Abstract
Erythritol is a naturally abundant sweetener gaining more and more importance especially within the food industry. It is widely used as sweetener in calorie-reduced food, candies, or bakery products. In research focusing on sugar alternatives, erythritol is a key issue due to its, compared to other polyols, challenging production. It cannot be chemically synthesized in a commercially worthwhile way resulting in a switch to biotechnological production. In this area, research efforts have been made to improve concentration, productivity, and yield. This mini review will give an overview on the attempts to improve erythritol production as well as their development over time.
Keywords: Erythritol, Sugar alcohols, Polyols, Sweetener, Sugar, Sugar alternatives
Introduction
Because of today’s lifestyle, the number of people suffering from diabetes mellitus and obesity is increasing. The desire of the customers to regain their health created a whole market of non-sugar and non-caloric or non-nutrient foods. An important part of this market is the production of sugar alcohols, the so-called polyols. The applications vary from food over cosmetics to pharmaceuticals. Whereas polyols like sorbitol, xylitol, mannitol, lactitol, and maltitol are already established and widely used as sugar alternatives for quite a while, erythritol is still developing its whole potential (Billaux et al. 1991; Goossens and Roper 1994). As the production of erythritol is more difficult than of the other polyols, intensive research was performed to optimize its production in terms of improving erythritol concentration, productivity rate, and/or yield. Reports on erythritol reflect the scientific and commercial history of erythritol production. Early studies collected data and information about naturally producing organisms. Later investigations focused on media and cultivation optimization as well as metabolic pathway engineering to increase the amount of produced erythritol. Then, research split into two directions. One focused the discovery of alternative, suitable organisms, like bacteria or filamentous fungi, to open up the range of optimization parameters. The other research direction focused on metabolic pathway engineering or genetic engineering to improve yield and productivity as well as to allow the use of inexpensive and abundant substrates. This review will present the history of erythritol production-related research from a more commercial viewpoint moving towards sustainability and fundamental research.
Erythritol
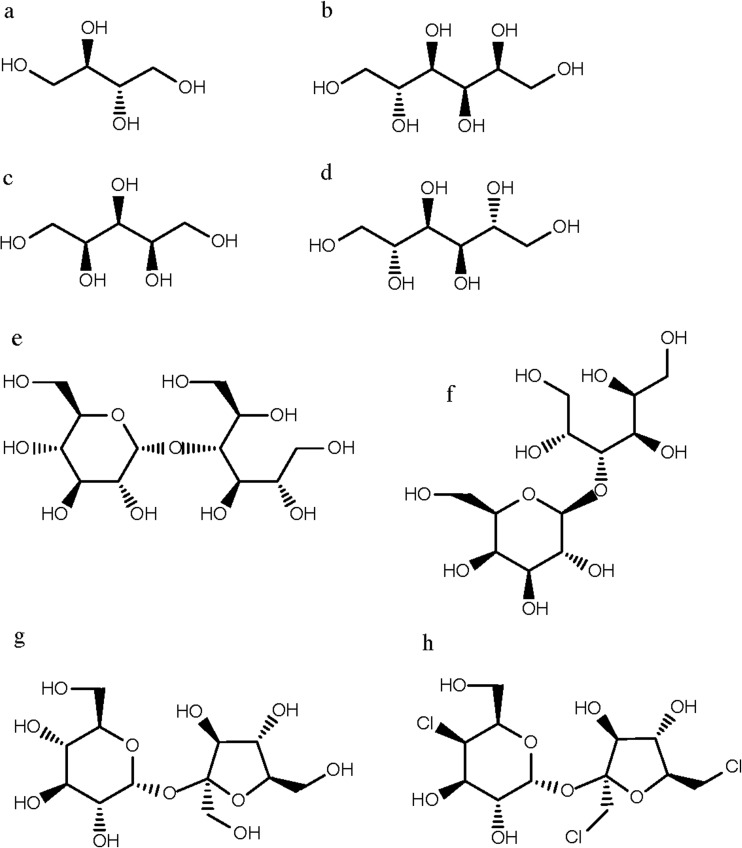
Erythritol ((2R,3S)-Butan-1,2,3,4-tetrol) belongs to the family of sugar alcohols also known as polyols, which are formed due to hydrolysation processes of the aldehyde or ketone group in various carbohydrates (Billaux et al. 1991). The chemical structure of erythritol and the other sweeteners discussed in this review are provided in Fig. 1. Polyols are naturally abundant in fruits and vegetables, like grapes and mushrooms as well as in fermented foods like soy sauce (Bernt et al. 1996; Shindou et al. 1988; Yoshida et al. 1986). The most valuable properties of these sugar alcohols are their sweetness and low calorie content combined with being non-cariogenic (Mäkinen 1994). For an overview on these properties, see Table 1. Within the sugar alcohols, erythritol plays a somehow extraordinary part. It consists of only four carbon atoms and has therefore the smallest molecular weight of all sugar alcohols, which is associated with slightly different physical and chemical properties. Erythritol is also a symmetrical molecule and therefore exists only in one form, the meso-form (Fig. 1). It forms anhydrous crystals with a moderate sweetness of 60–80% of sucrose (Goossens and Gonze 1996) (Table 1). However, as an advantage, it can be mixed with more intense sugars due to the absence of any aftertaste (Barbieri et al. 2014; Bernt et al. 1996; Moon et al. 2010). But due to the high production costs of erythritol compared to more intense sweeteners, it is not primarily chosen for its sweetness synergy. As a more important feature, erythritol can improve the mouth feeling and can mask certain unwanted aftertastes such as astringency and the irritant effect of intense sweeteners (de Cock 2012). When dissolved, erythritol exhibits a strong cooling effect due to its high negative heat of solution (Park et al. 2005). Along with the artificial sweetener sucralose, it is the only polyol that is non-caloric, providing no energy to the body. The majority of erythritol cannot be metabolized by the human body and is excreted unmodified into the urine without changing blood glucose and insulin levels (de Cock 2012; Efsa Panel on Dietetic Products and Allergies 2011; Grabitske and Slavin 2008). The latter is a stand-alone property of erythritol among the commonly used polyols and allows its usage as sweetener in specialized food for diabetics or people suffering obesity (Wheeler and Pi-Sunyer 2008). It also means that a severe disadvantage of other polyols, namely sorbitol and xylitol, leading to diarrhea is eliminated (Bernt et al. 1996; de Cock 2012). Only a little amount, less than 10%, undergoes a reversible metabolic reaction like the dehydration to d- or l-erythrulose (Moon et al. 2010; Park et al. 2005, 2016). Finally, erythritol is also a free radical scavenger with the ability to potentially exercise its anti-oxidant activity while circulating the body before it is excreted into the urine (de Cock 2012; den Hartog et al. 2010). For an overview on the biological effectiveness and reported side effects of the sweeteners discussed in this review, see Table 2.
Fig. 1.
Chemical structures of sweeteners discussed in this review, namely a erythritol, b sorbitol, c xylitol, d mannitol, e maltitol, f lactitol, g sucrose, and h sucralose
Table 1.
Properties of sweeteners discussed in this review
| Sweetener | Systematic name | Synonyms | Glycemic index1 | Caloric value (kcal/g)1 | Sweetness2 |
|---|---|---|---|---|---|
| Sucrose | (2R,3R,4S,5S,6R)-2-[(2S,3S,4S,5R)-3,4-dihydroxy-2,5-bis(hydroxymethyl)oxolan-2-yl]oxy-6-(hydroxymethyl)oxane-3,4,5-triol | Sugar Saccharose |
65.03 | 3.9 | 1.0 |
| Erythritol | (2R,3S)-Butane-1,2,3,4-tetrol | Erythrit Meso-erythritol Tetrahydroxybutane |
0.0 | 0.2 | 0.6–0.8 |
| Xylitol | D-erythro-pentitol | Xylit Birkenzucker |
13.0 | 2.4 | 1.0 |
| Mannitol | D-Mannitol | Mannite | 0.0 | 1.6 | 0.5–0.7 |
| Sorbitol | D-Glucitol | D-Glucitol syrup Sorbit Sorbol |
9.0 | 2.7 | 0.5–0.7 |
| Maltitol | 4-O-α-d-Glucopyranosyl-d-glucitol | Dried maltitol syrup Hydrogenated maltose Maltitol syrup powder |
35.0 | 2.1 | 0.9 |
| Lactitol | 4-O-β-l-Galactopyranosyl-l-glucitol | Lactit Lactobiosit Lactositol |
6.0 | 1.9 | 0.3–0.4 |
| Sucralose | (1 → 6)-Dichloro-(1 → 6)-dideoxy-β-D-fructofuranosyl-4-chloro-4-deoxy-α-D-galactopyranoside | Trichlorosucrose TGS Splenda |
0.0 | 0.0 | 320.0–1000 |
Table 2.
Biological effectiveness and reported side effects of polyols (Grembecka 2015) and sucralose discussed in this review
| Sweetener | Biological effectiveness | Reported side effects |
|---|---|---|
| Erythritol | Non-caloric Non-glycemic Non-cariogenic High digestive tolerance Free radical scavenger Non-acidogenicity Anti-oxidative and endothelium-protective properties Increases malabsorption of fructose |
Non-observed Symptoms of overconsumption are flatulence and laxation |
| Xylitol | Low calorie index Low glycemic index Non-cariogenic, improves dental health Increases saliva production, which helps in treating xerostomia Protects salivary proteins, has a protein-stabilizing effect Improves breath odor Reduces infections in the mouth and nasopharynx Anti-ketogenic—decreases serum-free fatty acid levels and improves peripheral glucose utilization Favors absorption of calcium and B vitamins Inhibits growth of yeast, including Candida albicans Decreases glycation of proteins, reduces AGEs Helps to maintain healthy gut function |
Temporary laxation and gastrointestinal discomfort |
| Mannitol | Low calorie index Reduces the rise in blood glucose and insulin levels Non-cariogenic When inhaled, helpful in mucus and cough clearance in asthmatics and other hypersecretory diseases |
In amounts greater than 20 mg/kg body weight may cause abdominal pain, excessive gas (flatulence), loose stools or diarrhea |
| Sorbitol | Reduced calorie value Low glycemic index Non-cariogenic |
Osmotic diarrhea as a result of intestinal malabsorption when ingested dose is greater than 50 g per day Consumption of 20–30 g/day results in abdominal pain |
| Maltitol | Reduced calorie value Low glycemic index Non-cariogenic Increases mineral bioavailability in human and rats Combination with short-chain fructo-oligosaccharides in sugar-free food product formulations results in lower postprandial glycemic responses |
Abdominal bloating and laxative effect when consumed in large quantities |
| Lactitol | Reduced calorie value Non-cariogenic Increases the growth of probiotic bacteria Reduces the population of putrefactive bacteria Lowers the intestinal pH Role in treating encephalopathy and constipation Increases mineral bioavailability in human and rats |
Bloating and flatulence after an intake more than 20 g in a single dose |
| Sucralose | Non-caloric Non-glycemic Non-carcinogenic Poorly absorbed and no dechlorination Not accumulated in fat; readily eliminated1 |
Not recommended for fructose-intolerant persons Induces glucose-intolerance by altering gut microbiota2 |
Applications
Although it was firstly isolated in 1852, it took until 1990 for erythritol to become present on the Japanese market as a new natural sweetener (Boesten et al. 2015). The range of applications for erythritol is still growing. It can currently be found on its own, or in combination with other polyols in foods, cosmetics, and pharmaceuticals.
To date, the use of erythritol in foods has been approved in more than 60 countries, including Europe, the USA, Japan, Canada, Mexico, Brazil, Argentina, Turkey, Russia, China, India, Australia and New Zealand (Boesten et al. 2015; de Cock 2012). Within the food sector, erythritol is mainly utilized as sweetener to balance the finished product with regard to its sensory characteristics, such as flavor, color, and texture. Erythritol can therefore be used to produce no-sugar added, reduced-sugar, or sugar-free alternatives. Erythritol as sugar replacement can be found as tabletop sweetener, in beverages, chewing gum, chocolate, candies, and in bakery products (de Cock 2012). Due to its mild sweetness, it allows a volume-for-volume replacement of sugar, whereas for example, sucralose that has a much higher sweetness needs fillers and even then has a noticeably different texture in baked products. With regard to sucralose, it also needs to be considered that it is a chemically synthesized substance that does not naturally occur in nature. As a consequence, it currently accumulates in the environment due to the lack of sufficient natural degradation mechanisms (Lubick 2008).
Polyols are commonly used within the personal care industry like the cosmetic or toiletries sector. They are more and more incorporated as excipients in the manufacture of care products like toothpaste, mouthwashes, creams and lotions, make-up, perfumes, or deodorants. Due to its humectant function as well as its pleasant taste, its sweetness and its non-cariogenic properties, erythritol can be used as base for toothpaste and mouthwash recipes (EPA European association of polyol producers 2017). It gives toothpastes the required viscosity and humectancy. Additionally, erythritol inhibits the growth of Streptococcus mutans and acts as caries limiting in combination with xylitol (de Cock et al. 2016; Grembecka 2015). Further, it was found that a 3-year consumption of erythritol-containing candies by 7- to 8-year-old children resulted in reduced plaque growth, lower levels of plaque acetic acid, and propionic acid (Grembecka 2015; Runnel et al. 2013).
Erythritol can be used in a wide range of solid and liquid formulations, including granulated powders, tablets, tablet coating, consumer-friendly lozenges, medicated chewing gum, syrups, and as mentioned before, as oral care products (Michaud and Haest 2003). For pharmaceutical use, its interaction with water and its high stability in temperature and in acid or alkaline environments is a key (Grembecka 2015). Because of its properties, erythritol as excipient offers good flowability and stability, making it an ideal carrier for actives in sachets and capsules. More and more active ingredients are derived from biotechnological processes, which are often very efficient but also extremely reactive. Using the non-reducing sugar erythritol instead of lactose, which is the most commonly used pharmaceutical excipient, the unwanted reaction between the amino groups of the active and the reducing sugar can be prevented. Therefore, lactose is more often being replaced by erythritol. Besides this, non hygroscopic polyols need to be used when a very water-sensitive active has to be reformulated (EPA European association of polyol producers 2017).
Production—history and development
To extract erythritol from its natural sources, like fruits or vegetables, is not practical because of their low erythritol contents. And in contrast to the other polyols, erythritol is not favored being produced via chemical synthesis. The needed high temperatures as well as the nickel catalyst result in a cost-ineffective reaction with a low product yield (Park et al. 2005; Pfeifer et al. 1960). When in 1950, traces of erythritol were found in the residue of Cuban blackstrap molasses fermented by yeast, a flourishing new possibility opened up: the biotechnological production of erythritol (Deng et al. 2012). Please see Table 3 for an overview on the process parameters of the following discussed strategies or microorganisms.
Table 3.
Process parameters for erythritol production of the discussed genetically modified microorganisms
| Microorganism | Genetic modification | Substrate | Concentration (g/l) | Yield (g/g) | Productivity (g/l/h) | Reference |
|---|---|---|---|---|---|---|
| Yeast | ||||||
| T. megachiliensis SN-G42 | Mutagenesis | Glucose | 164.8 | nd1 | 2 | (Ishizuka et al. 1989), (Sawada et al. 2009) |
| C. magnoliae JH110 | Mutagenesis | Glucose | 200 | 0.43 | 1.2 | (Kohl et al. 2003) |
| C. magnoliae 12-2 | Mutagenesis | Glucose | 20.3 | nd | nd | (Ghezelbash et al. 2014) |
| T. oedocephalis HOG1 | Δhog1 | Glucose | 56.8 | 0.28 | nd | (Li et al. 2016) |
| Y. lipolytica MK1U- pADUTSuc2 | suc2 | Sucrose | 52 | 0.26 | 0.9 | (Mirończuk et al. 2015) |
| Y. lipolytica AMM pADUTSuc2 92h | suc2 | Sucrose | 73 | 0.37 | 1.0 | (Mirończuk et al. 2015) |
| Y. lipolytica AMM pADUTSuc2 144h | suc2 | Sucrose | 113.9 | 0.57 | 0.8 | (Mirończuk et al. 2015) |
| Y. lipolytica AJD pADUTSuc2 | suc2 | Sucrose | 59.5 | 0.32 | 0.9 | (Mirończuk et al. 2015) |
| Y. lipolytica AIB pAD-UTGUT1 | gut1 | Sucrose and glycerol | 82.2 | 0.55 | 0.87 | (Rakicka et al. 2017) |
| Y. lipolytica AMM pAD-GND1 | gnd1 | Glycerol | 40.2 | 0.4 | 0.43 | (Mirończuk et al. 2017) |
| Y. lipolytica AMM pAD-ZWF1 | zwf1 | Glycerol | 42.5 | 0.43 | 0.45 | (Mirończuk et al. 2017) |
| Y. lipolytica AMM pAD-TLK1 | tkl1 | Glycerol | 51.1 | 0.51 | 0.54 | (Mirończuk et al. 2017) |
| Y. lipolytica ΔYALI0F01606g | eyk1 | Glycerol | nd | 0.49 | 0.59 | (Carly et al. 2017) |
| Bacteria | ||||||
| O. oeni | Glucose | nd | nd | nd | (Ortiz et al. 2013) | |
| L. sanfranciscencis | Glucose | nd | nd | nd | (Ortiz et al. 2013) | |
| Synechocystis PCC6803 | err1/e4pp | None2 | nd | nd | nd | (van der Woude et al. 2016) |
| Filamentous fungi | ||||||
| T. reesei | err1 | Wheat straw | nd | nd | nd | (Jovanovic et al. 2013) |
1nd means not determined
2The strain is cultivated under moderate intensity white-light illumination or high intensity illumination for production via photosynthesis
Production in yeast
The production of erythritol was first observed in yeasts and yeast-like fungi. A strain probably belonging to the genus Torula was able to convert 35–40% of utilized glucose into erythritol. However, at the very beginning, it became clear, that although the production of erythritol is inherent in yeast, the fermentation conditions influence the yield in a large scale (Hajny et al. 1964). To understand the connection between metabolism and the fermentation parameters used, it is important to have a look at the natural production of erythritol in yeast. Erythritol is produced via the pentose phosphate pathway, in which d-erythrose-4-phosphate is dephosphorylated to d-erythrose and then reduced to erythritol. This pathway serves various purposes: the production and provision of reduction power in form of NADPH for cellular reactions, the production of precursors for the nucleotide and amino acid biosynthesis, and on its own as compatible solute to protect and stabilize enzymes facilitating cellular functions under osmotic conditions (Brown 1978; Moon et al. 2010). Its function as osmo-protectant is the reason for using osmophilic yeasts such as Moniliella pollinis, Trichosporonoides megachiliensis, Aureobasidium sp., Trigonopsis variabilis, Trichosporon sp., Torula sp., and Candida magnoliae as production strains (Chattopadhyay et al. 2014; Grembecka 2015; Lin et al. 2010). They are mainly producing erythritol when encountering salt or osmotic stress (Yang et al. 2015). However, unfavorable fermentation conditions can lead to the production of glycerol at the expense of erythritol formation because glycerol is the main osmolyte in yeasts. Consequently, the next important task was finding methods to increase the erythritol yield and minimize the reaction shift towards glycerol production. Two strategies, namely random mutagenesis and fermentation optimization, were implemented. Random mutagenesis by UV and chemical mutagens revealed mutants producing more erythritol and less by-products than their parental strains due to improved activities and expression levels of key enzymes involved in the pentose phosphate pathway (Park et al. 2016). Several high-yield mutation strains such as T. megachiliensis SNG-42, C. magnoliae JH110, or C. magnoliae 12-2 obtained by ultraviolet and chemical mutagenesis are currently used for erythritol production (Li et al. 2016). Besides random mutagenesis, research moved further to targeted gene editing as more and more knowledge about the regulation of erythritol could be obtained. The HOG1 gene encodes a mitogen-activated protein kinase (MAK) that plays an important role in osmo-adaptation and therefore might influence the production of glycerol and erythritol. The created Trichosporonoides oedocephalis HOG1 knockout mutant produced 1.4-fold more erythritol than its parental strain. Also, the glycerol production was decreased by 71.23% (Li et al. 2016).
Although erythritol-producing strains are able to convert glucose or fructose into erythritol, much higher productivities and yields were achieved by regulating the initial glucose concentration using a fed-batch fermentation with optimized media and vitamin or mineral supplementation (Moon et al. 2010; Park et al. 2016). Optimizing the fermentation parameters, such as controlling the dissolved oxygen and stirring during the process, could reduce the undesired accumulation of glycerol (Li et al. 2016). Additionally, the role of osmotic and salt stress became more important to improve erythritol yields. It was found that a DNA sequence corresponding to the putative response stress element within the erythrose reductase gene from C. magnolia is upregulated under osmotic and salt stress conditions. This stress can be caused by high concentrations of sugar, potassium chloride, or sodium chloride in the fermentation broth (Park et al. 2011).
Side by side, while the erythritol production methods were getting more polished and perfected, the search for other carbon sources than glucose or fructose as substrate began. The yeast Yarrowia lipolytica can convert pure or crude glycerol into different substances including polyols (Dobrowolski et al. 2016). Glycerol is a renewable feedstock and the main by-product of the bio-diesel production (Mirończuk et al. 2015; Moon et al. 2010). Hence, recombinant strains of Y. lipolytica that express the SUC2 gene from Saccharomyces cerevisiae were generated. By expression of this gene, the organism gains the ability to utilize sucrose, which is the main content of molasses, an agro-industrial by-product (Mirończuk et al. 2015). Such approaches are exemplarily reflecting the trend of using inexpensive raw materials for the conversion into value-added products. In addition to inserting the SUC2 gene, metabolic engineering by overexpressing the GUT1 gene leads to sucrose and glycerol utilization with efficient erythritol production. The GUT1 gene functions as glycerol kinase in the phosphorylative glycerol catabolic pathway in yeast. The enzyme is important for its ability to assimilate glycerol that somehow interferes with erythritol formation and complicates downstream processing (Rakicka et al. 2017). Even though, the strain has been genetically modified to produce more erythritol, the metabolic pathway in Y. lipolytica has never been described before. Now, a new study showed the formation of erythritol via pentose phosphate pathway trying additionally to identify the key genes involved (Mirończuk et al. 2017). The overexpression of genes involved in the pentose phosphate pathway, transketolase TKL1, and two dehydrogenases ZWF1 and GND1 revealed a twofold higher erythritol synthesis for the transketolase overexpression strain, showing the importance of this gene for erythritol biosynthesis (Mirończuk et al. 2017). Quite recently, another gene EYK1, coding for an erythrulose kinase, was found to enhance erythritol production (Carly et al. 2017).
Production in bacteria
Not only the desire for more cost-friendly substrates but also the possibility to use other production host organisms than yeasts is growing. The heterofermentative lactic acid bacteria Oenococcus oeni produces erythritol in an alternative pathway for NADPH reoxidation during anaerobic glucose metabolism (Veiga-da-Cunha et al. 1993). The formation of erythritol differs from the synthesis in yeasts: glucose-6-phosphate is converted into fructose-6-phoshate. After cleavage into erythrose-4-phosphate and acetyl-phosphate, the erythrose-4-phosphate is first reduced to erythritol-4-phosphate and then dephosphorylated to erythritol (Ortiz et al. 2013; Veiga-da-Cunha et al. 1993). Lactobacillus sanfranciscencis, another LAB strain isolated from sourdough, produces erythritol under stress conditions as an additional metabolic product (Ortiz et al. 2013). An interesting aspect of using functional lactic starter cultures with the ability to produce polyols may be the production of novel fermented food naturally sweetened with these low-calorie sugars.
A totally different approach to improve sustainability in erythritol production was performed using a genetic engineered cyanobacteria strain: Synechocystis PCC6803. Several characterized erythrose reductases and erythrose-4-phosphatases from fungi were introduced into the genome of Synechocystis PCC6803. The expressed enzymes can then convert erythrose-4-phosphate into erythritol. Erythrose-4-phosphate is inherent and is built as a key intermediate of the CO2-fixing Calvin Benson Bassham cycle (van der Woude et al. 2016).
Production in filamentous fungi
Erythrose reductase is the key enzyme for the production of erythritol and a lot of research has been done on the characterization and purification of various reductases in yeasts (Deng et al. 2012). In Trichoderma reesei, a filamentous fungus industrially used due to its ability to secrete vast amounts of cellulases and hemicellulases, the erythrose reductase is also naturally present and has already been characterized (Jovanovic et al. 2013). The erythritol production is, like in yeast, part of the pentose phosphate pathway. d-Erythrose-4-phosphate is first dephosphorylated and then further reduced to erythritol. This reaction is NADPH-dependent and reversible (Jovanovic et al. 2013). Although the production of erythritol in this strain is naturally not as high as in yeasts, the organism has other advantages. It is capable of growing on lignocellulosic material (Acebal et al. 1986) and therefore can utilize cheap biowaste materials as sole carbon source. This can be used for the production of erythritol. The strain can perfectly grow and produce erythritol on wheat straw. It was reported that an erythrose reductase overexpression strain leads to a clearly higher erythritol formation on pretreated wheat straw (Jovanovic et al. 2014). With further substrate optimization, strain improvement, pathway engineering, and fermentation optimization, this might be a promising way to bypass the utilization of expensive fermentation substrates.
Conclusion
Over time, different approaches have been applied to increase the production of erythritol. Because the demand of erythritol increased in a short time, the commercial availability became most important. First priority was a fast optimization of the fermentation parameters for the cultivation of known erythritol-producing organisms and random mutagenesis. Although the enzymes were soon known, and in some cases have already been characterized, just little research focused on the regulation of the expression of the corresponding genes. Only in the last few years, hypotheses about gene regulation started to be enlightened by targeted gene editing. The knowledge achieved from this research turned out to be useful for targeted genetic strain improvements and opened up new parameters for further fermentation optimization. Later on, the question about ecological sustainability arose. Is it justifiable to produce a sugar substitute using a sugar as feedstock, thereby creating a product that is furthermore expensive? The research work performed on the industrial cellulase and hemicellulase production strain T. reesei tried to combine all different optimization approaches. The organism is able to degrade lignocellulosic material and can therefore utilize renewable and cheap material, like wheat straw as starting material. The substrate can be pretreated to facilitate analysis and speeding up the degradation by T. reesei. The key enzyme for the synthesis of erythritol is naturally present in T. reesei and has been successfully expressed and characterized in Escherichia coli. Finally, leads an overexpression of this gene to an increase in erythritol synthesis. The ongoing research focuses on getting a whole picture about the pathway of erythritol synthesis, the enzymes involved, and the influence of certain production parameters like osmotic pressure with the aim to gain a value-added product from cheap renewable biomaterial.
Acknowledgements
Open access funding provided by Austrian Science Fund (FWF).
Funding
This work was supported by a grant from the Austrian Science Fund (FWF): P26733 given to ARMA.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
References
- Acebal C, Castillon MP, Estrada P, Mata I, Costa E, Aguado J, Romero D, Jimenez F. Enhanced cellulase production from Trichoderma reesei QM9414 on physically treated wheat straw. Appl Microbiol Biotechnol. 1986;24(3):218–223. doi: 10.1007/BF00261540. [DOI] [Google Scholar]
- Barbieri G, Barone C, Bhagat A, Caruso G, Conley ZR, Parisi S. Sweet compounds in foods: sugar alcohols the influence of chemistry on new foods and traditional products. Cham: Springer International Publishing; 2014. pp. 51–59. [Google Scholar]
- Bernt WO, Borzelleca JF, Flamm G, Munro IC. Erythritol: a review of biological and toxicological studies. Regul Toxicol Pharmacol. 1996;24(2 Pt 2):S191–S197. doi: 10.1006/rtph.1996.0098. [DOI] [PubMed] [Google Scholar]
- Billaux MS, Flourie B, Jacquemin C, Messing B. Sugar alcohols. In: Marie S, Piggott JR, editors. Handbook of sweeteners. Boston: Springer US; 1991. pp. 72–103. [Google Scholar]
- Boesten DMPHJ, den Hartog GJM, de Cock P, Bosscher D, Bonnema A, Bast A. Health effects of erythritol. Nutrafoods. 2015;14(1):3–9. doi: 10.1007/s13749-014-0067-5. [DOI] [Google Scholar]
- Brown A. Compatible solutes and extreme water stress in eukaryotic microorganisms. Adv Microb Physiol. 1978;17:181–242. doi: 10.1016/S0065-2911(08)60058-2. [DOI] [PubMed] [Google Scholar]
- Canada H (2016) Sugar alcohols (polyols) and polydextrose used as sweeteners in foods—food safety—Health Canada. Publisher. https://www.canada.ca/en/health-canada/services/food-nutrition/food-safety/food-additives/sugar-substitutes/sugar-alcohols-polyols-polydextrose-used-sweeteners-foods-food-safety.html. Accessed 9.11.2017 2017
- Carly F, Gamboa-Melendez H, Vandermies M, Damblon C, Nicaud JM, Fickers P. Identification and characterization of EYK1, a key gene for erythritol catabolism in Yarrowia lipolytica. Appl Microbiol Biotechnol. 2017;101(17):6587–6596. doi: 10.1007/s00253-017-8361-y. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay S, Raychaudhuri U, Chakraborty R. Artificial sweeteners—a review. J Food Sci Technol. 2014;51(4):611–621. doi: 10.1007/s13197-011-0571-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Cock P. Erythritol sweeteners and sugar alternatives in food technology. Hoboken: Wiley-Blackwell; 2012. pp. 213–241. [Google Scholar]
- de Cock P, Makinen K, Honkala E, Saag M, Kennepohl E, Eapen A. Erythritol is more effective than xylitol and sorbitol in managing oral health endpoints. Int J Dent. 2016;2016:1–15. doi: 10.1155/2016/9868421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Hartog GJ, Boots AW, Adam-Perrot A, Brouns F, Verkooijen IW, Weseler AR, Haenen GR, Bast A. Erythritol is a sweet antioxidant. Nutrition. 2010;26(4):449–458. doi: 10.1016/j.nut.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Deng H, Han Y, Liu Y, Jia W, Zhou Z. Identification of a newly isolated erythritol-producing yeast and cloning of its erythrose [corrected] reductase genes. J Ind Microbiol Biotechnol. 2012;39(11):1663–1672. doi: 10.1007/s10295-012-1162-5. [DOI] [PubMed] [Google Scholar]
- Dobrowolski A, Mitula P, Rymowicz W, Mironczuk AM. Efficient conversion of crude glycerol from various industrial wastes into single cell oil by yeast Yarrowia lipolytica. Bioresour Technol. 2016;207:237–243. doi: 10.1016/j.biortech.2016.02.039. [DOI] [PubMed] [Google Scholar]
- Efsa Panel on Dietetic Products N, Allergies Scientific opinion on the substantiation of health claims related to the sugar replacers xylitol, sorbitol, mannitol, maltitol, lactitol, isomalt, erythritol, D-tagatose, isomaltulose, sucralose and polydextrose and maintenance of tooth mineralisation by decreasing tooth demineralisation (ID 463, 464, 563, 618, 647, 1182, 1591, 2907, 2921, 4300), and reduction of post-prandial glycaemic responses (ID 617, 619, 669, 1590, 1762, 2903, 2908, 2920) pursuant to article 13(1) of regulation (EC) no 1924/2006. EFSA J. 2011;9(4):2076. doi: 10.2903/j.efsa.2011.2076. [DOI] [Google Scholar]
- EPA European association of polyol producers (2017) Publisher. http://www.polyols-eu.org/cosmetics Accessed 20.08.17
- Ghezelbash GR, Nahvi I, Malekpour A. Erythritol production with minimum by-product using Candida magnoliae mutant. Prikl Biokhim Mikrobiol. 2014;50(3):324–328. [PubMed] [Google Scholar]
- Goossens J, Gonze M. Nutritional properties and applications of erythritol: a unique combination? In: Grenby TH, editor. Advances in sweeteners. Boston: Springer US; 1996. pp. 150–186. [Google Scholar]
- Goossens J, Roper H (1994) Erythritol: a new sweetener. Confectionery Prod 60(3):182–184
- Grabitske HA, Slavin JL. Low-digestible carbohydrates in practice. J Am Diet Assoc. 2008;108(10):1677–1681. doi: 10.1016/j.jada.2008.07.010. [DOI] [PubMed] [Google Scholar]
- Grembecka M. Sugar alcohols—their role in the modern world of sweeteners: a review. Eur Food Res Technol. 2015;241(1):1–14. doi: 10.1007/s00217-015-2437-7. [DOI] [Google Scholar]
- Hajny GJ, Smith JH, Garver JC. Erythritol production by a yeastlike fungus. Appl Microbiol. 1964;12:240–246. doi: 10.1128/am.12.3.240-246.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuka H, Wako K, Kasumi T, Sasaki T. Breeding of a mutant of Aureobasidium sp. with high erythritol production. J Ferment Bioeng. 1989;68(5):310–314. doi: 10.1016/0922-338X(89)90003-2. [DOI] [Google Scholar]
- Jovanovic B, Mach RL, Mach-Aigner AR. Characterization of erythrose reductases from filamentous fungi. AMB Express. 2013;3(1):43. doi: 10.1186/2191-0855-3-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic B, Mach RL, Mach-Aigner AR. Erythritol production on wheat straw using Trichoderma reesei. AMB Express. 2014;4(1):34. doi: 10.1186/s13568-014-0034-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl ES, Leet TH, Lee DY, Kim HJ, Ryu YW, Seo JH. Scale-up of erythritol production by an osmophilic mutant of Candida magnoliae. Biotechnol Lett. 2003;25(24):2103–2105. doi: 10.1023/B:BILE.0000007076.64338.ce. [DOI] [PubMed] [Google Scholar]
- Li L, Yang T, Guo W, Ju X, Hu C, Tang B, Fu J, Gu J, Zhang H. Construction of an efficient mutant strain of Trichosporonoides oedocephalis with HOG1 gene deletion for production of erythritol. J Microbiol Biotechnol. 2016;26(4):700–709. doi: 10.4014/jmb.1510.10049. [DOI] [PubMed] [Google Scholar]
- Lin S-J, Wen C-Y, Wang P-M, Huang J-C, Wei C-L, Chang J-W, Chu W-S. High-level production of erythritol by mutants of osmophilic Moniliella sp. Process Biochem. 2010;45(6):973–979. doi: 10.1016/j.procbio.2010.03.003. [DOI] [Google Scholar]
- Livesey G. Health potential of polyols as sugar replacers, with emphasis on low glycaemic properties. Nutr Res Rev. 2003;16(2):163–191. doi: 10.1079/NRR200371. [DOI] [PubMed] [Google Scholar]
- Lubick N. Artificial sweetener persists in the environment. Environ Sci Technol. 2008;42(9):3125. doi: 10.1021/es087043g. [DOI] [PubMed] [Google Scholar]
- Magnuson BA, Roberts A, Nestmann ER. Critical review of the current literature on the safety of sucralose. Food Chem Toxicol. 2017;106(Pt A):324–355. doi: 10.1016/j.fct.2017.05.047. [DOI] [PubMed] [Google Scholar]
- Mäkinen KK (1994) Sugar alcohols. In: Goldberg I (eds) Functional foods: designer foods, pharmafoods, nutraceuticals. Spinger US, New York, pp 219–241
- Michaud J, Haest G (2003) Erythritol: a new multipurpose excipient. PUblisher. http://www.pharmtech.com/erythritol-new-multipurpose-excipient?id=&sk=&date=&%0A%09%09%09&pageID=2. Accessed Aug 2017
- Mirończuk AM, Rakicka M, Biegalska A, Rymowicz W, Dobrowolski A. A two-stage fermentation process of erythritol production by yeast Y. lipolytica from molasses and glycerol. Bioresour Technol. 2015;198:445–455. doi: 10.1016/j.biortech.2015.09.008. [DOI] [PubMed] [Google Scholar]
- Mirończuk AM, Biegalska A, Dobrowolski A. Functional overexpression of genes involved in erythritol synthesis in the yeast Yarrowia lipolytica. Biotechnol Biofuels. 2017;10(1):77. doi: 10.1186/s13068-017-0772-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon HJ, Jeya M, Kim IW, Lee JK. Biotechnological production of erythritol and its applications. Appl Microbiol Biotechnol. 2010;86(4):1017–1025. doi: 10.1007/s00253-010-2496-4. [DOI] [PubMed] [Google Scholar]
- Ortiz ME, Bleckwedel J, Raya RR, Mozzi F. Biotechnological and in situ food production of polyols by lactic acid bacteria. Appl Microbiol Biotechnol. 2013;97(11):4713–4726. doi: 10.1007/s00253-013-4884-z. [DOI] [PubMed] [Google Scholar]
- Park YC, Lee DY, Lee DH, Kim HJ, Ryu YW, Seo JH. Proteomics and physiology of erythritol-producing strains. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;815(1–2):251–260. doi: 10.1016/j.jchromb.2004.10.065. [DOI] [PubMed] [Google Scholar]
- Park EH, Lee HY, Ryu YW, Seo JH, Kim MD. Role of osmotic and salt stress in the expression of erythrose reductase in Candida magnoliae. J Microbiol Biotechnol. 2011;21(10):1064–1068. doi: 10.4014/jmb.1105.05029. [DOI] [PubMed] [Google Scholar]
- Park YC, Oh EJ, Jo JH, Jin YS, Seo JH. Recent advances in biological production of sugar alcohols. Curr Opin Biotechnol. 2016;37:105–113. doi: 10.1016/j.copbio.2015.11.006. [DOI] [PubMed] [Google Scholar]
- Pfeifer VF, Sohns VE, Conway HF, Lancaster EB, Dabic S, Griffin EL. Two stage process for dialdehyde starch using electrolytic regeneration of periodic acid. Ind Eng Chem. 1960;52(3):201–206. doi: 10.1021/ie50603a020. [DOI] [Google Scholar]
- Rakicka M, Biegalska A, Rymowicz W, Dobrowolski A, Mironczuk AM. Polyol production from waste materials by genetically modified Yarrowia lipolytica. Bioresour Technol. 2017;243:393–399. doi: 10.1016/j.biortech.2017.06.137. [DOI] [PubMed] [Google Scholar]
- Runnel R, Makinen KK, Honkala S, Olak J, Makinen PL, Nommela R, Vahlberg T, Honkala E, Saag M. Effect of three-year consumption of erythritol, xylitol and sorbitol candies on various plaque and salivary caries-related variables. J Dent. 2013;41(12):1236–1244. doi: 10.1016/j.jdent.2013.09.007. [DOI] [PubMed] [Google Scholar]
- Sawada K, Taki A, Yamakawa T, Seki M. Key role for transketolase activity in erythritol production by Trichosporonoides megachiliensis SN-G42. J Biosci Bioeng. 2009;108(5):385–390. doi: 10.1016/j.jbiosc.2009.05.008. [DOI] [PubMed] [Google Scholar]
- Shindou T, Sasaki Y, Miki H, Eguchi T, Hagiwara K, Ichikawa T (1988) Determination of erythritol in fermented foods by high performance liquid chromatography. J Food Hyg Safe Sci Jpn 29(6):419–422
- Suez J, Korem T, Zeevi D, Zilberman-Schapira G, Thaiss CA, Maza O, Israeli D, Zmora N, Gilad S, Weinberger A, Kuperman Y, Harmelin A, Kolodkin-Gal I, Shapiro H, Halpern Z, Segal E, Elinav E. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature. 2014;514(7521):181–186. doi: 10.1038/nature13793. [DOI] [PubMed] [Google Scholar]
- van der Woude AD, Perez Gallego R, Vreugdenhil A, Puthan Veetil V, Chroumpi T, Hellingwerf KJ. Genetic engineering of Synechocystis PCC6803 for the photoautotrophic production of the sweetener erythritol. Microb Cell Factories. 2016;15(1):60. doi: 10.1186/s12934-016-0458-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiga-da-Cunha M, Santos H, Van Schaftingen E. Pathway and regulation of erythritol formation in Leuconostoc oenos. J Bacteriol. 1993;175(13):3941–3948. doi: 10.1128/jb.175.13.3941-3948.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler ML, Pi-Sunyer FX. Carbohydrate issues: type and amount. J Am Diet Assoc. 2008;108(4 Suppl 1):S34–S39. doi: 10.1016/j.jada.2008.01.024. [DOI] [PubMed] [Google Scholar]
- Yang LB, Dai XM, Zheng ZY, Zhu L, Zhan XB, Lin CC. Proteomic analysis of erythritol-producing Yarrowia lipolytica from glycerol in response to osmotic pressure. J Microbiol Biotechnol. 2015;25(7):1056–1069. doi: 10.4014/jmb.1412.12026. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Hayashi J, Sugahara T (1986) Studies on free sugars, free sugar alcohols and organic acids of wild mushrooms. J Jpn Soc Food Sci Technol 33:426–433