Abstract
Brettanomyces bruxellensis is a common and significant wine spoilage microorganism. B. bruxellensis strains generally detain the molecular basis to produce compounds that are detrimental for the organoleptic quality of the wine, including some classes of volatile phenols that derive from the sequential bioconversion of specific hydroxycinnamic acids such as ferulate and p-coumarate. Although B. bruxellensis can be detected at any stage of the winemaking process, it is typically isolated at the end of the alcoholic fermentation (AF), before the staring of the spontaneous malolactic fermentation (MLF) or during barrel aging. For this reason, the endemic diffusion of B. bruxellensis leads to consistent economic losses in the wine industry. Considering the interest in reducing sulfur dioxide use during winemaking, in recent years, biological alternatives, such as the use of tailored selected yeast and bacterial strains inoculated to promote AF and MLF, are actively sought as biocontrol agents to avoid the “Bretta” character in wines. Here, we review the importance of dedicated characterization and selection of starter cultures for AF and MLF in wine, in order to reduce or prevent both growth of B. bruxellensis and its production of volatile phenols in the matrix.
Keywords: Brettanomyces bruxellensis, Wine, Saccharomyces, malolactic fermentation (MLF), Lactic acid bacteria
Introduction
The success of winemaking in terms of safety and quality considerably depends on the metabolism of microorganisms present on the grapes and during the fermentation process (Grangeteau et al. 2017, Liu et al. 2017). Several microbial species can cause depreciation of wine since they produce detrimental compounds that negatively affect wine aroma and flavors (Suárez et al. 2007). Among the spoilage microorganisms, the yeast Brettanomyces bruxellensis is generally considered one of the most relevant in term of depreciation potential. This species, because of its ability to survive during the winemaking process, within several years, has become a major oenological concern worldwide (Di Toro et al. 2015, Steensels et al. 2015, Capozzi et al. 2016). This species can persist through the harsh conditions, such as ethanol concentrations associated to the alcoholic fermentation (AF) and increasing additions of sulfur dioxide (SO2). Brettanomyces strains are well suited to surviving on all surfaces, in and around the winery: winery walls, presses, and fermentation tanks as well as within the barrels used for aging (Fugelsang 1997). Furthermore, the biofilms formed by B. bruxellensis causes important problems, as microbial cells in biofilms often showed an increased resistance to stressing conditions, including chemical cleaning agents and sanitisers (Oelofse et al. 2008).
Brettanomyces bruxellensis is able to live in environments uninhabited by other microorganisms, due to the “desolation” of these media, because the simultaneous presence of different stressors, e.g., high ethanol content, low pH, and starvation (Smith and Divol 2016). The genome sequencing has revealed genes allowing for the utilization of a varied range of substrates (Curtin and Pretorius 2014, Crauwels et al. 2015). In grape must, Saccharomyces cerevisiae is strongly adapted and easily dominates B. bruxellensis. In contrast, B. bruxellensis is well adapted to the physico-chemical conditions characterizing wines after the AF (Nardi et al. 2010). The mechanisms regarding either the growth in wine of B. bruxellensis or how it can outcompete all other yeasts after AF are nowadays not fully understood.
The risk of microbial spoilage can be minimize by the application of good cellar hygienic practices such as the reduction of the latent phase between the end of AF, the good performed malolactic fermentation (MLF), and the early wine stabilization. For years, SO2 has been employed as chemical preservative by winemakers for its antioxidant and microbiostatic properties (Divol et al. 2012, Zuehlke et al. 2013), and it is the most commonly added preservative to grape must and wine to limit the development of B. bruxellensis and other unwanted microorganisms (Couto et al. 2005, Oelofse et al. 2008). The response of B. bruxellensis to SO2 has been extensively studied (Longin et al. 2016) and various surviving strategies have been reported including sulfur reduction, acetaldehyde production, active sulfur efflux, and ability of this yeast to enter in a viable but not culturable (VBNC) state (Serpaggi et al. 2012; Divol et al. 2012; Capozzi et al. 2016). During the VBNC state, the yeast cells are able to remain viable while temporarily losing their ability to proliferate on culture media (Capozzi et al. 2016). Moreover, different strains display a range of sensitivity to SO2 (Louw et al. 2016), also in terms of SO2-induced VBNC state (Capozzi et al. 2016). In addition, investigations from Curtin et al. (2011) showed that B. bruxellensis isolates exhibit strain-dependent tolerance to sulphite. Considering human consumption, it is important to underline how these preservative molecules are usually linked to adverse effects in wine consumers, including allergic reactions, asthma and headaches (Guerrero and Cantos-Villar 2015). Several physico-chemical approaches have been tested to avoid undesired proliferation in wine contaminated by B. bruxellensis, providing an overview of these applications and underlining the main pros and cons about their use in oenology (Table 1).
Table 1.
Possible treatments for the control of B. bruxellensis in wine
| Treatment | Benefits | Disadvantages | Reference |
|---|---|---|---|
| Heat | Destroys microorganisms | Only used in barrels | Fabrizio et al. 2015 |
| Filtration | Reduces the number of cells by physical separation | Loss of color and aroma | Duarte et al. 2017 |
| Protein clarification | Reduces the number of cells by flocculation | Loss of color and aroma | Murat and Dumeau 2003 |
| SO2 | Inhibits cell proliferation. Prevents the ethylphenols formation and oxidation | Microbial resistance. Adverse effects in human health | Guerrero and Cantos-Villar 2015 |
| Chitosan | Inhibits cell proliferation. Prevents the ethylphenols formation | Loss of color. Only from fungal origin is permitted | Portugal et al. 2014 |
| Dimethyl dicarbonate | Inhibits cell proliferation. Prevents the ethylphenols formation | High costs. Dosing machine is needed | Renouf et al. 2008 |
| High pressure | Eliminates cells | High costs. Pressure and time dependent | van Wyk and Silva 2017 |
| Pulsed electric fields | Eliminates cells | High costs. | Puertolas et al. 2009 |
Biological alternatives are increasingly explored, including the use of starter cultures tailored to control spoilage microorganisms (García-Moruno and Muñoz 2012, Oro et al. 2014). Since the first developments of starter cultures technology in the wine sector, a particular attention has been deserved on the exploitation of intraspecific biodiversity within species responsible for AF (S. cerevisiae) and for MLF (Oenococcus oeni and Lactobacillus plantarum) (Berbegal et al. 2016). Moreover, in the last decade, several studies suggested the oenological application of strains/species belonging to the heterogeneous class of non-Saccharomyces yeasts (de Benedictis et al. 2011; Tristezza et al. 2016b; Berbegal et al. 2017b; Petruzzi et al. 2017). These species offer new opportunities to develop biotechnological solutions to cope with specific problems, hence improving the quality and safety of wines (Petruzzi et al. 2017). The aim of this review is to draw up a record of the current knowledge on the use of tailored starter cultures against B. bruxellensis yeast and their application in winemaking conditions.
Chemistry and B. bruxellensis development in wine: the production of off-flavors
The undesirable compounds most commonly associated with B. bruxellensis in wine contaminations are 4-vinylphenol, 4-vinylguaiacol, 4-ethylphenol (4-EP), and 4-ethylguaiacol (4-EG) (Chatonnet et al. 1995, Harris et al. 2008). The production of high concentrations of 4-EP are associated with unpleasant aromas described as “stable,” “horse sweat,” or “leather” (Chatonnet et al. 1995, Steensels et al. 2015). In last years, the formation of these compounds has been deeply studied and several reviews that highlight this topic have been published (Suárez et al. 2007, Wedral et al. 2010).
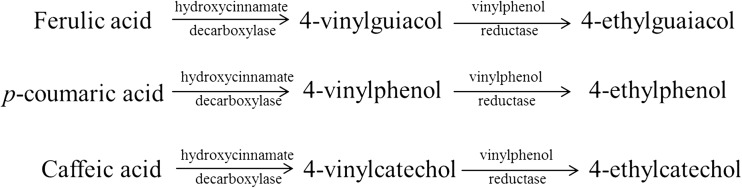
The origin of volatile phenols involves the sequential action of two enzymes on a hydroxycinnamic acid (ferulic, p-coumaric, or caffeic acid) substrate. In the first step, the hydroxycinnamate decarboxylase transforms the hydroxycinnamic acids into vinylphenols (Edlin et al. 1998), and then, the vinylphenol reductase reduced them to ethyl derivatives (Dias et al. 2003) (Fig. 1).
Fig. 1.
Formation of ethylphenols from their hydroxycinnamic precursors
Recent studies have demonstrated that B. bruxellensis is not the only microorganism that can produce 4-EP and 4-EG and that the capacity to produce these compounds is a strain-specific feature (Conterno et al. 2010). Several other microorganisms, including some lactic acid bacteria (LAB) and non-Saccharomyces yeasts, are able to produce volatile phenols (Chatonnet et al. 1995, Fras et al. 2014). What differentiates B. bruxellensis from the other microorganisms is its capacity to synthetize the highest amounts of these ethylphenols (Dias et al. 2003, Malfeito-Ferreira 2011). Different concentrations of 4-EP and 4-EG appear in wine depending on the variety of grape used, vinicultural conditions, and winemaking practices (Wedral et al. 2010). 4-EG are associated with descriptive expressions such as “bacon” or “smoked” (Suárez et al. 2007). Another ethylphenol produced by B. bruxellensis is the 4-ethylcatechol (4-EC), which has the caffeic acid as precursor and it is denoted by its medicinal aroma. 4-EC has, usually, a lower detection threshold than other ethyl phenols (Loureiro and Malfeito-Ferreira 2006). B. bruxellensis can metabolize only the free-form of p-coumaric, caffeic, and ferulic acids. Therefore, the conversion of coutaric acid by the cinnamyl esterase enzyme to p-coumaric acid by other microorganisms can contribute to the increased production of ethyl phenols by B. bruxellensis (Schopp et al. 2013).
Controlling volatile phenol formation
Preventing the increase of the concentrations of precursors of volatile phenols
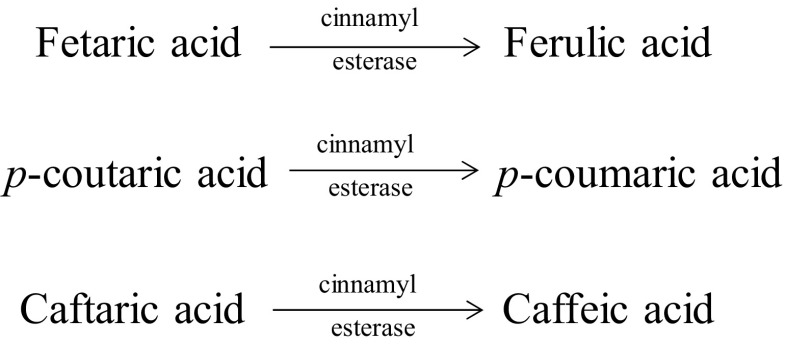
Ferulic, p-coumaric, and caffeic acids are naturally present in grape must and are typically found as esters of tartaric acid (fetaric, coutaric, and caftaric acids, respectively). During winemaking, these tartaric acid esters can be hydrolyzed, forming free hydroxycinnamic acids (Nagel and Wulf 1979). B. bruxellensis can metabolize only the free-form of these hydroxycinnamic acids. Therefore, the conversion of, for example, coutaric acid by the cinnamyl esterase enzyme to p-coumaric acid by other microorganisms as LAB can contribute to the increased production of ethylphenols by B. bruxellensis by increasing the concentration of ethylphenol precursors (Schopp et al. 2013) (Fig. 2).
Fig. 2.
Formation of free hydroxycinnamic acids from their esters of tartaric acid precursors
A possible strategy to reduce the precursors of ethylphenols is the use of S. cerevisiae strains with hydroxycinnamate decarboxylase (HCDC+) activity and able to carry out the AF (Suárez-Lepe and Morata 2012). The vinilphenols formed are able to spontaneously react with grape anthocyanins producing vinylphenolic pyranoanthocyanins. These molecules are stable pigments under oenological conditions, which can reduce the concentration of ethylphenol precursors (Romero and Bakker 2000; Bakker and Timberlake 1997). Morata et al. (2013) fermented grape musts using HCDC+ yeast strains, previously treated with cinnamylesterases in order to quickly release the grape hydroxycinnamic acids. The treated musts showed lower contents of 4-EP than those fermented by employing HCDC strains. The reduction in the ethylphenol content was due to the transformation of hydroxycinnamic acids in stable vinylphenolic pyranoanthocyanins pigments (Morata et al. 2013).
Studies from Hernández et al. (2007) and Cabrita et al. (2008) demonstarted that an increase in free hydroxycinnamic acids concentrations in wine at the end of the MLF was recorded. Nevertheless, Burns and Osborne (2013) observed an increase in p-coumaric and caffeic acids after MLF, and in this case, the fermentation was carried out by a commercial O. oeni strain. Chescheir et al. (2015) examined 10 commercial O. oeni strains for their ability to degrade tartaric acids—hydroxycinnamic acids—in Pinot noir wine. All strains completed MLF but one strain was able to degrade the caftaric and coutaric acids, thus increasing the amounts of caffeic and p-coumaric acids (Chescheir et al. 2015). The augmented free hydroxycinnamic acid content in wines significantly increased the production of 4-EP and 4-EG during growth of an inoculated B. bruxellensis strain. These studies confirm the importance of the inoculation of appropriately selected strains of S. cerevisiae and LAB to carry out the AF and MLF in order to control the volatile phenol precursors.
Performing spontaneous MLF increases the spoilage potential of B. bruxellensis in wine. Indeed, indigenous wine LAB associated with MLF may be able to degrade tartaric acid–hydroxycinnamic acids. Therefore, the selection criteria for commercial malolactic starters include the inability to degrade tartaric acid—hydroxycinnamic acids—in order to ensure satisfactory organoleptic properties of the final wine. Additional research to identify the genes encoding the O. oeni tartaric acid—hydroxycinnamic acid esterase—would enable a more efficient selection of wine LAB strains usable as commercial cultures.
Preventing the volatile phenol formation by lactic acid bacteria
Even though B. bruxellensis is not the only microorganism able to produce significant amounts of ethylphenols (Chatonnet et al. 1992), other microbes are capable to synthetize volatile phenols. Some LAB, such as Pediococcus and Lactobacillus are also able to produce volatile phenols from free hydroxycinnamic acid as p-coumaric, caffeic, and ferulic acids (Couto et al. 2006, Fras et al. 2014). For instance, Lactobacillus brevis and Pediococcus pentosaceus are able to produce significant amounts of 4-VP, but only traces of ethylphenols. L. plantarum is the only bacteria able to produce significant amounts of 4-EP (Chatonnet et al. 1995). Madsen et al. (2016) investigated the effect of two commercial O. oeni strains, with or without cinnamoyl esterase activity, on the contents of the hydroxycinnamic acids (p-coumaric and ferulic acid) in wine. Moreover, the authors studied the formation of volatile phenols 4-ethylphenol and 4-ethylguaiacol during a period of 6 months in Cabernet Sauvignon wines inoculated with two different B. bruxellensis strains. The authors suggested that the level of volatile phenols in wine was mainly associated with B. bruxellensis strain rather than the cinnamoyl esterase activity of O. oeni (Madsen et al. 2016).
Couto et al. (2006) studied the ability of 35 different strains of LAB to produce volatile phenols in culture medium. Results showed that 37% of the strains were capable of producing volatile phenols from p-coumaric acid, and that 9% could produce 4-EP. Chatonnet et al. (1997) studied the influence of polyphenolic compounds on the production of volatile phenols by LAB and found that tannins affect either the L. plantarum growth or the phenolic compound production, although synthesis of volatile phenols by B. bruxellensis was unaffected.
In order to avoid the formation of volatile phenols by LAB, a preventive approach is to carry out a safe and controlled MLF, by using commercial starters unable to form these undesirable compounds. However, the induction of MLF by commercial starters is not always successful because wine is a very harsh environment (Ruiz et al. 2010). The employment of autochthonous starter cultures that are well adapted to the conditions of a specific wine-producing area has been suggested (Ruiz et al. 2010). This feature may represent a concrete opportunity, if we consider that a huge number of studies have been performed on the characterization of autochthonous O. oeni and L. plantarum associated to spontaneous MLF in regional wines (Garofalo et al. 2015, Sun et al. 2016, Berbegal et al. 2016, Berbegal et al. 2017a, Brizuela et al. 2017).
Inhibiting Brettanomyces bruxellensis growth using non-Saccharomyces yeasts
The world wine market has an increase interest in new yeast strains with novel properties (Mylona et al. 2016, Petruzzi et al. 2017). Numerous studies on the influence of non-Saccharomyces yeast in winemaking have highlighted the oenological and technological relevance of these yeast species (Comitini et al. 2011, Tristezza et al. 2016b). Recently, some commercial yeast manufacturers have already included non-Saccharomyces yeast starters in their oenological products (Petruzzi et al. 2017). Strains of non-Saccharomyces yeasts have also shown potential for producing killer toxins with a broader spectrum of activity, inhibiting species within the non-Saccharomyces and the Saccharomyces genera (Petruzzi et al. 2017). Killer yeast strains have the characteristic of secreting toxins of proteinaceous nature that are lethal to sensitive yeast cells. The killer phenomenon in yeasts was first discovered in S. cerevisiae (Bevan and Makower 1963) and, then, reported to be present in many other yeast genera or species (Marquina et al. 2002, Liu et al. 2017).
Since the first record of a killer toxin inhibiting an apiculate yeast (Ciani and Fatichenti 2001), several studies focusing on yeast killer toxins have been conducted with the aim to contrast spoilage wine yeasts such as B. bruxellensis. Mehlomakulu et al. (2014) identified from the wine yeast Candida pyralidae two killer toxins, CpKT1 and CpKT2, that showed to possess a specific killer activity against several B. bruxellensis strains. A similar action was described for the killer toxins isolated from T. delbrueckii (Villalba et al. 2016), Ustilago maydis (Santos et al. 2011), Klyveromyces wickerhami and Pichia anomala (Comitini et al. 2004), and Pichia membranifaciens (Belda et al. 2017) (Table 2). These killer toxins were both active at oenological conditions, confirming their potential use as a biocontrol tool in winemaking process. Under winemaking conditions, the killer toxin Kwkt was efficient and comparable to the use of SO2 in inhibiting B. bruxellensis (Comitini and Ciani 2011). Killer toxins Kwkt and Pikt maintain their killer activity for 10 days in wine (Comitini et al. 2004). The killer toxins active against B. bruxellensis were stable at acidic pH ranges and at temperatures between 20 and 25 °C, which were compatible with winemaking conditions. Besides, these killer toxins were applied in trial fermentations without affecting the population of S. cerevisiae (Santos et al. 2009, Comitini and Ciani 2011, Santos et al. 2011). In addition, the metabolic by-products ethyl acetate and 4-ethylphenol were not detected and volatile acidity was reduced, confirming the antimicrobial efficiency of these killer toxins (Comitini and Ciani 2011, Santos et al. 2011).
Table 2.
Killer toxins secreted by non-Saccharomyces yeast against B. bruxellensis that have potential application in wine industry
| Yeast/filamentous fungus specie | Killer toxin | Mode of action | Reference |
|---|---|---|---|
| Kluyveromyces wickerhamii | Kwkt | – | (Comitini and Ciani 2011) |
| Pichia anomala | Pikt | – | (Comitini et al. 2004) |
| Pichia membranifaciens | PMTK2 | Cell cycle arrest/apoptosis | (Belda et al. 2017) |
| Candida pyralidae | CpKT1 | Cell Wall and membrane disruption | (Mehlomakulu et al. 2014) |
| Candida pyralidae | CpKT2 | – | (Mehlomakulu et al. 2014) |
| Ustilago maydis | KP6 | K+ depletion | (Santos et al. 2011) |
| Torulospora delbrueckii | TdKT | Cell wall disruption and apoptotic death processes | (Villalba et al. 2016) |
Other biological methods to control B. bruxellensis using non-Saccharomyces-specific strains have been recently investigated. For example, Oro et al. (2014) showed that Metschnikowia pulcherrima secretes pulcherriminic acid, which is an inhibitory to the growth of B. bruxellensis. Moreover, cell-to-cell contact and quorum sensing have been investigated as mechanisms involved in non-Saccharomyces-mixed fermentation. In this regard, quorum sensing was recently examined in H. uvarum, Torulaspora pretoriensis, Zygosaccharomyces bailii, Candida zemplinina, and B. bruxellensis. Results indicated species-specific kinetics for the production of 2-phenylethanol, tryptophol, and tyrosol, considered the main molecules involved in the quorum sensing mechanism (Zupan et al. 2013, Avbelj et al. 2016).
Inhibiting Brettanomyces bruxellensis growth using malolactic starters
Using selected yeasts and an appropriate yeast nutrition, winemakers safeguard a rapid, effective, and complete AF, which prevents the development of spoilage microorganisms (Abrahamse and Bartowsky 2012). However, one of the critical points during the winemaking process in which undesired microorganisms such as B. bruxellensis can develop is the period ranging from the end of AF to the start of MLF. At this stage, there are still some nutrients available to the spoilage microorganisms and, at the same time, microbial competitors are missing, considering that the indigenous LAB consortium is not yet established. Early inoculation with LAB after AF has been suggested as a useful way to control the proliferation of B. bruxellensis. Investigations from Gerbaux et al. (2009) showed that MLF began much sooner in Pinot Noir wines inoculated with two different wine bacteria, which contributed to a shorter duration for the winemaking process and significantly reduced the concentrations of volatile phenols (Gerbaux et al. 2009). Moreover, the inoculation of selected wine bacteria at the beginning of the AF is a solution to shorten the time-lapse between AF and MLF and thereby prevent the development of B. bruxellensis. Yeast and bacteria co-inoculation permits a reduction in overall vinification time and this is generally advantageous to the winery from an economical perspective (Abrahamse and Bartowsky 2012, Cañas et al. 2015). The wine is microbiologically stable, reducing the contamination by spoilage microorganisms, and this permits an earlier addition and reduced amounts of SO2 (Renouf and Murat 2008, Gerbaux et al. 2009). In this case, the importance to assess a microbial-compatibility before their utilization in industrial vinification is crucial (Alexandre et al. 2004, Tristezza et al. 2016a).
Recent studies have been performed by co-inoculating yeasts with commercial LAB strains in red grape must (Abrahamse and Bartowsky 2012, Muñoz et al. 2014, Tristezza et al. 2016a). Muñoz et al. (2014) investigated the inoculation of one commercial O. oeni strain with two S. cerevisiae strains following three different inoculation strategies: simultaneous, 3 days after the yeast inoculation or when AF was close to its end. Early bacterial inoculations with each of the two yeast strains allowed for the rapid development of the bacterial populations and the MLF duration was reduced to 6 days. Abrahamse and Bartowsky (2012) and Tristezza et al. (2016a) evaluated the interactions between commercial yeast and O. oeni strains. Their results indicated that simultaneous yeast and bacteria inoculation at the beginning of AF reduced the duration of the process and simultaneously lowered volatile acidity. Similar results were obtained when experiments were carried out with autochthonous O. oeni strains co-inoculated with S. cerevisiae (Izquierdo Cañas et al. 2012, Cañas et al. 2015
Conclusion
The use of starter cultures for the control of fermentative processes and production of wine with standardized quality is well recognized. Nevertheless, here, we highlighted a further role of selected cultures on (i) the control of development of the spoilage yeast B. bruxellensis and (ii) to prevent volatile phenol formation. Handling the winemaking process by promoting AF and MLF through selected starter cultures inoculation is a crucial point to avoid the development of spoilage microorganisms. Inoculation with selected LAB to induce and accelerate MLF has been reported to be an effective biotechnological tool able to prevent B. bruxellensis contamination. However, an important stage in the malolactic bacteria selection must consider their capacity to inhibit the production of free hydroxycinnamic acids without producing volatile phenols. Besides, appropriate inoculation strategies such as co-inoculation and early or sequential inoculation right after AF could be an effective approaches to prevent the development of B. bruxellensis.
Furthermore, investigations on non-Saccharomyces yeasts possibly denoted by killer yeast activity will supply interesting alternative tools for controlling B. bruxellensis. However, killer toxins from non-Saccharomyces have not yet characterized as well as those of S. cerevisiae, and further investigation should be performed in order to identify their genetic origin, mode of action, and how to employ them at commercial and industrial scale.
Acknowledgements
This research was supported by the Apulia Region in the framework of the Projects “Sviluppo di approcci microbiologici innovativi per il miglioramento della qualità di vini tipici Regionali—NEWINE (Bando “Ricerca e sperimentazione in Agricoltura”; project code PRS_042), “Biotecnologie degli alimenti per l'innovazione e la competitività delle principali filiere regionali: estensione della conservabilità e aspetti funzionali—BIOTECA” (Bando “Aiuti a Sostegno Cluster Tecnologici Regionali”; project code QCBRAJ6), and “Innovazioni di processo e di prodotto nel comparto dei vini spumanti da vitigni autoctoni pugliesi”—IPROVISP (Bando “Aiuti a Sostegno Cluster Tecnologici Regionali”; project code VJBKVF4). Carmen Berbegal was supported by “Programa Atracció de Talent VLC-Campus 2015 de la Universitat de València.” Pasquale Russo was supported by a grant of the Apulian Region in the framework of “Peform Tech (Puglia Emerging Food Technology)” project (practice code LPIJ9P2). Vittorio Capozzi was supported by Fondo di Sviluppo e Coesione 2007–2013—APQ Ricerca Regione Puglia “Programma regionale a sostegno della specializzazione intelligente e della sostenibilità sociale ed ambientale—FutureInResearch.” The open access option was financed by the Department of Sciences of Agriculture, Food and Environment of the University of Foggia (‘Bando per il finanziamento della pubblicazione dei lavori scientifici su riviste di categoria Q1/A’), within the Springer Open Choice/Open Access Programme.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All authors of this paper have read and approved the final version submitted. The contents of this manuscript have not been copyrighted or published previously. No procedures performed in these studies have been conducted in human participants and/or animals.
Availability of supporting data
No supporting data are provided.
References
- Abrahamse CE, Bartowsky EJ. Timing of malolactic fermentation inoculation in Shiraz grape must and wine: influence on chemical composition. World J Microbiol Biotechnol. 2012;28:255–265. doi: 10.1007/s11274-011-0814-3. [DOI] [PubMed] [Google Scholar]
- Alexandre H, Costello PJ, Remize F, Guzzo J, Guilloux-Benatier M. Saccharomyces cerevisiae-Oenococcus oeni interactions in wine: current knowledge and perspectives. Int J Food Microbiol. 2004;93:141–154. doi: 10.1016/j.ijfoodmicro.2003.10.013. [DOI] [PubMed] [Google Scholar]
- Avbelj M, Zupan J, Raspor P. Quorum-sensing in yeast and its potential in wine making. Appl Microbiol Biotechnol. 2016;100:7841–7852. doi: 10.1007/s00253-016-7758-3. [DOI] [PubMed] [Google Scholar]
- Bakker J, Timberlake CF. Isolation, identification, and characterization of new color-stable anthocyanins occurring in some red wines. J Agric Food Chem. 1997;45:35–43. doi: 10.1021/jf960252c. [DOI] [Google Scholar]
- Belda I, Ruiz J, Alonso A, Marquina D, Santos A (2017) The biology of Pichia membranifaciens killer toxins. Toxins (Basel) 9 [DOI] [PMC free article] [PubMed]
- Berbegal C, Peña N, Russo P, Grieco F, Pardo I, Ferrer S, Spano G, Capozzi V. Technological properties of Lactobacillus plantarum strains isolated from grape must fermentation. Food Microbiol. 2016;57:187–194. doi: 10.1016/j.fm.2016.03.002. [DOI] [PubMed] [Google Scholar]
- Berbegal C, Benavent-Gil Y, Navascués E, Calvo A, Albors C, Pardo I, Ferrer S. Lowering histamine formation in a red Ribera del Duero wine (Spain) by using an indigenous O. oeni strain as a malolactic starter. Int J Food Microbiol. 2017;244:11–18. doi: 10.1016/j.ijfoodmicro.2016.12.013. [DOI] [PubMed] [Google Scholar]
- Berbegal C, Spano G, Tristezza M, Grieco F, Capozzi V (2017b) Microbial resources and innovation in the wine production sector. S Afr J Enol Viticult Vit 38
- Bevan E, Makower M. The physiological basis of the killer character in yeast. Proc Int Congr Genet. 1963;1:202–203. [Google Scholar]
- Brizuela NS, Bravo-Ferrada BM, La Hens DV, Hollmann A, Delfederico L, Caballero A, Tymczyszyn EE, Semorile L. Comparative vinification assays with selected Patagonian strains of Oenococcus oeni and Lactobacillus plantarum. LWT Food Sci Technol. 2017;77:348–355. doi: 10.1016/j.lwt.2016.11.023. [DOI] [Google Scholar]
- Burns TR, Osborne JP. Impact of malolactic fermentation on the color and color stability of pinot noir and merlot wine. Am J Enol Viticult. 2013;64:370–377. doi: 10.5344/ajev.2013.13001. [DOI] [Google Scholar]
- Cabrita MJ, Torres M, Palma V, Alves E, Patao R, Costa Freitas AM. Impact of malolactic fermentation on low molecular weight phenolic compounds. Talanta. 2008;74:1281–1286. doi: 10.1016/j.talanta.2007.08.045. [DOI] [PubMed] [Google Scholar]
- Cañas PM, Romero EG, Perez-Martin F, Sesena S, Palop ML. Sequential inoculation versus co-inoculation in Cabernet Franc wine fermentation. Food Sci Technol Int. 2015;21:203–212. doi: 10.1177/1082013214524585. [DOI] [PubMed] [Google Scholar]
- Capozzi V, Di Toro MR, Grieco F, Michelotti V, Salma M, Lamontanara A, Russo P, Orrù L, Alexandre H, Spano G. Viable but not culturable (VBNC) state of Brettanomyces bruxellensis in wine: new insights on molecular basis of VBNC behaviour using a transcriptomic approach. Food Microbiol. 2016;59:196–204. doi: 10.1016/j.fm.2016.06.007. [DOI] [PubMed] [Google Scholar]
- Chatonnet P, Dubourdie D, Boidron J-N, Pons M. The origin of ethylphenols in wines. J Sci Food Agric. 1992;60:165–178. doi: 10.1002/jsfa.2740600205. [DOI] [Google Scholar]
- Chatonnet P, Dubourdieu D, Boidron JN. The influence of Brettanomyces/Dekkera sp. yeasts and lactic acid bacteria on the ethylphenol content of red wines. Am J Enol Viticult. 1995;46:463–468. [Google Scholar]
- Chatonnet P, Viala C, Dubourdieu D. Influence of polyphenolic components of red wines on the microbial synthesis of volatile phenols. Am J Enol Viticult. 1997;48:443–448. [Google Scholar]
- Chescheir S, Philbin D, Osborne JP. Impact of Oenococcus oeni on wine hydroxycinnamic acids and volatile phenol production by Brettanomyces bruxellensis. Am J Enol Viticult. 2015;66:357–362. doi: 10.5344/ajev.2015.14108. [DOI] [Google Scholar]
- Ciani M, Fatichenti F. Killer toxin of Kluyveromyces phaffii DBVPG 6076 as a biopreservative agent to control apiculate wine yeasts. Appl Environ Microbiol. 2001;67:3058–3063. doi: 10.1128/AEM.67.7.3058-3063.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comitini F, Ciani M. Kluyveromyces wickerhamii killer toxin: purification and activity towards Brettanomyces/Dekkera yeasts in grape must. FEMS Microbiol Lett. 2011;316:77–82. doi: 10.1111/j.1574-6968.2010.02194.x. [DOI] [PubMed] [Google Scholar]
- Comitini F, De Ingeniis J, Pepe L, Mannazzu I, Ciani M. Pichia anomala and Kluyveromyces wickerhamii killer toxins as new tools against Dekkera/Brettanomyces spoilage yeasts. FEMS Microbiol Lett. 2004;238:235–240. doi: 10.1111/j.1574-6968.2004.tb09761.x. [DOI] [PubMed] [Google Scholar]
- Comitini F, Gobbi M, Domizio P, Romani C, Lencioni L, Mannazzu I, Ciani M. Selected non-Saccharomyces wine yeasts in controlled multistarter fermentations with Saccharomyces cerevisiae. Food Microbiol. 2011;28:873–882. doi: 10.1016/j.fm.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Conterno L, Fondazione E, Henick-Kling T (2010) 12—Brettanomyces/Dekkera off-flavours and other wine faults associated with microbial spoilage—Reynolds, Andrew G. Managing Wine Quality. Woodhead Publishing, pp 346–387
- Couto JA, Neves F, Campos F, Hogg T. Thermal inactivation of the wine spoilage yeasts Dekkera/Brettanomyces. Int J Food Microbiol. 2005;104:337–344. doi: 10.1016/j.ijfoodmicro.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Couto JA, Campos FM, Figueiredo AR, Hogg TA. Ability of lactic acid bactera to produce volatile phenols. Am J Enol Viticult. 2006;57:166–171. [Google Scholar]
- Crauwels S, Van Assche A, de Jonge R, Borneman AR, Verreth C, Troels P, De Samblanx G, Marchal K, Van de Peer Y, Willems KA, Verstrepen KJ, Curtin CD, Lievens B. Comparative phenomics and targeted use of genomics reveals variation in carbon and nitrogen assimilation among different Brettanomyces bruxellensis strains. Appl Microbiol Biotechnol. 2015;99:9123–9134. doi: 10.1007/s00253-015-6769-9. [DOI] [PubMed] [Google Scholar]
- Curtin CD, Pretorius IS. Genomic insights into the evolution of industrial yeast species Brettanomyces bruxellensis. FEMS Yeast Res. 2014;14:997–1005. doi: 10.1111/1567-1364.12198. [DOI] [PubMed] [Google Scholar]
- Curtin C, Kennedy E, Henschke PA. Genotype-dependent sulphite tolerance of Australian Dekkera (Brettanomyces) bruxellensis wine isolates. Lett Appl Microbiol. 2011;55:56–61. doi: 10.1111/j.1472-765X.2012.03257.x. [DOI] [PubMed] [Google Scholar]
- De Benedictis M, Bleve G, Grieco F, Tristezza M, Tufariello M, Grieco F. An optimized procedure for the enological selection of non-Saccharomyces starter cultures. Antonie Van Leeuwenhoek. 2011;99:189–200. doi: 10.1007/s10482-010-9475-8. [DOI] [PubMed] [Google Scholar]
- Di Toro MR, Capozzi V, Beneduce L, Alexandre H, Tristezza M, Durante M, Tufariello M, Grieco F, Spano G. Intraspecific biodiversity and ‘spoilage potential’ of Brettanomyces bruxellensis in Apulian wines. LWT Food Sci Technol. 2015;60:102–108. doi: 10.1016/j.lwt.2014.06.059. [DOI] [Google Scholar]
- Dias L, Dias S, Sancho T, Stender H, Querol A, Malfeito-Ferreira M, Loureiro V. Identification of yeasts isolated from wine-related environments and capable of producing 4-ethylphenol. Food Microbiol. 2003;20:567–574. doi: 10.1016/S0740-0020(02)00152-1. [DOI] [Google Scholar]
- Divol B, du Toit M, Duckitt E. Surviving in the presence of sulphur dioxide: strategies developed by wine yeasts. Appl Microbiol Biotechnol. 2012;95:601–613. doi: 10.1007/s00253-012-4186-x. [DOI] [PubMed] [Google Scholar]
- Duarte FL, Coimbra L,Baleiras-Couto M (2017) Filter media comparison for the removal of Brettanomyces bruxellensis from wine. Am J Enol Viticult
- Edlin DAN, Narbad A, Gasson MJ, Dickinson JR, Lloyd D. Purification and characterization of hydroxycinnamate decarboxylase from Brettanomyces anomalus. Enzym Microb Technol. 1998;22:232–239. doi: 10.1016/S0141-0229(97)00169-5. [DOI] [Google Scholar]
- Fabrizio V, Vigentini I, Parisi N, Picozzi C, Compagno C, Foschino R. Heat inactivation of wine spoilage yeast Dekkera bruxellensis by hot water treatment. Lett Appl Microbiol. 2015;61:186–191. doi: 10.1111/lam.12444. [DOI] [PubMed] [Google Scholar]
- Fras P, Campos FM, Hogg T, Couto JA. Production of volatile phenols by Lactobacillus plantarum in wine conditions. Biotechnol Lett. 2014;36:281–285. doi: 10.1007/s10529-013-1351-y. [DOI] [PubMed] [Google Scholar]
- Fugelsang KC. Wine microbiology. New York: Chapman & Hall; 1997. [Google Scholar]
- García-Moruno E, Muñoz R. Does Oenococcus oeni produce histamine? Int J Food Microbiol. 2012;157:121–129. doi: 10.1016/j.ijfoodmicro.2012.05.013. [DOI] [PubMed] [Google Scholar]
- Garofalo C, El Khoury M, Lucas P, Bely M, Russo P, Spano G, Capozzi V. Autochthonous starter cultures and indigenous grape variety for regional wine production. J Appl Microbiol. 2015;118:1395–1408. doi: 10.1111/jam.12789. [DOI] [PubMed] [Google Scholar]
- Gerbaux V, Briffox C, Dumont A, Krieger S. Influence of inoculation with malolactic bacteria on volatile phenols in wines. Am J Enol Viticult. 2009;60:233. [Google Scholar]
- Grangeteau C, Roullier-Gall C, Rousseaux S, Gougeon RD, Schmitt-Kopplin P, Alexandre H, Guilloux-Benatier M. Wine microbiology is driven by vineyard and winery anthropogenic factors. Microb Biotechnol. 2017;10:354–370. doi: 10.1111/1751-7915.12428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero RF, Cantos-Villar E. Demonstrating the efficiency of sulphur dioxide replacements in wine: a parameter review. Trends Food Sci Technol. 2015;42:27–43. doi: 10.1016/j.tifs.2014.11.004. [DOI] [Google Scholar]
- Harris V, Ford CM, Jiranek V, Grbin PR. Dekkera and Brettanomyces growth and utilisation of hydroxycinnamic acids in synthetic media. Appl Microbiol Biotechnol. 2008;78:997–1006. doi: 10.1007/s00253-007-1328-7. [DOI] [PubMed] [Google Scholar]
- Hernández T, Estrella I, Pérez-Gordo M, Alegría EG, Tenorio C, Ruiz-Larrea F, Moreno-Arribas MV. Contribution of malolactic fermentation by Oenococcus oeni and Lactobacillus plantarum to the changes in the nonanthocyanin polyphenolic composition of red wine. J Agric Food Chem. 2007;55:5260–5266. doi: 10.1021/jf063638o. [DOI] [PubMed] [Google Scholar]
- Izquierdo Cañas PM, Perez-Martin F, Garcia Romero E, Sesena Prieto S, Palop Herreros Mde L. Influence of inoculation time of an autochthonous selected malolactic bacterium on volatile and sensory profile of Tempranillo and Merlot wines. Int J Food Microbiol. 2012;156:245–254. doi: 10.1016/j.ijfoodmicro.2012.03.033. [DOI] [PubMed] [Google Scholar]
- Liu Y, Rousseaux S, Tourdot-Marechal R, Sadoudi M, Gougeon R, Schmitt-Kopplin P, Alexandre H. Wine microbiome: a dynamic world of microbial interactions. Crit Rev Food Sci Nutr. 2017;57:856–873. doi: 10.1080/10408398.2014.983591. [DOI] [PubMed] [Google Scholar]
- Longin C, Degueurce C, Julliat F, Guilloux-Benatier M, Rousseaux S, Alexandre H. Efficiency of population-dependent sulfite against Brettanomyces bruxellensis in red wine. Food Res Int. 2016;89:620–630. doi: 10.1016/j.foodres.2016.09.019. [DOI] [PubMed] [Google Scholar]
- Loureiro V, Malfeito-Ferreira M (2006) 13 - Dekkera/Brettanomyces spp. - Blackburn, Clive de W. Food Spoilage Microorganisms. Woodhead Publishing, pp 354–398
- Louw M, du Toit M, Alexandre H, Divol B. Comparative morphological characteristics of three Brettanomyces bruxellensis wine strains in the presence/absence of sulfur dioxide. Int J Food Microbiol. 2016;238:79–88. doi: 10.1016/j.ijfoodmicro.2016.08.040. [DOI] [PubMed] [Google Scholar]
- Madsen MG, Edwards NK, Petersen MA, Mokwena L, Swiegers JH, Arneborg N (2016) Influence of Oenococcus oeni and Brettanomyces bruxellensis on hydroxycinnamic acids and volatile phenols of aged wine. Am J Enol Viticult
- Malfeito-Ferreira M. Yeasts and wine off-flavours: a technological perspective. Ann Microbiol. 2011;61:95–102. doi: 10.1007/s13213-010-0098-0. [DOI] [Google Scholar]
- Marquina D, Santos A, Peinado JM. Biology of killer yeasts. Int Microbiol. 2002;5:65–71. doi: 10.1007/s10123-002-0066-z. [DOI] [PubMed] [Google Scholar]
- Mehlomakulu NN, Setati ME, Divol B. Characterization of novel killer toxins secreted by wine-related non-Saccharomyces yeasts and their action on Brettanomyces spp. Int J Food Microbiol. 2014;188:83–91. doi: 10.1016/j.ijfoodmicro.2014.07.015. [DOI] [PubMed] [Google Scholar]
- Morata A, Vejarano R, Ridolfi G, Benito S, Palomero F, Uthurry C, Tesfaye W, Gonzalez C, Suarez-Lepe JA. Reduction of 4-ethylphenol production in red wines using HCDC+ yeasts and cinnamyl esterases. Enzym Microb Technol. 2013;52:99–104. doi: 10.1016/j.enzmictec.2012.11.001. [DOI] [PubMed] [Google Scholar]
- Muñoz V, Beccaria B, Abreo E. Simultaneous and successive inoculations of yeasts and lactic acid bacteria on the fermentation of an unsulfited Tannat grape must. Braz J Microbiol. 2014;45:59–66. doi: 10.1590/S1517-83822014000100009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murat ML, Dumeau F. Impact of fining on population levels of certain spoilage micro-organisms in red wines. Aust N Z Grapegrow Winemak. 2003;478:92–94. [Google Scholar]
- Mylona AE, Del Fresno JM, Palomero F, Loira I, Bañuelos MA, Morata A, Calderón F, Benito S, Suárez-Lepe JA. Use of Schizosaccharomyces strains for wine fermentation—effect on the wine composition and food safety. Int J Food Microbiol. 2016;232:63–72. doi: 10.1016/j.ijfoodmicro.2016.05.023. [DOI] [PubMed] [Google Scholar]
- Nagel CW, Wulf LW. Changes in the anthocyanins, flavonoids and hydroxycinnamic acid esters during fermentation and aging of merlot and cabernet sauvignon. Am J Enol Viticult. 1979;30:111–116. [Google Scholar]
- Nardi T, Remize F, Alexandre H. Adaptation of yeasts Saccharomyces cerevisiae and Brettanomyces bruxellensis to winemaking conditions: a comparative study of stress genes expression. Appl Microbiol Biotechnol. 2010;88:925–937. doi: 10.1007/s00253-010-2786-x. [DOI] [PubMed] [Google Scholar]
- Oelofse A, Pretorius IS, Toit MD (2008) Significance of Brettanomyces and Dekkera during winemaking: a synoptic review. S Afr J Enol Viticult 29
- Oro L, Ciani M, Comitini F. Antimicrobial activity of Metschnikowia pulcherrima on wine yeasts. J Appl Microbiol. 2014;116:1209–1217. doi: 10.1111/jam.12446. [DOI] [PubMed] [Google Scholar]
- Petruzzi L, Capozzi V, Berbegal C, Corbo MR, Bevilacqua A, Spano G, Sinigaglia M (2017) Microbial resources and enological significance: opportunities and benefits. Front Microbiol 8 [DOI] [PMC free article] [PubMed]
- Portugal C, Sáenz Y, Rojo-Bezares B, Zarazaga M, Torres C, Ruiz-Larrea F. Brettanomyces susceptibility to antimicrobial agents used in winemaking: in vitro and practical approaches. Eur Food Res Technol. 2014;238:641–652. doi: 10.1007/s00217-013-2143-2. [DOI] [Google Scholar]
- Puertolas E, Lopez N, Condon S, Raso J, Alvarez I. Pulsed electric fields inactivation of wine spoilage yeast and bacteria. Int J Food Microbiol. 2009;130:49–55. doi: 10.1016/j.ijfoodmicro.2008.12.035. [DOI] [PubMed] [Google Scholar]
- Renouf V, Murat ML. Using malolactic starters for improved control of Brettanomyces risks. Aust N Z Grapegrow Winemak. 2008;528:56–64. [Google Scholar]
- Renouf V, Strehaiano P, Lonvaud-Funel A. Effectiveness of dimethlydicarbonate to prevent Brettanomyces bruxellensis growth in wine. Food Control. 2008;19:208–216. doi: 10.1016/j.foodcont.2007.03.012. [DOI] [Google Scholar]
- Romero C, Bakker J. Effect of storage temperature and pyruvate on kinetics of anthocyanin degradation, vitisin a derivative formation, and color characteristics of model solutions. J Agric Food Chem. 2000;48:2135–2141. doi: 10.1021/jf990998l. [DOI] [PubMed] [Google Scholar]
- Ruiz P, Izquierdo PM, Seseña S, Palop ML. Selection of autochthonous Oenococcus oeni strains according to their oenological properties and vinification results. Int J Food Microbiol. 2010;137:230–235. doi: 10.1016/j.ijfoodmicro.2009.11.027. [DOI] [PubMed] [Google Scholar]
- Santos A, San Mauro M, Bravo E, Marquina D. PMKT2, a new killer toxin from Pichia membranifaciens, and its promising biotechnological properties for control of the spoilage yeast Brettanomyces bruxellensis. Microbiol. 2009;155:624–634. doi: 10.1099/mic.0.023663-0. [DOI] [PubMed] [Google Scholar]
- Santos A, Navascués E, Bravo E, Marquina D. Ustilago maydis killer toxin as a new tool for the biocontrol of the wine spoilage yeast Brettanomyces bruxellensis. Int J Food Microbiol. 2011;145:147–154. doi: 10.1016/j.ijfoodmicro.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Schopp LM, Lee J, Osborne JP, Chescheir SC, Edwards CG. Metabolism of nonesterified and esterified hydroxycinnamic acids in red wines by Brettanomyces bruxellensis. J Agric Food Chem. 2013;61:11610–11617. doi: 10.1021/jf403440k. [DOI] [PubMed] [Google Scholar]
- Serpaggi V, Remize F, Recorbet G, Gaudot-Dumas E, Sequeira-Le Grand A, Alexandre H. Characterization of the “viable but nonculturable” (VBNC) state in the wine spoilage yeast Brettanomyces. Food Microbiol. 2012;30:438–447. doi: 10.1016/j.fm.2011.12.020. [DOI] [PubMed] [Google Scholar]
- Smith BD, Divol B. Brettanomyces bruxellensis, a survivalist prepared for the wine apocalypse and other beverages. Food Microbiol. 2016;59:161–175. doi: 10.1016/j.fm.2016.06.008. [DOI] [PubMed] [Google Scholar]
- Steensels J, Daenen L, Malcorps P, Derdelinckx G, Verachtert H, Verstrepen KJ. Brettanomyces yeasts—from spoilage organisms to valuable contributors to industrial fermentations. Int J Food Microbiol. 2015;206:24–38. doi: 10.1016/j.ijfoodmicro.2015.04.005. [DOI] [PubMed] [Google Scholar]
- Suárez R, Suárez-Lepe JA, Morata A, Calderón F. The production of ethylphenols in wine by yeasts of the genera Brettanomyces and Dekkera: a review. Food Chem. 2007;102:10–21. doi: 10.1016/j.foodchem.2006.03.030. [DOI] [Google Scholar]
- Suárez-Lepe JA, Morata A. New trends in yeast selection for winemaking. Trends Food Sci Technol. 2012;23:39–50. doi: 10.1016/j.tifs.2011.08.005. [DOI] [Google Scholar]
- Sun SY, Gong HS, Liu WL, Jin CW. Application and validation of autochthonous Lactobacillus plantarum starter cultures for controlled malolactic fermentation and its influence on the aromatic profile of cherry wines. Food Microbiol. 2016;55:16–24. doi: 10.1016/j.fm.2015.11.016. [DOI] [PubMed] [Google Scholar]
- Tristezza M, di Feo L, Tufariello M, Grieco F, Capozzi V, Spano G, Mita G. Simultaneous inoculation of yeasts and lactic acid bacteria: effects on fermentation dynamics and chemical composition of Negroamaro wine. LWT Food Sci Technol. 2016;66:406–412. doi: 10.1016/j.lwt.2015.10.064. [DOI] [Google Scholar]
- Tristezza M, Tufariello M, Capozzi V, Spano G, Mita G, Grieco F. The oenological potential of Hanseniaspora uvarum in simultaneous and sequential co-fermentation with Saccharomyces cerevisiae for industrial wine production. Front Microbiol. 2016;7:670. doi: 10.3389/fmicb.2016.00670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalba ML, Susana Sáez J, del Monaco S, Lopes CA, Sangorrín MP. TdKT, a new killer toxin produced by Torulaspora delbrueckii effective against wine spoilage yeasts. Int J Food Microbiol. 2016;217:94–100. doi: 10.1016/j.ijfoodmicro.2015.10.006. [DOI] [PubMed] [Google Scholar]
- Wedral D, Shewfelt R, Frank J. The challenge of Brettanomyces in wine. LWT Food Sci Technol. 2010;43:1474–1479. doi: 10.1016/j.lwt.2010.06.010. [DOI] [Google Scholar]
- van Wyk S, Silva FV. High pressure inactivation of Brettanomyces bruxellensis in red wine. Food Microbiol. 2017;63:199–204. doi: 10.1016/j.fm.2016.11.020. [DOI] [PubMed] [Google Scholar]
- Zuehlke JM, Petrova B, Edwards CG. Advances in the control of wine spoilage by Zygosaccharomyces and Dekkera/Brettanomyces. Annu Rev Food Sci Technol. 2013;4:57–78. doi: 10.1146/annurev-food-030212-182533. [DOI] [PubMed] [Google Scholar]
- Zupan J, Avbelj M, Butinar B, Kosel J, Šergan M, Raspor P. Monitoring of quorum-sensing molecules during minifermentation studies in wine yeast. J Agric Food Chem. 2013;61:2496–2505. doi: 10.1021/jf3051363. [DOI] [PubMed] [Google Scholar]