Abstract
Intraocular pressure (IOP) is the most consistent risk factor for progressive vision loss in glaucoma. Cats with recessively inherited feline congenital glaucoma (FCG) exhibit elevated IOP with gradual, painless progression of glaucoma similar to humans and are studied as a model of glaucoma in humans and animals. Here, post-natal development of IOP was characterized in normal domestic cats and in cats with FCG caused by a homozygous LTBP2 mutation. Rebound tonometry (TonoVet®, ICare Oy, Finland) was used to measure IOP non-invasively, 2–3 times weekly in 63 FCG and 33 normal kittens, of both sexes, from eyelid opening until 3–6 months of age. IOPs in the left and right eyes of both FCG and normal kittens were compared by paired t-test and linear regression. One-way ANOVA and Tukey-Kramer post-tests were used to compare IOP of cats grouped by age and disease status. A p-value <0.05 was considered significant. In the second week of life, mean IOP was 7.16 mmHg (SD=1.3) in normal kittens and 8.72 mmHg (SD=1.4) in kittens with FCG. Mean IOP at age 10 weeks was significantly higher in FCG (19.8 mmHg; 95% CI= 17.7, 21.9mmHg) than in normal kittens (13.2 mmHg; 95% CI= 11.9, 14.5mmHg). At 3 months of age, IOP in normal cats reached adult values while IOP in FCG cats continued to increase through at least six months of age. These results provide ranges for normal IOP values in young kittens and confirm that IOP is significantly higher than normal by 10wks of age in this spontaneous feline glaucoma model.
Keywords: Intraocular pressure, glaucoma, feline, anterior chamber, LTBP2 mutation, aqueous outflow
Glaucomas are a group of diseases categorized by vision loss due to cell damage in the optic nerve and retina. Currently the second leading cause of blindness, it has been projected that glaucoma will affect nearly 80 million people by 2020 (Quigley and Broman, 2006). The most common underlying risk factor for the development of glaucoma is elevated intraocular pressure (IOP) (Leske et al., 2007). In many cases, inadequate drainage of aqueous humor through the trabecular meshwork leads to increased IOP, which is associated with damage to the optic nerve, nerve fiber degeneration and retinal cell apoptosis, resulting in permanent loss of vision. Although glaucoma is a disease that is most common in older adults, it is also an important cause of vision loss in pediatric patients. A colony of domestic cats with recessively inherited feline congenital glaucoma (FCG) has been established as an ortholog and model of human primary congenital glaucoma (PCG) (Rutz-Mendicino et al., 2011). In both human and feline cases of PCG, abnormal development of the structures of the conventional aqueous outflow pathway causes build-up of aqueous humor pressure (Bouhenni et al., 2012). A study of ocular pathology in humans with congenital glaucoma suggested that thick subcanalicular tissue is indicative of immature trabecular meshwork development in glaucomatous infants (Tawara and Inomata, 1981). Cats and dogs are born altricial, with relatively under-developed eyes, and the first time they open their eyelids is around 7 to 14 days of age. During the normal process of postnatal ocular development in cats, the trabecular meshwork forms by a rearrangement of the cells and extracellular matrix in the primitive connective tissue, which fills the anterior chamber angle recess (Richardson et al., 1985). The remodeling of these tissues allows for the appearance of intercellular spaces, which later form the inter-trabecular channels within the ciliary cleft. An arrest in this normal maturation process has been identified in cats homozygous for a mutation in LTBP2, causal for FCG (Kuehn et al., 2016).
There have been only very limited published studies of IOP development in normal and glaucomatous immature animals. In a study of dogs, Verboven et al., (2014) found that IOP increases until the age of about 10 to 11 weeks in that species. However, similar published data are lacking for cats, a species of particular interest as a model for human PCG. In the current study, IOP measurements were obtained in order to characterize and compare IOP development in both glaucomatous and normal domestic kittens from eyelid opening until six months of age. It was hypothesized that IOP would increase over the first few weeks of life, in parallel with ongoing post-natal development of ocular tissues and aqueous humor production, and that the increase in IOP would be greater in cats with FCG than in normal cats.
Intraocular pressure was measured using rebound tonometry in 96 domestic cats (Felis catus; 63 FCG cats homozygous for the LTBP2 mutation described above (36 male, 27 female) and 33 normal cats (17 male, 16 female)). All procedures were carried out in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, the National Institutes of Health guide for the care and use of laboratory animals and with the approvals of the University of Wisconsin-Madison and Iowa State University Institutional Animal Care and Use Committees. A Tonovet® rebound tonometer was used (TonoVet®, ICare Oy, Finland) as this tonometer type is recognized as the most consistently accurate tonometer in cats (Mclellan et al., 2013). Since IOP in cats is known to show circadian variation (Del Sole et al., 2007; Sigle et al., 2011), time of day of IOP measurement was consistent throughout the study. All IOP measurements were acquired between 7 am and 11 am. During the post-natal period, kittens were closely monitored to detect the earliest eyelid opening that would permit IOP measurements and data were collected from eyelid opening until 6 months of age or older. IOP was measured in triplicate and the 3 values were averaged to provide a single value at each time point for each eye, on 2–3 occasions per week. This procedure is performed without sedative or topical anesthetic and is well tolerated by most cats.
Symmetry between the left (OS) and right (OD) eyes in subjects was confirmed by linear correlation using Sigmaplot (Sigmaplot v.12, San Diego, CA, USA). To limit effects of high between-eye correlation in subsequent analyses, the mean IOP values from both eyes were averaged to provide a single IOP value at each time point, for each subject (Barbeito and Herse, 1991). Mean IOP data vs age at measurement were plotted for both glaucomatous and normal subjects (GraphPad Prism v6.04, La Jolla, CA, USA). Mean weekly IOPs were compared between each time point and between groups by Analysis of Variance (ANOVA), with Tukey-Kramer multiple comparisons post-test as appropriate, from 2 weeks until 12 weeks of age. Thereafter, between-group comparisons were made for mean IOPs obtained at 2, 3, 4, 5, and 6 months of age. A p value <0.05 was considered statistically significant.
In both normal and glaucomatous kittens, trends in IOP were highly symmetric between right and left eyes, and IOP in right and left eyes was positively correlated (y=0.823x+ 1.5957, r2=0.7347 and y=0.8455x+ 2.9476, r2=0.7058, for normal and FCG cats, respectively). Although, IOP in the right eye was significantly higher than in the left eye over the 6 month course of data collection (p<0.05), the difference in mean IOP between the right and left eyes was only 0.4 mmHg in glaucomatous cats and 0.8 mmHg in normal cats, which is not considered clinically significant.
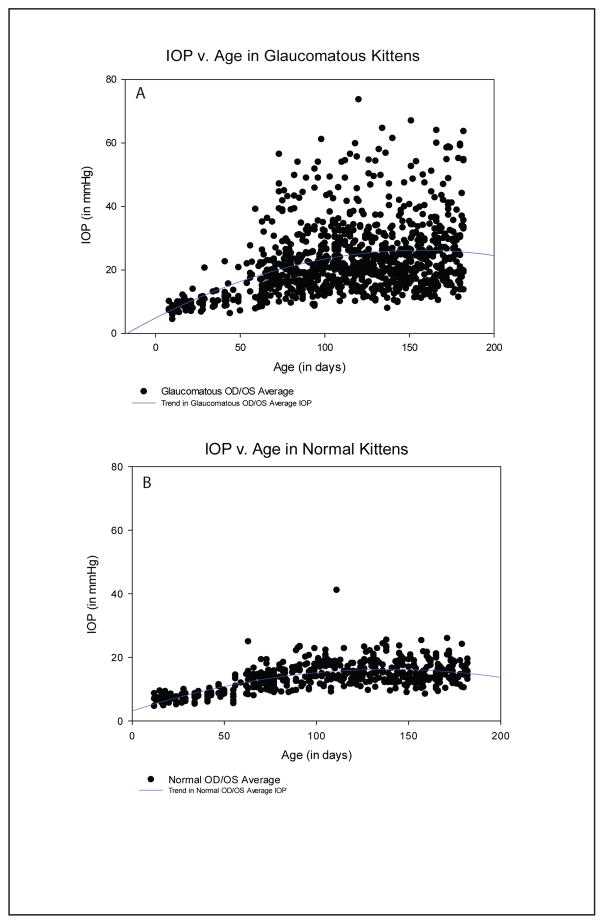
A quadratic curve was determined to provide the line of best fit on the scatterplots of mean IOP versus post-natal age in both normal and FCG kittens. Intraocular pressure gradually increased throughout the first 6 months of life in FCG (Figure 1A) in contrast to normal kittens, in which IOP plateaued relatively early in life, by ~3 months of age (Figure 1B). Curve fitting identified quadratic polynomial curves, describing the IOP data sets for glaucomatous and normal kittens. The mean IOP for the glaucomatous group followed the equation (y = 4.8819 + 0.2728x − 0.0009x2), while mean IOP for the normal group followed the equation (y = 3.1253 + 0.1881x − 0.0007x2). However, the r2 value of the quadratic equation was higher in normal cats (r2 − 0.4548), than in FCG cats (r2 − 0.1659). The lower r2 value in FCG cats can be attributed to the higher variability in IOP between and within individuals in the FCG group compared to the normal cats.
Figure 1.
Scatterplots depicting the trends in both glaucomatous (A) and normal (B) kittens from eyelid opening until 6 months of age. IOP in normal cats reached adult-like values at 10 weeks of age while IOP increased through 6 months of age in FCG cats. Trends in glaucomatous IOP (A) follow the equation (y = 4.8819 + 0.2728x − 0.0009x2), while trends in normal IOP (B) follow the equation (y = 3.1253 + 0.1881x − 0.0007x2).
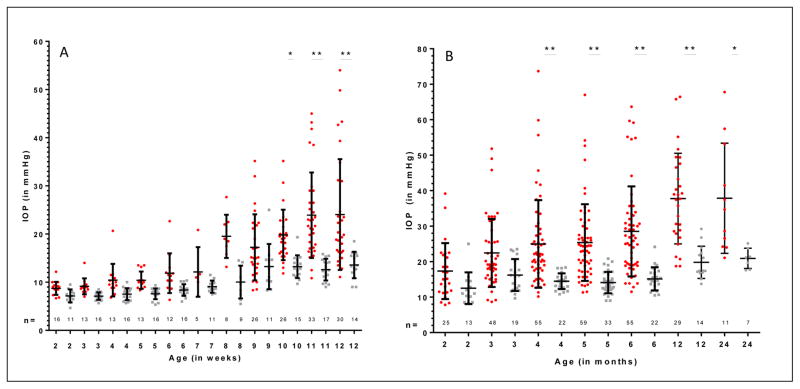
Mean IOP in kittens with FCG was significantly higher than in normal kittens, beginning at 10 weeks of age, with a mean difference in IOP of 6.6 mmHg (p<0.05) (Figure 2A). At 10 weeks, the mean (±SD) IOP was 13.2±2.4 mmHg in normal kittens (n=15) and 19.8±5.2 mmHg in FCG kittens (n=26). Mean IOP was significantly higher at 8 weeks of age and above when compared to 2 weeks of age in FCG. Although mean IOP increased between 2 and 12 weeks of age in normal kittens, increases were not statistically significant. The mean increase in IOP from 2–12 weeks of age was 15.3 mmHg (95% CI= 9.2, 21.4) in FCG kittens, but only 6.4 mmHg (95% CI= 1.5, 14.4) in normal kittens. The difference in IOP between 2 and 12 weeks of age in FCG kittens was extremely significant (p<0.001) (Figure 2A).
Figure 2.
A) Mean IOP values at weekly intervals during the first 12 weeks of age in normal and FCG cats. B) Mean IOP values at monthly intervals between 2 and 24 months of age in normal cats and FCG cats. The red dots represent data for individual FCG cats while the gray dots represent individual normal cats (many data points are superimposed) Error bars represent standard deviation. Statistically significant differences in mean IOP between normal cats and FCG cats for the same week (2A) or month (2B) are depicted by asterisks (*=p<0.05; **=p<0.001).
IOP remained significantly higher in FCG vs. age-matched normal cats at 4, 5 and 6 months of age. Mean IOP was 10.5, 11.3 and 13.4 mmHg higher in FCG vs. normal kittens at 4 (p<0.05), 5 (p<0.001) and 6 months (p<0.001), respectively (Figure 2B). Mean IOP was 11.2 mmHg higher at 6 months vs. 2 months of age (p<0.001) in FCG cats, and increased another 3.1mmHg between 5 and 6 months of age.
In this cross sectional study, trends in IOP based on age were established in both normal and glaucomatous kittens. IOP is very low in both glaucomatous and normal neonatal kittens at the time of eyelid opening at 5–7 days of age. As kittens mature, the gradual increase in IOP is likely a result of maturation of mechanisms for aqueous humor production and this process occurs in tandem with the development of aqueous humor outflow pathways in normal kittens. Results of this study suggest that by 10–12 weeks of age, adult levels of aqueous humor production are reached in both FCG and normal cats, however the aqueous humor outflow pathway only reaches functional maturity in the normal cats. Thereafter, IOP was significantly higher in FCG vs. normal cats and continued to increase through 6 months of age in FCG, and up to 1 year of age in a subset of FCG cats. This observation is supported by previously described morphological evidence of developmental arrest in the maturation of trabecular meshwork and ciliary cleft in FCG cats. (Kuehn et al., 2016). However, the precise anatomic and physiologic basis for the observed heterogeneity in IOP profile between cats homozygous for the same causal mutation remains unknown. Detailed histomorphological and clinical studies of conventional aqueous outflow pathways in affected animals with distinct IOP profiles are ongoing at the time of writing.
Normal IOP values in our cohort of cats were slightly lower than the mean IOP (20.7 mmHg) reported for clinically normal young adult cats in another study using the same type of tonometer (Rusanen et al., 2010). One reason for this discrepancy could be that in our colony, IOPs are measured frequently, beginning early in life. Thus the cats in our study are habituated to the process of rebound tonometry, early in life. In non-conditioned cats, such as those examined by Rusanen et al., (2010), which are unaccustomed to rebound tonometry and handling, higher stress levels can result in higher IOPs.
One limitation of the current study was a lack of complete longitudinal data sets from eyelid opening to 6 months of age in all cats. Therefore, data presented in this study were, by necessity, cross-sectional and may not fully capture inter-individual variability in IOP development in cats with glaucoma. In addition, the rebound tonometer used has been validated in adult cats, but not in very young kittens (Mclellan et al., 2013). Compared to mature cats, kittens have smaller eyes and thinner corneas (Moodie et al., 2001). Thus it is conceivable that tonometry readings may not be as accurate in kittens aged only 1 or 2 weeks compared with readings done in adult cats.
In summary, by 10 weeks of age, previously identified abnormalities in aqueous humor outflow pathway development were associated with significantly higher IOP in cats with FCG compared with normal cats. In addition, the current study established reference IOP values and provided data in order to better understand IOP development in normal and FCG kittens. These data will not only be of diagnostic value to veterinarians, but will also be of value in future studies that examine processes involved in eye development and glaucomatous damage in animal subjects with FCG. More detailed morphological analyses of these developmental processes are underway in normal and FCG cats. To our knowledge, there is currently very limited published data examining the development of outflow pathways and IOP in normal human infants and in human patients with congenital glaucoma. Further studies are indicated to examine the development of IOP and aqueous humor outflow pathways across species.
Highlights.
IOP is significantly higher by 10 weeks of age in cats with feline congenital glaucoma vs. normal kittens
Feline IOP is low early in life, reaching adult values in normal cats by 3 months of age and continuing to increase in a feline congenital glaucoma model through 6–12 months of age.
This study provides reference values for development of IOP in both normal cats and cats with feline congenital glaucoma
Acknowledgments
We are indebted to the following individuals who assisted with IOP data collection: Allison Ludwig, Lauren Rutkowski, Owen Bowie and Mary E. Mohr. Dr. Ralph Moller Trane provided advice on appropriate statistical analyses.
Funding: This work was supported by the National Institutes of Health [National Eye Institute grant numbers K08 EY018609 and P30 EY16665]; new faculty startup funds from the University of Wisconsin-Madison, and unrestricted funds to the Department of Ophthalmology and Visual Sciences, University of Wisconsin-Madison from Research to Prevent Blindness.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barbeito R, Herse PR. Problem of between-eye correlation for statistical hypothesis-testing - rabbit corneal thickness. Optometry and Vision Science. 1991;68:73–76. doi: 10.1097/00006324-199101000-00011. [DOI] [PubMed] [Google Scholar]
- Bouhenni RA, Dunmire J, Sewell A, Edward DP. Animal models of glaucoma. Journal of Biomedicine and Biotechnology. 2012:11. doi: 10.1155/2012/692609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Sole MJ, Sande PH, Bernades JM, Aba MA, Rosenstein RE. Circadian rhythm of intraocular pressure in cats. Veterinary Ophthalmology. 2007;10:155–161. doi: 10.1111/j.1463-5224.2007.00525.x. [DOI] [PubMed] [Google Scholar]
- Kuehn MH, Lipsett KA, Menotti-Raymond M, Whitmore SS, Scheetz TE, David VA, O’Brien SJ, Zhao ZY, Jens JK, Snella EM, Ellinwood NM, McLellan GJ. A mutation in LTBP2 causes congenital glaucoma in domestic cats (Felis catus) PloS One. 2016;11:2, e0154412. doi: 10.1371/journal.pone.0154412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leske MC, Heijl A, Hyman L, Bengtsson B, Dong LM, Yang ZM, Grp E. Predictors of long-term progression in the early manifest glaucoma trial. Ophthalmology. 2007;114:1965–1972. doi: 10.1016/j.ophtha.2007.03.016. [DOI] [PubMed] [Google Scholar]
- McLellan GJ, Kemmerling JP, Kiland JA. Validation of the TonoVet (R) rebound tonometer in normal and glaucomatous cats. Veterinary Ophthalmology. 2013;16:111–118. doi: 10.1111/j.1463-5224.2012.01038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moodie KL, Hashizume N, Houston DL, Hoopes PJ, Demidenko E, Trembly BS, Davidson MG. Postnatal development of corneal curvature and thickness in the cat. Veterinary Ophthalmology. 2001;4:267–272. doi: 10.1046/j.1463-5216.2001.00198.x. [DOI] [PubMed] [Google Scholar]
- Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. British Journal of Ophthalmology. 2006;90:262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson TM, Marks MS, Ausprunk DH, Miller M. A morphologic and morphometric analysis of the aqueous outflow system of the developing cat eye. Experimental Eye Research. 1985;41:31–51. doi: 10.1016/0014-4835(85)90092-2. [DOI] [PubMed] [Google Scholar]
- Rusanen E, Florin M, Hässig M, Spiess BM. Evaluation of a rebound tonometer (Tonovet®) in clinically normal cat eyes. Veterinary Ophthalmology. 2010;13:31–36. doi: 10.1111/j.1463-5224.2009.00752.x. [DOI] [PubMed] [Google Scholar]
- Rutz-Mendicino MM, Snella EM, Jens JK, Gandolfi B, Carlson SA, Kuehn MH, McLellan GJ, Ellinwood NM. Removal of potentially confounding phenotypes from a Siamese-derived feline glaucoma breeding colony. Comparative Medicine. 2011;61:251–257. [PMC free article] [PubMed] [Google Scholar]
- Sigle KJ, Camano-Garcia G, Carriquiry AL, Betts DM, Kuehn MH, McLellan GJ. The effect of dorzolamide 2% on circadian intraocular pressure in cats with primary congenital glaucoma. Veterinary Ophthalmology. 2011;14:48–53. doi: 10.1111/j.1463-5224.2011.00913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tawara A, Inomata H. Developmental immaturity of the trabecular meshwork in congenital glaucoma. American Journal of Ophthalmology. 1981;92:508–525. doi: 10.1016/0002-9394(81)90644-9. [DOI] [PubMed] [Google Scholar]
- Verboven C, Djajadiningrat-Laanen SC, Teske E, Boeve MH. Development of tear production and intraocular pressure in healthy canine neonates. Veterinary Ophthalmology. 2014;17:426–431. doi: 10.1111/vop.12196. [DOI] [PubMed] [Google Scholar]