Abstract
Background
Relationships between birthweight and future obesity risk remain unclear.
Objective
To assess associations between birthweight and later obesity in a nationally representative cohort of early school-aged children.
Methods
We used linear and logistic regression to evaluate 10,186 term- or preterm children in the Early Childhood Longitudinal Study-Kindergarten Cohort 2011 for relationshps between birthweight and later obesity and change in BMI-z-score from kindergarten-to-second grade. All analyses were adjusted for sex, race/ethnicity, parental education, and household income.
Results
Compared to children born normal birthweight (NBW), high birthweight (HBW) term children and large-for-gestational-age (LGA) preterm children had significantly greater BMI-z-scores from kindergarten-to-second grade (p<0.001). Term children born HBW had higher odds of obesity by kindergarten (adjusted odds ratios [aOR] 1.91, p<0.0001). Among preterm children, odds of obesity was higher among LGA children starting in first grade (aOR 2.34, p<0.05) and among small-for-gestational age children in second grade (aOR 2.26, p<0.05). Compared to NBW children, HBW children had greater change in BMI-z-score between kindergarten-first grade (p<0.01).
Conclusions
HBW term and LGA preterm children had increased adjusted odds of obesity in school-age compared to their NBW counterparts. Physicians may provide counseling early in life for families of large infants to help prevent future obesity.
Keywords: obesity, childhood, birthweight, small-for-gestational age, large-for-gestational age
INTRODUCTION
Childhood obesity has reached startling proportions in the US, doubling in children and quadrupling in adolescents over the past 30 years.1 Even though levels have stabilized recently, 16.9% of US children are obese and additional 14.9% overweight,2 with similar trends worldwide.3 Strategically targeting higher risk populations for more intensive education and prevention from a young age may help individuals avoid later complications resulting from excess weight.4
One potential predictor of later obesity is birthweight, with relationships possible for both lower and higher birthweights. Formulated over 20 years ago, the developmental origins of adult disease theory (sometimes referred to as the Barker Hypothesis), postulated that early developmental influences, particularly in utero, permanently alter a person’s physiology and metabolism.5,6 Researchers have observed that compared to children with normal birthweight (NBW) children born with low birthweight (LBW, <2500g) have a subsequent predisposition to truncal obesity later in life.7 One study demonstrated that infants born small but experiencing rapid “catch-up growth” in their first two years grew to be heavier and with a higher body fat percentage than their peers at age five,8 with another group categorizing this weight rebound as “catch-up fat.”9 Another study suggested that preterm birth status may carry additional metabolic consequences.10 Alternatively, other groups have demonstrated relationships between higher birthweight and later BMI11,12 and adiposity.13 Together, this suggests potential for U-shaped relationship between birthweight and later weight gain, with both those of low and high birthweight having higher odds of obesity.
Previous studies on this topic have preceeded the current obesity epidemic,7,8,11 lacked power or national representation of their cohorts,7, 13 did not take gestational age into account12 or focused on BMI at specific times rather than tracking change or future projection.12,14 The goals of the current study were to evaluate relationships between birthweight and gestational age with 1) the BMI-z-score of children at kindergarten, 1st and 2nd grade 2) the change in their BMI-z-score over time and 3) the odds of becoming overweight or obese during early school years. We analyzed data from a sizeable cohort of children followed longitudinally as part of the Early Childhood Longitudinal Survey-Kindergarten Cohort 2011 (ECLS-K:2011). We hypothesized that term children born LBW and HBW and preterm children born small for gestational age (SGA) or large for gestational age (LGA) would not only have a higher BMI-z-score, but also exhibit a greater change over time and increased odds of being obese in early schoolage.
METHODS
Cohort Description
The ECLS-K:2011 is an ongoing study sponsored by the National Center for Education Statistics (NCES) of the US Department of Education (https://nces.ed.gov/ecls/kindergarten2011.asp). The NCES ethics review board approved the study. The children in the cohort comprise a nationally-representative sample using multi-stage probability sampling of children attending kindergarten within schools. Participation was completely voluntary and all parents gave informed consent. The study enrolled 18,174 children beginning full or part-day kindergarten in the fall of 2010.15 Follow-up evaluations were performed in the spring of kindergarten, and then in both spring and fall of each subsequent school year. The current analysis included data through spring of second grade.
Trained field staff obtained data from participating children and their parents or caregivers. The majority of parent interviews were conducted by telephone, with additional interviews conducted in person for those without access. These interviews followed a validated computer-assisted interview template from which parents were presented multiple-choice questions.15 During these interviews, parents identified the child’s sex and race/ethnicity. Race/ethnicity was grouped into five categories: white, African-American, Asian, Hispanic, and other. Parents were also asked their highest education level achieved and the family’s income.
Anthropometry Measures
Trained researchers using a standardized protocol and including a digital scale and stadiometer obtained direct measurements of height and weight.16 Children were dressed in light clothing without shoes. Measurements were taken twice; if these were within 5% of each other, their average was used, otherwise a third measurement was taken and the three measurements averaged. From these measures, BMI was calculated and converted to age- and sex-specific percentiles and z-scores using the Centers for Disease Control and Prevention growth charts. Weight categories included normal weight (<85th percentile), overweight (85th-95th percentile), and obese (≥95th percentile).
Categories of Gestation and Birthweight
Parents reported whether their child was born more than 2 weeks before the expected due date. For children who were born >2 weeks early, parents were then asked how many days or weeks early the child was born; this response was subtracted from 40 weeks to calculate the child’s gestational age (e.g., 4 weeks early=36 weeks gestation). These data were used to categorize children to being term (≥37 weeks gestation) or preterm <37 weeks gestation). Birthweight was obtained from parental report. The term/preterm categories were then further broken down into three weight categories. For preterm infants (with known gestational age), we utilized the Fenton z-score calculator17 to calculate z-scores of size for gestational age and broke these into categories of small for gestational age (SGA <10th percentile), average for gestational age (AGA, 10th-90th percentile), and large for gestational age (LGA >90th percentile). For term children, because of the way the gestational age information were acquired, we had incomplete gestational age information, knowing only whether the child was >38 weeks or between 37 and 38 weeks. We thus divided term children into high birthweight (HBW) ≥4500g, normal birthweight (NBW) 2500 – 4500g, low birthweight (LBW) <2500g.
Statistics
All analyses were run using SAS 9.4 (SAS Institute, Research Triangle, NC). Linear and logistic regressions were used to examine relationships between gestational age and weight with future weight and weight-gain over time. We adjusted for gestational age, baseline BMI, sex, race/ethnicity, highest parental education, and household income. SAS survey procedures were used to account for the complex sampling design and nonresponses in the ECLS-K, and appropriate weights provided by the NCES were used to give population estimates. For linear regressions, gestational ages and sizes were assessed as linear variables and as categories when calculating adjusted means of BMI-z-score. To compare weight status and BMI-z-score between kindergarten, first, and second grade, we used the height and weight measurements from Spring of each year. In longitudinal linear regressions, our outcomes (in separate analyses) were BMI-z-score and change in BMI-z-score (between kindergarten and first grade, between first and second grade), which was adjusted for baseline BMI-z-score. For longitudinal analyses, we restricted our analysis to those who had complete data at the kindergarten, first, and second grade. In logistic regressions, we evaluated odds of overweight and obesity separately for preterm infants SGA vs AGA and LGA vs AGA as well as for term infants with LBW vs NBW and HBW vs NBW. In calculating adjusted mean values, we entered for continuous variables the mean of that variable and for categorical variables, the percent of the sample in each category.
RESULTS
Demographics
Of the 18,174 children originally enrolled in ECLS-K(2011), we analyzed data from a total of 10,186 participants who had complete data regarding BMI-z-score, gestational ages/weights, and sociodemographic variables. The analytic cohort was comprised of 828 preterm children and 9,358 term children. Compared to excluded participants, included participants had higher income, higher education, higher birthweight (among term but not preterm children), were more likely to be white or Hispanic and were less likely to be black, Asian or other race/ethnicity (Supplementary Table 1). Included and excluded participants were similar with respect to sex and proportion preterm/term. Overall demographic characteristics of those included are show in Supplementary Table 2.
Associations between birthweight and later overweight and obesity
Table 1 illustrates weight status during kindergarten, first, and second grades for each gestational age and weight category. Prevalence of overweight and obesity among children was high, and increased over time, rising such that the greatest proportions of obesity were observed in second graders. By 2nd grade, obesity prevalence among children born preterm who were SGA, AGA, and LGA was 28.0%, 14.2% and 27.8%, respectively, while obesity prevalence in 2nd grade among term children who were LBW, NBW, and HBW was 16.8%, 15.8%, and 23.1%.
Table 1.
Proportion of overweight and obese by gestational age and weight.
| Total number (weighted % by category) | Preterm Infants [<37 wks] (weighted % by category) | Total number (weighted % by category) | Term Infants [>37 wks] (weighted % by category) | |||||
|---|---|---|---|---|---|---|---|---|
| SGA (<10th%) |
AGA (10–90th %) |
LGA (>90th %) |
LBW (<2500g) |
NBW (2500–4000g) |
HBW (>4000g) |
|||
| KINDERGARTEN | ||||||||
| Total number | 828 | 9,358 | ||||||
| Normal weight (weighted % by category) |
598 (72.9) | 69.7 | 74.7 | 58.0 | 6,653 (70.9) | 73.4 | 72.2 | 59.5 |
| Overweight (weighted % by category) |
127 (15.2) | 13.0 | 14.6 | 23.3 | 1,474 (15.9) | 14.8 | 15.4 | 20.1 |
| Obese (weighted % by category) |
103 (12.0) | 17.3 | 10.7 | 18.6 | 1,231 (13.2) | 11.9 | 12.4 | 20.4 |
| Chi Square | <0.0001 | <0.0001 | ||||||
| 1ST GRADE | ||||||||
| Total number | 828 | 9,358 | ||||||
| Normal weight (weighted % by category) |
596 (73.1) | 63.1 | 75.8 | 55.9 | 6,599 (70.2) | 70.1 | 71.6 | 58.8 |
| Overweight (weighted % by category) |
110 (13.0) | 21.3 | 11.6 | 18.1 | 1,429 (15.4) | 15.8 | 14.8 | 19.9 |
| Obese (weighted % by category) |
122 (14.0) | 15.5 | 12.6 | 26.0 | 1,330 (14.5) | 14.1 | 13.6 | 21.3 |
| Chi Square | <0.0001 | <0.0001 | ||||||
| 2ND GRADE | ||||||||
| Total number | 828 | 9,358 | ||||||
| Normal weight (weighted % by category) |
572 (69.2) | 58.5 | 71.4 | 58.5 | 6,433 (68.7) | 69.9 | 69.8 | 59.3 |
| Overweight (weighted % by category) |
115 (14.3) | 13.5 | 14.5 | 13.8 | 1,379 (14.7) | 13.3 | 14.5 | 17.6 |
| Obese (weighted % by category) |
141 (16.5) | 28.0 | 14.2 | 27.8 | 1,546 (16.6) | 16.8 | 15.8 | 23.1 |
| Chi Square | <0.0001 | <0.0001 | ||||||
Table 2 provides adjusted odds ratios (aOR) for overweight/obesity or obesity at each of the three grade levels for SGA and LGA compared to AGA for preterm and LBW and HBW compared to NBW for term children. All analyses were adjustment for sex, race/ethnicity and SES factors, with additional adjustment for gestational age among preterm children. Among preterm children, LGA children had a higher odds of later overweight/obesity at each of the grade levels (aOR’s 2.04–2.46) but only a greater odds of obesity at grades 1 (aOR 2.34) and 2 (aOR 2.26), while SGA children only had a greater odds of obesity at 2nd grade (aOR 2.06). Among term children, HBW children had an elevated odds of overweight/obesity and obesity at each of the grades, with aOR of 1.68–1.87 for overweight/obese and 1.69–1.91 for obesity. Among term children there were no significant associations between LBW and future overweight/obese or obesity.
Table 2.
Logistic regression of birthweight on overweight and obesity status for preterm and term children at kindergarten, first-grade, and second-grade.
| Preterm (<37 weeks) | Term (≥ 37 weeks)* | |||||||
|---|---|---|---|---|---|---|---|---|
| SGA (vs AGA)† |
p-value | LGA (vs AGA)† |
p-value | LBW (vs NBW)† |
p-value | HBW (vs NBW)† |
p-value | |
| KINDERGARTEN | ||||||||
| Overweight (or obese) | ||||||||
| Model 1 (adjusted for sex, race/ethnicity, SES‡) | 1.03 (0.55–1.95) | 0.9250 | 2.00 (1.14–3.50) | 0.0153 | 0.83 (0.68, 1.01) | 0.0601 | 1.87 (1.64, 2.13) | <0.0001 |
| Model 2 (Model 1 adjustments plus gest. age*) | 0.98 (0.51–1.91) | 0.9616 | 2.04 (1.15–3.61) | 0.0143 | ||||
| Obese | ||||||||
| Model 1 (adjusted for sex, race/ethnicity, SES‡) | 1.44 (0.63, 3.29) | 0.3839 | 1.75 (0.86, 3.54) | 0.1213 | 0.80 (0.58, 1.11) | 0.1812 | 1.91 (1.58, 2.30) | <0.0001 |
| Model 2 (Model 1 adjustments plus gest. age*) | 1.43 (0.60, 3.41) | 0.4225 | 1.78 (0.88, 3.61) | 0.1103 | ||||
| FIRST-GRADE | ||||||||
| Overweight (or obese) | ||||||||
| Model 1 (adjusted for sex, race/ethnicity, SES‡) | 1.57 (0.92, 2.67) | 0.0957 | 2.42 (1.39, 4.24) | 0.0019 | 0.93 (0.74, 1.16) | 0.4983 | 1.90 (1.63, 2.20) | <0.0001 |
| Model 2 (Model 1 adjustments plus gest. age*) | 1.51 (0.88, 2.60) | 0.1336 | 2.46 (1.39, 4.33) | 0.0019 | ||||
| Obese | ||||||||
| Model 1 (adjusted for sex, race/ethnicity, SES‡) | 1.08 (0.51, 2.27) | 0.8391 | 2.34 (1.19, 4.63) | 0.0143 | 0.88 (0.65, 1.18) | 0.3910 | 1.83 (1.51, 2.21) | <0.0001 |
| Model 2 (Model 1 adjustments plus gest. age*) | 1.09 (0.50, 2.36) | 0.8254 | 2.34 (1.18, 4.65) | 0.0152 | ||||
| SECOND-GRADE | ||||||||
| Overweight (or obese) | ||||||||
| Model 1 (adjusted for sex, race/ethnicity, SES‡) | 1.52 (0.90, 2.57) | 0.1198 | 1.69 (0.95, 3.02) | 0.0758 | 0.86 (0.68, 1.01) | 0.2103 | 1.68 (1.47, 1.93) | <0.0001 |
| Model 2 (Model 1 adjustments plus gest. age*) | 1.43 (0.83, 2.46) | 0.2022 | 1.74 (0.96, 3.14) | 0.0680 | ||||
| Obese | ||||||||
| Model 1 (adjusted for sex, race/ethnicity, SES‡) | 2.04 (1.04, 4.00) | 0.0382 | 2.25 (1.16, 4.36) | 0.0164 | 0.92 (0.70, 1.21) | 0.5639 | 1.69 (1.44, 1.98) | <0.0001 |
| Model 2 (Model 1 adjustments plus gest. age*) | 2.06 (1.03, 4.09) | 0.0405 | 2.26 (1.16, 4.39) | 0.0162 | ||||
Data presented as odds ratio and 95% confidence interval.
Socioeconomic status variables included highest parental education level and household income.
Gestational age data not available for children born ≥38 weeks.
WHO categories from Blencowe Lancet 2012: [1: <28 weeks; 2: 28–32 weeks; 3: 32–35 weeks; 4: 35–37 weeks; 5: ≥ 37 weeks]
Abbreviations: AGA, average for gestational age; LGA, large for gestational age; SGA, small for gestational age; NBW, normal birthweight; HBW, high birthweight, LBW, low birth weight.
Longitudinal association of Gestational Age/Weight and BMI-z-score
Using linear regression of birthweight as a predictor of later BMI-z-score, adjusted for potential confounders, we found significant relationships in both preterm and term children at each time point (Supplmentary Table 3). We next assessed for differences in the change in BMI-z-score over time, assessed as adjusted mean change-in-BMI-z-score between kindergarten and 1st grade and between 1st and 2nd grade (Supplementary Table 4). Among children born at term, there was a significant positive relationship between birthweight and change in BMI-z-score between 1st and second grade (p<0.01).
Finally, Figure 1 provides mean BMI-z-scores by weight categories, adjusted for parent education, family income, sex, race and (among preterm children) gestational category. Among children born preterm, at all three grade levels, LGA children had significantly greater BMI-z-scores than those born AGA or SGA (all p<0.001), while there were no significant differences between those born AGA and SGA. Among term children, at all three grade levels, HBW children had significantly greater BMI-z-scores than those born NBW or LBW (p<0.001); NBW children had higher BMI-z-scores at each of the time points as well (p<0.01). Among term children, those born LBW had a lower BMI z-scores than NBW children by 0.18, 0.17, and 0.16 points in kindergarten, first grade, and second grade respectively (p<0.01). At these ages this corresponds to differences in BMI of ~0.4, ~0.3, and ~0.2 and differences in weight of ~0.2, ~0.26, and ~0.35 kg respectively. Regarding change in BMI z-score over time, there were no significant differences among preterm children by birthweight category. Among term children, those born HBW (compared to NBW) had a higher change in BMI-z-score between kindergarten and 1st grade (+0.029 vs. −0.020, p=0.035).
Figure 1. Mean BMI Z-scores over time by birthweight categories.
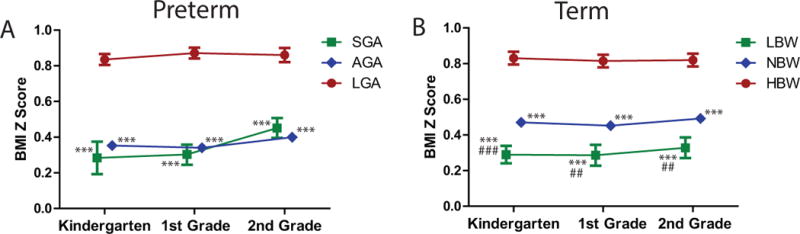
Data are shown for A. preterm children born small-for-gestational age (SGA), average for gestational age (AGA) or large-for-gestational-age (LGA) and B. term children born with low birthweight (LBW), normal birthweight (NBW) or high birthweight (HBW) at kindergarten, 1st and 2nd grade. All analyses were are adjusted for race, sex, parental education, income and gestational category. Error bars denote standard error. Inter-group differences: * p < 0.05 vs. LGA/HBW. ** p < 0.01 vs. LGA/HBW. *** p < 0.001 vs. LGA/HBW. # p < 0.05 vs. AGA/NBW. ## p < 0.01 vs. AGA/NBW. ### p < 0.001 vs. AGA/NBW.
DISCUSSION
These data are unique in demonstrating in a recent, nationally-representative cohort of children followed during the current obesity epidemic, that among birthweight categories, high birthweight is most strongly associated with subsequent childhood overweight and obesity. At each grade level and for both preterm and term children, children who were heavy as infants remained heavier than children born at normal birthweight. However, contrary to our original hypothesis, we did not see consistently higher BMI-z-scores or more rapid weight-gain among children born with lower birthweight—though by 2nd grade, preterm children born SGA had a 2-fold increase in odds of obesity compared to AGA counterparts. This association between lower birthweight and subsequent obesity status did not appear to be true for children born at term, among whom LBW children had lower subsequent BMI-z-scores than NBW’s, without differences in weight-gain. Thus, while lower birthweight has previously been linked to later cardiovascular disease5,6 and excess metabolic risk,18 we did not note a clear link between lower birthweight and more rapid weight-gain in early school age children, even in the current obesigenic environment. This may imply either a predominance of other metabolic changes besides weight-gain contributing to later cardiovascular risk or that the influence of low birthweight on later unhealthy weight-gain may occur after age 8. Overall, these data demonstrate long-term obesity risks among children of high birthweight, who bear 70–130% higher odds of overweight or obesity compared to children of normal birthweight. These findings may have strong implications for pediatricians regarding their counseling technique and content, with increased attention and follow up on children born at higher birthweights, and thus at increased risk for future obesity.
The underlying factors that link higher birthweight and later obesity are unclear but likely include genetic factors,19 maternal and fetal nutrition,20 and shared lifestyle practices (such as dietary and sedentary behaviors) associated with both maternal over-nutrition and later child over-nutrition.21 In the case of genetic factors and lifestyle practices, these may also contribute ongoing weight-gain, potentially underlying observations that children with early rapid weight-gain are more likely to have prediabetes and cardiovascular disease in later life.22,23 While some lifestyle factors contributing to maternal weight-gain and early childhood weight-gain are related to socio-economic status, our findings were present following adjustment for parental income and education, suggesting influences beyond SES. Unfortunately, we lacked data regarding these lifestyle factors, and future studies will be needed to assess relative contributions of lifestyle factors associated with both higher birthweight and later weight-gain.
Factors linking low birthweight and later metabolic risk are proposed to include epigenic changes including DNA methylation and histone modification that may occur in both conditions of placental insufficiency, poor maternal nutrition or even post-natal challenges.5,6,24 The role of prematurity per se is uncertain, though preterm status has been associated with with a greater degree of insulin resistance in later childhood.10 Thus, it may be notable that we found that lower size-for-gesational-age was associated with risk of obesity by 2nd grade among children born preterm. There is potential that the combination of preterm status and lower for gestational age was instrumental in weight accumulation by 2nd grade. Further following for progression of these associations in this cohort is warranted.
The data in the current analysis add longitudinal information to previous studies from either local cohorts or children born prior to the current obesity epidemic. In a nationally-representative cohort of children born in 1993, Cunningham et al. found that higher birthweight was associated with a higher prevalence of obesity by age 5, though they were not able to assess this relationship among preterm children.11 In a cohort from Boston born from 1999–2002, Perng et al. found that higher birthweight was associated with both higher amounts of both lean mass and fat mass at approximately 8 years old—suggesting that the higher BMI-z-scores in these children likely consist of some degree of unhealthy weight-gain,13 potentially related to “catch-up fat.”9 Our study supports that infants born large have greater odds of overweight and obesity through 2nd grade at a minimum, as well as greater increase in BMI-z-score as compared to those born at small and normal weights.
Knowledge of these obesity risks may help guide pediatricians regarding which patients to target for intervention. In this case, parents of children born with higher birthweight could be counselled from a younger age to practice lifestyle habits likely to restrain further unhealthy weight accumulation such as reduced TV exposure,16 avoidance of sugar-sweetened beverages and juice,25,26 receiving adequate sleep,27 and a higher level of physical activity.28 While these practices should be targeted at all children, underscoring their importance to childhren at higher risk, particularly early in life while these habits are more plastic, may help avoid obesity and related risks in adulthood.29,30
This study had several limitations including the observational study design with potential for selection bias. We also lacked comprehensive data on the entire population, including factors related to maternal health and lifestyle practices, which may have helped determine intrinsic vs. extrinsic variables behind these associations. While we adjusted for SES at kindergarden entry, we were unable to take into account SES over time. Our predictors relied on parental recall of birthweight and prematurity. However, this study had many strengths, including longitudinal following of a recent large, nationally-representative cohort. We were able to adjust for multiple potential confounders such as race/ethnicity with potential relationships to birthweight and obesity.1
Conclusion
In conclusion, we found strong associations between high birthweight and future childhood overweight and obesity. Infants with higher birthweights had increased odds of obesity from kindergarten through second grade as compared to children with normal and lower birthweight. Given the current high prevalence of obesity and declining time for physician counseling, our findings may have implications for better physician preparation in nutrition and weight control recommendations. Special attention should be paid early in life to those infants born large for their age to help prevent later obesity and its associated health problems.
Supplementary Material
What is already known on this subject
Childhood obesity has become a recent epidemic both in the U.S. and globally.
Both high and low birthweight have been suggested to be linked to risk for future obesity, though previous studies have lacked power, national representation, or were performed before the current obesity epidemic.
What this study adds
High birthweight in children born preterm or term was a predictor of future obesity from kindergarden through second grade and more rapid weight gain in the interval between kindergarten and first grade.
Contrary to previous hypotheses, we did not observe consistently higher BMI z-scores or more rapid weight gain in children born at lower birthweights; an exception to this was that among preterm children, those born small-for-gestational age were twice as likely as average-for-gestational age children to be obese by 2nd grade.
Acknowledgments
Funding source: This work was supported by NIH grant 1R01HL120960 (MJG and MDD) and a Doris Duke Charitable Foundation Clinical Scientist Development Award (RJS).
Abbreviations
- AGA
average for gestational age
- aOR
adjusted odds ratio
- ECSL-K
Early Childhood Longitudinal Survey-Kindergarten
- HBW
high birthweight
- LBW
low birthweight
- LGA
large for gestational age
- NBW
normal birthweight
- NCES
National Center for Education Statistics
- SGA
small for gestational age
Footnotes
Conflict of interst statement: The authors have no conflicts of interest.
References
- 1.Ogden CL, Carroll MD, Lawman HG, Fryar CD, Kruszon-Moran D, Kit BK, et al. Trends in Obesity Prevalence Among Children and Adolescents in the United States, 1988–1994 Through 2013–2014. JAMA. 2016;315(21):2292–2299. doi: 10.1001/jama.2016.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Flegal KM. Prevalence of obesity in the United States. JAMA. 2014;312(2):189–190. doi: 10.1001/jama.2014.6228. [DOI] [PubMed] [Google Scholar]
- 3.Olds T, Maher C, Zumin S, Péneau S, Lioret S, Castetbon K, et al. Evidence that the prevalence of childhood overweight is plateauing: data from nine countries. Int J Pediatr Obes. 2011;6(5–6):342–360. doi: 10.3109/17477166.2011.605895. [DOI] [PubMed] [Google Scholar]
- 4.DeBoer MD. Obesity, systemic inflammation, and increased risk for cardiovascular disease and diabetes among adolescents: A need for screening tools to target interventions. Nutrition. 2013;29(2):379–386. doi: 10.1016/j.nut.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hales CN, Barker DJ. The thrifty phenotype hypothesis. Br Med Bull. 2001;60:5–20. doi: 10.1093/bmb/60.1.5. [DOI] [PubMed] [Google Scholar]
- 6.Hanson M, Godfrey KM, Lillycrop KA, Burdge GC, Gluckman PD. Developmental plasticity and developmental origins of non-communicable disease: Theoretical considerations and epigenetic mechanisms. Progress in Biophysics & Molecular Biology. 2011;106(1):272–280. doi: 10.1016/j.pbiomolbio.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 7.Byberg L, McKeigue PM, Zethelius B, Lithell HO. Birth weight and the insulin resistance syndrome: association of low birth weight with truncal obesity and raised plasminogen activator inhibitor-1 but not with abdominal obesity or plasma lipid disturbances. Diabetologia. 2000;43(1):54–60. doi: 10.1007/s001250050007. [DOI] [PubMed] [Google Scholar]
- 8.Ong KK, Ahmed ML, Emmett PM, Preece MA, Dunger DB. Association between postnatal catch-up growth and obesity in childhood: prospective cohort study. BMJ. 2000;320(7240):967–971. doi: 10.1136/bmj.320.7240.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dulloo AG, Jacquet J, Seydoux J, Montani JP. The thrifty ‘catch-up fat’ phenotype: its impact on insulin sensitivity during growth trajectories to obesity and metabolic syndrome. Int J Obes (Lond) 2006;30(Suppl 4):S23–35. doi: 10.1038/sj.ijo.0803516. [DOI] [PubMed] [Google Scholar]
- 10.Hofman PL, Regan F, Jackson WE, Jefferies C, Knight DB, Robinson EM, et al. Premature birth and later insulin resistance. N Engl J Med. 2004;351(21):2179–2186. doi: 10.1056/NEJMoa042275. [DOI] [PubMed] [Google Scholar]
- 11.Cunningham SA, Kramer MR, Narayan KM. Incidence of childhood obesity in the United States. N Engl J Med. 2014;370(5):403–411. doi: 10.1056/NEJMoa1309753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mitchell EA, Stewart AW, Braithwaite I, Hancox RJ, Murphy R, Wall C, et al. Birth weight and subsequent body mass index in children: an international cross-sectional study. Pediatr Obes. 2016 doi: 10.1111/ijpo.12138. [DOI] [PubMed] [Google Scholar]
- 13.Perng W, Hajj H, Belfort MB, Rifas-Shiman SL, Kramer MS, Gillman MW, et al. Birth Size, Early Life Weight Gain, and Midchildhood Cardiometabolic Health. J Pediatr. 2016;173:122–130.e121. doi: 10.1016/j.jpeds.2016.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loos RJ, Beunen G, Fagard R, Derom C, Vlietinck R. Birth weight and body composition in young adult men–a prospective twin study. Int J Obes Relat Metab Disord. 2001;25(10):1537–1545. doi: 10.1038/sj.ijo.0801743. [DOI] [PubMed] [Google Scholar]
- 15.Mulligan GM, Hastedt S, McCarroll JC. First-Time Kindergartners in 2010–11: First Findings From the Kindergarten Rounds of the Early Childhood Longitudinal Study, Kindergarten Class of 2010–11 (ECLS-K:2011) Washington, DC: U.S. Department of Education, National Center for Education Statistics; 2012. [Google Scholar]
- 16.Peck T, Scharf RJ, Conaway MR, DeBoer MD. Viewing as little as 1 hour of TV daily is associated with higher change in BMI between kindergarten and first grade. Obesity (Silver Spring) 2015;23(8):1680–1686. doi: 10.1002/oby.21132. [DOI] [PubMed] [Google Scholar]
- 17.Fenton TR, Nasser R, Eliasziw M, Kim JH, Bilan D, Sauve R. Validating the weight gain of preterm infants between the reference growth curve of the fetus and the term infant. BMC Pediatr. 2013;13:92. doi: 10.1186/1471-2431-13-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Curhan GC, Willett WC, Rimm EB, Spiegelman D, Ascherio AL, Stampfer MJ. Birth weight and adult hypertension, diabetes mellitus, and obesity in US men. Circulation. 1996;94(12):3246–3250. doi: 10.1161/01.cir.94.12.3246. [DOI] [PubMed] [Google Scholar]
- 19.Horikoshi M, Yaghootkar H, Mook-Kanamori DO, Sovio U, Taal HR, Hennig BJ, et al. New loci associated with birth weight identify genetic links between intrauterine growth and adult height and metabolism. Nat Genet. 2013;45(1):76–82. doi: 10.1038/ng.2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barker DJ, Gluckman PD, Godfrey KM, Harding JE, Owens JA, Robinson JS. Fetal nutrition and cardiovascular disease in adult life. Lancet. 1993;341(8850):938–941. doi: 10.1016/0140-6736(93)91224-a. [DOI] [PubMed] [Google Scholar]
- 21.Mizutani T, Suzuki K, Kondo N, Yamagata Z. Association of maternal lifestyles including smoking during pregnancy with childhood obesity. Obesity (Silver Spring) 2007;15(12):3133–3139. doi: 10.1038/oby.2007.373. [DOI] [PubMed] [Google Scholar]
- 22.Bhargava SK, Sachdev HS, Fall CH, Osmond C, Lakshmy R, Barker DJ, et al. Relation of serial changes in childhood body-mass index to impaired glucose tolerance in young adulthood. N Engl J Med. 2004;350(9):865–875. doi: 10.1056/NEJMoa035698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barker DJ, Osmond C, Forsen TJ, Kajantie E, Eriksson JG. Trajectories of growth among children who have coronary events as adults. N Engl J Med. 2005;353(17):1802–1809. doi: 10.1056/NEJMoa044160. [DOI] [PubMed] [Google Scholar]
- 24.DeBoer MD, Lima AA, Oría RB, Scharf RJ, Moore SR, Luna MA, et al. Early childhood growth failure and the developmental origins of adult disease: do enteric infections and malnutrition increase risk for the metabolic syndrome? Nutr Rev. 2012;70(11):642–653. doi: 10.1111/j.1753-4887.2012.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeBoer MD, Scharf RJ, Demmer RT. Sugar-sweetened beverages and weight gain in 2- to 5-year-old children. Pediatrics. 2013;132(3):413–420. doi: 10.1542/peds.2013-0570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shefferly A, Scharf RJ, DeBoer MD. Longitudinal evaluation of 100% fruit juice consumption on BMI status in 2–5-year-old children. Pediatr Obes. 2016;11(3):221–227. doi: 10.1111/ijpo.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scharf RJ, DeBoer MD. Sleep timing and longitudinal weight gain in 4- and 5-year-old children. Pediatr Obes. 2014 doi: 10.1111/ijpo.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boone-Heinonen J, Markwardt S, Fortmann SP, Thornburg KL. Overcoming birth weight: can physical activity mitigate birth weight-related differences in adiposity? Pediatr Obes. 2016;11(3):166–173. doi: 10.1111/ijpo.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gardner DS, Hosking J, Metcalf BS, Jeffery AN, Voss LD, Wilkin TJ. Contribution of early weight gain to childhood overweight and metabolic health: a longitudinal study (EarlyBird 36) Pediatrics. 2009;123(1):e67–73. doi: 10.1542/peds.2008-1292. [DOI] [PubMed] [Google Scholar]
- 30.Whitaker RC, Wright JA, Pepe MS, Seidel KD, Dietz WH. Predicting obesity in young adulthood from childhood and parental obesity. New England Journal of Medicine. 1997;337(13):869–873. doi: 10.1056/NEJM199709253371301. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


