Abstract
The goal of the NIH Science of Behavior Change (SOBC) Common Fund Program is to provide the basis for an experimental medicine approach to behavior change that focuses on identifying and measuring the mechanisms that underlie behavioral patterns we are trying to change. This paper frames the development of the program within a discussion of the substantial disease burden in the U.S. attributable to behavioral factors, and details our strategies for breaking down the disease- and condition-focused silos in the behavior change field to accelerate discovery and translation. These principles serve as the foundation for our vision for a unified science of behavior change at the NIH and in the broader research community.
Keywords: Science of behavior change, Experimental medicine approach, Mechanisms of behavior change, Self-regulation, Stress, Interpersonal processes
It is now widely appreciated that poor health behaviors, including smoking, alcohol and substance abuse, poor diet, lack of exercise, and failure to adhere to medical regimens, account for a substantial proportion of disease burden in the United States. Recent estimates suggest that human behavior accounts for between 40 and 50% of the risk associated with deaths before the age of 75 in the United States (National Research Council, 2011; Schroeder, 2007). Moreover, it has been demonstrated repeatedly that changes in harmful health behaviors lead to improved outcomes. Yet, despite widespread awareness that improved health behaviors would be broadly beneficial, it remains exceptionally difficult for people to initiate and maintain behavior change, and it seems clear that we need to develop new and more effective ways to design behavioral interventions to increase their short-term efficacy and their longer-term benefits.
One point to address at the outset, however, is that we do have compelling evidence that we can cause relatively long-term behavior change, and that such changes do lead to substantial health benefits. One notable success in the behavior change field is the Diabetes Prevention Program (DPP), funded by the National Institutes of Health (The National Institute of Diabetes and Digestive and Kidney Diseases). The DPP was a large multisite clinical trial aimed at discovering whether either an intensive lifestyle intervention (ILI) or use of the oral diabetes drug metformin (Glucophage) could prevent or delay the onset of type 2 diabetes (compared to a placebo) in insulin-sensitive participants at high risk of conversion. The DPP found that both the ILI and metformin arms delayed conversion (indeed, the trial was stopped early based on Data Safety and Monitoring Board recommendations because the effects were so large). While both interventions led to significant weight loss, reduction in diabetes risk in the ILI program (58 percent) exceeded that in the metformin arm (31 percent), and the ILI program also changed levels of physical activity over the long term. During a mean follow-up of 15 years, diabetes incidence compared to the placebo group was reduced by 27% in the ILI group compared to 18% in the metformin group with declining between-group differences over time (Diabetes Prevention Program Research Group, 2015). Behavior change research can claim similar successes in managing anxiety (see recent review by Kaczkurkin & Foa, 2015), treating cocaine abuse and alcohol dependence (e.g., Carroll et al., 2009 & 2014), reducing conduct problems in children and adolescents (Furlong et al., 2013; Henggeler & Sheidow, 2012), and other significant public health challenges.
Despite these successes, initiating and maintaining behavior change remains a tremendous challenge, even in the presence of a health-related “wake-up call” where a seemingly modest behavior change—adherence to a prescribed medication–is indicated. For example, patients who have had a myocardial infarction (MI; commonly known as a heart attack) should be prescribed a statin, and they should continue to take this medication for the rest of their lives. Although it is difficult to imagine a more “teachable moment”, the rate of adherence to this regimen is remarkably poor. Even MI patients in Canada, where the comprehensive health system makes prescriptions available to anyone who needs them, have only a 50% statin adherence rate after two years (Jackevicius, Mamdani, & Tu, 2002). Moreover, this is not an isolated finding; we know that up to half of patients are nonadherent to medication regimens for a wide variety of chronic conditions including hypertension, diabetes mellitus, and dyslipidemia (Choudhry et al., 2011; Cramer, 2004; Yeaw, Benner, Sian, & Smith, 2009). Even with decades of work on behavior change supported by NIH and other funding agencies, behavior change remains tremendously difficult. This suggests we may need a new approach to behavior change research.
This paper describes a promising approach to behavior change research proposed by the Science of Behavior Change Program, an initiative supported by the National Institutes of Health (NIH) Common Fund. Our hope is that the Science of Behavior Change Program can catalyze the development of a unified and cumulative science of behavior change that leads to meaningful advances in public health.
1. The science of behavior change program
In 2008, a team of behavioral scientists working at institutes and centers across the NIH began to meet and discuss what the main impediments to a unified science of behavior change really were. Guided by research advances and expert-recommendations from their respective fields, the team identified three main divisions within the science that had reinforced silos in the field. First, insights from basic science, including emerging transdisciplinary domains of behavioral science, were rarely applied mechanistically in behavior change intervention development. Second, there was an NIH-wide pattern where most problem behaviors were typically studied and attacked from the point of view of a specific clinical endpoint (often corresponding to a specific “disease” institute) rather than from the perspective of finding common causes, with the predictable result that even researchers who studied closely related endpoints (e.g., drug and alcohol abuse) or potential common drivers of poor health behaviors (e.g., deleterious responses to chronic stress) often worked independently, attended different meetings, and therefore failed to capitalize on opportunities for generalization within clinical science. Third, there remains an artificial separation between basic science, where mechanistic intervention targets can be identified, and clinical science, which seeks to modify the activity of those targets to affect clinical endpoints. This last barrier appears to have been especially detrimental to efforts to develop mechanistically-informed behavioral interventions.
Building on these insights, as well as others derived from a major trans-NIH meeting (See: https://commonfund.nih.gov/behaviorchange/meetings/sobc061509/index) held in June, 2009, program staff from 17 Institutes and Centers across NIH proposed the founding of the Science of Behavior Change (SOBC) program, which was approved for support by the NIH Common Fund in 2010. The goal of SOBC from its inception has been to confront the disciplinary silos in the field of behavior change intervention development and thereby transform the field. Through a range of activities, SOBC seeks to create a unified, mechanisms-focused, science of behavior change that will transform how scientists tackle the substantial behavioral contributions to a wide range of health and disease outcomes.
1.1. The first phase of SOBC – 2010–2014
The first phase of SOBC, supported by the NIH Common Fund, aimed to capitalize on emerging basic science to accelerate investigation of common mechanisms of behavior change applicable across a broad range of health behaviors. It focused on mechanisms rather than clinical endpoints, on bringing basic and clinical researchers into closer contact, and on supporting a diverse group of lab and field studies to search for common behavior change mechanisms and examine how they’re engaged in in different contexts. The goal was to achieve a better understanding of how and why interventions work and to leverage that knowledge to improve intervention designs.
The program also supported trans-NIH meetings designed to break down disciplinary boundaries, start collaborations, and expand perspectives. Two of these were particularly ground-breaking for the initiative. The first was entitled “Revisiting Pasteur’s Quadrant: Use-inspired Basic Research” (See: https://commonfund.nih.gov/behaviorchange/meetings/sobc102012/index) and focused on how to promote the testing of basic mechanistic hypotheses within ongoing applied behavior change intervention research. A core principle of SOBC is that studies of mechanisms of change should be a standard feature of all phases of a clinical trial (Czajkowski et al., 2015; Onken, Carroll, Shoham, Cuthbert, & Riddle, 2014; Riddle & Ferrer, 2015). The notion is that if, in the context of a trial, you can learn something about how and why behavior change worked or did not—you have a chance of advancing basic understanding of the mechanisms of change as well as accelerating the translation of basic science to clinical settings. While it may seem obvious that intervention research should be designed to test hypotheses about how and why interventions work, this is typically not done (as we discuss below). This meeting revealed the value of designing research to explicitly test mechanisms of interventions and exploiting optimal methods to test these mechanisms.
The second trans-NIH meeting “Harnessing Neuroplasticity for Behavior Change” (See: https://commonfund.nih.gov/behaviorchange/meetings/sobc092013/index) investigated the value added when neurobiological variables are included as moderators or mediators of desirable behavioral outcomes in behavior change interventions. This meeting proved helpful in clarifying when neurobiological findings could serve as appropriate “assays” or intermediate biomarkers for behavior change interventions, as indicators of who will respond to interventions, or even as potential targets for behavioral interventions when the substrates and circuits are known. This meeting produced an organizing framework for classifying behavior change studies involving neurobiological assays and for evaluating mechanistic causal evidence in behavior change research at both the neurobiological and behavioral level of analysis (Fig. 1). Overall, these meetings have helped to create a new climate where basic researchers tackle behavior change questions that are relevant to, and studied within, the clinical context of intervention development research.
Fig. 1.
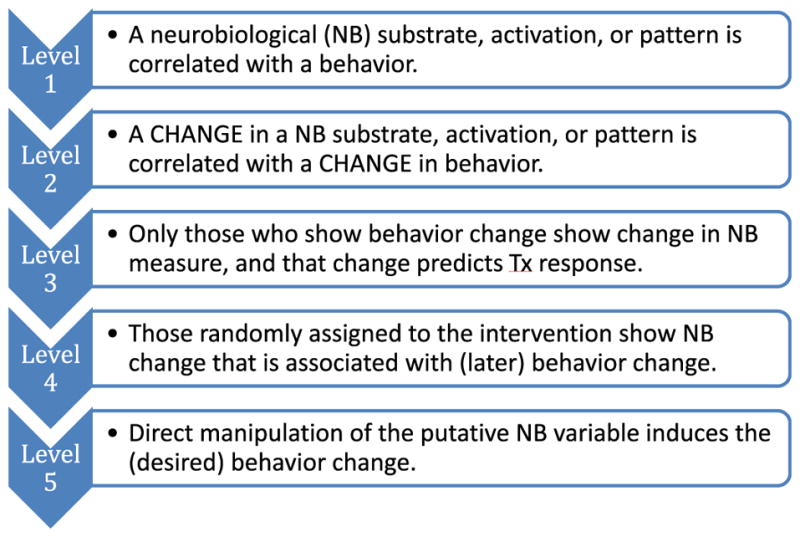
Proposed continuum of research on neurobiological variables in behavior change research.
1.2. The state of NIH science on behavior change mechanisms circa 2014
A fundamental principle guiding the SOBC program is that interventions to change health behaviors ought to be guided by a hypothesis about why the behavior exists and how best to change it (i.e., a mechanisms-of-change hypothesis). That hypothesis might be explicit—and explicitly tested in a lab or field study—or it may be implicit in the choice of intervention, but not directly tested. Without explicitly testing whether our interventions actually engage their putative targets, behavior change research has proceeded slowly and inefficiently (Czajkowski et al., 2015; Onken, Carroll, Shoham, Cuthbert, & Riddle, 2014). Similarly, there are often striking similarities between interventions meant to change health behaviors across a variety of clinical endpoints. Many clusters of unhealthy behaviors are known to co-occur, raising the possibility of underlying shared causal factors.
Mechanisms of behavior change1 – malleable targets that play a role in initiating or maintaining behavior change - can be described at different levels of analysis. Using the example of targeting executive function to bring about smoking cessation, the causal mechanism might be conceptualized in neuroscience as enhanced prefrontal-parietal brain activity and connectivity; in behavioral economics as choice of a long-term benefit over an immediate reward; in family-based therapy as increase in parental monitoring; or in sociology as increasing compliance with anti-smoking social norms. Any of these specific mechanisms could be the putative target of an intervention for smoking cessation, but they also reveal that a wide array of measures or assays could capture target engagement at different levels of analysis. Identifying, validating, and cross-calibrating multiple assays that can measure change in the same underlying target will facilitate comparison of results across studies, and will accelerate progress.
Promoting explicit testing of mechanisms in behavior change research is long overdue. In general, behavior change interventions have not been based on explicit tests of specific target engagement using well-validated assays. Instead, behavior change interventions tend to combine multiple components meant to engage a variety of targets, whether specified or not. Moreover, few intervention studies are designed to test whether the intervention actually engages the (multiple) target(s) it is meant to engage, and whether engagement of the target(s) produces the desired behavior change. As a result, even successful intervention studies do not generally inform behavior change research beyond the (often very specific) context in which they are tested. Furthermore, successful multi-component interventions are rarely adopted in their entirety for use in other settings or for other conditions; instead, interventions are unpacked, and components are adopted based on setting-specific factors rather than a mechanistic understanding of what individual components are targeting. Because we might not have realized why the complex intervention worked in the first place, this approach is slow and costly, requiring new and expensive clinical trials to test even the most incremental changes in intervention strategy. In fact, components of interventions that actually do nothing are difficult to identify and eliminate.
Work during the first phase of SOBC set the stage for a mechanisms-focused, Experimental Medicine Approach (described below) as an alternative to the inefficient multi-component intervention, “black box” approach. This Experimental Medicine Approach seeks to develop interventions that engage targets hypothesized to be putative mechanisms of change, and includes explicit tests of both target engagement and behavior change.
To illustrate the need for this paradigm shift, and the need for more efficiency in our current approaches, the SOBC Working Group conducted two portfolio analyses to provide a snapshot of the state of NIH science on mechanisms of behavior change circa 2014. Our first portfolio analysis estimated the extent to which hypotheses about mechanisms of change and tests of target engagement were being included in behavioral and social intervention research supported by selected NIH Institutes and Centers. We identified 902 behavioral or social intervention projects funded between 2008 and 2014 at nine NIH Institutes and Centers (ICs) representing a sub-set of ICs participating in the SOBC program. Using project abstracts, each was coded by stage of intervention development, with 44 percent supporting the early development and pilot testing of behavioral or social science-based interventions, and 56 percent supporting efficacy or effectiveness testing of established interventions. Using methods and analyses sections from the grant applications, a sub-set of the funded intervention grants was coded for inclusion of mechanisms of behavior change (n = 438). Of those supporting early intervention development, a full 54 percent did not consider mechanisms of behavior change when optimizing the intervention. Among those supporting intervention efficacy testing, 37 percent had no test of mechanisms of behavior change, and 34 percent had only an exploratory test of mechanisms of behavior change (Fig. 2). This means that the majority of studies are not validating target engagement or testing for causal mechanisms at the stage/phase of intervention development when studies are adequately powered and designed to conduct these tests. An optimal behavior change intervention development pipeline should address this gap, establishing the expectation that efficacy and effectiveness studies include tests of causal mechanisms.
Fig. 2.
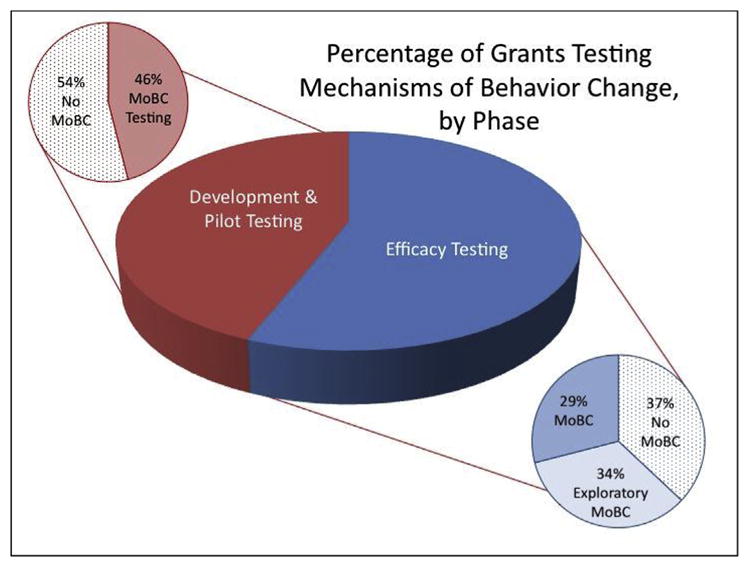
Percentage of grants testing mechanisms of behavior change (MoBC), by phase.
Our second portfolio analysis examined the specificity of assessment of behavior change targets and behavioral phenotypes in the domain of self-regulation. We hypothesized that the array of measures that purport to tap a basic behavioral mechanism of self-control and self-regulation would be broad and complex, raising the possibility that either (1) more than one mechanism is involved, depending on the behavior in question, but also that (2) there might be a few optimal ways to measure the broad, underlying construct that are applicable across multiple behavioral domains. If this turned out to be the case, then there would be merit in conducting work to determine the most appropriate assessments for behavior change science.
The portfolio analysis identified 294 research and career development projects funded by NIH between 2009 and 2014 addressing “self-regulation” or “self-control.” Among these were 117 R01 projects, which we subjected to further analysis, classifying them on the following dimensions: (1) type of science: interventions, basic science, or a hybrid of basic and applied; (2) the behavioral domain addressed (e.g. eating/obesity, substance abuse, child development, etc.); and (3) measures employed.
Of projects identified, 45 percent were interventions, 44 percent were basic behavioral science, and 12 percent a hybrid of basic and translational research. The analysis revealed a high degree of overlap between projects studying self-regulation and self-control, both in terms of measures used and in terms of the behavioral domains in which these constructs are posited to play a role. Self-regulation was invoked as a key construct in studies of problems as wide-ranging as cognitive and social development, school readiness, poverty-related adversity, homelessness, obesity, HIV, addictive behaviors, sleep, conduct disorder, risky sexual behavior, depression, anxiety disorder, ADHD, eating disorders, drinking and driving, physical activity, asthma, diabetes, cancer communication, mindfulness, stress, and resilience. A similar wide range of behaviors was found in the set of grants examining self-control, including obesity, school performance, socioemotional development, eating disorders, conduct disorder, smoking, sexual behavior, and decision-making. Notably, although the constructs of self-regulation or self-control were central to these projects, fifteen of the identified projects failed to measure the appropriate construct at all.
Supporting our hypothesis about the variety of measures used to assess these constructs, we found that within these grants, self-regulation is measured in dozens of ways using a range of approaches, with a similar and overlapping set of measures included in projects focused on self-control. Common measures include: (1) self-reports (including a variety of personality and emotion regulation assessments, self-reports of behaviors, daily diary); (2) other-reports (including teacher, parent or informant reports of emotional regulatory skills, temperament, behavior, emotion management); (3) behavior coding from free observation (e.g. family environment, classroom behavior, social interactions, eating behavior); (4) behavioral paradigms (a range of laboratory situations in which behavior is observed, including delay of gratification, disappointing gift, still face paradigm; as well as tasks such as the Iowa Gambling Task); (5) computerized tasks for risk assessment, inhibition, etc. (including the Balloon Analogue Risk Task, Stop-Signal Task, Monetary Incentive tasks, delay discounting); (6) neuropsychological tests including, primarily, a range of assessments of different facets of executive function (including aspects of the Wechsler Adult Intelligence Scale, Attention Network Task, go/no-go, Stroop) as well as tests of motor inhibition; (7) neuroimaging & event-related potential (ERP) assessments (including prefrontal cortical modulation of subcortical activation, and properties of various control networks); and (8) physiological assessments (heart rate variability, hypothalamic-pituitary-adrenal-axis and other stress biomarkers).
This analysis revealed the constructs of self-regulation and self-control reside in a broad conceptual ontology, while figuring centrally as hypothesized mechanisms, targets, or behavioral phenotypes in research on health behavior and behavior change across a wide range of conditions and developmental phases. The constructs appear to be indexing multiple mechanisms and processes, some likely distinct, others overlapping, and for which the developmental trajectory is not fully mapped out. This suggests—at a minimum—the need for more cross-validation to confirm findings by repeating experimental manipulations in one project using an independent assay technique from other research, as well as cross-calibration, to permit comparisons across projects where self-regulation/self-control has been measured differently, and where re-assessment is not possible. The overarching goal of such work would be to determine the extent to which various measures of self-control/self-regulation are tapping distinct or overlapping mechanisms, and whether measures are performing similarly across populations, laboratories, and age groups. Further work is needed to determine which measures are appropriate for which contexts, which are redundant, and which truly assess targets that are engaged by interventions in ways that are meaningfully related to behavior change.
Together, these analyses supported our view that a coordinated effort to focus on hypothesis-driven, mechanisms-focused behavior change research on targets likely common to multiple behaviors and clinical endpoints was overdue.
1.3. Second phase of SOBC – an experimental medicine approach to behavior change
In its first phase, SOBC had made substantial progress in reframing the key scientific question in the behavior change field from “Is this (specific) intervention efficacious?” to “How do our interventions work?” The answer to the first question will always be “Yes” - at least in the statistical (p-value) sense - if our intervention is intensive enough and our sample size is large. But it is the answer to the second question that has promise to transform the way we conduct behavior change interventions. Answering this question will require a significant, collaborative effort in the development of validated measures that get to the heart of mechanism both during the process of intervention development and during the conduct of clinical trials.
With renewed Common Fund support, a second phase of SOBC began in 2015 to support work to identify key targets of behavioral interventions, measure them well and validate them in multiple contexts. The overriding SOBC goal remains to transform behavioral intervention designs by promoting basic research on the initiation, personalization and maintenance of behavior change. In this second phase of SOBC, we are implementing an Experimental Medicine Approach to behavior change and developing the tools required to implement this approach. Achieving this overall goal will require success in each of following areas: (1) Identifying specific intervention targets whose engagement can be verified and that have promise to drive behavior change across multiple endpoints; (2) Developing appropriate assays to measure target engagement; (3) Pilot-testing the role of putative targets of change across multiple clinical endpoints; (4) Systematically improving trial designs to incorporate measures of target engagement throughout the intervention-target-clinical endpoint pathway.
An Experimental Medicine Approach seeks to answer the question: “What are the processes/mechanisms that drive behavior change?” This approach has three essential requirements. First, behavior change research must be driven by hypotheses about specific malleable targets (processes such as self-regulation or stress reactivity) that if altered, can lead to changes in behavior. Second, it requires experimental methods or interventions for engaging those targets. Third, valid measures of those processes are needed. These measures will allow us to see that the target has indeed been “engaged” and, ultimately, that its engagement is related to a change in the behavioral outcomes we observe.
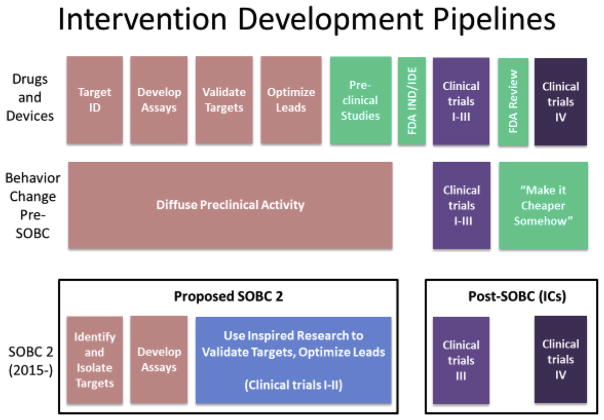
As a parallel, in the Experimental Medicine Approach to medication testing, drugs are used as clinical manipulations and the immediate goal is not to develop a treatment but to identify or verify a target. Using such proof of concept studies, drug developers can determine the ability of a drug to act on a target and affect a biological process or endpoint related to a clinical disorder, such as demonstrating that the new compound occupies relevant neural receptors or produces relevant changes in brain activity. As indicated in Fig. 3, SOBC intends to adapt a similar approach used for the development of drugs and devices. One key difference between the drugs and devices pipeline and the proposed SOBC pipeline is that in most cases in vitro or pre-clinical animal approaches will not exist for behavioral interventions, which will require use-inspired research to validate targets in clinical contexts, bringing this basic research of what we know and what we can measure directly into the clinical enterprise.
Fig. 3.
Intervention pipeline for behavior change pre- and post-SOBC, as compared to the pipeline for drug development in biomedicine.
Extrapolating from the portfolio analysis presented earlier, the NIH behavioral and social intervention research portfolio does not systematically include the steps proposed in the SOBC intervention development pipeline. One premise of the proposed intervention development pipeline for the second phase of SOBC is that interventions could be more potent if early intervention development activities optimized interventions based on their ability to engage a target responsible for change (i.e., mechanisms of behavior change). Our analysis suggests that currently this approach is only very rarely taken.
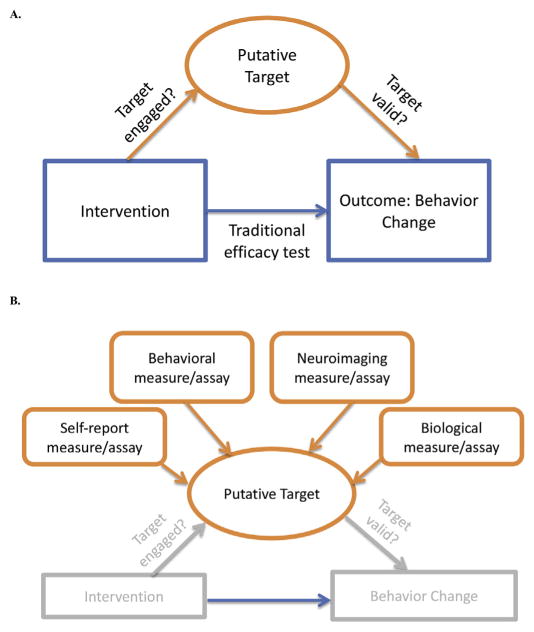
In the Experimental Medicine Approach to behavior change, putative intervention targets represent mechanisms or processes that are hypothesized to be measurable, malleable, and to play a causal role in producing behavior change. This approach has implications for intervention development in that interventions are developed in a way that allows for testing hypotheses about how behavior change is achieved, in order to understand the processes or mechanisms responsible for change. This can be contrasted with traditional efficacy testing (Fig. 4A). So rather than ask, “Does mindfulness lead to improved adherence to an exercise program?” We ask, “Does mindfulness improve my self-monitoring (as hypothesized), and do changes in self-monitoring result in improved exercise adherence?” In addition, to test whether an intervention “works” as hypothesized, valid measures of target engagement are needed. The Experimental Medicine Approach also has implications for measures development. Measures at multiple levels—behavioral, biological— are being tested or developed in our program to provide a convergence of evidence, increasing support for hypotheses and confidence that measures are valid (Fig. 4B). Approaches to manipulate or engage these targets, to demonstrate that they are malleable, and to optimize target engagement, are also required.
Fig. 4.
Experimental medicine approach to behavior change.
The second phase of SOBC supports set of projects that together constitute an SOBC Network. This Network is identifying intervention targets in several key domains (self-regulation, stress reactivity and stress resilience, interpersonal and social processes) that are considered relevant to multiple health behaviors and health and clinical endpoints. Teams are testing the extent to which they can engage specific targets in these broad domains – and identifying which targets are critical mediators of behavior change. This work is currently being conducted by teams consisting of basic, translational, and clinical scientists working together to develop and validate assays of the target mechanism, and interventions to engage that mechanism. Within teams, assays and interventions are being developed and tested for multiple clinical endpoints. Across teams, collaboration among researchers working on the same clinical endpoint or target domain is facilitated by a Resource and Coordinating Center. The SOBC Network is developing and building a registry of studies and trials including the mediators, moderators, measures, and effect sizes, so that the research community can have access to this information, thereby accelerating science and preventing unnecessary duplication. Ultimately, we seek broad adoption of these principles in the behavior change field(s) to insure sustainability of the SOBC approach at end of our 10 years of Common Fund support.
2. The SOBC target classes
An organizing principal for the current phase of SOBC is the identification of three broad classes of intervention targets that are conceptually distinct from each other but highly relevant to understanding the mechanisms by which behavior is changed. Three target classes of Self-regulation, Stress Resilience and Stress Reactivity, and Interpersonal and Social Processes were identified during the first phase of SOBC as being both central to behavior change and ready to contribute to an evidence-based approach to the design of behavioral interventions. Identification of these three areas relied foremost on the strength of existing research demonstrating their promise, their relevance across multiple clinical endpoints, and their fit within the Experimental Medicine Approach currently being proposed.
2.1. Self-regulation
As revealed in our portfolio analysis, described above, the domain of self-regulation is broad. It encompasses a wide range of behavioral and psychological constructs and processes, including, but not limited to: conscientiousness, self-control, response inhibition, impulsivity/impulse control, behavioral disinhibition, temporal discounting, emotion regulation, cognitive control (including goal selection, updating, representation and maintenance; response selection, inhibition or suppression; and performance or conflict monitoring), cognitive/emotional homeostasis, effort modulation, and flexible adaptation (Duckworth & Steinberg, 2015; Heatherton, 2011; Kotabe & Hofmann, 2015; Mischel et al., 2011; Miyake & Friedman, 2012; Vohs & Baumeister, 2011).
Measures of these processes have been developed at many levels of analysis and across a diverse set of scientific fields, using techniques such as self-report instruments, field-based approaches (e.g., ecological momentary assessment), and direct assessments of cognitive (e.g., stop-signal task) and behavioral (e.g., temporal discounting tasks) components of self-regulation, as well as indirect measures such as the effect of reappraisal strategies on emotional function (e.g., Bickel, Koffarnus, Moody, & Wilson, 2014; Congdon et al., 2012; Gross & John, 2003). A variety of other-report and observational approaches exist, such as teacher, parent or informant reports of emotional regulatory skills, temperament, and behavior; and behavioral coding from free observation of the family environment, classroom behavior, social interactions, or eating behavior (e.g., Cooper, Balsis, & Oltmanns, 2014; Drake, Belsky, & Fearon, 2014; Lakes, 2013). Also common are a range of neuroimaging and electrophysiological assessments, ranging from assessment of properties of prefrontal-parietal and prefrontal-subcortical control networks to measures of heart rate variability.
Despite a lack of a consistent ontology for self-regulation, many intervention approaches that purport to engage or change self-regulatory processes have been developed and tested. Relatively comprehensive behavioral intervention approaches include, for example, Cognitive-Behavioral Therapy and Mindfulness-Based Stress Reduction. More focal, targeted behavioral intervention approaches include attention modification/bias training, central executive training, and emotion regulation strategies. It is unclear the extent to which many of these are different names for the same thing or incorporate subsets of key, overlapping features (Tougas, Hayden, McGrath, Huguet, & Rozario, 2015).
The complexity of self-regulation at the psychological and behavioral level is reflected at the neurobiological level. A range of region-level brain targets have been implicated in self-regulation, which may function as components of one or more interconnected circuits or networks (Beauchaine, 2015; Braver, 2012; George & Koob, 2010; Helfinstein et al., 2013; Ochsner & Gross, 2008; Posner & Rothbart, 1998; Stoeckel et al., 2017). Biologically-based interventions targeting these networks include pharmacological interventions, neurofeedback, Transcranial Magnetic Stimulation, and Deep Brain Stimulation. Systems neuroscience approaches involving computational modeling may hold great utility for developing a functional ontology of self-regulation mechanisms and identifying common mechanisms across multiple laboratory paradigms.
Given the wide range of sub-components implicated, it has proven difficult to measure self-regulation consistently in the laboratory, in clinical trials, or in large scale observational studies (Duckworth & Kern, 2011; Hamilton et al., 2015; Morean et al., 2014). Work needs to be done to determine the most appropriate assessments for behavior change science. The overarching goal of such work would be to determine the extent to which various measures of self-regulation are tapping distinct or overlapping mechanisms involved in behavior change, and whether measures are performing similarly across populations, context, laboratories, and age groups. Through a series of ongoing studies, the SOBC Network will develop an ontology of self-regulation that addresses the variety of targets in this heterogeneous domain, identify and validate assays for a broad range of targets, and test the ability of target-oriented interventions to improve specific health behaviors.
2.2. Stress resilience and stress reactivity
Stress is defined as a real or perceived imbalance between environmental demands and an individual’s capacity to adapt to these requirements (Koolhaas et al., 2011). Stressors, or stress exposures, are potential or actual threats or challenges to an individual. The taxonomy for stressors includes, for example, major traumatic events; acute, novel, or unpredictable situations; repeated or chronic challenges; and daily “hassles.” Individual responses to stressors vary in nature, quality, and temporal characteristics. The initial and acute response to a stressor includes stress reactivity and recovery of those systems, with different time courses for distinct components (e.g., neural, physiological, cognitive affective, and behavioral) of the response (Linden, Earle, Gerin, & Christenfeld, 1997). Stress resilience refers to the dynamic multidimensional process encompassing positive adaptation within the context of the stressor or adversity (Bonanno & Diminich, 2013; Kalisch, Müller, & Tüscher, 2015).
A range of behavioral and psychological processes fall within the broad domain of stress reactivity and stress resilience, including perseverative cognition; cognitive flexibility; anticipatory or prolonged activation; perceptions of threat, challenge, and safety; negative and positive affect/emotions and cognitions; controllability; elasticity in affective and physiological response systems; and, emotional regulatory strategies. Likewise, numerous neurobiological processes and circuits are implicated within the central and peripheral nervous systems.
Stress reactivity and stress resilience are believed to be causal mechanisms or crucial intermediate phenotypes in the promotion of health and/or development of disease. Individual differences in patterns of stress reactivity and stress resilience affect multiple health behaviors, including medical regimen adherence, substance use, risky sexual behaviors, exercise, and food choice, and are associated with multiple adverse physical, mental, behavioral and social outcomes (Hackett & Steptoe, 2017; McEwen & Stellar, 1993; Miller, Chen & Parker, 2011; Piazza, Charles, Sliwinski, Mogle, & Almeida, 2013; Schneiderman, Ironson, & Siegel, 2005; Segerstrom & Miller, 2004; Sin, Graham-Engeland, Ong, & Almeida, 2015; Steptoe & Kivimaki, 2012). The current SOBC Network investigators will validate stress assays in the laboratory and real-world settings to allow improved assessment of the impact of variability of different components of stress responses on health behavior and the ability to improve behavior through manipulation of stress response.
2.3. Interpersonal and social processes
Finally, most of human behavior takes place in a social context. Individuals are embedded in multiple social contexts (e.g., families, households, schools, neighborhoods, workplaces) and webs of relationships (e.g., spouses, children, friends, coworkers). Since processes in these interpersonal and social contexts shape behavior formation, maintain current behaviors, and have the potential to reinforce or deter behavior change efforts, interventions that target these processes can be powerful levers for behavior change (Martire, Schulz, Helgeson, Small, & Saghafi, 2010; Pietromonaco & Collins, 2017; Smith & Christakis, 2008).
Interpersonal and social processes encompass a broad class of potential targets of behavior change. This broad class of targets can be unpacked into multiple targets that have varying degrees of conceptual overlap with each other and can be grouped in different ways. For example, the following promising targets for behavior change could be considered related or overlapping concepts within the broad categories of: culture (acculturation, collectivist vs. individualist, cultural orientation, workplace culture); social-emotional processes (affection, dyadic coping, emotional/social contagion, emotional social support, empathy, expressed emotion, hostility, social emotion regulation, social threat attenuation); social identity (self-affirmation, sense of belonging, social self-identity); social relationships (attachment, caregiving, family hierarchies, exclusion, homophily, instrumental social support, rejection, social isolation, stigmatization/shame, discrimination); social shaping (linking individual outcomes to group-level consequences, recasting, role modeling, parental monitoring or supervision, positive reinforcement, setting expectations, social/group norms, social reinforcement); and power (coercion/force, criticism, institutional social control, over-protectiveness).
Interpersonal and social processes have been measured in a variety of ways, but work is needed to develop and test measures that can be used to verify engagement of specific interpersonal or social targets. Measures that may serve as starting points include those that are: observational (e.g., coding the content of verbal or nonverbal interactions); self-reports (e.g., questionnaires, interviews); field-based (e.g., ecological momentary assessment); population-level (e.g., ethnography); physiological (e.g., heart rate and respiration); neural (e.g., fMRI, PET, EEG); neuroendocrine (e.g., oxytocin and cortisol); and immunological (e.g., cytokine and granulocyte assays) (Coan, Schaefer & Davidson, 2006; Crowell et al., 2014; Ferrer & Helm, 2013; Janicki, Kamarck, Shiffman, & Gwaltney, 2006; Kiecolt-Glaser, Gouin, & Hantsoo, 2010; Levenson & Gottman, 1983; Rilling & Sanfey, 2011; Roche, Pincus, Rebar, Conroy, & Ram, 2014; Snyder, Heyman, & Haynes, 2005). Given the wide range of interpersonal and social processes implicated in health behavior change, as well as the overlap among targets, it has proven difficult to measure these targets consistently in the laboratory, in clinical trials, or in large scale observational studies. SOBC is supporting the development of valid and reliable measures of interpersonal and social processes that may facilitate behavior change. Such measures will allow researchers to develop new or refine existing interventions designed to engage interpersonal and social targets, and to more precisely assess whether interventions are effectively engaging such targets – and whether such engagement is related to short-term health behaviors or proxies for health. This work can ultimately lead to future, large-scale interventions designed to engage interpersonal and social targets related to short-term health behavior change, in service of facilitating more sustained initiation and maintenance of health behaviors.
2.4. Conceptual and methodological overlap of network research
While each of the three domains above will be studied individually, they do not exist purely independently in vivo. Despite the conceptual overlap for self-regulation, stress, and interpersonal processes, though, the casual relationships between them have not been established. The SOBC network projects have the potential to elucidate the interactions between these domains that impact behavior change. For example, examining the impact of stressors on temporal discounting links the study of stress reactivity and self-regulation. Accounting for the impact of interpersonal conflict on stress can clarify the role of the latter as a mediator for the effect of conflict on health behavior. As the toolkit of targets and assays grows, additional investigations of these relationships will be possible.
3. Conclusion
Multiple high-profile analyses conducted over decades have shown that human behavior accounts for a large proportion of variance in preventable premature deaths in the United States—to such an extent that the United States is at a disadvantage compared to other developed countries (National Research Council and Institute of Medicine, 2013). Yet science has not yet delivered a unified understanding of basic mechanisms of behavior change across a broad range of health-related behaviors, limiting progress in the development and translation of effective and efficacious behavioral interventions. The SOBC program seeks to develop a unified science of behavior change that will increase the potential return on investment in the form of dollars and lives saved.
Promoting explicit testing of mechanisms in behavior change research is long overdue. At present, only a small number of behavioral interventions verify target engagement and examine the relationship between target engagement and outcome. Thus, we really do not know how these interventions work or how they might be optimized or adapted to meet the needs of special populations or developed for other clinical endpoints. The SOBC Network is well poised to create and validate new measures of target engagement, given advances in measurement theory, progress in data collection that ranges from improvements in neuroimaging to technological advances enabling real-time measurement, and the examples of other successful trans-NIH measurement creation efforts including PROMIS and the NIH Toolbox.
Our long-term goal is to systematically apply rigorous, mechanisms-focused methods to improve the efficacy and effectiveness of behavior change interventions via a more unified science of behavior change. We believe this will come through a transdisciplinary transformation of how behavior change science is conducted. The SOBC Program seeks to play a strategic role in furthering this progress by supporting innovative investigator-initiated research on mechanisms of behavior change across domains and diseases; documenting the current state-of-the-science upon which the field can build; and contributing more strongly toward a long-term paradigm shift in focusing on mechanisms of behavior change across basic, translational, and clinical research. A widespread implementation of an Experimental Medicine approach is a feasible and critical step in an effort to transform the behavioral interventions landscape.
Footnotes
Basic mechanisms of behavior change include mechanisms at the social, contextual, behavioral, psychological, neurobiological and genetic levels of analysis. Mechanisms of interest are those that may play a role in initiating or maintaining behavior change (including adherence to behavioral and biomedical regimens) over time. Examples of potential mechanisms of change include: (1) Mechanisms of decision-making including risk perception, temporal discounting, susceptibility to framing effects, and cognitive or affective heuristics and biases; (2) Mechanisms of control and self-monitoring, including executive function, metacognition, interoceptive awareness, and emotion regulation; (3) Mechanisms of social and cultural transmission of behaviors and of interpersonal transaction, such as contagion, mimicry, modeling, norms, peer effects, competition; (4) Structural mechanisms such as features of choice architectures, defaults, mechanisms of institutional (including healthcare systems and providers) or cultural practice, environmental affordances; (5) Neurobiological and genetic mechanisms related to these behavioral, psychological, social or environmental processes, including those associated with individual differences in biophysiologic capacity or psychological resilience/vulnerability.
Disclaimer
The content is solely the responsibility of the authors and does not represent the official views of the NIH or federal government.
References
- Beauchaine TP. Future directions in emotion dysregulation and youth psychopathology. Journal of Clinical Child & Adolescent Psychology. 2015;44(5):875–896. doi: 10.1080/15374416.2015.1038827. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Koffarnus MN, Moody L, Wilson AG. The behavioral- and neuro-economic process of temporal discounting: A candidate behavioral marker of addiction. Neuropharmacology. 2014;76:518–527. doi: 10.1016/j.neuropharm.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonanno GA, Diminich ED. Annual Research Review: Positive adjustment to adversity–trajectories of minimal-impact resilience and emergent resilience. Journal of Child Psychology and Psychiatry. 2013;54(4):378–401. doi: 10.1111/jcpp.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS. The variable nature of cognitive control: A dual mechanisms framework. Trends in Cognitive Sciences. 2012;16(2):106–113. doi: 10.1016/j.tics.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Ball SA, Martino S, Nich C, Babuscio TA, Rounsaville BJ. Enduring effects of a computer-assisted training program for cognitive behavioral therapy: A six-month follow-up of CBT4CBT. Drug Alcohol Depend. 2009 Feb 1;100(1–2):178–181. doi: 10.1016/j.drugalcdep.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Kiluk BD, Nich C, Gordon MA, Portnoy GA, Martino DR, Ball SA. Computer-assisted delivery of cognitive-behavioral therapy: Efficacy and durability of CBT4CBT among cocaine-dependent individuals maintained on methadone. The American Journal of Psychiatry. 2014;171:436–444. doi: 10.1176/appi.ajp.2013.13070987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhry NK, Avorn J, Glynn RJ, Antman EM, Schneeweiss S, Toscano M, et al. Full coverage for preventive medications after myocardial infarction. The New England Journal of Medicine. 2011;365(22):2088–2097. doi: 10.1056/NEJMsa1107913. [DOI] [PubMed] [Google Scholar]
- Coan JA, Schaefer HS, Davidson RJ. Lending a hand: Social regulation of the neural response to threat. Psychological Science. 2006;17:1032–1039. doi: 10.1111/j.1467-9280.2006.01832.x. [DOI] [PubMed] [Google Scholar]
- Congdon E, Mumford JA, Cohen JR, Galvan A, Canli T, Poldrack RA. Measurement and reliability of response inhibition. Frontiers in Psychology. 2012;21:3–37. doi: 10.3389/fpsyg.2012.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper LD, Balsis S, Oltmanns TF. Aging: Empirical contribution. A longitudinal analysis of personality disorder dimensions and personality traits in a community sample of older adults: Perspectives from selves and informants. Journal of Personality Disorders. 2014;28(1):151–165. doi: 10.1521/pedi.2014.28.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer JA. A systematic review of adherence with medications for diabetes. Diabetes Care. 2004;27(5):1218–1224. doi: 10.2337/diacare.27.5.1218. [DOI] [PubMed] [Google Scholar]
- Crowell SE, Baucom BR, Yaptangco M, Bride D, Hsiao R, McCauley E, et al. Emotion dysregulation and dyadic conflict in depressed and typical adolescents: Evaluating concordance across psychophysiological and observational measures. Biological Psychology. 2014;98:50–58. doi: 10.1016/j.biopsycho.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czajkowski S, Powell LH, Adler N, Naar-King S, Reynolds KD, Hunter CM, et al. From ideas to efficacy: The ORBIT model for developing behavioral treatments for chronic diseases. Health Psychology. 2015;34(10):971–982. doi: 10.1037/hea0000161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diabetes Prevention Program Research Group. Nathan DM, et al. Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: The Diabetes Prevention Program Outcomes Study. The Lancet Diabetes and Endocrinology. 2015;3:866–875. doi: 10.1016/S2213-8587(15)00291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake K, Belsky J, Fearon RM. From early attachment to engagement with learning in school: The role of self-regulation and persistence. Developmental Psychology. 2014;50(5):1350–1361. doi: 10.1037/a0032779. [DOI] [PubMed] [Google Scholar]
- Duckworth AL, Kern ML. A meta-analysis of the convergent validity of self-control measures. Journal of Research on Personality. 2011;45(3):259–268. doi: 10.1016/j.jrp.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth A, Steinberg L. Unpacking self-control. Child Development Perspectives. 2015;9(1):32–37. doi: 10.1111/cdep.12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer E, Helm JL. Dynamical systems modeling of physiological coregulation in dyadic interactions. International Journal of Psychophysiology. 2013;88:296–308. doi: 10.1016/j.ijpsycho.2012.10.013. [DOI] [PubMed] [Google Scholar]
- Furlong M, McGilloway S, Bywater T, Hutchings J, Smith SM, Donnelly M. Cochrane review: Behavioural and cognitive-behavioural group-based parenting programmes for early-onset conduct problems in children aged 3 to 12 years (Review) Evidence-based Child Health. 2013;8(2):318–692. doi: 10.1002/ebch.1905. [DOI] [PubMed] [Google Scholar]
- George O, Koob GF. Individual differences in prefrontal cortex function and the transition from drug use to drug dependence. Neuroscience and Bio-behavioral Reviews. 2010;35(2):232–247. doi: 10.1016/j.neubiorev.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JJ, John OP. Individual differences in two emotion regulation processes: Implications for affect, relationships, and wellbeing. Journal of Personality and Social Psychology. 2003;85:348–362. doi: 10.1037/0022-3514.85.2.348. [DOI] [PubMed] [Google Scholar]
- Hackett RA, Steptoe A. Type 2 diabetes mellitus and psychological stress - a modifiable risk factor. Nature Reviews Endocrinology. 2017;13(9):547–560. doi: 10.1038/nrendo.2017.64. [DOI] [PubMed] [Google Scholar]
- Hamilton KR, Mitchell MR, Wing VC, Balodis IM, Bickel WK, Fillmore M, et al. Choice impulsivity: Definitions, measurement issues, and clinical implications. Personality Disorders. 2015;6(2):182–198. doi: 10.1037/per0000099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF. Neuroscience of self and self-regulation. Annual Review of Psychology. 2011;62:363–390. doi: 10.1146/annurev.psych.121208.131616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfinstein SM, Schonberga T, Congdon E, Karlsgodt KH, Mumford JM, Sabb FW, et al. Predicting risky choices from brain activity patterns. Proceedings of the National Academy of Sciences. 2013;111(7):2470–2475. doi: 10.1073/pnas.1321728111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henggeler SW, Sheidow AJ. Empirically supported family-based treatments for conduct disorder and delinquency in adolescents. Journal of Marital and Family Therapy. 2012;38(1):30–58. doi: 10.1111/j.1752-0606.2011.00244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackevicius CA, Mamdani M, Tu JV. Adherence with statin therapy in elderly patients with and without acute coronary syndromes. Journal of the American Medical Association. 2002;288(4):462–467. doi: 10.1001/jama.288.4.462. [DOI] [PubMed] [Google Scholar]
- Janicki DL, Kamarck TW, Shiffman S, Gwaltney CJ. Application of ecological momentary assessment to the study of marital adjustment and social interactions during daily life. Journal of Family Psychology. 2006;20(1):168–172. doi: 10.1037/0893-3200.20.1.168. [DOI] [PubMed] [Google Scholar]
- Kaczkurkin AN, Foa EB. Cognitive-behavioral therapy for anxiety disorders: An update on the empirical evidence. Dialogues in Clinical Neuroscience. 2015;17(3):337–346. doi: 10.31887/DCNS.2015.17.3/akaczkurkin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalisch R, Müller MB, Tüscher O. A conceptual framework for the neurobiological study of resilience. Behavioral and Brain Sciences. 2015;38:1–79. doi: 10.1017/S0140525X1400082X. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Gouin JP, Hantsoo L. Close relationships, inflammation, and health. Neuroscience and Biobehavioral Reviews. 2010;35(1):33–38. doi: 10.1016/j.neubiorev.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolhaas JM, Bartolomucci A, Buwalda B, de Boer SF, Flügge G, Korte SM, et al. Stress revisited: A critical evaluation of the stress concept. Neuroscience and Biobehavioral Reviews. 2011;35(5):1291–1301. doi: 10.1016/j.neubiorev.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Kotabe HP, Hofmann W. On integrating the components of self-control. Perspectives in Psychological Science. 2015;10(5):618–638. doi: 10.1177/1745691615593382. [DOI] [PubMed] [Google Scholar]
- Lakes KD. Measuring self-regulation in a physically active context: Psychometric analyses of scores derived from an observer-rated measure of self-regulation. Mental Health and Physical Activity. 2013;8(3):189–196. doi: 10.1016/j.mhpa.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenson RW, Gottman JM. Marital interaction: Physiological linkage and affective exchange. Journal of Personality and Social Psychology. 1983;45(3):587–597. doi: 10.1037//0022-3514.45.3.587. [DOI] [PubMed] [Google Scholar]
- Linden W, Earle TL, Gerin W, Christenfeld N. Physiological stress reactivity and recovery: Conceptual siblings separated at birth? Journal of Psychosomatic Research. 1997;42(2):117–135. doi: 10.1016/s0022-3999(96)00240-1. [DOI] [PubMed] [Google Scholar]
- Martire LM, Schulz R, Helgeson VS, Small BJ, Saghafi EM. Review and meta-analysis of couple-oriented interventions for chronic illness. Annals of Behavioral Medicine. 2010;40(3):352–442. doi: 10.1007/s12160-010-9216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Stellar E. Stress and the individual. Mechanisms leading to disease. Archives of Internal Medicine. 1993;153(18):2093–2101. [PubMed] [Google Scholar]
- Miller GE, Chen E, Parker KJ. Psychological stress in childhood and susceptibility to the chronic diseases of aging: Moving toward a model of behavioral and biological mechanisms. Psychological Bulletin. 2011;137(6):959–997. doi: 10.1037/a0024768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mischel W, Ayduk O, Berman MG, Casey BJ, Gotlib IH, Jonides J, et al. ‘Willpower’ over the life span: Decomposing self-regulation. Social Cognitive and Affective Neuroscience. 2011;6(2):252–256. doi: 10.1093/scan/nsq081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A, Friedman NP. The nature and organization of individuals differences in executive functions: Four general conclusions. Current Directions in Psychological Science. 2012;21(1):8–14. doi: 10.1177/0963721411429458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morean ME, DeMartini KS, Leeman RF, Pearlson GD, Anticevic A, Krishnan-Sarin S, et al. Psychometrically improved, abbreviated versions of three classic measures of impulsivity and self-control. Psychological Assessment. 2014;26(3):1003–1020. doi: 10.1037/pas0000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council. International differences in mortality at older Ages: Dimensions and sources. Washington, DC: The National Academies Press; 2011. [PubMed] [Google Scholar]
- National Research Council and Institute of Medicine. U.S. Health in international Perspective: Shorter lives, poorer health. Panel on understanding cross-national health differences among high-income countries. In: Woolf Steven H, Aron Laudan., editors. Committee on population, division of behavioral and social sciences and education, and board on population health and public health practice, institute of medicine. Washington, DC: The National Academies Press; 2013. [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. Cognitive emotion regulation: Insights from social cognitive and affective neuroscience. Current Directions in Psychological Science. 2008;17(2):153–158. doi: 10.1111/j.1467-8721.2008.00566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onken LS, Carroll KM, Shoham V, Cuthbert BN, Riddle M. Reenvisioning clinical Science: Unifying the discipline to improve the public health. Clinical Psychological Science. 2014;22(1):22–34. doi: 10.1177/2167702613497932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza JR, Charles ST, Sliwinski MJ, Mogle J, Almeida DM. Affective reactivity to daily stressors and long-term risk of reporting a chronic physical health condition. Annals of Behavioral Medicine. 2013;45(1):110–120. doi: 10.1007/s12160-012-9423-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietromonaco PR, Collins NL. Interpersonal mechanisms linking close relationships to health. American Psychologist. 2017;72(6):531–542. doi: 10.1037/amp0000129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK. Attention, self-regulation and consciousness. Philosophical Transactions of the Royal Society of London B. 1998;353:1915–1927. doi: 10.1098/rstb.1998.0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle MW, Ferrer R. The science of behavior change. Association for Psychological Science Observer; 2015. https://www.psychologicalscience.org/observer/the-science-of-behavior-change#.WSLyyZLyt6k. [Google Scholar]
- Rilling JK, Sanfey AG. The neuroscience of social decision-making. Annual Review of Psychology. 2011;62:23–48. doi: 10.1146/annurev.psych.121208.131647. [DOI] [PubMed] [Google Scholar]
- Roche MJ, Pincus AL, Rebar AL, Conroy DE, Ram N. Enriching psychological assessment using a person-specific analysis of interpersonal processes in daily life. Assessment. 2014;21(5):515–528. doi: 10.1177/1073191114540320. [DOI] [PubMed] [Google Scholar]
- Schneiderman N, Ironson G, Siegel SD. Stress and health: Psychological, behavioral, and biological determinants. Annual Review of Clinical Psychology. 2005;1:607–628. doi: 10.1146/annurev.clinpsy.1.102803.144141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder SA. We can do better—improving the health of the American people. New England Journal of Medicine. 2007;357:1221–1228. doi: 10.1056/NEJMsa073350. [DOI] [PubMed] [Google Scholar]
- Segerstrom SC, Miller GE. Psychological stress and the human immune system: A meta-analytic study of 30 years of inquiry. Psychological Bulletin. 2004;130(4):601–630. doi: 10.1037/0033-2909.130.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sin NL, Graham-Engeland JE, Ong AD, Almeida DM. Affective reactivity to daily stressors is associated with elevated inflammation. Health Psychology. 2015;34(12):1154–1165. doi: 10.1037/hea0000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KP, Christakis NA. Social networks and health. Annual Review of Sociology. 2008;34:405–429. [Google Scholar]
- Snyder DK, Heyman RE, Haynes SN. Evidence-based approaches to assessing couple distress. Psychological Assessment. 2005;17(3):288–307. doi: 10.1037/1040-3590.17.3.288. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Kivimäki M. Stress and cardiovascular disease. Nature Reviews Cardiology. 2012;9(6):360–370. doi: 10.1038/nrcardio.2012.45. [DOI] [PubMed] [Google Scholar]
- Stoeckel LE, Birch LL, Heatherton T, Mann T, Hunter C, Czajkowski S, et al. Psychological and neural contributions to appetite self-regulation. Obesity. 2017;25(Suppl 1):S17–S25. doi: 10.1002/oby.21789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The National Institute of Diabetes and Digestive and Kidney Diseases. Diabetes prevention program. Retrieved from: https://www.niddk.nih.gov/about-niddk/research-areas/diabetes/diabetes-prevention-program-dpp/Pages/default.aspx.
- Tougas ME, Hayden JA, McGrath PJ, Huguet A, Rozario S. A systematic review exploring the social cognitive theory of self-regulation as a framework for chronic health condition interventions. PLoS One. 2015;10(8):e0134977. doi: 10.1371/journal.pone.0134977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vohs KD, Baumeister RF, editors. Handbook of self-regulation: Research, theory, and applications. 2. New York: Guilford Press; 2011. [Google Scholar]
- Yeaw J, Benner JS, Sian S, Smith DB. Comparing adherence and persistence across 6 chronic medication classes. Journal of Managed Care Pharmacy. 2009;15(9):728–740. doi: 10.18553/jmcp.2009.15.9.728. [DOI] [PMC free article] [PubMed] [Google Scholar]