Abstract
The role of the pathologist in the multidisciplinary management of women with gynecologic cancer has evolved substantially over the past decade. Pathologists’ evaluation of parameters such as pathologic stage, histologic subtype, grade and microsatellite instability, and their identification of patients at risk for Lynch syndrome have become essential components of diagnosis, prognostic assessment and determination of optimal treatment of affected women.
Despite the use of multimodality treatment and combination cytotoxic chemotherapy, the prognosis of women with advanced-stage gynecologic cancer is often poor. Therefore, expanding the arsenal of available systemic therapies with targeted therapeutic agents is appealing. Anti-angiogenic therapies, immunotherapy and poly ADP ribose polymerase (PARP) inhibitors are now routinely used for the treatment of advanced gynecologic cancer, and many more are under investigation. Pathologists remain important in the clinical management of patients with targeted therapy, by identifying potentially targetable tumors on the basis of their pathologic phenotype, by assessing biomarkers that are predictive of response to targeted therapy (e.g. microsatellite instability, PD1/PDL1 expression), and by monitoring treatment response and resistance. Pathologists are also vital to research efforts exploring novel targeted therapies by identifying homogenous subsets of tumors for more reliable and meaningful analyses, and by confirming expression in tumor tissues of novel targets identified in genomic, epigenetic or other screening studies.
In the era of precision gynecologic oncology, the roles of pathologists in the discovery, development and implementation of targeted therapeutic strategies remain as central as they are for traditional (surgery-chemotherapy-radiotherapy) management of women with gynecologic cancers.
Keywords: Cancer, Cervix, Endometrium, Gynecologic cancer, Ovary, Pathology, Precision medicine, Targeted therapy, Treatment
INTRODUCTION
In 2017 in the USA, it is estimated that 107,470 women will be diagnosed with gynecologic cancers, and that 31,600 women will die of gynecologic tumors (Table 1) [1]. This corresponds to 12.6% and 11.2%, respectively, of all cancers in women. The traditional management of women with gynecologic cancer largely rests upon surgery, cytotoxic chemotherapy and radiotherapy, singly or in combination as dictated by the clinical circumstances, with the stage of disease largely determining the need for adjuvant or first-line chemotherapy or radiation. In those with recurrent disease, the choice of cytotoxic chemotherapy is generally most dependent upon time since last platinum-based chemotherapy, with the platinum-free interval determining platinum sensitivity versus resistance. More recently, ever-increasing numbers of targeted therapies directed against a variety of molecular targets in gynecologic cancers and their microenvironments are being developed and used in women with these malignancies.
Table 1.
Estimated numbers of gynecologic tract cancers in 2017 (adapted from 1)
| Estimated new cases | Estimated deaths | |
|---|---|---|
| Ovary | 22,440 | 14,080 |
| Uterine corpus | 61,380 | 10,920 |
| Cervix | 12,820 | 4,210 |
| Vagina | 4,810 | 1,240 |
| Vulva | 6,020 | 1,150 |
| Total | 107,470 | 31,600 |
TRADITIONAL ROLES OF PATHOLOGY IN TREATMENT OF GYNECOLOGIC CANCERS
Pathologists have long played a central role in the multidisciplinary management of patients with gynecologic cancer by providing fundamental items of risk-stratification information that guide optimal treatment, such as pathologic stage of disease, histologic subtype and grade [2].
Pathologists are also key to assessment of other parameters that are useful in management. An important example of this is pathologic evaluation of DNA mismatch repair deficiency in endometrial cancer, which has become part of the standard of care for women with these tumors. DNA mismatch repair defects are found in 25–30% of endometrial cancers, and lead to a high-level microsatellite instability (MSI-H) phenotype [3–5]. A few MSI-H endometrial cancers are associated with Lynch syndrome-associated germline alterations in DNA mismatch repair genes (MLH1, PMS2, MSH2, MSH6) or EPCAM, but the majority are due to a sporadic epigenetic change, namely hypermethylation of the promoter region of MLH1, which leads to gene silencing.[3, 6, 7] Both germline and sporadic alterations are associated with loss of expression of protein products of the affected genes [3, 5]. Patients with tumors that exhibit loss of expression of MLH1/PMS2 by immunohistochemistry but which lack MLH1 promoter hypermethylation are likely to harbor germline MLH1 mutations as seen in Lynch syndrome. Should the presence of a MLH1 germline mutation be confirmed, they and their family members would be at increased risk for Lynch-syndrome-associated malignancies, and would require exploration of these risks, including personal and familial genetic counseling and consideration of increased cancer screening. In contrast, tumors that exhibit loss of expression of MLH1/PMS2 by immunohistochemistry and which show MLH1 promoter hypermethylation are likely to be sporadically hypermethylated tumors, and women with these tumors and their families do not have increased cancer risk [8, 9].
TARGETED THERAPY
During the past decade, there have been changes in histologic classification that affect surgical management, adjuvant therapies and prognostic assessment; recognition of areas of diagnostic difficulty (such as histologic subtyping of high-grade endometrial carcinomas); and discovery of molecular genetic alterations and genetically defined prognostic subgroups of gynecologic cancer. Most patients with early stage gynecologic cancer (when many endometrial cancers are diagnosed) have good clinical outcomes. Nevertheless, between 1987 and 2008, it is believed that the number of women who died from endometrial cancer in the US increased substantially, while relative survival has declined over the past decade [10]. The clinical course in patients with advanced-stage gynecologic cancer (which is frequent in ovarian cancer) is often aggressive and the prognosis is poor, despite the use of combination cytotoxic chemotherapy. Furthermore, many ovarian cancer patients who initially respond to chemotherapy suffer tumor recurrence and progressive resistance to therapy [11]. Therefore, there is a need for more effective therapeutic modalities for women with gynecologic cancers, in particular for those with advanced-stage disease.
Targeted therapies are a cornerstone of precision medicine, in which individual patients are treated using agents targeting molecules identified in that patient’s cancer or in the tumor’s microenvironment. Molecular targets include tumor-intrinsic signaling pathways, and aberrations in these pathways may result in expression of mutant/altered proteins, overexpression or loss of expression of normal proteins, and novel fusion proteins resulting from gene rearrangements. Other potential therapeutic targets in gynecologic cancers include homologous recombination deficiency, hormone receptors, angiogenesis and immunologic factors (Table 2).
Table 2.
A selected summary of targeted therapy in gynecologic cancer
| Targets | Class | Examples |
|---|---|---|
| Signaling pathways | PI3K/AKT/mTOR inhibitors MAPK inhibitors JAK1/JAK2 inhibitors NTRK/ROS1/ALK inhibitors |
Temsirolimus Trametinib* Ruxolitinib* Entrectinib* |
| Homologous recombination deficiency | PARP inhibitors | Olaparib, niraparib, rucaparib, veliparib* |
| Hormone receptors | Progesterone receptor Estrogen receptor Gonadotropin-releasing hormone agonists Androgen receptor |
Progestins Tamoxifen, aromatase inhibitors Leuprolide Enzalutamide* |
| Angiogenesis | Anti-VEGF/VEGFR | Bevacizumab, cediranib |
| Immunologic factors | Immune checkpoint inhibitors (e.g. anti- PD1 and anti-CTLA4) Adaptive T cells Vaccines |
Pembrolizumab, nivolumab*, ipilimumab* |
undergoing investigation
TARGETED THERAPY FOR GYNECOLOGIC CANCER
Several targeted therapies are currently available and approved by the US Food and Drugs Administration (FDA) and the European Medicines Agency (EMA) for patients with gynecologic cancer.
Anti-angiogenesis agents, such as bevacizumab (a monoclonal antibody which targets VEGF-A), have been shown to be effective in ovarian cancer when used in conjunction with standard platinum-based chemotherapy [12–15]. Recently, the FDA and EMA approved bevacizumab in combination with paclitaxel plus either cisplatin or topotecan as a treatment for patients with persistent, recurrent or metastatic cervical cancer, based on the extension of overall survival in the GOG-240 study [16]. Bevacizumab is FDA-approved for platinum-resistant ovarian cancer in combination with liposomal doxorubicin, topotecan, or paclitaxel based on results of the AURELIA trial [15]. It is FDA-approved for platinum-sensitive ovarian cancer in combination with carboplatin and gemcitabine based on results of the OCEANS trial [14] and also in combination with carboplatin and paclitaxel based on results of the GOG-0213 trial [17]. Bevacizumab has also shown promising activity in patients with low-grade serous ovarian cancer, a subtype of ovarian cancer that commonly has low response rates to conventional chemotherapy [18].
Poly ADP ribose polymerase (PARP) inhibition of cells containing a defect in homologous recombination pathways (e.g. those with BRCA1/2 mutations) results in the death of target tumor cells while sparing normal cells. Recently, both the FDA and EMA approved olaparib, a PARP inhibitor, as effective maintenance therapy in patients with platinum-sensitive ovarian cancer who are in complete or partial response following platinum-based chemotherapy; this follows its original approval in 2014 for the treatment of patients with deleterious or suspected deleterious germline BRCA-mutated advanced ovarian cancer who have been treated with three or more lines of chemotherapy [19]. Other FDA-approved PARP inhibitors that have shown objective responses include rucaparib (for patients with deleterious germline and/or somatic BRCA mutation-associated advanced ovarian cancer who have been treated with two or more chemotherapies [20]) and niraparib (for maintenance treatment of adult patients with recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer who are in complete or partial response to platinum-based chemotherapy [21]).
In endometrial cancer, POLE-mutated and mismatch repair deficient tumors have higher neoantigen load, increased tumor-infiltrating lymphocytes, and increased expression of several immune checkpoint genes [22, 23]. Immune checkpoint regulators such as programmed death receptor 1 (PD1) promote escape from tumor immune surveillance, and 80% of endometrial cancers express high levels of PD1, or its ligand, PDL1 [24]. Data suggest that POLE-mutated and mismatch repair deficient endometrial tumors might be excellent candidates for PD1-directed immune therapies [22]. Immune checkpoint blockade with the antibody pembrolizumab to PD-1 has shown responses in patients with POLE-mutated[23] and mismatch repair deficient cancers, including endometrial cancer [25]. Pembrolizumab has been approved by the FDA for metastatic cancers exhibiting mismatch repair deficiency, regardless of their histologic subtype. In endometrial cancer patients, pembrolizumab shows durable anti-tumor activity in a subset [26], including rare exceptional responses [23].
Endocrine therapy with progestins and tamoxifen for endometrial cancer is associated with overall response rates reported to range between 9% and 55% (summarized in [27]). Even though patients with lower grade tumors express estrogen and/or progesterone receptors, the Cochrane review in 2010 reported no improvement in survival in women with advanced endometrial cancer who received hormone therapy [27]. Hormonal therapy (LHRH agonists, tamoxifen, aromatase inhibitors) for ovarian cancer has been evaluated in small trials, which have reported inconsistent results [28–30]. A Cochrane review of hormonal therapy in women with ovarian cancer reported overall response rates ranging from 0% to 56%, but there was insufficient data to analyze duration of response or survival [31]. The data in both Cochrane reviews were limited by the lack of any large randomized trials.
In addition to the aforementioned approved agents, several targeted therapies are currently under investigation or undergoing trials in patients with gynecologic cancers. A detailed description of these agents is beyond the scope of this review, but a selected few are summarized in Table 2. One of the challenges for many targeted therapies in advanced gynecologic cancers is the large number of genetic alterations which is often seen in advanced tumors [32] and which worsens due to selective pressures during tumor progression, especially following therapy. This phenomenon may result in derangements in multiple oncogenic or tumor-promoting pathways, and raises doubts that targeted agents directed at one or two genetic alterations would significantly alter outcomes. The acquisition of epigenetic alterations in tumors and adaptations of the tumor microenvironment compound the challenges in achieving durable responses with targeted agents. In attempts to circumvent this problem, combinations of agents targeting different pathways, or combinations of targeted agents with cytotoxic chemotherapy or immunotherapy are also undergoing trials, in addition to novel single therapeutic agents.
ROLES OF PATHOLOGY IN TARGETED THERAPY OF GYNECOLOGIC CANCERS
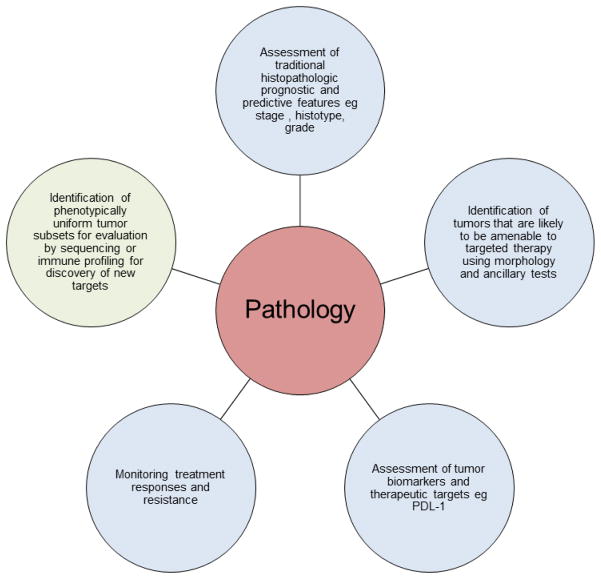
With the development and implementation of increasing numbers of targeted and other novel therapies, pathologists continue to have important roles in multidisciplinary teams managing patients with gynecologic cancer (Fig. 1).
Figure 1.
An overview of the roles of pathology in the management of women with gynecologic cancer.
Pathology and patient management
Certain pathologic features of gynecologic cancers are associated with genotype and underlying pathogenetic mechanisms, which may serve as targets for therapeutic agents. Identification by pathologists of these phenotypic features, a few examples of which are briefly discussed below, can be very helpful by focusing confirmatory testing of tumors that may be amenable to targeted agents.
Mismatch repair-deficient gynecologic tumors
Mismatch repair deficiency, in the setting of Lynch syndrome and occurring sporadically, has been reported in endometrial [4, 5, 33] and ovarian [33–35] carcinomas. In addition to the importance of pathologists screening endometrial cancers for mismatch repair deficiency to identify those women who are at risk for Lynch syndrome (as described earlier), this screen is also likely to become increasingly important for planning treatment. A recent study showed that microsatellite instability analysis is effective as a predictive biomarker for the effect of immune checkpoint inhibitors, including anti-PD1 antibody and anti-PDL1 antibody.16 MSI-H endometrial cancers show higher neoepitope levels and higher expression of PD-1 and PDL-1, when compared with microsatellite-stable cancer,13 and MSI-H ovarian clear cell carcinomas also show elevated expression of PDL-1 [35]. This suggests that microsatellite instability analysis may be a useful predictive biomarker for response to immunotherapy.
Histopathologic features that are significantly more commonly associated with MSI-H endometrial cancers than microsatellite-stable tumors include localization in the lower uterine segment, low-grade endometrioid histology, mucinous differentiation, tumor-infiltrating lymphocytes and peritumoral lymphocytes [9, 36–38]. Mucinous differentiation and tumor-infiltrating lymphocytes [38], and undifferentiated histology in a subset of cases [9] are features that characterize endometrial carcinomas with sporadic MLH1 promoter methylation. One study found that tumor-infiltrating lymphocyte counts of >40 per 10 high-power fields were associated with sensitivity and specificity of 85% and 46%, respectively in predicting microsatellite instability status in endometrioid endometrial carcinomas [36]. These features are readily assessable in routine histologic sections, and are helpful in guiding focused microsatellite instability testing of tumors harboring these morphologic hallmarks. In the same study, morphologic heterogeneity (presence of two or more clearly separate morphologic patterns, each constituting at least 10% of the tumor) was more frequent in microsatellite-unstable tumors, but the difference did not reach statistical significance [36].
Mismatch repair deficiency has been reported in approximately 10% of ovarian carcinomas [34, 39] of endometrioid [40], clear cell [35] and serous [33] histologic types. Although specific histologic features of ovarian endometrioid adenocarcinomas in one study did not correlate with mismatch repair status [40], a recent study of mismatch repair deficient clear cell carcinomas of ovary showed significantly higher number of CD8-positive and PD1-positive tumor-infiltrating lymphocytes with higher CD8+/CD4+ ratios compared with microsatellite stable tumors [35].
Microsatellite instability can also be assessed in histologic sections using immunohistochemistry. Detection of DNA mismatch repair deficiencies by immunohistochemistry using antibodies directed against MLH1, PMS2, MSH2 and MSH6 can effectively diagnose microsatellite instability in endometrial carcinomas [3, 5]. Indeed, many institutions have already implemented universal testing for DNA mismatch repair deficiency in women with newly diagnosed endometrial cancer using immunohistochemistry. Immunonohistochemistry is readily available and interpretable, both in specialized/tertiary institutions as well as smaller community hospitals and pathology laboratories. In larger institutions, there is a trend toward universal genomic profiling (including PCR-based microsatellite instability analysis) of newly diagnosed endometrial cancer. Concordance between the results of immunohistochemistry and PCR-based microsatellite instability analysis is high [41], and immunohistochemistry, allied with histopathologic assessment of microsatellite instability-associated morphological features, is a useful and cost-effective means of screening tumors for microsatellite instability. These approaches can rapidly identify those tumors that may be susceptible to immune checkpoint inhibitors. Expression of PDL1 can also be assessed by immunohistochemistry. However, this is an area of active study, and varying results have been reported with different antibodies, staining platforms and scoring criteria [42]. Until these elements are standardized after rigorous testing and validation in gynecologic cancers, it would be premature to use PDL1 immunohistochemistry as part of clinical management, except in the setting of investigative studies and clinical trials with clearly established parameters.
Gynecologic tumors with POLE mutations
The seminal Cancer Genome Atlas study described four major genomically-defined groups of endometrial cancers (POLE ultramutated, MSI hypermutated, copy-number-low, and copy-number-high). These groups were also clinically significant, as they correlated with progression-free survival, with POLE-mutated tumors having an excellent prognosis [43]. A subset of primary ovarian carcinomas also harbor POLE mutations [44–47].
Ovarian carcinomas with POLE mutations are characterized by endometrioid histology and rare cases show morphologic heterogeneity [44–46]. Features of endometrial carcinomas harboring POLE mutations that help in their identification are: occurrence in younger women; high grade; frequent lymphovascular space invasion; frequent endometrioid histology; conspicuous tumor-infiltrating lymphocytes and/or peri-tumoral lymphocytes, morphologic heterogeneity/ambiguity and bizarre/giant tumor cell nuclei [48, 49]. POLE mutations have also been reported in endometrial clear cell carcinomas [50], undifferentiated carcinomas [51] and carcinosarcomas [52]. Algorithms for identifying patients for adjuvant chemotherapy and radiation therapy rely on tumor grade, stage and lymphovascular space invasion, and based on these criteria, a significant proportion of patients with POLE-mutated tumors would receive adjuvant therapy [49]. However, their apparently high-risk characteristics belie their excellent outcomes [43]. This raises the possibility that aggressive adjuvant chemoradiation for these tumors may not be required.
In addition to their selection for immunotherapy (as discussed earlier), identification of patients with POLE-mutated tumors may be important to avoid over-treatment. The Leiden [53] and Vancouver [54] groups have proposed diagnostic algorithms for molecular classification of endometrial cancers. The Vancouver group algorithm is particularly applicable to diagnostic pathology specimens; it involves, in sequence, immunohistochemistry for DNA mismatch repair proteins, sequencing of mismatch-repair-intact tumors for POLE mutations, and immunohistochemistry for p53 in the POLE-wild-type tumors. This algorithm accurately classifies tumors as mismatch repair-deficient (MSI-H), POLE-mutated, p53-wild type (copy-number-low) and p53-aberrant (copy-number-high) [41, 54], and allied with morphologic assessment, may represent a useful means of classifying endometrial carcinomas into genomically distinct and clinically relevant subgroups.
Ovarian carcinomas associated with homologous recombination deficiency
Aberrations of homologous recombination repair are identified in approximately half of all high-grade serous carcinomas. Homologous recombination deficiency may be due to germline or somatic mutations in BRCA1/BRCA2, as well as mutations in genes such as ATM, BRIP1, CHEK2, RAD51 and PALB2 [55, 56]. Compared with homologous recombination-competent tumors, homologous recombination-deficient ovarian carcinomas are associated with significantly more frequent variant (solid, endometrioid, and transitional cell carcinoma, SET-like) morphology, greater mitotic activity, more tumor-infiltrating lymphocytes, and more frequent necrosis, and associated with a lower frequency of serous tubal intraepithelial carcinoma, younger patient age and improved survival. SET features are also more common in BRCA2-mutant tumors [57–59]. Pathologic recognition of homologous recombination-deficient tumors can be useful in identifying patients who might benefit from PARP inhibitors.
Tumors associated with mitogen-activated protein kinase (MAPK) pathway activation
Mutations in the mitogen-activated protein kinase (MAPK) pathway are commonly observed in gynecologic cancers. This pathway is potentially targetable using agents such as BRAF inhibitors and MEK inhibitors [60, 61].
KRAS mutations are associated with mucinous differentiation. KRAS mutations are common in endometrial cancer [43], and endometrial cancers with mucinous differentiation appear to be more frequently associated with KRAS mutations [62]. Primary ovarian mucinous carcinomas also frequently harbor KRAS mutations and ERBB2 amplifications [63]; the latter are also identified in endometrial serous carcinomas [64]. Although KRAS is not a direct molecular therapeutic target, identification of tumors that are likely to harbor KRAS mutations might make them amenable to therapy directed against other components of the MAPK/ERK pathway, such as members of the EGFR family.
KRAS and BRAF mutations have been reported in 17–36% [65–67] and 30–45% [66–68], respectively, of serous borderline tumors. Low-grade serous carcinomas of the ovary also harbor mutations in KRAS (16–41%) as well as BRAF (<10%). Tumors with V600E BRAF mutations have been found to have a better prognosis than those with wild-type BRAF [67–70]. These tumors can also bear other alterations that result in MAPK pathway activation [61]. While a phase II study of the MEK inhibitor selumetinib in patients with advanced low-grade serous ovarian cancer showed promising results, a phase III study comparing the MEK inhibitor binimetinib with physicians’ choice of chemotherapy (the MILO study [71]) recently closed after a planned interim analysis showed that the hazard ratio for progression-free survival crossed the predefined futility boundary. Additional analyses are ongoing in order to determine if any molecular biomarkers were associated with response in the patients treated in that study.
BRAF-mutated serous borderline tumors are characterized by a distinctive subpopulation of cells with abundant eosinophilic cytoplasm, well-defined cell borders and bland nuclei. In addition, the tumors exhibit cuboidal and columnar cells that line papillae and bud from their surfaces, leading to the appearance of individual cells and clusters of detached cells above the papillae [72]. The eosinophilic cells are highly correlated with BRAF mutation status in serous borderline tumors but are seen only in rare BRAF-mutated low-grade serous carcinomas [72]. Immunohistochemistry using a monoclonal antibody (VE1) specific for the BRAF V600E protein has been shown to be sensitive and specific for the detection of BRAFV600E mutation in ovarian serous tumors [73]. The eosinophilic cells in serous borderline tumors also exhibit markers of senescence [72], and the comparatively low frequency of BRAF mutations in low-grade serous carcinomas may indicate that BRAF mutation in serous borderline tumors identifies tumors at low risk of progression.
Value of pathologists in integrating genotype and phenotype for optimal patient management
Pathologists are ideally positioned to synthesize the clinico-pathologic phenotype with genomic data from molecular pathology. For instance, in the setting of high-grade adnexal serous carcinomas, accurate pathologic classification and assessment of morphologic features can help identify the presence of homologous recombination deficiency, suggest the need for germline testing for BRCA1/BRCA2 mutations, provide an indication of chemosensitivity to conventional agents, and offer the option of PARP inhibitors or clinical trials for other suitable targeted therapies. Other settings in which pathology-driven genotype-phenotype correlations are important in patient management are listed in Table 3.
Table 3.
Pathologic-molecular correlations in gynecologic cancers and their implications for patient management
| Histopathologic finding(s) | Ancillary pathologic test(s) (IHC/ISH) | Associated molecular abnormality | Intervention/Management implications |
|---|---|---|---|
| Endometrial: Lower uterine segment location; endometrioid or undifferentiated histotype; mucinous differentiation; tumor-infiltrating lymphocytes; peri-tumoral lymphocytes Ovarian: endometrioid or clear cell histotype; tumor- infiltrating lymphocytes |
Immunohistochemistry for MLH1, MSH2, MSH6, PMS2; MLH1 promoter methylation analysis | DNA mismatch repair deficiency due to: 1) germline mutations in MLH1, MSH2, MSH6, PMS2 or EPCAM 2) somatic mutations or MLH1 promoter methylation |
1) Genetic counseling and family screening for Lynch syndrome 2) Consider immune therapy e.g. checkpoint inhibitors for recurrent disease |
| Endometrial: High grade; frequent lymphovascular space invasion; endometrioid, clear cell, undifferentiated or carcinosarcoma histotype; conspicuous tumor- infiltrating lymphocytes and/or peri-tumoral lymphocytes; morphologic heterogeneity/ambiguity; bizarre/giant tumor cell nuclei Ovarian: endometrioid histotype; morphologic heterogeneity |
POLE mutations | Consider immune therapy e.g. checkpoint inhibitors for recurrent disease Consideration of avoidance of overtreatment in adjuvant setting |
|
| Ovarian: Variant (solid, endometrioid, and transitional cell carcinoma, SET-like) morphology; tumor-infiltrating lymphocytes; necrosis | Homologous recombination deficiency e.g. BRCA1/2 mutations | Consider germline BRCA1/BRCA2 testing and PARP inhibitor therapy in recurrent setting | |
| Ovarian: Serous borderline tumors with eosinophilic cells; cuboidal and columnar cells that line papillae and bud from their surfaces | BRAF VE1 immunohistochemistry | MAPK pathway activation | Presence of V600E BRAF mutation may portend improved prognosis |
Pathology and clinical trials of targeted agents
Based on morphologic assessment and judicious ancillary testing for biomarkers, as detailed above, allied with limited sequencing, pathologists can help identify phenotypically and genotypically homogenous sets of tumors, and optimize the selection of patients with these tumors for entry into clinical trials of suitable targeted agents. The example of high-grade endometrial carcinomas highlights the benefits of such an integrated pathologic-genomic-clinical approach. In the past, high-grade endometrial cancers were treated as a homogenous group. Pathologic refinements in morphologic grading and classification of these tumors, initially into type I and type II tumors, and subsequently into specific histotypes (endometrioid, serous, clear cell) improved risk stratification and allowed the formulation of informed treatment algorithms for these patients. However, morphologic limitations became apparent, particularly in high-grade endometrial carcinomas, which are difficult to subclassify reproducibly due to ambiguities in their morphologic features [74]. For example, 82 tumors diagnosed as FIGO grade 3 endometrioid carcinoma in TCGA study of endometrial carcinoma [43] were subsequently reviewed independently by two specialist gynecologic pathologists, who re-classified 20–25% of the tumors as serous carcinoma. The subsequent discovery of genomic classes of endometrial carcinoma [43] and their associations with phenotypic features [36, 37, 48, 49] have revealed that morphologically high-grade tumors include copy-number-high, MSI-H and POLE-mutated subsets, each of which is associated with specific pathologic features and clinical implications, as described earlier. Given the challenges in reproducible classification of high-grade tumors in particular, pathologic review by specialist gynecologic pathologists would be valuable for accurate assignation of histotype. Integration of the pathologic phenotype with judicious use of selected biomarkers [54] allows subcategorization of high-grade endometrial carcinomas into biologically homogenous subgroups in which specific therapeutic targets can be explored in clinical trials.
Pathology in monitoring response and resistance in tumors treated with targeted agents
Primary and acquired resistance, driven by intratumor heterogeneity as well as other tumor-specific and tumor microenvironmental factors, has been documented in a variety of tumors and represents a key challenge to delivering enduring responses to targeted therapy [75, 76]. Pathologic evaluation of tumor responses to targeted agents in the course of clinical trials can help quantify tumor responses and changes in the tumor microenvironment post-treatment. Allied with ancillary techniques such as immunohistochemistry and in-situ hybridization, pathologists can potentially help identify additional biomarkers of tumor sensitivity and resistance to therapy. Furthermore, increasing experience with ancillary testing (e.g. flow cytometry and single-cell sequencing) of cytologic specimens offers the promise of using cytologic material, such as effusions or cervicovaginal material, to monitor treatment response and resistance [77, 78].
Pathology in research and discovery of novel therapeutic targets
In the research setting, there are numerous studies and clinical trials attempting to identify or to implement novel targets for therapy in patients with gynecologic cancers.
Pathologists can help explore expression of novel targets identified in genomic, epigenetic or other screening studies in tumor tissues, using techniques such as immunohistochemistry and in-situ hybridization. Confirmation of tissue expression of protein products of mutated genes, or localization of their expression in specific tissues or tissue compartments can provide insights into the biology and mechanisms of action of these molecules, which in turn can aid in tailoring agents directed against them for optimal efficacy.
Correlative analyses of genotype or epigenetic or immune profiles with pathologic phenotypes can be helpful in fine-tuning the application of novel targeted therapies, by selecting for those patients who are most likely to benefit from agents targeting specific alterations whose histopathologic correlates are identified in their tumors (as described above).
Based on histopathologic evaluation and judicious ancillary testing, pathologists can help identify phenotypically homogenous subsets of tumors for analysis, which facilitates reliable and reproducible interpretation of molecular and genomic findings. A recent example is the case of ovarian small cell carcinoma of hypercalcemic type; the uniform histopathologic phenotype of these tumors led to the hypothesis that they harbor a common driver mutation. Subsequent sequencing analyses identified recurrent driver mutations in SMARCA4 [79]. BRG1, the protein product of SMARCA4, is a component of the SWI/SNF chromatin remodeling complex, and EZH2 inhibitors may prove to be useful therapeutic agents for these tumors.
The computer science term ‘Garbage In, Garbage Out’ [80], which refers to the reliance of the quality of post-analytical data upon the quality of the input, applies to research studies of biomarkers and therapeutic targets. The importance of rigor in the pre-analytical selection of tumors for molecular genetic analyses and clinical trials is highlighted by the following examples. In the Cancer Genome Atlas analysis of ovarian carcinomas [55], 96% of high-grade serous carcinomas were reported to harbor TP53 mutations. In this study, cases were included based on the original pathology diagnosis. Although specimens were subsequently reviewed in a centralized laboratory, specific histologic criteria that were used were not reported. A subsequent review of 14 of 15 TP53-wild-type cases from this cohort found that 5 specialized gynecologic pathologists rendered a unanimous diagnosis of high-grade serous carcinoma in only one (7%) case, which was associated with BRCA1 germline mutation and a homozygous TP53 deletion [81]. The authors concluded that all de novo high-grade serous carcinomas contain TP53 somatic mutations or deletions, with the exception of rare tumors that develop from an antecedent low-grade serous tumor. They proposed that molecular alterations of TP53 are the sine qua non for a diagnosis of ovarian high-grade serous carcinoma [81]. Similarly, in the TCGA studies of cervical carcinomas, adenocarcinomas were analyzed together as a single group [82], or subdivided into adenocarcinoma, clear cell carcinoma and serous carcinoma [83]. Since it is recognized that HPV-negative adenocarcinomas (such as gastric-type adenocarcinomas) appear to be biologically, clinically and pathologically distinct from HPV-associated adenocarcinomas [84, 85], analysis of adenocarcinomas without subclassification based on HPV status may not yield results that are universally applicable to all patients with cervical adenocarcinomas. Furthermore, a subset of HPV-negative carcinomas in this study were characterized by mutations in KRAS, ARID1A and PTEN [82], which are commonly seen in endometrioid tumors; this raises the possibility that at least some of these tumors might represent endometrioid adenocarcinomas arising in the corpus or lower uterine segment rather than being primary cervical adenocarcinomas. These examples illustrates the critical importance of high quality pathology review to ensure pathobiologically-informed selection and phenotypic homogeneity of tumors for study, particularly when attempting to define genotypic or immunophenotypic biomarkers that may be subsequent therapeutic targets.
CONCLUSION
In the era of precision medicine, increasing numbers of targeted therapies are in clinical use and undergoing trials in patients with gynecologic cancer. As described above, the roles of pathologists in the discovery, development and implementation of these novel therapeutic strategies are as central as they are for traditional (surgery-chemotherapy-radiotherapy) management of women with gynecologic cancers. This underscores the importance of pathology as a key component of multidisciplinary approaches to research and deployment of targeted therapy and precision gynecologic oncology.
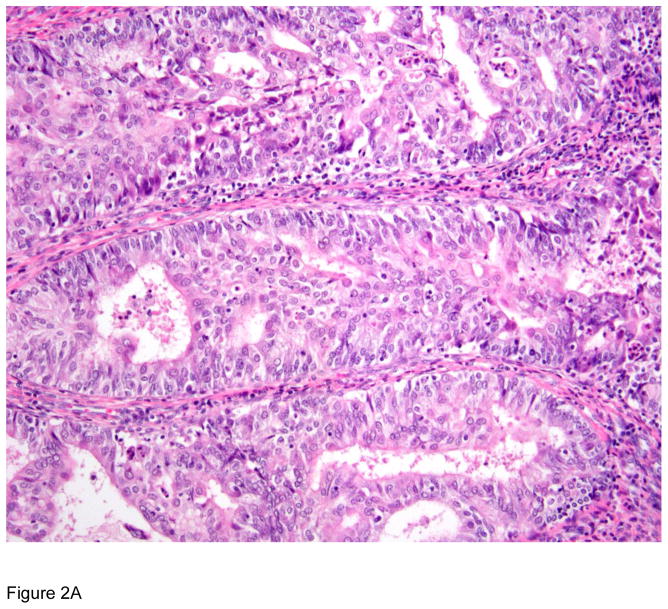
Figure 2.
Pathologic correlates of molecular/genetic alterations in gynecologic cancers. a) Mismatch repair deficient endometrial carcinoma exhibiting low-grade endometrioid histology, tumor-infiltrating lymphocytes (lymphocytes infiltrating neoplastic epithelium) and peritumoral lymphocytes (lymphocytes present adjacent to tumor). b) POLE-mutated endometrial carcinoma showing conspicuous tumor-infiltrating lymphocytes and peri-tumoral lymphocytes and bizarre/giant tumor cell nuclei.
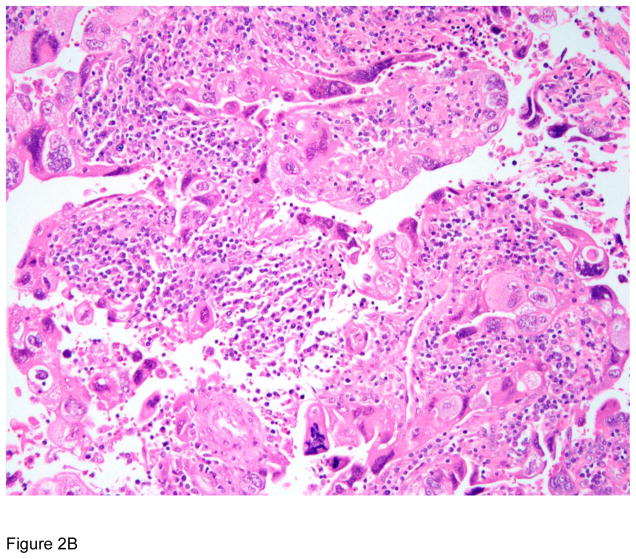
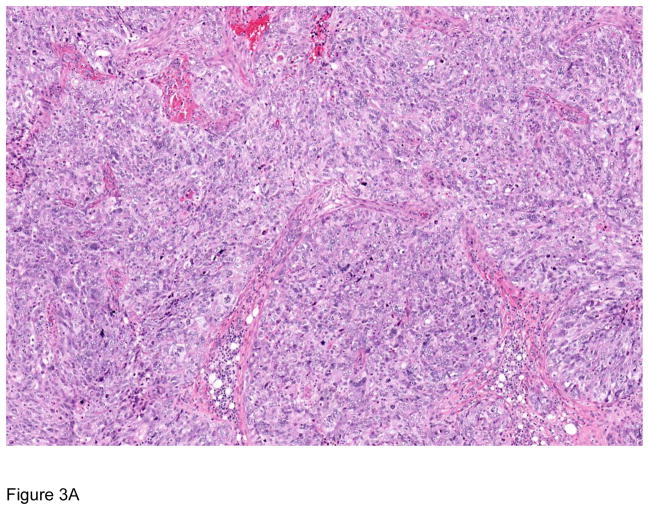
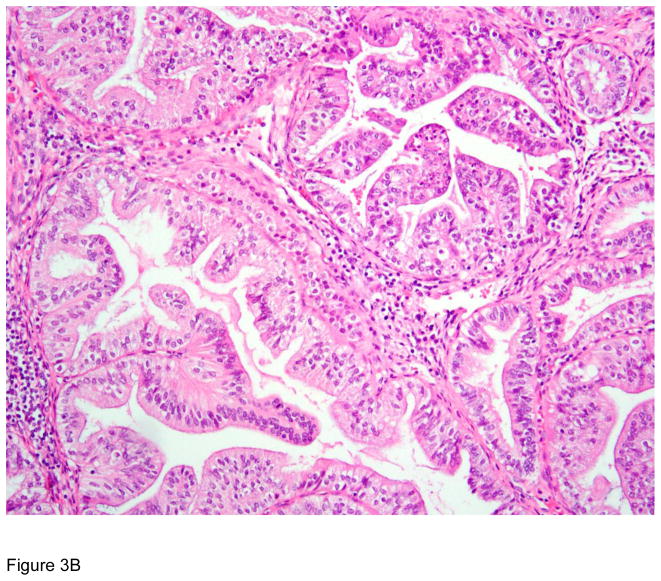
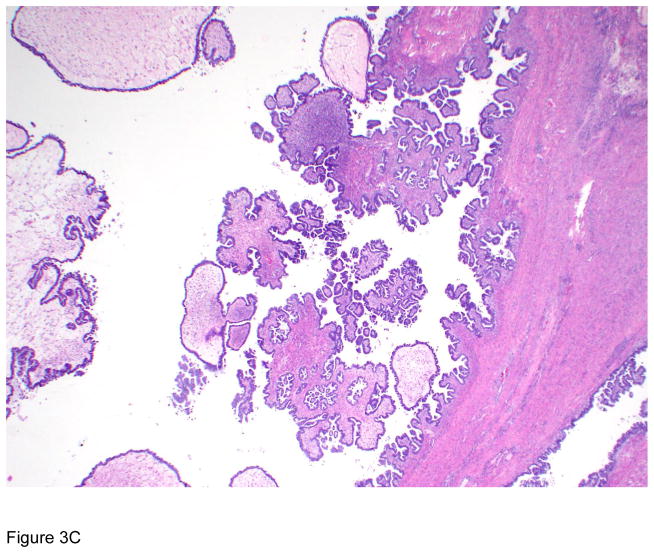
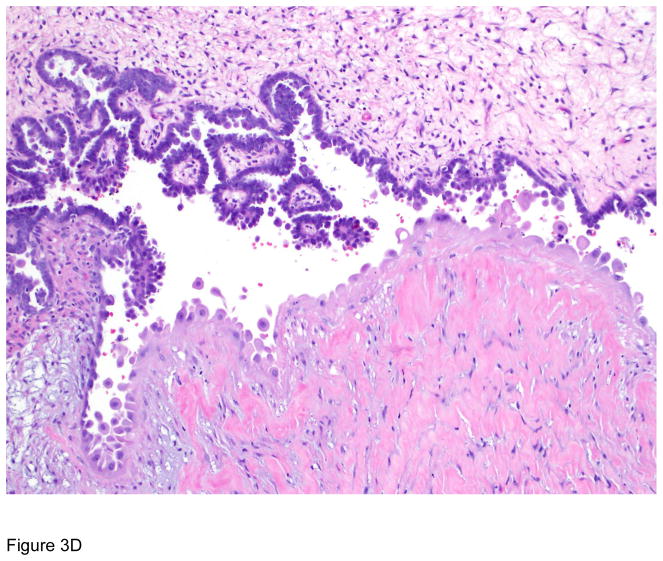
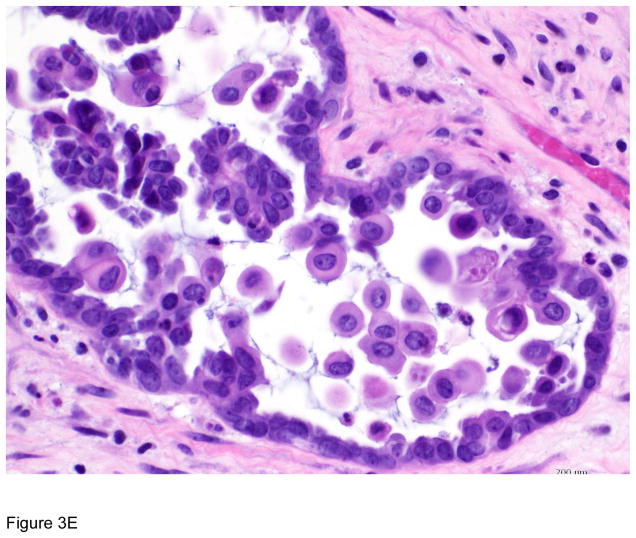
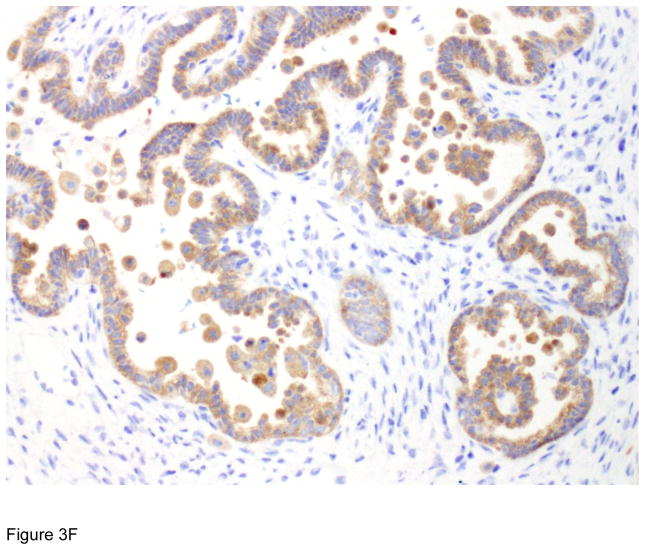
Figure 3.
Pathologic correlates of molecular/genetic alterations. a) Homologous recombination-deficient high-grade serous carcinoma exhibiting solid, endometrioid, and transitional cell carcinoma (SET)-like morphology. b) Endometrioid adenocarcinoma with mucinous differentiation, which is more frequently associated with KRAS mutations. c–f) BRAF-mutated serous borderline tumor showing complex papillary architecture (c), a subpopulation of cells with abundant eosinophilic (pink) cytoplasm (d, e). Cells bud from the epithelial surface, leading to the appearance of individual cells and clusters of detached cells (e). Immunohistochemistry for BRAF VE1 shows overexpression, which correlates with the presence of V600E BRAF mutation (f).
Highlights.
Pathologists play a central role in the management (including targeted therapy) of women with gynecologic cancer
Pathology is important in identification of targetable tumors based on morphologic features and biomarkers
Pathology is key to monitoring therapeutic response, and to discovery of novel biomarkers and therapeutic targets
Acknowledgments
This work was supported in part through the NIH/NCI Cancer Center Support Grant P30 CA008748. R. Grisham receives research funding from the Ovarian Cancer Research Fund Alliance, Cycle for Survival, and Conquer Cancer Foundation.
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Kong TW, Chang SJ, Paek J, Lee Y, Chun M, Ryu HS. Risk group criteria for tailoring adjuvant treatment in patients with endometrial cancer: a validation study of the Gynecologic Oncology Group criteria. J Gynecol Oncol. 2015;26:32–9. doi: 10.3802/jgo.2015.26.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Leeuw WJ, Dierssen J, Vasen HF, Wijnen JT, Kenter GG, Meijers-Heijboer H, et al. Prediction of a mismatch repair gene defect by microsatellite instability and immunohistochemical analysis in endometrial tumours from HNPCC patients. J Pathol. 2000;192:328–35. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH701>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 4.Peiro G, Diebold J, Lohse P, Ruebsamen H, Lohse P, Baretton GB, et al. Microsatellite instability, loss of heterozygosity, and loss of hMLH1 and hMSH2 protein expression in endometrial carcinoma. Hum Pathol. 2002;33:347–54. doi: 10.1053/hupa.2002.32220. [DOI] [PubMed] [Google Scholar]
- 5.Peterson LM, Kipp BR, Halling KC, Kerr SE, Smith DI, Distad TJ, et al. Molecular characterization of endometrial cancer: a correlative study assessing microsatellite instability, MLH1 hypermethylation, DNA mismatch repair protein expression, and PTEN, PIK3CA, KRAS, and BRAF mutation analysis. Int J Gynecol Pathol. 2012;31:195–205. doi: 10.1097/PGP.0b013e318231fc51. [DOI] [PubMed] [Google Scholar]
- 6.Esteller M, Levine R, Baylin SB, Ellenson LH, Herman JG. MLH1 promoter hypermethylation is associated with the microsatellite instability phenotype in sporadic endometrial carcinomas. Oncogene. 1998;17:2413–7. doi: 10.1038/sj.onc.1202178. [DOI] [PubMed] [Google Scholar]
- 7.Niessen RC, Hofstra RM, Westers H, Ligtenberg MJ, Kooi K, Jager PO, et al. Germline hypermethylation of MLH1 and EPCAM deletions are a frequent cause of Lynch syndrome. Genes Chromosomes Cancer. 2009;48:737–44. doi: 10.1002/gcc.20678. [DOI] [PubMed] [Google Scholar]
- 8.Buttin BM, Powell MA, Mutch DG, Rader JS, Herzog TJ, Gibb RK, et al. Increased risk for hereditary nonpolyposis colorectal cancer-associated synchronous and metachronous malignancies in patients with microsatellite instability-positive endometrial carcinoma lacking MLH1 promoter methylation. Clin Cancer Res. 2004;10:481–90. doi: 10.1158/1078-0432.ccr-1110-03. [DOI] [PubMed] [Google Scholar]
- 9.Broaddus RR, Lynch HT, Chen LM, Daniels MS, Conrad P, Munsell MF, et al. Pathologic features of endometrial carcinoma associated with HNPCC: a comparison with sporadic endometrial carcinoma. Cancer. 2006;106:87–94. doi: 10.1002/cncr.21560. [DOI] [PubMed] [Google Scholar]
- 10.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 11.Oronsky B, Ray CM, Spira AI, Trepel JB, Carter CA, Cottrill HM. A brief review of the management of platinum-resistant-platinum-refractory ovarian cancer. Med Oncol. 2017;34:103. doi: 10.1007/s12032-017-0960-z. [DOI] [PubMed] [Google Scholar]
- 12.Burger RA, Brady MF, Bookman MA, Fleming GF, Monk BJ, Huang H, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011;365:2473–83. doi: 10.1056/NEJMoa1104390. [DOI] [PubMed] [Google Scholar]
- 13.Perren TJ, Swart AM, Pfisterer J, Ledermann JA, Pujade-Lauraine E, Kristensen G, et al. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med. 2011;365:2484–96. doi: 10.1056/NEJMoa1103799. [DOI] [PubMed] [Google Scholar]
- 14.Aghajanian C, Blank SV, Goff BA, Judson PL, Teneriello MG, Husain A, et al. OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol. 2012;30:2039–45. doi: 10.1200/JCO.2012.42.0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pujade-Lauraine E, Hilpert F, Weber B, Reuss A, Poveda A, Kristensen G, et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: The AURELIA open-label randomized phase III trial. J Clin Oncol. 2014;32:1302–8. doi: 10.1200/JCO.2013.51.4489. [DOI] [PubMed] [Google Scholar]
- 16.Tewari KS, Sill MW, Long HJ, 3rd, Penson RT, Huang H, Ramondetta LM, et al. Improved survival with bevacizumab in advanced cervical cancer. N Engl J Med. 2014;370:734–43. doi: 10.1056/NEJMoa1309748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coleman RL, Brady MF, Herzog TJ, Sabbatini P, Armstrong DK, Walker JL, et al. Bevacizumab and paclitaxel-carboplatin chemotherapy and secondary cytoreduction in recurrent, platinum-sensitive ovarian cancer (NRG Oncology/Gynecologic Oncology Group study GOG-0213): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2017;18:779–91. doi: 10.1016/S1470-2045(17)30279-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grisham RN, Iyer G, Sala E, Zhou Q, Iasonos A, DeLair D, et al. Bevacizumab shows activity in patients with low-grade serous ovarian and primary peritoneal cancer. Int J Gynecol Cancer. 2014;24:1010–4. doi: 10.1097/IGC.0000000000000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ledermann J, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G, et al. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: a preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. Lancet Oncol. 2014;15:852–61. doi: 10.1016/S1470-2045(14)70228-1. [DOI] [PubMed] [Google Scholar]
- 20. [Accessed 13 September 2017]; https://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm533891.htm.
- 21. [Accessed 13 September 2017]; https://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm548487.htm.
- 22.Howitt BE, Shukla SA, Sholl LM, Ritterhouse LL, Watkins JC, Rodig S, et al. Association of Polymerase e-Mutated and Microsatellite-Instable Endometrial Cancers With Neoantigen Load, Number of Tumor-Infiltrating Lymphocytes, and Expression of PD-1 and PD-L1. JAMA Oncol. 2015;1:1319–23. doi: 10.1001/jamaoncol.2015.2151. [DOI] [PubMed] [Google Scholar]
- 23.Mehnert JM, Panda A, Zhong H, Hirshfield K, Damare S, Lane K, et al. Immune activation and response to pembrolizumab in POLE-mutant endometrial cancer. J Clin Invest. 2016;126:2334–40. doi: 10.1172/JCI84940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gatalica Z, Snyder C, Maney T, Ghazalpour A, Holterman DA, Xiao N, et al. Programmed cell death 1 (PD-1) and its ligand (PD-L1) in common cancers and their correlation with molecular cancer type. Cancer Epidemiol Biomarkers Prev. 2014;23:2965–70. doi: 10.1158/1055-9965.EPI-14-0654. [DOI] [PubMed] [Google Scholar]
- 25.Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409–13. doi: 10.1126/science.aan6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ott PA, Bang YJ, Berton-Rigaud D, Elez E, Pishvaian MJ, Rugo HS, et al. Safety and Antitumor Activity of Pembrolizumab in Advanced Programmed Death Ligand 1-Positive Endometrial Cancer: Results From the KEYNOTE-028 Study. J Clin Oncol. 2017;35:2535–41. doi: 10.1200/JCO.2017.72.5952. [DOI] [PubMed] [Google Scholar]
- 27.Kokka F, Brockbank E, Oram D, Gallagher C, Bryant A. Hormonal therapy in advanced or recurrent endometrial cancer. Cochrane Database Syst Rev. 2010:CD007926. doi: 10.1002/14651858.CD007926.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwartz PE, Chambers JT, Kohorn EI, Chambers SK, Weitzman H, Voynick IM, et al. Tamoxifen in combination with cytotoxic chemotherapy in advanced epithelial ovarian cancer. A prospective randomized trial. Cancer. 1989;63:1074–8. doi: 10.1002/1097-0142(19890315)63:6<1074::aid-cncr2820630606>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 29.Kothari R, Argenta P, Fowler J, Carter J, Shimp W. Antiestrogen therapy in recurrent ovarian cancer resulting in 28 months of stable disease: a case report and review of the literature. Arch Oncol. 2010;18:32–5. doi: 10.2298/AOO1002032K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yokoyama Y, Mizunuma H. Recurrent epithelial ovarian cancer and hormone therapy. World J Clin Cases. 2013;1:187–90. doi: 10.12998/wjcc.v1.i6.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams CJ. Tamoxifen for relapse of ovarian cancer. Cochrane Database Syst Rev. 2001:CD001034. doi: 10.1002/14651858.CD001034. [DOI] [PubMed] [Google Scholar]
- 32.Abdallah BY, Horne SD, Kurkinen M, Stevens JB, Liu G, Ye CJ, et al. Ovarian cancer evolution through stochastic genome alterations: defining the genomic role in ovarian cancer. Syst Biol Reprod Med. 2014;60:2–13. doi: 10.3109/19396368.2013.837989. [DOI] [PubMed] [Google Scholar]
- 33.Hause RJ, Pritchard CC, Shendure J, Salipante SJ. Classification and characterization of microsatellite instability across 18 cancer types. Nat Med. 2016;22:1342–50. doi: 10.1038/nm.4191. [DOI] [PubMed] [Google Scholar]
- 34.Sood AK, Holmes R, Hendrix MJ, Buller RE. Application of the National Cancer Institute international criteria for determination of microsatellite instability in ovarian cancer. Cancer Res. 2001;61:4371–4. [PubMed] [Google Scholar]
- 35.Howitt BE, Strickland KC, Sholl LM, Rodig S, Ritterhouse LL, Chowdhury D, et al. Clear cell ovarian cancers with microsatellite instability: A unique subset of ovarian cancers with increased tumor-infiltrating lymphocytes and PD-1/PD-L1 expression. Oncoimmunology. 2017;6:e1277308. doi: 10.1080/2162402X.2016.1277308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shia J, Black D, Hummer AJ, Boyd J, Soslow RA. Routinely assessed morphological features correlate with microsatellite instability status in endometrial cancer. Hum Pathol. 2008;39:116–25. doi: 10.1016/j.humpath.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 37.Rabban JT, Calkins SM, Karnezis AN, Grenert JP, Blanco A, Crawford B, et al. Association of tumor morphology with mismatch-repair protein status in older endometrial cancer patients: implications for universal versus selective screening strategies for Lynch syndrome. Am J Surg Pathol. 2014;38:793–800. doi: 10.1097/PAS.0000000000000177. [DOI] [PubMed] [Google Scholar]
- 38.Sloan EA, Moskaluk CA, Mills AM. Mucinous Differentiation With Tumor Infiltrating Lymphocytes Is a Feature of Sporadically Methylated Endometrial Carcinomas. Int J Gynecol Pathol. 2017;36:205–16. doi: 10.1097/PGP.0000000000000315. [DOI] [PubMed] [Google Scholar]
- 39.Aysal A, Karnezis A, Medhi I, Grenert JP, Zaloudek CJ, Rabban JT. Ovarian endometrioid adenocarcinoma: incidence and clinical significance of the morphologic and immunohistochemical markers of mismatch repair protein defects and tumor microsatellite instability. Am J Surg Pathol. 2012;36:163–72. doi: 10.1097/PAS.0b013e31823bc434. [DOI] [PubMed] [Google Scholar]
- 40.Huang HN, Lin MC, Tseng LH, Chiang YC, Lin LI, Lin YF, et al. Ovarian and endometrial endometrioid adenocarcinomas have distinct profiles of microsatellite instability, PTEN expression, and ARID1A expression. Histopathology. 2015;66:517–28. doi: 10.1111/his.12543. [DOI] [PubMed] [Google Scholar]
- 41.McConechy MK, Talhouk A, Li-Chang HH, Leung S, Huntsman DG, Gilks CB, et al. Detection of DNA mismatch repair (MMR) deficiencies by immunohistochemistry can effectively diagnose the microsatellite instability (MSI) phenotype in endometrial carcinomas. Gynecol Oncol. 2015;137:306–10. doi: 10.1016/j.ygyno.2015.01.541. [DOI] [PubMed] [Google Scholar]
- 42.Sholl LM, Aisner DL, Allen TC, Beasley MB, Borczuk AC, Cagle PT, et al. Programmed Death Ligand-1 Immunohistochemistry--A New Challenge for Pathologists: A Perspective From Members of the Pulmonary Pathology Society. Arch Pathol Lab Med. 2016;140:341–4. doi: 10.5858/arpa.2015-0506-SA. [DOI] [PubMed] [Google Scholar]
- 43.Cancer Genome Atlas Research N. Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497:67–73. doi: 10.1038/nature12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hoang LN, McConechy MK, Kobel M, Anglesio M, Senz J, Maassen M, et al. Polymerase Epsilon Exonuclease Domain Mutations in Ovarian Endometrioid Carcinoma. Int J Gynecol Cancer. 2015;25:1187–93. doi: 10.1097/IGC.0000000000000492. [DOI] [PubMed] [Google Scholar]
- 45.Zou Y, Liu FY, Liu H, Wang F, Li W, Huang MZ, et al. Frequent POLE1 p.S297F mutation in Chinese patients with ovarian endometrioid carcinoma. Mutat Res. 2014;761:49–52. doi: 10.1016/j.mrfmmm.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 46.Teer JK, Yoder S, Gjyshi A, Nicosia SV, Zhang C, Monteiro ANA. Mutational heterogeneity in non-serous ovarian cancers. Sci Rep. 2017;7:9728. doi: 10.1038/s41598-017-10432-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Parra-Herran C, Lerner-Ellis J, Xu B, Khalouei S, Bassiouny D, Cesari M, et al. Molecular-based classification algorithm for endometrial carcinoma categorizes ovarian endometrioid carcinoma into prognostically significant groups. Mod Pathol. 2017 doi: 10.1038/modpathol.2017.81. [DOI] [PubMed] [Google Scholar]
- 48.Hussein YR, Weigelt B, Levine DA, Schoolmeester JK, Dao LN, Balzer BL, et al. Clinicopathological analysis of endometrial carcinomas harboring somatic POLE exonuclease domain mutations. Mod Pathol. 2015;28:505–14. doi: 10.1038/modpathol.2014.143. [DOI] [PubMed] [Google Scholar]
- 49.Bakhsh S, Kinloch M, Hoang LN, Soslow RA, Kobel M, Lee CH, et al. Histopathological features of endometrial carcinomas associated with POLE mutations: implications for decisions about adjuvant therapy. Histopathology. 2016;68:916–24. doi: 10.1111/his.12878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.DeLair DF, Burke KA, Selenica P, Lim RS, Scott SN, Middha S, et al. The genetic landscape of endometrial clear cell carcinomas. J Pathol. 2017 doi: 10.1002/path.4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kobel M, Hoang LN, Tessier-Cloutier B, Meng B, Soslow RA, Stewart CJR, et al. Undifferentiated Endometrial Carcinomas Show Frequent Loss of Core Switch/Sucrose Nonfermentable Complex Proteins. Am J Surg Pathol. 2017 doi: 10.1097/PAS.0000000000000941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hoang LN, Kinloch MA, Leo JM, Grondin K, Lee CH, Ewanowich C, et al. Interobserver Agreement in Endometrial Carcinoma Histotype Diagnosis Varies Depending on The Cancer Genome Atlas (TCGA)-based Molecular Subgroup. Am J Surg Pathol. 2017;41:245–52. doi: 10.1097/PAS.0000000000000764. [DOI] [PubMed] [Google Scholar]
- 53.Stelloo E, Nout RA, Osse EM, Jurgenliemk-Schulz IJ, Jobsen JJ, Lutgens LC, et al. Improved Risk Assessment by Integrating Molecular and Clinicopathological Factors in Early-stage Endometrial Cancer-Combined Analysis of the PORTEC Cohorts. Clin Cancer Res. 2016;22:4215–24. doi: 10.1158/1078-0432.CCR-15-2878. [DOI] [PubMed] [Google Scholar]
- 54.Talhouk A, Hoang LN, McConechy MK, Nakonechny Q, Leo J, Cheng A, et al. Molecular classification of endometrial carcinoma on diagnostic specimens is highly concordant with final hysterectomy: Earlier prognostic information to guide treatment. Gynecol Oncol. 2016;143:46–53. doi: 10.1016/j.ygyno.2016.07.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cancer Genome Atlas Research N. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–15. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ledermann JA, Drew Y, Kristeleit RS. Homologous recombination deficiency and ovarian cancer. Eur J Cancer. 2016;60:49–58. doi: 10.1016/j.ejca.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 57.Soslow RA, Han G, Park KJ, Garg K, Olvera N, Spriggs DR, et al. Morphologic patterns associated with BRCA1 and BRCA2 genotype in ovarian carcinoma. Mod Pathol. 2012;25:625–36. doi: 10.1038/modpathol.2011.183. [DOI] [PubMed] [Google Scholar]
- 58.Howitt BE, Hanamornroongruang S, Lin DI, Conner JE, Schulte S, Horowitz N, et al. Evidence for a dualistic model of high-grade serous carcinoma: BRCA mutation status, histology, and tubal intraepithelial carcinoma. Am J Surg Pathol. 2015;39:287–93. doi: 10.1097/PAS.0000000000000369. [DOI] [PubMed] [Google Scholar]
- 59.Ritterhouse LL, Nowak JA, Strickland KC, Garcia EP, Jia Y, Lindeman NI, et al. Morphologic correlates of molecular alterations in extrauterine Mullerian carcinomas. Mod Pathol. 2016;29:893–903. doi: 10.1038/modpathol.2016.82. [DOI] [PubMed] [Google Scholar]
- 60.Combe P, Chauvenet L, Lefrere-Belda MA, Blons H, Rousseau C, Oudard S, et al. Sustained response to vemurafenib in a low grade serous ovarian cancer with a BRAF V600E mutation. Invest New Drugs. 2015;33:1267–70. doi: 10.1007/s10637-015-0297-4. [DOI] [PubMed] [Google Scholar]
- 61.Grisham RN, Sylvester BE, Won H, McDermott G, DeLair D, Ramirez R, et al. Extreme Outlier Analysis Identifies Occult Mitogen-Activated Protein Kinase Pathway Mutations in Patients With Low-Grade Serous Ovarian Cancer. J Clin Oncol. 2015;33:4099–105. doi: 10.1200/JCO.2015.62.4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xiong J, He M, Jackson C, Ou JJ, Sung CJ, Breese V, et al. Endometrial carcinomas with significant mucinous differentiation associated with higher frequency of k-ras mutations: a morphologic and molecular correlation study. Int J Gynecol Cancer. 2013;23:1231–6. doi: 10.1097/IGC.0b013e31829ea82f. [DOI] [PubMed] [Google Scholar]
- 63.Anglesio MS, Kommoss S, Tolcher MC, Clarke B, Galletta L, Porter H, et al. Molecular characterization of mucinous ovarian tumours supports a stratified treatment approach with HER2 targeting in 19% of carcinomas. J Pathol. 2013;229:111–20. doi: 10.1002/path.4088. [DOI] [PubMed] [Google Scholar]
- 64.Buza N, Hui P. Marked heterogeneity of HER2/NEU gene amplification in endometrial serous carcinoma. Genes Chromosomes Cancer. 2013;52:1178–86. doi: 10.1002/gcc.22113. [DOI] [PubMed] [Google Scholar]
- 65.Mok SC, Bell DA, Knapp RC, Fishbaugh PM, Welch WR, Muto MG, et al. Mutation of K-ras protooncogene in human ovarian epithelial tumors of borderline malignancy. Cancer Res. 1993;53:1489–92. [PubMed] [Google Scholar]
- 66.Mayr D, Hirschmann A, Lohrs U, Diebold J. KRAS and BRAF mutations in ovarian tumors: a comprehensive study of invasive carcinomas, borderline tumors and extraovarian implants. Gynecol Oncol. 2006;103:883–7. doi: 10.1016/j.ygyno.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 67.Wong KK, Tsang YT, Deavers MT, Mok SC, Zu Z, Sun C, et al. BRAF mutation is rare in advanced-stage low-grade ovarian serous carcinomas. Am J Pathol. 2010;177:1611–7. doi: 10.2353/ajpath.2010.100212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Grisham RN, Iyer G, Garg K, Delair D, Hyman DM, Zhou Q, et al. BRAF mutation is associated with early stage disease and improved outcome in patients with low-grade serous ovarian cancer. Cancer. 2013;119:548–54. doi: 10.1002/cncr.27782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Farley J, Brady WE, Vathipadiekal V, Lankes HA, Coleman R, Morgan MA, et al. Selumetinib in women with recurrent low-grade serous carcinoma of the ovary or peritoneum: an open-label, single-arm, phase 2 study. Lancet Oncol. 2013;14:134–40. doi: 10.1016/S1470-2045(12)70572-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gershenson DM, Sun CC, Wong KK. Impact of mutational status on survival in low-grade serous carcinoma of the ovary or peritoneum. Br J Cancer. 2015;113:1254–8. doi: 10.1038/bjc.2015.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. [Accessed on 13 September 2017]; https://clinicaltrials.gov/ct2/show/NCT01849874.
- 72.Zeppernick F, Ardighieri L, Hannibal CG, Vang R, Junge J, Kjaer SK, et al. BRAF mutation is associated with a specific cell type with features suggestive of senescence in ovarian serous borderline (atypical proliferative) tumors. Am J Surg Pathol. 2014;38:1603–11. doi: 10.1097/PAS.0000000000000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bosmuller H, Fischer A, Pham DL, Fehm T, Capper D, von Deimling A, et al. Detection of the BRAF V600E mutation in serous ovarian tumors: a comparative analysis of immunohistochemistry with a mutation-specific monoclonal antibody and allele-specific PCR. Hum Pathol. 2013;44:329–35. doi: 10.1016/j.humpath.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 74.Gilks CB, Oliva E, Soslow RA. Poor interobserver reproducibility in the diagnosis of high-grade endometrial carcinoma. Am J Surg Pathol. 2013;37:874–81. doi: 10.1097/PAS.0b013e31827f576a. [DOI] [PubMed] [Google Scholar]
- 75.Seoane J, De Mattos-Arruda L. The challenge of intratumour heterogeneity in precision medicine. J Intern Med. 2014;276:41–51. doi: 10.1111/joim.12240. [DOI] [PubMed] [Google Scholar]
- 76.Wu D, Wang DC, Cheng Y, Qian M, Zhang M, Shen Q, et al. Roles of tumor heterogeneity in the development of drug resistance: A call for precision therapy. Semin Cancer Biol. 2017;42:13–9. doi: 10.1016/j.semcancer.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 77.Rossi ED, Bizzarro T, Longatto-Filho A, Gerhard R, Schmitt F. The diagnostic and prognostic role of liquid-based cytology: are we ready to monitor therapy and resistance? Expert Rev Anticancer Ther. 2015;15:911–21. doi: 10.1586/14737140.2015.1053874. [DOI] [PubMed] [Google Scholar]
- 78.Tang Y, Wang Z, Li Z, Kim J, Deng Y, Li Y, et al. High-throughput screening of rare metabolically active tumor cells in pleural effusion and peripheral blood of lung cancer patients. Proc Natl Acad Sci U S A. 2017;114:2544–9. doi: 10.1073/pnas.1612229114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jelinic P, Mueller JJ, Olvera N, Dao F, Scott SN, Shah R, et al. Recurrent SMARCA4 mutations in small cell carcinoma of the ovary. Nat Genet. 2014;46:424–6. doi: 10.1038/ng.2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.The Hammond Times. 1957. Work With New Electronic ‘Brains’ Opens Field For Army Math Experts. [Google Scholar]
- 81.Vang R, Levine DA, Soslow RA, Zaloudek C, Shih Ie M, Kurman RJ. Molecular Alterations of TP53 are a Defining Feature of Ovarian High-Grade Serous Carcinoma: A Rereview of Cases Lacking TP53 Mutations in The Cancer Genome Atlas Ovarian Study. Int J Gynecol Pathol. 2016;35:48–55. doi: 10.1097/PGP.0000000000000207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cancer Genome Atlas Research N, Albert Einstein College of M, Analytical Biological S, Barretos Cancer H, Baylor College of M, Beckman Research Institute of City of H et al. Integrated genomic and molecular characterization of cervical cancer. Nature. 2017;543:378–84. doi: 10.1038/nature21386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ojesina AI, Lichtenstein L, Freeman SS, Pedamallu CS, Imaz-Rosshandler I, Pugh TJ, et al. Landscape of genomic alterations in cervical carcinomas. Nature. 2014;506:371–5. doi: 10.1038/nature12881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Karamurzin YS, Kiyokawa T, Parkash V, Jotwani AR, Patel P, Pike MC, et al. Gastric-type Endocervical Adenocarcinoma: An Aggressive Tumor With Unusual Metastatic Patterns and Poor Prognosis. Am J Surg Pathol. 2015;39:1449–57. doi: 10.1097/PAS.0000000000000532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Murali R, Burke KA, De Filippo MR, Berman SH, Park KJ, Weigelt B. The genomic landscape of gastric-type cervical adenocarcinomas. Int J Gynecol Cancer. 2016;26:412. [Google Scholar]