Abstract
REV-ERBα is a nuclear heme receptor, transcriptional repressor and critical component of the molecular clock that drives daily rhythms of metabolism. Evidence reveals that REV-ERBα also plays an important regulatory role in clock-dependent lung physiology and inflammatory responses. We hypothesize that cigarette smoke (CS) exposure influences REV-ERBα abundance in the lungs, facilitating a pro-inflammatory phenotype. To determine the impact of REV-ERBα activation in the CS-induced inflammatory response we treated primary human small airway epithelial cells (SAECs) with CS extract (CSE) or lipopolysaccharide (LPS) in the absence or presence of pre-treatment with the REV-ERBα agonist GSK 4112. We also exposed adult C57BL/6J (WT) and Rev-erbα global KO mice to CS (10 and 30 days) and measured pro-inflammatory cytokine release. Our data reveal that pre-treatment with GSK 4112 reduced CSE/LPS induced pro-inflammatory cytokines release from both SAECs and mouse lung fibroblasts (MLFs). Furthermore, REV-ERBα KO mice show a greater inflammatory response to 10 and 30 days of CS, including increased neutrophil lung influx, pro-inflammatory cytokine (IL-6, MCP-1 and KC) release, and pro-senescence marker (p16) when compared to WT mice. These data demonstrate that REV-ERBα is a critical regulator of CS-induced lung inflammatory responses.
Keywords: Oxidants, REV-ERBα, molecular clock, cellular senescence, cytokines, COPD
Graphical abstract
Cigarette smoke (CS) causes reduction in nuclear receptor REV-ERBα and its knockout mice exposed to acute CS showed increased lung inflammatory and pro-senescence responses in vivo.
1. Introduction
The nuclear heme receptor REV-ERBα is an integral part of the molecular clock that drives daily rhythms of behavior, metabolism and inflammatory-immune responses [1–3]. REV-ERBα may play an important regulatory role in clock-dependent lung physiology and inflammatory responses. REV-ERBα acts as part of a repressor complex, binding to and interacting with HDAC3 and NCoR. As part of the molecular clock, REV-ERBα (and its paralog REV-ERBβ) regulates the timing and amplitude of brain and muscle arnt like protein 1 (BMAL1) [4]. BMAL1 binds to and interacts with the CLOCK protein, forming the primary activator complex of the circadian clock. REV-ERB acts in concert with, but antagonistic to, the activators retinoic acid-like orphan receptor alpha/gamma (RORα/γ). Together, these proteins (REV-ERB and ROR) form what is commonly referred to as the “stabilizing loop” of the molecular clock [5–8]. We have shown that the mRNA and protein abundance of Rev-erbα was reduced in both airway and alveolar cellular compartments of lungs from emphysematous mice and patients with chronic obstructive pulmonary disease (COPD), particularly during exacerbations [9]. Though these data would suggest that REV-ERBα plays an important role in the clock-mediated response to cigarette smoke (CS)-induced lung inflammation, there is currently little to no appreciation of how REV-ERBα contributes to the processes of DNA damage/repair and stress-induced premature senescence (SIPS) during the development of COPD. We hypothesize that the CS-mediated reduction in REV-ERBα abundance potentiates lung cellular senescence and inflammatory responses. Further, we postulated that activation of REV-ERBα/β with small molecule agonists would dampen the inflammo-senescent response to CS in the lung.
2. Materials and Methods
All the key biological and/or chemical resources that are used in this study were validated and authenticated (methods and resources) and are of scientific standard from commercial sources. We used a rigorous and unbiased approach throughout execution of the experimental plan (e.g. in vitro cells and in vivo mouse samples) and during analysis of the data so as to ensure that our data are reproducible. Our results adhere to NIH standards of reproducibility and scientific rigor. All experiments for animal studies were performed in accordance with the standards established by the United States Animal Welfare Act, as set forth by the National Institutes of Health guidelines. The research protocol for mouse studies was approved by the University Committee on Animal Research Committee of the University of Rochester, Rochester, NY.
2.1 Cell Culture
Primary mouse lung fibroblasts were isolated and digested with Liberase (Roche) for 1 h at room temperature as described previously [10]. Cells were washed using RPMI medium, and initially grown in DMEM-F12 containing 10% FBS for 10 days with media change on alternate days under low oxygen concentration (3%). The primary mouse lung fibroblasts were grown in Eagle’s minimum essential medium with FBS (10%). Human primary small airway epithelial cells (SAECs) from COPD patients were obtained from Lonza and cultured as described by us previously [10].
2.2 Cell treatment
SAEC and MLF were treated with CS extract (CSE: 0.25%) or LPS (1 μg/ml) alone or pre-treated with and without GSK 4112 (20 μM) for 2 or 6 hours. After 24 hrs media was recovered and the levels of pro-inflammatory cytokines IL-6 and IL-8 was measured.
2.3. Cigarette smoke exposure
These mice were housed under a 12:12 light-dark (LD) cycle with lights on at 6 a.m. and fed with a regular diet and water ad libitum unless otherwise indicated. For CS exposure, mice were kept in a standard 12:12 L:D cycle with lights on from 6 am-6 pm throughout the experiment. C57BL/6J (WT) littermates WT and Rev-erbα global KO mice (males and females, 3–5 months old) obtained from Ronald Evans, PhD, at Salk Institute for Biological Studies, La Jolla, CA [11]., were exposed to CS at a concentration of ~250–300 Total Particular Matter (TPM mg/m3) for 2 hrs each day for 10 (acute) and 30 (sub-chronic) days of exposure for 5 hrs each day (5 days/week) using the Baumgartner smoking machine [12]. We have previously determined that CS exposure to mice at 3–10 days induces lung inflammatory responses [10]. In this experiment, we have exposed mice for 30 days as a model of sub-chronic exposure to validate and extend the CS responses we observed with our acute exposure model.
2.4. Differential inflammatory cell counts
Cytospin slides were prepared at 50,000 cells/slide and differential cell counts (~500 cells/slide) were performed on cytospin-prepared slides stained with Diff-Quik (Dade Behring, Newark, DE, USA) for 10 days Air and CS exposed mice.
Flow cytometric analysis of immune inflammatory cells was performed using cell-type specific monoclonal antibodies in one month exposed samples. Briefly, the BAL cell were re-suspended in 1 ml 1x PBS for cell counting using cellometer to determine the total cell counts/ml. Approximately 2.0–4.0 × 105 cells were stained in 1x PBS using cell-type specific markers for 30 min., then washed and re-suspended in 0.1 ml of 1x PBS for analysis. Markers, such as LY6B.2 Alexa fluor 488 -conjugated antibody for neutrophils (Novus Biologicals Cat# NBP213077AF488), F4/80 PE-conjugated antibody for macrophages (BioLegend Cat #123109) were used. Flow cytometry data acquisition was performed on a BD Accuri flow cytometer (BD Accuri C6 software) and analyzed using the FlowJo software.
2.5. Pro-inflammatory cytokine analysis
Levels of pro-inflammatory mediators (IL-6, MCP-1, MIP-2 and KC) in bronchoalveolar lavage fluid (BALF) from 10 or 30 days exposure experiments or conditioned media from SAECs and MLFs were assayed by using ELISA kit according to the manufacturers’ instructions (R&D Systems, Minneapolis, MN, USA) for mouse and human cells (Life Technologies, MA).
2.6. Immunoblotting
Immunoblotting was used to measure the level of cellular senescence markers (p16 and p21) in air- and CS-exposed mouse lung tissue extracts. Lung tissues were excised at the time of sacrifice and snap frozen. Protein was extracted and subjected to PAGE. Blots were exposed to anti-p21 (Santa Cruz, SC-397) or anti-p16 (Santa Cruz, sc-54309) primary antibodies overnight at 4C (1:1000), followed by secondary antibody incubation for 1 h at room temperature (1:5000 dilutionECL (Bio-Rad) was used for detection and images were taken with Bio-Rad (ChemiDoc MP, Imaging system), and normalized against their respective β-actin bands using the densitometry.
2.6. Statistical analysis
Data are presented as means ± SD. Statistical analysis of significance was calculated using one-way Analysis of Variance (ANOVA) for multigroup comparisons using GraphPad Prism 6. All of the data are presented as mean ± SEM. P < 0.05 is considered a threshold for significance.
3. Results
3.1. Pre-treatment of primary human SAECs and MLFs with REV-ERBα agonist GSK 4112 reduces pro-inflammatory cytokine release following exposure to CSE in vitro
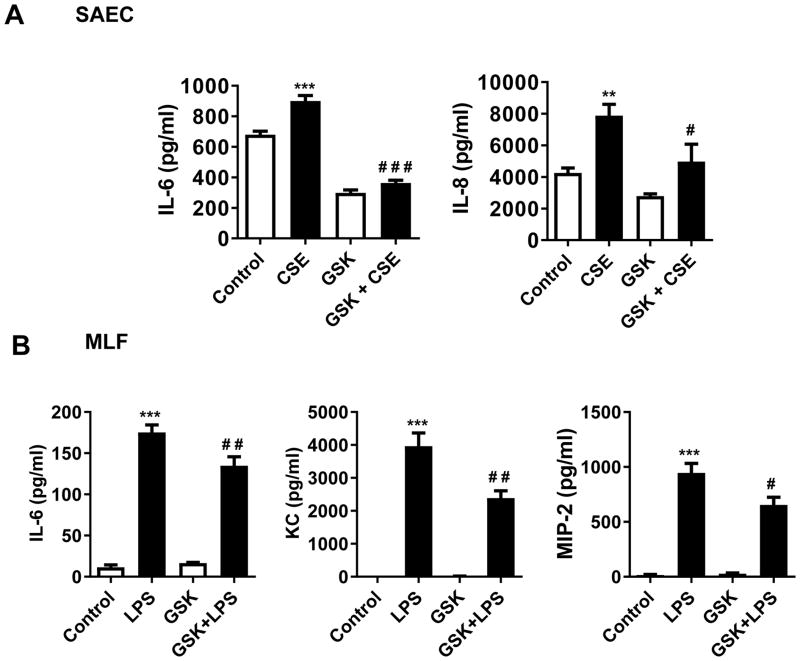
We pre-treated human small airway epithelial cells (SAEC) obtained from patients with COPD and primary mouse lung fibroblasts (MLF) with the REV-ERBα agonist GSK 4112 [12] (20 μM/ml for 2 or 6 hrs) prior to CSE (0.25%) or LPS (1 μg/ml) exposure, and measured inflammatory responses 24h post-treatment. We found that pre-treatment with GSK 4112 agonist significantly inhibited IL-6 and IL-8 release in SAECs and IL-6, KC and MIP-2 release in MLFs (Fig. 1).
Fig. 1. REV-ERBα agonist attenuates pro-inflammatory response to CSE and LPS in vitro.
(A) Human small airway epithelial cells (SAEC) from chronic obstructive pulmonary disease (COPD) patients were pre-treated with veh (DMSO) or REV-ERBα agonist (GSK4112: 20 μM/ml) for 6 h followed by treatment with CSE (0.25%) for 24 h. (B) Mouse lung fibroblasts (MLF) from C57BL/6J mice were pre-treated with veh or GSK4112 for 2 h followed by treatment with LPS (1 μg/ml) for 24 h. CSE-induced IL-6, and IL-8 and LPS-induced IL-6, KC, and MIP-2 release in conditioned medium was measured by ELISA after 24 h treatment. Data are shown as mean ± SEM, n=3–6 per group. ***P<0.01, ***P<0.001 vs. respective control; #P<0.05, # # P<0.01, # # # P<0.001 vs. CSE or LPS.
3.2. CS induced inflammatory response in Rev-erba KO mice
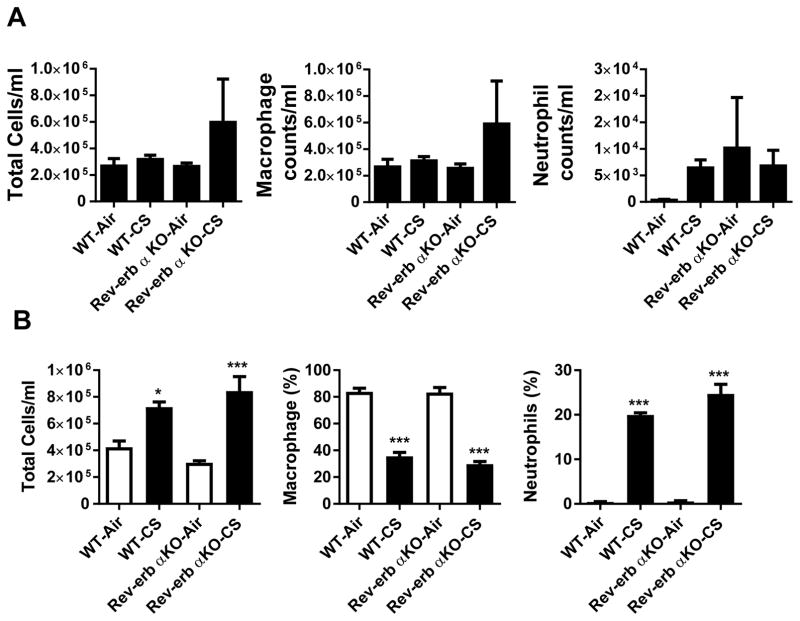
We have determined the role of REV-ERBα in regulating lung inflammatory responses to acute and sub-chronic CS exposures. Increased inflammatory responses were observed in Rev-erbα KO mice as compared to WT mice following both acute and sub-chronic CS exposures. Total inflammatory cells and neutrophils (number or % of total cells) were increased after both 10 day acute and one month of sub-chronic CS exposures in WT and Rev-erbα KO mice (Fig. 2A–B). Moreover, one month of CS exposure produced a greater response in Rev-erbα KO mice relative to WT animals (Fig 2B) as depicted in % of counted cells.
Fig. 2. Lung inflammatory cell influx in CS exposed Rev-erbα KO and WT mice.
Wild-type littermates and Rev-erbα KO mice were exposed to room air, 10 days (acute) or 30 days (sub-chronic) of CS. Total cell counts per ml in BAL, macrophages, and neutrophils in WT or REV-ERBa KO mice exposed to room air, (A) acute CS (10 days) or (B) sub-chronic CS (30 days). In (A and B) data are presented as total cell counts per ml in BAL fluid while in (B) macrophage and neutrophil levels are presented as a percentage of total cells. For 10 days CS exposure, the number of total cells in BALF was counted in at least 500 cells stained with Diff Quik on a hemocytometer. For one month CS exposure, the cell counts were determined using a flow cytometer (See Methods). In A and B, data are shown as mean ± SEM, n=4–6 per group for one month Air and CS exposures, and n=3–8 per group except for Rev-erbα KO and Air n=2 per group for 10 days. *P<0.05 and ***P<0.001 vs WT-Air or Rev-erbα KO-Air.
3.3. CS exposure increased pro-inflammatory cytokines in Rev-erbα KO mice
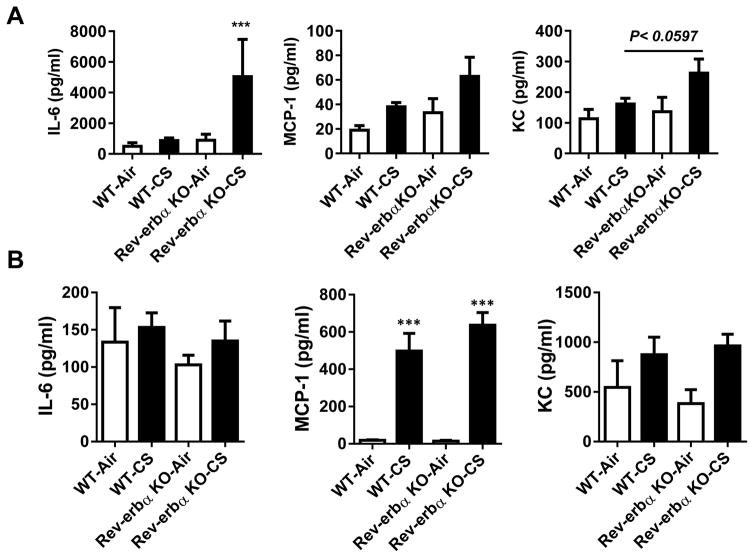
Rev-erbα KO mice show increased inflammatory responses to acute 10 days CS exposure as compared to WT mice (Fig. 3A). This was associated with a significant increase in IL-6 (P < 0.05) and an apparent trend towards increases in MCP-1 and KC (P = 0.0597) in BALF from CS exposed Rev-erbα KO mice exposed to CS vs air groups after 10 days of CS exposures. Similar data were found after 30 days of sub-chronic exposure in lungs for a trend in increased MCP-1 and KC by CS exposures in Rev-erbα KO mice compared to air exposed groups (Fig 3B).
Fig. 3. Rev-erbα KO mice show pro-inflammatory responses to CS exposure.
Wild-type littermates and Rev-erbα KO mice were exposed to acute CS exposure for (A) 10 days or (B) sub-chronic for 30 days. Levels of pro-inflammatory mediators (IL-6, MCP-1 and KC) were measured in BAL fluid obtained from acute 10 d and 30 d air- or CS-exposed mice by ELISA. Some of the analyses were repeated based on availability of the samples to reproduce the findings. Data are shown as mean ± SEM, n=4–6 per group for one month Air and CS exposures, and n=3–8 per group except for Rev-erbα KO and Air n=2–4 per group for 10 days. P<0.059 vs WT Air group, and ***P<0.001 vs WT-Air or Rev-erbα KO-Air.
3.4. CS exposure significantly increased pro-senescence markers in Rev-erbα KO mice
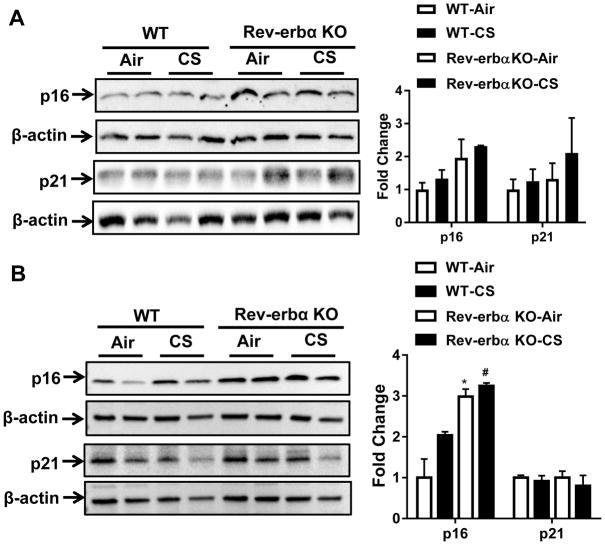
Immunoblot analysis of p16 and p21 in lung tissue homogenates from mice after 10 and 30 days of air or CS exposure show increased p16 without appreciable increase in p21 in Rev-erbα KO mice compared to air (Fig. 4A–B).
Fig. 4. Rev-erbα KO mice show increased cellular senescence markers in response to acute CS exposure.
Wild-type littermates and Rev-erbα KO mice were exposed to acute CS exposure for (A) 10 days (acute) or (B) sub-chronic for 30 days. Immunoblot analysis of pro-senescence markers p16 and p21 in lung tissue homogenates from WT and Rev-erbα KO mice. After densitometry analysis, levels of p16 and p21 were normalized against respective loading control β-actin. Data are shown as mean ± SEM, n=2–4 per group. *P < 0.05 vs WT; # P < 0.05 vs WT-CS.
4. Discussion
The heme receptor REV-ERBα, encoded by Nr1d1, regulates the expression of genes involved in circadian rhythms [13, 14] metabolism [2, 3, 15] and inflammatory responses [16–19]. REV-ERBα and RORα provide stability and precision the timing of the molecular clock [20]. Not surprisingly, abnormal molecular clock function has been shown to hasten the appearance of chronic diseases associated with inflammation and aging [21, 22]. We have shown that the mRNA and protein levels of REV-ERBα were reduced in lung tissue (airway epithelium and type II cells) of mice with emphysema, and in COPD patients without any change in REV-ERBβ [9, 23] when compared to healthy nonsmokers. We hypothesize that CS exposure reduces the abundance of REV-ERBα specifically, which is associated with increased lung inflammatory and senescence responses.
We found that pre-treatment with the REV-ERB agonist GSK 4112 significantly inhibited pro-inflammatory cytokine release in SAECs and MLFs following CSE or LPS exposure in vitro. These data are particularly exciting, as they suggest a general anti-inflammatory effect of enhanced REV-ERB signaling across multiple pro-inflammatory mediators (CSE and LPS).
Our data show that REV-ERBα deficient mice exhibit inflammatory and senescence responses in the lung. We have shown that the protein levels of REV-ERBα were reduced in mouse lung with emphysema and in lungs of COPD patients [9]. This is consistent with the finding that the expression of Nr1d1 (encoding REV-ERBα) in mouse lung tissue is suppressed by CS [13]. Acute and sub-chronic CS exposures in Rev-erbα KO mice show a phenotype of increased pro-inflammatory mediators release (IL-6, MCP-1 and KC). This increase may be associated with upregulating their gene transcription by decreased recruitment of NcoR1/HDAC3 co-repressors [6], as CS is shown to downregulate HDAC2 and disruption of HDAC repressor complex in lungs [24, 25]. This is one of the mechanisms for CS-induced lung inflammatory response by REV-ERBα.
Our data further suggest that CS-mediated REV-ERBα reduction may be permissive for the development of cellular senescence and inflammatory responses. However, the molecular mechanism through which REV-ERBα regulates the development and amplitude of chronic CS-induced lung cellular senescence and inflammatory responses remain unknown. Furthermore, it has yet to be determined whether selective activation of REV-ERBα in the lungs attenuates CS-induced lung inflammation and senescence responses in a long-term model of CS exposures. Further, development of improved agonists (e.g. SR series developed by Burris and colleagues; [7]) should provide more detailed and accurate information regarding its anti-inflammatory effects in vivo as the currently available agonist GSK 4112 has some solubility and bioavailability issues in mouse.
Persistent DNA damage results in SIPS and senescence-associated secretory phenotype (SASP) by forming senescence-associated heterochromatin foci [26, 27]. CS-induced DNA damage response may cause an inflammatory phenotype in senescent cells [28, 29]. Our data reveal that REV-ERBα KO mice exhibit enhanced lung cellular pro-senescence and inflammatory responses in the lung. Our assertion is that suppression of REV-ERBα levels in the lungs produced by chronic CS enhances the development of cellular senescence and increases the magnitude of inflammatory responses that eventually lead to COPD/emphysema. One of the reasons for this anti-inflammatory response involves interaction of REV-ERBα with RORα on pro-inflammatory genes [6, 18, 30, 31], and disruption of this complex may culminate in various pro-inflammatory, DNA damage, and pro-senescence responses.
Overall, REV-ERBα plays an important role in regulating CS-induced lung inflammatory responses. Our findings have great translational potential for the development of pharmacological therapies based on targeting REV-ERBα (clock based treatment) to ameliorate lung inflammatory and cellular senescence responses in COPD progression and exacerbations.
Acknowledgments
Funding Sources:
This study was supported by the NIH 1R01HL085613, 1R01HL133404, 2R01 HL085613, 1R01HL137738, and 1R56ES027012 (to I. Rahman).
We thank to Ms. Janice Gerloff and Meimei Yin, PhD for their technical assistance.
Abbreviations
- BMAL1
brain and muscle ARNT-like 1
- BALF
bronchoalveolar lavage fluid
- CLOCK
circadian locomoter output cycles protein kaput
- COPD
chronic obstructive pulmonary disease
- Rev-Erbα (Nr1d1)
nuclear receptor subfamily 1 group D member 1
- ROR
Retinoid-Related Orphan Receptor
- SASP
stress-induced secretory phenotype
- SIPS
stress-induced premature senescence
Footnotes
Author contributions
I.K.S., K.R., M.T.S., and IR conceived and designed the experiments; I.K.S. and K.R., performed the experiments and analyzed the data; I.K.S., M.T.S. and I.R. drafted this research article; I.K.S., M.T.S., and I.R. edited and revised this manuscript.
Conflicts of interest
No conflicts of interest, financial or otherwise, are declared by the author(s).
References
- 1.Preitner N, Damiola F, Lopez-Molina L, Zakany J, Duboule D, Albrecht U, Schibler U. The orphan nuclear receptor REV-ERBalpha controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell. 2002;110:251–260. doi: 10.1016/s0092-8674(02)00825-5. [DOI] [PubMed] [Google Scholar]
- 2.Cho H, Zhao X, Hatori M, Yu RT, Barish GD, Lam MT, Chong LW, DiTacchio L, Atkins AR, Glass CK, Liddle C, Auwerx J, Downes M, Panda S, Evans RM. Regulation of circadian behaviour and metabolism by REV-ERB-alpha and REV-ERB-beta. Nature. 2012;485:123–127. doi: 10.1038/nature11048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Solt LA, Wang Y, Banerjee S, Hughes T, Kojetin DJ, Lundasen T, Shin Y, Liu J, Cameron MD, Noel R, Yoo SH, Takahashi JS, Butler AA, Kamenecka TM, Burris TP. Regulation of circadian behaviour and metabolism by synthetic REV-ERB agonists. Nature. 2012;485:62–68. doi: 10.1038/nature11030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu AC, Tran HG, Zhang EE, Priest AA, Welsh DK, Kay SA. Redundant function of REV-ERBalpha and beta and non-essential role for Bmal1 cycling in transcriptional regulation of intracellular circadian rhythms. PLoS Genet. 2008;4:e1000023. doi: 10.1371/journal.pgen.1000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sundar IK, Yao H, Sellix MT, Rahman I. Circadian molecular clock in lung pathophysiology. Am J Physiol Lung Cell Mol Physiol. 2015;309:L1056–1075. doi: 10.1152/ajplung.00152.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Journiac N, Jolly S, Jarvis C, Gautheron V, Rogard M, Trembleau A, Blondeau JP, Mariani J, Vernet-der Garabedian B. The nuclear receptor ROR(alpha) exerts a bi-directional regulation of IL-6 in resting and reactive astrocytes. Proc Natl Acad Sci U S A. 2009;106:21365–21370. doi: 10.1073/pnas.0911782106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kojetin DJ, Burris TP. REV-ERB and ROR nuclear receptors as drug targets. Nat Rev Drug Discov. 2014;13:197–216. doi: 10.1038/nrd4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duez H, Staels B. The nuclear receptors Rev-erbs and RORs integrate circadian rhythms and metabolism. Diab Vasc Dis Res. 2008;5:82–88. doi: 10.3132/dvdr.2008.0014. [DOI] [PubMed] [Google Scholar]
- 9.Yao H, Sundar IK, Huang Y, Gerloff J, Sellix MT, Sime PJ, Rahman I. Disruption of SIRT1-mediated Control of Circadian Molecular Clock and Inflammation in Chronic Obstructive Pulmonary Disease. Am J Respir Cell Mol Biol. 2015;53:782–792. doi: 10.1165/rcmb.2014-0474OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahmad T, Sundar IK, Lerner CA, Gerloff J, Tormos AM, Yao H, Rahman I. Impaired mitophagy leads to cigarette smoke stress-induced cellular senescence: implications for chronic obstructive pulmonary disease. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2015;29:2912–2929. doi: 10.1096/fj.14-268276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cho H, Zhao X, Hatori M, Yu RT, Barish GD, Lam MT, Chong LW, DiTacchio L, Atkins AR, Glass CK, Liddle C, Auwerx J, Downes M, Panda S, Evans RM. Regulation of circadian behaviour and metabolism by REV-ERB-alpha and REV-ERB-beta. Nature. 2012;485:123–127. doi: 10.1038/nature11048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sundar IK, Nevid MZ, Friedman AE, Rahman I. Cigarette smoke induces distinct histone modifications in lung cells: implications for the pathogenesis of COPD and lung cancer. Journal of proteome research. 2014;13:982–996. doi: 10.1021/pr400998n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vasu VT, Cross CE, Gohil K. Nr1d1, an important circadian pathway regulatory gene, is suppressed by cigarette smoke in murine lungs. Integr Cancer Ther. 2009;8:321–328. doi: 10.1177/1534735409352027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Demaria M, Ohtani N, Youssef SA, Rodier F, Toussaint W, Mitchell JR, Laberge RM, Vijg J, Van Steeg H, Dolle ME, Hoeijmakers JH, de Bruin A, Hara E, Campisi J. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev Cell. 2014;31:722–733. doi: 10.1016/j.devcel.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woldt E, Sebti Y, Solt LA, Duhem C, Lancel S, Eeckhoute J, Hesselink MK, Paquet C, Delhaye S, Shin Y, Kamenecka TM, Schaart G, Lefebvre P, Neviere R, Burris TP, Schrauwen P, Staels B, Duez H. Rev-erb-alpha modulates skeletal muscle oxidative capacity by regulating mitochondrial biogenesis and autophagy. Nature medicine. 2013;19:1039–1046. doi: 10.1038/nm.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lam MT, Cho H, Lesch HP, Gosselin D, Heinz S, Tanaka-Oishi Y, Benner C, Kaikkonen MU, Kim AS, Kosaka M, Lee CY, Watt A, Grossman TR, Rosenfeld MG, Evans RM, Glass CK. Rev-Erbs repress macrophage gene expression by inhibiting enhancer-directed transcription. Nature. 2013;498:511–515. doi: 10.1038/nature12209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gibbs JE, Blaikley J, Beesley S, Matthews L, Simpson KD, Boyce SH, Farrow SN, Else KJ, Singh D, Ray DW, Loudon AS. The nuclear receptor REV-ERBalpha mediates circadian regulation of innate immunity through selective regulation of inflammatory cytokines. Proc Natl Acad Sci U S A. 2012;109:582–587. doi: 10.1073/pnas.1106750109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sato S, Sakurai T, Ogasawara J, Takahashi M, Izawa T, Imaizumi K, Taniguchi N, Ohno H, Kizaki T. A Circadian Clock Gene, Rev-erbalpha, Modulates the Inflammatory Function of Macrophages through the Negative Regulation of Ccl2 Expression. J Immunol. 2014;192:407–417. doi: 10.4049/jimmunol.1301982. [DOI] [PubMed] [Google Scholar]
- 19.Yang G, Wright CJ, Hinson MD, Fernando AP, Sengupta S, Biswas C, La P, Dennery PA. Oxidative stress and inflammation modulate Rev-erbalpha signaling in the neonatal lung and affect circadian rhythmicity. Antioxidants & redox signaling. 2014;21:17–32. doi: 10.1089/ars.2013.5539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Albrecht U. Timing to perfection: the biology of central and peripheral circadian clocks. Neuron. 2012;74:246–260. doi: 10.1016/j.neuron.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 21.Brown SA, Schmitt K, Eckert A. Aging and circadian disruption: causes and effects. Aging (Albany NY) 2011;3:813–817. doi: 10.18632/aging.100366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sellix MT, Evans JA, Leise TL, Castanon-Cervantes O, Hill DD, DeLisser P, Block GD, Menaker M, Davidson AJ. Aging differentially affects the re-entrainment response of central and peripheral circadian oscillators. J Neurosci. 2012;32:16193–16202. doi: 10.1523/JNEUROSCI.3559-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hwang JW, Sundar IK, Yao H, Sellix MT, Rahman I. Circadian clock function is disrupted by environmental tobacco/cigarette smoke, leading to lung inflammation and injury via a SIRT1-BMAL1 pathway. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2014;28:176–194. doi: 10.1096/fj.13-232629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang SR, Chida AS, Bauter MR, Shafiq N, Seweryniak K, Maggirwar SB, Kilty I, Rahman I. Cigarette smoke induces proinflammatory cytokine release by activation of NF-kappaB and posttranslational modifications of histone deacetylase in macrophages. American journal of physiology Lung cellular and molecular physiology. 2006;291:L46–57. doi: 10.1152/ajplung.00241.2005. [DOI] [PubMed] [Google Scholar]
- 25.Yao H, Rahman I. Role of histone deacetylase 2 in epigenetics and cellular senescence: implications in lung inflammaging and COPD. American journal of physiology Lung cellular and molecular physiology. 2012;303:L557–566. doi: 10.1152/ajplung.00175.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coppe JP, Patil CK, Rodier F, Sun Y, Munoz DP, Goldstein J, Nelson PS, Desprez PY, Campisi J. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6:2853–2868. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodier F, Munoz DP, Teachenor R, Chu V, Le O, Bhaumik D, Coppe JP, Campeau E, Beausejour CM, Kim SH, Davalos AR, Campisi J. DNA-SCARS: distinct nuclear structures that sustain damage-induced senescence growth arrest and inflammatory cytokine secretion. J Cell Sci. 2011;124:68–81. doi: 10.1242/jcs.071340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodier F, Coppe JP, Patil CK, Hoeijmakers WA, Munoz DP, Raza SR, Freund A, Campeau E, Davalos AR, Campisi J. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nature cell biology. 2009;11:973–979. doi: 10.1038/ncb1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu ZH, Shi Y, Tibbetts RS, Miyamoto S. Molecular linkage between the kinase ATM and NF-kappaB signaling in response to genotoxic stimuli. Science. 2006;311:1141–1146. doi: 10.1126/science.1121513. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y, Fang B, Emmett MJ, Damle M, Sun Z, Feng D, Armour SM, Remsberg JR, Jager J, Soccio RE, Steger DJ, Lazar MA. GENE REGULATION. Discrete functions of nuclear receptor Rev-erbalpha couple metabolism to the clock. Science. 2015;348:1488–1492. doi: 10.1126/science.aab3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morioka N, Tomori M, Zhang FF, Saeki M, Hisaoka-Nakashima K, Nakata Y. Stimulation of nuclear receptor REV-ERBs regulates tumor necrosis factor-induced expression of proinflammatory molecules in C6 astroglial cells. Biochem Biophys Res Commun. 2016;469:151–157. doi: 10.1016/j.bbrc.2015.11.086. [DOI] [PubMed] [Google Scholar]