Abstract
Objective
To qualitatively and quantitatively characterize third trimester growth patterns in fetuses/neonates with growth restriction using Individualized Growth Assessment.
Methods
Serial fetal size measurements from 73 fetuses with proven growth restriction were evaluated using a novel composite parameter, the Fetal Growth Pathology Score [FGPS1]. Third trimester FGPS1 measurements plotted against fetal age were examined for patterns. Identified patterns were characterized using the four components of the FGP1 [head circumference {HC}, abdominal circumference {AC}, femur diaphysis length {FDL}, estimated weight {EWT}]. A secondary characterization using age of onset, duration and magnitude of the growth abnormality process was also performed. Frequencies and magnitudes of abnormal values in different FGPS1 patterns were compared.
Results
Five growth restriction patterns were found in 70/73 {95.9%} of the cases, with progressive worsening [Pattern 1{37.0%}] and abnormal growth identified only at last scan [Pattern 2 {27.4%}] being the most common. These two patterns were usually statistically different from each other and the other three with respect to size parameter abnormalities and abnormal growth process characteristics [MANOVA]. Growth abnormalities in all parameters of the FGPS1 contributed to five abnormality patterns although AC and EWT were most important. The age of onset, duration and magnitude were similar between patterns except for Pattern 2, which had a late onset and a short duration [GLM + contrasts].
Conclusions
Our study represents the first detailed evaluation of third trimester growth restriction using methods that consider the growth potential of each fetus. Five distinctive and repetitive patterns were found, suggesting that fetal growth restriction evolves in different ways. Further research is needed to determine the relationships of these patterns to physiological/biochemical changes and adverse outcomes associated with growth restriction.
Keywords: Individualized growth assessment, longitudinal growth study, SGA
INTRODUCTION
Fetal growth assessment is based on the belief that this process can indirectly characterize the quality of the intrauterine environment that is sustaining fetal development. This concept is supported by studies that show an association between abnormal growth outcomes and increased rates of both perinatal complications and long-term neurobehavioral development abnormalities (1–3). Current obstetrical practice typically defines normal and abnormal growth outcomes in terms of population-based birth weight standards (2, 4). Prenatal assessment of growth has been primarily focused on monitoring fetal weight estimates derived from fetal biometry (5) although other investigators have used abdominal circumference measurements (4). However, the majority of individuals with perinatal or postnatal complications have no apparent evidence of a growth abnormality using conventional standards (6).
These observations suggest that fetal or neonatal weight categorizations alone are limited in their ability to identify individuals at risk for subsequent adverse outcomes. Some investigators have proposed the addition of pathophysiological or biochemical evaluations to the prenatal assessment of risk (4,7). However, such studies have not provided definitive evidence, on an individualized basis, that such evaluations result in adequate predictive capability (8,9).
Another explanation for these findings could be that the optimal size parameter, or combination of size parameters, has not been tested. With respect to fetal and neonatal growth assessment, it has been shown that different individuals express their growth abnormalities in different ways (10,11). For example, in neonates 13 types of growth restriction and 11 types of macrosomia were identified based on which of five anatomical parameters were abnormal (11). Composite parameter studies of fetal growth abnormalities in the third trimester have shown similar variability in fetal growth restriction (12,13). These results suggest that relying on a single parameter is not precise enough for the identification of growth problems that predict subsequent adverse outcomes, either alone or in combination with physiological and/or biochemical assessment.
The limited performance of growth assessments might also be the result of using population-based (14) instead of individualized standards (15) for evaluating fetal biometry. The former includes growth potential variability between individuals and does not correct for a number of confounding variables. However, growth evaluation using individualized standards [each fetus serving as its own control] is now possible using Individualized Growth Assessment [IGA] (10). Recent IGA studies have shown good agreement between prenatal and postnatal growth assessments as well as between biometric and placental evaluations of neonatal growth outcomes (16,17).
To date, there have been no IGA studies that characterize fetal growth patterns for small-for-gestational-age (SGA) newborns. The current investigation uses a novel Fetal Growth Pathology Score [FGPS1], based on multiple size parameters, to comprehensively describe patterns of abnormal growth in individual fetuses during the third trimester.
METHODS
Sample
This investigation was carried out in 73 fetuses from a retrospective, multicenter growth study of SGA cases described in detail previously (16). Fetuses were chosen because there was definitive evidence of growth restriction based on their Fetal Growth Pathology Score [FGPS1: head circumference {HC}, abdominal circumference {AC}, femur diaphysis length {FDL}, estimated weight {EWT}] calculated at the end of the third trimester (16) and their average negative pathological Growth Potential Realization Index [av –pGPRI: neonatal head circumference {HC}, crown-heel length {CHL} and weight {WT}] (17). Birth weights were below the 5th percentile in 48/73 [65.8 %] and between the 10th and 5th percentiles in 25/73 [34.2 %] (16).
Fetal Biometry
Ultrasound assessments of fetal age and growth in these SGA cases have been described in detail previously (16). A brief description of these procedures is presented below.
Fetal age was determined from crown-rump length measurements before 12 weeks, MA, or measurements of the biparietal diameter [BPD], HC, AC and FDL before 16 weeks, MA, as previously described (16).
Serial ultrasound examinations were carried out from 14 weeks, MA, to delivery as described in detail previously (16). A majority of cases [68.5%] had at least three scans in both the second and third trimesters. Measurements of BPD, HC, AC and FDL were obtained at each examination and used to calculate fetal weight estimates (16).
Neonatal Assessment
Neonatal measurements of WT, HC and CHL were obtained within 24 hours of delivery using electronic scales and a tape measure (16).
Data Analysis
The initial publication of results obtained with the Fetal Growth Pathology Score in SGA cases (16) provided a detailed description of the data analyses used in this investigation. A summary of these procedures and additions specific for this investigation are presented below.
Individualized Growth Assessment
Anatomical measurements (2–5) made before 28 weeks, MA, were used to calculate second trimester growth rates for BPD, HC, AC and FDL in each fetus. These growth rates were used to specify Rossavik size models for each anatomical parameter in each fetus using the Individualized Growth Assessment Program [iGAP, http://igap.research.bcm.edu]. These models generated expected third trimester size trajectories for HC, AC, and FDL, to which actual measurements were compared and percent deviations [%Dev] were calculated. For estimated weight [EWT], expected and measured values for the set of anatomical parameters used in the weight estimation procedure were obtained at each time point and converted to expected EWT and measured EWT values, respectively. These data were used to calculate % Dev values for EWT. % Dev values were compared to appropriate reference ranges (18) and negative pathological % Dev [−%Devp] values were calculated (16). For each anatomical parameter, the −%Devp values obtained at all third trimester time points studied were averaged to give an anatomical parameter Prenatal Growth Assessment Score [−apPGAS] value (19). At each time point, −% Devp values for HC, AC, FDL and EWT were averaged to give a negative, individual, composite Prenatal Growth Assessment Score [−icPGAS] value (19). The composite moving average of all available −%Devp values, defined as the Fetal Growth Pathology Score [FGPS1] (16), was determined for each third trimester time point and plotted against fetal age using iGAP. FGPS1 values at the last third trimester scan were compared to −0.19% for determination of prenatal growth outcomes (16).
Rossavik size models were also used to generate predicted values for WT, HC and CHL at the Growth Cessation Age (20). Neonatal measurements of these anatomical parameters were compared their predicted values and Growth Potential Realization Index [GPRI] values were calculated. These values were compared to the appropriate reference ranges (20) and the negative, pathological GPRI [−pGPRI] values were determined (16). These –pGPRI values were averaged and the average compared to −0.69% to determine the neonatal growth outcomes (16).
Assessment of third trimester growth restriction patterns
Growth restriction patterns
Individual plots of third trimester FGPS1 values were examined for differences in patterns and a classification system based on pattern shape was developed.
General characteristics of growth restriction patterns
Differences between patterns were evaluated with respect to the incidences in different BW categories, number of third trimester scans, last-scan-to-delivery interval and birth age. Third trimester growth pathology [FGPS1 values at end of third trimester] and neonatal growth pathology [average –pGPRI values] in different pattern groups were also compared. All comparisons were made using the GLM + contrasts procedure.
Anatomical components of growth restriction patterns
Since the FGPS1 is average of third trimester −%Devp values for HC, AC, FDL and EWT, differences in the characteristics of the four components between patterns could exist. To test this hypothesis, the set of –apPGAS values [−hcPGAS, −acPGAS, −fdlPGAS, −ewtPGAS] for each fetus were studied after sub-classification according to pattern type [the three unclassifiable fetuses were excluded from the analysis]. The initial evaluation of −apPGAS value sets was to determine the incidence of abnormal values based on previously established reference ranges (19).
Differences in −apPGAS values between patterns was also evaluated using balanced Multivariate Analyses of Variance [MANOVA] (21), a variance evaluation procedure used when there are two or more types of measurements defining a group. Statistical significance was determined from the values for Wilks’ Lamda, Pillai’s Trace, Hotelling-Lawley Trace and Roy’s Greatest Root (21). A statistically significant MANOVA was followed by pair-wise MANOVAs to determine which differences between patterns were responsible for the overall significant difference. The GLM Procedure with paired contrasts was applied to the four −apPGASs separately to determine differences between patterns.
Pathological processes associated with different growth restriction patterns
Age of onset, duration and magnitude were used to characterize the growth restriction pattern in each fetus. The methods utilized for estimating these parameters are given in the Appendix. Although subject to error, these estimates represent the first attempt to determine the fundamental properties of any abnormal fetal growth process. Again, fetuses were separated into subgroups based on pattern type and analyzed using MANOVA. Statistical significance was again followed by pair-wise MANOVAs to identify differences. The GLM Procedure with paired contrasts was utilized to identify differences in age of onset, duration and magnitude between patterns.
These statistical procedures were carried out with SAS [version 9.4, Chicago, IL]. A p-value less than 0.05 [adjusted for multiple comparisons when indicated] was used to identify statistically significant differences in all analyses.
RESULTS
Growth restriction patterns — qualitative characteristics
Inspection of the 73 plots of FGPS1 values against fetal age revealed five types of patterns that included 95.9% of the cases [Table 1].
Table 1.
General Characteristics of Fetal Growth Pathology Score 1 (FGPS1) Patterns
| Pattern1 | N (%) | 3rd Tri Scans2 | FGPS13 | LS-Del Interval4 | Birth Age | Average −pGPRI5 | |
|---|---|---|---|---|---|---|---|
| % | weeks | weeks | % | ||||
| 1 | 27 (37.0) | median | 3 | −1.82 | 1.1 | 37.3 | −2.95 |
| 100% range | 2 to 6 | −7.47 to −0.53 | 0.0 to 6.7 | 33.1 to 40.8 | −10.41 to −0.84 | ||
| 2 | 20 (27.4) | median | 3 | −0.41 | 0.7 | 37.3 | −2.44 |
| 100% range | 2 to 5 | −2.15 to −0.21 | 0.0 to 4.1 | 34.6 to 39.8 | −5.36 to −0.70 | ||
| 3 | 9 (12.3) | median | 3 | −1.51 | 1.0 | 37.3 | −2.35 |
| 100% range | 2 to 5 | −5.67 to −0.46 | 0.1 to 5.9 | 34.7 to 40.8 | −5.70 to −1.09 | ||
| 4 | 8 (11.0) | median | 4 | −1.36 | 0.8 | 37.6 | −3.44 |
| 100% range | 3 to 4 | −2.18 to −0.93 | 0.1 to 5.6 | 35.7 to 39.3 | −5.59 to −0.99 | ||
| 5 | 6 (8.2) | median | 2.5 | −0.70 | 0.4 | 38.6 | −2.35 |
| 100% range | 2 to 6 | −0.91 to −0.32 | 0.0 to 3.7 | 34.4 to 39.5 | −3.93 to −0.73 |
Unclassifiable cases: 3 (4.1%)
3rd Tri: third trimester
FGPS1; Fetal Growth Pathology Score 1
LS-Del: last scan-to-delivery
−pGPRI: negative, pathological Growth Potential Realization Index
FGPS1 for Pattern 1 significantly different from those of Patterns 2 and 5 [GLM + contrasts]
FGPS1 for Pattern 2 significantly different from that of Pattern 3 [GLM + contrasts]
Pattern 1 [Figure 1a], seen in 27/73 [37.0%, Table 1] of the cases, showed a persistent decrease in the FGPS1 throughout the third trimester.
Figure 1. Third trimester Fetal Growth Pathology Score 1 [FGPS1] patterns in growth restricted fetuses.
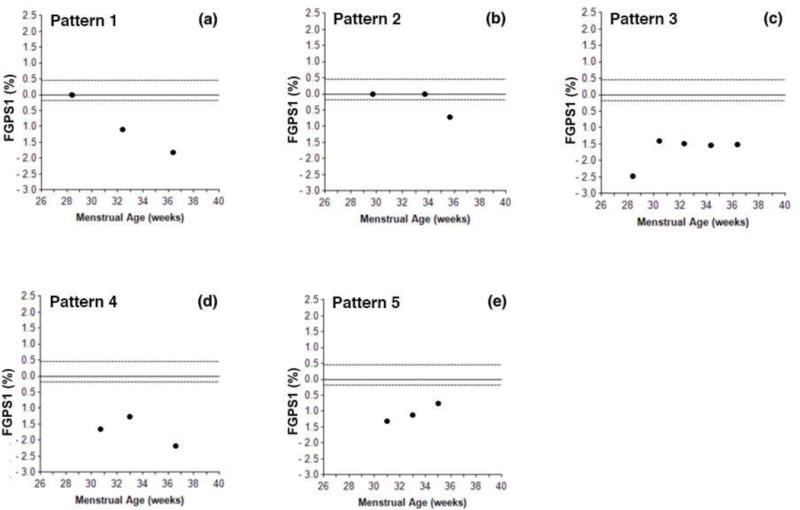
This figure illustrates the five types of patterns found when serial FGPS1 measurements were plotted against fetal age at the time of scan. Pattern 1 showed FGPS1 values that became progressively more negative throughout the third trimester. Pattern 2 showed a single negative value at the last scan [70% were within two weeks of delivery]. Pattern 3 had an initial negative FGPS1 value that then leveled off and remained approximately constant. After the initial negative value, Pattern 4 showed evidence of recovery, followed by subsequent progression of the growth restriction process. In Pattern 5, the initial negative value was followed by continuous regression back toward normal during the rest of the third trimester.
Pattern 2 [Figure 1b], seen in 20/73 [27.4%, Table 1] of the cases, had an abnormally low FGPS1 value at the last scan in the third trimester, 70% of which were within two weeks of delivery.
Pattern 3 [Figure 1c], seen in 9/73 [12.3%, Table 1] of the cases, was characterized by a set of nearly equal abnormal FGPS1 values at different third trimester time points.
Pattern 4 [Figure 1d], seen in 8/73 [11.0%, Table 1] of the cases, showed an initial, abnormally low FGPS1 value, followed by a partial recovery and then a further progression of the abnormality later in the third trimester.
Pattern 5 [Figure 1e], seen in 6/73 [8.2%, Table 1] of the cases, was characterized by an initial abnormal FGPS1 value, followed by one or more values that were less negative than the previous one. This pattern is consistent with progressive recovery from the growth abnormality. However, in no case did the FGPS1 return to a normal value.
Finally, there were 3/73 [4.1%, Table 1] of the cases that did not have one of these five patterns. The three patterns were different from each other and considered unclassifiable.
Growth restriction patterns – general characteristics
All five patterns were found in pregnancies at moderate risk [BW<10th to 5th percentiles] and significant risk [BW<5th percentile] for fetal growth restriction [FGR]. The proportion of significant risk cases varied from 62.5% [Pattern 4] to 66.7% [Pattern 3], mirroring the 67% for all 73 cases (16). There were no statistically significant differences between patterns with respect to number of third trimester scans, last scan-to-delivery interval, birth age or average −pGPRI values [Table 1]. However, small sample size and the considerable variability in pattern groups made detection of such differences difficult. With respect to the FGPS1, a significant difference among patterns was found but only between Pattern1 and Patterns 2 or 5 and between Patterns 2 and 3 [Table 1]. Significant correlations between FGPS1 and average −pGPRI values were found for Pattern 1 [r=0.67; p<0.0001] and Pattern 2 [r=0.52; p=0.019], the only patterns with sufficient sample size.
Growth restriction patterns — characteristics of anatomic parameters
Each of the five growth restriction patterns, defined by −hcPGAS, −acPGAS, −fdlPGAS and −ewtPGAS, were evaluated with respect to the number of abnormal values seen for each anatomical parameter [Table 2]. In all patterns [except Pattern 5], the highest incidences of abnormal values were found for −acPGAS and its closely associated −ewtPGAS, which were near or equal to 100%. However, 6/70 [8.6 %] and 3/70 [4.3 %] of −acPGAS and −ewtPGAS values, respectively, were normal. Approximately one-half of the −hcPGAS and −fdlPGAS values were abnormal except for Pattern 2 where the incidences for these two anatomical parameters were 35% and 20%, respectively. Pattern 5 had the highest incidences of −hcPGAS and −fdlPGAS abnormalities at 66.7% and 50%, respectively.
Table 2.
Incidences of Abnormal Negative Anatomical Parameter Modified Prenatal Growth Assessment Scores in FGPS1 Patterns
| Pattern | N | −hcPGAS1 | −acPGAS2 | −fdlPGAS3 | −ewtPGAS4 |
|---|---|---|---|---|---|
| Abnormal (%) | Abnormal (%) | Abnormal (%) | Abnormal (%) | ||
| 1 | 27 | 13 (48.1) | 27 (100.0) | 12 (44.4) | 26 (96.3) |
| 2 | 20 | 7 (35.0) | 17 (85.0) | 4 (20.0) | 19 (95.0) |
| 3 | 9 | 4 (44.4) | 9 (100.0) | 4 (44.4) | 9 (100.0) |
| 4 | 8 | 5 (62.5) | 8 (100.0) | 4 (50.0) | 7 (87.5) |
| 5 | 6 | 4 (66.7) | 3 (50.0) | 3 (50.0) | 6 (100%) |
Definition of Prenatal Growth Assessment Score (19)
−hcPGAS: negative head circumference Prenatal Growth Assessment Score
−acPGAS: negative abdominal circumference Prenatal Growth Assessment Score
−fdlPGAS: negative femur diaphysis length Prenatal Growth Assessment Score
−ewtPGAS: negative estimated weight Prenatal Growth Assessment Score
The MANOVA evaluation of growth restriction patterns indicated that there were significant differences between patterns [p-values <0.01 for 4 statistics]. However, pair-wise MANOVA assessment found that the significant differences were limited to Pattern 1 vs. Pattern 2 (p<0.001) or Pattern 5 (p=0.008), due to differences in −acPGAS [p<0.001] and −ewtPGAS [p<0.001]. For −acPGAS, statistically significant differences were found between Pattern 1 and Pattern 2 [p<0.001], Pattern 3 [p=0.022], Pattern 4 [p=0.046] and Pattern 5 [p<0.001]. A similar analysis for −ewtPGAS indicated significant differences between Pattern 1 and Pattern 2 [p<0.001], Pattern 4 [p=0.028] and Pattern 5 [p=0.0035]. The difference between Pattern 2 and Pattern 3 was also statistically significant [p=0.041].
Growth restriction patterns — abnormal growth process characteristics
Using the definitions and rules given in the Appendix, estimates of the age of onset, duration and magnitude were obtained in each of the 70 fetuses with defined growth restriction patterns (Table 4). Despite no previous use of these procedures, the estimates appear reasonable as illustrated by the age of onset estimates [before 26 weeks: 3/70 {4.3%}; before 28 weeks: 11/70 {15.7%}]. MANOVA evaluation of the five growth restriction patterns represented by their ages of onset, durations and magnitudes indicated the presence of significant differences [p<0.001 for all four statistics] between patterns. Pair-wise comparisons found that Pattern 1 was different from all other patterns except Pattern 3. Pattern 2 was different from all other patterns. The GLM Procedure with contrasts applied to age of onset and duration of growth pathology showed significant differences between Pattern 2 and the other four patterns [all p-values <0.001] for both of these growth pathology characteristics. For Magnitude, the GLM procedure found significant differences between Pattern 1 and Pattern 2 [p=0.016], Pattern 4 [p=0.036] and Pattern 5 [p=0.001]. Magnitude was significantly correlated with average −pGPRI values in Pattern 1 [r=0.67, p<0.001] and Pattern 2 [r=0.49, p=0.027], the only patterns with sufficient sample size.
Table 4.
Basic Characteristics of Growth Restriction Processes in FGPS1 Patterns
| Pattern | N | Onset | Duration | Magnitude | |
|---|---|---|---|---|---|
| weeks | weeks | % | |||
| 1 | 27 | median | 29.4 | 6.7 | −2.40 |
| 100% range | 24.7 to 33.6 | 3.5 to 13.8 | −7.47 to −0.71 | ||
| 2 | 20 | median | 34.7 | 2.6 | −1.41 |
| 100% range | 30.0 to 37.9 | 0.8 to 5.2 | −6.46 to −0.37 | ||
| 3 | 9 | median | 30.1 | 6.9 | −1.51 |
| 100% range | 27.4 to 33.4 | 5.0 to 9.9 | −5.67 to −0.62 | ||
| 4 | 8 | median | 29.1 | 9.2 | −1.58 |
| 100% range | 24.7 to 31.0 | 5.1 to 11.4 | −2.18 to −1.16 | ||
| 5 | 6 | median | 29.0 | 6.0 | −0.70 |
| 100% range | 28.1 to 32.6 | 4.4 to 11.0 | −0.91 to −0.32 |
Onset for Pattern 2 significantly different from those for all other patterns [GLM + contrasts]
Duration for Pattern 2 significantly different from those for all other patterns [GLM + contrasts]
DISCUSSION
Principal findings
Our most significant finding was the identification of five, previously unknown, types of third trimester growth restriction patterns using the Fetal Growth Pathology Score. These empirically generated patterns differed significantly from each other, were seen repeatedly in individual fetuses and have plausible biological interpretations. They strongly suggest that third trimester FGR evolves differently in different fetuses. Given such specific pattern characteristics, it is unlikely that they will be related to the same biochemical/physiological changes or perinatal/long-term adverse outcomes associated with growth restriction.
All patterns occurred with similar frequencies in fetuses with moderate and significant risk for FGR, the majority [~ 65%] being in the significant risk category. Their general characteristics were quite similar except for the FGPS1 values. Pattern 1 values were significantly higher than those for Patterns 2 and 5. Of particular interest was the lack of significant differences in average −pGPRI values, perhaps due to the small sample sizes, lack of soft tissue measures and considerable intra-pattern variability.
Quantitative evaluations of pattern components, either the incidences of abnormalities [Table 2] or abnormality magnitudes [Table 3], indicate differences between patterns. Although all four anatomical parameters contributed to growth pathology in different fetuses, the most consistent were −acPGAS and −ewtPGAS. The largest difference in incidence [100% vs. 50%] was seen for −acPGAS between Pattern 1 and Pattern 5. Growth pathology magnitudes were also greater for Pattern 1 compared to Pattern 5 with three of the four anatomical parameters [HC, AC, EWT].
Table 3.
Negative Anatomical Parameter Modified Prenatal Growth Assessment Scores in FGPS1 Patterns
| Pattern | N | −hcPGAS1 | −acPGAS2 | −fdlPGAS3 | −ewtPGAS4 | |
|---|---|---|---|---|---|---|
| % | % | % | % | |||
| 1 | 27 | median | −0.70 | −2.28 | 0.00 | −4.40 |
| 100% range | −3.38 to 0.00 | −10.30 to −0.79 | −3.53 to 0.00 | −18.48 to 0.00 | ||
| 2 | 20 | median | 0.00 | −0.65 | 0.00 | −1.02 |
| 100% range | −0.92 to 0.00 | −2.69 to 0.00 | −0.26 to 0.00 | −5.15 to 0.00 | ||
| 3 | 9 | median | −0.50 | −1.44 | 0.00 | −3.56 |
| 100% range | −3.50 to 0.00 | −3.42 to −0.18 | −3.26 to 0.00 | −13.78 to −1.25 | ||
| 4 | 8 | median | −0.39 | −1.71 | −0.03 | −3.13 |
| 100% range | −1.41 to 0.00 | −3.34 to −0.63 | −2.82 to 0.00 | −5.32 to 0.00 | ||
| 5 | 6 | median | −0.36 | −0.14 | −0.02 | −1.55 |
| 100% range | −1.96 to 0.00 | −0.60 to 0.00 | −1.34 to 0.00 | −2.18 to −0.58 |
Definition of Prenatal Growth Assessment Score (19)
−hcPGAS: negative head circumference Prenatal Growth Assessment Score
−acPGAS: negative abdominal circumference Prenatal Growth Assessment Score
−fdlPGAS: negative femur diaphysis length Prenatal Growth Assessment Score
−ewtPGAS: negative estimated weight Prenatal Growth Assessment Score
−acPGAS Pattern 1 significantly different from those for all other patterns [GLM + contrasts]
−ewtPGAS Pattern 1 significantly different from those for other patterns except Pattern 3 [GLM + contrasts]
−ewtPGAS Pattern 2 significantly different from that for Pattern 3 [GLM + contrasts]
Pattern 2 was clearly different from the other patterns as it had the lowest abnormality incidences and magnitudes for −hcPGAS and −fdlPGAS [suggesting a primary soft tissue growth problem}] and a late onset and short duration. The FGPS1 for this pattern includes the history of the pathological process [only one abnormality in a set of about three scans] while the Magnitude is the average size of the abnormality over the duration of the abnormal process [only measurable in one scan] {(16), Appendix}. The mean FGPS1 value [−0.41%] and the mean Magnitude value [−1.41%] for Pattern 2 illustrate the effect of these differences in definition.
Previous studies
The relationship between fetal growth trajectories and subsequent cognitive development has been studied with growth mixture modeling (22). In 1,059 cases at risk for growth restriction, serial measurements of BPD, AC and FDL identified four stable [71.4%] and three shifting [28.6%] growth patterns. Author-defined groups [Group Big + Medium {50.1%} and Group Small + Medium-to-Small {24.2%}] were compared with respect to cognitive development. Significantly lower mental performance index scores [at one year] and lower performance IQ and verbal IQ scores [at five years] were found in the latter group.
Barker et al (23) evaluated growth pathology in a SGA [BW<10th percentile] cohort using growth mixture models. Using estimated fetal weight, a very small sub-group [37/1116 {3.3%}] with a decreased rate of growth was identified. Significant incidences of abnormal Doppler findings, prematurity [BA: 32.0 ± 3.4 SD wks] and adverse neonatal outcomes were found in this subgroup.
Strengths and limitations
A major strength of our study is that it utilizes cases in which growth restriction was verified in both the fetus and neonate. Parameters used to classify third trimester growth [FGPS1] and neonatal growth outcomes [av −pGPRI] include multiple anatomical parameters which correct for most confounding variables (16). These parameters have been shown to effectively detect abnormal growth in the fetus and neonate (16) and the neonatal assessment is carried out without the use of any third trimester information (20). Additional strengths are the relatively large sample size for an IGA study and the comprehensive sonographic evaluation of second and third trimester growth.
An important limitation is the lack of soft tissue measurements, particularly in the neonate, due to the retrospective nature of this investigation. Although using conventional biometric measurements makes the results more widely applicable, it has been shown (20) that soft tissue parameters [e.g. abdominal circumference, thigh circumference] are more sensitive indicators of neonatal growth pathology. Therefore, the magnitude of growth pathology in the neonate is probably underestimated. A second limitation is the lack of data on physiologic evaluations, perinatal complications or long-term neurobehavioral development for correlation with FGPS1 patterns. This greatly reduces our ability to determine the clinical significance of pattern differences. Third, maternal biomarkers related to placental function such as Placental Insulin-like Growth Factor [PlGF] (24) were not studied and could play an important role in determining growth pathology evolution.
CONCLUSIONS
Our study represents the first detailed evaluation of third trimester growth restriction based on individualized assessment and demonstrates that this pathological process evolves in different ways. As defined by the FGPS1, five distinct patterns in 70/73 {95.9%} of the cases were found. Qualitative and quantitative assessments of the anatomical parameters used in FGPS1 calculations [HC, AC, FDL, EWT] indicated that all parameters contributed to the five growth abnormality patterns but AC and EWT were most important. The primary characteristics of fetal growth restriction [onset, duration, magnitude] were similar except in Pattern 2, which had a late onset and short duration. Further research is needed to clarify the relationships between these patterns and physiologic change, placental biomarkers or adverse outcomes in small fetuses.
Acknowledgments
Rose Torno Chair at Mount Sinai Hospital, University of Toronto, to Professor John Kingdom. This research was supported (in part) by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS. R. Romero contributed to this work as part of his official duties as an employee of the United States Federal Government.
APPENDIX
Basic Characteristics of Growth Abnormalities
Third trimester fetal growth abnormalities were evaluated based on three primary characteristics of this dynamic process: age of onset, duration, and magnitude. These characteristics generally determine the effect of the growth abnormality on secondary phenomena such as physiological status and perinatal outcomes. The development of a Fetal Growth Abnormality Score [FGAS] provides a means for estimating these three characteristics. For this investigation, the following estimation procedures were used to quantify fetal growth pathology in this manner:
Age of Onset
Calculation of the FGAS utilizes pathological Percent Deviations [%Devp], statistics that have assigned values of zero if the %Dev values are within their reference ranges (16). Therefore, in serial measurements of the FGPS, a non-zero value indicates that growth pathology has started. If a previous FGPS value is zero, the pathological process started at some time point between the two scans. Since the actual age at onset cannot be known, two methods of estimation have been defined. If there is a previous zero value for the FGPS and the interval between scans is four weeks or less, the age of onset is defined as the age at the mid-point between scans. If there is no previous zero FGPS value or the interval between scans is more than four weeks, the age of onset is considered to be two weeks before the age at which the first non-zero FGPS value was obtained.
Duration
With the age of onset defined, the duration can be estimated if the end of the growth abnormality can be determined. Clearly, a fetal growth abnormality ends with delivery. Previous studies (16) have found evidence of continued growth abnormalities in the last week [non-zero individual composite Prenatal Growth Assessment Score {icPGAS} values] before delivery or no such evidence [zero icPGAS values]. Since the actual age at the end of the growth abnormality cannot be known, three methods of estimation have been defined. If the icPGAS value is zero at a given scan and there are no subsequent non-zero icPGAS values, the age at this scan is taken as the end of the growth abnormality. If the icPGAS is non-zero and the last-scan-to-delivery is less than two weeks, the fetal age at delivery is taken as the end of the growth abnormality. For last-scan-to-delivery intervals of more than two weeks, the end age is two weeks after the age at the last scan. The duration of the growth abnormality is the difference between the estimates of the onset and end ages.
Magnitude
In IGA, the magnitude of 3rd trimester growth pathology for a specified anatomical parameter is the average %Devp value calculated over all 3rd trimester time points [apPGAS]. For a set of anatomical parameters at a given time point, it is the average of the %Devp values obtained at that time point for the set of anatomical parameters [icPGAS] (19). To be consistent with these other definitions, the Magnitude of a growth abnormality is estimated from the average of the icPGAS values obtained for all time points included in the time interval specified by the Duration of the growth abnormality.
The estimates of onset, duration and magnitude defined above are logical and consistent with previous IGA definitions but are still arbitrary. However, this study indicates that results obtained using these definitions are reasonable. Further investigation is required before it can be determined if they will need revision.
Footnotes
Disclosure statement: None of the other authors have disclosed a conflict of interest.
References
- 1.American College of Obstetricians and Gynecologists. ACOG Practice bulletin no. 134: fetal growth restriction. Obstet Gynecol. 2013;121:1122–1133. doi: 10.1097/01.AOG.0000429658.85846.f9. [DOI] [PubMed] [Google Scholar]
- 2.Sharma D, Farahbakhsh N, Shastri S, Sharma P. Intrauterine growth restriction – part 2. J Matern Fetal Neonatal Med. 2016;29:4037–4018. doi: 10.3109/14767058.2016.1154525. [DOI] [PubMed] [Google Scholar]
- 3.Murray E, Fernandes M, Fazel M, Kennedy SH, Villar J, Stein A. Differential effect of intrauterine growth on childhood neurodevelopment: a systematic review. BJOG. 2015;122:1062–1072. doi: 10.1111/1471-0528.13435. [DOI] [PubMed] [Google Scholar]
- 4.Figueras F, Gardosi J. Intrauterine growth restriction: new concepts in antenatal surveillance, diagnosis and management. Am J Obstet Gynecol. 2011;204:288–300. doi: 10.1016/j.ajog.2010.08.055. [DOI] [PubMed] [Google Scholar]
- 5.Nyberg DA, Abuhamad A, Ville Y. Ultrasound assessment of abnormal fetal growth. Sem Perinatol. 2004;28:3–22. doi: 10.1053/j.semperi.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 6.McIntire DD, Bloom SL, Casey BM, Leveno KJ. Birth weight in relation to morbidity and mortality among newborn infants. N Engl J Med. 1999;340:1234–1238. doi: 10.1056/NEJM199904223401603. [DOI] [PubMed] [Google Scholar]
- 7.Hernandez-Andrade E. Optimal strategies for managing fetal growth restriction. Minerva Ginecol. 2015;67:47–63. [PubMed] [Google Scholar]
- 8.Unterscheider J, Daly S, Geary MP, Kennelly MM, McAuliffe FM, O’Donoghue K, Hunter A, Morrison JJ, Burke G, Dicker P, Tully EC, Malone FD. Optimizing the definition of intrauterine growth restriction: the multicenter prospective PORTO study. Am J Obstet Gynecol. 2013;208:290.e1–e6. doi: 10.1016/j.ajog.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 9.Unterscheider J, Daly S, Geary MP, Kennelly MM, McAuliffe FM, O’Donoghue K, Hunter A, Morrison JJ, Burke G, Dicker P, Tully EC, Malone FD. Predictable progressive Doppler deterioration in IUGR: does it really exist? Am J Obstet Gynecol. 2013;209:539.e1–e7. doi: 10.1016/j.ajog.2013.08.039. [DOI] [PubMed] [Google Scholar]
- 10.Deter RL. Individualized Growth Assessment: evaluation of growth using each fetus as its own control. Semin Perinatol. 2004;28:23–32. doi: 10.1053/j.semperi.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 11.Deter RL, Spence L. Identification of macrosomic, normal and intrauterine growth retarded neonates using the modified Neonatal Growth Assessment Score. Fetal Diagn Ther. 2004;19:58–67. doi: 10.1159/000074262. [DOI] [PubMed] [Google Scholar]
- 12.Deter RL, Stefos T, Harrist RB, Hill RM. Detection of intrauterine growth retardation in twins using individualized growth assessment: II. Evaluation of third-trimester growth and prediction of growth outcome at birth. J Clin Ultrasound. 1992;20:579–585. doi: 10.1002/jcu.1870200903. [DOI] [PubMed] [Google Scholar]
- 13.Deter RL, Xu B, Milner LL. Prenatal prediction of neonatal growth status in twins using individualized growth assessment. J Clin Ultrasound. 1996;24:53–59. doi: 10.1002/(SICI)1097-0096(199602)24:2<53::AID-JCU1>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 14.Papageorghiou AT, Ohuma EO, Altman DG, et al. International standards for fetal growth based on serial ultrasound measurements: the Fetal Growth Longitudinal Study of the INTERGROWTH-21st Project. Lancet. 2014;384:869–879. doi: 10.1016/S0140-6736(14)61490-2. [DOI] [PubMed] [Google Scholar]
- 15.Deter RL, Lee W, Sangi-Haghpeykar H, Li J, Tarca AL, Yeo L, Romero R. Personalized third trimester fetal growth evaluation: Comparison of individualized growth assessment, percentile line and conditional probability methods. J Matern Fetal Neonatal Med. 2016;29:177–185. doi: 10.3109/14767058.2014.995083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deter RL, Lee W, Kingdom JCP, Romero R. Fetal growth pathology score: A novel ultrasound parameter for individualized assessment of third trimester growth abnormalities. J Matern Fetal Neonatal Med. 2017 Mar 20;:1–11. doi: 10.1080/14767058.2017.1300646. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deter RL, Levytska K, Lee W, Melamed N, Kingdom JCP. Classifying neonatal growth outcomes: Use of birth weight, placental evaluation and individualized growth assessment. J Matern Fetal Neonatal Med. 2016;29:3939–3949. doi: 10.3109/14767058.2016.1157576. [DOI] [PubMed] [Google Scholar]
- 18.Deter RL, Lee W, Sangi-Haghpeykar H, Tarca AL, Yeo L, Romero R. Individualized fetal growth assessment: Critical evaluation of key concepts in the specification of third trimester growth trajectories. J Matern Fetal Neonatal Med. 2014;27:537–542. doi: 10.3109/14767058.2013.833904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deter RL, Lee W, Sangi-Haghpeykar H, Tarca AL, Yeo L, Romero R. A modified prenatal growth assessment score for the evaluation of fetal growth in the third trimester using single and composite biometric parameters. J Matern Fetal Neonatal Med. 2015;28:745–754. doi: 10.3109/14767058.2014.934218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deter RL, Lee W, Sangi-Haghpeykar H, Tarca AL, Yeo L, Romero R. Fetal growth cessation in late pregnancy: Its impact on predicted size parameters used to classify small for gestational age neonates. J Matern Fetal Neonatal Med. 2015;28:755–765. doi: 10.3109/14767058.2014.934219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harris RJ. A primer of multivariate statistics. Academic Press; Orlando: 1985. pp. 147–199. [Google Scholar]
- 22.von Ehrenstein OS, Mikolajczyk RT, Zhang J. Timing and trajectories of fetal growth related to cognitive development in childhood. Am J Epidemiol. 2009;170:1388–1395. doi: 10.1093/aje/kwp296. [DOI] [PubMed] [Google Scholar]
- 23.Barker ED, McAuliffe FM, Alderdice F, Untersheider J, Daly S, Geary MP, Kennelly MM, O’Donoghue K, Hunter A, Morrison JJ, Burke G, Dicker P, Tully EC, Malone FD. Growth trajectories in classifying fetal growth restriction. Obstet Gynecol. 2013;122:248–254. doi: 10.1097/AOG.0b013e31829ca9a7. [DOI] [PubMed] [Google Scholar]
- 24.Benton SJ, McCowan LM, Heazell AEP, Grynspan D, Hutcheon JA, Senger C, Burke O, Chan Y, Harding JE, Yockell-Lelievre J, Hu Y, Chappell LC, Griffin MJ, Shennan AH, Magee LA, Gruslin A, von Dadelszen P. Placental growth factor as a marker of fetal growth restriction caused by placental dysfunction. Placenta. 2016;42:1–8. doi: 10.1016/j.placenta.2016.03.010. [DOI] [PubMed] [Google Scholar]


