Abstract
Glucocorticoids have strong effects on diverse human activities through the glucocorticoid receptor (GR). Sirtuin 1 (SIRT1) is a NAD+-dependent histone deacetylase and promotes longevity by influencing intermediary metabolism and other regulatory activities including mitochondrial function. In this study, we examined the effects of SIRT1 on GR-mediated transcriptional activity. We found that SIRT1 enhanced GR-induced transcriptional activity on exogenous and endogenous glucocorticoid-responsive genes, whereas knockdown of SIRT1 attenuated it. This effect of SIRT1 was independent to its deacetylase activity, as the SIRT1 mutant defective in this activity (H363Y) enhanced GR transcriptional activity, and the compounds inhibiting or activating the SIRT1 deacetylase activity did not influence it. RNA-seq analysis revealed that SIRT1 knockdown influenced ~30% of the glucocorticoid-responsive transcriptome for most of which it acted as an enhancer for positive/negative effects of this hormone. SIRT1 physically interacted with GR, and was attracted to GR-bound glucocorticoid response elements in a glucocorticoid-dependent fashion. SIRT1 cooperatively activated GR transcriptional activity with the PPARγ coactivator-1α also in its deacetylase activity-independent fashion. Thus, SIRT1 is a novel transcriptional enhancer of GR-induced transcriptional activity possibly by functioning as a scaffold for the transcriptional complex formed on GR.
Keywords: glucocorticoids, longevity, protein-protein interaction, transcriptome
1. INTRODUCTION
Chronic exposure to stress increases susceptibility to various diseases including obesity, insulin resistance and overt diabetes mellitus, and significantly accelerates the process of aging in humans and several other animals (Chrousos and Kino, 2007, Spiers, Chen, Sernia et al., 2014, Haussmann and Heidinger, 2015). Substantial part of these effects of stress is mediated by glucocorticoid hormones chronically over-secreted from the adrenal glands as end-effectors of the stress-responsive hypothalamic-pituitary-adrenal (HPA) axis (Haussmann and Heidinger, 2015, Nader, Chrousos and Kino, 2010). In contrast, diurnally fluctuating physiologic levels of glucocorticoids are essential for human life through their homeostatic actions on many important biological activities, including intermediary metabolism in the liver, muscle and adipose tissues, electrolyte handling, vascular tone regulation, immune activity and cognitive functions of the central nervous system (Chrousos and Kino, 2007, Nader et al., 2010, Chrousos and Kino, 2009, Franchimont, Kino, Galon et al., 2002, Nicolaides, Galata, Kino et al., 2010). Thus, glucocorticoids are essential and required for human survival and longevity. These diverse actions of glucocorticoids are mediated by the glucocorticoid receptor (GR), a ligand-dependent transcription factor and a member of the nuclear hormone (NR)/steroid hormone (SR) receptor family (Kino, 2000, Mackeh, Marr, Fadda et al., 2017). Human GR consists of 777 amino acids and comprises 3 structural/functional domains, the N-terminal immunogenic (NTD), middle DNA-binding (DBD) and C-terminal ligand-binding (LBD) domain (Kino, 2000). Upon binding to glucocorticoids, GR dissociates from several heat shock proteins and translocates into the nucleus. Inside the nucleus, GR binds glucocorticoid response elements (GREs) located in the regulatory region of glucocorticoid-responsive genes, and alters their transcriptional activity by attracting/communicating with numerous cofactor molecules, including various histone acetyltransferases (HATs) and/or deacetylases (HDACs) (Chrousos and Kino, 2005). These molecules respectively acetylate/deacetylate several lysine residues of the histone tails, alter chromatin accessibility, and enhance/repress the transcriptional activity of glucocorticoid-responsive genes (Heery, Kalkhoven, Hoare et al., 1997, McKenna, Lanz and O’Malley, 1999, Tessarz and Kouzarides, 2014). In addition to histones, HATs/HDACs acetylate/deacetylate GR itself and/or components of the transcriptional complex attracted to GREs-bound GRs, and further modulate the transcriptional activity of their associating genes (Kino, 2000, Chrousos and Kino, 2005).
Sirtuin 1 (SIRT1), a member of the sirtuin protein family and a mammalian homolog of the yeast Silent Information Regulator 2 (Sir2), is a NAD+-dependent class III HDAC (Finkel, Deng and Mostoslavsky, 2009). It has diverse physiologic roles including regulation of the intermediary metabolism of glucose and fat, mitochondrial energy production, immune activity and circadian rhythms by deacetylating histones and various non-histone molecules (Finkel et al., 2009, Chang and Guarente, 2014, Moore, Dai and Faller, 2012). SIRT1 mediates anti-aging effects of the calorie restriction from lower to higher organisms by ameliorating insulin resistance and by increasing mitochondrial activity, thus chemical compounds either stimulating or inhibiting the deacetylase activity of SIRT1 have attracted large scientific interest (Bordone and Guarente, 2005, Milne, Lambert, Schenk et al., 2007). In addition to these actions, SIRT1 regulates the transcriptional activity of several NRs by deacetylating their specific lysine residues (Feige and Auwerx, 2008). For example, SIRT1 deacetylates androgen receptor, thyroid hormone receptor β1, liver X receptor (LXR) and peroxisome proliferator-activating receptor γ (PPARγ), and modulates their downstream biologic actions (Feige and Auwerx, 2008, Dai, Ngo, Forman et al., 2007, Popov, Wang, Shirley et al., 2007, Suh, Sieglaff, Zhang et al., 2013, Li, Zhang, Blander et al., 2007, Picard, Kurtev, Chung et al., 2004). SIRT1 also deacetylates PPARγ coactivator-1α (PGC1α) upon physical interaction to this molecule (Rodgers, Lerin, Gerhart-Hines et al., 2008). This unique coactivator plays a central role in mitochondrial biogenesis and thermoregulation as well as glucose, fatty acid and cholesterol metabolism in part by regulating the actions of several NRs, such as PPARγ, GR and LXR (Oberkofler, Schraml, Krempler et al., 2003, Puigserver and Spiegelman, 2003). Thus, SIRT1 can indirectly regulate the transcriptional activity of these PGC1α-associating NRs through cooperating with this coactivator.
Because glucocorticoids/GR and SIRT1 have strong and overlapping effects on various human activities and SIRT1 has strong regulatory actions on several NRs, we investigated whether SIRT1 also influences the transcriptional activity of GR. We found that SIRT1 directly interacts with GR through the latter’s DBD and modulates its transcriptional activity in its deacetylase activity-independent and a gene-specific fashion. These results suggest that SIRT1 influences GR activities, which further contributes to the diverse actions of SIRT1 on human physiology and pathophysiology.
2. MATERIALS AND METHODS
2.1. Plasmids and Reagents
pBK/CMV-SIRT1 was constructed by subcloning full-length human SIRT1 cDNA into pBK/CMV (Stratagene, La Jolla, CA). pCDNA3.1His/C-SIRT1 and pGEX4T3-SIRT1 were created by inserting the corresponding SIRT1 cDNA fragments from pBK/CMV-SIRT1 into pCDNA3.1His/C (Invitrogen, Carlsbad, CA) and pGEX4T3 (GE Healthcare Bio-Science Corp., Piscataway, NJ), respectively. pBK/CMV-SIRT1-H363Y, which expresses a SIRT1 mutant with histidine to tyrosine substitution at amino acid position 363, was constructed with PCR-assisted site-directed mutagenesis using pBK/CMV-SIRT1 as a template. SIRT1 H363Y is defective in deacetylase activity (Kim, Nguyen, Dobbin et al., 2007). pTagRFP-C-SIRT1, which expresses the human SIRT1 N-terminally fused with the red fluorescent protein (RFP), was constructed by subcloning SIRT1 cDNA into pTagRFP-C (BioVision, Inc., Milpitas, CA). The human GRα-expressing pRShGRα and its control pRSerbA−1, and pGR107 that expresses the human GRα under the control of the SP6 promoter were gifts from R. M. Evans (Salk Institute, La Jolla, CA). pGEX-4T3-GRα full-length (FL), NTD, DBD, and LBD, which express corresponding portions of the human GRα fused with GST were previously reported (Nader, Chrousos and Kino, 2009, Fadda, Syed, Mackeh et al., 2017). pEGFP-C1-GRα, which expresses the human GRα N-terminally fused with enhanced green fluorescent protein (EGFP), was also previously reported (Habib, Sadoun, Nader et al., 2017). pCDNA4-MYC-PGC-1α was purchased from Addgene (Cambridge, MA). pODLO-MMTV-Luc, which expresses firefly luciferase under the control of the synthetic GREs from the glucocorticoid-responsive mouse mammary tumor virus (MMTV) promoter was a gift from J. N. Miner (Ligand Pharmaceuticals, Inc, San Diego, CA). pRL-TK and pSV40-β-Gal, which respectively express renilla luciferase under the control of the herpes simplex virus thymidine kinase promoter and β-galactosidase under the control of the simian virus 40 promoter, were purchased from Promega Corp (Madison, WI). Compounds SRT1720 (SIRT1 activator) and EX-527 (SIRT1 inhibitor) were purchased from Selleckchem (Houston, TX) and Sigma-Aldrich (St. Louis, MO), respectively.
2.2. Cell culture, transfection, and reporter assay
Human cervical cancer HeLa cells, colon cancer HCT116 cells and hepatocellular cancer HepG2 cells were previously reported (Nader, Ng, Wang et al., 2012). HeLa and HepG2 cells were maintained in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum, 100 U/mL of penicillin, and 100 μg/mL of streptomycin. HCT116 cells were maintained in McCoy’s 5A medium with the same supplements. HCT116 cells do not express endogenous GR, whereas HeLa and HepG2 cells have endogenous and functional GR (Nader et al., 2012, Kino, Ichijo, Amin et al., 2007).
For reporter assays, cells were transfected with 0.125 μg or 0.25 μg of pBK/CMV-SIRT1 (wild type or H363Y mutant) together with pODLO-MMTV-Luc and pRL-TK (for HeLa or HepG2 cells), or pSV40-β-Gal in the presence of pRShGRα (for HCT116 cells) using Lipofectamine LTX (Invitrogen) or polyethylenimine (Polysciences, Inc., Warrington, PA) in 24-well plates. Control plasmids were used to account for the same amounts of plasmid DNA. For HeLa cells, some experiments using pCDNA4-MYC-PGC-1α in addition to the plasmids mentioned above were also performed. Twenty-four hours after the transfection, media were changed and the cells were treated with 10−6 M of dexamethasone (DEX) for an additional 3 and 8 hours for HeLa and HCT116 cells, and for HepG2 cells, respectively. In some experiments, 10 nmol of control or SIRT1-directing small interfering RNA (siRNA) (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) were also transfected using Lipofectamin™ RNAiMAX (Invitrogen). Twenty-four hours after siRNA transfection, reporter plasmids were further transfected with indicated plasmids, and the cells were treated with 10−6 M of DEX for the time periods indicated above. The SIRT1 inhibitor (EX-527) and activator (SRT1720) were also used in some experiments to examine the impact of the SIRT1 enzymatic activity. Practically, HeLa cells were transfected with SIRT1-expressing plasmid together with pODLO-MMTV-Luc and pRL-TK. The cells were then treated with EX-527 (2.5 mM) or SRT1720 (0.16 mM) for 10 hours. They were further treated with 10−6 M of DEX or vehicle for an additional 4 hours at the time-point passing 6 hours after addition of EX-527 or SRT1720. Lysates obtained from transfected/treated cells were analyzed for firefly and renilla luciferase activities by using the Dual Luciferase Assay kit (Promega Corp.) or β-galactosidase activity by using the Galacto-Light Plus™ System (Applied Biosystems, Foster City, CA) in the GloMax Luminometer (Promega Corp.) (Hill, Suzuki, Segars et al., 2016).
2.3. Regular immunoprecipitation and Western blotting
For regular immunoprecipitation assays, HeLa cells were transfected with control or SIRT1 siRNA as mentioned above. Twenty-four hours after the transfection, the cells are incubated with 10−6 M of DEX for 3 hours, and were treated with 1% formaldehyde for 1 min to cross-link proteins (Klockenbusch and Kast, 2010, De Martino, Bhattachryya, Alesci et al., 2004). Nuclear fraction was isolated from the cells using the membrane extraction buffer (Pierce Inc., Rockford, IL), and subsequent sonication was performed to lyse the cells in a buffer containing 50 mM Tris-HCl [pH 7.4], 150 mM NaCl, 0.1% sodium dodecyl sulfate, 1% Nonidet P-40, 0.5% sodium deoxycholate, and 1 Tablet/50 mL Complete Tablet (Roche, Indianapolis, IN). Immunoprecipitation was carried out with anti-GR or -SIRT1 antibody, or rabbit control IgG, and the antibody/protein complex was precipitated with protein A/G magnetic beads (Pierce Inc.) according to the manufacturer’s instruction. Samples were run on 4%–12% Bis-Tris gels together with 5% of input used for the immunoprecipitation reactions. Western blotting was then performed for GR or SIRT1 using their specific antibodies, and their protein bands were visualized using the horseradish-conjugated secondary antibody and the Amersham ECL Detection Reagents (GE Healthcare Bio-Sciences Corp., Pittsburgh, PA). Western blotting was also performed for the lysates obtained for luciferase assays by using anti-GR, -SIRT1, -α-tubulin or -β-actin antibody.
2.4. GST pull-down assay
35S-labeled human GR and SIRT1 were generated with in vitro transcription/translation reactions (Promega Corp.) using pR107 and pCDNA3.1His/C-SIRT1 as templates, respectively, and were tested for interaction with GST-GRs or GST-SIRT1 immobilized on glutathione-sepharose beads (GE Healthcare Bio-Science Corp.) in the presence or absence of 10−5 M of DEX in the buffer containing 50 mM Tris-HCl [pH 8.0], 50 mM NaCl, 1 mM EDTA, 0.1% NP-40, 10% glycerol and 0.1 mg/ml BSA at 4°C for 1.5 hour, as previously described (Hill et al., 2016, Kino, Gragerov, Kopp et al., 1999). Three μg of GST-fused protein was applied into each reaction. For the reactions employing GST-GRs, no DEX was added, as these bacterially produced fusion proteins do not respond to glucocorticoids (Nader et al., 2009, Hill et al., 2016). After vigorous washing with the buffer, proteins were eluted and separated on 8 or 4–12% Bis-Tris gels together with 1% of input used for pull-down reactions. Gels were fixed, treated with Enlightening (NEN Life Science Products, Inc., Boston, MA), dried and exposed to film.
2.5. Subcellular localization of GR and SIRT1
HeLa cells were transfected with pEGFP-C1-GRα and pTagRFP-C-SIRT1, and were cultured overnight. The cells were then treated with 10−6 M of DEX or vehicle ethanol for 2 hours. The cells were then incubated for 2 minutes with 2 drops of the NucBlue Live ReadyProbes Reagent (Thermo Fisher Scientific, Waltham, MA) to stain nuclear DNA with Hoechst® 33342. Subcellular localization of EGFP-fused GRα and RFP-fused SIRT1, and nuclear staining with Hoechst® 33342 were recorded under an inverted fluorescence microscope (Leica DM IRB, Wetzlar, Germany) equipped with a PeCon temperature controller (PeCon GmbH, Erbach, Germany), as previously described (Kino, Souvatzoglou, De Martino et al., 2003).
2.6. RNA sampling and sequencing (RNA-seq)
HeLa cells were transfected with control or SIRT1 siRNA as described above, and were treated with 10−6 M of DEX or vehicle ethanol for 3 hours. Total RNA was then purified using the RNeasy Mini Kit (Qiagen, Hilden, Germany). Depletion of ribosomal RNAs from total RNA was performed using the Ribo-Zero Gold rRNA Removal Kit (Epicentre, Madison, WI), and sequencing libraries were constructed using the Library Builder Whole Transcriptome Core Kit according to the manufacturer’s instructions (Thermo Fisher Scientific). Libraries were sequenced on a SOLiD 5500xl System to generate 75-bp forward reads (Thermo Fisher Scientific).
2.7. RNA-seq data analysis
RNA-seq data were aligned to the reference hg19-GRCh37 assembly obtained from the UCSC Genome Browser (https://genome.ucsc.edu/cgi-bin/hgGateway?db=hg19) using TopHat (http://ccb.jhu.edu/software/tophat/index.shtml) with default parameters, and HTSeq (http://www-huber.embl.de/users/anders/HTSeq/doc/overview.html) was used to count reads aligned to each genomic feature. The output files were used to identify differentially expressed genes in DESeq (http://bioconductor.org/packages/release/bioc/html/DESeq.html).
2.8. SYBR Green real-time PCR
HeLa cells were transfected with control or SIRT1 siRNA and treated with or without 10−6 M of DEX similar to the samples for RNA-seq. Some HeLa and HepG2 cells were also transfected with pBK/CMV-SIRT1 and/or pCDNA4-MYC-PGC-1α, and were treated with 10−6 M of DEX. Total RNA was then purified using the RNeasy Mini Kit (Qiagen). cDNA was synthesized using the TaqMan Reverse Transcription Reagents and oligo-dT as a primer (Applied Biosystems). PCR was performed in the 7500 Real-time PCR System (Applied Biosystems), as previously described (Kino, Tiulpakov, Ichijo et al., 2005, Kino, Jaffe, Amin et al., 2010, Ng, Li, Pavlakis et al., 2013). Amplification was performed in a two-step cycle: 95°C for 10 min, then 45 cycles of the reaction consisting of denaturing at 95°C for 15 sec and annealing/extension at 60°C for 1 min. Primer pairs for quantifying mRNA levels of the growth arrest and DNA damage inducible β (GADD45B), glucocorticoid-induced leucine zipper (GILZ), heparin-binding EGF-like growth factor (HBEGF), metallothionein 2A (MT2A), myocardin (MYOCD), protein phosphatase 1 regulatory subunit 3C (PPP1R3C), prostaglandin-endoperoxide synthase 2 (PTGS2), zinc finger protein 36 (ZFP36) and ribosomal protein, large, P0 (RPLP0) genes were designed so that the sequence spanning between a forward and a reverse primer contains at least one intron. Their sequences are listed in Supplemental Table 1. Ct values of the examined genes were normalized with those of RPLP0, and fold changes were obtained by using the comparative Ct method (Papadopoulou, Siamatras, Delgado-Morales et al., 2015).
2.9. Chromatin immunoprecipitation (ChIP) assay
ChIP was performed by using the Pierce Magnetic ChIP Kit (Pierce Inc.) in HeLa cells according to the modified instruction from the manufacturer (Fadda et al., 2017). Briefly, HeLa cells were grown on 10-cm dishes, transfected with control or SIRT1 siRNA, and were treated with or without 10−6 M of DEX similar to the samples for RNA-seq. The cells were then fixed with 1% formaldehyde for 1 min to cross-link DNA and associated proteins, and were lysed with the membrane extraction buffer. Obtained whole homogenates were centrifuged to precipitate cell nuclei. They were then resuspended in the IP dilution buffer containing protease/phosphatase inhibitors, and the micrococcal nuclease digestion was performed to shear DNA. The nuclei were then resuspended in the ChIP dilution buffer (16.7 mM Tris-HCl [pH 8.1], 167 mM NaCl, 0.01% SDS, 1.1% Triton X-100, 1.2 mM EDTA and protease inhibitors), and were sonicated using the Bioruptor sonicator (Diagenode, Denville, NJ) to break the nuclear membrane completely. They were subsequently incubated with anti-GR or anti-SIRT1 antibody or control IgG overnight at 4°C, and the antibody/protein/DNA complex was collected with ChIP Grade Protein A/G Magnetic Beads (Pierce Inc.). The samples were washed with the low salt immune complex wash buffer (20 mM Tris-HCl [pH 8.1], 150 mM NaCl, 0.1% SDS, 1% Triton X-100 and 2 mM EDTA), high salt immune complex wash buffer (20 mM Tris-HCl [pH 8.1], 500 mM NaCl, 0.1% SDS, 1% Triton X-100 and 2 mM EDTA), LiCl immune complex wash buffer (10 mM Tris-HCl [pH 8.1], 0.25 M LiCl, 1% IGEPAL-CA630, 1% deoxycholic acid and 1 mM EDTA), and 1X TE (10 mM Tris-HCl [pH 8.0] and 1 mM EDTA). The protein/DNA complex was finally eluted with the elution buffer (1% SDS and 0.1 M NaHCO3), and the protein-DNA cross-link was reversed by incubating with 0.2 M NaCl at 65°C for 40 min. Liberated DNA was treated with 10 mM EDTA and 80 μg/ml proteinase K (Sigma-Aldrich, St. Louis, MI, USA) at 65°C for 1.5 hour, and was precipitated with ethanol. ChIP assays for examining the association of GR or SIRT1 to GILZ GREs were performed using anti-GR or -SIRT1 antibody, or rabbit control IgG. The DNA fragment spanning the reported GILZ GREs was amplified from the DNA samples using the primer pairs shown in Supplemental Table 1 in the SYBR Green real-time PCR using the 7500 Real-time PCR System with the same amplification protocol described above (Nader et al., 2009). Obtained Ct values were normalized for those with control IgG, and their relative precipitation was demonstrated as percent precipitation compared to the corresponding input.
2.10. Statistical analysis
RNA-seq was performed in triplicate in one experiment. All other experiments were performed in triplicate and were repeated at least 3 times, and their representative results were shown in Figures. For RNA-seq, false discovery rate (FDR)-adjusted p-values were calculated. Other statistical analyses were performed using the Student t-test with 2-tailed value for 2 comparisons or one-way ANOVA with Bonferroni correction for multiple comparisons in the GraphPad Prism 6 (GraphPad Software, San Diego, CA). Statistical significance was set at p<0.05.
3. RESULTS
3.1. SIRT1 enhances GR transcriptional activity in its deacetylase activity-independent fashion
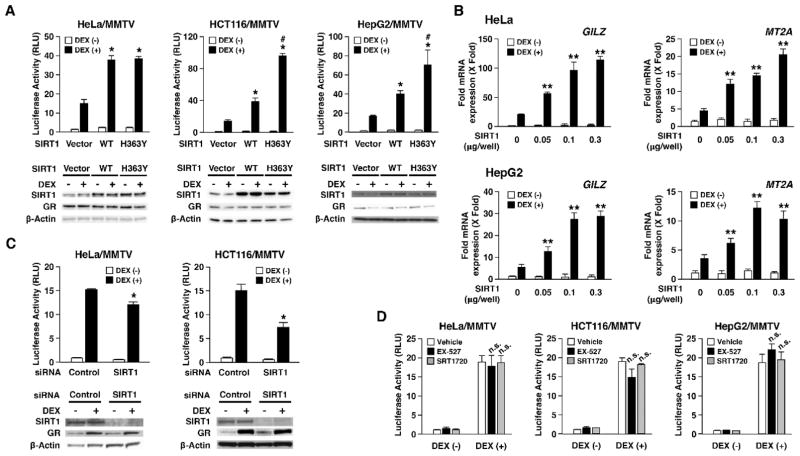
To examine the effect of SIRT1 on GR-mediated transcriptional activity, we first expressed SIRT1 in three human cell lines, HeLa, HCT116 and HepG2, along with transfection of the glucocorticoid-responsive MMTV GREs-driven luciferase reporter plasmid. Overexpression of SIRT1 enhanced GR-induced transcriptional activity in a DEX-dependent fashion in all cell lines employed (Fig. 1A). We next examined the effects of SIRT1 on two endogenous glucocorticoid-responsive genes, GILZ and MT2A, which have functional GREs in their regulatory region (Sasse, Zuo, Kadiyala et al., 2015, Asselin-Labat, David, Biola-Vidamment et al., 2004). SIRT1 dose-dependently enhanced DEX-stimulated mRNA expression of these genes in HeLa and HepG2 cells (Fig. 1B). In HeLa and HCT116 cells, knockdown of endogenous SIRT1 with its siRNA significantly reduced GR transcriptional activity on the MMTV promoter, indicating that endogenous SIRT1 is required for GR to stimulate its transcriptional activity (Fig. 1C, top panels). The SIRT1 siRNA used in this analysis strongly suppressed expression of the SIRT1 protein (Fig 1C, bottom panels). As SIRT1 is a HDAC, we examined whether the enhancing effect of SIRT1 on GR transcriptional activity is dependent on its deacetylase activity. Deacetylase activity-defective SIRT1 H363Y mutant demonstrated the enhancing activity comparable to that of the wild-type SIRT1 in HeLa cells (Fig. 1A, left panel), whereas the same mutant showed an even stronger enhancing effect in HCT116 and HepG2 cells (Fig. 1A, middle and right panels). These results suggest that the enhancing effect of SIRT1 on GR is independent to its deacetylase activity. To confirm this unexpected finding, we treated these cells with the chemical compounds either inhibiting (EX-527) or activating (SRT1720) the deacetylase activity of SIRT1, and examined GR transcriptional activity in HeLa, HCT116 and HepG2 cells (Fig. 1D). We found that neither EX-527 nor SRT1720 changed GR-induced transcriptional activity, supporting our finding that SIRT1 enhances GR transcriptional activity in its deacetylase activity-independent fashion.
Figure 1. SIRT1 enhances GR transcriptional activity in its deacetylase activity-independent fashion.
A: Both wild type SIRT1 and its deacetylase activity-defective mutant enhance GR transcriptional activity on the MMTV promoter in three cell lines.
HeLa, HCT116 and HepG2 cells were transfected with SIRT1 (wild type: WT or its deacetylase activity-defective mutant: H363Y)-expressing plasmid together with pODLO-MMTV-Luc and pRL-TK (HeLa and HepG2), or pSV40-β-Gal (HCT116 cells). pRShGRα was also included for HCT116 cells. Bars represent mean ± SEM values of the firefly luciferase activity normalized for renilla luciferase activity or β-galactosidase activity in the presence or absence of 10−6 M of DEX. *: p<0.05, compared to the condition with control vector transfection and DEX treatment. #: p<0.05, compared to the condition with SIRT1 WT transfection and DEX treatment. Results of Western blotting for SIRT1 and GR in the samples used for reporter assays are shown in the bottom panels.
B: SIRT1 dose-dependently enhances GR transcriptional activity on endogenous glucocorticoid-responsive genes.
HeLa (top panels) and HepG2 (bottom panels) cells were transfected with indicated amounts of SIRT1-expressing plasmid. pBK/CMV was used to account for the same amounts of plasmid DNA. Bars represent mean ± SEM values of the fold mRNA expression of GILZ or MT2A normalized for RPLP0 in the presence or absence of 10−6 M of DEX. **: p<0.01, compared the conditions obtained in the presence of DEX (multiple comparison).
C: SIRT1 knockdown reduced GR transcriptional activity on the MMTV promoter.
HeLa and HCT116 cells were transfected with control or SIRT1 siRNA in the presence of pODLO-MMTV-Luc, and pRL-TK (HeLa cells) or pSV40-β-Gal (HCT116 cells). pRShGRα was included for HCT116 cells. Bars represent mean ± SEM values of the firefly luciferase activity normalized for renilla luciferase activity or β-galactosidase activity in the presence or absence of 10−6 M of DEX. *: p<0.05, compared to the condition under control siRNA transfection and DEX treatment. Results of Western blotting for SIRT1 and GR in the samples used for reporter assays are shown in the bottom panels.
D: Compounds activating or inhibiting the deacetylase activity of SIRT1 do not influence the enhancing effect of SIRT1 on GR transcriptional activity.
HeLa, HCT116 and HepG2 cells were transfected with SIRT1-expressing plasmid together with pODLO-MMTV-Luc, and pRL-TK (HeLa and HepG2 cells) or pSV40-β-Gal (HCT116 cells). pRShGRα was included for HCT116 cells. Cells were then treated with the SIRT1 inhibitor EX-527 (2.5 mM) or the SIRT1 activator SRT1720 (0.16 mM) for 10 hours. Six hours after addition of these compounds, cells were treated with 10−6 M of DEX or vehicle for 4 hours. Bars represent mean ± SEM values of the firefly luciferase activity normalized for renilla luciferase activity or β-galactosidase activity. n.s.: not significant, compared to the condition under DEX treatment without SIRT1 activator or inhibitor.
3.2. GR and SIRT1 physically interact with each other
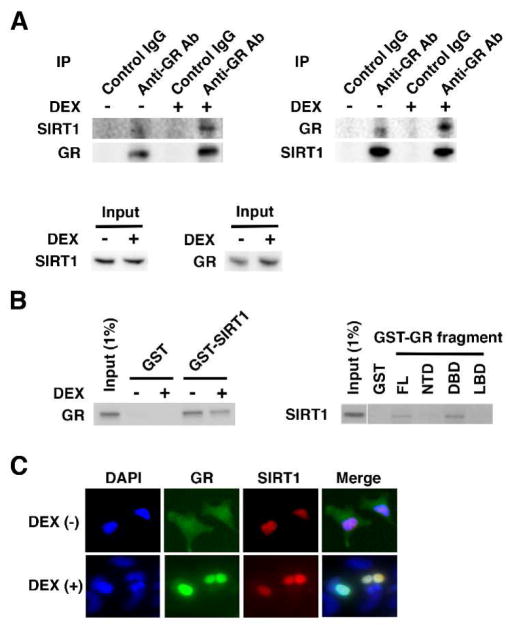
We next examined physical interaction between SIRT1 and GR in vivo. In the co-immunoprecipitation assays using anti-GR or -SITR1 antibody, SIRT1 and GR were reciprocally co-precipitated with each other in a DEX-dependent fashion in HeLa cells (Fig. 2A). To test further direct interaction between SIRT1 and GR, we performed GST pull-down assays by using in vitro translated and radiolabeled SIRT1 or GR together with GST-fused GR or -SIRT1 (Fig. 2B). SIRT1 physically interacted with GST-fused GR FL and DBD, but not with GR NTD and LBD. We did not use DEX for this GST pull-down assay, as bacterially produced GST-GRs do not respond to glucocorticoids (Hill et al., 2016, De Martino et al., 2004). The glucocorticoid-responsive radiolabelled GR produced with reticulocyte lysates interacted with GST-SIRT1 in a DEX-independent fashion, in contrast to our findings obtained in the co-immunoprecipitation assays (Habib et al., 2017, De Martino et al., 2004). We further examined their co-localization in HeLa cells by employing EGFP-fused GR and RFP-fused SIRT1. The former fusion protein translocated from the cytoplasm to the nucleus in response to DEX, whereas the latter stayed constitutively in the nucleus regardless of the presence or absence of DEX, indicating that these two proteins co-localize in the nucleus in the presence of DEX (Fig. 2C). Taken together, these results indicate that GR physically interacts with SIRT1 at its DBD in the nucleus.
Figure 2. GR and SIRT1 physically interact with each other in vivo and in vitro.
A: SIRT1 and GR are co-precipitated with each other in a DEX-dependent fashion in HeLa cells.
HeLa cells were treated with 10−6 M of DEX, and co-immunoprecipitation was carried out using anti-GR or -SIRT1 antibody, or control IgG. GR and SIRT1 were visualized with their specific antibodies in Western blotting. Five percent of the samples used for co-immunoprecipitation reactions were loaded as input. Ab: antibody, IP: immunoprecipitation
B: SIRT1 and GR interact with each other in GST pull-down assays.
In vitro translated and radiolabelled SIRT1 and GR were incubated with bacterially produced GST or indicated GST-fused GRs or SIRT1. For the experiment using radiolabelled GR, 10−5 M of DEX or vehicle was added to reactions. One percent of the labelled SIRT1 or GR added to the reactions was loaded as input. FL: full length, NTD: N-terminal domain, DBD: DNA-binding domain, LBD: ligand-binding domain.
C: SIRT1 and GR co-localize in the nucleus upon DEX treatment in HeLa cells.
HeLa cells were transfected with EFGP-fused GR- and RFP-fused SIRT1-expressing plasmids and were treated with or without 10−6 M of DEX. Images of DAPI, EGFP (GR) and RFP (SIRT1) were recorded using the inverted microscope.
3.3. SIRT1 differentially regulates glucocorticoid-responsive transcriptome by acting as a transcriptional enhancer
To further address functional interaction between SIRT1 and GR in a genome-wide fashion, we performed RNA-seq analyses in which SIRT1 was knocked down with its siRNA in HeLa cells (Fig. 3). Successful knockdown of SIRT1 with the siRNA was again confirmed in these cells: no SIRT1 protein expression and significant reduction (~5% of the basal level) of its RNA was observed upon SIRT1 knockdown respectively in Western blotting and in SYBR Green real-time PCR (Supplemental Fig. 1). Approximately 26,000 transcripts were successfully detected in all conditions for which SIRT1 knockdown and/or DEX treatment were performed (Supplemental Table 2). DEX regulated 190 and 152 genes, respectively, upon control and SIRT1 knockdown, and these gene groups demonstrated significant overlap (Fig. 3A, left panel). Under the control knockdown, DEX positively and negatively regulated ~60 and ~40% of the entire DEX-responding genes, respectively. SIRT1 knockdown little influenced the percentages of the genes positively/negatively responding to DEX, indicating that SIRT1 does not change transcriptional direction of the DEX effects on glucocorticoid-responsive genes (Fig. 3A, right panel). Among these DEX-responding genes, we found that 126 and 113 genes were positively regulated under control and SIRT1 knockdown respectively, whereas 64 and 39 genes negatively responded to these treatments (Fig. 3B). Among the genes positively or negatively regulated by DEX under control knockdown (126 and 64 genes, respectively), SIRT1 further modulated their expression positively or negatively (Fig. 3C). We then analyzed details of the SIRT1-mediated regulation on these genes, and found that a substantial number of glucocorticoid-responsive genes was further regulated by SIRT1 (Fig. 3D and Supplemental Table 3 and 4); Among 126 genes positively regulated by DEX under control knockdown, 27 genes (21.4%) were further regulated by SIRT1; SIRT1 knockdown influenced basal expression of 13 genes in the absence of DEX, whereas 14 genes did not respond to this treatment in the absence of DEX. We focused on the latter genes, as they are considered as “pure” SIRT1-responding, glucocorticoid-responsive genes (regulation by SIRT1 is absolutely dependent on the GR activation by DEX), and found that 13 out of the 14 genes were down-regulated by SIRT1 knockdown (Fig. 3D, top panel). These results indicate that SIRT1 generally acts as an enhancer for the transactivation activity of GR, consistent with our findings observed in reporter assays. We also performed the same analysis on the 64 genes negatively responding to DEX, and found that SIRT1 knockdown up-regulated the transcriptional activity of all 7 genes repressed by DEX (Fig. 3D, bottom panel), again indicating that SIRT1 functions as an enhancer for the repressive action of GR. We confirmed the above results by employing several glucocorticoid-responsive genes in reconstituted real-time PCR assays (Supplemental Fig. 2). Taken together, our analyses revealed that SIRT1 influences GR-induced transcriptional activity on a substantial number of the glucocorticoid-responsive genes by functioning as an enhancer for both transactivational and transrepressive effects of GR.
Figure 3. SIRT1 modulates glucocorticoid-responsive transcriptome.
A: SIRT1 knockdown alters DEX-responsive transcriptome in HeLa cells.
Venn diagram for DEX-responding genes in the presence or absence of SIRT1 knockdown (SIRT1-KD) is shown in the left panel, whereas percentages of the genes positively (Positive) or negatively (Negative) responding to DEX in the presence or absence of SIRT1-KD are shown in the right panel.
B: SIRT1 knockdown modulates the transcriptome positively or negatively responding to DEX in HeLa cells.
Venn diagrams for the genes positively (left panel) or negatively (right panel) responding to DEX in the presence or absence of SIRT1-KD are shown.
C: SIRT1 differentially regulates DEX-responsive transcriptiome in HeLa cells.
Scatter plot in the left panel shows the effect of SIRT1-KD on DEX-responsive transcriptome. The Y-axis shows the log2 values of the fold changes in response to DEX in the absence of SIRT1-KD, whereas the X-axis indicates the log2 values of the fold changes in response to DEX in the presence of SIRT1-KD. Black dots indicate the genes insignificantly responding to SIRT-KD. Colored dots (coordinated with right table) indicate significant genes. The right table demonstrates numbers of the genes in each color-coded subset shown in the left panel.
D: SIRT1 knockdown modulates mRNA expression of the genes positively or negatively responding to DEX in HeLa cells.
Pie charts created based on the RNA-seq data and representing the genes positively (top) or negatively (bottom) responding to DEX (126 and 64 genes, respectively) demonstrate the number of such genes responsive (27 and 23 genes) or unresponsive (99 and 41 genes) to SIRT1-KD. The middle bar graphs indicate the number of the genes, which respond (Yes)/do not respond (No) to SIRT1-KD in the DEX-untreated basal condition, whereas the right bar graphs demonstrate the number of the genes further up- or down-regulated by SIRT1-KD under DEX treatment in a fraction of the genes not responding to SIRT1-KD in the absence of DEX.
3.4. SIRT1 is attracted to GREs of a glucocorticoid-responsive gene
We showed that SIRT1 enhanced GR transcriptional activity on two glucocorticoid-responsive GILZ and MT2A genes (Fig. 1B). These genes were also found among the genes regulated by SIRT1 in RNA-seq and subsequent SYBR Green real-time PCR analysis (Supplemental Table 3 and Supplemental Fig. 2). Since GILZ has functional GREs within its regulatory region (Asselin-Labat et al., 2004), we examined the attraction of SIRT1 to GILZ GREs to explore the mechanism(s) underlying SIRT1-mediated regulation of GR transcriptional activity. Both endogenous SIRT1 and GR were attracted to GILZ GREs in a DEX-dependent fashion (Fig. 4). SIRT1 knockdown did not change the attraction of GR to GILZ GREs. These results suggest that SIRT1 acts as a coactivator of GR via attraction to GREs through GR, but not as a molecule that influences the association of GR to this DNA sequence.
Figure 4. SIRT1 is attracted to GILZ GREs in a glucocorticoid-dependent fashion.
HeLa cells were transfected with control or SIRT1 siRNA, and were treated with 10−6 of DEX. Attraction of GR and SIRT1 to GILZ GREs was examined with ChIP assays respectively using anti-GR and -SIRT1 antibody, and with the subsequent SYBR Green real-time PCR using specific primers for GILZ GREs (Supplemental Table 1). Bars represent mean ± SEM values of the GR (left panel) or SIRT1 (right panel) association to GILZ GREs expressed as percentages to input. *: p<0.05, n.s.: not significant, compared with the two conditions indicated. Expression of the GR or SIRT1 protein in the samples used for ChIP assays was examined with Western blotting and is shown in the bottom panel.
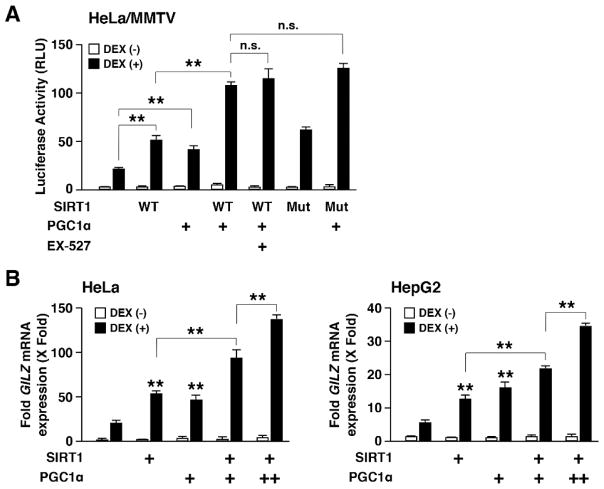
3.5. SIRT1 and PGC1α cooperatively enhance GR-induced transcriptional activity
NR coactivators interact with ligand-activated and DNA-bound receptors and form a large protein complex including other cofactors, general transcription factors and RNA polymerase II to stimulate the transcription of NR-responsive genes (McKenna et al., 1999). Therefore, we hypothesized that SIRT1 might cooperate with other coactivators on GREs-bound GR. Among them, PGC1α, a GR coactivator important for thermoregulation and energy metabolism, was shown to interact directly with SIRT1, and SIRT1 deacetylates this protein (Rodgers et al., 2008, Knutti, Kaul and Kralli, 2000, Nemoto, Fergusson and Finkel, 2005). We thus examined influence of SIRT1 and PGC1α on the transcriptional activity of GR in HeLa cells, and found that they cooperatively enhanced it (Fig. 5A). PGC1α potentiated SIRT1-induced enhancement of GR transcriptional activity on the GILZ gene in a dose-dependent fashion in HeLa and HepG2 cells (Fig. 5B). The cooperation between SIRT1 and PGC1α was observed with deacetylase activity-defective SIRT1 H363Y mutant as well as under the treatment with deacetylase inhibitor EX-527 (Fig. 5A), indicating that neither the deacetylase activity of SIRT1 nor its deacetylation of PGC1α is required for their regulation of GR transcriptional activity. Rather, these results indicate that SIRT1 and PCG1α cooperate with each other in the coactivator complex formed on DNA-bound GR in which SIRT1 might act as a scaffold connecting GR and other cofactors including PGC1α through protein-protein interactions.
Figure 5. SIRT1 and PGC1α cooperatively enhance GR transcriptional activity in the former’s deacetylase activity-independent fashion.
A: SIRT1 and PGC1α enhance GR transcriptional activity in the former’s deacetylase activity-independent fashion on the MMTV promoter in HeLa cells.
HeLa cells were transfected with wild type (WT) SIRT1, its deacetylase activity-defective H363Y mutant (Mut) and/or PGC1α-expressing plasmids together with pODLO-MMTV-Luc and pRL-TK. The SIRT1 inhibitor EX-527 (2.5 mM) was also added in some conditions. Bars represent mean ± SEM values of the firefly luciferase activity normalized for renilla luciferase activity in the presence or absence of 10−6 M of DEX. *: p<0.05, **: p<0.01, n.s.; not significant, compared with the two conditions indicated.
B: PGC1α dose-dependently potentiates SIRT1-induced enhancement of GR transcriptional activity on the endogenous GILZ gene in HeLa and HepG2 cells.
HeLa and HepG2 cells were transfected with SIRT1- and/or PGC1α-expressing plasmids (+: 0.05 μg/well, ++: 0.20 μg/well), and were treated with 10−6 M of DEX. Control plasmid was used to account for the same amounts of plasmid DNA. GILZ mRNA expression was examined in the SYBR Green real-time PCR using its specific primer pair. Bars indicate mean ± SEM of the fold expression of GILZ mRNA compared to the condition obtained under control plasmid transfection in the absence of DEX treatment. **: p<0.01, compared to the condition in the absence of SIRT/PGC1α transfection but with DEX (those shown above data bars) or the 2 conditions indicated.
4. DISCUSSION
In this study, we investigated the crosstalk between SIRT1 and GR at the cellular and molecular levels. We found that SIRT1 enhances the transcriptional activity of GR in the reporter assays using three representative cell lines, whereas it acts as an enhancer for both transactivational and transrepressive actions of GR in transcriptome analysis. At a molecular level, SIRT1 physically interacts with GR, is attracted to GILZ GREs, and co-localizes with GR in the nucleus upon DEX treatment. Further, SIRT1 cooperates with PGC1α for regulating GR transcriptional activity in its deacetylase-independent fashion.
SIRT1 influences GR-induced transcriptional activity in its deacetylase activity-independent fashion, as the SIRT1 mutant defective in this activity (H363Y) demonstrated the effects equivalent to the wild type protein, and the chemical compounds activating or inhibiting its deacetylase activity showed no effects on SIRT1-mediated enhancement of GR transcriptional activity. Although the deacetylase activity-independent effects of SIRT1 on GR we report here are different from known deacetylase activity-dependent actions of SIRT1 on some other NRs, the action independent to its deacetylase activity was reported previously. For example, SIRT1 protects cerebellar granule neurons from the pro-apoptotic effect of low potassium levels in its deacetylase activity-independent fashion (Pfister, Ma, Morrison et al., 2008). Some HDACs also have their deacetylase activity-independent actions on their interacting molecules (Lahm, Paolini, Pallaoro et al., 2007, Lee, Sengupta, Villagra et al., 2006, Sun, Feng, Fang et al., 2013); The deacetylase activity of HDAC8 is not required for this protein to protect the human ever-shorter telomeres 1B protein from ubiquitin-mediated degradation (Lee et al., 2006). Further, the transcriptional repressive action of HDAC3 is not sensitive to HDAC inhibitors for some responsive genes, indicating that this effect of HDAC3 is independent to its deacetylase activity (Sun et al., 2013).
We found that SIRT1 physically interacts with GR DBD and is attracted to GILZ GREs together with GR in a DEX-dependent fashion. These results indicate that SIRT1 acts as a GR coactivator attracted to GREs-bound GR through its physical interaction to GR. Since SIRT1 cooperates with PGC1α for enhancing the transcriptional activity of GR also in its deacetylase activity-independent fashion, SIRT1 appears to act as a transcriptional scaffold for the transcriptional complex attracted to GREs-bound GR, possibly by strengthening the multi-protein interaction supported by mutual physical communication between component proteins, such as GR/SIRT1, GR/PGC1α and SIRT1/PGC1α (Knutti et al., 2000, Nemoto et al., 2005) (Fig. 6). Since physical interaction of HDAC3 with the nuclear receptor corepressor (NCoR)/silencing mediator for retinoid or thyroid-hormone receptors (SMRT) is necessary for its deacetylase activity-independent transcriptional regulation (Sun et al., 2013), our results together with this reported finding suggest that some HDACs including SIRT1 have an ability to modulate the transcriptional activity of responsive genes possibly by supporting the formation/stability of the transcriptional complex. In fact, deacetylase activity-dependent and -independent actions of HDACs may cooperate with each other in the net transcriptional regulation promoted by these HDACs in a context-specific fashion, such as the transcription factors/NRs to be attracted, the target genes for regulation and/or the tissues/cells where these HDACs are expressed. We found that the deacetylase activity-defective SIRT1 mutant demonstrated even a stronger enhancing effect on GR transcriptional activity than wild type SIRT1 in HCT116 cells and HepG2 cells, suggesting that the repressive action of SIRT1 through its deacetylase activity may be functional for these cells in addition to its enhancing effect independent to this enzymatic activity.
Figure 6. Schematic diagrams of the SIRT1 actions on GR transcriptional activity.
SIRT1 potentiates both transactivational (left) and transrepressing (right) actions of GR on glucocorticoid-responsive genes in its deacetylase activity-independent fashion possibly by facilitating (left) and suppressing (right) formation/accumulation of the transcriptional complex including PGC1α through mutual physical interaction. SIRT1 may enhance both the transactivational and transrepressive action of GR that bind DNA either directly via GREs or indirectly through interaction with other transcription factors. GREs: glucocorticoid response elements, TF; transcription factor, TFREs: transcription factor response elements.
In glucocorticoid-responsive transcriptome analysis in HeLa cells, we found that SIRT1 influenced the transcriptional activity of ~30% of the entire glucocorticoid-responsive genes, indicating its strong regulatory activity on glucocorticoid-sensitive transcriptome. In addition, SIRT1 tends to enhance positive or negative regulatory actions of DEX to the same directions that this steroid exerts. Therefore, SIRT1 appears to act as an “enhancer” of both transactivational and transrepressive actions of GR (Fig. 6). The hypothetical scaffolding action of SIRT1 we proposed above may be the underlying mechanism(s) for its enhancing action on the genes transactivated by GR, whereas other mode(s) of actions may be operational for the genes transrepressed by this receptor. Although it is still not clear how GR represses the transcriptional activity of other transcription factors (Kino, 2000), inhibition of the functional transcriptional complex formation by SIRT1 might be one of the potential mechanisms for the latter action of this protein. Since GR may regulate indirectly some of the glucocorticoid-responsive genes we identified to be sensitive to SIRT1, the “enhancer” action of SIRT1 appears to be functional not only on the GREs-associated GR, but also that indirectly bound to DNA via other transcription factors.
Physiological or pathological implications of the SIRT1-mediated regulation of the GR activity particularly in connection with longevity is still unknown. Physiologic levels of glucocorticoids are generally beneficial for humans, whereas chronic elevation of their serum levels causes numerous adverse effects, leading to shortening of life (Chrousos and Kino, 2007, Spiers et al., 2014, Nader et al., 2010). Indeed, physiologic amounts of glucocorticoids appear to be required for maintaining proper functions of mitochondria in part by stimulating expression of PGC1α and some mitochondrial component proteins (Puigserver and Spiegelman, 2003, Weber, Bruck, Mikes et al., 2002, Scheller and Sekeris, 2003, Amat, Solanes, Giralt et al., 2007), thus enhancement of this action of glucocorticoids by SIRT1 may support its longevity-promoting effects. In contrast, excess glucocorticoids increase oxidative stress in their target cells/tissues, and enhancement of this glucocorticoid actions by SIRT1 may compromise its beneficial effects on longevity (Spiers et al., 2014). Further, chronic use of excess glucocorticoids accelerates atherosclerosis by causing insulin resistance and hyperlipidemia, which also impact favorable effects of SIRT1 on longevity (Chrousos and Kino, 2007). Therefore, it is important to explore the exact actions of SIRT1 on glucocorticoids/GR functions in various organs/tissues as well as in different physiologic/pathologic situations to reveal the overall effects of SIRT1 on longevity through modulation of the glucocorticoids/GR regulatory network.
In conclusion, SIRT1 acts as an enhancer of GR, augmenting both transactivational and transrepressive actions of this receptor in its deacetylase activity-independent fashion. The molecular interaction between SIRT1 and GR may potentially influence diverse actions of these proteins and further contribute to the control of human longevity. Further intensive work will warrant this important research area.
Supplementary Material
SIRT1 enhances glucocorticoid receptor (GR) transcriptional activity.
This activity augments both transactivating and transrepressing actions of GR.
The activity is independent to its deacetylase activity.
SIRT1 physically interacts with GR bound on the glucocorticoid response elements.
Acknowledgments
We thank Drs. R. M. Evans and J. N. Miner for providing us with their plasmids, the Molecular Genomics Core of the National Institute of Child Health and Human Development for running sequencing samples, Dr. A. H. DeCherney for supporting this study, and Mr. E. K. Zachman for technical support.
6. FUNDING
This study was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health (Z01 HD008732-05 HNT), and the Intramural Research Program of the Sidra Medical and Research Center. S. Suzuki was supported by an Internal Grant of the Asahikawa Medical University.
Footnotes
DISCLOSURE SUMMARY: The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chrousos GP, Kino T. Glucocorticoid action networks and complex psychiatric and/or somatic disorders. Stress. 2007;10:213–9. doi: 10.1080/10253890701292119. [DOI] [PubMed] [Google Scholar]
- 2.Spiers JG, Chen HJ, Sernia C, Lavidis NA. Activation of the hypothalamic-pituitary-adrenal stress axis induces cellular oxidative stress. Front Neurosci. 2014;8:456. doi: 10.3389/fnins.2014.00456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haussmann MF, Heidinger BJ. Telomere dynamics may link stress exposure and ageing across generations. Biol Lett. 2015:11. doi: 10.1098/rsbl.2015.0396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nader N, Chrousos GP, Kino T. Interactions of the circadian CLOCK system and the HPA axis. Trends Endocrinol Metab. 2010;21:277–86. doi: 10.1016/j.tem.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chrousos GP, Kino T. Glucocorticoid signaling in the cell. Expanding clinical implications to complex human behavioral and somatic disorders. Ann N Y Acad Sci. 2009;1179:153–66. doi: 10.1111/j.1749-6632.2009.04988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Franchimont D, Kino T, Galon J, Meduri GU, Chrousos G. Glucocorticoids and inflammation revisited: the state of the art. NIH clinical staff conference. Neuroimmunomodulation. 2002;10:247–60. doi: 10.1159/000069969. [DOI] [PubMed] [Google Scholar]
- 7.Nicolaides NC, Galata Z, Kino T, Chrousos GP, Charmandari E. The human glucocorticoid receptor: molecular basis of biologic function. Steroids. 2010;75:1–12. doi: 10.1016/j.steroids.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kino T. Glucocorticoid Receptor. In: De Groot LJ, et al., editors. Endotext. MDText.com, Inc; South Dartmouth MA: 2000. [Google Scholar]
- 9.Mackeh R, Marr AK, Fadda A, Kino T. C2H2-type zinc finger proteins: evolutionarily old and new partners of the nuclear hormone receptors. Nucl Recept Signal. 2017 doi: 10.1177/1550762918801071. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chrousos GP, Kino T. Intracellular glucocorticoid signaling: a formerly simple system turns stochastic. Sci STKE. 2005;2005:pe48. doi: 10.1126/stke.3042005pe48. [DOI] [PubMed] [Google Scholar]
- 11.Heery DM, Kalkhoven E, Hoare S, Parker MG. A signature motif in transcriptional co-activators mediates binding to nuclear receptors. Nature. 1997;387:733–6. doi: 10.1038/42750. [DOI] [PubMed] [Google Scholar]
- 12.McKenna NJ, Lanz RB, O’Malley BW. Nuclear receptor coregulators: cellular and molecular biology. Endocr Rev. 1999;20:321–44. doi: 10.1210/edrv.20.3.0366. [DOI] [PubMed] [Google Scholar]
- 13.Tessarz P, Kouzarides T. Histone core modifications regulating nucleosome structure and dynamics. Nat Rev Mol Cell Biol. 2014;15:703–8. doi: 10.1038/nrm3890. [DOI] [PubMed] [Google Scholar]
- 14.Finkel T, Deng CX, Mostoslavsky R. Recent progress in the biology and physiology of sirtuins. Nature. 2009;460:587–91. doi: 10.1038/nature08197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang HC, Guarente L. SIRT1 and other sirtuins in metabolism. Trends Endocrinol Metab. 2014;25:138–45. doi: 10.1016/j.tem.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moore RL, Dai Y, Faller DV. Sirtuin 1 (SIRT1) and steroid hormone receptor activity in cancer. J Endocrinol. 2012;213:37–48. doi: 10.1530/JOE-11-0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bordone L, Guarente L. Calorie restriction, SIRT1 and metabolism: understanding longevity. Nat Rev Mol Cell Biol. 2005;6:298–305. doi: 10.1038/nrm1616. [DOI] [PubMed] [Google Scholar]
- 18.Milne JC, Lambert PD, Schenk S, Carney DP, Smith JJ, Gagne DJ, Jin L, Boss O, Perni RB, Vu CB, Bemis JE, Xie R, Disch JS, Ng PY, Nunes JJ, Lynch AV, Yang H, Galonek H, Israelian K, Choy W, Iffland A, Lavu S, Medvedik O, Sinclair DA, Olefsky JM, Jirousek MR, Elliott PJ, Westphal CH. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450:712–6. doi: 10.1038/nature06261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feige JN, Auwerx J. Transcriptional targets of sirtuins in the coordination of mammalian physiology. Curr Opin Cell Biol. 2008;20:303–9. doi: 10.1016/j.ceb.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dai Y, Ngo D, Forman LW, Qin DC, Jacob J, Faller DV. Sirtuin 1 is required for antagonist-induced transcriptional repression of androgen-responsive genes by the androgen receptor. Mol Endocrinol. 2007;21:1807–21. doi: 10.1210/me.2006-0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Popov VM, Wang C, Shirley LA, Rosenberg A, Li S, Nevalainen M, Fu M, Pestell RG. The functional significance of nuclear receptor acetylation. Steroids. 2007;72:221–30. doi: 10.1016/j.steroids.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suh JH, Sieglaff DH, Zhang A, Xia X, Cvoro A, Winnier GE, Webb P. SIRT1 is a direct coactivator of thyroid hormone receptor β1 with gene-specific actions. PLoS One. 2013;8:e70097. doi: 10.1371/journal.pone.0070097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li X, Zhang S, Blander G, Tse JG, Krieger M, Guarente L. SIRT1 deacetylates and positively regulates the nuclear receptor LXR. Mol Cell. 2007;28:91–106. doi: 10.1016/j.molcel.2007.07.032. [DOI] [PubMed] [Google Scholar]
- 24.Picard F, Kurtev M, Chung N, Topark-Ngarm A, Senawong T, Machado De Oliveira R, Leid M, McBurney MW, Guarente L. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-γ. Nature. 2004;429:771–6. doi: 10.1038/nature02583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodgers JT, Lerin C, Gerhart-Hines Z, Puigserver P. Metabolic adaptations through the PGC-1α and SIRT1 pathways. FEBS Lett. 2008;582:46–53. doi: 10.1016/j.febslet.2007.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oberkofler H, Schraml E, Krempler F, Patsch W. Potentiation of liver X receptor transcriptional activity by peroxisome-proliferator-activated receptor γ co-activator 1α. Biochem J. 2003;371:89–96. doi: 10.1042/BJ20021665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Puigserver P, Spiegelman BM. Peroxisome proliferator-activated receptor-γ coactivator 1α (PGC-1α): transcriptional coactivator and metabolic regulator. Endocr Rev. 2003;24:78–90. doi: 10.1210/er.2002-0012. [DOI] [PubMed] [Google Scholar]
- 28.Kim D, Nguyen MD, Dobbin MM, Fischer A, Sananbenesi F, Rodgers JT, Delalle I, Baur JA, Sui G, Armour SM, Puigserver P, Sinclair DA, Tsai LH. SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer’s disease and amyotrophic lateral sclerosis. EMBO J. 2007;26:3169–79. doi: 10.1038/sj.emboj.7601758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nader N, Chrousos GP, Kino T. Circadian rhythm transcription factor CLOCK regulates the transcriptional activity of the glucocorticoid receptor by acetylating its hinge region lysine cluster: potential physiological implications. FASEB J. 2009;23:1572–83. doi: 10.1096/fj.08-117697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fadda A, Syed N, Mackeh R, Papadopoulou A, Suzuki S, Jithesh PV, Kino T. Genome-wide Regulatory Roles of the C2H2-type Zinc Finger Protein ZNF764 on the Glucocorticoid Receptor. Sci Rep. 2017;7:41598. doi: 10.1038/srep41598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Habib T, Sadoun A, Nader N, Suzuki S, Liu W, Jithesh PV, Kino T. AKT1 has dual actions on the glucocorticoid receptor by cooperating with 14-3-3. Mol Cell Endocrinol. 2017;439:431–443. doi: 10.1016/j.mce.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 32.Nader N, Ng SS, Wang Y, Abel BS, Chrousos GP, Kino T. Liver x receptors regulate the transcriptional activity of the glucocorticoid receptor: implications for the carbohydrate metabolism. PLoS One. 2012;7:e26751. doi: 10.1371/journal.pone.0026751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kino T, Ichijo T, Amin ND, Kesavapany S, Wang Y, Kim N, Rao S, Player A, Zheng YL, Garabedian MJ, Kawasaki E, Pant HC, Chrousos GP. Cyclin-dependent kinase 5 differentially regulates the transcriptional activity of the glucocorticoid receptor through phosphorylation: clinical implications for the nervous system response to glucocorticoids and stress. Mol Endocrinol. 2007;21:1552–68. doi: 10.1210/me.2006-0345. [DOI] [PubMed] [Google Scholar]
- 34.Hill MJ, Suzuki S, Segars JH, Kino T. CRTC2 Is a coactivator of GR and couples GR and CREB in the regulation of hepatic gluconeogenesis. Mol Endocrinol. 2016;30:104–17. doi: 10.1210/me.2015-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klockenbusch C, Kast J. Optimization of formaldehyde cross-linking for protein interaction analysis of non-tagged integrin β1. J Biomed Biotechnol. 2010;2010:927585. doi: 10.1155/2010/927585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Martino MU, Bhattachryya N, Alesci S, Ichijo T, Chrousos GP, Kino T. The glucocorticoid receptor and the orphan nuclear receptor chicken ovalbumin upstream promoter-transcription factor II interact with and mutually affect each other’s transcriptional activities: implications for intermediary metabolism. Mol Endocrinol. 2004;18:820–33. doi: 10.1210/me.2003-0341. [DOI] [PubMed] [Google Scholar]
- 37.Kino T, Gragerov A, Kopp JB, Stauber RH, Pavlakis GN, Chrousos GP. The HIV-1 virion-associated protein vpr is a coactivator of the human glucocorticoid receptor. J Exp Med. 1999;189:51–62. doi: 10.1084/jem.189.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kino T, Souvatzoglou E, De Martino MU, Tsopanomihalu M, Wan Y, Chrousos GP. Protein 14-3-3σ interacts with and favors cytoplasmic subcellular localization of the glucocorticoid receptor, acting as a negative regulator of the glucocorticoid signaling pathway. J Biol Chem. 2003;278:25651–6. doi: 10.1074/jbc.M302818200. [DOI] [PubMed] [Google Scholar]
- 39.Kino T, Tiulpakov A, Ichijo T, Chheng L, Kozasa T, Chrousos GP. G protein β interacts with the glucocorticoid receptor and suppresses its transcriptional activity in the nucleus. J Cell Biol. 2005;169:885–96. doi: 10.1083/jcb.200409150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kino T, Jaffe H, Amin ND, Chakrabarti M, Zheng YL, Chrousos GP, Pant HC. Cyclin-dependent kinase 5 modulates the transcriptional activity of the mineralocorticoid receptor and regulates expression of brain-derived neurotrophic factor. Mol Endocrinol. 2010;24:941–52. doi: 10.1210/me.2009-0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ng SS, Li A, Pavlakis GN, Ozato K, Kino T. Viral infection increases glucocorticoid-induced interleukin-10 production through ERK-mediated phosphorylation of the glucocorticoid receptor in dendritic cells: potential clinical implications. PLoS One. 2013;8:e63587. doi: 10.1371/journal.pone.0063587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Papadopoulou A, Siamatras T, Delgado-Morales R, Amin ND, Shukla V, Zheng YL, Pant HC, Almeida OF, Kino T. Acute and chronic stress differentially regulate cyclin-dependent kinase 5 in mouse brain: implications to glucocorticoid actions and major depression. Transl Psychiatry. 2015;5:e578. doi: 10.1038/tp.2015.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sasse SK, Zuo Z, Kadiyala V, Zhang L, Pufall MA, Jain MK, Phang TL, Stormo GD, Gerber AN. Response element composition governs correlations between binding site affinity and transcription in glucocorticoid receptor feed-forward loops. J Biol Chem. 2015;290:19756–69. doi: 10.1074/jbc.M115.668558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Asselin-Labat ML, David M, Biola-Vidamment A, Lecoeuche D, Zennaro MC, Bertoglio J, Pallardy M. GILZ, a new target for the transcription factor FoxO3, protects T lymphocytes from interleukin-2 withdrawal-induced apoptosis. Blood. 2004;104:215–23. doi: 10.1182/blood-2003-12-4295. [DOI] [PubMed] [Google Scholar]
- 45.Knutti D, Kaul A, Kralli A. A tissue-specific coactivator of steroid receptors, identified in a functional genetic screen. Mol Cell Biol. 2000;20:2411–22. doi: 10.1128/mcb.20.7.2411-2422.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nemoto S, Fergusson MM, Finkel T. SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1α. J Biol Chem. 2005;280:16456–60. doi: 10.1074/jbc.M501485200. [DOI] [PubMed] [Google Scholar]
- 47.Pfister JA, Ma C, Morrison BE, D’Mello SR. Opposing effects of sirtuins on neuronal survival: SIRT1-mediated neuroprotection is independent of its deacetylase activity. PLoS One. 2008;3:e4090. doi: 10.1371/journal.pone.0004090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lahm A, Paolini C, Pallaoro M, Nardi MC, Jones P, Neddermann P, Sambucini S, Bottomley MJ, Lo Surdo P, Carfi A, Koch U, De Francesco R, Steinkuhler C, Gallinari P. Unraveling the hidden catalytic activity of vertebrate class IIa histone deacetylases. Proc Natl Acad Sci U S A. 2007;104:17335–40. doi: 10.1073/pnas.0706487104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee H, Sengupta N, Villagra A, Rezai-Zadeh N, Seto E. Histone deacetylase 8 safeguards the human ever-shorter telomeres 1B (hEST1B) protein from ubiquitin-mediated degradation. Mol Cell Biol. 2006;26:5259–69. doi: 10.1128/MCB.01971-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sun Z, Feng D, Fang B, Mullican SE, You SH, Lim HW, Everett LJ, Nabel CS, Li Y, Selvakumaran V, Won KJ, Lazar MA. Deacetylase-independent function of HDAC3 in transcription and metabolism requires nuclear receptor corepressor. Mol Cell. 2013;52:769–82. doi: 10.1016/j.molcel.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weber K, Bruck P, Mikes Z, Kupper JH, Klingenspor M, Wiesner RJ. Glucocorticoid hormone stimulates mitochondrial biogenesis specifically in skeletal muscle. Endocrinology. 2002;143:177–84. doi: 10.1210/endo.143.1.8600. [DOI] [PubMed] [Google Scholar]
- 52.Scheller K, Sekeris CE. The effects of steroid hormones on the transcription of genes encoding enzymes of oxidative phosphorylation. Exp Physiol. 2003;88:129–40. doi: 10.1113/eph8802507. [DOI] [PubMed] [Google Scholar]
- 53.Amat R, Solanes G, Giralt M, Villarroya F. SIRT1 is involved in glucocorticoid-mediated control of uncoupling protein-3 gene transcription. J Biol Chem. 2007;282:34066–76. doi: 10.1074/jbc.M707114200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.