4 % of men were seropositive to ≥1 of the 14 cutaneous HPV types, and 39.0 % were positive for ≥1 β-HPV types. Seroprevalence was 8.9, 30.9, 28.6 and 9.4 % for α-HPV 27, γ-HPV 4, µ-HPV 1 and ν-HPV 41, respectively. In multivariate analyses, seropositivity for any cutaneous HPV type was associated with higher education [adjusted odds ratio (AOR) 1.75; 95 % confidence interval (CI) 1.08–2.83], and seropositivity of any β-HPV type was significantly associated with increasing age (AOR 1.72; 95 % CI 1.12–2.63, for men aged 31–44 years vs men aged 18–30 years). Other factors associated with various type-specific cutaneous HPV seropositivity included country, circumcision and lifetime number of male sexual partners. These data indicate that exposure to cutaneous HPV is common. Future studies are needed to assess the role of cutaneous HPV in diseases.
Keywords: Cutaneous HPV, Seroprevalence, the HIM Study, serology
Introduction
Human papillomavirus (HPV) is a non-enveloped dsDNA virus that infects epithelial cells of the skin and mucus membranes. Over 200 types of HPV have been recognized and fully sequenced, and the majority of these types fall within three genera: alpha (α), predominantly found on mucosal surfaces; and beta (β) and gamma (γ), which are commonly found on skin and in hair follicles (Bernard et al., 2010; Sichero et al., 2014).
Mucosal HPV types have been studied extensively with respect to their aetiological role in the development of genital warts (Lacey et al., 2006; Steben & Garland, 2014) and several cancers (Parkin & Bray, 2006; Walboomers et al., 1999). However, emerging evidence suggests that cutaneous HPV infection may increase the risk for squamous cell carcinoma (SCC) of the skin (Feltkamp et al., 2003; Iannacone et al., 2012; Karagas et al., 2006, 2010; Waterboer et al., 2008). Several studies have reported associations between cutaneous HPV serum antibodies and a higher risk for SCC (Andersson et al., 2008; Iannacone et al., 2012; Karagas et al., 2010; Struijk et al., 2006); while the exact mechanism is unclear, it is hypothesized that cutaneous HPV infection might modify the effect of UV-induced DNA damage and apoptosis, leading to the accumulation of mutations and SCC (Iannacone et al., 2012). Furthermore, cutaneous HPV types have also been detected on the surface of external genital lesions (Pierce Campbell et al., 2013) and penile cancer precursors in non-UV-exposed regions of the genital skin. Two hospital-based case–control studies have reported the presence of cutaneous HPV types in nearly all penile intraepithelial neoplasia cases (Kreuter et al., 2008; Wieland et al., 2000).
Cutaneous HPV DNA is abundantly found on the surfaces of skin and persists in the hair follicles of healthy individuals (Plasmeijer et al., 2009). This ubiquity and abundance of the DNA on skin may largely reflect viral colonization or deposits of viral DNA fragments due to the shedding of keratinocytes. Prevalence based on DNA detection varies considerably between the various types of specimens collected from the skin, e.g. skin swab versus biopsy. Serological studies measuring antibodies against the type-specific L1 major capsid protein of HPV provide evidence of a host immune response to previous or recent infection and indicate cumulative exposure to HPV over time. Prevalence based on serology is also not dependent on the anatomical site of HPV infection. However, there are two important limitations to HPV serology studies. Not all infected individuals develop antibodies against HPV infection, and antibodies may diminish over time.
Despite its potential role in the development of SCC and penile intraepithelial neoplasia, very little is known about the epidemiology of cutaneous HPV infection among men. The objective of the current study was to estimate the seroprevalence of cutaneous HPV types, including β (types 5/8/12/14/17/22/23/24/38/47), α (type 27), γ (type 4), µ (type 1) and ν (type 41), in a sub-cohort of healthy men from the HPV Infection in Men (HIM) study and to investigate the factors associated with the seropositivity.
Results
Significant differences in socio-demographic and behavioural characteristics were observed by country for all the listed variables in Table 1 (P values <0.05), except the number of male anal sex partners in the past 6 months among those reporting ever having had a male sex partner. Participants in the sub-cohort (600 randomly selected men) were similar (P value >0.05) to the full HIM study cohort (>4000 men) on all baseline socio-demographic and sexual behavioural characteristics.
Table 1. Participant characteristics by country of residence (USA, Brazil, Mexico).
| Characteristic | Country | P value* | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Overall (n=598) |
USA (n=184) |
Brazil (n=200) |
Mexico (n=214) |
||||||
| n | % | n | % | n | % | n | % | ||
| Age (years) | |||||||||
| 18–30 | 259 | 43.3 | 113 | 61.4 | 74 | 37.0 | 72 | 33.6 | <0.001 |
| 31–44 | 256 | 42.8 | 50 | 27.2 | 99 | 49.5 | 107 | 50.0 | |
| 45–70 | 83 | 13.9 | 21 | 11.4 | 27 | 13.5 | 35 | 16.4 | |
| Race | |||||||||
| White | 269 | 45.0 | 111 | 60.3 | 146 | 73.0 | 12 | 5.6 | <0.001 |
| Black | 78 | 13.0 | 40 | 21.7 | 38 | 19.0 | 0 | 0 | |
| Asian | 17 | 2.8 | 15 | 8.2 | 2 | 1.0 | 0 | 0 | |
| American Indian/Alaskan Native | 9 | 1.5 | 0 | 0 | 9 | 4.5 | 0 | 0 | |
| Other | 218 | 36.5 | 17 | 9.2 | 4 | 2.0 | 197 | 92.1 | |
| Not reported | 7 | 1.2 | 1 | 0.5 | 1 | 0.5 | 5 | 2.3 | |
| Ethnicity | |||||||||
| Hispanic | 282 | 47.2 | 22 | 12.0 | 49 | 24.5 | 211 | 98.6 | <0.001 |
| Non-Hispanic | 305 | 51.0 | 162 | 88.0 | 143 | 71.5 | 0 | 0 | |
| Not reported | 11 | 1.8 | 0 | 0 | 8 | 4.0 | 3 | 1.4 | |
| Education (years) | |||||||||
| ≤12 | 287 | 48.0 | 44 | 23.9 | 112 | 56.0 | 131 | 61.2 | <0.001 |
| 13–15 | 159 | 26.6 | 94 | 51.1 | 45 | 22.5 | 20 | 9.3 | |
| ≥16 | 149 | 24.9 | 45 | 24.5 | 43 | 21.5 | 61 | 28.5 | |
| Not reported | 3 | 0.5 | 1 | 0.5 | 0 | 0 | 2 | 0.9 | |
| Marital status | |||||||||
| Single/never married | 259 | 43.3 | 125 | 67.9 | 89 | 44.5 | 45 | 21.0 | <0.001 |
| Married/cohabitating | 278 | 46.5 | 37 | 20.1 | 84 | 42.0 | 157 | 73.4 | |
| Divorced/separated/widowed | 58 | 9.7 | 21 | 11.4 | 25 | 12.5 | 12 | 5.6 | |
| Not reported | 3 | 0.5 | 1 | 0.5 | 2 | 1.0 | 0 | 0 | |
| Smoking status | |||||||||
| Never | 339 | 56.7 | 111 | 60.3 | 129 | 64.5 | 99 | 46.3 | 0.003 |
| Former | 113 | 18.9 | 30 | 16.3 | 32 | 16.0 | 51 | 23.8 | |
| Current | 146 | 24.4 | 43 | 23.4 | 39 | 19.5 | 64 | 29.9 | |
| Alcohol (no. of drinks per month) | |||||||||
| 0 | 141 | 23.6 | 29 | 15.8 | 55 | 27.5 | 57 | 26.6 | 0.016 |
| 1–30 | 279 | 46.7 | 82 | 44.6 | 89 | 44.5 | 108 | 50.5 | |
| 31–60 | 58 | 9.7 | 22 | 12.0 | 18 | 9.0 | 18 | 8.4 | |
| ≥61 | 110 | 18.4 | 48 | 26.1 | 34 | 17.0 | 28 | 13.1 | |
| Not reported | 10 | 1.7 | 3 | 1.6 | 4 | 2.0 | 3 | 1.4 | |
| Circumcision | |||||||||
| No | 389 | 65.1 | 32 | 17.4 | 165 | 82.5 | 192 | 89.7 | <0.001 |
| Yes | 209 | 34.9 | 152 | 82.6 | 35 | 17.5 | 22 | 10.3 | |
| Sexual orientation | |||||||||
| MSW | 524 | 87.6 | 167 | 90.8 | 154 | 77.0 | 203 | 94.9 | <0.001 |
| MSM | 51 | 8.5 | 6 | 3.3 | 37 | 18.5 | 8 | 3.7 | |
| MSWM | 15 | 2.5 | 4 | 2.2 | 9 | 4.5 | 2 | 0.9 | |
| Missing | 8 | 1.3 | 7 | 3.8 | 0 | 0 | 1 | 0.5 | |
| No. of female LTP | |||||||||
| 0 | 60 | 10.0 | 29 | 15.8 | 15 | 7.5 | 16 | 7.5 | <0.001 |
| 1–3 | 126 | 21.1 | 42 | 22.8 | 26 | 13.0 | 58 | 27.1 | |
| 4–18 | 246 | 41.1 | 63 | 34.2 | 74 | 37.0 | 109 | 50.9 | |
| ≥19 | 126 | 21.1 | 41 | 22.3 | 65 | 32.5 | 20 | 9.3 | |
| Not reported | 40 | 6.7 | 9 | 4.9 | 20 | 10.0 | 11 | 5.1 | |
| No. of male anal LTP | |||||||||
| 0 | 507 | 84.8 | 176 | 95.7 | 135 | 67.5 | 196 | 91.6 | <0.001 |
| 1 | 28 | 4.7 | 1 | 0.5 | 17 | 8.5 | 10 | 4.7 | |
| 2 or more | 58 | 9.7 | 7 | 3.8 | 43 | 21.5 | 8 | 3.7 | |
| Not reported | 5 | 0.8 | 0 | 0 | 5 | 2.5 | 0 | 0 | |
| No. of female sex partners in the past 6 months† | |||||||||
| 0 | 115 | 21.4 | 26 | 16.8 | 34 | 18.4 | 55 | 27.8 | <0.001 |
| 1 | 241 | 44.8 | 80 | 51.6 | 74 | 40.0 | 87 | 43.9 | |
| ≥2 | 162 | 30.1 | 49 | 31.6 | 72 | 38.9 | 41 | 20.7 | |
| Not reported | 20 | 3.7 | 0 | 0 | 5 | 7.6 | 15 | 7.6 | |
| No. of male sex partners in the past 6 months† | 0.133 | ||||||||
| 0 | 55 | 60.4 | 2 | 25.0 | 39 | 60.0 | 14 | 77.8 | |
| 1 | 13 | 14.3 | 2 | 25.0 | 9 | 13.9 | 2 | 11.1 | |
| ≥2 | 21 | 23.1 | 4 | 50.0 | 16 | 24.6 | 1 | 5.6 | |
| Not reported | 2 | 2.2 | 0 | 0 | 1 | 1.5 | 1 | 5.6 | |
LTP, lifetime sex partners; MSM, men having sex with men; MSW, men having sex with women; MSWM, men having sex with women and men.
*P value is from the chi-square test. When the expected frequency in ≥1 cells was ≤5 then Fisher’s exact test was used. Significant P values are highlighted in bold.
†Among those reporting ever having a female/male sex partner.
Overall, 65.4 % participants were seropositive to one or more cutaneous HPV type (Table 2). The seroprevalence was 39.0 % for at least one β-cutaneous HPV type, 8.9 % for α-cutaneous type 27, 30.9 % for γ-HPV type 4, 28.6 % for µ-HPV type 1 and 9.4 % for ν-HPV type 41 (Table 2; Fig. S1a–d, available in the online Supplementary Material). The highest overall type-specific seroprevalences were observed for γ-HPV type 4 (30.9 %), µ-HPV type 1 (28.6 %) and β-HPV type 8 (20.4 %). No significant difference (P value=0.140) in the seroprevalence of any-HPV was observed by country (Brazil, 70.0 %; USA, 65.4 %; Mexico, 60.7 %). Significant differences in cutaneous HPV seroprevalence by country were observed for any β-HPV, as well as for each of the HPV type-specific seroprevalences listed, with the exceptions of HPV 27 and HPV 41 (Table 2). Using the Holm–Bonferroni method, we observed significantly higher grouped β-HPV seroprevalence among men in Brazil compared to Mexico (P value <0.001).
Table 2. Type-specific cutaneous HPV seroprevalence among men residing in the USA, Brazil and Mexico.
A total of 600 subjects were randomly selected at baseline from the HIM study to participate in the cutaneous HPV seroprevalence study. Seroprevalence data for two subjects were not available; therefore, these subjects were excluded from the analysis. The chi-square test was used to calculate P values; except for any-HPV, α-HPV 27 and ν-HPV 41, all other HPV types differed by country at a P value <0.05.
| Type of HPV | Seroprevalence (%) | |||
|---|---|---|---|---|
| Overall (n=598) | USA (n=184) | Brazil (n=200) | Mexico (n=214) | |
| Any-HPV | 65.4 | 65.8 | 70.0 | 60.7 |
| Any β-HPV | 39.0 | 39.1 | 48.5 | 29.9 |
| β-HPV 5 | 7.7 | 6.5 | 15.0 | 1.9 |
| β-HPV 8 | 20.4 | 21.2 | 29.5 | 11.2 |
| β-HPV 12 | 6.0 | 3.3 | 13.0 | 1.9 |
| β-HPV 14 | 5.7 | 3.8 | 11.0 | 2.3 |
| β-HPV 17 | 13.0 | 10.9 | 19.5 | 8.9 |
| β-HPV 22 | 6.9 | 6.5 | 11.0 | 3.3 |
| β-HPV 23 | 12.2 | 12.0 | 19.5 | 5.6 |
| β-HPV 24 | 7.7 | 7.1 | 12.5 | 3.7 |
| β-HPV 38 | 13.9 | 14.1 | 21.0 | 7.0 |
| β-HPV 47 | 12.4 | 13.0 | 16.5 | 7.9 |
| α-HPV 27 | 8.9 | 9.2 | 11.5 | 6.1 |
| γ-HPV 4 | 30.9 | 33.2 | 37.5 | 22.9 |
| µ-HPV 1 | 28.6 | 37.5 | 34.5 | 15.4 |
| ν-HPV 41 | 9.4 | 7.6 | 12.0 | 8.4 |
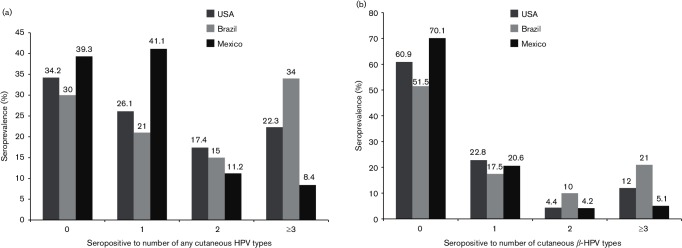
Averaged across all three countries, approximately 21.2 % of men had antibodies to three or more HPV types, 14.4 % to two HPV types, 29.8 % to only one HPV type and 34.6 % did not have antibodies against any type. Seropositivity to multiple HPV infections was statistically different by country (overall P value <0.001) and remained significant after adjusting for pairwise comparisons (P value <0.05). Men in Brazil were more likely to be seropositive to three or more HPV types (34.0 %) compared to men from the USA (22.3 %) and Mexico (8.4 %) (Fig. 1a, b).
Fig. 1.
(a) Seropositivity for zero, one, two and three or more cutaneous HPV types by country. If a person was negative for all 14 genotypes, he was categorized in the seropositivity in the ‘0 HPV’ group. If a person was positive for only one genotype, he was categorized in the seropositivity to ‘1 HPV’ group; if he was positive for two genotypes, he was categorized in the ‘2 HPV’ group; if he was positive for three or more genotypes, he was categorized in the ‘≥3’ group. The chi-square P value represents differences in each category by country. Distributions of seropositivity to 0, 1, 2 and ≥3 HPV types significantly differed by country in all categories (for each group the overall P value was <0.05). (b) Seropositivity for zero, one, two and three or more β-HPV types by country. If a person was negative for all 10 β genotypes, he was categorized in the seropositivity in the ‘0 β-HPV’ group. If a person was positive for only one β genotype, he was categorized in the seropositivity to ‘1 β-HPV’ group; if he was positive for two β genotypes, he was categorized in the ‘2 β-HPV’ group; if he was positive for three or more β genotypes, he was categorized in the ‘≥ 3 β-HPV’ group. The chi-square P value represents differences in each category by country. Distributions of seropositivity to zero, one, two and three or more β-HPV types significantly differed by country in all categories (for each group the overall P value was <0.05).
Factors associated with seropositivity to cutaneous HPV types (any-HPV, any β-HPV, γ-HPV4, µ-HPV, β-HPV 8, β-HPV17, β-HPV 23, β-HPV 38 and β-HPV 47) are presented in Tables 3 and 4, and for HPV types with seroprevalence of <10 %, type-specific logistic regression models are reported in Tables S1 and S2. In the multivariate model, adjusting for all variables listed in Table 3, country was significantly associated with seropositivity to β-HPV 47 [adjusted odds ratio (AOR) 2.67; 95 % confidence interval (CI) 1.19–5.99], with men from Brazil more likely to be seropositive than men from the USA. Compared to the youngest age group (18–30 years), mid-adult aged men (31–44 years) were significantly more likely to be seropositive for any β-HPV (AOR 1.72; 95 % CI 1.12–2.63), β-HPV 8 (AOR 2.28; 95 % CI 1.33–3.91), β-HPV 38 (AOR 2.01; 95 % CI 1.09–3.72) and β-HPV 47 (AOR 2.00; 95 % CI 1.03–3.88), and men in the oldest age group (45–73 years) were more likely to be seropositive for β-HPV 8 and β-HPV 47. Higher educational attainment was significantly associated with any-HPV (AOR 1.75 for ≥16 years of education compared to ≤12 years of education, 95 % CI 1.08–2.83) and γ-HPV 4 (AOR 1.77 for ≥16 years of education compared to ≤12 years of education, 95 % CI 1.10–2.82). Circumcised men were more likely to be seropositive for γ-HPV 4 (AOR 1.72; 95 % CI 1.02–2.89) and β-HPV 47 (AOR 2.37; 95 % CI 1.21–4.67). Similarly, having two or more lifetime male sex partners was significantly independently associated with seropositivity for β-HPV 17 (AOR 2.22; 95 % CI 1.06–4.62) and β-HPV 23 (AOR 2.39; 95 % CI 1.07–5.33).
Table 3. Factors independently associated with any cutaneous HPV, β-, γ- and µ-HPV seropositivity among men residing in the USA, Brazil and Mexico.
The final model was selected through backward stepwise elimination with a significance level of P value ≤0.1 for retention in the model. Country, age, race, marital status, education, smoking, alcohol consumption, circumcision, number of female LTP and number of male LTP were loaded to the model, while forcing country and age (design factors) to stay in the model. Significant results are highlighted in bold. LTP, lifetime sex partners.
| Characteristic | Any-HPV* | Any β-HPV* | γ-HPV 4 | µ-HPV 1 | ||||
|---|---|---|---|---|---|---|---|---|
| OR | AOR (95 % CI) | OR | AOR (95 % CI) | OR | AOR (95 % CI) | OR | AOR (95 % CI) | |
| Country | ||||||||
| USA | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Brazil | 1.21 | 1.41 (0.76–2.63) | 1.46 | 1.44 (0.80–2.61) | 1.21 | 1.76 (0.95–3.26) | 0.88 | 1.17 (0.63–2.17) |
| Mexico | 0.81 | 0.75 (0.31–1.85) | 0.66 | 0.40 (0.16–0.99) | 0.60 | 0.81 (0.31–2.17) | 0.30 | 0.47 (0.17–1.29) |
| Age (years) | ||||||||
| 18–30 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 31–44 | 1.07 | 1.20 (0.79–1.83) | 1.54 | 1.72 (1.12–2.63) | 1.04 | 1.09 (0.70–1.71) | 0.87 | 1.12 (0.70–1.79) |
| 45–70 | 1.08 | 1.04 (0.58–1.85) | 1.54 | 1.63 (0.91–2.92) | 1.51 | 1.39 (0.77–2.53) | 1.02 | 1.33 (0.71–2.49) |
| Race | ||||||||
| White | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Black | 0.63 | 0.56 (0.32–0.98) | 0.80 | 0.72 (0.41–1.28) | 0.59 | 0.58 (0.31–1.06) | 0.69 | 0.64 (0.35–1.17) |
| Asian/American Indian/Alaskan Native | 1.46 | 1.70 (0.59–4.88) | 1.28 | 1.47 (0.60–3.59) | 0.85 | 1.06 (0.42–2.68) | 2.38 | 2.78 (1.12–6.89) |
| Other | 0.70 | 1.01 (0.44–2.30) | 0.58 | 1.53 (0.65–3.60) | 0.50 | 0.86 (0.35–2.14) | 0.35 | 0.70 (0.27–1.80) |
| Education (years) | ||||||||
| ≤12 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 13–15 | 1.08 | 0.89 (0.55–1.44) | 1.26 | 1.19 (0.74–1.93) | 1.22 | 1.06 (0.64–1.76) | 1.63 | 0.91 (0.54–1.53) |
| ≥16 | 1.75 | 1.75 (1.08–2.83) | 1.53 | 1.46 (0.93–2.30) | 1.91 | 1.77 (1.10–2.82) | 1.50 | 1.41 (0.86–2.32) |
| Circumcision | ||||||||
| No | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Yes | 1.15 | 1.36 (0.80–2.30) | 1.12 | 1.23 (0.74–2.04) | 1.57 | 1.72 (1.02–2.89) | 1.96 | 1.50 (0.88–2.56) |
| No. of female LTP | ||||||||
| 0 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 1–3 | 0.69 | 0.69 (0.34–1.40) | 0.73 | 0.90 (0.46–1.79) | 0.98 | 0.94 (0.45–1.98) | 1.10 | 1.24 (0.59–2.62) |
| 4–18 | 0.79 | 0.79 (0.41–1.55) | 0.93 | 1.03 (0.54–1.94) | 1.30 | 1.26 (0.63–2.49) | 1.12 | 1.25 (0.62–2.52) |
| ≥19 | 0.69 | 0.56 (0.27–1.15) | 0.98 | 0.77 (0.38–1.54) | 1.48 | 1.03 (0.49–2.18) | 1.10 | 0.85 (0.39–1.84) |
| No. of male LTP | ||||||||
| 0 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 1 | 0.86 | 0.86 (0.36–2.09) | 1.77 | 1.91 (0.80–4.56) | 0.76 | 0.69 (0.26–1.84) | 0.69 | 0.69 (0.24–1.97) |
| ≥2 | 1.74 | 1.27 (0.63–2.54) | 2.34 | 1.81 (0.97–3.38) | 1.40 | 1.05 (0.55–2.02) | 1.23 | 0.85 (0.43–1.68) |
*The any-HPV category included men seropositive for at least one HPV genotype, and the any β-HPV category included men seropositive for at least one β-HPV type.
Table 4. Factors independently associated with β cutaneous HPV seropositivity among men residing in the USA, Brazil, and Mexico.
The final model was selected through backward stepwise elimination with a significance level of P value ≤0.1 for retention in the model. Country, age, race, marital status, education, smoking, alcohol consumption, circumcision, number of female LTP and number of male LTP were loaded to the model, while forcing country and age (design factors) to stay in the model. Significant results are highlighted in bold. LTP, lifetime sex partners.
| β-HPV 8 | β-HPV 17 | β-HPV 23 | β-HPV 38 | β-HPV 47 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristics | OR | AOR (95%CI) | OR | AOR (95%CI) | OR | AOR (95%CI) | OR | AOR (95%CI) | OR | AOR (95%CI) |
| Country | ||||||||||
| U.S. | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Brazil | 1.56 | 1.24 (0.62–2.48) | 1.99 | 1.98 (0.87–4.55) | 1.78 | 1.37 (0.58–3.26) | 1.62 | 1.10 (0.49–2.44) | 1.32 | 2.67 (1.19–5.99) |
| Mexico | 0.47 | 0.52 (0.16–1.71) | 0.80 | 0.81 (0.19–3.53) | 0.44 | 0.28 (0.06–1.26) | 0.46 | 0.20 (0.06–0.75) | 0.58 | 0.42 (0.11–1.64) |
| Age, Years | ||||||||||
| 18-30 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 31-44 | 2.13 | 2.28 (1.33–3.91) | 1.68 | 1.86 (0.98–3.51) | 1.81 | 1.79 (0.90–3.53) | 1.86 | 2.01 (1.09–3.72) | 1.34 | 2.00 (1.03–3.88) |
| 45-73 | 2.45 | 2.83 (1.41–5.65) | 1.90 | 1.95 (0.85–4.48) | 1.19 | 1.23 (0.47–3.26) | 1.66 | 1.77 (0.77–4.05) | 2.41 | 3.16 (1.41–7.06) |
| Race | ||||||||||
| White | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Black | 0.73 | 0.64 (0.33–1.25) | 0.91 | 0.83 (0.38–1.83) | 0.65 | 0.68 (0.28–1.66) | 0.78 | 0.80 (0.37–1.73) | 0.84 | 0.85 (0.38–1.89) |
| Asian/American Indian/Alaska Native | 0.79 | 1.02 (0.35–3.01) | 1.32 | 1.40 (0.43–4.56) | 3.58 | 4.75 (1.71–13.14) | 1.42 | 2.23 (0.77–6.44) | 1.04 | 1.04 (0.28–3.83) |
| Other | 0.33 | 0.57 (0.19–1.71) | 0.53 | 0.93 (0.24–3.65) | 0.36 | 1.25 (0.31–5.12) | 0.43 | 1.48 (0.44–5.02) | 0.55 | 2.29 (0.63–8.25) |
| Education | ||||||||||
| ≤12 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 13-15 | 0.90 | 0.82 (0.45–1.51) | 1.10 | 1.32 (0.66–2.62) | 1.53 | 1.25 (0.58–2.69) | 1.07 | 0.99 (0.50–1.96) | 1.43 | 1.63 (0.82–3.26) |
| ≥16 | 1.50 | 1.28 (0.74–2.21) | 1.25 | 1.17 (0.61–2.28) | 2.02 | 2.02 (1.00–4.07) | 1.80 | 1.56 (0.83–2.92) | 1.54 | 1.36 (0.69–2.66) |
| Circumcision | ||||||||||
| No | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Yes | 1.21 | 1.09 (0.60–1.98) | 1.05 | 1.38 (0.68–2.77) | 1.03 | 1.24 (0.59–2.59) | 0.94 | 0.81 (0.40–1.63) | 1.70 | 2.37 (1.21–4.67) |
| No. of Female LTP | ||||||||||
| 0 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 1-3 | 0.55 | 0.69 (0.30–1.60) | 0.78 | 1.10 (0.43–2.80) | 0.80 | 1.13 (0.37–3.47) | 1.02 | 1.74 (0.60–5.02) | 0.88 | 1.02 (0.38–2.73) |
| 4-18 | 0.88 | 0.90 (0.42–1.91) | 0.69 | 0.83 (0.34–2.01) | 1.05 | 1.53 (0.55–4.23) | 1.34 | 2.02 (0.75–5.40) | 1.01 | 0.94 (0.38–2.31) |
| ≥19 | 1.07 | 0.63 (0.28–1.45) | 0.78 | 0.63 (0.24–1.67) | 1.26 | 1.10 (0.37–3.33) | 1.43 | 1.23 (0.43–3.56) | 0.95 | 0.58 (0.21–1.59) |
| No. of Male LTP | ||||||||||
| 0 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 1 | 0.98 | 0.86 (0.29–2.52) | 0.95 | 0.61 (0.13–2.77) | 2.39 | 2.43 (0.80–7.45) | 1.24 | 1.14 (0.35–3.68) | 0.56 | 0.58 (0.13–2.69) |
| ≥2 | 2.96 | 1.80 (0.92–3.51) | 3.27 | 2.22 (1.06–4.62) | 2.78 | 2.39 (1.07–5.33) | 2.84 | 1.97 (0.94–4.16) | 1.52 | 0.95 (0.41–2.24) |
Discussion
This is, to the best of our knowledge, the first study to estimate cutaneous HPV seroprevalence in a healthy population of men from three countries. We have reported the seroprevalence of 14 cutaneous HPV types and factors associated with grouped and individual HPV seroprevalence in 600 men from the HIM study residing in the USA, Mexico and Brazil. More than 65 % of men were seropositive for one or more cutaneous HPV infection type, and more than 39 % to one or more β-HPV type. Elevated risk of cutaneous HPV seropositivity was observed among men who were mid-adult and older ages, from Brazil, had attained ≥16 years of education, were circumcised, and reported two or more lifetime male sex partners.
Previous to our population-based study, other estimates of cutaneous HPV seroprevalence were available only from control series of case–control studies of skin cancer. Due to differences in the populations studied, age distribution and the number of HPV types assessed, a direct comparison with the published literature is not possible. A cross-sectional study (Iannacone et al., 2010) of cutaneous HPV seroprevalence among men and women undergoing routine skin cancer screening in Tampa, FL, USA, reported an overall seroprevalence for any-HPV of 96.0 %, and 76.0 % for any β-HPV in men, which is higher than what we observed at 65.4 and 39.0 %, respectively. Differences between this and the cross-sectional study (Iannacone et al., 2010) may be due to the older age of the study population in the cross-sectional study, the recruitment from a skin cancer screening clinic and that analysis of the overall seroprevalence included 36 types of HPV compared with 14 types in our study. Type-specific seroprevalence estimates in the cross-sectional study (Iannacone et al., 2010) were also slightly higher for γ-HPV 4 (46.0 vs 39.0 % in our study), µ-HPV 1 (37.0 vs 28.6 % in our study) and ν-HPV 41 (14.0 vs 9.4 % in our study). The ubiquity of the cutaneous HPV suggests that cutaneous HPV might be a commensal component of the microbiological flora of the skin (Antonsson et al., 2000; Hannigan & Grice, 2013). One case–control study of SCC (Andersson et al., 2008) from Sweden and Austria reported seroprevalence estimates for controls that were comparable to those in this study for any β-HPV (42.0 %) and slightly higher seroprevalence estimates for type-specific HPV; however, the study population in that case–control study was older and the number of β-HPV tested was eight compared to 10 in our study. Some of the type-specific seroprevalence estimates in our study were also comparable with seroprevalence estimates reported from the control groups of a systematic review (Bzhalava et al., 2013) of 15 studies examining the associations between cutaneous HPV and skin cancer: β-HPV 8, 22.8 vs 20.4 % in our study; β-HPV 23, 12.4 vs 12.2 %; α-HPV 27, 12.5 vs 8.9%; γ-HPV 4, 33.7 vs 30.9 %; µ-HPV 1, 27.1 vs 28.6 %; and ν-HPV 41, 11.8 vs 9.4 %.
The increase in seroprevalence with increasing age observed in our study is consistent with studies of cutaneous HPV DNA detection conducted in the general population (Boxman et al., 2001; de Koning et al., 2009). Boxman et al. (2001) found strong associations between increasing age and cutaneous HPV DNA prevalence in the general population, with 29 % DNA prevalence of any cutaneous HPV among 25–39-year-olds, 42 % in 40–59-year-olds and 65 % in 60–79-year-olds. A study by de Koning et al. (2009) demonstrated a marginally significant association between increasing age and the DNA prevalence of grouped β-HPV in the general population in Italy and Australia. However, neither study assessed associations between age and individual HPV types. Furthermore, few skin cancer case–control studies have assessed factors associated with cutaneous HPV seroprevalence in controls. Struijk et al. (2003) and Hampras et al. (2014) reported a significant association between increasing age and cutaneous HPV DNA prevalence. However, Iannacone et al. (2012) did not report an association between age and seropositivity to cutaneous HPV. In multiple studies of mucosal HPV seroprevalence among men, in general, seroprevalence has been shown to increase with age (Dunne et al., 2009; Hariri et al., 2008; Liu et al., 2016; Lu et al., 2011). However, among women the peak of seroprevalence is noted at 25–40 years, which decreases or plateaus with older age (Tiggelaar et al., 2012). Cutaneous HPV seroprevalence in the current study also varied across geographical regions, with men in Brazil having the highest seroprevalence and men in Mexico the lowest for certain β-HPV types. This is consistent with our previous findings where we reported higher seroprevalence among Brazilian men for mucosal HPV genotypes (Lu et al., 2011). Other multi-centre studies have also reported a geographical variation of cutaneous HPV DNA prevalence (de Koning et al., 2009; Sampogna et al., 2012; Sichero et al., 2014). It is possible that the observed geographical variation is reflective of the differences in individual immune responses, environmental factors, sexual behaviours and other unobserved factors. The observed difference in our study is unlikely to be due to specimen collection or antibody testing techniques as study protocols were uniformly applied across the three study centres, and the serum specimens were tested using the same assay in a single laboratory.
A pattern of elevated risk of cutaneous HPV seropositivity among circumcised men was observed, specifically for γ-HPV 4, and β types 5, 14, 24 and 47. To our knowledge, this is the first study to compare the seropositivity of cutaneous HPV between circumcised and uncircumcised men. In a previous study of the HIM study population, Sichero et al. (2014) did not find cutaneous HPV DNA to be associated with circumcision. Similarly, Albero et al. (2014) did not find an association between circumcision and the DNA prevalence of any mucosal HPV, oncogenic HPV and unclassified HPV types, but found a weak inverse association for non-oncogenic HPV types. However, Lu et al. (2011) reported an association between the seroprevalence of mucosal HPV type 11 and circumcision, with lower seroprevalence among circumcised men. These findings suggest that the role of circumcision and HPV prevalence is complex, and may depend on HPV type (cutaneous or mucosal), anatomical site of infection and whether prevalence is based on DNA detection or antibody response. Although the inverse association of circumcision with mucosal HPV prevalence has been reported in other populations (Albero et al., 2012; Giuliano et al., 2009), the role of circumcision and cutaneous HPV prevalence is largely unknown. One possible explanation for this positive association could be the keratinization of the glans penis after circumcision. At birth, the glans is covered by the foreskin or prepuce, which is often removed during infancy by circumcision (Warren, 2010). The glans is non-keratinized before circumcision and becomes keratinized after circumcision (Taylor et al., 1996; Warren, 2010). In contrast to mucosal HPV, cutaneous HPVs mainly infect skin and keratinized epithelium. Alternatively, it is also possible that circumcised penile tissues might have a different immunogenic response to HPV than uncircumcised penile tissues. However, men in Brazil were mostly uncircumcised, but had a higher prevalence of HPV DNA and seroprevalence. It is also possible that the observed difference in seropositivity between circumcised and uncircumcised men is due to residual confounding from life-style differences between these men. Cutaneous HPV DNA has been commonly detected on normal skin (de Koning et al., 2009), on skin tumours (Ekstrom et al., 2011), in the oral cavity (Bottalico et al., 2011) and in the anogenital tract (Sichero et al., 2014). Given that the vast majority of studies have examined cutaneous HPV from non-genital anatomical sites, other modes of transmission, besides skin to skin contact, might warrant further investigations.
Education was significantly associated with any-HPV, γ-HPV type 4 and β-HPV type 24. Men with higher education were more likely to be seropositive, an association also observed in the study of Farzan et al. (2013) for some types of cutaneous HPV and in the study of Molano et al. (2002) for mucosal HPV types. It is likely that education functions as a marker of lifestyle characteristics that may influence cutaneous HPV acquisition and natural history. It is possible that men with higher education have more opportunities of meeting new partners (Chandra et al., 2011) and increased skin to skin contact, which could result in a higher level of exposure to HPV. Seropositivity to β types 17, 23 and 24 was positively associated with the number of male lifetime sex partners. This finding is consistent with previous studies of mucosal HPV (Hariri et al., 2008; Lu et al., 2011; Svare et al., 2002), and could be explained by a higher number of sexual partners increasing the probability of viral exposure, transmission and risk of infection.
The major strengths of this study are its large sample size, estimation of prevalence based on antibodies against L1 HPV capsid protein in a single laboratory simultaneously by multiplex serology, and the availability of detailed demographic and sexual behaviour data. We also acknowledge some limitations. Considering the cross-sectional nature of the current study, causal associations could not be determined. Another limitation is that the measurement of antibodies against L1 viral protein does not necessarily represent current infection with HPV. Furthermore, due to lack of information of the serodynamics of cutaneous HPV, the impact of seroconversion, clearance of infections and waning of antibodies with time could not be assessed; however, these are limitations inherent to all seroprevalence studies. Despite its limitations, the current study provides important data, and contributes to the understanding of the distribution and associated factors of cutaneous HPV, which is largely unknown. To validate these results, future large prospective studies of cutaneous HPV with repeated measures are recommended to evaluate the aetiological role of cutaneous HPV in the development of skin lesions including skin cancer, and benign and malignant diseases of the genitalia. Also, future studies should focus on identifying the viral mechanism and pathway of pathogenesis for cutaneous HPV infections in different epithelial sites, which are currently largely unknown.
Methods
Study population.
This study included a sub-cohort of 600 men randomly selected from the HIM study, a prospective cohort of the natural history of HPV infections in men. The HIM study population and methods have been described previously in detail (Giuliano et al., 2008, 2011). Briefly, between July 2005 and September 2009, the HIM study enrolled over 4000 men aged 18–70 years at baseline from Tampa, FL, USA; Sao Paulo, Brazil; and Cuernavaca, Mexico. Men were recruited from the general population, universities and organized health-care systems. Eligibility criteria included no previous history of penile cancer, anal cancer, anogenital warts or human immunodeficiency virus; no current history or treatment for sexually transmitted infections; no current discharges from the penis or burning sensation during urination; and no history of prior or current participation in HPV vaccine trails. A total of 3695 HIM study participants who provided a serum sample and completed the questionnaire at baseline were eligible for the current study. The study was approved by the Institutional Review Boards of the University of South Florida (Tampa, FL, USA), the Ludwig Institute for Cancer Research and Centro de Referência e Treinamento DST/AIDS-SP (Sao Paulo, Brazil) and the Instituto Nacional de Salud Pública de Mexico (Cuernavaca, Mexico). All participants had provided written informed consent.
Specimen and data collection.
At the baseline visit, HIM study participants provided detailed information on socio-demographics, lifestyle, and medical and sexual history. Archived baseline serum samples from the 600 participants were tested for seroreactivity to the L1 protein of 14 cutaneous HPV types, including β-types (5/8/12/14/17/22/23/24/38/47), α-type 27, γ-type 4, µ-type 1 and ν-type 41. Only 14 types were tested due to limited funds, and were carefully selected based on previous reports of their association with cutaneous SCC (Iannacone et al., 2012; Waterboer et al., 2008) and their detection on the surface of external genital lesions (Pierce Campbell et al., 2013). The antibody detection method was based on a glutathione S-transferase capture ELISA, in combination with fluorescent bead technology, as previously described (Sehr et al., 2002; Waterboer et al., 2005). To define type-specific HPV seropositivity, cut-off values [200 median fluorescence intensity (MFI) units] were applied, as previously described (Casabonne et al., 2007; Michael et al., 2008). Serology results for two subjects were inadequate and were, therefore, excluded from analysis, resulting in a final sample size of 598 men.
Statistical analysis.
Baseline participant characteristics, including demographic, lifestyle and sexual behaviour factors, were compared between participants from the USA, Brazil and Mexico using the chi-square or Fisher’s exact test for categorical variables (Table 1). Participants in the randomly selected sub-cohort (n=600) were also compared to the full HIM study cohort (>4000 men) on all baseline socio-demographic and sexual behavioural characteristics listed in Table 1. Seropositivity to any cutaneous HPV (any-HPV) was defined as the proportion of men who were seropositive to at least one of the 14 types of HPV included in this study, and seropositivity to any β-HPV was defined as the proportion of men who were seropositive to at least one of the 10 β-HPV types. Type-specific and grouped seroprevalence was calculated for the overall study population and by country. Holms–Bonferroni correction of P values was used to account for post hoc multiple comparisons (Holm, 1979). Seropositivity to one, two and three or more of the 14 cutaneous types was also estimated. To assess factors associated with seroprevalence of type-specific, any β-HPV and any-HPV in univariate analyses, logistic regression was used to estimate odds ratios (ORs) and their 95 % CI values. As seroprevalence was low (<10 %) for some HPV types, type-specific logistic regression models were reported only for those types with a seroprevalence of 10 % or higher. To assess factors associated with cutaneous HPV seroprevalence, the factors listed in Table 1 were considered in modelling associations with any-HPV and any β-HPV seroprevalence in multivariate models. A backward stepwise elimination logistic regression procedure was conducted, using significance at a P value ≤0.1 for retention. Country and age were forced into the model due to study design. The final multivariate model included country, age, race, education, circumcision, number of female lifetime sex partners and number of male lifetime sex partners. Using these adjustment factors, separate multivariate models were estimated for seropositivity to any-HPV, any β-HPV and type-specific HPV. All analyses were performed using sas 9.3.
Acknowledgements
We thank the HIM study teams and participants in the USA (Moffitt Cancer Center, Tampa, FL), Brazil (Centro de Referência e Treinamento em DST/AIDS, Fundação Faculdade de Medicina Instituto do Câncer do Estado de São Paulo, Ludwig Institute for Cancer Research, São Paulo) and Mexico (Instituto Mexicano del Seguro Social, Instituto Nacional de Salud Pública, Cuernavaca).
Footnotes
Two supplementary tables and one supplementary figure are available with the online Supplementary Material.
Supplementary Data
References
- Albero G., Castellsagué X., Giuliano A. R., Bosch F. X.(2012). Male circumcision and genital human papillomavirus: a systematic review and meta-analysis. Sex Transm Dis 39104–113. 10.1097/OLQ.0b013e3182387abd [DOI] [PubMed] [Google Scholar]
- Albero G., Castellsagué X., Lin H. Y., Fulp W., Villa L. L., Lazcano-Ponce E., Papenfuss M., Abrahamsen M., Salmerón J., et al. (2014). Male circumcision and the incidence and clearance of genital human papillomavirus (HPV) infection in men: the HPV Infection in Men (HIM) cohort study. BMC Infect Dis 1475. 10.1186/1471-2334-14-75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson K., Waterboer T., Kirnbauer R., Slupetzky K., Iftner T., de Villiers E. M., Forslund O., Pawlita M., Dillner J.(2008). Seroreactivity to cutaneous human papillomaviruses among patients with nonmelanoma skin cancer or benign skin lesions. Cancer Epidemiol Biomarkers Prev 17189–195. 10.1158/1055-9965.EPI-07-0405 [DOI] [PubMed] [Google Scholar]
- Antonsson A., Forslund O., Ekberg H., Sterner G., Hansson B. G.(2000). The ubiquity and impressive genomic diversity of human skin papillomaviruses suggest a commensalic nature of these viruses. J Virol 7411636–11641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard H. U., Burk R. D., Chen Z., van Doorslaer K., zur Hausen H., de Villiers E. M.(2010). Classification of papillomaviruses (PVs) based on 189 PV types and proposal of taxonomic amendments. Virology 40170–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottalico D., Chen Z., Dunne A., Ostoloza J., McKinney S., Sun C., Schlecht N. F., Fatahzadeh M., Herrero R., et al. (2011). The oral cavity contains abundant known and novel human papillomaviruses from the Betapapillomavirus and Gammapapillomavirus genera. J Infect Dis 204787–792. 10.1093/infdis/jir383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxman I. L., Russell A., Mulder L. H., Bavinck J. N., ter Schegget J., Green A., Collaborators of the Nambour Prevention Study (2001). Association between epidermodysplasia verruciformis-associated human papillomavirus DNA in plucked eyebrow hair and solar keratoses. J Invest Dermatol 1171108–1112. 10.1046/j.1523-1747.2001.01499.x [DOI] [PubMed] [Google Scholar]
- Bzhalava D., Guan P., Franceschi S., Dillner J., Clifford G.(2013). A systematic review of the prevalence of mucosal and cutaneous human papillomavirus types. Virology 445224–231. [DOI] [PubMed] [Google Scholar]
- Casabonne D., Michael K. M., Waterboer T., Pawlita M., Forslund O., Burk R. D., Travis R. C., Key T. J., Newton R.(2007). A prospective pilot study of antibodies against human papillomaviruses and cutaneous squamous cell carcinoma nested in the Oxford component of the European prospective investigation into cancer and nutrition. Int J Cancer 1211862–1868. [DOI] [PubMed] [Google Scholar]
- Chandra A., Mosher W. D., Copen C., Sionean C.(2011). Sexual Behavior, Sexual Attraction, and Sexual Identity in the United States: Data from the 2006–2008 National Survey of Family Growth. National Health Statistics Reports, no. 36 Hyattsville, MD: National Center for Health Statistics. [PubMed] [Google Scholar]
- de Koning M. N., Weissenborn S. J., Abeni D., Bouwes Bavinck J. N., Euvrard S., Green A. C., Harwood C. A., Naldi L., Neale R., et al. (2009). Prevalence and associated factors of betapapillomavirus infections in individuals without cutaneous squamous cell carcinoma. J Gen Virol 901611–1621. 10.1099/vir.0.010017-0 [DOI] [PubMed] [Google Scholar]
- Dunne E. F., Nielson C. M., Hagensee M. E., Papenfuss M. R., Harris R. B., Herrel N., Gourlie J., Abrahamsen M., Markowitz L. E., Giuliano A. R.(2009). HPV 6/11, 16, 18 seroprevalence in men in two US cities. Sex Transm Dis 36671–674. 10.1097/OLQ.0b013e3181bc094b [DOI] [PubMed] [Google Scholar]
- Ekstrom J., Bzhalava D., Svenback D., Forslund O., Dillner J.(2011). High throughput sequencing reveals diversity of human papillomaviruses in cutaneous lesions. Int J Cancer 1292643–2650. [DOI] [PubMed] [Google Scholar]
- Farzan S. F., Waterboer T., Gui J., Nelson H. H., Li Z., Michael K. M., Perry A. E., Spencer S. K., Demidenko E., et al. (2013). Cutaneous alpha, beta and gamma human papillomaviruses in relation to squamous cell carcinoma of the skin: a population-based study. Int J Cancer 1331713–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltkamp M. C., Broer R., di Summa F. M., Struijk L., van der Meijden E., Verlaan B. P., Westendorp R. G., ter Schegget J., Spaan W. J., Bavinck J. N. B.(2003). Seroreactivity to epidermodysplasia verruciformis-related human papillomavirus types is associated with nonmelanoma skin cancer. Cancer Res 632695–2700. [PubMed] [Google Scholar]
- Giuliano A. R., Lazcano-Ponce E., Villa L. L., Flores R., Salmeron J., Lee J.-H., Papenfuss M. R., Abrahamsen M., Jolles E., et al. (2008). The human papillomavirus infection in men study: human papillomavirus prevalence and type distribution among men residing in Brazil, Mexico, and the United States. Cancer Epidemiol Biomarkers Prev 172036–2043. 10.1158/1055-9965.EPI-08-0151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliano A. R., Lazcano E., Villa L. L., Flores R., Salmeron J., Lee J. H., Papenfuss M., Abrahamsen M., Baggio M. L., Silva R.(2009). Circumcision and sexual behavior: factors independently associated with human papillomavirus detection among men in the HIM study. Int J Cancer 1241251–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliano A. R., Lee J. H., Fulp W., Villa L. L., Lazcano E., Papenfuss M. R., Abrahamsen M., Salmeron J., Anic G. M., et al. (2011). Incidence and clearance of genital human papillomavirus infection in men (HIM): a cohort study. Lancet 377932–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampras S. S., Giuliano A. R., Lin H. Y., Fisher K. J., Abrahamsen M. E., Sirak B. A., Iannacone M. R., Gheit T., Tommasino M., Rollison D. E.(2014). Natural history of cutaneous human papillomavirus (HPV) infection in men: the HIM study. PLoS One 9e104843. 10.1371/journal.pone.0104843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannigan G. D., Grice E. A.(2013). Microbial ecology of the skin in the era of metagenomics and molecular microbiology. Cold Spring Harb Perspect Med 3a015362. 10.1101/cshperspect.a015362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri S., Dunne E. F., Sternberg M., Unger E. R., Meadows K. S., Karem K. L., Markowitz L. E.(2008). Seroepidemiology of human papillomavirus type 11 in the United States: results from the third National Health and Nutrition Examination Survey, 1991–1994. Sex Transm Dis 35298–303. [DOI] [PubMed] [Google Scholar]
- Holm S.(1979). A simple sequentially rejective multiple test procedure. Scand J Stat 665–70. [Google Scholar]
- Iannacone M. R., Michael K. M., Giuliano A. R., Waterboer T., Pawlita M., Rollison D. E.(2010). Risk factors for cutaneous human papillomavirus seroreactivity among patients undergoing skin cancer screening in Florida. J Infect Dis 201760–769. 10.1086/650466 [DOI] [PubMed] [Google Scholar]
- Iannacone M. R., Gheit T., Waterboer T., Giuliano A. R., Messina J. L., Fenske N. A., Cherpelis B. S., Sondak V. K., Roetzheim R. G., et al. (2012). Case-control study of cutaneous human papillomaviruses in squamous cell carcinoma of the skin. Cancer Epidemiol Biomarkers Prev 211303–1313. 10.1158/1055-9965.EPI-12-0032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karagas M. R., Nelson H. H., Sehr P., Waterboer T., Stukel T. A., Andrew A., Green A. C., Bavinck J. N. B., Perry A., Spencer S.(2006). Human papillomavirus infection and incidence of squamous cell and basal cell carcinomas of the skin. J Natl Cancer Inst 98389–395. [DOI] [PubMed] [Google Scholar]
- Karagas M. R., Waterboer T., Li Z., Nelson H. H., Michael K. M., Bavinck J. N., Perry A. E., Spencer S. K., Daling J., et al. (2010). Genus beta human papillomaviruses and incidence of basal cell and squamous cell carcinomas of skin: population based case-control study. BMJ 341c2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuter A., Brockmeyer N. H., Weissenborn S. J., Gambichler T., Stucker M., Altmeyer P., Pfister H., Wieland U., German Competence Network, HIV/AIDS (2008). Penile intraepithelial neoplasia is frequent in HIV-positive men with anal dysplasia. J Invest Dermatol 1282316–2324. [DOI] [PubMed] [Google Scholar]
- Lacey C. J., Lowndes C. M., Shah K. V.(2006). Burden and management of non-cancerous HPV-related conditions: HPV-6/11 disease. Vaccine 24S35–S41. 10.1016/j.vaccine.2006.06.015 [DOI] [PubMed] [Google Scholar]
- Liu G., Markowitz L. E., Hariri S., Panicker G., Unger E. R.(2016). Seroprevalence of 9 human papillomavirus types in the United States 2005-2006. J Infect Dis 213191–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B., Viscidi R. P., Lee J. H., Wu Y., Villa L. L., Lazcano-Ponce E., da Silva R. J., Baggio M. L., Quiterio M., et al. (2011). Human papillomavirus (HPV) 6, 11, 16, and 18 seroprevalence is associated with sexual practice and age: results from the multinational HPV Infection in Men study (HIM study). Cancer Epidemiol Biomarkers Prev 20990–1002. 10.1158/1055-9965.EPI-10-1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael K. M., Waterboer T., Sehr P., Rother A., Reidel U., Boeing H., Bravo I. G., Schlehofer J., Gartner B. C., Pawlita M.(2008). Seroprevalence of 34 human papillomavirus types in the German general population. PLoS Pathog 4e1000091. 10.1371/journal.ppat.1000091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molano M., Posso H., Weiderpass E., van den Brule A. J., Ronderos M., Franceschi S., Meijer C. J., Arslan A., Munoz N., Study H. P. V. S. G. H.(2002). Prevalence and determinants of HPV infection among Colombian women with normal cytology. Br J Cancer 87324–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin D. M., Bray F.(2006). The burden of HPV-related cancers. Vaccine 24S11–S25. 10.1016/j.vaccine.2006.05.111 [DOI] [PubMed] [Google Scholar]
- Pierce Campbell C. M., Messina J. L., Stoler M. H., Jukic D. M., Tommasino M., Gheit T., Rollison D. E., Sichero L., Sirak B. A., et al. (2013). Cutaneous human papillomavirus types detected on the surface of male external genital lesions: a case series within the HPV Infection in Men study. J Clin Virol 58652–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plasmeijer E. I., Struijk L., Bouwes Bavinck J. N., Feltkamp M. C.(2009). Epidemiology of cutaneous human papillomavirus infections. Cancer Treat Res 146143–157. 10.1007/978-0-387-78574-5_13 [DOI] [PubMed] [Google Scholar]
- Sampogna F., Bavinck J. N., Pawlita M., Abeni D., Harwood C. A., Proby C. M., Feltkamp M. C., Euvrard S., Naldi L., et al. (2012). Factors associated with the seroprevalence of 26 cutaneous and two genital human papillomavirus types in organ transplant patients. J Gen Virol 93165–174. [DOI] [PubMed] [Google Scholar]
- Sehr P., Muller M., Hopfl R., Widschwendter A., Pawlita M.(2002). HPV antibody detection by ELISA with capsid protein L1 fused to glutathione S-transferase. J Virol Methods 10661–70. 10.1016/S0166-0934(02)00134-9 [DOI] [PubMed] [Google Scholar]
- Sichero L., Pierce Campbell C. M., Fulp W., Ferreira S., Sobrinho J. S., Baggio M., Galan L., Silva R. C., Lazcano-Ponce E., et al. (2014). High genital prevalence of cutaneous human papillomavirus DNA on male genital skin: the HPV Infection in Men study. BMC Infect Dis 14677. 10.1186/s12879-014-0677-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steben M., Garland S. M.(2014). Genital warts. Best Pract Res Clin Obstet Gynaecol 281063–1073. 10.1016/j.bpobgyn.2014.07.002 [DOI] [PubMed] [Google Scholar]
- Struijk L., Bouwes Bavinck J. N., Wanningen P., van der Meijden E., Westendorp R. G., ter Schegget J., Feltkamp M. C.(2003). Presence of human papillomavirus DNA in plucked eyebrow hairs is associated with a history of cutaneous squamous cell carcinoma. J Invest Dermatol 1211531–1535. 10.1046/j.1523-1747.2003.12632.x [DOI] [PubMed] [Google Scholar]
- Struijk L., Hall L., van der Meijden E., Wanningen P., Bavinck J. N., Neale R., Green A. C., ter Schegget J., Feltkamp M. C.(2006). Markers of cutaneous human papillomavirus infection in individuals with tumor-free skin, actinic keratoses, and squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev 15529–535. [DOI] [PubMed] [Google Scholar]
- Svare E. I., Kjaer S. K., Worm A. M., Osterlind A., Meijer C. J., van den Brule A. J.(2002). Risk factors for genital HPV DNA in men resemble those found in women: a study of male attendees at a Danish STD clinic. Sex Transm Infect 78215–218. 10.1136/sti.78.3.215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. R., Lockwood A. P., Taylor A. J.(1996). The prepuce: specialized mucosa of the penis and its loss to circumcision. Br J Urol 77291–295. 10.1046/j.1464-410X.1996.85023.x [DOI] [PubMed] [Google Scholar]
- Tiggelaar S. M., Lin M. J., Viscidi R. P., Ji J., Smith J. S.(2012). Age-specific human papillomavirus antibody and deoxyribonucleic acid prevalence: a global review. J Adolesc Health 50110–131. 10.1016/j.jadohealth.2011.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walboomers J. M., Jacobs M., V, Manos M. M., Bosch F., X, Kummer J. A., Shah K., V, Snijders P. J., Peto J., Meijer C. J., Munoz N.(1999). Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 18912–19. [DOI] [PubMed] [Google Scholar]
- Warren J.(2010). Physical effects of circumcision. Genital Autonomy, 75–79. New York, NY: Springer. [Google Scholar]
- Waterboer T., Sehr P., Michael K. M., Franceschi S., Nieland J. D., Joos T. O., Templin M. F., Pawlita M.(2005). Multiplex human papillomavirus serology based on in situ-purified glutathione S-transferase fusion proteins. Clin Chem 511845–1853. [DOI] [PubMed] [Google Scholar]
- Waterboer T., Abeni D., Sampogna F., Rother A., Masini C., Sehr P., Michael K. M., Pawlita M.(2008). Serological association of beta and gamma human papillomaviruses with squamous cell carcinoma of the skin. Br J Dermatol 159457–459. 10.1111/j.1365-2133.2008.08621.x [DOI] [PubMed] [Google Scholar]
- Wieland U., Jurk S., Weissenborn S., Krieg T., Pfister H., Ritzkowsky A.(2000). Erythroplasia of queyrat: coinfection with cutaneous carcinogenic human papillomavirus type 8 and genital papillomaviruses in a carcinoma in situ. J Invest Dermatol 115396–401. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.