Summary
The interleukin-1 (IL-1) family of cytokines and receptors is unique in immunology because the IL-1 family and Toll-like receptor (TLR) families share similar functions. More than any other cytokine family, the IL-1 family is primarily associated with innate immunity. More than 95% of living organisms use innate immune mechanisms for survival whereas less than 5% depend on T-and B-cell functions. Innate immunity is manifested by inflammation, which can function as a mechanism of host defense but when uncontrolled is detrimental to survival. Each member of the IL-1 receptor and TLR family contains the cytoplasmic Toll-IL-1-Receptor (TIR) domain. The 50 amino acid TIR domains are highly homologous with the Toll protein in Drosophila. The TIR domain is nearly the same and present in each TLR and each IL-1 receptor family. Whereas IL-1 family cytokine members trigger innate inflammation via IL-1 family of receptors, TLRs trigger inflammation via bacteria, microbial products, viruses, nucleic acids, and damage-associated molecular patterns (DAMPs). In fact, IL-1 family member IL-1a and IL-33 also function as DAMPs. Although the inflammatory properties of the IL-1 family dominate in innate immunity, IL-1 family member can play a role in acquired immunity. This overview is a condensed update of the IL-1 family of cytokines and receptors.
Keywords: acquired immunity, cytokines, host defense, immunity, inflammation, innate immunity
1 | THE IL-1 FAMILY OF CYTOKINES AND THE INNATE IMMUNE SYSTEM
There are 11 members of the IL-1 family of cytokines (Figure 1) and 10 members of the IL-1 family of receptors (Table 1). More than any other cytokine family, the interleukin-1 family members are closely linked to damaging inflammation; however, the same members also function to increase nonspecific resistance to infection and development of the immune response to foreign antigens. These inflammatory and host defense properties are now termed ‘innate immunity’, and augmenting response to antigens is now called ‘acquired immunity’. What characterizes innate immunity is the lack of specificity. Indeed, the numerous biological properties of the IL-1 family are nonspecific. The importance of IL-1 family members to the innate response became evident upon the discovery that the cytoplasmic domain of the IL-1 receptor type I (IL-1R1) is also found in the Toll protein of the fruit fly.1 The functional domain of the cytosolic component of IL-1R12 is termed the Toll interleukin-1 receptor (TIR) domain. In fact, the TIR domain is highly homologous to the TIR domains of all Toll-like receptors (TLR). Thus, fundamental inflammatory responses such as the induction of cyclooxygenase type 2, production of multiple cytokines and chemokines, increased expression of adhesion molecules, or synthesis of nitric oxide are indistinguishable responses to both IL-1 and TLR ligands.
FIGURE 1.
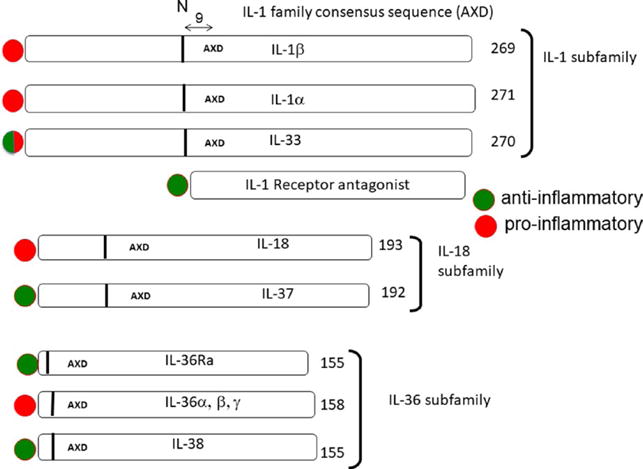
The 3 subfamilies of the IL-1 family. The number of amino acids is indicated at the end of each cytokine precursor. The IL-1 family consensus sequence AXD is indicated for each cytokine, and the 9 amino acids preceding the AXD site is indicated by a vertical bar. The vertical bar indicates the location of the optimal N-terminus. A red circle represents the members with primary pro-inflammatory properties, whereas a green circle represents cytokines that are anti-inflammatory. Because IL-1Ra has a signal peptide and is readily secreted, there is no AXD site. Adapted from6
TABLE 1.
IL-1 family of ligands and receptors
| IL-1 family | Specific receptor | Coreceptor | Function |
|---|---|---|---|
| IL-1α, IL-1β | IL-1R1 | IL-1R3 | Pro-inflammatory |
| IL-1β | IL-1R2 | IL-1R3 | Anti-inflammatory |
| IL-1Ra | IL-1R1 | NA | Anti-inflammatory |
| IL-18 | IL-1R5 | IL-1R7 | Pro-inflammatory |
| IL-33 | IL-1R4 | IL-1R3 | Pro-inflammatory |
| IL-36α, β, γ | IL-1R6 | IL-1R3 | Pro-inflammatory |
| IL-36Ra | IL-1R6 | IL-1R3 | Anti-inflammatory |
| IL-37 | IL-1R5 | IL-1R8 | Anti-inflammatory |
| IL-38 | IL-1R6 | IL-1R9 | Anti-inflammatory |
NA, not applicable.
Both the TLR and IL-1 families nonspecifically augment antigen recognition and activate lymphocyte function. The lymphocyte-activating function of purified IL-1 was first described in 19793 and is now termed a fundamental property of the acquired immune response. IL-1β is the most studied member of the IL-1 family due to its role in mediating autoinflammatory diseases.4 Unquestionably, IL-1β evolved to assist host defense against infection5 and this landmark study established how a low dose of recombinant IL-1β protects mice against lethal bacterial infection in the absence of neutrophils. Although we now accept the concept that cytokines like IL-1β served millions of years of evolution to protect the host, in the antibiotic and antiviral therapies era of today, we view cytokines also as the cause of disease due to acute or chronic inflammation.
2 | ORGANIZATION OF THE IL-1 FAMILY OF CYTOKINES AND RECEPTORS
2.1 | The IL-1 family consensus sequence
The IL-1 family of cytokines and receptors broadly affects a broad spectrum of immunological and inflammatory responses.6 As shown in Figure 1, the 11 members of the IL-1 family are divided into 3 subfamilies based on the IL-1 consensus sequence and the primary ligand binding receptor. With the exception of IL-1Ra, all members of the IL-1 family lack a signal peptide and are not readily secreted. They are found diffusely in the cytoplasm as precursors, and each precursor contains a three-amino acid conserved consensus sequence A-X-D, in which A may be any aliphatic amino acid, followed by any amino acid (X) and then D for aspartic acid.7 As depicted in Figure 1, nine amino acids before the consensus sequence is the N-terminal amino acid, which provides the optimal folding of the cytokine into the barrel shape for receptor binding. In the case of the IL-1β precursor, nine amino acids before the consensus motif (Leu-Arg-Asp) is the caspase-1 cleavage site creating the N-terminus for optimal IL-1β bioactivity. Truncation of IL-36α, IL-36β and IL-36γ to the 9th amino acid before the A-X-D motif results in a 1000-old increase in bioactivity.7
2.2 | The IL-1 family of receptors
As shown in Table 1, there are 10 members of the IL-1 family of receptors.9 IL-1R1 binds IL-1α, IL-1β, and IL-1Ra. IL-1R3 (formerly IL-1R accessory protein) is the coreceptor for forming a trimeric signaling complex with IL-1α or IL-1β. As shown in Figure 2, in the resting state, IL-1R1 and IL-1R3 are present on the cell membrane. Once IL-1 (either IL-1α or IL-1β) binds to IL-1R1, a structural change occurs that allows IL-1R3 to bind to IL-1R1. There is no direct contact of the ligand(s) to the IL-1R3 coreceptor. The trimeric complex allows for the approximation of the TIR domains of each receptor chain. MyD88 then binds to the TIR domains. The binding of MyD88 triggers a cascade of kinases that produce a strong pro-inflammatory signal leading to activation of NFκB.
FIGURE 2.
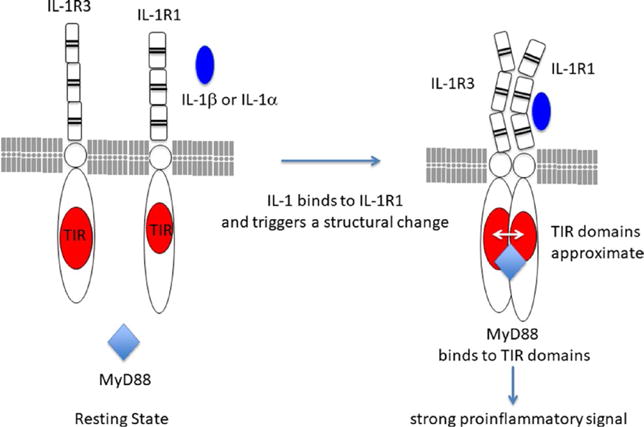
Resting and activation state of IL-1 receptors. In the resting state (left), the ligand binding chain, IL-1R1, is present on all nucleated cells. IL-1R3 is also present in the same cell. Both receptors are integral membrane proteins. Upon the binding of either IL-1α or IL-1β to the third Ig domain of IL-1R1, the receptor undergoes a structural change (left). This structural change allows the coreceptor, IL-1R3, to bind and form a trimeric complex. The intracellular TIR domains of IL-1R1 and IL-1R3 approximate and MyD88 now binds to the TIR domains. MyD88 becomes phosphorylated and other kinases (IL-1 receptor activated kinases (IRAKs) 1–4, data not shown) participate in a strong pro-inflammatory signal to the nucleus
IL-1R3 also exists as a soluble receptor form. The term ‘soluble’ is used to describe the liberated extracellular domain of membrane receptors. A common mechanism for forming soluble receptors is cleavage from the cell surface; however, the soluble form of IL-1R3 is also made by the liver.10,11 IL-1R2, a decoy receptor for IL-1β,12 lacks a cytoplasmic domain, does not signal, but rather sequesters IL-1β. IL-1R2 exists as an integral membrane protein but also as a soluble form. Although soluble IL-1R2 binds IL-1β in the extracellular space, neutralization of IL-1β activity is greatly enhanced by the forming a complex with soluble IL-1R3. Intracellularly, IL-1R2 associates with the IL-1α precursor and prevents the release and subsequent extracellular processing of the precursor by calpain. IL-1R3 is also the coreceptor for IL-33, IL-36α, IL-36β, or IL-36γ. The ligand binding receptor for IL-33 is IL-1R4, formerly ST2. The IL-36 receptor (IL-1R6) binds IL-36α, IL-36β, or IL-36γ but also IL-38.13
Importantly, the IL-1 family of receptors also contains anti-inflammatory receptors.14 These are IL-1R8 (formerly SIGIRR, TIR8), IL-1R9 (formerly TIGIRR-2, IL-1RAPL1), and IL-1R10 (formerly TIGIRR-1). In the case of IL-1R8, mice deficient in this receptor develop spontaneous inflammation, and when challenged with inflammatory agents, this mouse exhibits more severe disease.15 The IL-18 binding protein (IL-18BP) is not a classic receptor because it is a secreted protein; nevertheless, IL-18BP contains a single IgG domain similar to the extracellular IgG-like domains of the IL-18Rα but no cytoplasmic domain.16 The IL-18BP functions similarly as do other soluble receptors in the IL-1 receptor family; that is, they bind and neutralize the cognate ligand. In addition to the IL-18BP, there is soluble form of the IL-1R2. As an integral membrane protein, this receptor acts as a decoy receptor12 but like IL-18BP, soluble IL-1R2 will bind the cognate ligand in the extracellular compartment.
3 | IL-1α
3.1 | IL-1α is a dual-function cytokine
IL-1α is a ‘dual-function’ cytokine. Dual-function cytokines are found in the nucleus where they bind to DNA and serve a function; the same cytokine binds to its cell membrane receptor and initiates signal transduction. In the case of IL-1α, there is a nuclear localization sequence in the precursor region of the cytokine and IL-1α in the nucleus acts as a transcription factor.17 In that context, nuclear IL-1α functions to increase gene expression, for example the chemokine IL-8.17 Another member of the IL-1 family, IL-33, also is a dual-function cytokine. When the nuclear localization sequence of IL-33 in the mouse is deleted, the cytokine is found only in the cytosol; in these mice, overwhelming unresolved inflammation occurs early after birth, dominated by large numbers of eosinophils.18 Thus, nuclear localization of IL-33 acts as a sink for its pro-inflammatory properties. Nuclear translocation of IL-1α can also be a sink for its pro-inflammatory properties. For example, the IL-1α precursor shuttles between the cytosol and the nucleus within a few nanoseconds.19 When the cell is exposed to a proapoptotic signal, IL-1α leaves the cytosolic pool and rapidly migrates to the nucleus where it binds tightly to chromatin and fails to induce inflammation. In contrast, when the cell is exposed to a necrotic signal, IL-1α migrates from nucleus to the cytosol and the lysates of these cells are highly inflammatory.19 In general, when the precursor of IL-1α is released from necrotic cells, IL-1α is a DAMP and evokes a broad number of inflammatory reactions via the IL-1R1.20
3.2 | Constitutive IL-1α in health and disease
IL-1α is an unusual member of the IL-1 family because IL-1α is found constitutively present in epithelial and mesenchymal cell types of healthy subjects, whereas IL-1β is primarily induced under disease conditions. Endothelial cells contain IL-1α—the cytokine present in vesicles comprising the endothelial membrane.21 These vesicles are termed ‘apoptotic bodies’ but despite the name, they are highly inflammatory and contribute to vasculitis.21 IL-1α is a classic DAMP and functions as an ‘alarmin’ because the IL-1α precursor itself is active.8 In cell death by necrosis, IL-1α is released into the extracellular space where it stimulates the production of chemokines resulting in the infiltration of neutrophils first followed by monocytes. This transition from neutrophils to monocytes in ischemic tissues has been demonstrated in cells exposed to hypoxic conditions where the IL-1α precursor is released.22
3.3 | Processing of IL-1α
From cell cultures producing IL-1α, the N-terminus was found at amino acid serine 113.23 Serine 113 is not the N-terminus as predicted by the consensus sequence. The processing of human IL-1α from endotoxin-stimulated macrophages required a calcium-dependent neutral protease.24 In a mouse macrophage cell line, inhibitors of calpain prevent the processing of the IL-1α precursor.25,26 Copper is also required for the processing and release of IL-1α via S100A13 in both human macrophages and mouse fibroblasts.27,28 One can conclude that there may be more than one N-terminus for bioactive IL-1α, as there is also for IL-33 as described below.
3.4 | Membrane IL-1α
The precursor of IL-1α can be found on the surface of several cells, particularly on monocytes and B lymphocytes, where it is termed membrane IL-1α.29 Membrane IL-1α is biologically active30; thus, membrane IL-1α is an integral membrane protein. Membrane IL-1α plays an important role in inflammation as mice deficient in IL-1α exhibit reduced inflammation in models in which cell death and the release of intracellular IL-1α do not take place.31 Constitutively expressed membrane IL-1α is critical for several IFN-γ activities. Using the WISH epithelial cell line, what was considered to be intrinsic IFN-γ activities depended largely on constitutively expressed IL-1α. IFN-γ activities were inhibited antibodies to IL-1α, but not to IL-1β.32
3.5 | Studies in IL-1α-deficient mice
Mice deficient in IL-1α are born healthy and develop normally. Following subcutaneous injection of turpentine, which induces a local inflammatory response, wildtype and IL-1α-deficient mice develop fever and acute phase proteins, whereas IL-1β-deficient mice do not.33 In addition, although the induction of glucocorticoids after turpentine injection was suppressed in IL-1β-deficient mice, this suppression was not observed in IL-1α-deficient mice. Expression of IL-1β mRNA in the brain decreased 1.5-fold in IL-1α-deficient mice, whereas expression of IL-1α mRNA decreased more than 30-fold in IL-1β-deficient mice. These data suggest that IL-1β exerts greater control over the production of IL-1α than does IL-1α over the production of IL-1β. In caspase-1-deficient mice, IL-1α production is also reduced,34 suggesting that production of IL-1α is under the control of IL-1β.
4 | IL-1β
4.1 | Historical background
Human IL-1β was first purified to homogeneity in 1977 with a specific activity of producing a monophasic fever in rabbits at 10 ng/kg.35 This low specific activity was initially met with skepticism until the cDNA cloning of human IL-1β in 1984.36 With recombinant IL-1β available to study, during the following years, the multiple biological properties of IL-1β expanded greatly. Although the properties of recombinant IL-1β indicated the likely role in inflammation, a causative role for IL-1β in disease was shown in clinical trials using specific neutralization of IL-1β by monoclonal antibodies as reviewed in.37 More recent clinical trials using neutralizing anti-IL-1β antibodies have revealed a pivotal role for IL-1β in the pathogenesis of atherosclerosis38 and progression of cancer.39
4.2 | Transcription, translation, and synthesis of IL-1β
Although nearly all microbial products induce IL-1β via TLR ligands, IL-1 induces itself both in vivo and in monocytes in vitro.40 Other studies supporting this concept have been reported.41,42 Following LPS stimulation in human blood monocytes, IL-1β mRNA levels rise rapidly within 15 minutes, reach peak levels at 4 hours but begin to decline, thereafter 4 hours due to the half-life of the mRNA or the action of microRNA. However, using IL-1 itself as a stimulant, IL-1β mRNA levels are sustained for over 24 hours compared to microbial stimulants.43 The inactive IL-1β precursor accumulates in the cytosol until processed by the activation of nucleotide-binding domain and leucine-rich repeat pyrin containing protein-3 (NLRP3) and caspase-1 into an active cytokine.
4.3 | The NLRP3 inflammasome-dependent processing and secretion of IL-1β
Regardless of the stimulus, in monocytes and macrophages, specific inhibitors of caspase-1 reduce the secretion of mature IL-1β, while precursor IL-1β accumulates inside the cell. The rate-limiting step in the processing and secretion of IL-1β takes place with activation of the inflammasome. Several intracellular proteins form a complex with NLRP3, also termed cryopyrin. NLRP3 was initially discovered in patients with ‘familial cold autoinflammatory syndrome’, genetic disease characterized by constitutional symptoms, fevers, and elevated acute phase proteins following exposure to cold.44 Assembly of the inflammasome components with inactive procaspase-1 takes place following a fall in intracellular potassium. ATP activation of the P2X7 receptor opens the potassium channel, and simultaneously as potassium levels fall, caspase-1 is activated by the inflammasome.45–48
The cleavage of the IL-1β precursor by active caspase-1 can take place in the specialized secretory lysosomes or in the cytoplasm. However, more than one pathway seems available for processed IL-1β to exit the cell. These include exocytosis of the secretory lysosomes,45,46 shedding of plasma membrane microvesicles, direct release via transporters or multivesicular bodies containing exosomes,49 or a process termed pyroptosis.50 In general, the release of processed IL-1β takes place before there is a significant release of lactate dehydrogenase,51 although in vitro cell death eventually takes place. Pyroptosis is a caspase-1-dependent form of cell death and employed by certain bacteria using Ipaf, a member of the NLR family of intracellular receptors.52 An increase in intracellular calcium is also required for the mature IL-1β to exit the cell, which is phospholipase C dependent.46
4.4 | Non-caspase-1 processing of IL-1 β
A caspase-1 site exists for the IL-1β, IL-18, IL-33, and IL-37 precursors. However, for each of these cytokines, non-caspase-1 mechanisms also exist and generate active forms. For example, sterile inflammation induces fever, elevated IL-6, and increased production of hepatic acute phase proteins. These responses are absent in mice deficient in IL-1β but present in mice deficient in caspase-1.53 Sterile inflammation is often associated with neutrophilic infiltration, and neutrophils produce IL-1β. Because neutrophils are short-lived cells dying within hours upon emigration, release of the IL-1β precursor from intracellular stores is not unexpected. Therefore, processing of the IL-1β precursor extracellularly into an active cytokine has been reported for the common neutrophil protease, proteinase-3.54 Proteinase-3 also contributes to the processing of IL-18.55 Other proteases such as elastase, matrix metalloprotease 9, and granzyme A process the IL-1β precursor extracellularly. In addition, a mast cell chymase generates active IL-1β.
4.5 | Effects in mice deficient in IL-1β
After 10 years of continuous breeding, mice deficient in IL-1β exhibit no spontaneous disease. However, upon challenge, IL-1β-deficient mice exhibit specific differences from their wildtype controls. The most dramatic is the response to local inflammation induced by a subcutaneous injection of turpentine. Within the first 24 hours, IL-1β-deficient mice injected with turpentine do not manifest an acute phase response, do not develop anorexia, have no circulating IL-6 and no fever.53,56 These findings are consistent with those reported in the same model using anti-IL-1R type I antibodies in wildtype mice.56 IL-1β-deficient mice also have reduced inflammation due to zymosan-induced peritonitis.57 In contrast, IL-1β-deficient mice have elevated febrile responses to LPS, IL-1β, or IL-1α compared to wildtype mice.58 IL-1β-deficient mice injected with LPS have little or no expression of leptin mRNA or protein.59
5 | IL-1 RECEPTOR ANTAGONIST (IL-1Ra)
5.1 | Historical Background of IL-1Ra
IL-1Ra was first reported as a suppressor factor specifically inhibiting IL-1-mediated thymocyte proliferation.60 Subsequent to this observation, others reported the presence of a ‘specific’ inhibitor of IL-1 bioactivity in supernatants of human monocytes61 and in the serum and urine of children with systemic juvenile arthritis.62 In 1987, the IL-1 ‘inhibitor’ isolated from the urine was shown to prevent the binding of IL-1 to cells63 and this study provided the evidence for the mechanism of action of the IL-1 inhibitor. The IL-1 inhibitor was purified in 1990,64 and the cDNA reported that same year.65 The term IL-1 inhibitor was changed to IL-1 receptor antagonist and used for the first time in that report.
5.2 | Pharmacological use of IL-1Ra
Natural IL-1Ra is glycosylated but recombinant IL-1Ra expressed in E. coli is not glycosylated; nevertheless, recombinant IL-1Ra (generic anakinra) is fully active in blocking the IL-1R1, and therefore, the activities of IL-1α and IL-1β. Anakinra is approved for the treatment of rheumatoid arthritis and cryopyrin-associated periodic syndrome (CAPS). However, anakinra is used to treat a large number of diverse but common diseases from rheumatoid arthritis, gout, and idiopathic pericarditis to rare diseases and hereditary diseases (reviewed in37).
5.3 | Anakinra and autoinflammation
Specific missense mutations in diseases such as familial Mediterranean fever (FMF), and CAPS have been described and result in periodic fevers with systemic and local inflammation. These diseases do not involve T-lymphocytes, which are characteristically the effector cells in autoimmune diseases. Therefore, gout, pericarditis, and heart failure are not autoimmune diseases but rather autoinflammatory syndromes. IL-1 is well established for its role in the pathogenesis of disorders of autoinflammation. In autoinflammatory diseases, the effector cell is a myeloid cell, characteristically a monocyte or macrophage.66 The myeloid compartment also mediates common diseases such a gout, pericarditis, and heart failure. Because of the safety and relative short duration, anakinra can be used as a diagnostic as well as a treatment for patients refractory to glucocorticoid treatment with undefined autoinflammatory signs and symptoms.67
5.4 | Anakinra and the treatment for acute and chronic inflammatory diseases
Sensorineural deafness is a prominent characteristic of persons with Muckle-Wells syndrome and persons with mutations in NLRP3.68 The first reports of efficacy of anakinra to improve hearing were in patients with Muckle-Wells syndrome69 and several other reports subsequently followed.70–78 In general, the reversal in sensorineural deafness with anakinra treatment was unexpected and signified the concept that hearing loss in autoinflammatory diseases was due to a reversible chronic inflammatory response and not due to the loss of neuronal function. Anakinra has been administered to patients with an acute myocardial infarction.79,80 Anakinra treatment resulted in a significant reduction in the medium level of CRP.80 Twelve weeks following the myocardial infarctions, patients exhibited improved functional status.81 Additional studies examined the effect of anakinra on heart failure in patients with poor exercise tolerance and signs of systemic inflammation.81 The study established a role for treating patients with anakinra for refractory heart failure. Pericarditis can be a manifestation of an inherited autoinflammatory disorder such as TRAPS, FMF, and CAPS, and there are case reports of successful treatment with anakinra.70,82 Patients with adult-onset Still’s disease (AOSD) also have bouts of pericarditis83 that respond to anakinra. A summary of case reports that anakinra was highly effective in treating pericarditis was published in 2011.84
5.5 | Anakinra enters the brain
The first findings that anakinra administered peripherally reduced severity in a disease with central nervous system manifestations were reported in 2006 by Goldbach-Mansky in children with neonatal onset multi-inflammatory disease (NOMID).41 Twelve children were treated with 1–2 mg/kg of subcutaneous anakinra daily and the median cerebrospinal fluid (CSF) levels of anakinra rose to 1136 pg/mL after 3 months of treatment.41 This was the first evidence in humans that anakinra passes the blood-brain barrier. Intravenous anakinra was administered to patients admitted to the hospital within 6 hours of the signs of an acute thrombotic stroke.85 The dose, 2 mg/kg/hour for 72 hours, was the same dose used to treat septic shock. Although the study was not powered for significantly improved neurological outcome, the subgroup of patients with cortical infarcts performed better compared to the placebo group. A role for IL-1 in seizure disorders is based on the innovative studies of Vezzani and Barfai.86 Studies have examined circulating cytokines in patients with recurrent seizures and find elevated levels of IL-6, IL-1Ra in the postictal period.87 In one study, elevated IL-1β has also been observed in the intracellular ictal period in patients with recurrent temporal lobe epilepsy.88 Anakinra has been administered to patients with a severe seizure disorder termed febrile infection-related epilepsy (FIRE) syndrome and resulted in a cessation of the seizures.89
5.6 | Anakinra for macrophage activation syndrome
The use of anakinra in macrophage activation syndrome (MAS) is primarily in patients with systemic juvenile idiopathic arthritis (sJIA). Similar to sJIA, some patients with adult-onset Still’s disease (AOSD) also develop MAS. There are several reports of successful treatment with anakinra use in sJIA and AOSD. There are three randomized, placebo-controlled trials of anakinra to reduce all-cause 28-day mortality in patients with a diagnosis of septic shock.90–92 A re-analysis of the data from the third trial was performed subsequently.93 The analysis revealed that patients with fever, disseminated intravascular coagulation, hepatobiliary dysfunction, cytopenia, and hyperferritinemia were features of MAS. Data were available for 763 adults from the original study cohort, randomized to receive either anakinra or placebo. Concurrent hepatobiliary dysfunction/disseminated intravascular coagulation was noted in 43 patients (5.6% of total; 18–75 years old; 47% women). Treatment with anakinra improved 28-day survival: 65.4% anakinra vs 35.3% placebo), with hazard ratio for death 0.28 (0.11–0.71; P = .0071).93
5.7 | Anakinra to treat pre–multiple myeloma
The first study to use a IL-1-blocking therapy to treat cancer is in patients with a diagnosis of premyeloma. Anakinra treatment was assessed in patients with smoldering or indolent myeloma, and first reported by Lust and coworkers in 2009.94 The treatment was the standard dose of 100 mg daily of anakinra plus a weekly low dose of 20 mg of oral dexamethasone. The rationale for using anakinra in these patients is to reduce progression to overt myeloma, a disease consistently fatal even with advanced chemotherapeutics and bone marrow transplantation. The mechanism of action of IL-1 blockade is based on data that IL-1β released from bone marrow plasma cells induces IL-6 production from marrow stromal cells; furthermore, IL-6 is a growth factor for the malignant plasma cell.95 As the amount of IL-1β released from malignant plasma cells is low, anakinra would reduce IL-6 with greater efficacy compared to neutralizing IL-6. In addition, experimental data revealed that although anakinra was highly effective in reducing IL-1β-induced IL-6 in vitro, adding dexamethasone resulted in the death of the plasma cells.
A follow-up assessment of the treated cohort of patients has recently been reported.96 A reduction in serum C-reactive protein (CRP) level of 40 or greater percentage from baseline was used as a biomarker of response to anakinra. Of the 47 patients, 22 patients did not have this 40% fall in CRP; the median progression-free survival was 11 months. However, in 25 patients with a fall in CRP of 40% or greater, the median progression-free survival was 104 months (P < .001). Moreover, the median overall survival of the responders has not been reached, whereas the overall survival of those patients without a 40% reduction in CRP was 7.9 years (P < .001).
5.8 | IL-1 and host defense against infection
Since its introduction in 2002 for the treatment of rheumatoid arthritis, anakinra has had a remarkable record of safety.97,98 It is estimated that over 150 000 patients have received anakinra, some treated daily for over 10 years. As with all ‘biologics’, increased routine infections are reported for anakinra. Nevertheless, anakinra has the safety benefit of short duration. A large number of animal studies including primates given live bacteria and treated to anakinra demonstrate greater survival with anakinra compared to infected animals treated with the vehicle. In humans, anakinra has been administered to patients with active infections99,100 and in over 1000 patients during the sepsis trials. Although anakinra in trials of septic shock did not reach a statistically significant reduction in all-cause 28-day mortality, a subgroup of patients with a likely diagnosis of MAS and treated with anakinra survived compared to placebo-treated patients with MAS. By comparison, in patients treated with anti-TNF-α-blocking therapies, there is well-known risk of several opportunistic infections. Reactivation of latent Mycobacterium tuberculosis in patients receiving anti-TNF-α therapies can be 25 times higher than in untreated persons101 and is often the disseminated form, similar to that observed in HIV-1-infected patients. In contrast, opportunistic infections in patients treated with anakinra are rare,102 including populations at high risk for reactivation of M. tuberculosis infections.103
6 | IL-33
6.1 | Background
For many years, the IL-1 receptor family member ST2 (proper nomenclature IL-1R4) was studied for its function in the Th2 paradigm of allergic diseases without knowledge of its specific ligand.104 In 2005, using in silico research methods, a new member of the IL-1 family was identified as the ligand binding to IL-1R4; it was termed IL-33.105 Following binding of IL-33, IL-1R4 undergoes a conformational change, similar to that of IL-1α or IL-1β binding to the IL-1R1.106 The conformational changes in IL-1R4 allow for IL-1R3 to bind to IL-1R4 to form the heterotrimer.107 IL-1R3 binds to the side of IL-1R4108 and there is no contact of IL-33 with IL-1IL-1R3. The TIR domains of IL-1R4 and IL-1R3 approximate and signaling is initiated via MyD88, IL-1 receptor activated kinases (IRAKs), and NFκB. As shown in Figure 1, IL-33 belongs to the IL-1 subfamily.
6.2 | Processing of IL-33
Cell source of IL-33 The IL-33 precursor contains a caspase-1 cleavage site at aspartic acid 111 and this site was thought to provide a mechanism similar to that of IL-1β and IL-18, that is processing intracellularly by caspase-1 and secretion of the active cytokine. In fact, caspase-1 inactivates IL-33.109 Similar to the IL-1α precursor, full-length IL-33 (amino acids 1–270) is active, and thus, IL-33 is considered an alarmin that is released upon cell damage and active as a precursor. Processing takes place extracellularly by enzymes such as neutrophil elastase and cathepsins.110 Processing by these and mast cell enzymes results in significantly greater activity of IL-33.110 There are at least 3 mature forms of IL-33 resulting from mast cell proteases that are significantly more potent than the precursor, and each activates group 2 innate lymphoid cells.111 There is also a short splice variant of IL-33,112 which lacks exon 3. The recombinant splice variant precursor was active on human mast cells, and anti-IL-1R4 was effective in reducing the activity of recombinant IL-33 splice variant.
6.3 | Nuclear function of IL-33
Girard and coworkers reported that in endothelial cells, IL-33 translocated to the nucleus.113 The nuclear translocation of the N-terminal domain of IL-33 is similar to that of IL-1α. IL-1α shuttles between the cytosol and the nucleus19; both IL-1α and IL-33 binding to chromatin acts to sequester the cytokine and reduce inflammation. When the nuclear localization sequence of IL-33 was deleted, transgenic mice expressing the mutated IL-33 develop overwhelming lethal inflammation with massive infiltration of eosinophils in the organs.18 Thus, similar to nuclear IL-1α, chromatin binding of IL-33 acts as a sink to prevent inflammation. This observation would be consistent with a pro-inflammatory role for IL-33 in rheumatoid arthritis.
IL-33 is an anti-inflammatory cytokine. Although IL-33 acts primarily as a pro-inflammatory cytokine in murine models of human rheumatoid arthritis, IL-33 has anti-inflammatory properties. For example, IL-33 exerts anti-inflammatory properties in a model of heart disease, obesity, and uveitis. It is possible that the anti-inflammatory properties of IL-33 are due to a shift to the Th2 paradigm. Using collagen-induced arthritis in mice, multiple administrations of recombinant IL-33 reduced the pathological changes in the joints only when the mice were treated during the induction rather than the treatment phase of the model.114 The protective properties of IL-33 were associated with increased circulating Th2 cytokines, decreased IFN-γ production, increased numbers of eosinophils, and type 2 innate lymphoid cells.114 In addition, IL-33 expanded the population of Fox3+ T-regulatory cells. Another mechanism for the anti-inflammatory properties of IL-33 appears to be due to the requirement for IL-1R8.115 In addition to a role for IL-33 in the Th2 paradigm, there are also studies revealing a role for IL-33 in classic Th1 diseases such as inflammatory bowel disease116 and rheumatoid arthritis.117,118 IL-33 also increases the production of IFN-γ.119
7 | IL-18 AND IL-18 BINDING PROTEIN
IL-18 was first described in 1989 as ‘IFN-γ-inducing factor’ isolated in the serum following intraperitoneal injection of endotoxin. Days before the injection, the mice had been pretreated with Proprionibacterium acnes, which stimulates the reticuloendothelial system, particularly the Kupffer cells of the liver. With purification from mouse livers and molecular cloning of ‘IFN-γ-inducing factor’ in 1995,120 the name was changed to IL-18. Unexpectedly, the new cytokine was related to IL-1β. Similar to IL-1β, IL-18 is first synthesized as an inactive precursor and without a signal peptide, remains as an intracellular cytokine. The tertiary structure of the IL-18 precursor is closely related to the IL-37 precursor and the intron-exon borders of the IL-18 and IL-37 genes suggest a close association. Since 1995, many studies have used neutralization of endogenous IL-18 or IL-18-deficient mice to demonstrate the role for this cytokine in promoting inflammation and immune responses (reviewed in121–123). However, the biology of IL-18 is hardly the recapitulation of IL-1β. There are several unique and specific differences between IL-18 and IL-1β. For example, in healthy human subjects and also in healthy mice, gene expression for IL-1β in blood mononuclear cells and hematopoietic cells is absent and there is no evidence that the IL-1β precursor is constitutively present in epithelial cells.124 In contrast, the IL-18 precursor is present in blood monocytes from healthy subjects and in the epithelial cells of the entire gastrointestinal tract.
7.1 | Processing of the IL-18 precursor by caspase-1
The IL-18 precursor has a molecular weight of 24 000 and is processed by caspase 1, which cleaves the precursor into an active mature molecule of 17 200. As with the processing of IL-1β, inactive procaspase-1 is first converted into active caspase-1 by the (NLRP3) inflammasome. Following cleavage of the IL-18 precursor by active caspase-1, mature IL-18 is secreted from the monocyte/macrophage, although over 80% of the IL-18 precursor remains unprocessed inside the cell. Importantly, any phenotypic characteristic of caspase-1-deficient mice should be studied whether the deficiency is due to reduced IL-1β or IL-18 activity. For example, the caspase-1-deficient mouse is resistant to dextran sulfate sodium (DSS)-induced colitis125 but the IL-1β-deficient mouse is susceptible in the same disease model.126 As neutralizing antibodies to IL-18 are protective in the DSS colitis model, caspase-1 deficiency appears to prevent processing of IL-18125,127 rather than IL-1β.
Similar to IL-1α and IL-33, the IL-18 precursor is constitutively expressed in endothelial cells, keratinocytes, and intestinal epithelial cells throughout the gastrointestinal tract. Macrophages and dendritic cells are the primary sources for the release of active IL-18, whereas the inactive precursor remains in the intracellular compartment of mesenchymal cells. Also, similar to IL-1α and IL-33, the IL-18 precursor is released from dying cells and processed extracellularly, most likely by neutrophil proteases such as proteinase-3.55
7.2 | Signal transduction by IL-18 receptors
IL-18 forms a signaling complex by binding to the IL-18 alpha chain (IL-18Rα, now IL-1R5), which is the ligand binding chain for mature IL-18; however, this binding is of low affinity. In cells that express the coreceptor, termed IL-18 receptor beta chain (IL-18Rβ, now IL-1R7), a high-affinity complex is formed, which then signals. The complex of IL-18 with the IL-1R5 and IL-1R7 chains is similar to that formed by other members of the IL-1 family with the coreceptor, IL-1R3. Following the formation of the heterodimer, TIR domains approximate and a cascade of sequential recruitment of MyD88, the four IRAKs and TNF receptor activating factor-6 followed by the degradation of IκB and release of NFκB are nearly identical as that for IL-1.128 However, there are differences between IL-1 and IL-18 signaling. With few exceptions, IL-1α or IL-1β is active on cells in the low nanogram/mL range and often in the picogram/mL range. In contrast, IL-18 activation of cells expressing the two IL-18 receptor chains requires 10–20 ng/mL and sometime higher levels.129,130
7.3 | Role of IL-18 in the production of IFN-γ
Together with IL-12, IL-18 participates in the Th1 paradigm. This property of IL-18 is due to its ability to induce IFN-γ either with IL-12 or with IL-15. Without IL-12 or IL-15, IL-18 does not induce IFN-γ. IL-12 or IL-15 increases the expression of IL-18Rβ, which is essential for IL-18 signal transduction. Importantly, without IL-12 or IL-15, IL-18 plays a role in Th2 diseases.131 The importance of IL-18 as an immunoregulatory cytokine is derived from its prominent biological property of inducing IFN-γ from NK cells. Membrane IL-18 is expressed in 30–40% of M-CSF-primed macrophages but LPS treatment is necessary for the release of membrane IL-18.132 A major immunoregulating role for IL-18 is on the NK cell. Several human autoimmune diseases are associated with elevated production of IFN-γ and IL-18. Diseases such as systemic lupus erythematosus (SLE), rheumatoid arthritis, type 1 diabetes, Crohn’s disease, psoriasis, and graft-vs-host disease are thought to be mediated, in part, by IL-18.
7.4 | IL-18, IL-17, and γ/δ T-cell activation
The role for IL-17 in the pathogenesis of autoimmune diseases has been studied in animal models but also validated in humans treated with either neutralizing antibodies to IL-17 or the IL-17 receptor. Is there a role for IL-18 in the production of IL-17? Attention has focused on a role for IL-18 in Th17 responses primarily because both IL-1β and IL-18 are processed into active cytokines via caspase-1. Using a model for multiple sclerosis termed experimental autoimmune encephalomyelitis (EAE),133 a role for IL-18 was studied. As expected, using the adjuvant of M. tuberculosis plus the myelin-derived immunogen for EAE, bone marrow-derived mouse dendritic cells released IL-1β and IL-18, which was dependent on caspase-1.134 The primed dendritic cells induced IL-17 from T cells, which when transferred to nonimmunized mice resulted in the encephalomyelitis. However, the disease did not develop when the dendritic cells were exposed to a caspase-1 inhibitor.134 Treating the mice with either IL-1β or IL-18 restored the ability of the T-cell transfer to induce the disease. Moreover, treating the recipient mice with the caspase-1 inhibitor reduced the disease as well as reduced the production of IL-17 from CD4-positive T cells as well as from gamma-delta T cells. Gamma-delta T cells produce IL-17 when stimulated with IL-18 plus IL-23, as these T cells express high levels of the IL-18 receptor alpha chain. Thus, similar to caspase-1-dependent IL-1β, IL-18 induces T cells to produce IL-17 and promote autoimmune responses to specific antigens.
7.5 | Pro-inflammatory properties of IL-18
In the absence of IL-12 or IL-15, IL-18 exhibits characteristics of other pro-inflammatory cytokines of the IL-1 family, such as increases in cell adhesion molecules, nitric oxide synthesis, and chemokine production. Inhibition of IL-18 activity takes place with the administration of either neutralizing anti-IL-18 antibodies or the IL-18 binding protein. In general, inhibition of IL-18 activity results in a reduction in disease severity. Blocking IL-18 activity reduces metastasis in a mouse model of melanoma; this is due to a reduction in IL-18-induced expression of vascular cell adhesion molecule-1.135 The induction of fever, a well-studied property of IL-1α and IL-1β as well as acute phase proteins, TNF-α and IL-6, is not a significant property of IL-18. Injection of IL-18 into mice or rabbits does not cause fever.136,137 In a clinical study of intravenous IL-18 dosing in patients with cancer, chills, and fevers were not common and were Grade 1 (low fevers). IL-1 and TNF-α fever in humans is observed in all patients at doses of 10 ng/kg, whereas IL-18 fevers were observed in 3 of 21 patients and only at doses of 100 and 200 μg/kg.138
Unlike IL-1 and TNF-α, IL-18 does not induce cyclooxygenase-2 and hence there is no production of prostaglandin E2.130,139 IL-18 has been administered to humans for the treatment of cancer in order to increase the activity and expansion of cytotoxic T cells. Not unexpectedly and similar to several cytokines, the therapeutic focus on IL-18 has shifted from its use as an immune stimulant to inhibition of its activity.122,140
7.6 | IL-18 in heart disease
Heart disease includes coronary vessel disease with associated myocardial infarction, postviral myocardopathies, autoimmune heart disease, and chronic heart failure. Increasing numbers of animal and clinical studies indicate a role for IL-18 in heart disease. The myocardium of patients with ischemic heart failure expresses the alpha chain of the IL-18 receptor and elevated levels of circulating IL-18 are associated with death.141 Daily administration of IL-18 results in ventricular hypertrophy, increased collagen,142 and elevated left ventricular diastolic pressure in mice.143 In a model of myocardial suppression associated with septic shock, mice injected with LPS and a neutralizing antibody to murine IL-18 were protected.144 In another study, human atrial muscle strips were obtained from patients undergoing by-pass surgery and the atrial tissue exposed to ischemia ex vivo. The addition of IL-18BP to the perfusate improved contractile function from 35% of control to 76% with IL-18BP.145 Serum from patients with heart failure intravenously administered to mice results in myocardial suppression. However, using mice deficient in IL-18 or IL-18R5 did not develop the suppression.146 Mice pretreated with IL-18BP or caspase-1 inhibition also were protected. Although myocardial suppression in mice is induced by IL-1β, it was not expected that IL-18 was required. Thus, IL-1β induces heart failure in mice by an IL-18-dependent pathway. As the IL-18 precursor is present in myocardiocytes and the endothelium, caspase-1 is induced by IL-1β, which then processes the IL-18 precursor into an active cytokine. Another role for IL-18 in heart is a model of isoproterenol-induced cardiomyocyte hypertrophy. Mice transgenic for IL-18BP are protected.147
7.7 | IL-18 as a protective cytokine
There are a growing number of studies supporting a protective role for IL-18. Mice deficient in caspase-1 experience increased disease severity when subjected to DSS colitis and that administration of exogenous IL-18 restored mucosal healing in these mice.148 Mice deficient in NLRP3 are more susceptible to DSS colitis, which is thought to be due to decreased IL-18.149 Mice deficient in NLRP6 are also more vulnerable to DSS150,151 and the susceptibility appears to be due to the lack of sufficient IL-18. A protective role for IL-18 is not limited to the gastrointestinal tract. In the eye, a condition resembling ‘wet macular degeneration’ worsens with antibodies to IL-18.152 In a mouse model of ‘wet’ age-related macular degeneration, the disease was worse in mice deficient in NLRP3, but not in IL-1RI-deficient mice.152 Therefore, IL-18 rather than IL-1α or IL-1β was protective, and upon the administration of IL-18, the disease severity improved.
Starting at 16 weeks of age, IL-18-deficient mice start to overeat, become obese, and exhibit lipid abnormalities; there is increased atherosclerosis, insulin resistance, and diabetes mellitus reminiscent of the metabolic syndrome.153 IL-1R7-deficient mice also develop a similar phenotype. The higher body weight is attributed to enhanced food intake, in which the IL-18-deficient mice begin to diverge from wildtype animals at a relatively early age, and to reach values 30%–40% higher than that of wildtype mice. Others have observed similar findings.154 A striking finding was an increase of more than 100% in the percentage of adipose tissue in the IL-18-deficient animals that was accompanied by fat deposition in the arterial walls. The insulin resistance in these mice is corrected by exogenous recombinant IL-18. This unexpected and unique mechanism is responsible for the higher food intake in the IL-18-deficient animals, which appears to be due to a central nervous system and loss of appetite control. IL-18-deficient mice eat throughout the day, whereas wildtype mice eat once, nocturnally.
8 | IL-18 BINDING PROTEIN
8.1 | Background on IL-18BP
The discovery of the IL-18BP took place during the search for the soluble receptors for IL-18.16 With a clear signal peptide, IL-18BP is a constitutively secreted protein. IL-18BP has an exceptionally high affinity for IL-18 of 400 pM, although recent studies indicate an affinity for IL-18 of 5 pM. IL-18BP is present in the serum of healthy humans at a 20-fold molar excess compared to IL-18155; thus, IL-18BP may contribute to a default mechanism by which a Th1 response to foreign organisms is blunted. Although IL-18BP is readily secreted, it falls into the functional category of being a shed soluble receptor. IL-18BP contains only one IgG domain, whereas the IL-1R2 contains three domains. In this regard, the single IgG domain of IL-18BP is similar to IL-1R8 (SIGIRR), which also has a single IgG domain and also functions as a decoy receptor. The salient property of IL-18BP in immune responses is in downregulating Th1 responses by binding to IL-18 and thus reducing the induction of IFN-γ.131 As IL-18 also affects Th2 responses, IL-18BP also has properties controlling a Th2 cytokine response.131
8.2 | The concept of free IL-18
Because a single IL-18BP molecule binds a single IL-18 molecule, one can calculate bound vs free IL-18 in a mixture of both molecules.155 If one examines immunologically mediated diseases where IFN-γ plays a pathological role such as Wegener’s granulomatosis and SLE, one must consider the level of free IL-18 compared to IL-18 bound to IL-18BP. In fact, in these diseases, both IL-18BP and IL-18 are high156,157 but the level of IL-18BP is not sufficiently high enough to neutralize IL-18, and therefore, the level of free IL-18 is higher than in healthy subjects. In MAS where IFN-γ plays a pathological role, both IL-18BP and IL-18 are also high but the clinical and hematological abnormalities correlate with elevated free IL-18.158
A unique property of IL-18BP is that the molecule also binds IL-37159 and in doing so, enhances the ability of IL-18BP to inhibit the induction of IFN-γ by IL-37. IL-37 binds to the IL-18Rα with a very low affinity but in mice expressing human IL-37, a profound anti-inflammatory effect is observed,160 particularly of LPS-induced cytokines and dendritic cell maturation.160 Human IL-37-expressing mice are also resistant to colitis.161 Thus, the anti-inflammatory property of IL-37 can be affected by the concentration of IL-18BP. As the concentration of IL-18BP increases and binds IL-37, there is the possibility that IL-37 becomes less available as an anti-inflammatory cytokine. Indeed, this has been observed in mice injected with IL-18BP. At low dosing of IL-18BP, there is reduced inflammation in a model of rheumatoid arthritis but as the dosing of IL-18BP increases, the anti-inflammatory properties of IL-18BP are lost.162 Table 2 summarizes several disease states in which IL-18 and IL-18BP are measured, and in some studies, the level of free IL-18 has been reported.
TABLE 2.
Levels of IL-18 and IL-18BP in human disease
| Disease | IL-18a | IL-18BPb | Free IL-18a | Reference |
|---|---|---|---|---|
| Sepsis | 500–2000 | ND | ND | 237 |
| Sepsis | 250–10 000 | 22.5 | 250–3000 | 155 |
| Sepsis | 592–1755 | ND | ND | 238 |
| Trauma | 300–600 | ND | ND | 239 |
| Schizophrenia | 518 | 10 | 253 | 240 |
| Ulcerative colitis | 274 | ND | ND | 241 |
| Ulcerative colitis | 393 | 4.7 | 250 | 242 |
| Crohn’s disease | 387 | ND | ND | 241 |
| Crohn’s disease | 546 | 5 | 340 | 242 |
| Wegener’s disease | 240 | 14.5 | 84 | 156 |
| Rheumatoid arthritis | 230–400 | ND | ND | 243 |
| SLE | 700 | 7.5 | 408 | 244 |
| SLE | 400 | 15 | 167 | 157 |
| SLE | 135–560 | ND | ND | 245 |
| MAS | 2200 | 35 | 660 | 158 |
| Systemic JIA | 1600–78 000 | ND | ND | 246 |
| Systemic JIA | 130 000 | ND | ND | 247 |
| Adult-onset Still’s disease | 1000–6000 | ND | ND | 248 |
| Adult-onset Still’s disease | 1000–5000 | ND | ND | 249 |
| Myocardial infarction | 238 | ND | ND | 250 |
| Myocardial infarction | 355 | ND | ND | 251 |
| Myocardial infarction | 346–2068 | ND | ND | 252 |
| Coronary artery disease | 356 | 13.7 | 125 | 253 |
| Coronary artery disease | 195–322 | 19–29 | ND | 254 |
| Metabolic syndrome | 380 | ND | ND | 255 |
| Acute kidney injuryc | 500 | ND | ND | 256 |
| Acute kidney injuryc | 2000 | ND | ND | 257 |
| Acute kidney injuryc | >360 | ND | ND | 258 |
| Acute kidney injuryc | 884 | ND | ND | 259 |
| Acute kidney injuryc | 1265–2873 | ND | ND | 260 |
ND, not determined.
Levels in pg/mL, range or mean.
Levels in ng/mL, range or mean.
Urine levels (mean in pg/mL).
SLE, systemic lupus erythematosus; MAS, macrophage activation syndrome; JIA, juvenile idiopathic arthritis.
8.3 | Regulation of IL-18BP
IL-18BP is highly regulated at the level of gene expression, and unexpectedly, IFN-γ increases gene expression and synthesis of IL-18BP.163,164 Therefore, IFN-γ driving an increase in the natural and potent inhibitor of IL-18 falls into the category of a negative feedback loop. The concept is supported by clinical data showing that patients being treated with IFN-α for hepatitis have elevated levels of IL-18BP.165,166 IL-27, like IFN-γ, functions as both a pro- and an anti-inflammatory cytokine and both may accomplish their roles as anti-inflammatory cytokines at the level of increased production of IL-18BP. In the skin, IL-27 also acts through a negative feedback loop for inflammation. IL-27 is acting, as is IFN-γ, by the induction of IL-18BP gene expression and synthesis.167
8.4 | Viral IL-18BP
Natural neutralization of human IL-18 by IL-18BP takes place during a common viral infection. In Molluscum contagiosum infection, characterized by raised but bland skin eruptions, there are large numbers of viral particles in the epithelial cells of the skin but histologically there are few inflammatory or immunologically active cells in or near the lesions. Clearly, the virus fails to elicit an inflammatory or immunological response. Amino acid similarity exists between human IL-18BP and a gene found in various members of the poxviruses; the greatest degree of homology is in Molluscum contagiosum gene.168 The ability of viral IL-18BP to reduce the activity of mammalian IL-18 likely explains the lack of inflammatory and immune cells in the virally infected tissues and provides further evidence for the natural ability of IL-18BP to interfere with IL-18 activity.
8.5 | IL-18 in the hemophagocytic syndromes
In the case of familial hemophagocytic lymphohistiocytosis or MAS, gene expression for IL-18 is upregulated in peripheral mononuclear cells169,170 and serum IL-18 is unusually elevated.158,171–174 Although levels of IL-18 in the circulation are below 1 ng/mL in inflammatory diseases such as severe sepsis, in the active phase of MAS, serum IL-18 is usually in the range of 5–7 ng/ml, and in familial hemophagocytic lymphohistiocytosis complicating XIAP gene mutations as well as in MAS complicating sJIA, levels of circulating IL-18 can be in 20–30 nanograms per milliliter range.158,175–177 However, it is necessary to calculate the level of free IL-18 as IL-18BP is present in the circulation in health and disease.155 In patients with MAS, free IL-18 but not IL-12 concentrations significantly correlated with clinical status and the biologic markers of MAS such as anemia (P < .001), hypertriglyceridemia, and hyperferritinemia (P < .01) and also with markers of Th1 lymphocyte or macrophage activation, such as elevated concentrations of IFN-γ and soluble IL-2 and TNF-α receptor concentrations.158 Patients with a mutation in NLRC4 experience a life-threatening hyperinflammation state, mostly manifested by intractable colitis. Free IL-18 is markedly elevated and treatment with IL-18BP provides resolution of the inflammatory state.178
9 | IL-37
IL-37 is an unexpected member of the IL-1 family. IL-37, a member of the IL-18 subfamily, was first reported in 2000 using in silico research179 and given the name for IL-1 family 7 (IL-1F7); this name was changed to IL-37 during the revision of interleukin-1 family nomenclature.160 There are 4 IL-37 isoforms due to exon deletions and IL-37b is the most complete form.180 Most studies with recombinant IL-37 use IL-37b, although isoform IL-37a has also been studied.181 IL-37 is unique in the IL-1 family in that it broadly suppresses innate but also acquired immunity.182,183 Another distinguishing characterization of IL-37 is that that there is no homologue for IL-37 in the mouse, but also not in the chimpanzee.180 IL-37 is often elevated in humans with inflammatory and autoimmune diseases where the cytokine likely functions to limit inflammation. For example, compared to levels of IL-37 in healthy subjects, IL-37 is elevated in persons with Grave’s disease,184 rheumatoid arthritis,185,186 ankylosing spondylitis,187 psoriasis,188 and SLE.189 However, in calcific aortic valve disease,190 asthma,191,192 insulin resistance,193 and allergic rhinitis,194 levels of the mRNA as well as the IL-37 protein cytokine are lower than in tissues from healthy subjects, suggesting a relative deficient state. An important concept to emerge from such studies is that relative deficiencies of endogenous IL-37 seem to contribute to the severity of inflammation.
9.1 | The IL-37 signaling complex: requirement for IL-1R5 and IL-1R8
As shown in Table 1, IL-18 binds to IL-1R5 (IL-18Rα) and recruits IL-1R7. Crystallization reveals that following the binding of IL-18 to IL-1R5, IL-1R7 is recruited to IL-1R5 and not to IL-18. The trimeric complex signals a pro-inflammatory response.195,196 Although IL-37 also binds to IL-1R5,159,197,198 IL-37 does not recruit IL-1R7; rather, IL-37 recruits IL-1R8. IL-1R8 (formerly TIR8, SIGIRR) is an anti-inflammatory receptor and deficiency of IL-1R8 results in increased inflammation in many models of human disease as reviewed in.14 The TIR domain of IL-1R8 is mutated (termed TIRb),15,199 and thus, IL-1R8 functions as a sink for MyD88. By depriving MyD88 from binding to native TIR domain, there is a weak or no signal. The IL-37 signaling complex suppresses the phosphorylation of several inflammatory kinases,180 particularly mTOR.182 A reduction in mTOR and an increase in AMP kinase reverse the Warburg effect. Indeed, recombinant IL-37 in mice spares the metabolic cost of inflammation.200
9.2 | Translocation of IL-37 to the nucleus
Similar to IL-1α and IL-33, IL-37 translocates to the nucleus.201 Fifteen hours after LPS stimulation, IL-37 is detected in nuclei202 and IL-1β, IL-1α, TNF-α, IL-6, and chemokines are substantially reduced (72%–98%) compared to cells transfected with an empty plasmid.182,201 The nuclear translocation of IL-37 requires active caspase-1.202 Mutation of the caspase-1 recognition aspartic acid in position 20 to alanine prevents nuclear translocation and also prevents IL-37 from suppressing LPS-induced IL-6.203 These data reveal an unexpected role for caspase-1 in enabling the nuclear translocation of IL-37 and subsequent suppression of inflammation. Intracellular IL-37 forms a complex with Smad3, an anti-inflammatory kinase downstream of the TGF-β receptor; Smad3 is associated with the immunosuppressive and anti-inflammatory properties of TGF-β. A proteomics-based search for intracellular proteins that interact with Smad3 identified IL-37.204 IL-37 immunoprecipitates with phosphorylated Smad3 and the IL-37-Smad3 complex has been observed in the perinuclear space.182 Thus, following cleavage by caspase-1, the carboxyl domain of IL-37 binds to Smad3 and the complex moves to the nucleus. It is likely that Smad3 provides the anti-inflammatory portfolio for IL-37.
9.3 | Production of IL-37
The IL-37 protein is expressed by human blood monocytes, tissue macrophages, synovial cells, tonsillar B cells, plasma cells, T cells, neoplastic cells as well as epithelial cells of the skin, kidney, and intestine. IL-37 is not constitutively expressed by blood monocytes from healthy humans but rather requires stimulation by IL-1β and TLR agonists but unexpectedly by TGF-β182; in human keratinocytes, beta-defensin-3 induces IL-37.205 The abundance of IL-37 transcripts is low in human blood monocytes and dendritic cells due to an instability sequence in IL37. This instability sequence limits mRNA half-life of IL-37.206 Indeed, despite having a CMV promoter, resting mRNA levels for IL-37 in IL37-tg mice is either absent or low but rapidly increases with inflammation.161,182,203,207 Even in stimulated human blood monocytes, the level of IL-37 protein is low at 10–20 pg/million cells. Despite the low production of endogenous IL-37, the use of recombinant IL-37 reveals that the cytokine is highly active. Picomolar concentrations of recombinant IL-37 are required to reduce LPS-induced IL-1β, IL-6, and TNF production in vitro.208
9.4 | Processing and release
Similar to other members of the IL-1 family, the IL-37 precursor contains no classical signal peptide. In endotoxin-stimulated human blood monocytes, the addition of exogenous ATP results in a modest release of processed IL-37203 but most of the secreted IL-37 is in the precursor form. Also, similar to IL-1α and IL-33, both precursor and processed forms of IL-37 are active.197,207,208 The precursor of IL-37 in the extracellular space likely reflects an unorthodox mechanism to exit the cell, which is unrelated to cell death.
9.5 | IL37-tg mice
IL37-tg were generated using the full-length precursor cDNA of IL37b isoform driven by the CMV promoter for constitutive expression,182 as this promoter is commonly used to drive expression in most cells. IL37-tg mice breed normally and have no obvious phenotype. However, compared to WT mice, IL37-tg mice are protected against LPS challenge, exhibiting significantly less hypothermia, acidosis, hyperkalemia, hepatitis, and dehydration in addition to lower levels of cytokines.182 IL37-tg mice are also protected against spinal cord injury,207 DSS-induced colitis,161 coronary artery ligation,209 acute renal ischemia,210 and metabolic syndrome.193
9.6 | A role for IL-37 in human disease
Increased levels of IL-37 have been reported in several human disease conditions by measuring mRNA levels in cells derived from subjects affected by a specific disease as well as the concentrations of IL-37 in the circulation. In addition, immunohistochemistry of tissues and production from cells in vitro are also reported. For example, IL-37 is present in colonic tissues from patients with inflammatory bowel disease, but is absent in biopsies from healthy subjects.211,212 IL-37 is elevated in patients with rheumatoid arthritis.185,186,213,214 These and similar studies support the concept that infection or inflammation increases IL-37 expression, which is an appropriate response to limit disease severity. Anti-inflammatory mediators such as IL-1 receptor antagonist, soluble TNF-α receptors and the IL-18 binding protein are also elevated in several of these diseases.
In contrast, in some diseases, the opposite is observed; that is, there is a lower level of IL-37 compared to level in healthy subjects. A low level of IL-37 likely contributes to increased disease severity.190 Patients with a wide range of inflammatory diseases and low levels of IL-37 may reflect a failure to have sufficient levels to combat inflammation. In obesity, increased levels of IL-37 in human adipose tissue are associated with less insulin resistance193 and therefore are consistent with a functional role for IL-37 in limiting inflammation. In HIV-1, the elevated levels of IL-37 can be interpreted in two ways; the elevation is an appropriate response to limit the inflammation of a chronic infection or the elevation contributes to the immunosuppression of HIV-1.215
10 | IL-36
10.1 | The IL-36 subfamily
There are 5 ligands that bind to the IL-36 receptor (IL-1R6): IL-36α, IL-36β, IL-36γ, the IL-36 receptor antagonist (IL-36Ra), and IL-38. The binding of IL-38 to the IL-36R was reported in 201213 and confirmed in 2016.216 IL-36α, IL-36β, IL-36γ, and IL-36Ra were reported in 2000–2001 by in silico research.179,217,218 The IL-36 receptor (IL-1R6) was previously known as IL-1 receptor-related protein 2.219 IL-36 is part of the IL-1 gene cluster located on chromosome 2q13 in a stretch of 360 kb with IL-1 and IL-37.219 The IL-36 subfamily is primarily found in the skin. For example, keratinocytes synthesize and release large amounts of IL-36γ when stimulated with polyinosinic–polycytidylic acid (poly I:C).220 Flagellin also stimulates IL-36γ but the IL-36 remains intracellular.220 However, the release of IL-36γ from keratinocytes was dependent on caspase-1.220
10.2 | Processing of IL-36
The original observation of the activity of IL-36 revealed that the IL-1 family consensus sequence played a critical role in the conversion of low-activity IL-36 to a highly active cytokine. As shown in Figure 1, there are few amino acids at the N-terminus of IL-36 prior to the AXD site. Towne and colleagues demonstrated a major gain in function of IL-36α, β, γ and IL-36Ra with removal of the 9 amino acids preceding the AXD site.7 Neutrophil proteases, which are prominent in psoriatic skin, contribute to the N-terminal processing of the IL-36 ligands.221 Proteinase-3, cathepsin G, and elastase increased activity by 500-fold.221
10.3 | Role of IL-36 in disease
A great deal of attention focused on IL-36 when it was reported that a mutation in IL-36Ra resulted in pustular psoriasis.222–224 Indeed, IL-36 is expressed primarily in the skin and particularly in psoriatic skin. In human psoriasis, there is the expression of IL-36α and IL-36γ, but not IL-36β.225 IL-36α· IL-36β, and IL-36γ are elevated in the lesional skin of patients with hidradenitis suppurativa.226 There is also a role for IL-36 in rheumatoid arthritis. IL-36 is found in the synovium of patients with rheumatoid arthritis and levels correlate with IL-1β and chemokines. In the mouse DSS colitis model and also in humans with Crohn’s colitis, only IL-36α and IL-36γ correlate with disease severity and not with IL-36β.225 In the epidermis, LL37 is a major inducer of chemokines in the skin; moreover, the antimicrobial peptide LL37 can act as an alarmin. In keratinocytes, blocking IL-36γ with siRNA or IL-36 receptor suppressed LL37-mediated production of IL-8 as well as CXCL10.227 Elevated circulating levels as well as salivary glands of IL-36a have been reported in patients with Sjogren’s syndrome.228 Histologically, α/β and γ/δ T cells were identified in the salivary glands of the patients.
11 | IL-38
11.1 | The functional role of IL-38
IL-1 family member 10 was renamed IL-38 with the new IL-1 family nomenclature.160 IL-38 also was discovered by in silico research from the large databases of the human genome. IL-38 was an orphan cytokine in the IL-1 family until the discovery that recombinant human IL-38 bound to the IL-36 receptor.13 Interest in IL-38 increased with the large genome-wide association analysis study (GWAS) in over 66 000 subjects with elevated C-reactive protein. In the search for missense mutations, IL-38 was deemed a high-risk cytokine.229 IL-38 belongs to the IL-36 subfamily (see Figure 1 and Table 1). Using killed Candida albicans yeast cells, recombinant human IL-38 stimulated the released IL-17 and IL-22 from human peripheral blood mononuclear cells (PBMC).230 The reduction in IL-22 and IL-17 by IL-38 was similar to that caused by the naturally occurring IL-36 receptor antagonist (IL-36Ra). IL-38 and IL-36Ra have similar biological effects on immune activation.
Others have studied the role of IL-38 in suppressing inflammation by apoptotic cells. When endogenous IL-38 was suppressed in apoptotic cells, IL-6 increased in macrophages present in the coculture.216 These data support the concept that IL-38 is anti-inflammatory, consistent with previous studies.13 The increase in inflammation following silencing of endogenous IL-38 from apoptotic cells also resulted in increased IL-17.216 The IL-38 precursor was processed in apoptotic cells but both the processed and truncated IL-38 suppressed IL-6 production. The anti-inflammatory property of IL-38 required IL-1R9,216 also termed IL-1R accessory protein-like-1 and three immunoglobulin IL-1-related receptor-2 (TIGIRR-2). IL-1R9 is X-linked and males with mutations in IL-1R9 have severe developmental impairment.231
11.2 | IL-38 in disease
IL-38 appears to play a role in SLE. Circulating levels of IL-38 were measured in 142 patients with SLE and 16% were positive (range 63–6000 pg/mL) and were significantly higher than in samples from healthy subjects.232 The elevated levels were observed in patients with renal and central nervous system forms of active SLE.232 Blocking endogenous IL-38 in PBMC from healthy subjects resulted in increased IL-6 and other pro-inflammatory cytokines.232 These findings are consistent with previous studies on the ability of recombinant human IL-38 to suppress IL-17 and IL-22.13
11.3 | IL-38 emerges as an anti-inflammatory cytokine member of the IL-1 family
Treating the lupus-prone MRL/lpr mice with recombinant human IL-38 reduced several manifestations of the disease, including the proteinuria and skin lesions.233 Recombinant IL-38 administered to the MRL/lpr mice also reduced the circulating levels of IL-17 and IL-22. Humans with asthma have elevated IL-38 in the circulation, and the levels negatively correlated with the numbers of Treg lymphocytes.234 In addition to lupus and asthma, IL-38 is also elevated in the circulation and synovial membranes of patients with rheumatoid arthritis.235 Using an adenoviral expression of human IL-38, mice with collagen-induced arthritis showed reduced inflammation.235 A similar reduction in the severity of the joint inflammation was observed using antigen-induced arthritis.235 Similar to the anti-inflammatory members of the IL-1 family, IL-1Ra, IL-37, and IL-36Ra, IL-38 are elevated in disease. Does the elevated level reflect the severity of the disease as a biomarker or is the IL-38 functional in reducing the disease? For example, in patients admitted to the hospital for ST-elevated myocardial infarction (STEMI), the levels of circulating IL-38 reached peak levels at 24 hours.236 In addition, levels of IL-38 gene expression were elevated in circulating PBMC. In STEMI patients who were treated with re-perfusion, the levels of IL-38 were markedly lower compared with the untreated group, suggesting that IL-38 is a biomarker for the intensity of inflammation in this population.236
Acknowledgments
These studies are supported by NIH Grant AI15614.
Funding information
NIH Grant, Grant/Award Number: AI15614
Footnotes
CONFLICT OF INTEREST
The author declares no conflict of interest.
This article is part of a series of reviews covering The IL-1 cytokine and receptor family appearing in Volume 281 of Immunological Reviews.
References
- 1.Gay NJ, Keith FJ. Drosophila Toll and IL-1 receptor. Nature. 1991;351:355–356. doi: 10.1038/351355b0. [DOI] [PubMed] [Google Scholar]
- 2.Heguy A, Baldari CT, Macchia G, Telford JL, Melli M. Amino acids conserved in interleukin-1 receptors (IL-1Rs) and the Drosophila toll protein are essential for IL-1R signal transduction. J Biol Chem. 1992;267:2605–2609. [PubMed] [Google Scholar]
- 3.Rosenwasser LJ, Dinarello CA, Rosenthal AS. Adherent cell function in murine T-lymphocyte antigen recognition. IV. Enhancement of murine T-cell antigen recognition by human leukocytic pyrogen. J Exp Med. 1979;150:709–714. doi: 10.1084/jem.150.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood. 2011;117:3720–3732. doi: 10.1182/blood-2010-07-273417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van der Meer JWM, Barza M, Wolff SM, Dinarello CA. A low dose of recombinant interleukin 1 protects granulocytopenic mice from lethal gram-negative infection. Proc Natl Acad Sci USA. 1988;85:1620–1623. doi: 10.1073/pnas.85.5.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garlanda C, Dinarello CA, Mantovani A. The interleukin-1 family: Back to the future. Immunity. 2013;39:1003–1018. doi: 10.1016/j.immuni.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Towne JE, Renshaw BR, Douangpanya J, et al. Interleukin-36 (IL-36) ligands require processing for full agonist (IL-36alpha, IL-36beta, and IL-36gamma) or antagonist (IL-36Ra) activity. J Biol Chem. 2011;286:42594–42602. doi: 10.1074/jbc.M111.267922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim B, Lee Y, Kim E, et al. The interleukin-1alpha precursor is biologically active and is likely a key alarmin in the IL-1 family of cytokines. Front Immunol. 2013;4:391. doi: 10.3389/fimmu.2013.00391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boraschi D, Tagliabue A. The interleukin-1 receptor family. Semin Immunol. 2013;25:394–407. doi: 10.1016/j.smim.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 10.Jensen LE, Whitehead AS. Expression of alternatively spliced interleukin-1 receptor accessory protein mRNAs is differentially regulated during inflammation and apoptosis. Cell Signal. 2003;15:793–802. doi: 10.1016/s0898-6568(03)00039-1. [DOI] [PubMed] [Google Scholar]
- 11.Jensen LE, Muzio M, Mantovani A, Whitehead AS. IL-1 signaling cascade in liver cells and the involvement of a soluble form of the IL-1 receptor accessory protein. J Immunol. 2000;164:5277–5286. doi: 10.4049/jimmunol.164.10.5277. [DOI] [PubMed] [Google Scholar]
- 12.Colotta F, Re F, Muzio M, et al. Interleukin-1 type II receptor: A decoy target for IL-1 that is regulated by IL-4. Science. 1993;261:472–475. doi: 10.1126/science.8332913. [DOI] [PubMed] [Google Scholar]
- 13.Gow NA, van de Veerdonk FL, Brown AJ, Netea MG. Candida albicans morphogenesis and host defence: Discriminating invasion from colonization. Nat Rev Microbiol. 2012;10:112–122. doi: 10.1038/nrmicro2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garlanda C, Riva F, Bonavita E, Mantovani A. Negative regulatory receptors of the IL-1 family. Semin Immunol. 2013;25:4087–4415. doi: 10.1016/j.smim.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 15.Garlanda C, Riva F, Bonavita E, Gentile S, Mantovani A. Decoys and regulatory “receptors” of the IL-1/Toll-like receptor superfamily. Front Immunol. 2013;4:180–192. doi: 10.3389/fimmu.2013.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Novick D, Kim S-H, Fantuzzi G, Reznikov L, Dinarello CA, Rubinstein M. Interleukin-18 binding protein: A novel modulator of the Th1 cytokine response. Immunity. 1999;10:127–136. doi: 10.1016/s1074-7613(00)80013-8. [DOI] [PubMed] [Google Scholar]
- 17.Werman A, Werman-Venkert R, White R, et al. The precursor form of IL-1alpha is an intracrine proinflammatory activator of transcription. Proc Natl Acad Sci USA. 2004;101:2434–2439. doi: 10.1073/pnas.0308705101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bessa J, Meyer CA, de Vera Mudry MC, et al. Altered subcellular localization of IL-33 leads to non-resolving lethal inflammation. J Autoimmun. 2014;55:33–41. doi: 10.1016/j.jaut.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 19.Cohen I, Rider P, Carmi Y, et al. Differential release of chromatin-bound IL-1alpha discriminates between necrotic and apoptotic cell death by the ability to induce sterile inflammation. Proc Natl Acad Sci USA. 2010;107:2574–2579. doi: 10.1073/pnas.0915018107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Di Paolo NC, Shayakhmetov DM. Interleukin-1a and the inflammatory process. Nat Immunol. 2016;17:906–913. doi: 10.1038/ni.3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berda-Haddad Y, Robert S, Salers P, et al. Sterile inflammation of endothelial cell-derived apoptotic bodies is mediated by interleukin-1alpha. Proc Natl Acad Sci USA. 2011;108:20684–20689. doi: 10.1073/pnas.1116848108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rider P, Carmi Y, Guttman O, et al. IL-1alpha and IL-1beta recruit different myeloid cells and promote different stages of sterile inflammation. J Immunol. 2011;187:4835–4843. doi: 10.4049/jimmunol.1102048. [DOI] [PubMed] [Google Scholar]
- 23.Lomedico PT, Gubler R, Hellmann CP, et al. Cloning and expression of murine interleukin-1 cDNA in Escherichia coli. Nature. 1984;312:458–462. doi: 10.1038/312458a0. [DOI] [PubMed] [Google Scholar]
- 24.Kobayashi Y, Yamamoto K, Saido T, Kawasaki H, Oppenheim JJ, Matsushima K. Identification of calcium-activated neutral protease as a processing enzyme of human interleukin 1 alpha. Proc Natl Acad Sci USA. 1990;87:5548–5552. doi: 10.1073/pnas.87.14.5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kavita U, Mizel SB. Differential sensitivity of interleukin-1 alpha and -beta precursor proteins to cleavage by calpain, a calcium-dependent protease. J Biol Chem. 1995;270:27758–27765. doi: 10.1074/jbc.270.46.27758. [DOI] [PubMed] [Google Scholar]
- 26.Carruth LM, Demczuk S, Mizel SB. Involvement of a calpain-like protease in the processing of the murine interleukin 1 alpha precursor. J Biol Chem. 1991;266:12162–12167. [PubMed] [Google Scholar]
- 27.Prudovsky I, Mandinova A, Soldi R, et al. The non-classical export routes: FGF1 and IL-1alpha point the way. J Cell Sci. 2003;116:4871–4881. doi: 10.1242/jcs.00872. [DOI] [PubMed] [Google Scholar]
- 28.Mandinova A, Soldi R, Graziani I, et al. S100A13 mediates the copper-dependent stress-induced release of IL-1alpha from both human U937 and murine NIH 3T3 cells. J Cell Sci. 2003;116:2687–2696. doi: 10.1242/jcs.00471. [DOI] [PubMed] [Google Scholar]
- 29.Kurt-Jones EA, Beller DI, Mizel SB, Unanue ER. Identification of a membrane-associated interleukin-1 in macrophages. Proc Natl Acad Sci USA. 1985;82:1204–1208. doi: 10.1073/pnas.82.4.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaplanski G, Farnarier C, Kaplanski S, et al. Interleukin-1 induces interleukin-8 from endothelial cells by a juxtacrine mechanism. Blood. 1994;84:4242–4248. [PubMed] [Google Scholar]
- 31.Kamari Y, Werman-Venkert R, Shaish A, et al. Differential role and tissue specificity of interleukin-1alpha gene expression in atherogenesis and lipid metabolism. Atherosclerosis. 2007;195:31–38. doi: 10.1016/j.atherosclerosis.2006.11.026. [DOI] [PubMed] [Google Scholar]
- 32.Hurgin V, Novick D, Werman A, Dinarello CA, Rubinstein M. Antiviral and immunoregulatory activities of IFN-gamma depend on constitutively expressed IL-1alpha. Proc Natl Acad Sci USA. 2007;104:5044–5049. doi: 10.1073/pnas.0611608104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horai R, Asano M, Sudo K, et al. Production of mice deficient in genes for interleukin (IL)-1a, IL-1b, IL-1a/b, and IL-1 receptor antagonist shows that IL-1b is crucial in turpentine-induced fever development and glucocorticoid secretion. J Exp Med. 1998;187:1463–1475. doi: 10.1084/jem.187.9.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuida K, Lippke JA, Ku G, et al. Altered cytokine export and apoptosis in mice deficient in interleukin-1b converting enzyme. Science. 1995;267:2000–2003. doi: 10.1126/science.7535475. [DOI] [PubMed] [Google Scholar]
- 35.Dinarello CA, Renfer L, Wolff SM. Human leukocytic pyrogen: Purification and development of a radioimmunoassay. Proc Natl Acad Sci USA. 1977;74:4624–4627. doi: 10.1073/pnas.74.10.4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Auron PE, Webb AC, Rosenwasser LJ, et al. Nucleotide sequence of human monocyte interleukin 1 precursor cDNA. Proc Natl Acad Sci USA. 1984;81:7907–7911. [PubMed] [Google Scholar]
- 37.Dinarello CA, van der Meer JW. Treating inflammation by blocking interleukin-1 in humans. Semin Immunol. 2013;25:469–484. doi: 10.1016/j.smim.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 39.Ridker PM, MacFadyen JG, Thuren T, et al. Effect of interleukin-1beta inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390:1833–1842. doi: 10.1016/S0140-6736(17)32247-X. [DOI] [PubMed] [Google Scholar]
- 40.Dinarello CA, Ikejima T, Warner SJ, et al. Interleukin 1 induces interleukin 1. I. Induction of circulating interleukin 1 in rabbits in vivo and in human mononuclear cells in vitro. J Immunol. 1987;139:1902–1910. [PubMed] [Google Scholar]
- 41.Goldbach-Mansky R, Dailey NJ, Canna SW, et al. Neonatal-onset multisystem inflammatory disease responsive to interleukin-1beta inhibition. N Engl J Med. 2006;355:581–592. doi: 10.1056/NEJMoa055137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Greten FR, Arkan MC, Bollrath J, et al. NF-kappaB is a negative regulator of IL-1beta secretion as revealed by genetic and pharmacological inhibition of IKKbeta. Cell. 2007;130:918–931. doi: 10.1016/j.cell.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schindler R, Ghezzi P, Dinarello CA. IL-1 induces IL-1. IV. IFN-gamma suppresses IL-1 but not lipopolysaccharide-induced transcription of IL-1. J Immunol. 1990;144:2216–2222. [PubMed] [Google Scholar]
- 44.Hoffman HM, Mueller JL, Broide DH, Wanderer AA, Kolodner RD. Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle-Wells syndrome. Nat Genet. 2001;29:301–305. doi: 10.1038/ng756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Andrei C, Dazzi C, Lotti L, Torrisi MR, Chimini G, Rubartelli A. The secretory route of the leaderless protein interleukin 1beta involves exocytosis of endolysosome-related vesicles. Mol Biol Cell. 1999;10:1463–1475. doi: 10.1091/mbc.10.5.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Andrei C, Margiocco P, Poggi A, Lotti LV, Torrisi MR, Rubartelli A. Phospholipases C and A2 control lysosome-mediated IL-1 beta secretion: Implications for inflammatory processes. Proc Natl Acad Sci USA. 2004;101:9745–9750. doi: 10.1073/pnas.0308558101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gardella S, Andrei C, Costigliolo S, Olcese L, Zocchi MR, Rubartelli A. Secretion of bioactive interleukin-1beta by dendritic cells is modulated by interaction with antigen specific T cells. Blood. 2000;95:3809–3815. [PubMed] [Google Scholar]
- 48.Perregaux DG, McNiff P, Laliberte R, Conklyn M, Gabel CA. ATP acts as an agonist to promote stimulus-induced secretion of IL-1 beta and IL-18 in human blood. J Immunol. 2000;165:4615–4623. doi: 10.4049/jimmunol.165.8.4615. [DOI] [PubMed] [Google Scholar]
- 49.Qu Y, Franchi L, Nunez G, Dubyak GR. Nonclassical IL-1 beta secretion stimulated by P2X7 receptors is dependent on inflammasome activation and correlated with exosome release in murine macrophages. J Immunol. 2007;179:1913–1925. doi: 10.4049/jimmunol.179.3.1913. [DOI] [PubMed] [Google Scholar]
- 50.Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: Host cell death and inflammation. Nat Rev Microbiol. 2009;7:99–109. doi: 10.1038/nrmicro2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brough D, Rothwell NJ. Caspase-1-dependent processing of pro-interleukin-1beta is cytosolic and precedes cell death. J Cell Sci. 2007;120:772–781. doi: 10.1242/jcs.03377. [DOI] [PubMed] [Google Scholar]
- 52.Suzuki T, Franchi L, Toma C, et al. Differential regulation of caspase-1 activation, pyroptosis, and autophagy via Ipaf and ASC in Shigella-infected macrophages. PLoS Pathog. 2007;3:e111. doi: 10.1371/journal.ppat.0030111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fantuzzi G, Ku G, Harding MW, et al. Response to local inflammation of IL-1 beta-converting enzyme-deficient mice. J Immunol. 1997;158:1818–1824. [PubMed] [Google Scholar]
- 54.Coeshott C, Ohnemus C, Pilyavskaya A, et al. Converting enzyme-independent release of TNFa and IL-1b from a stimulated human monocytic cell line in the presence of activated neutrophils or purified proteinase-3. Proc Natl Acad Sci USA. 1999;96:6261–6266. doi: 10.1073/pnas.96.11.6261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sugawara S, Uehara A, Nochi T, et al. Neutrophil proteinase 3-mediated induction of bioactive IL-18 secretion by human oral epithelial cells. J Immunol. 2001;167:6568–6575. doi: 10.4049/jimmunol.167.11.6568. [DOI] [PubMed] [Google Scholar]
- 56.Zheng H, Fletcher D, Kozak W, et al. Resistance to fever induction and impaired acute-phase response in interleukin-1b deficient mice. Immunity. 1995;3:9–19. doi: 10.1016/1074-7613(95)90154-x. [DOI] [PubMed] [Google Scholar]
- 57.Fantuzzi G, Sacco S, Ghezzi P, Dinarello CA. Physiological and cytokine responses in interleukin-1b-deficient mice after zymosan-induced inflammation. Am J Physiol. 1997;273:R400–R406. doi: 10.1152/ajpregu.1997.273.1.R400. [DOI] [PubMed] [Google Scholar]
- 58.Alheim K, Chai Z, Fantuzzi G, et al. Hyperresponsive febrile reactions to interleukin (IL) 1alpha and IL-1beta, and altered brain cytokine mRNA and serum cytokine levels, in IL-1beta-deficient mice. Proc Natl Acad Sci USA. 1997;94:2681–2686. doi: 10.1073/pnas.94.6.2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Faggioni R, Fantuzzi G, Fuller J, Dinarello CA, Feingold KR, Grunfeld C. IL-1b mediates leptin induction during inflammation. Am J Physiol. 1998;274:R204–R208. doi: 10.1152/ajpregu.1998.274.1.R204. [DOI] [PubMed] [Google Scholar]
- 60.Dinarello CA, Rosenwasser LJ, Wolff SM. Demonstration of a circulating suppressor factor of thymocyte proliferation during endotoxin fever in humans. J Immunol. 1981;127:2517–2519. [PubMed] [Google Scholar]
- 61.Arend WP, Joslin FG, Massoni RJ. Effects of immune complexes on production by human monocytes of interleukin 1 or an interleukin 1 inhibitor. J Immunol. 1985;134:3868–3875. [PubMed] [Google Scholar]
- 62.Prieur AM, Kaufmann MT, Griscelli C, Dayer JM. Specific interleukin-1 inhibitor in serum and urine of children with systemic juvenile chronic arthritis. Lancet. 1987;2:1240–1242. doi: 10.1016/s0140-6736(87)91854-x. [DOI] [PubMed] [Google Scholar]
- 63.Seckinger P, Lowenthal JW, Williamson K, Dayer JM, MacDonald HR. A urine inhibitor of interleukin 1 activity that blocks ligand binding. J Immunol. 1987;139:1546–1549. [PubMed] [Google Scholar]
- 64.Hannum CH, Wilcox CJ, Arend WP, et al. Interleukin-1 receptor antagonist activity of a human interleukin-1 inhibitor. Nature. 1990;343:336–340. doi: 10.1038/343336a0. [DOI] [PubMed] [Google Scholar]
- 65.Eisenberg SP, Evans RJ, Arend WP, et al. Primary structure and functional expression from complementary DNA of a human interleukin-1 receptor antagonist. Nature. 1990;343:341–346. doi: 10.1038/343341a0. [DOI] [PubMed] [Google Scholar]
- 66.Dinarello CA, Simon A, van der Meer JW. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov. 2012;11:633–652. doi: 10.1038/nrd3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Harrison SR, McGonagle D, Nizam S, et al. Anakinra as a diagnostic challenge and treatment option for systemic autoinflammatory disorders of undefined etiology. JCI Insight. 2016;1:e86336. doi: 10.1172/jci.insight.86336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Aganna E, Martinon F, Hawkins PN, et al. Association of mutations in the NALP3/CIAS1/PYPAF1 gene with a broad phenotype including recurrent fever, cold sensitivity, sensorineural deafness, and AA amyloidosis. Arthritis Rheum. 2002;46:2445–2452. doi: 10.1002/art.10509. [DOI] [PubMed] [Google Scholar]
- 69.Rynne M, Maclean C, Bybee A, McDermott MF, Emery P. Hearing improvement in a patient with variant Muckle-Wells syndrome in response to interleukin 1 receptor antagonism. Ann Rheum Dis. 2006;65:533–534. doi: 10.1136/ard.2005.038091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kuemmerle-Deschner JB, Lohse P, Koetter I, et al. NLRP3 E311K mutation in a large family with Muckle-Wells syndrome – Description of a heterogeneous phenotype and response to treatment. Arthritis Res Ther. 2011;13:R196. doi: 10.1186/ar3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kuemmerle-Deschner JB, Tyrrell PN, Koetter I, et al. Efficacy and safety of anakinra therapy in pediatric and adult patients with the autoinflammatory Muckle-Wells syndrome. Arthritis Rheum. 2011;63:840–849. doi: 10.1002/art.30149. [DOI] [PubMed] [Google Scholar]
- 72.Kuemmerle-Deschner JB, Wittkowski H, Tyrrell PN, et al. Treatment of Muckle-Wells syndrome: Analysis of two IL-1-blocking regimens. Arthritis Res Ther. 2013;15:R64. doi: 10.1186/ar4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stew BT, Fishpool SJ, Owens D, Quine S. Muckle-Wells syndrome: A treatable cause of congenital sensorineural hearing loss. B-ENT. 2013;9:161–163. [PubMed] [Google Scholar]
- 74.Kitley JL, Lachmann HJ, Pinto A, Ginsberg L. Neurologic manifestations of the cryopyrin-associated periodic syndrome. Neurology. 2010;74:1267–1270. doi: 10.1212/WNL.0b013e3181d9ed69. [DOI] [PubMed] [Google Scholar]
- 75.Ahmadi N, Brewer CC, Zalewski C, et al. Cryopyrin-associated periodic syndromes: Otolaryngologic and audiologic manifestations. Otolaryngol Head Neck Surg. 2011;145:295–302. doi: 10.1177/0194599811402296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Klein AK, Horneff G. Improvement of sensoneurinal hearing loss in a patient with Muckle-Wells syndrome treated with anakinra. Klin Padiatr. 2011;222:266–268. doi: 10.1055/s-0029-1239527. [DOI] [PubMed] [Google Scholar]
- 77.Eungdamrong J, Boyd KP, Meehan SA, Latkowski JA. Muckle-Wells treatment with anakinra. Dermatol Online J. 2013;19:20720. [PubMed] [Google Scholar]
- 78.Gerard S, le Goff B, Maugars Y, Berthelot JM, Malard O. Lasting remission of a Muckle-Wells syndrome with CIAS-1 mutation using half-dose anakinra. Joint Bone Spine. 2007;74:659. doi: 10.1016/j.jbspin.2007.01.032. [DOI] [PubMed] [Google Scholar]
- 79.Abbate A, Kontos MC, Grizzard JD, et al. Interleukin-1 blockade with anakinra to prevent adverse cardiac remodeling after acute myocardial infarction (Virginia Commonwealth University Anakinra Remodeling Trial [VCU-ART] Pilot study) Am J Cardiol. 2010;105(1371–7):e1. doi: 10.1016/j.amjcard.2009.12.059. [DOI] [PubMed] [Google Scholar]
- 80.Abbate A, Van Tassell BW, Biondi-Zoccai G, et al. Effects of interleukin-1 blockade with anakinra on adverse cardiac remodeling and heart failure after acute myocardial infarction [from the Virginia Commonwealth University-Anakinra Remodeling Trial (2) (VCU-ART2) pilot study] Am J Cardiol. 2013;111:1394–1400. doi: 10.1016/j.amjcard.2013.01.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Van Tassell BW, Arena RA, Toldo S, et al. Enhanced interleukin-1 activity contributes to exercise intolerance in patients with systolic heart failure. PLoS ONE. 2012;7:e33438. doi: 10.1371/journal.pone.0033438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cantarini L, Lucherini OM, Cimaz R, Galeazzi M. Recurrent pericarditis caused by a rare mutation in the TNFRSF1A gene and with excellent response to anakinra treatment. Clin Exp Rheumatol. 2010;28:802. [PubMed] [Google Scholar]
- 83.Gerfaud-Valentin M, Jamilloux Y, Iwaz J, Seve P. Adult-onset Still’s disease. Autoimmun Rev. 2014;13:708–722. doi: 10.1016/j.autrev.2014.01.058. [DOI] [PubMed] [Google Scholar]
- 84.Scott IC, Vijay Hajela V, Hawkins PN, Lachmann HJ. A case series and systematic literature review of anakinra and immunosuppression in idiopathic recurrent pericarditis. J Cardiology Cases. 2011;4:e93–e97. doi: 10.1016/j.jccase.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Emsley HC, Smith CJ, Georgiou RF, et al. A randomised phase II study of interleukin-1 receptor antagonist in acute stroke patients. J Neurol Neurosurg Psychiatry. 2005;76:1366–1372. doi: 10.1136/jnnp.2004.054882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vezzani A, Maroso M, Balosso S, Sanchez MA, Bartfai T. IL-1 receptor/Toll-like receptor signaling in infection, inflammation, stress and neurodegeneration couples hyperexcitability and seizures. Brain Behav Immun. 2011;25:1281–1289. doi: 10.1016/j.bbi.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 87.Uludag IF, Bilgin S, Zorlu Y, Tuna G, Kirkali G. Interleukin-6, interleukin-1 beta and interleukin-1 receptor antagonist levels in epileptic seizures. Seizure. 2013;22:457–461. doi: 10.1016/j.seizure.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 88.Uludag IF, Duksal T, Tiftikcioglu BI, Zorlu Y, Ozkaya F, Kirkali G. IL-1beta, IL-6 and IL1Ra levels in temporal lobe epilepsy. Seizure. 2015;26:22–25. doi: 10.1016/j.seizure.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 89.Kenney-Jung DL, Vezzani A, Kahoud RJ, et al. Febrile infection-related epilepsy syndrome treated with anakinra. Ann Neurol. 2016;80:939–945. doi: 10.1002/ana.24806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Opal SM, Fisher CJJ, Dhainaut JF, et al. Confirmatory interleukin-1 receptor antagonist trial in severe sepsis: A phase III, randomized, double-blind, placebo-controlled, multicenter trial. Crit Care Med. 1997;25:1115–1124. doi: 10.1097/00003246-199707000-00010. [DOI] [PubMed] [Google Scholar]
- 91.Fisher CJJ, Slotman GJ, Opal SM, et al. Initial evaluation of human recombinant interleukin-1 receptor antagonist in the treatment of sepsis syndrome: A randomized, open-label, placebo-controlled multicenter trial. Crit Care Med. 1994;22:12–21. doi: 10.1097/00003246-199401000-00008. [DOI] [PubMed] [Google Scholar]
- 92.Fisher CJ, Jr, Dhainaut JF, Opal SM, et al. Recombinant human interleukin 1 receptor antagonist in the treatment of patients with sepsis syndrome. Results from a randomized, double-blind, placebo-controlled trial. Phase III rhIL-1ra Sepsis Syndrome Study Group. JAMA. 1994;271:1836–1843. [PubMed] [Google Scholar]
- 93.Shakoory B, Carcillo JA, Chatham WW, et al. Interleukin-1 receptor blockade is associated with reduced mortality in sepsis patients with features of macrophage activation syndrome: Reanalysis of a prior phase III trial. Crit Care Med. 2016;44:275–281. doi: 10.1097/CCM.0000000000001402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lust JA, Lacy MQ, Zeldenrust SR, et al. Induction of a chronic disease state in patients with smoldering or indolent multiple myeloma by targeting interleukin 1{beta}-induced interleukin 6 production and the myeloma proliferative component. Mayo Clin Proc. 2009;84:114–122. doi: 10.4065/84.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lust JA, Donovan KA. The role of interleukin-1 beta in the pathogenesis of multiple myeloma. Hematol Oncol Clin North Am. 1999;13:1117–1125. doi: 10.1016/s0889-8588(05)70115-5. [DOI] [PubMed] [Google Scholar]
- 96.Lust JA, Lacy MQ, Zeldenrust SR, et al. Reduction in C-reactive protein indicates successful targeting of the IL-1/IL-6 axis resulting in improved survival in early stage multiple myeloma. Am J Hematol. 2016;91:571–574. doi: 10.1002/ajh.24352. [DOI] [PubMed] [Google Scholar]
- 97.Fleischmann RM, Tesser J, Schiff MH, et al. Safety of extended treatment with anakinra in patients with rheumatoid arthritis. Ann Rheum Dis. 2006;65:1006–1012. doi: 10.1136/ard.2005.048371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mertens M, Singh JA. Anakinra for rheumatoid arthritis: A systematic review. J Rheumatol. 2009;36:1118–1125. doi: 10.3899/jrheum.090074. [DOI] [PubMed] [Google Scholar]
- 99.van de Veerdonk FL, Netea MG, Dinarello CA, van der Meer JW. Anakinra for the inflammatory complications of chronic granulomatous disease. Neth J Med. 2011;69:95. [PubMed] [Google Scholar]
- 100.Hennig S, Bayegan K, Uffmann M, Thalhammer F, Winkler S. Pneumonia in a patient with familial Mediterranean fever successfully treated with anakinra-case report and review. Rheumatol Int. 2010;32:1801–1804. doi: 10.1007/s00296-010-1429-y. [DOI] [PubMed] [Google Scholar]
- 101.Solovic I, Sester M, Gomez-Reino JJ, et al. The risk of tuberculosis related to tumour necrosis factor antagonist therapies: A TBNET consensus statement. Eur Respir J. 2011;36:1185–1206. doi: 10.1183/09031936.00028510. [DOI] [PubMed] [Google Scholar]
- 102.Fleischmann RM, Schechtman J, Bennett R, et al. Anakinra, a recombinant human interleukin-1 receptor antagonist (r-metHuIL-1ra), in patients with rheumatoid arthritis: A large, international, multicenter, placebo-controlled trial. Arthritis Rheum. 2003;48:927–934. doi: 10.1002/art.10870. [DOI] [PubMed] [Google Scholar]
- 103.Bresnihan B, Alvaro-Gracia JM, Cobby M, et al. Treatment of rheumatoid arthritis with recombinant human interleukin-1 receptor antagonist. Arthritis Rheum. 1998;41:2196–2204. doi: 10.1002/1529-0131(199812)41:12<2196::AID-ART15>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 104.Liew FY, Girard JP, Turnquist HR. Interleukin-33 in health and disease. Nat Rev Immunol. 2016;16:676–689. doi: 10.1038/nri.2016.95. [DOI] [PubMed] [Google Scholar]
- 105.Schmitz J, Owyang A, Oldham E, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 106.Casadio R, Frigimelica E, Bossu P, et al. Model of interaction of the IL-1 receptor accessory protein IL-1RAcP with the IL-1beta/IL-1R(I) complex. FEBS Lett. 2001;499:65–68. doi: 10.1016/s0014-5793(01)02515-7. [DOI] [PubMed] [Google Scholar]
- 107.Ali S, Huber M, Kollewe C, Bischoff SC, Falk W, Martin MU. IL-1 receptor accessory protein is essential for IL-33-induced activation of T lymphocytes and mast cells. Proc Natl Acad Sci USA. 2007;104:18660–18665. doi: 10.1073/pnas.0705939104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lingel A, Weiss TM, Niebuhr M, et al. Structure of IL-33 and its interaction with the ST2 and IL-1RAcP receptors–insight into heterotrimeric IL-1 signaling complexes. Structure. 2009;17:1398–1410. doi: 10.1016/j.str.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cayrol C, Girard JP. The IL-1-like cytokine IL-33 is inactivated after maturation by caspase-1. Proc Natl Acad Sci USA. 2009;106:9021–9026. doi: 10.1073/pnas.0812690106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lefrancais E, Roga S, Gautier V, et al. IL-33 is processed into mature bioactive forms by neutrophil elastase and cathepsin G. Proc Natl Acad Sci USA. 2012;109:1673–1678. doi: 10.1073/pnas.1115884109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lefrancais E, Duval A, Mirey E, et al. Central domain of IL-33 is cleaved by mast cell proteases for potent activation of group-2 innate lymphoid cells. Proc Natl Acad Sci USA. 2014;111:15502–15507. doi: 10.1073/pnas.1410700111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hong J, Bae S, Jhun H, et al. Identification of constitutively active interleukin 33 (IL-33) splice variant. J Biol Chem. 2011;286:20078–20086. doi: 10.1074/jbc.M111.219089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Carriere V, Roussel L, Ortega N, et al. IL-33, the IL-1-like cytokine ligand for ST2 receptor, is a chromatin-associated nuclear factor in vivo. Proc Natl Acad Sci USA. 2007;104:282–287. doi: 10.1073/pnas.0606854104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Biton J, Khaleghparast Athari S, Thiolat A, et al. In vivo expansion of activated foxp3+ regulatory T cells and establishment of a Type 2 immune response upon IL-33 treatment protect against experimental arthritis. J Immunol. 2016;197:1708–1719. doi: 10.4049/jimmunol.1502124. [DOI] [PubMed] [Google Scholar]
- 115.Bulek K, Swaidani S, Qin J, et al. The essential role of single Ig IL-1 receptor-related molecule/Toll IL-1R8 in regulation of Th2 immune response. J Immunol. 2009;182:2601–2609. doi: 10.4049/jimmunol.0802729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sedhom MA, Pichery M, Murdoch JR, et al. Neutralisation of the interleukin-33/ST2 pathway ameliorates experimental colitis through enhancement of mucosal healing in mice. Gut. 2013;62:1714–1723. doi: 10.1136/gutjnl-2011-301785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Macedo RB, Kakehasi AM, Melo de Andrade MV. IL33 in rheumatoid arthritis: Potential contribution to pathogenesis. Rev Bras Reumatol Engl Ed. 2016;56:451–457. doi: 10.1016/j.rbre.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 118.Kunisch E, Chakilam S, Gandesiri M, Kinne RW. IL-33 regulates TNF-alpha dependent effects in synovial fibroblasts. Int J Mol Med. 2012;29:530–540. doi: 10.3892/ijmm.2012.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Liang Y, Jie Z, Hou L, et al. IL-33 promotes innate IFN-gamma production and modulates dendritic cell response in LCMV-induced hepatitis in mice. Eur J Immunol. 2015;45:3052–3063. doi: 10.1002/eji.201545696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Okamura H, Nagata K, Komatsu T, et al. A novel costimulatory factor for gamma interferon induction found in the livers of mice causes endotoxic shock. Infect Immun. 1995;63:3966–3972. doi: 10.1128/iai.63.10.3966-3972.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Boraschi D, Dinarello CA. IL-18 in autoimmunity: Review. Eur Cytokine Netw. 2006;17:224–252. [PubMed] [Google Scholar]
- 122.Dinarello CA. Interleukin-18 and the pathogenesis of inflammatory diseases. Semin Nephrol. 2007;27:98–114. doi: 10.1016/j.semnephrol.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 123.Tsutsui H, Nakanishi K. Immunotherapeutic applications of IL-18. Immunotherapy. 2012;4:1883–1894. doi: 10.2217/imt.12.137. [DOI] [PubMed] [Google Scholar]
- 124.Puren AJ, Fantuzzi G, Dinarello CA. Gene expression, synthesis and secretion of IL-1b and IL-18 are differentially regulated in human blood mononuclear cells and mouse spleen cells. Proc Natl Acad Sci USA. 1999;96:2256–2261. doi: 10.1073/pnas.96.5.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Siegmund B, Lehr HA, Fantuzzi G, Dinarello CA. IL-1beta-converting enzyme (caspase-1) in intestinal inflammation. Proc Natl Acad Sci USA. 2001;98:13249–13254. doi: 10.1073/pnas.231473998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bersudsky M, Luski L, Fishman D, et al. Non-redundant properties of IL-1alpha and IL-1beta during acute colon inflammation in mice. Gut. 2014;63:598–609. doi: 10.1136/gutjnl-2012-303329. [DOI] [PubMed] [Google Scholar]
- 127.Siegmund B, Fantuzzi G, Rieder F, et al. Neutralization of interleukin-18 reduces severity in murine colitis and intestinal IFN-g and TNF-a production. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1264–R1273. doi: 10.1152/ajpregu.2001.281.4.R1264. [DOI] [PubMed] [Google Scholar]
- 128.Weber A, Wasiliew P, Kracht M. Interleukin-1 (IL-1) pathway. Sci Signal. 2010;3:cm1. doi: 10.1126/scisignal.3105cm1. [DOI] [PubMed] [Google Scholar]
- 129.Morel JC, Park CC, Woods JM, Koch AE. A novel role for interleukin-18 in adhesion molecule induction through NFkappa B and phosphatidylinositol (PI) 3-kinase-dependent signal transduction pathways. J Biol Chem. 2001;276:37069–37075. doi: 10.1074/jbc.M103574200. [DOI] [PubMed] [Google Scholar]
- 130.Lee JK, Kim SH, Lewis EC, Azam T, Reznikov LL, Dinarello CA. Differences in signaling pathways by IL-1beta and IL-18. Proc Natl Acad Sci USA. 2004;101:8815–8820. doi: 10.1073/pnas.0402800101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nakanishi K, Yoshimoto T, Tsutsui H, Okamura H. Interleukin-18 is a unique cytokine that stimulates both Th1 and Th2 responses depending on its cytokine milieu. Cytokine Growth Factor Rev. 2001;12:53–72. doi: 10.1016/s1359-6101(00)00015-0. [DOI] [PubMed] [Google Scholar]
- 132.Bellora F, Castriconi R, Doni A, et al. M-CSF induces the expression of a membrane-bound form of IL-18 in a subset of human monocytes differentiating in vitro toward macrophages. Eur J Immunol. 2012;42:1618–1626. doi: 10.1002/eji.201142173. [DOI] [PubMed] [Google Scholar]
- 133.Sutton C, Brereton C, Keogh B, Mills KH, Lavelle EC. A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. J Exp Med. 2006;203:1685–1691. doi: 10.1084/jem.20060285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lalor SJ, Dungan LS, Sutton CE, Basdeo SA, Fletcher JM, Mills KH. Caspase-1-processed cytokines IL-1beta and IL-18 promote IL-17 production by gammadelta and CD4 T cells that mediate autoimmunity. J Immunol. 2011;186:5738–5748. doi: 10.4049/jimmunol.1003597. [DOI] [PubMed] [Google Scholar]
- 135.Vidal-Vanaclocha F, Fantuzzi G, Mendoza L, et al. IL-18 regulates IL-1beta-dependent hepatic melanoma metastasis via vascular cell adhesion molecule-1. Proc Natl Acad Sci USA. 2000;97:734–739. doi: 10.1073/pnas.97.2.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gatti S, Beck J, Fantuzzi G, Bartfai T, Dinarello CA. Effect of interleukin-18 on mouse core body temperature. Am J Physiol Regul Integr Comp Physiol. 2002;282:R702–R709. doi: 10.1152/ajpregu.00393.2001. [DOI] [PubMed] [Google Scholar]
- 137.Li S, Goorha S, Ballou LR, Blatteis CM. Intracerebroventricular interleukin-6, macrophage inflammatory protein-1 beta and IL-18: Pyrogenic and PGE(2)-mediated? Brain Res. 2003;992:76–84. doi: 10.1016/j.brainres.2003.08.033. [DOI] [PubMed] [Google Scholar]
- 138.Robertson MJ, Mier JW, Logan T, et al. Clinical and biological effects of recombinant human interleukin-18 administered by intravenous infusion to patients with advanced cancer. Clin Cancer Res. 2006;12:4265–4273. doi: 10.1158/1078-0432.CCR-06-0121. [DOI] [PubMed] [Google Scholar]
- 139.Reznikov LL, Kim SH, Westcott JY, et al. IL-18 binding protein increases spontaneous and IL-1-induced prostaglandin production via inhibition of IFN-gamma. Proc Natl Acad Sci USA. 2000;97:2174–2179. doi: 10.1073/pnas.040582597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tak PP, Bacchi M, Bertolino M. Pharmacokinetics of IL-18 binding protein in healthy volunteers and subjects with rheumatoid arthritis or plaque psoriasis. Eur J Drug Metab Pharmacokinet. 2006;31:109–116. doi: 10.1007/BF03191127. [DOI] [PubMed] [Google Scholar]
- 141.Mallat Z, Heymes C, Corbaz A, et al. Evidence for altered interleukin 18 (IL)-18 pathway in human heart failure. FASEB J. 2004;18:1752–1754. doi: 10.1096/fj.04-2426fje. [DOI] [PubMed] [Google Scholar]
- 142.Platis A, Yu Q, Moore D, Khojeini E, Tsau P, Larson D. The effect of daily administration of IL-18 on cardiac structure and function. Perfusion. 2008;23:237–242. doi: 10.1177/0267659108101511. [DOI] [PubMed] [Google Scholar]
- 143.Woldbaek PR, Sande JB, Stromme TA, et al. Daily administration of interleukin-18 causes myocardial dysfunction in healthy mice. Am J Physiol Heart Circ Physiol. 2005;289:H708–H714. doi: 10.1152/ajpheart.01179.2004. [DOI] [PubMed] [Google Scholar]
- 144.Raeburn CD, Dinarello CA, Zimmerman MA, et al. Neutralization of IL-18 attenuates lipopolysaccharide-induced myocardial dysfunction. Am J Physiol Heart Circ Physiol. 2002;283:H650–H657. doi: 10.1152/ajpheart.00043.2002. [DOI] [PubMed] [Google Scholar]
- 145.Pomerantz BJ, Reznikov LL, Harken AH, Dinarello CA. Inhibition of caspase 1 reduces human myocardial ischemic dysfunction via inhibition of IL-18 and IL-1beta. Proc Natl Acad Sci USA. 2001;98:2871–2876. doi: 10.1073/pnas.041611398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Toldo S, Mezzaroma E, O’Brien L, et al. Interleukin-18 mediates interleukin-1-induced cardiac dysfunction. Am J Physiol Heart Circ Physiol. 2014;306:H1025–H1031. doi: 10.1152/ajpheart.00795.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Murray DR, Mummidi S, Valente AJ, et al. beta2 adrenergic activation induces the expression of IL-18 binding protein, a potent inhibitor of isoproterenol induced cardiomyocyte hypertrophy in vitro and myocardial hypertrophy in vivo. J Mol Cell Cardiol. 2012;52:206–218. doi: 10.1016/j.yjmcc.2011.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Dupaul-Chicoine J, Yeretssian G, Doiron K, et al. Control of intestinal homeostasis, colitis, and colitis-associated colorectal cancer by the inflammatory caspases. Immunity. 2010;32:367–378. doi: 10.1016/j.immuni.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 149.Hirota SA, Ng J, Lueng A, et al. NLRP3 inflammasome plays a key role in the regulation of intestinal homeostasis. Inflamm Bowel Dis. 2011;17:1359–1372. doi: 10.1002/ibd.21478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Elinav E, Strowig T, Kau AL, et al. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell. 2011;145:745–757. doi: 10.1016/j.cell.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chen CJ, Kono H, Golenbock D, Reed G, Akira S, Rock KL. Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nat Med. 2007;13:851–856. doi: 10.1038/nm1603. [DOI] [PubMed] [Google Scholar]
- 152.Doyle SL, Campbell M, Ozaki E, et al. NLRP3 has a protective role in age-related macular degeneration through the induction of IL-18 by drusen components. Nat Med. 2012;18:791–798. doi: 10.1038/nm.2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Netea MG, Joosten LA, Lewis E, et al. Deficiency of interleukin-18 in mice leads to hyperphagia, obesity and insulin resistance. Nat Med. 2006;12:650–656. doi: 10.1038/nm1415. [DOI] [PubMed] [Google Scholar]
- 154.Zorrilla EP, Sanchez-Alavez M, Sugama S, et al. Interleukin-18 controls energy homeostasis by suppressing appetite and feed efficiency. Proc Natl Acad Sci USA. 2007;104:11097–11102. doi: 10.1073/pnas.0611523104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Novick D, Schwartsburd B, Pinkus R, et al. A novel IL-18BP ELISA shows elevated serum il-18BP in sepsis and extensive decrease of free IL-18. Cytokine. 2001;14:334–342. doi: 10.1006/cyto.2001.0914. [DOI] [PubMed] [Google Scholar]
- 156.Novick D, Elbirt D, Dinarello CA, Rubinstein M, Sthoeger ZM. Interleukin-18 binding protein in the sera of patients with Wegener’s granulomatosis. J Clin Immunol. 2009;29:38–45. doi: 10.1007/s10875-008-9217-0. [DOI] [PubMed] [Google Scholar]
- 157.Novick D, Elbirt D, Miller G, Dinarello CA, Rubinstein M, Sthoeger ZM. High circulating levels of free interleukin-18 in patients with active SLE in the presence of elevated levels of interleukin-18 binding protein. J Autoimmun. 2011;34:121–126. doi: 10.1016/j.jaut.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 158.Mazodier K, Marin V, Novick D, et al. Severe imbalance of IL-18/IL-18BP in patients with secondary hemophagocytic syndrome. Blood. 2005;106:3483–3489. doi: 10.1182/blood-2005-05-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Bufler P, Azam T, Gamboni-Robertson F, et al. A complex of the IL-1 homologue IL-1F7b and IL-18-binding protein reduces IL-18 activity. Proc Natl Acad Sci USA. 2002;99:13723–13728. doi: 10.1073/pnas.212519099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Dinarello C, Arend W, Sims J, et al. IL-1 family nomenclature. Nat Immunol. 2010;11:973. doi: 10.1038/ni1110-973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.McNamee EN, Masterson JC, Jedlicka P, et al. Interleukin 37 expression protects mice from colitis. Proc Natl Acad Sci USA. 2011;108:16711–16716. doi: 10.1073/pnas.1111982108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Banda NK, Vondracek A, Kraus D, et al. Mechanisms of inhibition of collagen-induced arthritis by murine IL-18 binding protein. J Immunol. 2003;170:2100–2105. doi: 10.4049/jimmunol.170.4.2100. [DOI] [PubMed] [Google Scholar]
- 163.Muhl H, Kampfer H, Bosmann M, Frank S, Radeke H, Pfeilschifter J. Interferon-gamma mediates gene expression of IL-18 binding protein in nonleukocytic cells. Biochem Biophys Res Commun. 2000;267:960–963. doi: 10.1006/bbrc.1999.2064. [DOI] [PubMed] [Google Scholar]
- 164.Hurgin V, Novick D, Rubinstein M. The promoter of IL-18 binding protein: Activation by an IFN-gamma-induced complex of IFN regulatory factor 1 and CCAAT/enhancer binding protein beta. Proc Natl Acad Sci USA. 2002;99:16957–16962. doi: 10.1073/pnas.262663399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kaser A, Novick D, Rubinstein M, et al. Interferon-alpha induces interleukin-18 binding protein in chronic hepatitis C patients. Clin Exp Immunol. 2002;129:332–338. doi: 10.1046/j.1365-2249.2002.01911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ludwiczek O, Kaser A, Novick D, et al. Plasma levels of interleukin-18 and interleukin-18 binding protein are elevated in patients with chronic liver disease. J Clin Immunol. 2002;22:331–337. doi: 10.1023/a:1020600230977. [DOI] [PubMed] [Google Scholar]
- 167.Wittmann M, Bachmann M, Doble R, Pfeilschifter J, Werfel T, Mühl H. IL-27 regulates IL-18 binding protein in skin resident cells. PLoS ONE. 2012;7:e38751. doi: 10.1371/journal.pone.0038751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Xiang Y, Moss B. Correspondence of the functional epitopes of poxvirus and human interleukin-18-binding proteins. J Virol. 2001;75:9947–9954. doi: 10.1128/JVI.75.20.9947-9954.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ogilvie EM, Khan A, Hubank M, Kellam P, Woo P. Specific gene expression profiles in systemic juvenile idiopathic arthritis. Arthritis Rheum. 2007;56:1954–1965. doi: 10.1002/art.22644. [DOI] [PubMed] [Google Scholar]
- 170.Sumegi J, Barnes MG, Nestheide SV, et al. Gene expression profiling of peripheral blood mononuclear cells from children with active hemophagocytic lymphohistiocytosis. Blood. 2011;117:e151–e160. doi: 10.1182/blood-2010-08-300046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Maeno N, Takei S, Imanaka H, et al. Increased interleukin-18 expression in bone marrow of a patient with systemic juvenile idiopathic arthritis and unrecognized macrophage-activation syndrome. Arthritis Rheum. 2004;50:1935–1938. doi: 10.1002/art.20268. [DOI] [PubMed] [Google Scholar]
- 172.Emmenegger U, Reimers A, Frey U, et al. Reactive macrophage activation syndrome: A simple screening strategy and its potential in early treatment initiation. Swiss Med Wkly. 2002;132:230–236. doi: 10.4414/smw.2002.09941. [DOI] [PubMed] [Google Scholar]
- 173.Nold-Petry CA, Lehrnbecher T, Jarisch A, et al. Failure of interferon gamma to induce the anti-inflammatory interleukin 18 binding protein in familial hemophagocytosis. PLoS ONE. 2010;5:e8663. doi: 10.1371/journal.pone.0008663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Honda K, Ohga S, Takada H, et al. Neuron-specific enolase in hemophagocytic lymphohistiocytosis: A potential indicator for macrophage activation? Int J Hematol. 2000;72:55–60. [PubMed] [Google Scholar]
- 175.Wada T, Muraoka M, Yokoyama T, Toma T, Kanegane H, Yachie A. Cytokine profiles in children with primary Epstein-Barr virus infection. Pediatr Blood Cancer. 2013;60:E46–E48. doi: 10.1002/pbc.24480. [DOI] [PubMed] [Google Scholar]
- 176.Fitzgerald AA, Leclercq SA, Yan A, Homik JE, Dinarello CA. Rapid responses to anakinra in patients with refractory adult-onset Still’s disease. Arthritis Rheum. 2005;52:1794–1803. doi: 10.1002/art.21061. [DOI] [PubMed] [Google Scholar]
- 177.Larroche C, Mouthon L. Pathogenesis of hemophagocytic syndrome (HPS) Autoimmunity Rev. 2004;3:69–75. doi: 10.1016/S1568-9972(03)00091-0. [DOI] [PubMed] [Google Scholar]
- 178.Canna SW, Girard C, Malle L, et al. Life-threatening NLRC4-associated hyperinflammation successfully treated with IL-18 inhibition. J Allergy Clin Immunol. 2017;139:1698–1701. doi: 10.1016/j.jaci.2016.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kumar S, McDonnell PC, Lehr R, et al. Identification and initial characterization of four novel members of the interleukin-1 family. J Biol Chem. 2000;275:10308–10314. doi: 10.1074/jbc.275.14.10308. [DOI] [PubMed] [Google Scholar]
- 180.Dinarello CA, Nold-Petry C, Nold M, et al. Suppression of innate inflammation and immunity by interleukin-37. Eur J Immunol. 2016;46:1067–1081. doi: 10.1002/eji.201545828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sakai N, Van Sweringen HL, Belizaire RM, et al. Interleukin-37 reduces liver inflammatory injury via effects on hepatocytes and non-parenchymal cells. J Gastroenterol Hepatol. 2012;27:1609–1616. doi: 10.1111/j.1440-1746.2012.07187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Nold MF, Nold-Petry CA, Zepp JA, Palmer BE, Bufler P, Dinarello CA. IL-37 is a fundamental inhibitor of innate immunity. Nat Immunol. 2010;11:1014–1022. doi: 10.1038/ni.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Nold-Petry CA, Lo CY, Rudloff I, et al. IL-37 requires the receptors IL-18Ralpha and IL-1R8 (SIGIRR) to carry out its multifaceted anti-inflammatory program upon innate signal transduction. Nat Immunol. 2015;16:354–365. doi: 10.1038/ni.3103. [DOI] [PubMed] [Google Scholar]
- 184.Li Y, Wang Z, Yu T, et al. Increased expression of IL-37 in patients with Graves’ disease and its contribution to suppression of proinflammatory cytokines production in peripheral blood mononuclear cells. PLoS ONE. 2014;9:e107183. doi: 10.1371/journal.pone.0107183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Xia T, Zheng XF, Qian BH, et al. Plasma interleukin-37 is elevated in patients with rheumatoid arthritis: Its correlation with disease activity and Th1/Th2/Th17-related cytokines. Dis Markers. 2015;2015:795043. doi: 10.1155/2015/795043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Yang L, Zhang J, Tao J, Lu T. Elevated serum levels of Interleukin-37 are associated with inflammatory cytokines and disease activity in rheumatoid arthritis. APMIS. 2015;123:1025–1031. doi: 10.1111/apm.12467. [DOI] [PubMed] [Google Scholar]
- 187.Chen B, Huang K, Ye L, et al. Interleukin-37 is increased in ankylosing spondylitis patients and associated with disease activity. J Transl Med. 2015;13:36. doi: 10.1186/s12967-015-0394-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Keermann M, Koks S, Reimann E, et al. Expression of IL-36 family cytokines and IL-37 but not IL-38 is altered in psoriatic skin. J Dermatol Sci. 2015;80:150–152. doi: 10.1016/j.jdermsci.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 189.Song L, Qiu F, Fan Y, et al. Glucocorticoid regulates interleukin-37 in systemic lupus erythematosus. J Clin Immunol. 2013;33:111–117. doi: 10.1007/s10875-012-9791-z. [DOI] [PubMed] [Google Scholar]
- 190.Zeng Q, Song R, Fullerton DA, et al. Interleukin-37 suppresses the osteogenic responses of human aortic valve interstitial cells in vitro and alleviates valve lesions in mice. Proc Natl Acad Sci USA. 2017;114:1631–1636. doi: 10.1073/pnas.1619667114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Charrad R, Berraies A, Hamdi B, Ammar J, Hamzaoui K, Hamzaoui A. Anti-inflammatory activity of IL-37 in asthmatic children: Correlation with inflammatory cytokines TNF-alpha, IL-beta, IL-6 and IL-17A. Immunobiology. 2016;221:182–187. doi: 10.1016/j.imbio.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 192.Lunding L, Webering S, Vock C, et al. IL-37 requires IL-18Ralpha and SIGIRR/IL-1R8 to diminish allergic airway inflammation in mice. Allergy. 2015;79:366–373. doi: 10.1111/all.12566. [DOI] [PubMed] [Google Scholar]
- 193.Ballak DB, van Diepen JA, Moschen AR, et al. IL-37 protects against obesity-induced inflammation and insulin resistance. Nat Commun. 2014;5:4711. doi: 10.1038/ncomms5711. [DOI] [PubMed] [Google Scholar]
- 194.Liu W, Deng L, Chen Y, et al. Anti-inflammatory effect of IL-37b in children with allergic rhinitis. Mediators Inflamm. 2014;2014:746846. doi: 10.1155/2014/746846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kimura T, Tsutsumi N, Arita K, et al. Purification, crystallization and preliminary X-ray crystallographic analysis of human IL-18 and its extracellular complexes. Acta Crystallogr F Struct Biol Commun. 2014;70:1351–1356. doi: 10.1107/S2053230X14016926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Tsutsumi N, Kimura T, Arita K, et al. The structural basis for receptor recognition of human interleukin-18. Nat Commun. 2014;5:5340. doi: 10.1038/ncomms6340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kumar S, Hanning CR, Brigham-Burke MR, et al. Interleukin-1F7B (IL-1H4/IL-1F7) is processed by caspase-1 and mature IL-1F7B binds to the IL-18 receptor but does not induce IFN-gamma production. Cytokine. 2002;18:61–71. doi: 10.1006/cyto.2002.0873. [DOI] [PubMed] [Google Scholar]
- 198.Pan G, Risser P, Mao W, et al. IL-1H, an interleukin 1-related protein that binds IL-18 receptor/IL-1Rrp. Cytokine. 2001;13:1–7. doi: 10.1006/cyto.2000.0799. [DOI] [PubMed] [Google Scholar]
- 199.Gong J, Wei T, Stark RW, et al. Inhibition of Toll-like receptors TLR4 and 7 signaling pathways by SIGIRR: A computational approach. J Struct Biol. 2010;169:323–330. doi: 10.1016/j.jsb.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 200.Cavalli G, Justice JN, Boyle KE, et al. Interleukin 37 reverses the metabolic cost of inflammation, increases oxidative respiration, and improves exercise tolerance. Proc Natl Acad Sci USA. 2017;114:2313–2318. doi: 10.1073/pnas.1619011114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Sharma S, Kulk N, Nold MF, et al. The IL-1 family member 7b translocates to the nucleus and down-regulates proinflammatory cytokines. J Immunol. 2008;180:5477–5482. doi: 10.4049/jimmunol.180.8.5477. [DOI] [PubMed] [Google Scholar]
- 202.Ross R, Grimmel J, Goedicke S, et al. Analysis of nuclear localization of interleukin-1 family cytokines by flow cytometry. J Immunol Methods. 2013;387:219–227. doi: 10.1016/j.jim.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 203.Bulau AM, Nold MF, Li S, et al. Role of caspase-1 in nuclear translocation of IL-37, release of the cytokine, and IL-37 inhibition of innate immune responses. Proc Natl Acad Sci USA. 2014;111:2650–2655. doi: 10.1073/pnas.1324140111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Grimsby S, Jaensson H, Dubrovska A, Lomnytska M, Hellman U, Souchelnytskyi S. Proteomics-based identification of proteins interacting with Smad3: SREBP-2 forms a complex with Smad3 and inhibits its transcriptional activity. FEBS Lett. 2004;577:93–100. doi: 10.1016/j.febslet.2004.09.069. [DOI] [PubMed] [Google Scholar]
- 205.Smithrithee R, Niyonsaba F, Kiatsurayanon C, et al. Human beta-defensin-3 increases the expression of interleukin-37 through CCR6 in human keratinocytes. J Dermatol Sci. 2014;77:46–53. doi: 10.1016/j.jdermsci.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 206.Bufler P, Gamboni-Robertson F, Azam T, Kim SH, Dinarello CA. Interleukin-1 homologues IL-1F7b and IL-18 contain functional mRNA instability elements within the coding region responsive to lipopolysaccharide. Biochem J. 2004;381:503–510. doi: 10.1042/BJ20040217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Coll-Miro M, Francos-Quijorna I, Santos-Nogueira E, et al. Beneficial effects of IL-37 after spinal cord injury in mice. Proc Natl Acad Sci USA. 2016;113:1411–1416. doi: 10.1073/pnas.1523212113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Li S, Neff CP, Barber K, et al. Extracellular forms of IL-37 inhibit innate inflammation in vitro and in vivo but require the IL-1 family decoy receptor IL-1R8. Proc Natl Acad Sci USA. 2015;112:2497–2502. doi: 10.1073/pnas.1424626112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Yousif NG, Li J, Yousif F, et al. Expression of IL-37 in mouse protects the myocardium against ischemic injury via modulation of NF-κB activation. Circulation. 2011;124(Suppl 21):A8603. [Google Scholar]
- 210.Yang Y, Zhang ZX, Lian D, Haig A, Bhattacharjee RN, Jevnikar AM. IL-37 inhibits IL-18-induced tubular epithelial cell expression of pro-inflammatory cytokines and renal ischemia-reperfusion injury. Kidney Int. 2014;87:396–408. doi: 10.1038/ki.2014.295. [DOI] [PubMed] [Google Scholar]
- 211.Imaeda H, Takahashi K, Fujimoto T, et al. Epithelial expression of interleukin-37b in inflammatory bowel disease. Clin Exp Immunol. 2013;172:410–416. doi: 10.1111/cei.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Weidlich S, Bulau AM, Schwerd T, et al. Intestinal expression of the anti-inflammatory interleukin-1 homologue IL-37 in pediatric inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2014;59:e18–e26. doi: 10.1097/MPG.0000000000000387. [DOI] [PubMed] [Google Scholar]
- 213.Zhao PW, Jiang WG, Wang L, Jiang ZY, Shan YX, Jiang YF. Plasma levels of IL-37 and correlation with TNF-alpha, IL-17A, and disease activity during DMARD treatment of rheumatoid arthritis. PLoS ONE. 2014;9:e95346. doi: 10.1371/journal.pone.0095346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Xia L, Shen H, Lu J. Elevated serum and synovial fluid levels of interleukin-37 in patients with rheumatoid arthritis: Attenuated the production of inflammatory cytokines. Cytokine. 2015;76:553–557. doi: 10.1016/j.cyto.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 215.Hojen JF, Rasmussen TA, Andersen KL, et al. Interleukin-37 expression is increased in chronic HIV-1-infected individuals and is associated with inflammation and the size of the total viral reservoir. Mol Med. 2015;21:337–345. doi: 10.2119/molmed.2015.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Mora J, Schlemmer A, Wittig I, et al. Interleukin-38 is released from apoptotic cells to limit inflammatory macrophage responses. J Mol Cell Biol. 2016;8:426–438. doi: 10.1093/jmcb/mjw006. [DOI] [PubMed] [Google Scholar]
- 217.Busfield SJ, Comrack CA, Yu G, et al. Identification and gene organization of three novel members of the IL-1 family on human chromosome 2. Genomics. 2000;66:213–216. doi: 10.1006/geno.2000.6184. [DOI] [PubMed] [Google Scholar]
- 218.Debets R, Timans JC, Homey B, et al. Two novel IL-1 family members, IL-1 delta and IL-1 epsilon, function as an antagonist and agonist of NF-kappa B activation through the orphan IL-1 receptor-related protein 2. J Immunol. 2001;167:1440–1446. doi: 10.4049/jimmunol.167.3.1440. [DOI] [PubMed] [Google Scholar]
- 219.Sharaf N, Nicklin MJ, di Giovine FS. Long-range DNA interactions at the IL-1/IL-36/IL-37 gene cluster (2q13) are induced by activation of monocytes. Cytokine. 2014;68:16–22. doi: 10.1016/j.cyto.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 220.Lian LH, Milora KA, Manupipatpong KK, Jensen LE. The double-stranded RNA analogue polyinosinic-polycytidylic acid induces keratinocyte pyroptosis and release of IL-36gamma. J Invest Dermatol. 2012;132:1346–1353. doi: 10.1038/jid.2011.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Henry CM, Sullivan GP, Clancy DM, Afonina IS, Kulms D, Martin SJ. Neutrophil-derived proteases escalate inflammation through activation of IL-36 family cytokines. Cell Rep. 2016;14:708–722. doi: 10.1016/j.celrep.2015.12.072. [DOI] [PubMed] [Google Scholar]
- 222.Onoufriadis A, Simpson MA, Pink AE, et al. Mutations in IL36RN/IL1F5 are associated with the severe episodic inflammatory skin disease known as generalized pustular psoriasis. Am J Hum Genet. 2011;89:432–437. doi: 10.1016/j.ajhg.2011.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Teoh YL, Tay YK. Generalized pustular psoriasis with a novel mutation of interleukin-36 receptor antagonist, responding to methotrexate. JAAD Case Rep. 2015;1:51–53. doi: 10.1016/j.jdcr.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Marrakchi S, Guigue P, Renshaw BR, et al. Interleukin-36-receptor antagonist deficiency and generalized pustular psoriasis. N Engl J Med. 2011;365:620–628. doi: 10.1056/NEJMoa1013068. [DOI] [PubMed] [Google Scholar]
- 225.Boutet MA, Bart G, Penhoat M, et al. Distinct expression of interleukin (IL)-36alpha, beta and gamma, their antagonist IL-36Ra and IL-38 in psoriasis, rheumatoid arthritis and Crohn’s disease. Clin Exp Immunol. 2016;184:159–173. doi: 10.1111/cei.12761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Hessam S, Sand M, Gambichler T, Skrygan M, Ruddel I, Bechara FG. IL-36 in hidradenitis suppurativa: Evidence for a distinctive pro-inflammatory role and a key factor in the development of an inflammatory loop. Br J Dermatol. 2017 doi: 10.1111/bjd.16019. [Epub ahead of print]. https://doi.org/10.1111/bjd.16019. [DOI] [PubMed]
- 227.Li N, Yamasaki K, Saito R, et al. Alarmin function of cathelicidin antimicrobial peptide LL37 through IL-36gamma induction in human epidermal keratinocytes. J Immunol. 2014;193:5140–5148. doi: 10.4049/jimmunol.1302574. [DOI] [PubMed] [Google Scholar]
- 228.Ciccia F, Accardo-Palumbo A, Alessandro R, et al. Interleukin-36alpha axis is modulated in patients with primary Sjogren’s syndrome. Clin Exp Immunol. 2015;181:230–238. doi: 10.1111/cei.12644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Dehghan A, Dupuis J, Barbalic M, et al. Meta-analysis of genome-wide association studies in >80 000 subjects identifies multiple loci for C-reactive protein levels. Circulation. 2011;123:731–738. doi: 10.1161/CIRCULATIONAHA.110.948570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.van de Veerdonk FL, Stoeckman AK, Wu G, et al. IL-38 binds to the IL-36 receptor and has biological effects on immune cells similar to IL-36 receptor antagonist. Proc Natl Acad Sci USA. 2012;109:3001–3005. doi: 10.1073/pnas.1121534109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Sana TR, Debets R, Timans JC, Bazan JF, Kastelein RA. Computational identification, cloning, and characterization of IL-1R9, a novel interleukin-1 receptor-like gene encoded over an unusually large interval of human chromosome Xq22.2–q22.3. Genomics. 2000;69:252–262. doi: 10.1006/geno.2000.6328. [DOI] [PubMed] [Google Scholar]
- 232.Rudloff I, Godsell J, Nold-Petry CA, et al. Interleukin-38 exerts anti-inflammatory functions and is associated with disease activity in systemic lupus erythematosus. Arthritis Rheumatol. 2015;67:3219–3225. doi: 10.1002/art.39328. [DOI] [PubMed] [Google Scholar]
- 233.Chu M, Tam LS, Zhu J, et al. In vivo anti-inflammatory activities of novel cytokine IL-38 in Murphy Roths Large (MRL)/lpr mice. Immunobiology. 2017;222:483–493. doi: 10.1016/j.imbio.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 234.Chu M, Chu IM, Yung EC, et al. Aberrant expression of novel cytokine IL-38 and regulatory T lymphocytes in childhood asthma. Molecules. 2016;21:E933. doi: 10.3390/molecules21070933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Boutet MA, Najm A, Bart G, et al. IL-38 overexpression induces anti-inflammatory effects in mice arthritis models and in human macrophages in vitro. Ann Rheum Dis. 2017;76:1304–1312. doi: 10.1136/annrheumdis-2016-210630. [DOI] [PubMed] [Google Scholar]
- 236.Zhong Y, Yu K, Wang X, Wang X, Ji Q, Zeng Q. Elevated Plasma IL-38 Concentrations in Patients with Acute ST-Segment Elevation Myocardial Infarction and Their Dynamics after Reperfusion Treatment. Mediators Inflamm. 2015;2015:490120. doi: 10.1155/2015/490120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Emmanuilidis K, Weighardt H, Matevossian E, et al. Differential regulation of systemic IL-18 and IL-12 release during postoperative sepsis: High serum IL-18 as an early predictive indicator of lethal outcome. Shock. 2002;18:301–305. doi: 10.1097/00024382-200210000-00002. [DOI] [PubMed] [Google Scholar]
- 238.Tsai MH, Chen YC, Yang CW, et al. Acute renal failure in cirrhotic patients with severe sepsis: Value of urinary interleukin-18. J Gastroenterol Hepatol. 2013;28:135–141. doi: 10.1111/j.1440-1746.2012.07288.x. [DOI] [PubMed] [Google Scholar]
- 239.Mommsen P, Frink M, Pape HC, et al. Elevated systemic IL-18 and neopterin levels are associated with posttraumatic complications among patients with multiple injuries: A prospective cohort study. Injury. 2009;40:528–534. doi: 10.1016/j.injury.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 240.Palladino I, Salani F, Ciaramella A, et al. Elevated levels of circulating IL-18BP and perturbed regulation of IL-18 in schizophrenia. J Neuroinflammation. 2012;9:206. doi: 10.1186/1742-2094-9-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Haas SL, Abbatista M, Brade J, Singer MV, Bocker U. Interleukin-18 serum levels in inflammatory bowel diseases: Correlation with disease activity and inflammatory markers. Swiss Med Wkly. 2009;139:140–145. doi: 10.4414/smw.2009.12482. [DOI] [PubMed] [Google Scholar]
- 242.Ludwiczek O, Kaser A, Novick D, Dinarello CA, Rubinstein M, Tilg H. Elevated systemic levels of free interleukin-18 (IL-18) in patients with Crohn’s disease. Eur Cytokine Netw. 2005;16:27–33. [PubMed] [Google Scholar]
- 243.Bokarewa M, Hultgren O. Is interleukin-18 useful for monitoring rheumatoid arthritis? Scand J Rheumatol. 2005;34:433–436. doi: 10.1080/03009740510026724. [DOI] [PubMed] [Google Scholar]
- 244.Favilli F, Anzilotti C, Martinelli L, et al. IL-18 activity in systemic lupus erythematosus. Ann N Y Acad Sci. 2009;1173:301–309. doi: 10.1111/j.1749-6632.2009.04742.x. [DOI] [PubMed] [Google Scholar]
- 245.Koenig KF, Groeschl I, Pesickova SS, Tesar V, Eisenberger U, Trendelenburg M. Serum cytokine profile in patients with active lupus nephritis. Cytokine. 2012;60:410–416. doi: 10.1016/j.cyto.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 246.Jelusic M, Lukic IK, Tambic-Bukovac L, et al. Interleukin-18 as a mediator of systemic juvenile idiopathic arthritis. Clin Rheumatol. 2007;26:1332–1334. doi: 10.1007/s10067-006-0474-0. [DOI] [PubMed] [Google Scholar]
- 247.Shimizu M, Yokoyama T, Yamada K, et al. Distinct cytokine profiles of systemic-onset juvenile idiopathic arthritis-associated macrophage activation syndrome with particular emphasis on the role of interleukin-18 in its pathogenesis. Rheumatology (Oxford) 2010;49:1645–1653. doi: 10.1093/rheumatology/keq133. [DOI] [PubMed] [Google Scholar]
- 248.Kawashima M, Yamamura M, Taniai M, et al. Levels of interleukin-18 and its binding inhibitors in the blood circulation of patients with adult-onset Still’s disease. Arthritis Rheum. 2001;44:550–560. doi: 10.1002/1529-0131(200103)44:3<550::AID-ANR103>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 249.Colafrancesco S, Priori R, Alessandri C, et al. IL-18 serum level in adult onset Still’s disease: A marker of disease activity. Int J Inflam. 2012;2012:156890. doi: 10.1155/2012/156890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Blankenberg S, Luc G, Ducimetiere P, et al. Interleukin-18 and the risk of coronary heart disease in European men: The Prospective Epidemiological Study of Myocardial Infarction (PRIME) Circulation. 2003;108:2453–2459. doi: 10.1161/01.CIR.0000099509.76044.A2. [DOI] [PubMed] [Google Scholar]
- 251.Narins CR, Lin DA, Burton PB, Jin ZG, Berk BC. Interleukin-18 and interleukin-18 binding protein levels before and after percutaneous coronary intervention in patients with and without recent myocardial infarction. Am J Cardiol. 2004;94:1285–1287. doi: 10.1016/j.amjcard.2004.07.114. [DOI] [PubMed] [Google Scholar]
- 252.Jefferis BJ, Papacosta O, Owen CG, et al. Interleukin 18 and coronary heart disease: Prospective study and systematic review. Atherosclerosis. 2011;217:227–233. doi: 10.1016/j.atherosclerosis.2011.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Thompson SR, Novick D, Stock CJ, et al. Free Interleukin (IL)-18 levels, and the impact of IL18 and IL18BP genetic variation, in CHD patients and healthy men. Arterioscler Thromb Vasc Biol. 2007;27:2743–2749. doi: 10.1161/ATVBAHA.107.149245. [DOI] [PubMed] [Google Scholar]
- 254.Opstad TB, Pettersen AA, Arnesen H, Seljeflot I. Circulating levels of IL-18 are significantly influenced by the IL-18 +183 A/G polymorphism in coronary artery disease patients with diabetes type 2 and the metabolic syndrome: An observational study. Cardiovasc Diabetol. 2011;10:110. doi: 10.1186/1475-2840-10-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Troseid M, Seljeflot I, Arnesen H. The role of interleukin-18 in the metabolic syndrome. Cardiovasc Diabetol. 2010;9:11. doi: 10.1186/1475-2840-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Parikh CR, Abraham E, Ancukiewicz M, Edelstein CL. Urine IL-18 is an early diagnostic marker for acute kidney injury and predicts mortality in the ICU. J Am Soc Nephrol. 2005;16:3046–3052. doi: 10.1681/ASN.2005030236. [DOI] [PubMed] [Google Scholar]
- 257.Vaidya VS, Waikar SS, Ferguson MA, et al. Urinary biomarkers for sensitive and specific detection of acute kidney injury in humans. Clin Transl Sci. 2008;1:200–208. doi: 10.1111/j.1752-8062.2008.00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Parikh CR, Mishra J, Thiessen-Philbrook H, et al. Urinary IL-18 is an early predictive biomarker of acute kidney injury after cardiac surgery. Kidney Int. 2006;70:199–203. doi: 10.1038/sj.ki.5001527. [DOI] [PubMed] [Google Scholar]
- 259.Sirota JC, Walcher A, Faubel S, et al. Urine IL-18, NGAL, IL-8 and serum IL-8 are biomarkers of acute kidney injury following liver transplantation. BMC Nephrol. 2013;14:17. doi: 10.1186/1471-2369-14-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Lin CY, Chang CH, Fan PC, et al. Serum interleukin-18 at commencement of renal replacement therapy predicts short-term prognosis in critically ill patients with acute kidney injury. PLoS ONE. 2013;8:e66028. doi: 10.1371/journal.pone.0066028. [DOI] [PMC free article] [PubMed] [Google Scholar]


