Abstract
Over the last few decades, hot melt extrusion (HME) has emerged as a successful technology for a broad spectrum of applications in the pharmaceutical industry. As indicated by multiple publications and patents, HME is mainly used for the enhancement of solubility and bioavailability of poorly soluble drugs. This review is focused on the recent reports on the solubility enhancement via HME and provides an update for the manufacturing/scaling up aspects of melt extrusion. In addition, drug characterization methods and dissolution studies are discussed. The application of process analytical technology (PAT) tools and use of HME as a continuous manufacturing process may shorten the drug development process; as a result, the latter is becoming the most widely utilized technique in the pharmaceutical industry. The advantages, disadvantages, and practical applications of various PAT tools such as near and mid-infrared, ultraviolet/visible, fluorescence, and Raman spectroscopies are summarized, and the characteristics of other techniques are briefly discussed. Overall, this review also provides an outline for the currently marketed products and analyzes the strengths, weaknesses, opportunities and threats of HME application in the pharmaceutical industry.
Keywords: Hot melt extrusion, Process analytical technology tool, SWOT analysis, Poorly soluble drug, Polymer, Continuous manufacturing, Dissolution
Graphical Abstract

Introduction
Over many decades, the poor solubility of APIs has been a major obstacle for the development of more efficient drug delivery methods. To overcome this problem, various strategies were proposed in the literature (Alam et al., 2012; Goke et al., 2017; Wen et al., 2015). Among these strategies, hot-melt extrusion (HME) represents a complementary pharmaceutical manufacturing technology, which is widely used by industrial and academic researchers for mitigating the solubility issues of APIs.
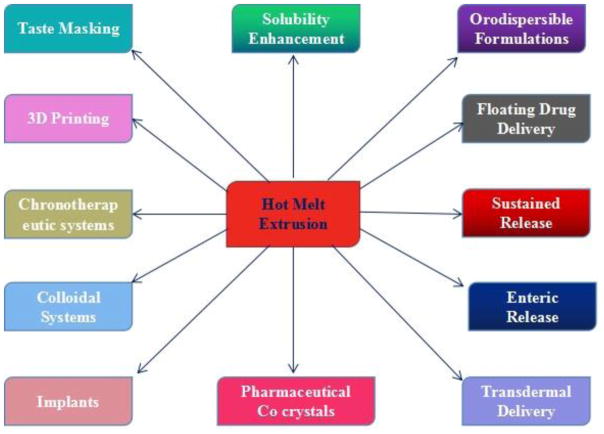
HME was first introduced in 1971 as a formulation technology platform for the pharmaceutical industry (El-Egakey et al., 1971). Since that time, not only HME was adopted by the industry, but it was also modified and customized for multiple applications (Langley et al., 2013). The versatility of HME for various drug delivery strategies such as transdermal applications (Qi and Craig, 2016), bio-adhesive functions (Palem et al., 2016), implants (Cosse et al., 2017), orodispersible formulations (Pimparade et al., 2017), pellets, gastroretentive systems (Vo et al., 2016) lipid nanoparticles (Bhagurkar et al., 2017; Patil et al., 2015), and other applications (Fig. 1) has made it a technology that is poised to shift the entire paradigm of pharmaceutical industry research and manufacturing. One of the most significant uses of HME is the improvement of the solubility of poorly soluble drugs obtained via advanced combinatory and high-throughput screening. The strengths, weaknesses, opportunities, and threats (SWOTs) of HME are listed in Table 1. Regardless of gaining popularity, HME faces challenges (perceived or real) from various issues such as thermal degradation of API and/or carrier at processing temperatures, recrystallization of API over time, and reproducibility of HME products. However, the above issues can be addressed by reducing the processing temperatures by use of plasticizers, reduction of residence time of materials during the extrusion process and drug melting point depression by co-crystallization, etc.
Fig. 1.
The various broad-spectrum applications of hot melt extrusion
Table 1.
S.W.O.T. analysis of pharmaceutical hot melt extrusion technology
| Strengths | Weaknesses |
|---|---|
|
|
| Opportunities | Threats |
|
|
HME can be considered an optimal technique for the processing of highly viscous materials without using any solvents and thus has been widely recognized as a green technology (Repka et al., 2008; Sarode et al., 2013). Its unique blending geometry promotes high-shear localized mixing while retaining the high throughput of the process. The HME screw configuration can be highly customized to tailor its shear level based on the formulation requirements (Haser et al., 2017; Morott et al., 2015). The continuous thinning, deformation, and elongation processes occurring in a very narrow space between the intermeshing elements facilitate the dissolution of drug molecules and/or their dispersion in a molten carrier (Haser et al., 2016). The high efficiency of distributive and dispersive mixing also allows contact between various chemical molecules at high frequencies without using any solvents that promote the formation of salt co-crystal species (Li et al., 2016; Liu et al., 2017). In addition, HME represents a high-throughput continuous process, which can be scaled up for industrial manufacture (Langley et al., 2013). The application of quality by design (QbD) and real-time monitoring techniques utilizing process analytical technology (PAT) strategies allows the development of HME as a continuous manufacturing (CM) platform (Islam et al., 2014; Tiwari et al., 2016), which can be used for more efficient and better controlled drug delivery (Chatterjee, 2012). This platform also offers many industrial benefits in the form of reduced labor, shorter operation times, lower investments, smaller facilities, and instrumentation (Bhagurkar et al., 2016). The concept of real-time release testing (RTRT) of pharmaceutical products proposed by the U.S. Food and Drug Administration (FDA) in 2004 promoted the use of process analytical tools for product development in the pharmaceutical industry. The resulting shift in the paradigm from batch testing to in-process testing (which improved the quality of product manufacturing) has been widely recognized and implemented. This article represents an integrated update of our previous review (Shah et al., 2013), which is written with an emphasis on the advances of HME for improved solubility and dissolution rates of poorly soluble drugs, thermodynamic aspects and mechanisms of drug/polymer solubilization, characterization techniques and its applicability as a CM technology for solid dispersions. This review also provides an overview of the current PAT tools employed in various pharmaceutical HME processes.
1. HME and solubilization enhancement
HME has been widely used to prepare amorphous solid dispersions for the improvement of solubility and dissolution rates of poorly soluble materials. During the melt extrusion process, the dissolution of APIs into the polymer matrix is accelerated under the influence of shear and heat. The amorphous solid dispersions produced via HME are expected to possess lower molecular mobilities and API molecules “freeze” inside a polymer matrix to inhibit the nucleation and crystallization processes (Baghel et al., 2016; Theil et al., 2017).
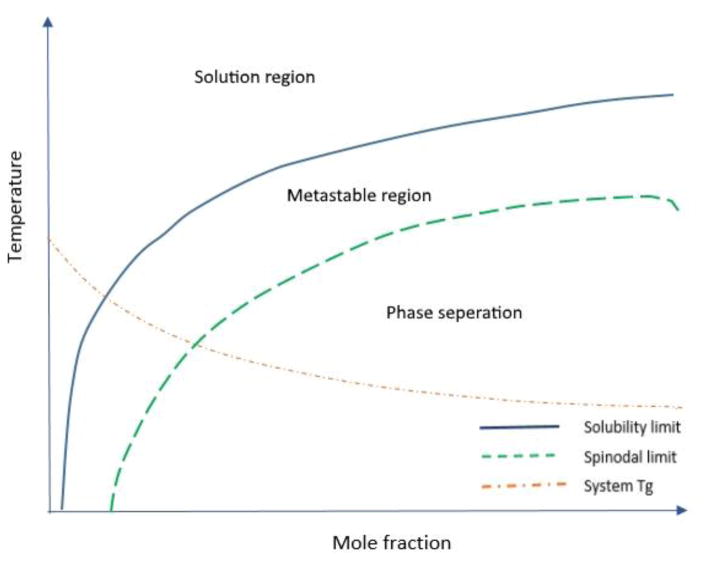
2.1. Thermodynamic phase diagram of a API/polymer binary mixture
A typical phase diagram depicted in Fig. 2 exhibits various drug-polymer solubility and miscibility phases of the drug-polymer binary mixture corresponding to different temperatures and drug/polymer ratios. The solubility limit and spinodal curves divide the diagram area into three distinct regions (solution region, metastable region, and phase separation region) of the solid dispersion (Tian et al., 2014). In the solution region, the drug and polymer form a thermodynamically stable single-phase system. In the metastable region, amorphous APIs are miscible with polymers. Theoretically, systems in this region are not stable and exhibit a tendency to transform into a more stable state; however, the related evolution process may be very slow. Thus, they can be considered relatively stable systems under specific conditions or within a relatively short period. In the phase separation region, two different phases exist: a drug-rich phase and a polymer-rich phase. The glass transition temperature (Tg) line in the phase diagram further divides each of the described regions into two sub-regions, and the systems existing above this line can reach a steady state much faster than their counterparts. Three different types of relaxations (α, β, and γ), which are directly related to molecular mobility, strongly depend on temperature. In the regions below the Tg, only beta and gamma relaxations (which are weaker than alpha relaxations) can be observed.
Fig. 2.
Typical phase diagram of API/polymer binary mixture
2.2. API/polymer solubility and miscibility measurements
The solubility of a drug in a polymer matrix is a very important parameter that must be considered during polymer selection. Estimating the drug/polymer miscibility is a difficult task since reaching equilibrium usually takes a very long time. Therefore, the determination of the API/polymer solubility and miscibility can only be performed under certain assumptions (Qian et al., 2010). The most popular approach to the determination of the drug/polymer miscibility is the application of the Flory-Huggins theory for the dispersions of small molecules in a polymer matrix (Marsac et al., 2009; Marsac et al., 2006; Tian et al., 2014).
Using the lattice theory, the mixing free energy of an API/polymer blend can be calculated via the Flory–Huggins equation (Eq. 1):
| (1) |
where ϕ is the volume fraction, N is the molecular volume, χ is the Flory-Huggins interaction parameter, R is the molar ideal gas constant, and T is the temperature.
When studying solid dispersions, the Flory-Huggins equation is primarily utilized for constructing free energy phase diagrams of API/polymer blends. The free energy of mixing (ΔGmix) can be calculated at a specified temperature with respect to the interaction parameter. Experimentally, the latter can be obtained via a melting point depression method, which utilizes the following relationship between the melting point and the interaction parameters (Marsac et al., 2006; Tian et al., 2013):
| (2) |
where ϕ is the volume fraction; m is the ratio of the volume of a polymer chain to the drug molecular volume; Tm and Tm0 are the melting points of the drug crystals in the drug/polymer mixture and pure drug, respectively; R is the gas constant, and ΔH is the heat of fusion of the drug. The value of the left side of Eq. (2) can be determined via differential scanning calorimetry (DSC), and the magnitudes of ϕ and m can be calculated from the molecular weights and ratio between the drug and the polymer components. As a result, the Flory-Huggins interaction parameters are obtained and an API/polymer phase diagram can be plotted. One drawback of this method is that the melting point depression is not always detectable, especially when the Tg of polymer carriers is close to or higher than the melting point of APIs. The application of this method is also limited when APIs possess a high melting point because the thermal degradation of components might occur during experiments.
Another method for determining the API/polymer solubility is using the Hansen–Hildebrand solubility parameters (δ), which contains three components taken into account, namely dispersive interactions (δd), polar interactions (δp), and hydrogen bonding (δh) (Stefanis and Panayiotou, 2008; Stefanis and Panayiotou, 2012). The three-dimensional solubility parameter is the sum of squares of the partial solubility parameters:
| (3) |
| (4) |
where Fdi is the molar attraction constant corresponding to the dispersion component, Fpi is the molar attraction constant related to the polar component, Ehi is the hydrogen bonding energy, and V is the molar volume (Bellantone et al., 2012; Tian et al., 2014; Vay et al., 2011).
In general, if the difference between the solubility parameters of the drug and polymer is greater than 10.0 MPa1/2, their miscibility is extremely low; however, when the differential solubility parameter is below 7 MPa1/2, the drug and polymer are considered highly miscible (Ghebremeskel et al., 2007; Sarode et al., 2013). Solubility parameters can only be utilized to qualitatively estimate the cohesive properties of drug/polymer mixtures. They do not reflect the drug/polymer extent of miscibility, but can be used to calculate the interaction parameter (χ) in the Flory-Huggins equation. It should be noted that the calculation of solubility parameters is highly likely to pose errors since the concept is based on simple organic structures. The effects of long-range orders and interactions within the polymer chains are not considered.
The recently proposed perturbed-chain statistical associating fluid theory (PC–SAFT) was utilized for constructing the phase diagrams of various API/polymer blends (Prudic et al., 2014; Prudic et al., 2015). To apply this method, the residual Helmholtz energy must be calculated by considering the repulsion forces, van der Waals attraction forces, and hydrogen bonding of molecules. Each API or polymer molecule is described as a chain consisting of multiple spherical segments. The total interaction is simulated using the following ve pure-component parameters: segment number, segment diameter, dispersion-energy parameter, association-volume parameter, and association energy parameter. The solubility of APIs in polymers can be predicted assuming the thermodynamic solid–liquid equilibrium of pure API species in the API/polymer phase. Hence, the API solubility in a polymer can be calculated using the following equation:
| (5) |
where T and R are the temperature and universal ideal gas constant, respectively. , and are the API melting temperature, API fusion heat, and the difference in the heat capacity between the solid and liquid APIs, respectively (all these parameters can be determined by DSC). Therefore, a phase diagram can be constructed by estimating the API solubility in the polymer at different conditions (Rask et al., 2016). The application of PC–SAFT to construct drug/polymer phase diagrams is relatively new.
Although the described methods can be used for the prediction of API-polymer interactions; however, a gap exists between the prediction and reality. Therefore, the utilization of experimental methods such as DSC, various spectroscopic and microscopic techniques, and X-ray diffraction (XRD) is required to confirm the miscibility of the API and polymer (Sarode et al., 2013).
2.3. Solubilization and homogenization of drug molecules in a polymer matrix via HME
HME promotes the solubilization/dispersion of APIs in a polymer matrix and it depends on various parameters such as the formulation components (the compositions of the API and polymer and their ratio), process conditions (temperature, pressure, torque, and shear force), and equipment (type, screw configuration, feed rate, and other parameters). If the APIs are considered a solute, and the soft polymer matrix, a solvent, the dispersion of API species in the molten polymer is similar to the process of mixing small molecules with a liquid solvent. Thus, the described mixture can form a thermodynamically stable solution, metastable (supersaturated) molecular dispersion, or a suspension. The dispersion and solubilization of APIs in a polymer matrix are relatively slow processes because of the high viscosity of molten polymers. HME lowers the viscosity of a system, while the vigorous shear of screws improves its homogeneity. Furthermore, the effect of thinning and mixing at the micro-scale increases the effectiveness of melt extrusion. Based on the relative comparison between the operating temperature and the melting temperature of API, the process of melt extrusion can occur in two distinct regimes: solubilization regime and miscible regime (DiNunzio et al., 2012). For both regimes, the processing temperature preferably should significantly exceed the Tg of the carrier excipients. In the solubilization regime, the processing temperature is lower than the melting point of API; as a result, the drug molecules have to overcome molecular bonds within the bulk API crystal lattice before diffusing into the molten polymer matrix. If the drug/polymer ratio is greater than the solubility, only a fraction of API molecules can be dissolved, while the rest remains dispersed across the polymer matrix in the crystalline form. The kinetics of the solubilization process can be described by the Noyes Whitney equation:
| (6) |
where dM is the amount of API dissolved within the specified time interval (dt), dM/dt is the solubilization rate, D is the diffusion coefficient, A is the interphase area, h is the diffusion distance, C0 is the solubility of API in the polymer, and Ct is the bulk concentration of API in the polymer matrix.
All parameters of the Noyes–Whitney equation are affected by the melt extrusion process, which ultimately increases the API solubilization in the polymer matrix (Shah et al., 2013). The increase in the processing temperature increases solubility C0 and diffusivity D and decreases the melt viscosity of the molten polymer, thus facilitating the diffusion and API/polymer mixing processes. Additionally, the shear and blending processes decrease the boundary thickness h, bulk concentration Ct, and, in certain cases, increase the interphase area A. The applied shear force can be adjusted by modifying the screw design, screw speed, and feed rate. Increasing HME residence time and using micronized materials can further enhance the API solubilization in the polymer matrix (Li et al., 2015).
In the miscible regime, the processing temperature is higher than the melting point of API species; as a result, the latter are melted quickly inside the barrel and form a liquid phase during the melt extrusion process. The liquid API phase can be easily divided, kneaded, blended, and dispersed inside the molten polymer, which drastically increases the interphase area and thus promotes the solubilization process. When the drug/polymer ratio is higher than the drug solubility, the coexistence of two phases (the drug-rich and polymer-rich ones) is observed. Because of the stability concerns, the miscible regime is only applicable to the drugs with low melting points and very limited for the thermolabile APIs. It was found that along with the processing parameters, excipients also play a crucial role in the success of the HME process. Hence, the API/carrier solubility, melt viscosity, and thermal stability parameters must be considered during excipient screening.
A polymer, in which APIs are inherently soluble, always represent a good choice for a drug carrier since it enhances both the dissolution rate and degree of solubilization, owing to the high diffusivity (D) and solubility (C0) values. In addition, when the API is highly miscible with the polymer, the melting point of the drug is decreased (Djuris et al., 2013), which ultimately facilitates the HME process. However, carrier species should be soluble in water to ensure proper release of APIs from the matrix (Janssens and Van den Mooter, 2009). This criterion limits the range of materials with good carrier properties because the hydrophobic nature of APIs decreases their solubility in hydrophilic polymers. Additionally, a good carrier should possess reasonable melt viscosity and thermal stability values. Increasing the temperature lowers the torque, and thus facilitates the melt extrusion process. However, it is limited by the thermal stability concerns. Adding plasticizers can lower Tg and thus improve the processability of the formulations (Crowley et al., 2007), but it may accelerate the crystallization and phase separation processes during storage.
2. Manufacturing aspects
In our earlier review, basic aspects of the utilized HME equipment (including processing parameters and screw configurations) were discussed. This current review focuses on the recent updates on the manufacturing issues and specific issues related to the HME process (Maniruzzaman and Nokhodchi, 2017; Patil et al., 2016).
3.1 HME scale-up strategies
Generally, commercial production implies large-scale processing and a high throughput rate of materials. The process feasibility and preserving the key process parameters are critical for maintaining high quality of the product. Different scale-up strategies for HME include power scale-up, volumetric scale-up, and heat transfer scale-up (Dreiblatt, 2012).
3.1.1 Power scale-up
Specific energy (SE) input and specific torque are critical parameters that play a pivotal role in the successful scale-up implementation. The HME mechanical energy input can be expressed as (Munjal et al., 2006):
| (7) |
Here E is the mechanical energy, N is the real screw speed, τ is the real torque; Emax is the maximum mechanical energy of the system, Nmax is the maximal screw speed, and τmax is the maximal torque. The values of Emax, Nmax, and τmax are predetermined by the instrument design (Breitenbach, 2006). SE = E/Q, where Q is the true throughput of the process (which equals the feed rate of the starved feeding system).
3.1.2 Volume scale-up
Volumetric scale-up requires maintaining the same filling level and residence time distribution during the process. For the geometrically similar extruders, the target process throughput can be calculated using the following equation (Rothen-Weinhold et al., 2000):
| (8) |
where QT is the final process feed rate, QM is the initial process feed rate, DT is the screw diameter after scale-up, DM is the screw diameter before scale-up, NT is the screw speed after scale-up, and NM is the screw speed before scale-up.
3.1.3 Heat transfer scale-up
Melt extrusion depends on the heat transfer inside the barrel; thus, the surface area of heat transfer is equivalent to the barrel surface area. The scale-up for heat transfer can be described by the following equation (Munjal et al., 2006):
| (9) |
Here QT is the target throughput, QM is the initial throughput, DT is the target screw diameter, DM is the initial screw diameter, NT is the screw speed after scale-up, and NM is the screw speed before scale-up.
Recently, Agrawal et al. (2016) have performed the successful scale-up studies of an insoluble drug (compound X) by formulating an amorphous solid dispersion containing Kollidon® K12 (PVP K12) and Kollidon® VA64 (vinyl pyrrolidine-vinyl acetate co-polymer) polymers, utilized volumetric and power scale-up. In this study, a scale-up from a 9-mm extruder (with a batch size of 5–20 g) to a 16-mm or 18-mm clinical scale extruder with a minimum batch size of 500 g was performed. For successful scale-up, the geometric similarity between extruders is very important. Therefore, due to the difference in the geometries of the 16-mm and 18-mm extruders, feed rate estimation was conducted via the volumetric approach to ensure that both extruders exhibited the same values of specific energy. The melt pressure was greater inside the 16-mm extruder due to the differences in the die and screw designs (the latter had a large diameter due to the larger screw flight of 3 mm). The smaller shear force observed for the 18-mm extruder resulted in a low level of impurities as compared to those obtained for the 9-mm and 16-mm extruders. Hence, for successful scale-up, both the equipment geometry and screw design must be considered along with the scale-independent parameters.
3.2. Process parameters
The process parameters play a significant role in ensuring the quality and stability of HME products. This review discusses various process parameters reported in the literature.
3.2.1. Residence time
The residence time (RT) of the HME process characterizes the heat energy produced over a specific period during the material shear. If a given API/carrier system is thermolabile, downstream feeding can be used to narrow the residence time distribution (RTD) which avoids material degradation (Repka et al., 2012). The RT in a starved area (where the extruder operates at a feed rate capacity below the conveying capacity, and the screw is not completely filled with the material) can be obtained via the following equation (Hughey et al., 2010):
| (10) |
where Q is the net flow rate, Ls is the zone length, a is the open cross-section of the screw, f is the degree of filling, and z is the head length.
For the extruder with the completely filled screw, the mean RT is equal to
| (11) |
where Q is the net flow rate, Lf is the length of the completely filled zone, and a is the open cross-section of the screw (Stroyer et al., 2006).
The total RT is equal to the sum of the mean RTs of all zones (Wang et al., 2004):
| (12) |
RTD also plays an important role in maintaining the product stability. The increased RT exposes the material to heat and shear for a longer period, which can cause degradation affecting the product quality and performance (Gao et al., 2012).
Finally, RT is related to the filling properties of a material via the following equation (Gryczke et al., 2011):
| (13) |
where RT is the residence time in seconds, L/D is the extruder ratio, SV is the specific volume of the material, SG is the specific gravity of the material, % fill is the degree of filling expressed as a fraction, and Q is the throughput expressed in kg/h.
Li et al. (2015) investigated the effect of the API particle size (API milling) and HME process parameters (such as screw design and residence time) on the dissolution rate of acetaminophen (APAP) combined with Soluplus® (polyvinyl caprolactam–polyvinyl acetate–polyethylene glycol graft copolymer) at a Soluplus®/APAP ratio of 70/30. Extrusion was performed at a temperature of 100 °C, which was below the melting point of APAP (169–170 °C). These researchers reported an increased dissolution rate for the milled API, which was attributed to the greater area of interaction with Soluplus® and shortened diffusion path lengths. The dissolution of API was increased for the screw configuration with a high number of reverse elements because of the increased holdup volume resulting in a longer residence time (corresponding to an increment from 130 s to 180 s).
3.2.2. Viscosity
The basic factors affecting the HME process are represented by the mechanical properties of the blend as well as the thermoplasticity, deformation characteristics, and softness of the utilized materials. Viscosity is a specific parameter that maintains the value of the process torque within certain limits.
It was observed that the molecular weight (M.W.) and chain length of a polymer could significantly affect the product quality since these parameters were related to the rheological behavior of the polymer and its interactions in the molten state (Sperling, 2005). The viscosity of a polymer is proportional to its M.W.; thus, the polymers with high M.W. exhibit high melt viscosity.
Melt viscosity also depends on the Tg of a polymeric carrier. In particular, the melt viscosity of an amorphous carrier above Tg can be characterized by the William–Landel–Ferry (WLF) equation (Lu et al., 2003):
| (14) |
Here C1 and C2 are the constants, η is the melt viscosity at a processing temperature, and ηTg is the melt viscosity at Tg.
3.2.3. Temperature, screw design, and speed
The other important parameters of HME are the screw design, speed, and temperature of operation. The careful optimization of the processing conditions should be performed since these parameters directly influence the quality of the final product (Dong and Choi, 2008).
Huang et al. (2017) studied the effect of the processing conditions on the melt extrusion of amorphous dispersions of Gliclazide (GLZ) (M.P. = 180–182 °C) in Soluplus®, AffiniSol™, and PVP VA64 polymers. Extrusion was conducted in the temperature range of 100–160 °C at screw speeds of 100, 200, and 300 RPM. The results of thermogravimetric analysis (TGA) studies revealed that GLZ was stable at temperatures below 165 °C.
Overall, the selection of suitable scale up methods for HME depends on the quality and stability of the final product. In fact, the difference in geometry of the equipment could be a potential factor taken into consideration since it can influence the total energy introduced into the extruder (and thus the product). The scale-up strategy should be systematically studied to establish a relation between quality product attributes and extrusion parameters by understanding their effect on scale-independent system parameters. The relation between the scale independent parameters residence time distribution, maximum shear rate, specific energy, degree of fill and temperature inside the barrel affect quality product attributes irrespective of equipment model and scale. It is crucial to note that each system parameter is affected by multiple extrusion parameters, such as the change in the feed rate and screw configuration directly affect the shear generated within the process (Liu et al., 2012a; Markarian, 2012). It is critical to establish a feasible scale up approach to develop a quality product. However, the type of method selected depends on the physico-chemical properties of the API and the excipients utilized.
3. Role of polymers in solubilization
This section of the review is focused on the broad applicability of the polymers reported in the literature with a special emphasis on the recent case studies on solubility enhancement. HME facilitates the enhancement of the solubility and dissolution rate of poorly soluble API species by distributing them in a suitable polymeric carrier system. The mechanisms of the improvement of API solubility involve the alteration of the physicochemical properties of APIs to enhance their solubility, inhibit recrystallization, and improve their stability in the extrudates (Fousteris et al., 2013).
The mixing of the drug and polymer components during HME converts the crystalline API into its amorphous form and prevents further drug recrystallization via either the non-covalent bonding between the drug and the polymer chain or steric hindrance (Crowley et al., 2007). Since the drug had been previously dissolved in the water-soluble carrier, its solubility in the GI fluid was improved as compared to that in its native crystalline state, owing to Gibb’s free energy and absence of a lattice energy barrier (Terife et al., 2012).
Krupa et al. (2017) prepared the extrudates of poorly soluble Tadalafil species using Soluplus®, lactitol, and mannitol carriers with drug loadings of 10, 20 and 30%. The extrusion temperature ranged between 110 and 160 °C depending on the Tg of the carrier. The Soluplus® extrudates were smooth and semi-transparent at low drug loads and exhibited a “creamy” white appearance at higher loads, suggesting partial dissolution of the drug species in the polymeric matrix. The mannitol extrudates were rough and porous, indicating that the native crystal structure of the mannitol affected the total crystallinity of the extrudates. Both the disintegration and drug release processes proceeded faster in the case of porous mannitol extrudates, while the milling procedure promoted the release of Soluplus® extrudates due to the destruction of the polymeric network.
Thiry et al. (2017a) investigated the effect of polymeric systems on in-vitro drug release and in-vivo absorption of a poorly soluble drug itraconazole (ITZ). The studied amorphous solid dispersions of ITZ contained a combination of Soluplus®, Ac-Di-Sol®, and β-cyclodextrins (βCD). It was reported that Soluplus® in combination with other components exhibited enhanced solubility as compared to that of plain Soluplus®. The in-vivo studies revealed significant improvement in the absorption of the Soluplus®-Ac-Di-Sol® and Soluplus®-cyclodextrin mixtures as compared to that of the marketed product Sporanox®, which could be explained by the formation of stable Soluplus® micelles preventing the re-precipitation of drug molecules.
Martinez-Marcos et al. (2016) reported HME-enhanced solubility of the high melting point drug albendazole (M.P. = 208 °C) containing hydrophilic polymer PVP K12. During miscibility studies, they found that the drug completely melted at 216 °C, and the polymer at 145 °C. The drug loads of 1, 5 and 10% extruded in the temperature range of 70–145 °C at 100 RPM resulted in improved solubility as compared to that of pure drug (its supersaturation (spring effect) was achieved at a concentration of extrudates equal to 10% drug load). The observed solubility enhancement was due to the amorphous conversion of drug molecules within the extrudates as indicated by scanning electron microscopy (SEM), DSC, and powder X-ray diffraction (PXRD) studies. Although the drug dissolution improved significantly, its complete release was not observed, which indicated the role of the polymer in controlling the drug release process.
Kate et al. (2016) prepared a solid dispersion of atovaquone (M.P. = 220 °C), a highly lipophilic drug with very low solubility (<0.2 μg/mL) via HME to improve its bioavailability. Its formulations were obtained by using a combination of hydrophilic carriers (PVP K30, HPMC E5, and PVP VA64) and surfactants/solubilizers (Tween 80®, Gelucire® 44/14, Poloxamer 188, Soluplus®, PEG 400, and PEG 6000) in the temperature range of 110–160 °C. In the dissolution studies, media containing a 40% isopropyl alcohol solution buffered to pH = 8 exhibited the highest rate of drug release for PVP VA 64 at a ratio between the drug and the polymer equal to 1:2. The authors reported that reduction in the glass transition temperature of PVP VA64 was observed after the addition of surfactants (such as Tween 80® and PEG 6000). These researchers reported the improved absorption profile characterized by an 8-fold dose reduction in an anti-malarial efficacy study, which provided various insights into the selection of hydrophilic polymers and surfactants for the improvement of drug solubility. These data demonstrated that one’s choice of polymer combinations as well as surfactant selection can solve a solubility problem with a very low soluble drug (<0.2 μg/mL).
Wang et al. (2015) formulated a solid dispersion to improve the solubility and bioavailability of Ginkgo biloba extract (GE) with a Kollidon® VA64/Kolliphor® RH40 in a ratio of 85:15 using hot melt techniques. The extrusion procedure was performed in the temperature range of 120–125 °C at a speed of 100 RPM and drug load of 25%. Both the DSC and XRD studies confirmed the amorphous conversion of GE, and its dissolution in 250 mL of 0.1 N HCl solution showed that the solubility of the solid dispersion of GE was significantly improved by a factor of 2–3 after 120 min, compared to pure GE and its corresponding physical mixture. This study illustrates an example of solubilizing plant extracts with melt extrusion techniques and proper selection of polymers.
Similarly, Fu et al. (2016) prepared an amorphous solid dispersion of nisoldipine (M.P = 151 °C) in Kollidon® VA64 at an extrusion temperature of 153 °C and ratio of 1:10 to improve the solubility and stability of the amorphous dispersion. Among the various formulations, the maximum dissolution (100% after 1 h) was achieved at a ratio between the drug and polymer equal to 1:10. Both the DSC and PXRD studies confirmed the absence of crystalline nisoldipine species in the final formulation, while stability testing conducted at temperatures of 40 °C and 60 °C and relatively humidity (RH) of 0% for up to 10 days showed no recrystallization. Furthermore, the stress studies conducted at a higher RH of 92.5% for 10 days revealed a shift in the carbonyl wavelength to a lower value, indicating the destruction of H bonds.
HME was also explored as a method for the development of co-amorphous systems containing heat- and shear-sensitive molecules. Lenz et al. (2017) studied the feasibility of the extrusion process for the enhancement of the solubility of indomethacin (IND, M.P. = 160 °C) by forming an arginine (AGN)-based co-amorphous system with and without copovidone (CP). Extrusion was conducted in the temperature range of 120–200 °C and different heating zones at a speed of 50–100 RPM. The resulting amorphous dispersions exhibited a common Tg of around 70 °C and a second Tg value at 116–117 °C corresponding to the phase separation of AGN and IND. Drug release testing performed under non-sink conditions demonstrated a higher solubility profile (by almost a factor of 2) and preservation of the supersaturated state for the copovidone-containing melt extrusion formulations indicating the effect of copovidone on the obtained co-amorphous system.
Pawar et al. (2017) developed a solid crystalline suspension of the poorly soluble Efavirenz drug (M.P. = 139 °C) using xylitol and mannitol as crystalline carriers. Extrusion was performed at the melting point of Efavirenz (138–140 °C) and screw speed of 100 RPM. Both the DSC and XRD studies confirmed the crystallinity of the obtained formulations, and Fourier transform infrared (FTIR) chemical imaging revealed the uniform distribution of API species in the hydrophilic carrier. The dissolution studies performed in 1000 mL of the 0.1 N HCl solution containing 0.2% of sodium lauryl sulfate (SLS) showed significant improvement in the drug release rates of the xylitol formulations as compared to that of pure drug. Regardless of the polyol structure, the drug release rate was improved by the hydrophilic environment provided by the crystalline carrier. The stability studies conducted for up to 12 months showed no significant differences in the drug release patterns of the mannitol-based formulations; however, the xylitol-based formulations were found to be unstable.
Lipid micro-domains (LMD) represent a novel technique for the preparation of solid dispersions, in which lipid molecules are adsorbed on the carrier surface. As a result, the lipid structure becomes disrupted by the disorder of the lipid chains, leading to the formation of a new microstructure. This newly created microstructure can accommodate drug molecules, producing a solid dispersion. Adler et al. (2016) fabricated LMDs using β-carotene (βC) drug, stearic acid (STA) used as acidic lipid and plasticizer interacting with the inorganic carrier Neusilin® US2 (aluminum magnesium silicate) and HPC polymer utilized for controlling the drug release rate. Extrusion was performed at a temperature of 160 °C and speed of 50 RPM for the most favorable formulation of 70/10/20% (HPC/STA/Neusilin® US2). The analysis of the extrudates showed that the interaction of STA with 5–20% Neusilin®US2 was proportional to the degree of the amorphous conversion of STA. The attenuated total reflectance FTIR studies revealed that the presence of βC did not disturb the interactions between STA and Neusilin® US2 species. The authors reported higher amorphousness and stability of the obtained drug due to the restricted movement of its molecules across the lipid chain structure.
Another approach to producing stable amorphous dispersions is to prepare solid solutions. In these solutions, the drug species are homogenously dispersed across the polymer matrix in a metastable state, which prevents the recrystallization of API. Fule et al. (2016) prepared a solid solution of artesunate (M.P. = 145–149 °C), a poorly soluble drug containing Soluplus® along with a combination of surfactants such as PEG 400, Lutrol F 127, TPGS, and Lutrol F 68. Both the DSC and XRD studies showed no signs of crystallization in the obtained extrudates, and the FTIR spectra revealed no changes in the system functionality even in the presence of surfactants. The observed significant enhancement of solubility occurred due to the structural disorder of the amorphous system and short-range intermolecular attractions. The results of in-vivo studies showed improved permeation through the GI membrane due to the presence of surfactants. After accelerated testing at 40 °C/75% RH for 6 months, the formulations were physically stable and maintained good content uniformity. Thus a “metastable” solid solution can be stabilized within an extrudate with selection of appropriate matrices.
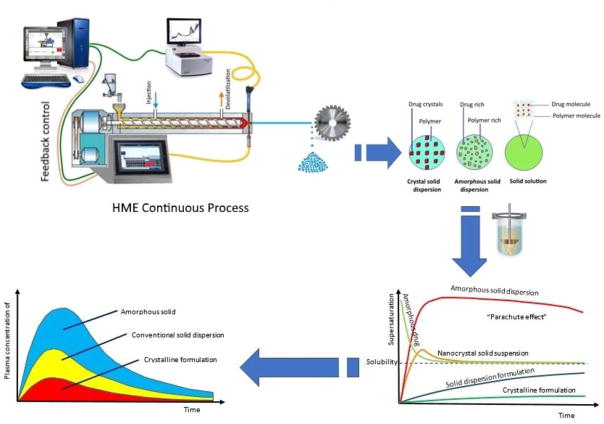
Thiry et al. (2017b) investigated ternary inclusion complexes of cyclodextrin to improve the solubility of poorly soluble ITZ by HME. The studied cyclodextrins (CD) were β-cyclodextrin (βCD), randomly methylated β-cyclodextrin (Rameb®), sulfobutylether-β-cyclodextrin (Captisol®), methyl-β-cyclodextrin (Crysmeb®), and hydroxypropyl-β-cyclodextrin (HPβCD) mixed with Soluplus® (SL) at a molar ratio of 1:1. Their extrusion was performed at a temperature of 180 °C and screw speed of 100 RPM. The observed shifts of the cyclodextrin FTIR peaks centered at 3300 cm−1 and DSC results confirmed the formation of a complex containing ITZ, CD, and SL species. The in-vitro release studies demonstrated a significantly higher solubility of all the obtained ternary cyclodextrin complexes as compared to that of pure drug. This phenomenon can be attributed to the high hydrophilicity of cyclodextrin along with the stabilizing effect of SL on the ternary complex. Many of the studies discussed above suggest the improvement of drug solubility and bioavailability, which is schematically illustrated in Fig. 3.
Fig. 3.
Schematic representation of various possible dispersions in the HME process
4. Characterization of HME-produced systems
The characterization, quality and stability of the extrudates of poorly soluble drugs prepared by HME can be predicted via physicochemical, thermal characterization and microscopic studies. However, the recent introduction of PAT tools facilitated the inline quantification, characterization, and monitoring of both the HME process and resulting products. The various characterization methods reported in the literature include DSC, PXRD, FTIR, Raman spectroscopy, nuclear magnetic resonance (NMR) spectroscopy, isothermal micro-calorimetry (IC), TGA, hot stage microscopy (HSM), SEM, and polarized light microscopy (PLM) techniques. Finally, the process efficiency and performance of the obtained product can be evaluated via content uniformity and in vitro dissolution studies. The above-mentioned physicochemical and thermal characterization methods are extensively discussed in earlier reviews (Maniruzzaman et al., 2012; Patil et al., 2015; Shah et al., 2013); therefore, this review provides updates for those recent reports with a special emphasis on the in vitro dissolution studies and PAT tools.
5.1. Physico-chemical characterization of extrudates
5.1.1. PXRD
The PXRD technique is used to determine whether the drug in polymer system is in the crystalline or amorphous state. The kinetics of crystallization investigated at various temperature and humidity conditions provides important information regarding product stability. Lu et al. (2016) developed an amorphous solid dispersion of felodipine in Soluplus® via HME at drug loads of 10, 20, and 30% w/w. At a drug load of 10%, its complete conversion into an amorphous system was reported. Similar results for the amorphous conversion in solid solutions were reported for a poorly soluble azithromycin dihydrate using Soluplus® and Kollidon® VA64 carriers (Jaiswar et al., 2016). Maddineni et al. (2015) produced a solid dispersion of poorly soluble nifedipine drug (M.P. = 172–174 °C) via HME using Kollidon® VA64 at temperatures below the M.P. of the drug (115–130 °C).
5.1.2. FTIR and near-infrared spectroscopy
FTIR is one of the most efficient vibrational spectroscopy techniques used for examining the drug-polymer interactions in physical mixtures and processed extrudates. It provides data describing the presence or absence of interactions in the system, which facilitates the selection of excipients for the final formulations. The type of interactions between the molecules represents the type of chemical bonding in the final product, which may affect the solubility properties of the formulation. Djuris et al. (2013) reported a study on the miscibility of carbamazepine (CBZ) and Soluplus® via FTIR. The absence of the API characteristic peaks in the FTIR spectra recorded for extrudates and appearance of a new peak at 1034 cm−1 indicate a high probability of hydrogen bond formation between the drug and the polymer. The obtained results were further confirmed by DSC and XRD studies. Nevertheless, the FTIR studies of the HME-based solid solution of artesunate in the water-soluble Soluplus® and Kollidon® VA64 polymers did not exhibit any changes in molecular stretching (although slight shifts of specific peaks were observed) and the resulting molecular dispersions significantly improved the dissolution properties of artesunate (Fule et al., 2016). Li et al. (2016) engineered a co-crystal suspension of ibuprofen and isonicotinamide via HME using xylitol as a molecular matrix carrier. The presence of an additional FTIR peak centered at 3317.93 cm−1 can be attributed due to the formation of supramolecular heteromeric synthon between the N pyridine group of isonicotinamide and the OH group of ibuprofen.
5.1.3. NMR spectroscopy
NMR spectroscopy is used to characterize the physical state of a component (crystalline or amorphous) based on the spin energy transitions in pure API, a physical mixture, or a formulation. It is frequently utilized for evaluating the stability of drug–polymer complexes by analyzing additional peaks and proton de-shielding (Hanafy et al., 2009; Stuart, 2005). NMR can also provide valuable information on the structural similarity of impurities and structural comparison of the unknown impurities with the known ones. Yang et al. (2016) analyzed the crystallinity and amorphous character of clotrimazole–PVP solid dispersions via NMR spectroscopy using cross polarizing and magic angle spinning techniques. They reported shorter spin relaxation time (SRT) of the cryo-milled samples, which indicated the presence of a crystalline component along with the amorphous one, while the broad C13 peaks obtained for the HME extrudates confirmed the complete amorphous conversion of API.
5.2. Thermal characterization
Thermal analysis provides information on the state of the drug in a dispersion system and is closely related to the dissolution behavior and stability of the final product (Stankovic et al., 2015).
5.2.1. DSC
DSC is a destructive technique that is used for the qualitative and quantitative analysis of thermal events in a physical mixture or final formulation. The observed melting transitions allow characterization of the crystallinity of the tested product. The selected temperature range strongly depends on the individual thermal properties (M.P./Tg) of the drug and polymers (Guo et al., 2014). Medarevic et al. (2016) reported absence of drug peaks in the physical mixture of carbamazepine and poloxamers which was attributed to the gradual dissolution of carbamazepine in the molten polymer during DSC scanning. It was hypothesized that after the temperature reached the melting point of the drug, its remaining quantity was very low to produce a detectable melting endotherm on the DSC curve.
Lamm et al. (2016) examined the phase transition properties of D-α-tocopherol polyethylene glycol 1000 succinate (TPGS 1000) mixed with copovidone via modulated DSC (mDSC) and reported melting point depression due to the difference in the melting points of the mixture components. Keating et al. (2017) formulated the dispersions of isoniazid (M.P=171°C) with Soluplus® and Eudragit® EPO for taste masking applications. From the DSC characterization of the produced extrudates, they observed that Soluplus® formed amorphous dispersions at drug loadings up to 20%, while Eudragit® EPO produced crystalline dispersions. Puri et al. (2017) evaluated the crystalline properties of the griseofulvin-maltodextrin extrusion-molded tablets at 40 °C/75% RH and observed that they are instable. Similarly, Genina et al., 2017 studied the distribution of ibuprofen and carvedilol in mesoporous silica and Soluplus® via DSC.
5.2.2. Thermogravimetric Analysis
Prior to HME processing, the elucidation of the thermal behavior of APIs and other excipients is required (Morott et al., 2015). This facilitates to determine the safe processing temperature range, in which a formulation can be extruded without any undesirable thermal events (Juluri et al., 2016; Tiwari et al., 2016; Vo et al., 2017). The Tg of the obtained extrudates represented the degree of solubility of the API in a specific carrier. Cifuentes et al. (2017) studied the effect of Mg content on the stability of poly-L-lactic acid/magnesium (PLA/Mg) composites. The TGA of the formulation revealed that the degree of degradation of the PLA–Mg composites was dependent on the Mg content.
5.3. Microscopic characterization
Microscopy is the most widely used method for studying the morphological characteristics of extrudates (such as surface morphology, crystalline/amorphous nature, and particle size).
5.3.1. SEM
SEM is utilized to observe the surface morphology, porosity of extrudates and surface changes during release studies (Almeida et al., 2011; Stankovic et al., 2013). Jaiswar et al. (2016) formulated a solid solution of azithromycin, which exhibited shiny appearance and the absence of crystallinity indicating good miscibility between the drug and carrier species. Lu et al. (2016) reported that after increasing drug load from 10% to 50%, the resulting formulations contained few crystalline particles indicating incomplete amorphous conversion of the drug, which was confirmed by its solubility data. Puri et al. (2017) observed that the morphological changes of the tablet surface observed during the stability studies were related to the moisture uptake by the tablet matrix and its subsequent effect on the formulation.
5.3.2. HSM
HSM represents another HME microscopic characterization tool, which visually provides information about the observed thermal events as a function of time and temperature. It can be utilized for studying the dissolution of drug and polymer species in the molten state and provides good preformulation input for the HME process and subsequent experimental design. HSM can serve as a complimentary technique to DSC since it characterizes the melt behavior of the material tested at a specific temperature (Li et al., 2006). Pawar et al. (2017) studied the crystalline dispersion of efavirenz in a carrier matrix using HSM. Djuris et al. (2013) investigated the effect of temperature on the recrystallization of API in carbamazepine-Soluplus® solid dispersions through HSM.
5.4. In vitro dissolution testing
Drug dissolution testing is an important tool that allows evaluation of the performance of extrudates. During HME, drug molecules are blended, sheared, and melted with hydrophilic polymers; therefore, the assessment of drug solubilization in the presence of polymers represents a significant parameter of the characterization of extrudates.
The dissolution testing of poorly soluble drugs is an important task that must be performed to develop a suitable, discriminating, and validated dissolution model of the correlation between the processes occurring in vitro and in vivo. According to the literature, the utilized dissolution media are characterized by different volumes and pH values. In most of the studies, surfactants (such as Tween 80® and SLS ones) were added to the dissolution media. In this review, various dissolution media used for the characterization of HME products are highlighted to provide an insight into the selection of suitable dissolution conditions. Table 2 lists different types of dissolution media and equipment as well as the testing times utilized for the release studies of several poorly soluble drugs.
Table 2.
In vitro dissolution conditions of selected poorly soluble drugs
| Drug | Carrier | Official dissolution media (USP 40) | Dissolution Conditions | Ref |
|---|---|---|---|---|
| Tadalafil | Soluplus®, Mannitol and Lactitol | 0.5% SLS, 1000 mL, type II, 50RPM | USP type II, 0.1 % SLS simulated gastric fluid, 900 mL, 50 RPM, DT: 2 h | (Krupa et al., 2017) |
| Indomethacin-Arginine | Copovidone | 1 volume of pH 7.2 phosphate buffer mixed with 4 volumes of water, 750 mL, type I, 100RPM (Indomethacin) and 0.1 N hydrochloric acid, 900 mL, type II, 100 RPM (Arginine USP 40 Dietary supplements) | USP type II, Non-sink dissolution medium (pH 4.5 phosphate buffer) or at pH 1.2 (using HCl)), 900 mL, 50 RPM, DT: 24 h | (Lenz et al., 2017) |
| Efavirenz | Xylitol and mannitol | 2.0% (w/v) SLS in water, 1000 mL, type II, 50RPM | USP type II, 0.2% SLS in 1000 mL of 0.1 N HCl, 50 RPM, DT: 1.5 h | (Pawar et al., 2017) |
| Itraconazole | Soluplus®/βCD and Ac- Di-Sol® | Not Available | USP type II, Biphasic dissolution media 400 mL of HCl and 0.1 M and 400 mL of octanol; 50 RPM, DT: 6 h | (Thiry et al., 2017a) |
| Felodipine | HPMC K15M + HPC | pH 6.5 phosphate buffer with 1% SLS, 500 mL, type II, 50 RPM | USP type II, 1% SLS in 0.1 N HCl, 900 mL, DT: 12 h | (Vo et al., 2017) |
| Azithromycin sulphate | Soluplus® and Kollidon® VA 64 | pH 6.0 sodium phosphate buffer, 900 mL,; type II, 50 RPM | USP type II, phosphate buffer pH 6 and water, 900 mL, 50 RPM, DT:1h | (Jaiswar et al., 2016) |
| Nisoldipine | Kollidon® VA 64 | Not Available | USP type II, distilled water, 900 mL, 50 RPM, DT: 1 h | (Fu et al., 2016) |
| Ibuprofen and isonicotinamide | Soluplus®, Eudragit® EPO, Xylitol | pH 7.2 phosphate buffer, 900mL, type II, 50 RPM, (Ibuprofen) Isonicotinamide - Not Available | USP type II, 600 mL of deionized water, 75 RPM, DT: 8 h | (Li et al., 2016) |
| Artesunate | Soluplus® and Kollidon® VA 64 | Not Available | Type I, 1000 mL of water, 50 RPM, DT: 2 h | (Fule et al., 2016) |
| Atovaquone | PVP K30, HPMC E5, PVP-VA64 and tween 80, Gelucire® 44/14, poloxamer 188, Soluplus®, PEG 400 and 6000 | Not Available | USP type II, 40% isopropyl alcohol buffered to pH 8, 900mL and multi media studies in pH 1.2 (with 1 % tween 80) and 6.8 media; 900 mL, DT: 2 h | (Kate et al., 2016) |
| Nifedipine | Kollidon® VA 64 | Simulated gastric fluid TS (without pepsin), 900 mL, type II, 50RPM | USP type II, pH 6.8 phosphate buffer with 1% w/v SLS, 900 mL, DT: 2 h | (Maddineni et al., 2015) |
| Suvorexant | PVP VA | Not Available | USP type II, 900 mL with 0.4% sodium dodecyl sulfate, DT: 1 h | (Kesisoglou et al., 2015) |
| Ginkgo biloba extract | Kollidon® VA64/Kolliph or ® RH40 | 0.1 N hydrochloric acid, 500 mL, type II, 75 RPM (Dietary Supplements USP 40) | USP type II, 250 mL of 0.1N HCl, 250 mL; 100 RPM, DT: 2 h | (Wang et al., 2015) |
| Naproxen | Eudragit®L 100-55, HPMC AS LF, HPMCP-HP-50, Tri ethyl cellulose | pH 7.4 phosphate buffer;900mL, type II, 50 RPM | USP type II, 750 mL of a 0.1 N solution of HCl followed by addition of 250 mL Na3PO4.12H2O (0.2 M) to make up to pH 6.8 (for naproxen); demineralized water (for Esomeprazole); 100RPM, DT: 10 h and 2 h for naproxen and esomeprazole | (Vynckier et al., 2015) |
| Ibuprofen | HPMC, Neusilin® and PEG 2000 | pH 7.2 phosphate buffer, 900mL; type II;50 RPM | USP type II, 900 mL of both 0.1 M HCl (pH 1.2) and 0.2 M dihydrogen-sodium-orthophosphate (pH adjusted with NaOH to 6.8), DT: 2 h | (Maniruzzaman et al., 2015) |
| Lornoxicam | Soluplus® | Not Available | USP type II, 900 mL phosphate buffer of pH 6.2, DT: 1 h | (Fule et al., 2014) |
| Olanzapine | PVP-VA/PVPK30/Soluplus® | 0.1N HCl; 900mL, type II, type II, 50RPM | USP type II, 900 mL of phosphate buffer pH 6.8, DT: 2 h | (Pina et al., 2014) |
| Osthole | PVP-VA or Eudragit® E PO or HPMC E5 or Soluplus® | Not Available | Chinese Pharmacopeia dissolution type II, 900 mL of pH 4.5 acetate buffer with 0.05% Tween 80, DT: 2 h | (Yun et al., 2014) |
| Felodipine | Soluplus® | pH 6.5 phosphate buffer with 1% SLS, 500mL, type II, 50 RPM | USP type II, pH 6.8 phosphate buffer and pH 6.8 phosphate buffer with pre-dissolved Soluplus®, 500ml, 100 RPM, DT: 12 h | (Lu et al., 2016) |
5.6. Micromeritics
Most commonly, extrudates are milled and formulated in the form of tablets or capsules. Before this procedure, the micromeritics of extrudate blends must be evaluated to achieve a clear understanding of the powder/granule behavior. Various traditional compendium methods are typically used for the evaluation of powder flow characteristics (including the angle of repose, bulk density, tapped density, Carr’s index, compressibility index, and Hausner ratio) (Shah et al., 2008). This evaluation can help to determine the most suitable dosage form for the milled extruded material flowing from a hopper.
5. HME and PAT tools
This section provides an overview of the current PAT tools for pharmaceutical HME, their advantages, disadvantages, and applications as summarized in Tables 3 & 4. Since each tool has specific applications, it is difficult to suggest a universal PAT method. Hence, the information provided in this section may help the reader to select an appropriate tool for their specific needs.
Table 3.
The various process analytical technology (PAT) tools for real time analysis of solid dispersions using HME processes
| PAT Tool | Advantages | Disadvantages | Application | Site of Probe | Analys is type | Ref |
|---|---|---|---|---|---|---|
| UV/VIS Spectroscopy | Cost Effective Technique | Low count rate results in error, Limited to Polymer and Food Industry | Residence Time Distribution, Particle size analysis, Quantification | Die | Inline | (Becker et al., 2017; Wang et al., 2008) |
| NIR Spectroscopy (Transmittance & Reflectance) | Noninvasive, Nondestructive, Fast, Continuous, No sample preparation | Data Pretreatment or Chemometrical Data analysis is needed | Molecular Interaction, Hydrogen Bonding, Quantification of API | Die | Inline/Online | (Apruzzese et al., 2003; Jamrogiewicz, 2012; Luypaert et al., 2007; Roggo et al., 2007; Tumuluri et al., 2004) |
| MIR Spectroscopy Attenuated Total Reflection (ATR) | Noninvasive, Nondestructive, Little or No sample preparation | Limited radiation path length & Penetration depth length, Polymer Industry | High density polymer blend composition, Residence Time Distribution | Modified Barrel or Special Gear pump flow cell | Online (Special adaptor) | (Coates et al., 2003; Haberstroh et al., 2002) |
| Raman Spectroscopy | Nondestructive, Minor sample preparation | Low signal intensity, Fluorescence due to impurities, Risk of sub sampling | Molecular interaction, API Quantification, Composition of Polymer or melt blends | Die | Inline | (Coates et al., 2003; Gala and Chauhan, 2015; Saerens et al., 2014a) |
| Fluorescence Spectroscopy | Quantification less than 1% w/w, Improved peak resolution and recording speed | Limited to developmental stage | Melt temperature measurements, Residence Time Distribution, simultaneous quantification of two APIs | Die | Inline | (Cogdill et al., 2007; Sievers, 1972; Zhong et al., 2011) |
| Parsum Probe | Can measure particles up to 20000/s & Wide particle size range 50–6000um | Not suitable for particle size less than 50um | Particle size analysis in Melt granulation | Die (Adaptor) | online | (Huang et al., 2010) |
Table 4.
Promising PAT tools for prospects in Pharmaceutical HME
| PAT Tool | Principle | Site & Type of Analysis | Applications | Advantages | Limitations | Ref |
|---|---|---|---|---|---|---|
| Terahertz Spectroscopy | Electromagnetic wave, Terahertz radiation 0.1 THz to 10 THz | Die & Inline | Differentiate Crystal Forms, Intermolecular Bonding, Refractive Index Measurement, | Insensitive to Thermal Interferences | High Cost, Complex System | (Strachan et al., 2004; Zeitler et al., 2006) |
| Ultrasonic Spectroscopy | Hi frequency acoustical wave | Die, Barrel & Inline or Online | API Quantification, Residence Time Distribution, Particle Size Distribution, Crystal Size Distribution, | Good Penetrability, Easy to change wavelength of ultrasonic wave | Probe adapter required | (Li et al., 2004; Shukla et al., 2010; Stelzer et al., 2013) |
| Dielectric Spectroscopy | Electric Field and measures energy associated with dipole orientation. | Die & Inline | Melt Rheology and Quantification | Useful to analyse physical/chemical structures of organic materials | Limited depth penetration measures at surface | (Gottwald and Scheler, 2005) |
| NMR Spectroscopy | Magnetic field | Next to Die & Online | Monitor API and Polymer interaction, Different polymorphs | Sensitive with small amounts of API | High temperature decreases magnetic field | (Gottwald and Scheler, 2005) |
| Rheometry | Capillary, Rotational & Oscillatory | Between screw and die & Inline/On line | Measures Viscosity, and linear viscoelastic behaviour, Reaction kinetics, Melt strength | Thermal degradation is also noted with reduction in viscosity | High shear rates influence morphology of product | (Covas et al., 2004; Rajan et al., 2010) |
Process analytical testing can be performed in-line, on-line, at-line, and off-line. The in-line and on-line measurements are useful for CM, which involves the direct real-time analysis of the entire process (here the word “in-line” represents the direct analysis of the studied process, and the word “on-line” – the analysis of the sample). The at-line and off-line analyses are typically used in batch-type manufacturing (“at-line” involves the examination of the removed sample at proximity, whereas “off-line” is the analysis of the removed sample performed in a different laboratory) (Saerens et al., 2014a). A conventional HME setup consists of an extruder connected to ancillary equipment to monitor the torque, screw speed, and melt pressure as well as the die and barrel temperatures. In general, the HME process involves the feeding of a physical mixture (containing an API, a polymer, and excipients) through the extruder hopper into the extruder barrel fixed with a specific screw design and maintained at a desired temperature. Both the thermal heat and shear generated in rotating screws melt the powder blend and convey it towards the die to produce the final extrudate. Over the decades, the obtained extrudates have been analyzed using various offline characterization techniques such as DSC, TGA, FTIR, and PXRD.
Typically, off-line testing is expensive, time consuming, and requires random sampling, which makes it a batch-type manufacturing process and therefore contradicts the interests of the pharmaceutical industry. The CM ability of the HME-coupled PAT tools converted them into an important technology that satisfies the requirement of the FDA to conduct real-time monitoring of product quality attributes. It also allows visualization of the material behavior and thus the development of high-quality HME products. The currently available PAT tools can provide a better understanding of the manufacturing process on-line and control its various processing parameters such as temperature, pressure, screw speed, and feed rate.
The spectroscopic PAT tools utilized in pharmaceutical HME include NIR, mid-infrared (MIR), Raman, and fluorescence spectroscopies. These tools can be integrated by using the probe in the barrel or die of extruder to monitor the process, detect molecular changes in the raw material characteristics and perform API quantification. However, the data obtained by these techniques are very complex and must be interpreted appropriately to draw meaningful conclusions. It was reported that multivariate data analysis (MVA) was utilized for processing such complex data to optimize the related process parameters. It should be noted that the PAT tools equipped with MVA reduce the process errors and provide a quality product (Challa and Potumarthi, 2013).
The vibrational NIR, MIR, and Raman spectroscopy techniques utilized in HME are fast, continuous, non-invasive, and non-destructive tools, which can analyze molecular interactions to better understand the nature of hydrogen bonding in solid dispersions. Saerens et al. (2012) reported the use of NIR for the in-line characterization of metoprolol tartarate extrudates with Kollidon® SR. The obtained information about molecular interactions suggested partial conversion of the API into an amorphous form, which was further confirmed by other analytical techniques. Khorasani et al. (2016) used near-infrared spectroscopy chemical imaging (NIR-CI) during HME and 3D printing to characterize the spatial distribution of the present components. The obtained data were analyzed using the NIR-CI technique complemented with the multivariate curve resolution-alternating least squares method, which could predict the spatial distribution of the drug in polymeric films. Saerens et al. (2011) reported the in-line analysis of the metoprolol tartarate concentration in a polymer matrix and related molecular interactions, which was performed by inserting a Raman probe into the die of the extruder. Chiu et al. (1997) reported the in-line measurements of melt viscosity using a viscometer at different screw speeds and temperatures. The obtained results were compared with the corresponding off-line data; they indicated that the utilized method was more suitable for the use in pharmaceutical HME.
The following PAT tools utilized in the polymer industry can serve as potential tools for pharmaceutical HME: terahertz spectroscopy, ultrasonic spectroscopy, dielectric spectroscopy, NMR spectroscopy, and rheometry (Hitzer et al., 2017; Maniruzzaman and Nokhodchi, 2017; Saerens et al., 2012; Saerens et al., 2014b). The use of PAT tools in HME represent a future strategy; however, the quality of the obtained product depends on the proper understanding of the process, accurate assessment of the melt temperature, and viscosity of the system, which can be determined using different probes. A thorough understanding of the various PAT tools described in this review may suggest a strategy for the optimization of the CM process.
6. Innovative applications of HME for solubility enhancement
The industrial use of HME technology is clearly stimulated by the availability of various types of pharmaceutical products in the global market (Tiwari et al., 2016). Apart from the development of traditional dosage forms, HME techniques have been further explored for various novel opportunities such as foam extrusion, co-crystallization, nano-systems, and reactive extrusion.
7.1. Pharmaceutical foam extrusion
HME is one of the most effective approaches to formulating solid dispersions for the enhancement of the dissolution and solubility properties of poorly soluble drugs. Sometimes, the processability and stability concerns limit the application of the HME technology. Supercritical and pressurized CO2-assisted extrusions are excellent approaches to improving both the extrusion processing window and stability of formulations. The presence of CO2 in a molten polymer lowers the viscosity of the resulting polymer blends (Nalawade et al., 2006) that allow their extrusion at lower temperatures and torque, while increasing the stability of the formulation. In addition, the injected CO2 acts as a temporary plasticizer during the melt extrusion process and is not present in the final product (Verreck et al., 2005). Therefore, the Tg of the system can be maintained sufficiently high that benefits the physical stability of formulations during storage. Furthermore, CO2 acts as a blowing agent and creates an internal porous foam structure of the extrudate, which increases the total surface area and ultimately improves the drug dissolution properties (Nagy et al., 2012; Verreck et al., 2006). Foam extrusion also facilitates the extrudate milling process because of its porous internal structure and low physical strength. This is especially helpful for minimizing the heat generated during milling that significantly softens the formulation and makes it particularly sticky inside the milling equipment (Ashour et al., 2016).
Foam extrusion is also exploited to develop floating drug delivery systems. Owing to its low density, the foam strand can be cut into pellets or mini-tablets to formulate buoyance dosage forms, which are able to prolong their residence time inside the stomach. Low boiling point solvents (Vo et al., 2016) and sodium bicarbonate (Fukuda et al., 2006; Vo et al., 2017) can be utilized as foaming agents, based on phase transition phenomena and thermal degradation, respectively. The floating dosage forms can be utilized to formulate controlled release drug delivery systems of narrow absorption window drugs.
7.2. Melt extrusion of co-crystals
Pharmaceutical co-crystals are crystalline molecular complexes that are largely formed by the hydrogen bonding between therapeutic molecules and co-formers (Williams et al., 2013). Co-crystals may exhibit entirely different physical properties as compared to those of the corresponding pure components. Their formation can potentially improve the solubility, stability, bioavailability, and mechanical properties of the active drug (Smith et al., 2011). Co-crystals are mainly formed via solvent-based crystallization and solid-based techniques. Recently, HME has been used as a promising technology for producing pharmaceutical co-crystals because of its scalability, absence of solvents, and cost effectiveness.
During the HME process, the high shear at a microscale continuously divide and blend the drug and co-former phases that facilitate a close contact between the API and co-former molecules and the formation of co-crystal bonds. In addition, the use of melt extruders enables varying temperature profiles, shear force, and residence time, which represent critical productivity parameters of the co-crystallization process. The ibuprofen and nicotinamide (1:1) co-crystals was successfully prepared via melt extrusion technology (Dhumal et al., 2010). The results obtained at high shear, high temperatures (above the formulation eutectic point), and low screw speed (long residence time) produced almost 100% pure co-crystals. The studied co-crystals were physically stable for a period of 6 months under ambient conditions.
The corresponding processes can be completed in a single step via HME, in which API, co-former, and polymer species are fed into the extruder barrel simultaneously or separately at different feed zones. In general, co-crystals can be formed in situ and dispersed in polymer matrices during the extrusion process (Li et al., 2016; Liu et al., 2012b). The formation of co-crystals reduces the melting point of the entire system, which allows reduction of the HME processing temperature. However, the effect of the co-former on the re-crystallization, stability, and moisture absorption of the obtained dispersion need to be investigated thoroughly. Hot melt twin-screw extrusion is an effective, environmentally friendly, and scalable technique for the continuous production of co-crystals (Boksa et al., 2014).
7.3. Nanocrystal solid dispersions formulated by melt extrusion
Reducing the size of API particles to the micro- or nano-scale and thus increasing their specific surface area is a well-established approach to the enhancement of the dissolution and apparent solubility of poorly soluble drugs (Blagden et al., 2007; Merisko-Liversidge and Liversidge, 2011). It can be achieved by drastically increasing the specific surface area and related energy of the system (Müller et al., 2001). As compared to amorphous solid dispersions, nanomaterials are more thermodynamically stable since drugs can exist in a lower energy state. However, they possess some limitations that are related to particle aggregation, morphological instability, and poor wettability. It is well known that aggregation occurs more often to high surface area materials, which results in an increase in the particle size and decrease in the free surface energy (Brough and Williams, 2013). To overcome these drawbacks, nanomaterials can be incorporated into solid dispersions. Engineering nanoparticles usually require the removal of a large amount of solvents prior to formulating various dosage forms. The corresponding processes are typically complex, time-consuming, less controllable, and difficult to scale-up.
Solid dispersions loaded with nanocrystals have been prepared in three steps, including the preparation of a nanosuspension, suspension injection, and solvent devolatilization. Aqueous nanosuspensions are obtained by dispersing powder nanomaterials (Khinast et al., 2013), wet ball milling (Baumgartner et al., 2014), or high-pressure homogenization (Ye et al., 2016) to form a nanosuspension in water. In the second step, the prepared nanosuspension is slowly injected into the extruder barrel to blend with the molten carrier. Presently, only Soluplus® has been used as a carrier for this application. In the last step, the utilized solvent is eliminated via devolatilization through the port located at the end of the barrel. Inside the barrel, the molten mass is continuously thinned on the screw surface that dramatically enhances its surface area and thus accelerates the evaporation process. This devolatilization technique shows promise for successful production of selected solid dispersions.
7.4. Melt extrusion of amorphous APIs
Converting APIs from the crystalline to the amorphous form is a widely used approach to enhance the dissolution and solubility of poorly soluble drugs. It has been reported that HME can be successfully used to formulate amorphous solid dispersions, in which amorphous drugs are dispersed inside a polymer matrix to enhance their physical stability. However, high processing temperatures limit the applications of this technique (especially for high melting point or heat-sensitive drugs).
A feasible solution to the problem is converting crystalline APIs to an amorphous form before extrusion. Since their values of Tg are usually lower than the corresponding melting points, the use of amorphous materials facilitates the extrusion process by conducting it at lower temperatures in the miscibility regime, which results in faster mixing of API species with the molten carrier. Among many polymorphic transformation techniques, solvent evaporation methods such as rotary evaporation, spray drying, and low-pressure evaporation are easy to perform in the laboratory and scale up.
In a study utilizing this approach, pre-engineered ITZ amorphous microparticles were used as a starting material for the extrusion process (Miller et al., 2007). The amorphous microparticles were prepared using a flash evaporation method, in which the solvent devolatilized under low pressure. The obtained amorphous solid dispersion flakes were milled into microparticles via ball milling before feeding into an extruder containing a low melting point polymer (polyethylene oxide) used as a second carrier. The properties of the amorphous micro-particles were preserved after extrusion and exhibited improved dissolution and bioavailability. In another study, a new compound was transformed from the crystalline to an amorphous state via a simple solvent evaporation method (Lakshman et al., 2008). The resulting amorphous drug served as a plasticizer during the melt extrusion process with the polymer carrier. The final product was characterized by higher solubility and bioavailability as compared to those of the crystalline API. The amorphous nature of API particles in the solid dispersion was preserved after the stability study with a duration of 12 months.
Despite the promising results reported in the published studies, the production of amorphous APIs prior to HME via solvent evaporation poses a risk of environmental pollution that makes the HME technique “less green”. Moreover, it raises a question whether the HME integration is necessary or not, since polymeric carriers can be introduced into the formulation to produce stable amorphous solid dispersions using solvent evaporation techniques.
7.5. Pre-emulsified extrusion of lipid nanoparticles
The hot melt extruder, which simultaneously provides heat and mechanical shear, can be utilized as a continuous emulsifying tool. The supplied solid contents of the formulations are melted inside the first several zones of the extruder barrel before mixing with the aqueous phase that is injected through the middle port of the barrel. The application of shear inside the twin-screw extruder forms a crude emulsion, which is subsequently transferred to a homogenizer for further particle size reduction. The emulsifying effect of the extruder can be optimized by establishing an appropriate temperature pattern for different zones, customizing the screw configuration, and varying the screw speed. Although the size of the final products is determined during the homogenization stage, the preparation of a suitable crude emulsion significantly contributes to the quality of the final product (Mehnert and Mader, 2001). By conjugating the extruder with a high-pressure homogenizer, a continuous process for the preparation of solid lipid nanoparticles loaded with fenofibrate was proposed (Patil et al., 2015). A solid lipid dispersion with particle sizes below 200 nm was obtained that significantly enhanced the solubility and oral bioavailability of fenofibrate. In another study, the combination of a sonication probe with an extruder was used to fabricate lipid nanoparticles loaded with lidocaine (Bhagurkar et al., 2017). The resulting lipid nanosuspension was formulated to a topical gel for cutaneous pain management, whose drug release profile exhibited extended characteristics governed by the lipid matrix. Although the emulsifying efficiency of the extrusion process is not as effective as that of high-speed homogenizers or colloidal mills, it can be a viable approach to the integration of continuous manufacturing processes.
7.6. Pharmaceutical reactive extrusion
The term “reactive extrusion” has been used in polymer science for several decades. It is considered an efficient method for continuous polymerization and chemical modification of polymers (Tzoganakis, 1989). HME produces heat and leads to microscale homogenization simultaneously; hence, it can be potentially used for melting/softening compounds and blending them together at a molecular level, and thus facilitates chemical reactions without using solvents. Therefore, melt extrusion is considered an environment friendly, green, and continuous process.
Salt formation is a popular approach to overcoming the poor solubility of drugs. It has been found that the solubility of salts is typically 100-fold higher than that of its non-ionized form (Williams et al., 2013). However, the preparation of salts at the industrial scale is a complex high-temperature process, which utilizes large amounts of organic solvents (Wermuth and Stahl, 2002) and thus raises serious environmental concerns. The recent advances in reactive extrusion can be exemplified by the synthesis of a salt of haloperidol and maleic acid via HME (Lee et al., 2017). A highly crystalline salt was obtained by extruding a physical mixture of haloperidol and maleic acid (1:1) at 60 °C. Screw configuration and processing temperature were critical factors for the salt formation. The salt nature of the product was confirmed via 1H NMR and single-crystal XRD, and its solubility (4.7 mg/mL) was significantly higher than that of haloperidol (14 μg/ml). The obtained salt was stable for 90 d at 25 °C and 75% RH.
HME can be also used for the dual functional application of reactive extrusion and ASD formulation. A physical mixture of naproxen and meglumine with a ratio 1:1 was extruded in the presence of polymeric carriers (povidone, copovidone, or Soluplus®) (Liu et al., 2017). The salt was formed in-situ and simultaneously dispersed into a polymer matrix inside the barrel to form an amorphous solid dispersion. The dissolution rates of the produced naproxen-meglumine salt-based ASDs were two times greater than those of their corresponding physical mixture and conventional naproxen ASDs. The amorphous nature of the salt was preserved after the storage at 40 °C and 75% RH for 4 months. Although the stability data of the last two described studies are not exciting, further investigation of the process may lead to utilization of these methods for HME-induced salt formation in the near future.
7. Marketed products
Since the early 1980s, the HME technique has gained popularity and emerged as a rapidly growing technology in the pharmaceutical industry due to its ability to enhance the bioavailability of poorly soluble drugs. Approximately 56% of all the HME-related patents were issued in the United States or Germany (Wilson et al., 2012). Various HME products available in the market were developed for oral delivery, the enhancement of solubility and bioavailability of poorly soluble drugs, immediate release (Dierickx et al., 2012; Fu et al., 2016), sustained and controlled release (Almeida et al., 2011; Ma et al., 2013), taste masking (Gryczke et al., 2011), enteric release (Schilling et al., 2010), targeted release products, implants (Johnson et al., 2010), and ocular inserts (Balguri et al., 2017).
The list of the marketed products for poorly soluble drugs that includes their dosage forms, carriers, indications, status, and manufacturers is provided in Table 5. Here, Noxafil® is composed of posaconazole, which is a weakly basic triazole antifungal agent with pH-dependent solubility (Haser et al., 2016). The HPMCAS polymer was extruded with posaconazole to develop a solid dispersion. The obtained extrudates were used to produce tablets or capsules containing 100 mg of posaconazole (Haser et al., 2016). The bioavailability study conducted under fed conditions exhibited significantly higher mean plasma drug concentrations for the solid dispersion as compared to those in the oral suspension. Similarly, under fasting conditions, the solid dispersion contained higher mean plasma drug concentrations than those of the oral suspension, which suggested that the developed product was not influenced by the food intake.
Table 5.
Marketed HME products of poorly soluble drugs summarized for indication, type of dosage form and polymer employed.
| Brand Name | API | Polymer | Indication | Dosage form | Manufacturer/Status | Ref |
|---|---|---|---|---|---|---|
| Gris-PEG | Griseofulvin | PEG | Onychomycosis | Oral Tablet (Crystalline dispersion) | Pedinol Pharmacal (Marketed) | (Ahmed et al., 2010) |
| Norvir® | Ritonavir | PEG-glyceride | Viral infection (HIV) | Oral Tablet (Amorphous dispersion) | AbbVie (Marketed) | (Sherman and Steinberg, 2011) |
| Kaletra® | Ritonavir, Lopinavir | PVP/PVA | Viral infection (HIV) | Oral Tablet (Amorphous dispersion) | AbbVie (Marketed) | (Klein et al., 2007) |
| Onmel® | Itraconazole | HPMC | Onychomycosis | Oral Tablet (Amorphous dispersion) | Merz North American, Inc. (Marketed) | (Baert et al., 2003) |
| Viekirapak | Ombitasvir, Paritaprevir, Ritonavir, Dasabuvir | PVP/VA copolymer | Anti-viral (HIV) | Oral Tablet (Amorphous dispersion) | AbbVie (Marketed) | (Miller et al., 2016) |
| Belsomra ® | Suvorexant | PVP/VA copolymer | Insomnia Treatment | Oral Tablet (Amorphous dispersion) | Merck (Marketed) | (Harmon and Variankav al, 2013) |
| Noxafil® | Posaconazole | HPMCAS | Anti-fungal | Oral Tablet (Amorphous dispersion) | Merck (Marketed) | (Haser et al., 2016) |
| Anacetrapib | Anacetrapib | PVP/VA copolymer | Cardiovascular disease | Oral Tablet (Amorphous dispersion) | Merck (Development) | (Haser et al., 2016) |
| Venclexta™ | Venetoclax | PVP/VA copolymer | Oncology | Oral Tablet (Amorphous dispersion) | AbbVie (Development) | (Haser et al., 2016) |
| Rezulin® | Troglitazone | PVP | Diabetes | Oral Tablet (Amorphous dispersion) | Wyeth (Withdrawn) | (Terife, 2013) |
ITZ is a weakly basic antifungal drug that exhibits pH-dependent solubility and variable bioavailability affected by the food intake (Haser et al., 2016; Shah et al., 2013). Using the Meltrex® technology, AbbVie produced an amorphous solid dispersion containing 40% ITZ and 60% HPMC, which is currently marketed under the name of Onmel® (Baert et al., 2003). According to a study conducted on fasted healthy volunteers, the AUC of Onmel® was 2.3 times greater than that of Sporanox®, indicating its improved bioavailability. Similarly, the Meltrex® technology was used to develop Kaletra® and Norvir® tablets, which are administered to treat HIV. Kaletra® is a combination of ritonavir and lopinavir, while Norvir® contains only ritonavir. For each of these drugs, the application of HME reduced the dosing frequency as compared to that of the soft gelatin capsule formulation (Shah et al., 2013).
Belsomra® is an HME-synthesized drug developed by Merck. The API in this product is suvorexant, which is a class II poorly water-soluble drug. Harmon and Variankaval (2013) extruded the API with a pH-independent vinylpyrrolidone-vinyl acetate copolymer (Kollidon® VA 64) and observed that its disintegration, dissolution, and absorption were affected by tablet hardness (Kesisoglou et al., 2015). The disintegration times observed for the tablets with hardness values of 14.8 kPa, 22.1 kPa, 32 kPa, and 38.1 kPa were 5.29, 12.11, 23.58, and 30.23 min, respectively. The softer tablets demonstrated 100% dissolution of the drug after a noticeably shorter period. However, the tablets with different hardnesses produced similar mean plasma drug concentration curves.
AbbVie recently received an FDA approval in 2016 for Venclexta™, which is one of the newest melt extrusion products on the market (Haser et al., 2016). Its API is venetoclax – an anticancer agent, which is used to treat chronic lymphocytic leukemia (CLL). Mixtures of Kollidon® VA 64 with various surfactants were extruded with venetoclax (a poorly soluble drug) to enhance its bioavailability and determine a therapeutically effective dose. The formulation selected for animal studies contained 12% venetoclax, 80% Kollidon VA 64, 7% Tween, and 1% Aerosil (Birtalan et al., 2011). These studies demonstrated improved bioavailability of venetoclax as compared to that of a reference lipid formulation. Venclexta™ is a novel and versatile drug produced by HME technology. Thus, the take-away from this example, as well as the Kaletra® outcome, is that simple formulations can be utilized via HME technology to produce marketed products.
Indeed, hot melt techniques can also be used to produce various medical devices and implants, such Zoladex™, Ozurdex®, Implanon®, and NuvaRing®. To round out the list, the melt-granulated product Eucreas® (containing a mixture of vildagliptin and metformin HCl) is used to treat diabetes, while Zithromax® is a melt congealing (taste masking) brand of azithromycin. From the above discussions on scale up strategies, types of polymers studied, with innovative applications and available PAT tools, it is evident that HME is a promising technology. Finally, the increasing number of marketed products and patents in recent years clearly suggests that HME has become a technology-leader within the pharmaceutical industry.
8. Conclusions
The poor solubility of newly engineered drugs is a crucial issue for improved drug delivery. Among the numerous suggested approaches, HME has been established as an efficient technology for the enhancement of solubility and bioavailability of poorly soluble drugs. The SWOT analysis of HME suggests significant advantages of this platform as compared to other techniques. Regardless of gaining popularity, HME processing may induce thermal degradation of API and carrier at certain processing temperatures, as well as recrystallization of API during storage of the HME product. However, these issues are addressed by reducing the processing temperatures by use of plasticizers, reduction of residence time of materials during extrusion and proper selection of polymer carriers. The use of various PAT tools coupled with HME represents promising CM methods. The recent PAT tools employed in the polymer industry can be utilized by pharmaceutical scientists to understand the nature of materials and processes for successful development of pharmaceutical products. The launch of commercial HME products into the market has confirmed the significant business potential of this technology for the pharmaceutical industry. These successes associated/coupled with the versatility of HME, defines the future potential for this paradigm-changing technology.
Acknowledgments
This work was partially supported by Grant Number P20GM104932 from the National Institute of General Medical Sciences (NIGMS), a component of NIH. The authors also thank the Pii Center for Pharmaceutical Technology.
ABBREVIATIONS
- AFM
Atomic force microscopy
- AGN
Arginine
- APAP
Acetaminophen
- API
Active pharmaceutical ingredient
- ASD
Amorphous solid dispersion
- AUC
Area under curve
- BLA
Betulinic acid
- CBZ
Carbamazepine
- CLL
Chronic lymphocytic leukemia
- CM
Continuous manufacturing
- CP
Copovidone
- CPP
Critical process parameters
- DSC
Differential scanning calorimetry
- FDA
Food and drug administration
- FTIR
Fourier transform infrared spectroscopy
- GE
Ginkgo biloba extract
- GI
Gastrointestinal
- GLZ
Gliclazide
- HIV
Human immunodeficiency virus
- HME
Hot melt extrusion
- HPC
Hydroxypropyl cellulose
- HPLC
High performance liquid chromatography
- HPMC
Hydroxypropylmethyl cellulose
- HPMCAS
Hydroxypropylmethyl cellulose acetate succinate
- HPβCD
hydroxypropyl-β-cyclodextrin
- HSM
Hot stage microscopy
- IC
Isothermal micro calorimetry
- IND
Indomethacin
- ITZ
Itraconazole
- LMD
Lipid micro domains
- mDSC
Modulated differential scanning calorimetry
- MIR
Mid-infrared
- MVA
Multivariate data analysis
- NIR-CI
Near-infrared spectroscopy chemical imaging
- NMR
Nuclear magnetic resonance
- PAA
Poly acrylic acid
- PAT
Process analytical technology
- PC-SAFT
Perturbed chain statistical associating fluid theory
- PEG
Polyethylene glycol
- PLA
Poly lactic acid
- PLM
Polarized light microscopy
- PSSA
Polystyrene sulfonic acid
- PVP
Polyvinyl pyrrolidine
- PXRD
Powder X-ray diffraction
- QbD
Quality by design
- RH
Relative humidity
- RT
Residence time
- RTD
Residence time distribution
- RTRT
Real-time release testing
- SE
Specific energy
- SEM
Scanning electron microscopy
- SG
Specific gravity
- SL
Soluplus
- SLS
Sodium lauryl sulfate
- SME
Specific mechanical energy
- STA
Stearic acid
- SWOT
Strengths, weaknesses, opportunities and threats
- Tg
Glass transition temperature
- TGA
Thermo gravimetric analysis
- TPGS
Tocopherol polyethylene glycol succinate
- VA
Vinyl alcohol
- WLF
William-Landel-Ferry
- XRD
X-ray diffraction
- βCD
β-Cyclodextrin
Footnotes
Declaration of interest
The authors report no potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adler C, Schönenberger M, Teleki A, Kuentz M. Molecularly designed lipid microdomains for solid dispersions using a polymer/inorganic carrier matrix produced by hot-melt extrusion. Int J Pharm. 2016;499:90–100. doi: 10.1016/j.ijpharm.2015.12.057. [DOI] [PubMed] [Google Scholar]
- Agrawal AM, Dudhedia MS, Zimny E. Hot Melt Extrusion: Development of an Amorphous Solid Dispersion for an Insoluble Drug from Mini-scale to Clinical Scale. AAPS PharmSciTech. 2016;17:133–147. doi: 10.1208/s12249-015-0425-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed HA, Alfredson TV, Birudaraj K, Brandl MT, Phuapradit W, Shah NH, Stefanidis D. Pharmaceutical composition and process. Google Patents 2010 [Google Scholar]
- Alam MA, Ali R, Al-Jenoobi FI, Al-Mohizea AM. Solid dispersions: a strategy for poorly aqueous soluble drugs and technology updates. Expert Opin Drug Deliv. 2012;9:1419–1440. doi: 10.1517/17425247.2012.732064. [DOI] [PubMed] [Google Scholar]
- Almeida A, Possemiers S, Boone M, De Beer T, Quinten T, Van Hoorebeke L, Remon JP, Vervaet C. Ethylene vinyl acetate as matrix for oral sustained release dosage forms produced via hot-melt extrusion. Eur J Pharm Biopharm. 2011;77:297–305. doi: 10.1016/j.ejpb.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Apruzzese F, Pato J, Balke S, Diosady L. In-line measurement of residence time distribution in a co-rotating twin-screw extruder. Food Research International. 2003;36:461–467. [Google Scholar]
- Ashour E, Kulkarni V, Almutairy B, Park JB, Shah SP, Majumdar S, Lian Z, Pinto E, Bi V, Durig T, Martin ST, Repka MA. Influence of pressurized carbon dioxide on ketoprofen-incorporated hot-melt extruded low molecular weight hydroxypropylcellulose. Drug Dev Ind Pharm. 2016;42:123–130. doi: 10.3109/03639045.2015.1035282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baert LEC, Verreck G, Thoné D. Antifungal compositions with improved bioavailability. Google Patents 2003 [Google Scholar]
- Baghel S, Cathcart H, O’Reilly NJ. Polymeric Amorphous Solid Dispersions: A Review of Amorphization, Crystallization, Stabilization, Solid-State Characterization, and Aqueous Solubilization of Biopharmaceutical Classification System Class II Drugs. J Pharm Sci. 2016;105:2527–2544. doi: 10.1016/j.xphs.2015.10.008. [DOI] [PubMed] [Google Scholar]
- Balguri SP, Adelli GR, Tatke A, Janga KY, Bhagav P, Majumdar S. Melt-Cast Noninvasive Ocular Inserts for Posterior Segment Drug Delivery. J Pharm Sci. 2017 doi: 10.1016/j.xphs.2017.07.017. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner R, Eitzlmayr A, Matsko N, Tetyczka C, Khinast J, Roblegg E. Nano-extrusion: a promising tool for continuous manufacturing of solid nano-formulations. Int J Pharm. 2014;477:1–11. doi: 10.1016/j.ijpharm.2014.10.008. [DOI] [PubMed] [Google Scholar]
- Becker W, Guschin V, Mikonsaari I, Teipel U, Kolle S, Weiss P. Turbidimetric method for the determination of particle sizes in polypropylene/clay-composites during extrusion. Anal Bioanal Chem. 2017;409:741–751. doi: 10.1007/s00216-016-0038-3. [DOI] [PubMed] [Google Scholar]
- Bellantone RA, Patel P, Sandhu H, Choi DS, Singhal D, Chokshi H, Malick AW, Shah N. A method to predict the equilibrium solubility of drugs in solid polymers near room temperature using thermal analysis. J Pharm Sci. 2012;101:4549–4558. doi: 10.1002/jps.23319. [DOI] [PubMed] [Google Scholar]
- Bhagurkar AM, Angamuthu M, Patil H, Tiwari RV, Maurya A, Hashemnejad SM, Kundu S, Murthy SN, Repka MA. Development of an ointment formulation using hot-melt extrusion technology. AAPS PharmSciTech. 2016;17:158–166. doi: 10.1208/s12249-015-0453-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhagurkar AM, Repka MA, Murthy SN. A Novel Approach for the Development of a Nanostructured Lipid Carrier Formulation by Hot-Melt Extrusion Technology. J Pharm Sci. 2017;106:1085–1091. doi: 10.1016/j.xphs.2016.12.015. [DOI] [PubMed] [Google Scholar]
- Birtalan E, Hoelig P, Lindley DJ, Sanzgiri YD, Tong P. Melt-extruded solid dispersions containing an apoptosis-inducing agent. Google Patents 2011 [Google Scholar]
- Blagden N, de Matas M, Gavan PT, York P. Crystal engineering of active pharmaceutical ingredients to improve solubility and dissolution rates. Adv Drug Deliv Rev. 2007;59:617–630. doi: 10.1016/j.addr.2007.05.011. [DOI] [PubMed] [Google Scholar]
- Boksa K, Otte A, Pinal R. Matrix-assisted cocrystallization (MAC) simultaneous production and formulation of pharmaceutical cocrystals by hot-melt extrusion. J Pharm Sci. 2014;103:2904–2910. doi: 10.1002/jps.23983. [DOI] [PubMed] [Google Scholar]
- Breitenbach J. Melt extrusion can bring new benefits to HIV therapy. Am J Drug Deliv. 2006;4:61–64. [Google Scholar]
- Brough C, Williams RO., 3rd Amorphous solid dispersions and nano-crystal technologies for poorly water-soluble drug delivery. Int J Pharm. 2013;453:157–166. doi: 10.1016/j.ijpharm.2013.05.061. [DOI] [PubMed] [Google Scholar]
- Challa S, Potumarthi R. Chemometrics-based process analytical technology (PAT) tools: applications and adaptation in pharmaceutical and biopharmaceutical industries. Appl Biochem Biotechnol. 2013;169:66–76. doi: 10.1007/s12010-012-9950-y. [DOI] [PubMed] [Google Scholar]
- Chatterjee S. FDA perspective on continuous manufacturing. IFPAC Annual Meeting; Baltimore, MD. 2012. pp. 34–42. [Google Scholar]
- Chiu SH, Yiu HC, Pong SH. Development of an in- line viscometer in an extrusion molding process. Journal of applied polymer science. 1997;63:919–924. [Google Scholar]
- Cifuentes S, Lieblich M, López F, Benavente R, González-Carrasco J. Effect of Mg content on the thermal stability and mechanical behaviour of PLLA/Mg composites processed by hot extrusion. Mater Sci Eng C. 2017;72:18–25. doi: 10.1016/j.msec.2016.11.037. [DOI] [PubMed] [Google Scholar]
- Coates P, Barnes S, Sibley M, Brown E, Edwards HG, Scowen I. In-process vibrational spectroscopy and ultrasound measurements in polymer melt extrusion. Polymer. 2003;44:5937–5949. [Google Scholar]
- Cogdill RP, Forcht RN, Shen Y, Taday PF, Creekmore JR, Anderson CA, Drennen JK. Comparison of terahertz pulse imaging and near-infrared spectroscopy for rapid, nondestructive analysis of tablet coating thickness and uniformity. J Pharm Innov. 2007;2:29–36. [Google Scholar]
- Cosse A, Konig C, Lamprecht A, Wagner KG. Hot Melt Extrusion for Sustained Protein Release: Matrix Erosion and In vitro Release of PLGA-Based Implants. AAPS PharmSciTech. 2017;18:15–26. doi: 10.1208/s12249-016-0548-5. [DOI] [PubMed] [Google Scholar]
- Covas J, Carneiro O, Costa P, Machado A, Maia J. Online monitoring techniques for studying evolution of physical, rheological and chemical effects along the extruder. Plastics, rubber and composites. 2004;33:55–61. [Google Scholar]
- Crowley MM, Zhang F, Repka MA, Thumma S, Upadhye SB, Battu SK, McGinity JW, Martin C. Pharmaceutical applications of hot-melt extrusion: part I. Drug Dev Ind Pharm. 2007;33:909–926. doi: 10.1080/03639040701498759. [DOI] [PubMed] [Google Scholar]
- Dhumal RS, Kelly AL, York P, Coates PD, Paradkar A. Cocrystalization and simultaneous agglomeration using hot melt extrusion. Pharm Res. 2010;27:2725–2733. doi: 10.1007/s11095-010-0273-9. [DOI] [PubMed] [Google Scholar]
- Dierickx L, Saerens L, Almeida A, De Beer T, Remon JP, Vervaet C. Co-extrusion as manufacturing technique for fixed-dose combination mini-matrices. Eur J Pharm Biopharm. 2012;81:683–689. doi: 10.1016/j.ejpb.2012.03.018. [DOI] [PubMed] [Google Scholar]
- DiNunzio JC, Zhang F, Martin C, McGinity JW. Melt extrusion, Formulating poorly water soluble drugs. Springer; 2012. pp. 311–362. [Google Scholar]
- Djuris J, Nikolakakis I, Ibric S, Djuric Z, Kachrimanis K. Preparation of carbamazepine–Soluplus® solid dispersions by hot-melt extrusion, and prediction of drug–polymer miscibility by thermodynamic model fitting. Eur J Pharm Biopharm. 2013;84:228–237. doi: 10.1016/j.ejpb.2012.12.018. [DOI] [PubMed] [Google Scholar]
- Dong Z, Choi DS. Hydroxypropyl methylcellulose acetate succinate: potential drug-excipient incompatibility. AAPS PharmSciTech. 2008;9:991–997. doi: 10.1208/s12249-008-9138-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreiblatt A. Technological Considerations Related to Scale- Up of Hot- Melt Extrusion Processes. Hot-Melt Extrusion: Pharmaceutical Applications. 2012:285–300. [Google Scholar]
- El-Egakey MA, Soliva M, Speiser P. Hot extruded dosage forms. I. Technology and dissolution kinetics of polymeric matrices. Pharm Acta Helv. 1971;46:31–52. [PubMed] [Google Scholar]
- Fousteris E, Tarantili PA, Karavas E, Bikiaris D. Poly (vinyl pyrrolidone)–poloxamer-188 solid dispersions prepared by hot melt extrusion. J Therm Anal Calorim. 2013;113:1037–1047. [Google Scholar]
- Fu Q, Fang M, Hou Y, Yang W, Shao J, Guo M, Li M, Li J, Wang Y, He Z. A physically stabilized amorphous solid dispersion of nisoldipine obtained by hot melt extrusion. Powder Technology. 2016;301:342–348. [Google Scholar]
- Fukuda M, Peppas NA, McGinity JW. Floating hot-melt extruded tablets for gastroretentive controlled drug release system. J Control Release. 2006;115:121–129. doi: 10.1016/j.jconrel.2006.07.018. [DOI] [PubMed] [Google Scholar]
- Fule R, Meer T, Amin P, Dhamecha D, Ghadlinge S. Preparation and characterisation of lornoxicam solid dispersion systems using hot melt extrusion technique. J Pharm Investig. 2014;44:41–59. [Google Scholar]
- Fule R, Paithankar V, Amin P. Hot melt extrusion based solid solution approach: Exploring polymer comparison, physicochemical characterization and in-vivo evaluation. Int J Pharm. 2016;499:280–294. doi: 10.1016/j.ijpharm.2015.12.062. [DOI] [PubMed] [Google Scholar]
- Gala U, Chauhan H. Principles and applications of Raman spectroscopy in pharmaceutical drug discovery and development. Expert Opin Drug Discov. 2015;10:187–206. doi: 10.1517/17460441.2015.981522. [DOI] [PubMed] [Google Scholar]
- Gao Y, Muzzio FJ, Ierapetritou MG. A review of the Residence Time Distribution (RTD) applications in solid unit operations. Powder technology. 2012;228:416–423. [Google Scholar]
- Genina N, Hadi B, Löbmann K. Hot melt extrusion (HME) as solvent-free technique for a continuous manufacturing of drug-loaded mesoporous silica. J Pharm Sci. 2017 doi: 10.1016/j.xphs.2017.05.039. (In Press) [DOI] [PubMed] [Google Scholar]
- Ghebremeskel AN, Vemavarapu C, Lodaya M. Use of surfactants as plasticizers in preparing solid dispersions of poorly soluble API: selection of polymer–surfactant combinations using solubility parameters and testing the processability. Int J Pharm. 2007;328:119–129. doi: 10.1016/j.ijpharm.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Goke K, Lorenz T, Repanas A, Schneider F, Steiner D, Baumann K, Bunjes H, Dietzel A, Finke JH, Glasmacher B, Kwade A. Novel strategies for the formulation and processing of poorly water-soluble drugs. Eur J Pharm Biopharm. 2017 doi: 10.1016/j.ejpb.2017.05.008. (In Press) [DOI] [PubMed] [Google Scholar]
- Gottwald A, Scheler U. Extrusion Monitoring of Polymer Melts Using a High- Temperature Surface- NMR Probe. Macromolecular Materials and Engineering. 2005;290:438–442. [Google Scholar]
- Gryczke A, Schminke S, Maniruzzaman M, Beck J, Douroumis D. Development and evaluation of orally disintegrating tablets (ODTs) containing Ibuprofen granules prepared by hot melt extrusion. Colloids Surf B Biointerfaces. 2011;86:275–284. doi: 10.1016/j.colsurfb.2011.04.007. [DOI] [PubMed] [Google Scholar]
- Guo Z, Lu M, Li Y, Pang H, Lin L, Liu X, Wu C. The utilization of drug-polymer interactions for improving the chemical stability of hot-melt extruded solid dispersions. J Pharm Pharmacol. 2014;66:285–296. doi: 10.1111/jphp.12145. [DOI] [PubMed] [Google Scholar]
- Haberstroh E, Jakisch L, Henssge E, Schwarz P. Real- Time Monitoring of Reactive Extrusion Processes by Means of In- Line Infrared Spectroscopy and Infrared Temperature Measurement. Macromolecular materials and engineering. 2002;287:203–208. [Google Scholar]
- Hanafy AFA, El-Egaky AM, Mortada SA, Molokhia AM. Development of implants for sustained release of 5-fluorouracil using low molecular weight biodegradable polymers. Drug Discov Ther. 2009;3:287–95. [PubMed] [Google Scholar]
- Harmon PA, Variankaval N. Solid dosage formulations of an orexin receptor antagonists. Google Patents 2013 [Google Scholar]
- Haser A, DiNunzio JC, Martin C, McGinity JW, Zhang F. Melt Extrusion, Formulating Poorly Water Soluble Drugs. Springer; 2016. pp. 383–435. [Google Scholar]
- Haser A, Huang S, Listro T, White D, Zhang F. An approach for chemical stability during melt extrusion of a drug substance with a high melting point. Int J Pharm. 2017;524:55–64. doi: 10.1016/j.ijpharm.2017.03.070. [DOI] [PubMed] [Google Scholar]
- Hitzer P, Bäuerle T, Drieschner T, Ostertag E, Paulsen K, van Lishaut H, Lorenz G, Rebner K. Process analytical techniques for hot-melt extrusion and their application to amorphous solid dispersions. Anal Bioanal Chem. 2017;409:4321–4333. doi: 10.1007/s00216-017-0292-z. [DOI] [PubMed] [Google Scholar]
- Huang J, Goolcharran C, Utz J, Hernandez-Abad P, Ghosh K, Nagi A. A PAT approach to enhance process understanding of fluid bed granulation using in-line particle size characterization and multivariate analysis. J Pharm Innov. 2010;5:58–68. [Google Scholar]
- Huang S, O’Donnell KP, Delpon de Vaux SM, O’Brien J, Stutzman J, Williams RO., 3rd Processing thermally labile drugs by hot-melt extrusion: The lesson with gliclazide. Eur J Pharm Biopharm. 2017;119:56–67. doi: 10.1016/j.ejpb.2017.05.014. [DOI] [PubMed] [Google Scholar]
- Hughey JR, DiNunzio JC, Bennett RC, Brough C, Miller DA, Ma H, Williams RO, McGinity JW. Dissolution enhancement of a drug exhibiting thermal and acidic decomposition characteristics by fusion processing: a comparative study of hot melt extrusion and KinetiSol® dispersing. AAPS PharmSciTech. 2010;11:760–774. doi: 10.1208/s12249-010-9431-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam MT, Maniruzzaman M, Halsey SA, Chowdhry BZ, Douroumis D. Development of sustained-release formulations processed by hot-melt extrusion by using a quality-by-design approach. Drug delivery and translational research. 2014;4:377–387. doi: 10.1007/s13346-014-0197-8. [DOI] [PubMed] [Google Scholar]
- Jaiswar DR, Jha D, Amin PD. Preparation and characterizations of stable amorphous solid solution of azithromycin by hot melt extrusion. J Pharm Investig. 2016;46:655–668. [Google Scholar]
- Jamrogiewicz M. Application of the near-infrared spectroscopy in the pharmaceutical technology. J Pharm Biomed Anal. 2012;66:1–10. doi: 10.1016/j.jpba.2012.03.009. [DOI] [PubMed] [Google Scholar]
- Janssens S, Van den Mooter G. Review: physical chemistry of solid dispersions. J Pharm Pharmacol. 2009;61:1571–1586. doi: 10.1211/jpp/61.12.0001. [DOI] [PubMed] [Google Scholar]
- Johnson TJ, Gupta KM, Fabian J, Albright TH, Kiser PF. Segmented polyurethane intravaginal rings for the sustained combined delivery of antiretroviral agents dapivirine and tenofovir. Eur J Pharm Sci. 2010;39:203–212. doi: 10.1016/j.ejps.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Juluri A, Popescu C, Zhou L, Murthy RN, Gowda VK, Kumar C, Pimparade MB, Repka MA, Murthy SN. Taste masking of griseofulvin and caffeine anhydrous using Kleptose Linecaps DE17 by hot melt extrusion. AAPS PharmSciTech. 2016;17:99–105. doi: 10.1208/s12249-015-0374-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kate L, Gokarna V, Borhade V, Prabhu P, Deshpande V, Pathak S, Sharma S, Patravale V. Bioavailability enhancement of atovaquone using hot melt extrusion technology. Eur J Pharm Sci. 2016;86:103–114. doi: 10.1016/j.ejps.2016.03.005. [DOI] [PubMed] [Google Scholar]
- Keating AV, Soto J, Tuleu C, Forbes C, Zhao M, Craig DQM. Solid state characterisation and taste masking efficiency evaluation of polymer based extrudates of isoniazid for paediatric administration. Int J Pharm. 2017 doi: 10.1016/j.ijpharm.2017.07.008. (In Press) [DOI] [PubMed] [Google Scholar]
- Kesisoglou F, Hermans A, Neu C, Yee KL, Palcza J, Miller J. Development of In vitro-In vivo Correlation for Amorphous Solid Dispersion Immediate-Release Suvorexant Tablets and Application to Clinically Relevant Dissolution Specifications and In-Process Controls. J Pharm Sci. 2015;104:2913–2922. doi: 10.1002/jps.24362. [DOI] [PubMed] [Google Scholar]
- Khinast J, Baumgartner R, Roblegg E. Nano-extrusion: a one-step process for manufacturing of solid nanoparticle formulations directly from the liquid phase. AAPS PharmSciTech. 2013;14:601–604. doi: 10.1208/s12249-013-9946-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khorasani M, Edinger M, Raijada D, Botker J, Aho J, Rantanen J. Near-infrared chemical imaging (NIR-CI) of 3D printed pharmaceuticals. Int J Pharm. 2016;515:324–330. doi: 10.1016/j.ijpharm.2016.09.075. [DOI] [PubMed] [Google Scholar]
- Klein CE, Chiu YL, Awni W, Zhu T, Heuser RS, Doan T, Breitenbach J, Morris JB, Brun SC, Hanna GJ. The tablet formulation of lopinavir/ritonavir provides similar bioavailability to the soft-gelatin capsule formulation with less pharmacokinetic variability and diminished food effect. J Acquir Immune Defic Syndr. 2007;44:401–410. doi: 10.1097/QAI.0b013e31803133c5. [DOI] [PubMed] [Google Scholar]
- Krupa A, Cantin O, Strach B, Wyska E, Tabor Z, Siepmann J, Wróbel A, Jachowicz R. In vitro and in vivo behavior of ground tadalafil hot-melt extrudates: How the carrier material can effectively assure rapid or controlled drug release. Int J Pharm. 2017;528:498–510. doi: 10.1016/j.ijpharm.2017.05.057. [DOI] [PubMed] [Google Scholar]
- Lakshman JP, Cao Y, Kowalski J, Serajuddin AT. Application of melt extrusion in the development of a physically and chemically stable high-energy amorphous solid dispersion of a poorly water-soluble drug. Mol Pharm. 2008;5:994–1002. doi: 10.1021/mp8001073. [DOI] [PubMed] [Google Scholar]
- Lamm MS, DiNunzio J, Khawaja NN, Crocker LS, Pecora A. Assessing Mixing Quality of a Copovidone-TPGS Hot Melt Extrusion Process with Atomic Force Microscopy and Differential Scanning Calorimetry. AAPS PharmSciTech. 2016;17:89–98. doi: 10.1208/s12249-015-0387-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley N, DiNunzio J, Repka MA. Melt Extrusion: Materials, Technology and Drug Product Design (AAPS Advances in the Pharmaceutical Sciences Series) Springer; 2013. [Google Scholar]
- Lee HL, Vasoya JM, Cirqueira ML, Yeh KL, Lee T, Serajuddin AT. Continuous Preparation of 1:1 Haloperidol-Maleic Acid Salt by a Novel Solvent-Free Method Using a Twin Screw Melt Extruder. Mol Pharm. 2017;14:1278–1291. doi: 10.1021/acs.molpharmaceut.7b00003. [DOI] [PubMed] [Google Scholar]
- Lenz E, Lobmann K, Rades T, Knop K, Kleinebudde P. Hot Melt Extrusion and Spray Drying of Co-amorphous Indomethacin-Arginine With Polymers. J Pharm Sci. 2017;106:302–312. doi: 10.1016/j.xphs.2016.09.027. [DOI] [PubMed] [Google Scholar]
- Li L, AbuBaker O, Shao ZJ. Characterization of poly (ethylene oxide) as a drug carrier in hot-melt extrusion. Drug Dev Ind Pharm. 2006;32:991–1002. doi: 10.1080/03639040600559057. [DOI] [PubMed] [Google Scholar]
- Li M, Gogos CG, Ioannidis N. Improving the API dissolution rate during pharmaceutical hot-melt extrusion I: Effect of the API particle size, and the co-rotating, twin-screw extruder screw configuration on the API dissolution rate. Int J Pharm. 2015;478:103–112. doi: 10.1016/j.ijpharm.2014.11.024. [DOI] [PubMed] [Google Scholar]
- Li M, Wilkinson D, Patchigolla K, Mougin P, Roberts KJ, Tweedie R. On-line crystallization process parameter measurements using ultrasonic attenuation spectroscopy. Crystal growth & design. 2004;4:955–963. [Google Scholar]
- Li S, Yu T, Tian Y, McCoy CP, Jones DS, Andrews GP. Mechanochemical Synthesis of Pharmaceutical Cocrystal Suspensions via Hot Melt Extrusion: Feasibility Studies and Physicochemical Characterization. Mol Pharm. 2016;13:3054–3068. doi: 10.1021/acs.molpharmaceut.6b00134. [DOI] [PubMed] [Google Scholar]
- Liu H, Zhu L, Wang P, Zhang X, Gogos CG. Effects of screw configuration on indomethacin dissolution behavior in Eudragit E PO. Polym Adv Technol. 2012a;31:331–342. [Google Scholar]
- Liu X, Lu M, Guo Z, Huang L, Feng X, Wu C. Improving the chemical stability of amorphous solid dispersion with cocrystal technique by hot melt extrusion. Pharm Res. 2012b;29:806–817. doi: 10.1007/s11095-011-0605-4. [DOI] [PubMed] [Google Scholar]
- Liu X, Zhou L, Zhang F. Reactive Melt Extrusion To Improve the Dissolution Performance and Physical Stability of Naproxen Amorphous Solid Dispersions. Mol Pharm. 2017;14:658–673. doi: 10.1021/acs.molpharmaceut.6b00960. [DOI] [PubMed] [Google Scholar]
- Lu G, Kalyon DM, Yilgör I, Yilgör E. Rheology and extrusion of medical- grade thermoplastic polyurethane. Polym Eng Sci. 2003;43:1863–1877. [Google Scholar]
- Lu J, Cuellar K, Hammer NI, Jo S, Gryczke A, Kolter K, Langley N, Repka MA. Solid-state characterization of Felodipine-Soluplus amorphous solid dispersions. Drug Dev Ind Pharm. 2016;42:485–496. doi: 10.3109/03639045.2015.1104347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luypaert J, Massart DL, Vander Heyden Y. Near-infrared spectroscopy applications in pharmaceutical analysis. Talanta. 2007;72:865–883. doi: 10.1016/j.talanta.2006.12.023. [DOI] [PubMed] [Google Scholar]
- Ma D, Djemai A, Gendron CM, Xi H, Smith M, Kogan J, Li L. Development of a HPMC-based controlled release formulation with hot melt extrusion (HME) Drug Dev Ind Pharm. 2013;39:1070–1083. doi: 10.3109/03639045.2012.702350. [DOI] [PubMed] [Google Scholar]
- Maddineni S, Battu SK, Morott J, Majumdar S, Murthy S, Repka MA. Influence of Process and Formulation Parameters on Dissolution and Stability Characteristics of Kollidon® VA 64 Hot-Melt Extrudates. AAPS PharmSciTech. 2015;16:444–454. doi: 10.1208/s12249-014-0226-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniruzzaman M, Boateng JS, Snowden MJ, Douroumis D. A review of hot-melt extrusion: process technology to pharmaceutical products. ISRN Pharm. 2012;2012:436763. doi: 10.5402/2012/436763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniruzzaman M, Nair A, Renault M, Nandi U, Scoutaris N, Farnish R, Bradley MS, Snowden MJ, Douroumis D. Continuous twin-screw granulation for enhancing the dissolution of poorly water soluble drug. Int J Pharm. 2015;496:52–62. doi: 10.1016/j.ijpharm.2015.09.025. [DOI] [PubMed] [Google Scholar]
- Maniruzzaman M, Nokhodchi A. Continuous manufacturing via hot-melt extrusion and scale up: regulatory matters. Drug discov today. 2017;22:340–351. doi: 10.1016/j.drudis.2016.11.007. [DOI] [PubMed] [Google Scholar]
- Markarian J. Scale-up Challenges in Hot-Melt Extrusion. Pharmaceutical Technology. 2012;36:88–92. [Google Scholar]
- Marsac PJ, Li T, Taylor LS. Estimation of drug-polymer miscibility and solubility in amorphous solid dispersions using experimentally determined interaction parameters. Pharm Res. 2009;26:139–151. doi: 10.1007/s11095-008-9721-1. [DOI] [PubMed] [Google Scholar]
- Marsac PJ, Shamblin SL, Taylor LS. Theoretical and practical approaches for prediction of drug-polymer miscibility and solubility. Pharm Res. 2006;23:2417–2426. doi: 10.1007/s11095-006-9063-9. [DOI] [PubMed] [Google Scholar]
- Martinez-Marcos L, Lamprou DA, McBurney RT, Halbert GW. A novel hot-melt extrusion formulation of albendazole for increasing dissolution properties. Int J Pharm. 2016;499:175–185. doi: 10.1016/j.ijpharm.2016.01.006. [DOI] [PubMed] [Google Scholar]
- Medarevic DP, Kachrimanis K, Mitric M, Djuris J, Djuric Z, Ibric S. Dissolution rate enhancement and physicochemical characterization of carbamazepine-poloxamer solid dispersions. Pharm Dev Technol. 2016;21:268–276. doi: 10.3109/10837450.2014.996899. [DOI] [PubMed] [Google Scholar]
- Mehnert W, Mader K. Solid lipid nanoparticles: production, characterization and applications. Adv Drug Deliv Rev. 2001;47:165–196. doi: 10.1016/s0169-409x(01)00105-3. [DOI] [PubMed] [Google Scholar]
- Merisko-Liversidge E, Liversidge GG. Nanosizing for oral and parenteral drug delivery: a perspective on formulating poorly-water soluble compounds using wet media milling technology. Advanced drug delivery reviews. 2011;63:427–440. doi: 10.1016/j.addr.2010.12.007. [DOI] [PubMed] [Google Scholar]
- Miller DA, McConville JT, Yang W, Williams RO, 3rd, McGinity JW. Hot-melt extrusion for enhanced delivery of drug particles. J Pharm Sci. 2007;96:361–376. doi: 10.1002/jps.20806. [DOI] [PubMed] [Google Scholar]
- Miller JM, Morris JB, Sever NE, Schmitt EA, Gao PX, Shi Y, Gao Y, Liepold B, Moosmann A, Pauli M. Solid antiviral dosage forms. Google Patents 2016 [Google Scholar]
- Morott JT, Pimparade M, Park JB, Worley CP, Majumdar S, Lian Z, Pinto E, Bi Y, Durig T, Repka MA. The Effects of Screw Configuration and Polymeric Carriers on Hot- Melt Extruded Taste- Masked Formulations Incorporated into Orally Disintegrating Tablets. J Pharm Sci. 2015;104:124–134. doi: 10.1002/jps.24262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller R, Jacobs C, Kayser O. Nanosuspensions as particulate drug formulations in therapy: rationale for development and what we can expect for the future. Advanced drug delivery reviews. 2001;47:3–19. doi: 10.1016/s0169-409x(00)00118-6. [DOI] [PubMed] [Google Scholar]
- Munjal M, ElSohly MA, Repka MA. Chemical stabilization of a Δ 9-tetrahydrocannabinol prodrug in polymeric matrix systems produced by a hot-melt method: Role of microenvironment pH. AAPS PharmSciTech. 2006;7:E114–E125. doi: 10.1208/pt070371. [DOI] [PubMed] [Google Scholar]
- Nagy ZK, Sauceau M, Nyúl K, Rodier E, Vajna B, Marosi G, Fages J. Use of supercritical CO2- aided and conventional melt extrusion for enhancing the dissolution rate of an active pharmaceutical ingredient. Polym Adv Technol. 2012;23:909–918. [Google Scholar]
- Nalawade SP, Picchioni F, Janssen L. Supercritical carbon dioxide as a green solvent for processing polymer melts: Processing aspects and applications. Progress in Polymer Science. 2006;31:19–43. [Google Scholar]
- Palem CR, Dudhipala NR, Battu SK, Repka MA, Rao Yamsani M. Development, optimization and in vivo characterization of domperidone-controlled release hot-melt-extruded films for buccal delivery. Drug Dev Ind Pharm. 2016;42:473–484. doi: 10.3109/03639045.2015.1104346. [DOI] [PubMed] [Google Scholar]
- Patil H, Feng X, Ye X, Majumdar S, Repka MA. Continuous production of fenofibrate solid lipid nanoparticles by hot-melt extrusion technology: a systematic study based on a quality by design approach. AAPS J. 2015;17:194–205. doi: 10.1208/s12248-014-9674-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil H, Tiwari RV, Repka MA. Hot-Melt Extrusion: from Theory to Application in Pharmaceutical Formulation. AAPS PharmSciTech. 2016;17:20–42. doi: 10.1208/s12249-015-0360-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawar JN, Fule RA, Maniruzzaman M, Amin PD. Solid crystal suspension of Efavirenz using hot melt extrusion: Exploring the role of crystalline polyols in improving solubility and dissolution rate. Mater Sci Eng C. 2017;78:1023–1034. doi: 10.1016/j.msec.2017.04.055. [DOI] [PubMed] [Google Scholar]
- Pimparade MB, Vo A, Maurya AS, Bae J, Morott JT, Feng X, Kim DW, Kulkarni VI, Tiwari R, Vanaja K. Development and Evaluation of an Oral Fast Disintegrating Anti-allergic Film Using Hot-melt Extrusion Technology. Eur J Pharm Biopharm. 2017;119:81–90. doi: 10.1016/j.ejpb.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pina MF, Zhao M, Pinto JF, Sousa JJ, Craig DQ. The Influence of Drug Physical State on the Dissolution Enhancement of Solid Dispersions Prepared Via Hot- Melt Extrusion: A Case Study Using Olanzapine. J Pharm Sci. 2014;103:1214–1223. doi: 10.1002/jps.23894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prudic A, Ji Y, Sadowski G. Thermodynamic phase behavior of API/polymer solid dispersions. Mol Pharm. 2014;11:2294–2304. doi: 10.1021/mp400729x. [DOI] [PubMed] [Google Scholar]
- Prudic A, Lesniak AK, Ji Y, Sadowski G. Thermodynamic phase behaviour of indomethacin/PLGA formulations. Eur J Pharm Biopharm. 2015;93:88–94. doi: 10.1016/j.ejpb.2015.01.029. [DOI] [PubMed] [Google Scholar]
- Puri V, Brancazio D, Desai PM, Jensen KD, Chun J-H, Myerson AS, Trout BL. Development of Maltodextrin-Based Immediate-Release Tablets Using an Integrated Twin-Screw Hot-Melt Extrusion and Injection-Molding Continuous Manufacturing Process. J Pharm Sci. 2017;106:3328–3336. doi: 10.1016/j.xphs.2017.06.020. [DOI] [PubMed] [Google Scholar]
- Qi S, Craig DQ. Hot melt extruded transdermal films based on amorphous solid dispersions in Eudragit RS PO: The inclusion of hydrophilic additives to develop moisture-activated release systems. Int J Pharm. 2016;514:270–281. doi: 10.1016/j.ijpharm.2016.06.137. [DOI] [PubMed] [Google Scholar]
- Qian F, Huang J, Hussain MA. Drug-polymer solubility and miscibility: Stability consideration and practical challenges in amorphous solid dispersion development. J Pharm Sci. 2010;99:2941–2947. doi: 10.1002/jps.22074. [DOI] [PubMed] [Google Scholar]
- Rajan VV, Wäber R, Wieser J. Online monitoring of the thermal degradation of POM during melt extrusion. Journal of applied polymer science. 2010;115:2394–2401. [Google Scholar]
- Rask MB, Knopp MM, Olesen NE, Holm R, Rades T. Influence of PVP/VA copolymer composition on drug-polymer solubility. Eur J Pharm Sci. 2016;85:10–17. doi: 10.1016/j.ejps.2016.01.026. [DOI] [PubMed] [Google Scholar]
- Repka MA, Majumdar S, Kumar Battu S, Srirangam R, Upadhye SB. Applications of hot-melt extrusion for drug delivery. Expert Opin Drug Deliv. 2008;5:1357–1376. doi: 10.1517/17425240802583421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repka MA, Shah S, Lu J, Maddineni S, Morott J, Patwardhan K, Mohammed NN. Melt extrusion: process to product. Expert Opin Drug Deliv. 2012;9:105–125. doi: 10.1517/17425247.2012.642365. [DOI] [PubMed] [Google Scholar]
- Roggo Y, Chalus P, Maurer L, Lema-Martinez C, Edmond A, Jent N. A review of near infrared spectroscopy and chemometrics in pharmaceutical technologies. J Pharm Biomed Anal. 2007;44:683–700. doi: 10.1016/j.jpba.2007.03.023. [DOI] [PubMed] [Google Scholar]
- Rothen-Weinhold A, Oudry N, Schwach-Abdellaoui K, Frutiger-Hughes S, Hughes GJ, Jeannerat D, Burger U, Besseghir K, Gurny R. Formation of peptide impurities in polyester matrices during implant manufacturing. Eur J Pharm Biopharm. 2000;49:253–257. doi: 10.1016/s0939-6411(00)00066-7. [DOI] [PubMed] [Google Scholar]
- Saerens L, Dierickx L, Lenain B, Vervaet C, Remon JP, De Beer T. Raman spectroscopy for the in-line polymer-drug quantification and solid state characterization during a pharmaceutical hot-melt extrusion process. Eur J Pharm Biopharm. 2011;77:158–163. doi: 10.1016/j.ejpb.2010.09.015. [DOI] [PubMed] [Google Scholar]
- Saerens L, Dierickx L, Quinten T, Adriaensens P, Carleer R, Vervaet C, Remon JP, De Beer T. In-line NIR spectroscopy for the understanding of polymer-drug interaction during pharmaceutical hot-melt extrusion. Eur J Pharm Biopharm. 2012;81:230–237. doi: 10.1016/j.ejpb.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Saerens L, Segher N, Vervaet C, Remon JP, De Beer T. Validation of an in-line Raman spectroscopic method for continuous active pharmaceutical ingredient quantification during pharmaceutical hot-melt extrusion. Anal Chim Acta. 2014a;806:180–187. doi: 10.1016/j.aca.2013.11.020. [DOI] [PubMed] [Google Scholar]
- Saerens L, Vervaet C, Remon JP, De Beer T. Process monitoring and visualization solutions for hot-melt extrusion: a review. J Pharm Pharmacol. 2014b;66:180–203. doi: 10.1111/jphp.12123. [DOI] [PubMed] [Google Scholar]
- Sarode AL, Sandhu H, Shah N, Malick W, Zia H. Hot melt extrusion (HME) for amorphous solid dispersions: predictive tools for processing and impact of drug-polymer interactions on supersaturation. Eur J Pharm Sci. 2013;48:371–384. doi: 10.1016/j.ejps.2012.12.012. [DOI] [PubMed] [Google Scholar]
- Schilling SU, Shah NH, Waseem Malick A, McGinity JW. Properties of melt extruded enteric matrix pellets. Eur J Pharm Biopharm. 2010;74:352–361. doi: 10.1016/j.ejpb.2009.09.008. [DOI] [PubMed] [Google Scholar]
- Shah RB, Tawakkul MA, Khan MA. Comparative evaluation of flow for pharmaceutical powders and granules. AAPS PharmSciTech. 2008;9:250–258. doi: 10.1208/s12249-008-9046-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah S, Maddineni S, Lu J, Repka MA. Melt extrusion with poorly soluble drugs. Int J Pharm. 2013;453:233–252. doi: 10.1016/j.ijpharm.2012.11.001. [DOI] [PubMed] [Google Scholar]
- Sherman EM, Steinberg JG. Heat-stable ritonavir tablets: a new formulation of a pharmacokinetic enhancer for HIV. Expert Opin Pharmacother. 2011;12:141–148. doi: 10.1517/14656566.2011.542151. [DOI] [PubMed] [Google Scholar]
- Shukla A, Prakash A, Rohani S. Online measurement of particle size distribution during crystallization using ultrasonic spectroscopy. Chem Eng Sci. 2010;65:3072–3079. [Google Scholar]
- Sievers A. Submillimetre Spectroscopy. In: Chantry GW, editor. A Guide to the Theoretical and Experimental Physics of the Far Infrared. Vol. 1971. Academic Press; New York: 1972. p. x.p. 386. illus. $18. American Association for the Advancement of Science. [Google Scholar]
- Smith AJ, Kavuru P, Wojtas L, Zaworotko MJ, Shytle RD. Cocrystals of quercetin with improved solubility and oral bioavailability. Mol Pharm. 2011;8:1867–1876. doi: 10.1021/mp200209j. [DOI] [PubMed] [Google Scholar]
- Sperling LH. Introduction to physical polymer science. John Wiley & Sons; 2005. [Google Scholar]
- Stankovic M, de Waard H, Steendam R, Hiemstra C, Zuidema J, Frijlink HW, Hinrichs WL. Low temperature extruded implants based on novel hydrophilic multiblock copolymer for long-term protein delivery. Eur J Pharm Sci. 2013;49:578–587. doi: 10.1016/j.ejps.2013.05.011. [DOI] [PubMed] [Google Scholar]
- Stankovic M, Frijlink HW, Hinrichs WL. Polymeric formulations for drug release prepared by hot melt extrusion: application and characterization. Drug discov today. 2015;20:812–823. doi: 10.1016/j.drudis.2015.01.012. [DOI] [PubMed] [Google Scholar]
- Stefanis E, Panayiotou C. Prediction of Hansen solubility parameters with a new group-contribution method. International Journal of Thermophysics. 2008;29:568–585. [Google Scholar]
- Stefanis E, Panayiotou C. A new expanded solubility parameter approach. Int J Pharm. 2012;426:29–43. doi: 10.1016/j.ijpharm.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Stelzer T, Pertig D, Ulrich J. Ultrasonic crystallization monitoring technique for simultaneous in-line measurement of liquid and solid phase. Journal of Crystal Growth. 2013;362:71–76. [Google Scholar]
- Strachan CJ, Rades T, Newnham DA, Gordon KC, Pepper M, Taday PF. Using terahertz pulsed spectroscopy to study crystallinity of pharmaceutical materials. Chem Phys Lett. 2004;390:20–24. [Google Scholar]
- Stroyer A, McGinity J, Leopold C. Solid state interactions between the proton pump inhibitor omeprazole and various enteric coating polymers. J Pharm Sci. 2006;95:1342–1353. doi: 10.1002/jps.20450. [DOI] [PubMed] [Google Scholar]
- Stuart B. Infrared spectroscopy. Wiley Online Library; 2005. [Google Scholar]
- Terife G. Foaming of amorphous drug delivery systems prepared by hot melt mixing and extrusion. New Jersey Institute of Technology; 2013. [Google Scholar]
- Terife G, Wang P, Faridi N, Gogos CG. Hot melt mixing and foaming of soluplus® and indomethacin. Polym Eng Sci. 2012;52:1629–1639. [Google Scholar]
- Theil F, Anantharaman S, Kyeremateng SO, van Lishaut H, Dreis-Kuhne SH, Rosenberg J, Magerlein M, Woehrle GH. Frozen in Time: Kinetically Stabilized Amorphous Solid Dispersions of Nifedipine Stable after a Quarter Century of Storage. Mol Pharm. 2017;14:183–192. doi: 10.1021/acs.molpharmaceut.6b00783. [DOI] [PubMed] [Google Scholar]
- Thiry J, Kok MG, Collard L, Frere A, Krier F, Fillet M, Evrard B. Bioavailability enhancement of itraconazole-based solid dispersions produced by hot melt extrusion in the framework of the Three Rs rule. Eur J Pharm Sci. 2017a;99:1–8. doi: 10.1016/j.ejps.2016.12.001. [DOI] [PubMed] [Google Scholar]
- Thiry J, Krier F, Ratwatte S, Thomassin J-M, Jerome C, Evrard B. Hot-melt extrusion as a continuous manufacturing process to form ternary cyclodextrin inclusion complexes. Eur J Pharm Sci. 2017b;96:590–597. doi: 10.1016/j.ejps.2016.09.032. [DOI] [PubMed] [Google Scholar]
- Tian Y, Booth J, Meehan E, Jones DS, Li S, Andrews GP. Construction of drug-polymer thermodynamic phase diagrams using Flory-Huggins interaction theory: identifying the relevance of temperature and drug weight fraction to phase separation within solid dispersions. Mol Pharm. 2013;10:236–248. doi: 10.1021/mp300386v. [DOI] [PubMed] [Google Scholar]
- Tian Y, Caron V, Jones DS, Healy AM, Andrews GP. Using Flory-Huggins phase diagrams as a pre-formulation tool for the production of amorphous solid dispersions: a comparison between hot-melt extrusion and spray drying. J Pharm Pharmacol. 2014;66:256–274. doi: 10.1111/jphp.12141. [DOI] [PubMed] [Google Scholar]
- Tiwari RV, Patil H, Repka MA. Contribution of hot-melt extrusion technology to advance drug delivery in the 21st century. Expert Opin Drug Deliv. 2016;13:451–464. doi: 10.1517/17425247.2016.1126246. [DOI] [PubMed] [Google Scholar]
- Tumuluri SVS, Prodduturi S, Crowley MM, Stodghill SP, McGinity JW, Repka MA, Avery BA. The Use of Near- Infrared Spectroscopy for the Quantitation of a Drug in Hot- Melt Extruded Films. Drug Dev Ind Pharm. 2004;30:505–511. doi: 10.1081/ddc-120037481. [DOI] [PubMed] [Google Scholar]
- Tzoganakis C. Reactive extrusion of polymers: a review. Polym Adv Technol. 1989;9:321–330. [Google Scholar]
- Vay K, Scheler S, Friess W. Application of Hansen solubility parameters for understanding and prediction of drug distribution in microspheres. Int J Pharm. 2011;416:202–209. doi: 10.1016/j.ijpharm.2011.06.047. [DOI] [PubMed] [Google Scholar]
- Verreck G, Decorte A, Heymans K, Adriaensen J, Cleeren D, Jacobs A, Liu D, Tomasko D, Arien A, Peeters J. The effect of pressurized carbon dioxide as a temporary plasticizer and foaming agent on the hot stage extrusion process and extrudate properties of solid dispersions of itraconazole with PVP-VA 64. Eur J Pharm Sci. 2005;26:349–358. doi: 10.1016/j.ejps.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Verreck G, Decorte A, Li H, Tomasko D, Arien A, Peeters J, Rombaut P, Van den Mooter G, Brewster ME. The effect of pressurized carbon dioxide as a plasticizer and foaming agent on the hot melt extrusion process and extrudate properties of pharmaceutical polymers. The Journal of supercritical fluids. 2006;38:383–391. [Google Scholar]
- Vo AQ, Feng X, Morott JT, Pimparade MB, Tiwari RV, Zhang F, Repka MA. A novel floating controlled release drug delivery system prepared by hot-melt extrusion. Eur J Pharm Biopharm. 2016;98:108–121. doi: 10.1016/j.ejpb.2015.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vo AQ, Feng X, Pimparade M, Ye X, Kim DW, Martin ST, Repka MA. Dual-mechanism gastroretentive drug delivery system loaded with an amorphous solid dispersion prepared by hot-melt extrusion. Eur J Pharm Sci. 2017;102:71–84. doi: 10.1016/j.ejps.2017.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vynckier AK, De Beer M, Monteyne T, Voorspoels J, De Beer T, Remon JP, Vervaet C. Enteric protection of naproxen in a fixed-dose combination product produced by hot-melt co-extrusion. Int J Pharm. 2015;491:243–249. doi: 10.1016/j.ijpharm.2015.06.010. [DOI] [PubMed] [Google Scholar]
- Wang SL, Lin SY, Chen TF, Cheng WT. Eudragit E accelerated the diketopiperazine formation of enalapril maleate determined by thermal FTIR microspectroscopic technique. Pharm Res. 2004;21:2127–2132. doi: 10.1023/b:pham.0000048206.62093.4e. [DOI] [PubMed] [Google Scholar]
- Wang W, Kang Q, Liu N, Zhang Q, Zhang Y, Li H, Zhao B, Chen Y, Lan Y, Ma Q, Wu Q. Enhanced dissolution rate and oral bioavailability of Ginkgo biloba extract by preparing solid dispersion via hot-melt extrusion. Fitoterapia. 2015;102:189–197. doi: 10.1016/j.fitote.2014.10.004. [DOI] [PubMed] [Google Scholar]
- Wang Y, Steinhoff B, Brinkmann C, Alig I. In-line monitoring of the thermal degradation of poly (l-lactic acid) during melt extrusion by UV–vis spectroscopy. Polymer. 2008;49:1257–1265. [Google Scholar]
- Wen H, Jung H, Li X. Drug Delivery Approaches in Addressing Clinical Pharmacology-Related Issues: Opportunities and Challenges. AAPS J. 2015;17:1327–1340. doi: 10.1208/s12248-015-9814-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wermuth CG, Stahl PH. Selected procedures for the preparation of pharmaceutically acceptable salts. Handbook of Pharmaceutical Salts: Properties, Selection, and Use. 2002:249–263. [Google Scholar]
- Williams HD, Trevaskis NL, Charman SA, Shanker RM, Charman WN, Pouton CW, Porter CJ. Strategies to address low drug solubility in discovery and development. Pharmacol Rev. 2013;65:315–499. doi: 10.1124/pr.112.005660. [DOI] [PubMed] [Google Scholar]
- Wilson M, Williams MA, Jones DS, Andrews GP. Hot-melt extrusion technology and pharmaceutical application. Ther Deliv. 2012;3:787–797. doi: 10.4155/tde.12.26. [DOI] [PubMed] [Google Scholar]
- Yang F, Su Y, Zhu L, Brown CD, Rosen LA, Rosenberg KJ. Rheological and solid-state NMR assessments of copovidone/clotrimazole model solid dispersions. Int J Pharm. 2016;500:20–31. doi: 10.1016/j.ijpharm.2016.01.026. [DOI] [PubMed] [Google Scholar]
- Ye X, Patil H, Feng X, Tiwari RV, Lu J, Gryczke A, Kolter K, Langley N, Majumdar S, Neupane D, Mishra SR, Repka MA. Conjugation of Hot-Melt Extrusion with High-Pressure Homogenization: a Novel Method of Continuously Preparing Nanocrystal Solid Dispersions. AAPS PharmSciTech. 2016;17:78–88. doi: 10.1208/s12249-015-0389-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun F, Kang A, Shan J, Zhao X, Bi X, Li J, Di L. Preparation of osthole-polymer solid dispersions by hot-melt extrusion for dissolution and bioavailability enhancement. Int J Pharm. 2014;465:436–443. doi: 10.1016/j.ijpharm.2014.02.040. [DOI] [PubMed] [Google Scholar]
- Zeitler JA, Newnham DA, Taday PF, Threlfall TL, Lancaster RW, Berg RW, Strachan CJ, Pepper M, Gordon KC, Rades T. Characterization of temperature-induced phase transitions in five polymorphic forms of sulfathiazole by terahertz pulsed spectroscopy and differential scanning calorimetry. J Pharm Sci. 2006;95:2486–2498. doi: 10.1002/jps.20719. [DOI] [PubMed] [Google Scholar]
- Zhong S, Shen YC, Ho L, May RK, Zeitler JA, Evans M, Taday PF, Pepper M, Rades T, Gordon KC. Non-destructive quantification of pharmaceutical tablet coatings using terahertz pulsed imaging and optical coherence tomography. Optics and Lasers in Engineering. 2011;49:361–365. [Google Scholar]