Abstract
Study design
Cross-sectional study of spine MR in a population, predominantly female, sample.
Objective
To determine the relationship between vertebral endplate defect and intervertebral disc degeneration (DD) in general population.
Summary of Background Data
Precise understanding of the mechanisms leading to DD development is lacking. In a degenerating disc, mechanical and structural changes lead to further worsening of disc integrity. Increasing attention has been paid to vertebral endplate defects as having a possible role in the etiopathogenesis of DD.
Methods
The study population comprised 831 twin volunteers from TwinsUK (mean age 54±8 years, 95.8% female). Lumbar T2-weighted magnetic resonance images were coded for endplate defects from 8310 endplates into six grades. Total endplate score (TEP score) was achieved by summing both endplate defect grades from the same disc level. DD was evaluated using two different classifications; Pfirrmann grading, and a quantitative trait for DD based on a 4-point grading systems. Multivariable regression analysis was used to determine relationships between the traits of interest and the known risk factors for DD, age and body mass index (BMI). A receiver operator curve for TEP score predicting DD was generated, and survival analysis paired with Cox proportional hazards models analysis performed.
Results
There was statistically significant association between DD and age and BMI. These associations lost significance when TEP score was included as predictor in multivariable model. TEP score was strongly and independently associated at every lumbar disc level with DD (Pfirmann p≤0.001; 4-point grading systems p<1e-16). A cut-off point score of 5 for TEP score was found above which there was a higher DD prevalence. Across all age subgroups, probabilities of having DD were significantly increased in those considered TEP score positive (≥5).
Conclusions
Our large, population-based study has shown that endplate defect was strongly and independently associated with DD at every lumbar disc level. These results provide a mechanism by which increasing age and BMI predispose to DD.
Keywords: Disc degeneration, endplate, intervertebral disc, lumbar spine, magnetic resonance imaging, Pfirrmann, TEP score, total endplate score, twin, TwinsUK
Introduction
One of the specific causes of low back pain (LBP) is thought to be intervertebral disc degeneration (DD) (1–5). Aging and metabolite transport problems can predispose a disc to degeneration (6, 7), and there is some biomechanical evidence that initial damage to the annulus fibrosus or endplate can both lead to disc decompression and degeneration (8). However, it remains unclear whether a structural defect within the disc annulus or endplate can act as an initiating factor for DD in humans, and which is the more important route. Lately, increasing attention has been paid to defects in the vertebral endplate as having a possible role in the etiopathogenesis of DD. DD is usually assessed using magnetic resonance imaging (MRI) as the gold standard, with a variety of different classifications (9, 10). Pfirrmann classification (9) takes into account decrease of the disc height and signal intensity but does not assess them separately. Other DD classifications have been developed to utilize components of DD more extensively (11).
The motion segment or functional spinal unit consists of two adjacent vertebrae, the intervertebral disc and all adjacent ligaments between them, of which the vertebral body endplate is thought to be the most vulnerable structure (6). The endplate is a bilayer structure between the vertebral body and the intervertebral disc, consisting of bony and cartilaginous layers (12).
The cartilage layer helps to equalize loading between the disc and vertebral body (13) and maintains a normal hydrostatic pressure within the nucleus by opposing water loss under load (14) it also regulates metabolite transport between the disc and vertebral body (7). Damage to the endplate leads to structural change affecting the motion segment and lower intradiscal pressure in the adjacent nucleus pulposus, inducing greater compressive stresses to the annulus fibrosus (7, 15, 16). Subsequently, according to evidence from cadaveric experiments (7), organ culture (17), and animal models (15), and living humans (18), this can lead to internal disc disruption and cell-mediated DD. Endplate fracture has also been shown to lead to necrotic and apoptotic cell death in the adjacent nucleus in human subjects (17), whereas in animal models endplate perforation induced disc degeneration resembling human disc degeneration (15). Hence, it seems reasonable to hypothesise that endplate defect could initiate disc degeneration in human lumbar spine.
Rajasekaran et al. (19) has reported that endplate defect was correlated with DD in a clinical sample of 47 patients and 26 volunteers. Endplate defect was evaluated on T1 scans and classified into six types according to severity of damage (type 1 to type 6) assessed on T1-weighted magnetic resonance scans. A Total Endplate score (TEP score) was derived from each disc by summing endplate defect scores of both rostral and caudal endplates of the disc. Strong correlation between progressive grades of endplate defect and disc degeneration was shown.
We hypothesised that endplate defect is a major initiating factor for DD not only in patients with back pain but in the general population. Accordingly, we examined existing MRI scans for endplate defects and examined the relationship between endplate defects and known risk factors for DD in TwinsUK, a large population sample highly phenotyped for medical conditions and lifestyle traits and shown to be similar to the singleton population (20). The purpose of our study was to characterise the relationship between endplate defect, other DD risk factors and DD graded by two different morphological classifications: i) Pfirrmann grading and ii) the quantitative trait for DD based on a 4-point grading system for disc height, signal change, posterior disc bulge, and anterior osteophytes in a larger population-based sample as reported previously (10).
Materials and Methods
Study population
The study population comprised part of the TwinsUK register of King’s College London (www.twinsuk.ac.uk) (21). The registry was founded in 1992 and comprises adult twins, the majority females. The baseline data and MRIs were collected between 1996 and 2000. Twins in the registry were recruited through national media campaigns and from other twin registers and had not been selected for any particular trait (10). Subjects in the registry were invited to take part in studies that cover a wide range of traits and common medical conditions and, where possible, were not aware of the precise hypothesis being tested. Clinical and demographic information on gender, body mass index (BMI), episodes of disabling low back pain and lumbar disc degeneration have been collected previously. All subjects have signed informed consent form. The study was approved by St. Thomas’ Hospital Ethics Committee.
Magnetic resonance imaging and grading
Magnetic resonance scans were performed at baseline using a Siemens (Munich, Germany) 1.0-T superconducting magnet. Sagittal images were obtained using a fast spin-echo sequence of time to recovery (TR)/time to echo (TE) 5000–4500/112 msec, with a slice thickness of 4 mm. Grading was performed on T2-weighted images. All MRI scans were performed at least 1 hour after rising in the morning, with no exercise or other rest allowed between getting up and the scan in order to avoid problems related to diurnal variation in disc height (22). Each twin pair was scanned at the same appointment.
Grading of Endplate Defects
Authors JHM and MR coded endplate defects in the baseline MRI scans from all 831 subjects (including 4155 discs and 8310 endplates). Endplate defect grade was evaluated on a scale of 1-6 according to Rajasekaran et al. 2008 (19) (Table 1). As in Rajasekaran et al. 2008 (19), total endplate scores for each disc were constructed by summing the endplate defect score of both rostral and caudal endplates in each functional spine unit. Inter-rater agreement for the coding of endplate defect was calculated using Cohen's weighted kappa and Pearson’s correlation.
Table 1.
Endplate defect score
| Grade 1 | Normal endplate, no breaks or defects |
| Grade 2 | Focal thinning of the endplate, no breaks or defects |
| Grade 3 | Focal disc marrow contacts, but with maintained endplate contour |
| Grade 4 | Endplate defects up to 25% of the endplate area |
| Grade 5 | Endplate defects up to 50% of the endplate area |
| Grade 6 | Extensive damaged endplates up to total destruction |
Agreement phase
An initial training phase was held in which an inter-rater agreement on endplate defect detection and grading of ≥0.85 was reached on at least 100 subjects and 1000 endplates. After the training phase, potential uncertainties were settled by discussion and consensus.
Grading of Disc Degeneration
Disc degeneration has been evaluated earlier using two different coding methods i) 4-point grading system for disc height, signal change, posterior disc bulge, and anterior osteophytes as by (23) reported in Sambrook et al. (10) and ii) Pfirrmann classification (9).
-
i)
The coding process has been previously described in details (10). Briefly, the following components were assessed; 1) disc signal intensity within the nucleus pulposus; 2) disc height loss; 3) posterior disc bulge; and 4) anterior osteophytes. Every component was evaluated from 0 to 3 from every lumbar disc level utilizing standardised atlas. An overall measure of degenerative change was determined as the arithmetic sum of the grades for each feature in the lumbar spine. Thus, a summary score had range 0-12 at every lumbar disc level.
-
ii)
Pfirrmann classified disc degeneration progressively according to severity from 1 (disc is homogeneous with bright hyperintense white signal intensity and normal disc height) to 5 (disc is inhomogeneous with a hypointense black signal intensity and no more detectable difference between the nucleus and annulus). Consistent with other studies (19), a score equal or greater than Pfirrmann 4 was chosen to define an abnormal, degenerate disc.
Statistical analysis
Critical scores were calculated for TEP score predicting DD on the same lumbar spine level using Receiver Operating Curves (ROC)-analysis. Cumulative link mixed models and linear mixed regression models were used to analyse association between endplate defect and DD adjusted for known risk factors (age and BMI) and family structure. Kaplan-Meier survival analysis and Cox proportional hazards models analysis were carried out to assess temporal dependence (by age subgroups) between endplate defect and DD. All calculations were done in R using packages “psych”, “survival”, “ordinal”, “lme4”, and “OptimalCutpoints”.
Results
The study sample consisted of 831 subjects having mean age 54±8 years, range 19-73 years 95.8% female including 4155 discs and 8310 endplates, as shown in Table 2. Inter-rater agreement for endplate defect between the two investigators showed Kappa values 0.72-0.94, with mean value of 0.86 and Pearson’s correlation between the two coders was 0.864, indicating good agreement.
Table 2.
Characteristics of the sample from TwinsUK.
| Zygosity | N | Age (mean±SD) | Age range | Gender (F/M) | BMI (median [IQR]) | 4-point DD sum score (mean±SD) | Actual range (Min, Max) | Pfirrmann sum score (mean±SD) | Actual range (Min, Max) | TEPS sum score (median [IQR]) | Actual range (Min, Max) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| MZ | 242 | 57 ± 8 | 32-73 | 238/4 | 24.0 (21.9, 26.5) | 15.4 (7.5) | 0, 45 | 16.2 (2.6) | 10, 22 | 23 (20, 29) | 10, 55 |
| DZ | 448 | 53 ± 8 | 19-71 | 420/28 | 24.2 (22.0, 27.1) | 12.7 (7.6) | 0, 48 | 15.8 (2.6) | 10, 24 | 22 (19, 29) | 10, 55 |
| Singleton | 141 | 52 ± 9 | 35-73 | 138/3 | 23.8 (22.1, 26.4) | 12.2 (6.8) | 0, 41 | 15.2 (2.7) | 10, 21 | 22 (18, 27) | 11, 51 |
| Total | 831 | 54 ± 8 | 19-73 | 796/35 | 24.1 (22.0, 26.9) | 13.4 (7.5) | 0, 48 | 15.8 (2.7) | 10-24 | 22 (19, 29) | 10-55 |
MZ = monozygotic, DZ = dizygotic, SD = standard deviation, F = female, M = male, BMI = body mass index, IQR = interquartile range, SUM SCORE= summary score, Min = minimum, smallest number of a set of values, Max = maximum, biggest number of a set of values
Disc degeneration score
Summative scores of 4-point grading system of (23) for disc height, signal change, disc bulge, and anterior osteophytes, and the Pfirrmann grading are shown in detail in Table 2.
Endplate defects and DD
Multivariable regression models revealed statistically significant associations between DD and known risk factors age (p≤0.0001) and BMI (p≤0.05) for all disc levels and by different disc codings. However, when TEP score was also included as predictor in the multivariable model, the strength of covariates such as age and BMI was mostly attenuated to non-significant, indicating endplate defect as the prevalent factor in DD. TEP score was strongly and independently associated with DD as graded with both Pfirmann (p≤0.001) and 4-point grading system methods (p<1e-16) at every lumbar disc level (Table 3).
Table 3.
Risk factors for disc degeneration including endplate damage, by lumbar level
| DD graded by Pfirrmann | |||||
| L1/L2 | L2/L3 | L3/L4 | L4/L5 | L5/S1 | |
| TEPS | 2.07e-7 | 4.66e-7 | 5.26e-13 | 1.23e-16 | 1.59e-19 |
| Sex | 0.010 | 0.793 | 0.704 | 0.829 | 0.839 |
| Age | 0.656 | 0.894 | 0.785 | 0.805 | 0.726 |
| BMI | 0.698 | 0.412 | 0.405 | 0.331 | 0.271 |
| DD graded by 4-point grading method | |||||
| TEPS | ≤1e-16 | ≤1e-16 | ≤1e-16 | ≤1e-16 | ≤1e-16 |
| Sex | 0.664 | 0.658 | 0.292 | 0.324 | 0.046 |
| Age | 0.538 | 0.535 | 0.723 | 0.128 | 0.054 |
| BMI | 0.971 | 0.928 | 0.822 | 0.265 | 0.662 |
Association was assessed using multivariable models with adjustment for twin pairing.
BMI = body mass index, TEPS = total endplate score
The prevalence of DD showed a significant correlation with higher TEP score. The ROC indicated a critical TEP score (cut-off point) of 5 after which there was a higher prevalence of DD graded with Pfirmann. The same TEP score cut-off point applied for each disc level (L1/L2, L2/L3, L3/L4, L4/L5, and L5/S1).
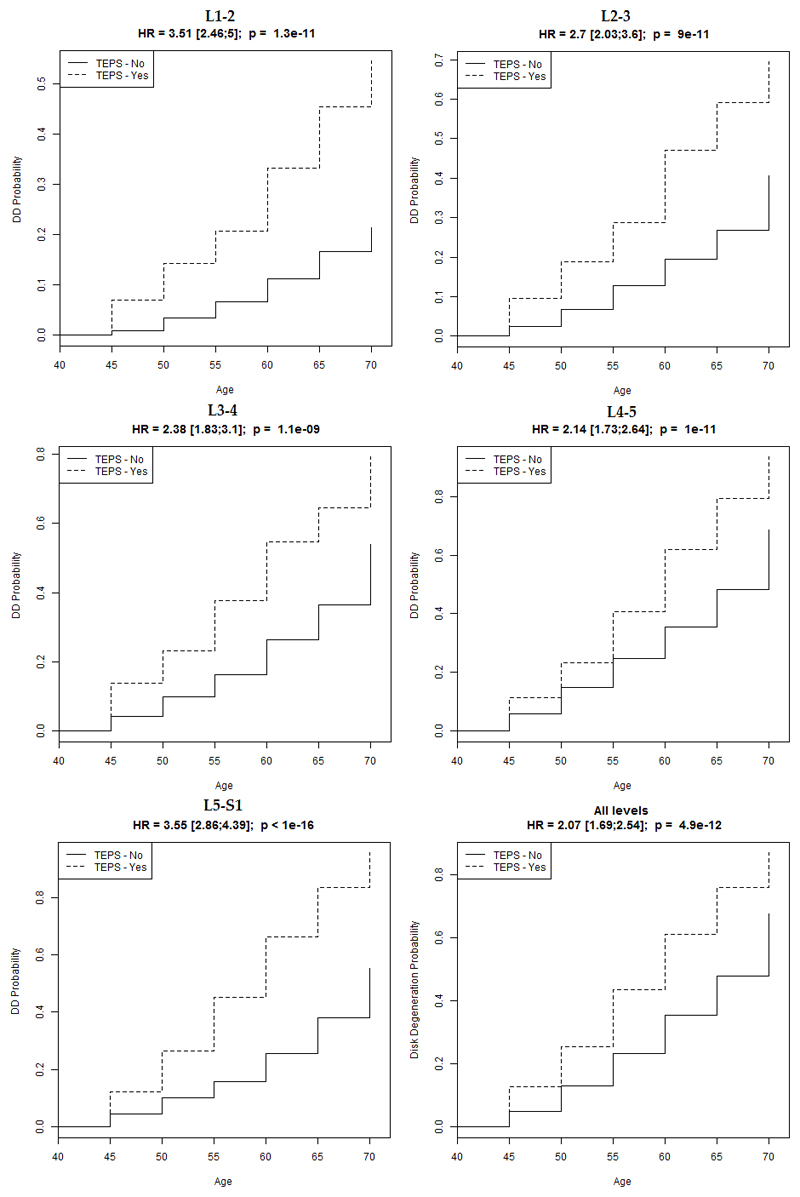
A survival analysis paired with Cox proportional hazards models analysis provided the probabilities of having DD by decade of age in subgroups with/without TEP score ≥5, and showed that probabilities of having DD are significantly increased in TEP score positive (≥5) age subgroups (Figure 2).
Figure 2.
Survival analysis paired with Cox proportional hazards models analysis. Probabilities of having DD are significantly increased with age in TEPS positive (≥5) subgroups at each disc level (denoted ‘TEPS – Yes’ in the Figure). The probabilities increase with age, and the influence of endplate defect is least at L4/L5. DD = disc degeneration by Pfirrmann, HR = hazard ratio, TEPS = total endplate score.
Discussion
This is the first study of a large population sample having spine MR imaging to examine the role of endplate defects in DD graded by different morphological classifications: i) Pfirrmann grading and ii) quantitative trait for DD based on a 4-point grading system for disc height, signal change, posterior disc bulge, and anterior osteophytes.
Our results showed endplate defects to be strongly and independently associated with DD graded by both Pfirrmann (p≤0.001) and 4-point grading methods (p<1e-16). Although it is not possible to infer causality in a cross-sectional study such as this, the strong dose-response relationship between the two traits of interest and the evidence from other sources concerning causal mechanisms (8) suggest that endplate (micro)fracture can indeed be an initiating factor in DD. While possible, it is unlikely that the reverse is true - that DD could cause endplate damage, because degenerate discs have a very low intradiscal pressure (24) so that compressive force is transmitted through the annulus, where it is more likely to cause collapse of the anterior cortex rather than endplate damage (6).
ROC analysis demonstrated a TEP score cut-off value of 5 over which the prevalence of DD was significantly higher. The association of TEP score with DD was low until TEP score of five, and after that there was a rapid response with a great increase of likelihood of DD for each unit increase in TEP score. Of interest, the same TEP score cut-off point was found for each disc level and is similar to the cut-off of six as observed in the previous clinical study (19). One possible explanation for the minor variation of the TEP score cut-off points in these studies can be that the TwinsUK is a large population-based registry consisting of subjects (twins) recruited through national media campaigns and from other twin registers, who were not selected for any particular trait. The recruitment of general, asymptomatic, volunteers was meant to allow to make use of the normal situation providing normative measurements and avoid potentially confounding variables such as local patterns of referral that may occur in a symptomatic population. An additional reason may be that Rajasekaran et al. used T1 scans to evaluate endplate defects, which allows better assessment of cartilage features.
Our results showed that it might be enough to have a single grade 3 endplate defect (with focal disc marrow contacts, normal contour of endplate maintained and no MC associated) at a single disc level to have adjacent disc degeneration. A recent longitudinal study of 90 patients showed DD, endplate defect and Modic change (MC) to be significantly associated with each other (25). A relatively small interruption in the structural integrity of the endplate can be enough to cause significant alterations to the mechanical environment of the disc (16) as well as in diffusion patterns essential to maintain disc nutrition and hydration of the nucleus pulposus (7, 15). Also, a herniating annulus fibrosus may pull some cartilage endplate off the subchondral bone (26), giving rise to the ‘erosion’ type of endplate defect (27). Anaerobic bacteria also may be able to enter the disc via an endplate defect and hence lead to disc degeneration by another route (28). Moreover, oedema and changes in the vertebral bone marrow adjacent to endplate (i.e. Modic change) could be linked with endplate defect (26).
As integrity of the endplate seems to be crucial for the maintenance of mechanical environment and proper nutrition of the avascular disc, it seems reasonable to conclude that endplate defects may indeed be an initiating factor and have a precipitating role for the whole cascade of events that lead to DD, and ultimately to the clinical manifestation of severe and disabling LBP, as shown previously (29).
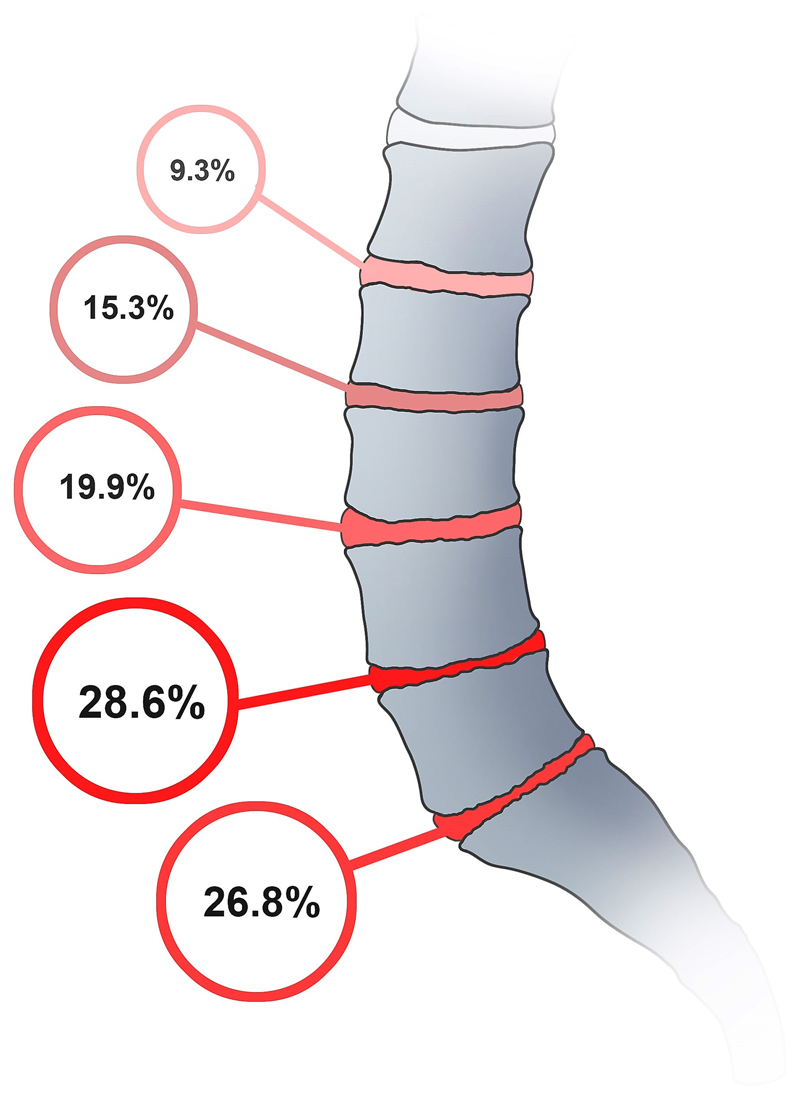
The finding that TEP score has a particularly great association with DD at upper lumbar levels, and a smaller, but still highly significant, association at L4/L5 (Figure 2), is consistent with experimental findings that disc decompression following endplate damage is greatest in the upper lumbar spine and particularly small at L4/L5 (16). Also, disc degeneration in the lower lumbar spine may be secondary to annulus failure (8) because the wedged lower lumbar discs are particularly vulnerable to prolapse in response to loading in bending and compression (30), and there is evidence that prolapse can initiate degenerative changes in displaced disc tissue (31). Some of these prolapses may involve endplate junction failure as well as annulus rupture (32). Figure 2 suggests that there is at least one other major cause of DD because the prevalence of DD is greatest at L4/L5 (28.6%) (Figure 3), precisely where the influence of endplate damage is least. Splitting the lumbar spine into upper and lower portions, as described by Mike Adams, may reveal differences in the aetiology of DD (8). Perhaps the disc herniates more commonly through endplate junction than the annulus fibrosus than previously thought.
Figure 3.
Prevalence of DD within the lumbar spine.
The limitations of this study include a study sample consisting mainly of female subjects but the previously held sex difference in DD has been questioned recently among researchers. In addition, due to missing MRI data in 10 subjects, the study population differs slightly from previous studies (29, 33). However, the generous size of the population sample can be considered a particular strength of this study. TwinsUK participants have been shown to be representative of singletons for a wide range of lifestyle and demographic traits (20). Another relevant strength is the thorough training phase undergone by the two evaluators (JHM and MR) leading to almost perfect inter-rater agreement for endplate defect: kappa value of 0.86, Pearson’s correlation 0.864 (correlation and kappa were employed in conjunction to uncover non-random examiner error, as in (34)). The other main limitation is that these are cross-sectional data, and strictly speaking longitudinal studies will be required to confirm the direction of relationship between the two traits.
Conclusion
To conclude, this study performed on a large population based sample confirmed that endplate defects are strongly and independently associated with DD and that this relationship is evident in adults across the age spectrum and at all lumbar levels. As the endplate is positioned exactly between the disc and the vertebral body in which relevant conditions as DD and MC occur, due to its structural contiguity, it could be that endplate defect is a major initiating factor for the whole cascade of events (that may include Modic change). A longitudinal study of twins’ MR spine scans and endplate is currently under way to confirm the order of events.
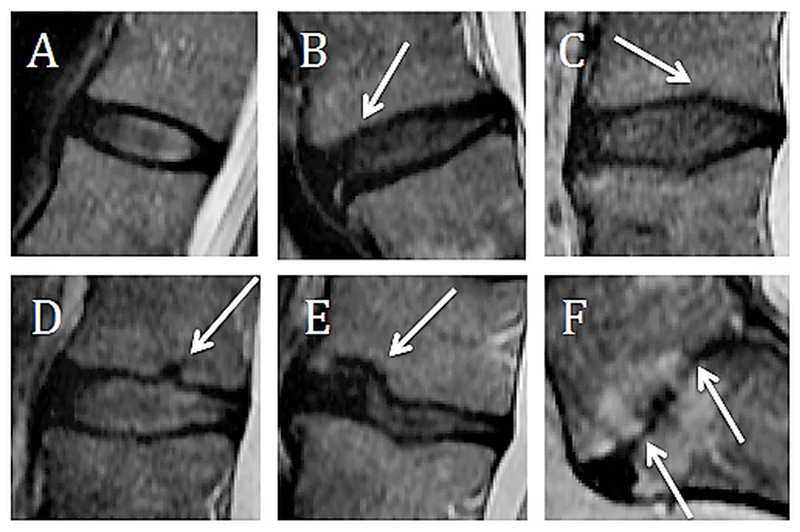
Figure 1.
End plate grading. A. Grade 1: Normal endplate, no breaks or defects, B. Grade 2: Focal thinning (white arrow) of the endplate, no breaks or defects, C. Grade 3: Focal disc marrow contacts (white arrow), but with maintained endplate contour, D. Grade 4: Endplate defects up to 25% of the endplate area (white arrow), E. Grade 5: Endplate defects up to 50% of the endplate area (white arrow), F. Grade 6: Extensive damaged endplates up to total destruction (white arrows).
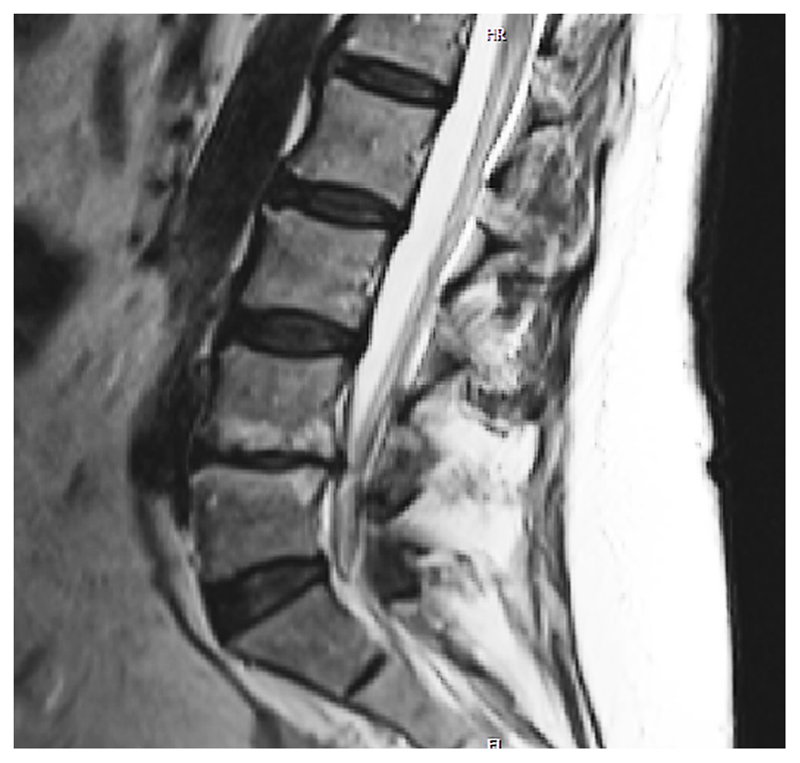
Figure 4.
MRI scan showing endplate defect grade VI both at the L4/L5 rostral and caudal endplates, with associated Modic changes (MC) over both rostral and caudal bone marrows adjacent to endplates and disc degeneration evaluated as Pfirrmann grade 5 and 4-point grading system grade 10. As the endplate is a fundamental part of the vertebral body-endplate-intervertebral disc motion segment, one could consider endplate defects to be an initiating factor not only for disc degeneration, but also for MC.
Acknowledgements
The authors would like to thank the twins who volunteered to take part in the study. The authors would also like to thank Prof. Michael A. Adams for his invaluable comments on the reporting of this study, and Ivan Barun MD, (ivan.i.barun@gmail.com), for the amazing work on figure 3.
The manuscript submitted does not contain information about medical device(s)/drug(s).
The EU FP7 project Pain_omics, the Wellcome Trust; European Community’s Seventh Framework Programme (FP7/2007-2013), and the National Institute for Health Research (NIHR) funds were received in support of this work.
Relevant financial activities outside the submitted work: board membership, payment for lectures, stocks.
References
- 1.Bendix T, Kjaer P, Korsholm L. Burned-out discs stop hurting: fact or fiction? Spine. 2008;33(25) doi: 10.1097/BRS.0b013e31818804b3. [DOI] [PubMed] [Google Scholar]
- 2.Livshits G, Popham M, Malkin I, Sambrook PN, MacGregor AJ, Spector T, et al. Lumbar disc degeneration and genetic factors are the main risk factors for low back pain in women: The UK Twin Spine Study. Ann Rheum Dis. 2011;70(10):1740–5. doi: 10.1136/ard.2010.137836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.MacGregor AJ, Andrew T, Sambrook PN, Spector TD. Structural, Psychological, and Genetic Influences on Low Back and Neck Pain: A Study of Adult Female Twins. Arthritis Care Res. 2004;51(2):160–7. doi: 10.1002/art.20236. [DOI] [PubMed] [Google Scholar]
- 4.Samartzis D, Cheung KMC. Lumbar Intervertebral Disk Degeneration. Orthop Clin North Am. 2011;42(4) doi: 10.1016/j.ocl.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Takatalo J, Karppinen J, Niinimäki J, Taimela S, Näyhä S, Mutanen P, et al. Does lumbar disc degeneration on magnetic resonance imaging associate with low back symptom severity in young finnish adults? Spine. 2011;36(25):2180–9. doi: 10.1097/BRS.0b013e3182077122. [DOI] [PubMed] [Google Scholar]
- 6.Adams MA, Roughley PJ. What is intervertebral disc degeneration, and what causes it? Spine. 2006;31:2151–2161. doi: 10.1097/01.brs.0000231761.73859.2c. [DOI] [PubMed] [Google Scholar]
- 7.Adams MA, Freeman BJC, Morrison HP, Nelson IW, Dolan P. Mechanical initiation of intervertebral disc degeneration. Spine. 2000;25:1625–1636. doi: 10.1097/00007632-200007010-00005. [DOI] [PubMed] [Google Scholar]
- 8.Adams MA, Dolan P. Intervertebral disc degeneration: evidence for two distinct phenotypes. J Anat. 2012;221(6):497–506. doi: 10.1111/j.1469-7580.2012.01551.x. Journal of Anatomy 221 497-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pfirrmann CWA, Metzdorf A, Zanetti M, Hodler J, Boos N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine. 2001;26(17):1873–8. doi: 10.1097/00007632-200109010-00011. [DOI] [PubMed] [Google Scholar]
- 10.Sambrook PN, MacGregor AJ, Spector TD. Genetic influences on cervical and lumbar disc degeneration: A magnetic resonance imaging study in twins. Arthritis Rheum. 1999;42(2):366–72. doi: 10.1002/1529-0131(199902)42:2<366::AID-ANR20>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 11.Sambrook PN, MacGregor AJ, Spector TD. Genetic influences on cervical and lumbar disc degeneration: A magnetic resonance imaging study in twins. Arthritis Rheum. 1999;42(2):366–72. doi: 10.1002/1529-0131(199902)42:2<366::AID-ANR20>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 12.Lotz JC, Fields AJ, Liebenberg EC. The role of the vertebral end plate in low back pain. Global Spine J. 2013 Jun;3(3):153–64. doi: 10.1055/s-0033-1347298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Setton LA, Zhu W, Weidenbaum M, Ratcliffe A, Mow VC. Compressive properties of the cartilaginous End-Plate of the baboon lumbar spine. J Orthop Res. 1993;11(2):228–39. doi: 10.1002/jor.1100110210. [DOI] [PubMed] [Google Scholar]
- 14.Rodriguez AG, Slichter CK, Acosta FL, Rodriguez-Soto AE, Burghardt AJ, Majumdar S, et al. Human disc nucleus properties and vertebral endplate permeability. Spine (Phila Pa 1976) 2011;36(7):512–20. doi: 10.1097/BRS.0b013e3181f72b94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holm S, Holm AK, Ekström L, Karladani A, Hansson T. Experimental Disc Degeneration Due to Endplate Injury. J Spinal Disord Tech. 2004;17(1):64–71. doi: 10.1097/00024720-200402000-00012. [DOI] [PubMed] [Google Scholar]
- 16.Dolan P, Luo J, Pollintine P, Landham PR, Stefanakis M, Adams MA. Intervertebral disc decompression following endplate damage: implications for disc degeneration depend on spinal level and age. Spine. 2013;38(17):1473–81. doi: 10.1097/BRS.0b013e318290f3cc. [DOI] [PubMed] [Google Scholar]
- 17.Haschtmann D, Stoyanov JV, Gédet P, Ferguson SJ. Vertebral endplate trauma induces disc cell apoptosis and promotes organ degeneration in vitro. Eur Spine J. 2008;17(2):289–99. doi: 10.1007/s00586-007-0509-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kerttula LI, Serlo WS, Tervonen OA, Pääkkö EL, Vanharanta HV. Post-Traumatic Findings of the Spine After Earlier Vertebral Fracture in Young Patients: Clinical and MRI Study. Spine (Phila Pa 1976) 2000;25(9):1104–8. doi: 10.1097/00007632-200005010-00011. [DOI] [PubMed] [Google Scholar]
- 19.Rajasekaran S, Venkatadass K, Naresh Babu J, Ganesh K, Shetty AP. Pharmacological enhancement of disc diffusion and differentiation of healthy, ageing and degenerated discs: Results from in-vivo serial post-contrast MRI studies in 365 human lumbar discs. Eur Spine J. 2008;17(5):626–43. doi: 10.1007/s00586-008-0645-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andrew T, Hart DJ, Snieder H, De Lange M, Spector TD, Macgregor AJ. Are twins and singletons comparable? A study of disease-related and lifestyle characteristics in adult women. Twin Res. 2001;4(6):464–77. doi: 10.1375/1369052012803. [DOI] [PubMed] [Google Scholar]
- 21.Livshits G, Popham M, Malkin I, Sambrook PN, MacGregor AJ, Spector T, et al. Lumbar disc degeneration and genetic factors are the main risk factors for low back pain in women: The UK Twin Spine Study. Ann Rheum Dis. 2011;70(10):1740–5. doi: 10.1136/ard.2010.137836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paajanen H, Lehto I, Alanen A, Erkintalo M, Komu M. Diurnal fluid changes of lumbar discs measured indirectly by magnetic resonance imaging. J Orthop Res. 1994;12(4):509–14. doi: 10.1002/jor.1100120407. [DOI] [PubMed] [Google Scholar]
- 23.Jarosz J, Bingham JB, Pemberton J, Sambrook PN, Spector TD. An atlas for scoring cervical and lumbar disc degeneration. London: Springer Verlag; 1997. [Google Scholar]
- 24.Sato K, Kikuchi S, Yonezawa T. In vivo intradiscal pressure measurement in healthy individuals and in patients with ongoing back problems. Spine (Phila Pa 1976) 1999;24(23):2468–74. doi: 10.1097/00007632-199912010-00008. [DOI] [PubMed] [Google Scholar]
- 25.Farshad-Amacker NA, Hughes A, Herzog RJ, Seifert B, Farshad M. The intervertebral disc, the endplates and the vertebral bone marrow as a unit in the process of degeneration. Eur Radiol. 2016 Oct 05; doi: 10.1007/s00330-016-4584-z. Published online. [DOI] [PubMed] [Google Scholar]
- 26.Lama P, Zehra U, Balkovec C, Claireaux HA, Flower L, Harding IJ, et al. Significance of cartilage endplate within herniated disc tissue. Eur Spine J. 2014;23:1869–77. doi: 10.1007/s00586-014-3399-3. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Videman T, Battié MC. ISSLS prize winner: Lumbar vertebral endplate lesions: Prevalence, classification, and association with age. Spine. 2012;37(17):1432–9. doi: 10.1097/BRS.0b013e31824dd20a. [DOI] [PubMed] [Google Scholar]
- 28.Albert HB, Lambert P, Rollason J, Sorensen JS, Worthington T, Pedersen MB, et al. Does nuclear tissue infected with bacteria following disc herniations lead to Modic changes in the adjacent vertebrae? Eur Spine J. 2013;22(4):690–6. doi: 10.1007/s00586-013-2674-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Määttä JH, Wadge S, MacGregor A, Karppinen J, Williams FMK. ISSLS prize winner: Vertebral endplate (modic) change is an independent risk factor for episodes of severe and disabling low back pain. Spine. 2015;40(15):1187–93. doi: 10.1097/BRS.0000000000000937. [DOI] [PubMed] [Google Scholar]
- 30.Adams MA, Hutton WC. Prolapsed intervertebral disc. A hyperflexion injury. Volvo Award 1981. Spine (Phila Pa 1976) 1982;7(3):184–91. [PubMed] [Google Scholar]
- 31.Lama P, Le Maitre CL, Dolan P, Tarlton JF, Harding IJ, Adams MA. Do intervertebral discs degenerate before they herniate, or after? Bone Joint J. 2013;95-B(8):1127–33. doi: 10.1302/0301-620X.95B8.31660. [DOI] [PubMed] [Google Scholar]
- 32.Rajasekaran S, Bajaj N, Tubaki V, Kanna RM, Shetty AP. ISSLS prize winner: The anatomy of failure in lumbar disc herniation: An in vivo, multimodal, prospective study of 181 subjects. Spine. 2013;38(17):1491–500. doi: 10.1097/BRS.0b013e31829a6fa6. [DOI] [PubMed] [Google Scholar]
- 33.Määttä JH, Kraatari M, Wolber L, Niinimäki J, Wadge S, Karppinen J, et al. Vertebral endplate change as a feature of intervertebral disc degeneration: a heritability study. Eur Spine J. 2014;23(9):1856–62. doi: 10.1007/s00586-014-3333-8. [DOI] [PubMed] [Google Scholar]
- 34.Hunt RJ. Percent agreement, Pearson's correlation, and kappa as measures of inter-examiner reliability. J Dent Res. 1986 Feb;65(2):128–30. doi: 10.1177/00220345860650020701. [DOI] [PubMed] [Google Scholar]