Abstract
Background/Aim: The kidney excretes waste materials and regulates important metabolic functions, and renal disorders constitute a significant medical problem and can result in fatalities. In the present study, mesenchymal stem cells derived from canine umbilical cord blood (cUCB-MSCs) were isolated and evaluated for their ability to improve renal function in a canine model of acute kidney injury (AKI). Materials and Methods: The canine AKI model was developed by i.v. injection of cisplatin and gentamycin into 14 male beagle dogs. cUCB-MSCs were administered into the renal corticomedullary junction following AKI induction. Survival time, clinical signs, blood analysis and histological parameters were analyzed. Results: The group treated with AKI plus cUCB-MSCs had decreased blood urea nitrogen and creatinine levels, and showed an extended life-span and improved histological manifestations. MSCs were detected around the tubules of these kidneys at the histological level. Conclusion: Taken together, our findings suggest that cUCB-MSCs could be an alternative therapeutic agent for canine AKI.
Keywords: Acute kidney injury, canine umbilical cord bloodmesenchymal stem cells, dog
Acute kidney injury (AKI) is a clinical disorder characterized by the sudden loss of kidney function, which includes excreting wastes and maintaining electrolyte and acid–base balance and body fluid levels (1). The clinical signs of AKI are sudden illness, vomiting, anorexia, weight loss, exercise intolerance, and eventually death. Recently reported mortality of AKI in dogs was 47-61% in a retrospective report (2). Factors that induce AKI include renal ischemia, nephrotoxic substances, infection, inflammation, and urinary tract obstruction by calculi, tumors, or strictures (3). A frequent cause of canine AKI is renal ischemia and exposure to nephrotoxins (4). Classic treatment for AKI includes fluid administration while monitoring urine output, use of diuretics (furosemide, dopamine, vasopressin), anti-nausea agent, gastroprotectants, phosphorus absorbent, antioxidants, sodium bicarbonate for metabolic acidosis, antidote for nephrotoxin, and antibiotics for infection (5). After nephron disruption, it is difficult for patients to overcome the disease without the aid of dialysis or renal transplantation (6-8). However, these approaches have considerable limitations, including high cost, difficulty in using these approaches in smaller animals owing to low total blood volume, immunological problems, and the low availability of donor kidneys (9,10). Although many dogs are brought to veterinary clinics or die due to AKI, little research to develop an effective treatment using a canine AKI model has been undertaken, to date. However, considering the urgent need to develop alternative treatments, studies of this aspect are on the rise.
In recent studies, stem cells have been suggested as alternative agents for the treatment of various degenerative diseases (11-13). Stem cells have distinctive characteristics: self-renewal and the ability to differentiate into various lineages. In particular, the use of adult stem cells for in vivo transplantation, unlike embryonic stem cells, is not associated with any ethical issues. Our previous study demonstrated the isolation and characterization of mesenchymal stem cells derived from canine umbilical cord blood (cUCB-MSCs) (14). Therefore, we aimed to investigate the therapeutic effects of cUCB-MSCs in a canine AKI model via cell transplantation.
Materials and Methods
Isolation and culture of cUCB-MSCs. Our previous study reported the isolation and culture of cUCB-MSCs (14). Briefly, canine umbilical cord blood was drawn and used for the isolation of mononuclear cells. The blood was collected in tubes that were treated with the anti-coagulant EDTA. Blood was diluted 1:1 with phosphate-buffered saline (PBS) (Cellgro, CA, USA). Density-gradient centrifugation was performed using 1.078 g/ml Ficoll-Paque (GE Healthcare, CA, USA) to collect the buffy coat layer. Mononucleated cells were seeded into T75 cell culture flasks (Nunc, NY, USA) at a density of 5×106 cells/ml. Three days after the cells were seeded, they were transferred to new flasks containing half the amount of low-glucose Dulbecco’s modified Eagle’s medium (LG-DMEM; Gibco BRL, CA, USA) with 10% fetal bovine serum (FBS; Gibco BRL). The adhered cells were trypsinized in preparation for transplantation into the canine AKI model.
Cell labelling with green fluorescent protein (GFP). cUCB-MSCs were labelled with GFP to enable tracking. Briefly (15), the ViraPower Lentiviral Packaging Mix (Invitrogen, Carlsbad, CA, USA) was used for generating lentiviruses. Lipofectamine 2000 (Invitrogen) was used for transfection of SHC003 (vector control) (Sigma, St. Louis, MO, USA) into 293FT cells (Invitrogen). Cell culture media were changed on the day after transfection, and supernatants were harvested 48 and 72 h after transfection. cUCB-MSCs were transduced with the Turbo-Green Fluorescent Protein (GFP)-Puror (Sigma) lentivirus at a multiplicity of infection of 10. Cell culture media were replaced with fresh culture media on the day after transduction. For selection, puromycin (Sigma) was added to the cell culture media at a final concentration of 3 mg/ml for 3 days. The presence of GFP-labelled cUCB-MSCs was confirmed with confocal laser microscopy (Eclipse TE2000; Nikon, Tokyo, Japan).
Differentiation assay using cUCB-MSCs. The multi-lineage differentiation assay was performed as previously described (14) to demonstrate the isolated cells were mesenchymal stem cells. Briefly, osteogenic differentiation was conducted with osteogenic induction medium (LG-DMEM containing 10% FBS, 100 nM dexamethasone, 50 μM ascorbic acid 2-phosphate, and 10 mM β-glycerophosphate; all obtained from Sigma-Aldrich, St. Louis, MO, USA). After 2 weeks of induction, the cells were stained with Alizarin Red S to confirm osteogenic differentiation; Alizarin Red S staining is particularly associated with calcium deposition. The cells were washed with PBS and fixed with 70% ice-cold ethanol for 1 h at 4˚C. After three washes with distilled water, the cells were stained using 40 mM Alizarin Red S (pH 4.2; Sigma-Aldrich) for 10 min at room temperature (22˚C). The unbound dye was rinsed-off the cells by five washes with distilled water.
Adipogenic differentiation was conducted with adipogenic induction medium containing 500 μM 3-isobutyl-1-metyl-xanthine, 1 μM dexamethasone, 60 μM indomethacin, and 5 μg/ml insulin (Sigma-Aldrich). After 3 weeks of induction, Oil Red O staining was conducted to confirm differentiation. The cells were fixed with 10% formalin for at least 1 h and rinsed with 60% isopropanol prior to incubation in freshly diluted Oil Red O for 10 min.
Chondrogenic differentiation was performed with chondrocyte differentiation medium (Lonza, Walkersville, MD, USA). The cells (5×105 cells/tube) were added to a 15-ml polypropylene tube with 1 ml chondrocyte differentiation medium and centrifuged to obtain a pellet. The pellet was cultured at 37˚C in a 5% CO2 incubator for 2 weeks. After differentiation, the round pellets were embedded in paraffin and cut into 3-μm sections, which were then stained with toluidine blue. The slide was deparaffinized and hydrated with distilled water. The slide was immersed in toluidine blue working solution for 1 min. For washing the unbound stain, the slide was rinsed using distilled water and continuously dehydrated with 95% and absolute alcohol. The slide was then cleared with xylene and covered with a coverslip using Canada balsam mounting medium (Sigma-Aldrich).
Design of animal study. All experiments were approved by and followed the policies and regulations of the Laboratory Animals Institutional Animal Care and Use Committee (IACUC) (SNU-070627-1; Seoul National University, Seoul, Korea).
A total of 14 dogs were enrolled in this study. They were male beagle dogs (age: 6 months to 1 year; weight: 10-15 kg) which were adopted from a beagle dog breeder (Cheonho-aegyeon, Gyeongi, Korea). All dogs were subjected to blood analysis to determine that they showed normal renal function. They were caged in a room maintained at constant temperature and provided a standard diet.
The experimental animals were divided into three different groups. Dogs in the AKI plus MSC group (n=3) were administered cUCB-MSCs via the bilateral renal corticomedullary junction following induction of AKI; dogs in the AKI plus PBS group (n=8) received a saline injection at the same site as the AKI plus MSC group after induction of AKI. Dogs in the non-AKI group (n=3) were not administered any agent. Dogs were randomly allocated to one of the three groups. After the end of experiment, all surviving AKI model dogs were euthanized using tiletamine hydrochloride and zolazepam hydrochloride (5 mg/kg; Zoletil; Virbac, Carros, France) and potassium chloride (2 mmol/kg; Daihan Pharm, Seoul, Korea).
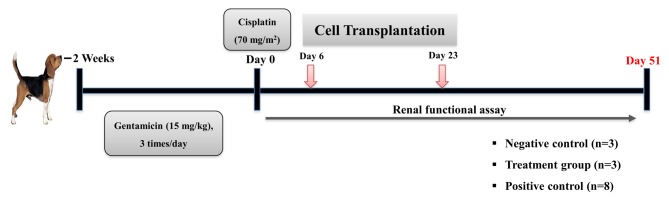
Generation of the canine AKI model. Renal damage was induced by intravenous injection of gentamicin (15 mg/kg; ShinPoong, Ansan, Gyeonggi, Korea) three times per day for 2 weeks, following which a single dose of cisplatin (70 mg/m2; Boryung, Seoul, Korea) was injected at the end of the last day of the gentamicin treatment period (Figure 1). Inducing experimental AKI can cause permanent damage to the kidneys, which was confirmed by clinical signs (vomiting, anorexia, depression), serum chemistry profile (elevation of serum blood urea nitrogen (BUN) and creatinine, normal or elevated phosphorus, electrolyte imbalance), ultrasonography (hyperechoic renal cortex, corticomedullar attenuation), and histological analysis after death.
Figure 1. Schematic overview of the experiment. The canine acute kidney injury model was induced by administration of gentamicin and cisplatin for two weeks. Transplantation with mesenchymal stem cells derived from canine umbilical blood was performed two times on day 6 and day 23. All animals were euthanized on day 51.
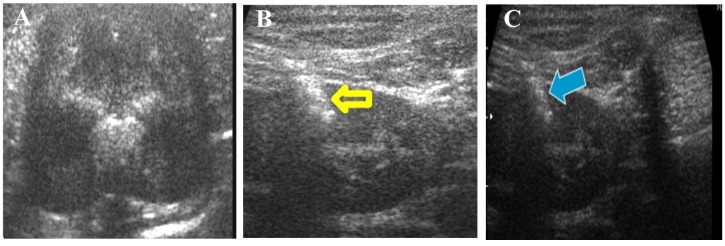
Transplantation of cUCB-MSCs. After induction of AKI, cUCB-MSCs were administered to the AKI plus MSC group (n=3) twice (days 6 and 23). Dogs in the AKI plus PBS group (n=8) received bilateral injections of PBS into the kidneys twice (days 6 and 23). To avoid unnecessary contamination during cell transplantation, we placed the animals under general anesthesia for complete immobilization, clipped the hair, and surgically prepared the entire clipped area. General anesthesia was administered by injecting tiletamine hydrochloride and zolazepam hydrochloride (Zoletil, 10 mg/kg; Virbac) into the quadriceps muscle. Ultrasound (Sono)-guided renal injection was performed using a microsyringe with a 1.5-inch 23-gauge needle (Figure 2). In the AKI plus MSC group, cUCB-MSCs (1×106 cells/30 μl PBS, bilaterally) were directly injected into one site of the renal corticomedullary junction within each kidney. Correspondingly, to induce the same damage caused by the injection procedure, 30 μl PBS was injected into the same site in the AKI plus PBS group (n=8). The non-AKI group (n=3) was not subjected to any procedure until these animals were euthanized. At 7 weeks after induction, all animals were euthanized and their kidneys were obtained for analysis.
Figure 2. Direct transplantation of canine umbilical cord blood-derived mesenchymal stem cells (cUCB-MSCs). A: Ultrasonographic image of the normal kidney. B: Phosphate-buffer solution was bilaterally injected into the kidneys under ultrasonographic guidance for the acute kidney injury (AKI)+PBS group (the yellow arrow indicates the injection needle). C: Cells were bilaterally transplanted into the kidneys for the AKI+MSC group under ultrasonographic guidance (the blue arrow indicates the injection needle).
Evaluation of renal function. Renal function was assessed based on BUN and creatinine levels (Vettest8008; IDEXX, Sungnam, Gyeongi, Korea). Blood samples were collected two or three times per week to determine the serum chemistry profile. The reference range of serum BUN was 8-30 mg/dl and that of serum creatinine was 0.5-1.5 mg/dl. Serum BUN and creatinine levels above the reference range were considered abnormal.
Detection of transplanted cUCB-MSCs. The presence of the administered stem cells was determined through necropsy at 51 days after injection of cUCB-MSCs. To facilitate the detection of transplanted cells, we generated green fluorescent protein (GFP)-labelled cUCB-MSCs by using a lentivirus-based transfection system. Histologically, we determined the presence of GFP-labelled cUCB-MSCs by confocal laser microscopy (Eclipse TE2000; Nikon). After necropsy, the kidneys were infused with a 30% sucrose (Sigma Aldrich) solution. After the kidneys were submerged, tissue from the kidney was collected and placed in an embedding mold fabricated from household aluminum foil, following which the mold was filled with fresh O.C.T. (Tissue-Tek; Sakura Finetech Co., Ltd., USA). The mold was rapidly transferred into the block, covered in cellophane and aluminum foil, and stored at −70˚C. Serial sections were obtained and stained with Hoechst 33238 (1 mg/ml) diluted 1:100 in PBS for nuclear staining and GFP-labelled cUCB-MSCs were counted.
Histological examination of the kidneys. After the experiment, the kidneys were harvested and fixed with 10% buffered formalin. The fixed tissues were embedded in paraffin wax and 4-μm sections were stained with hematoxylin and eosin (H&E) by standard procedures for histological examination, following which the sections were observed under a light microscope. In order to avoid subjective bias, histological evaluation for the degree of kidney damage was performed by a blinded pathologist.
Statistical analysis. All data were analysed using IBM SPSS Statistics 21.0 (IBM corp., Armonk, NY, USA) or GraphPad Prism 6 Software (GraphPad Inc., San Diego, CA, USA). Survival rate was analyzed with the log-rank test for trend. Statistical analysis of serum BUN and creatinine was performed by t-test. All data obtained from independent experiments are presented as mean±standard error of the mean (SEM).
Results
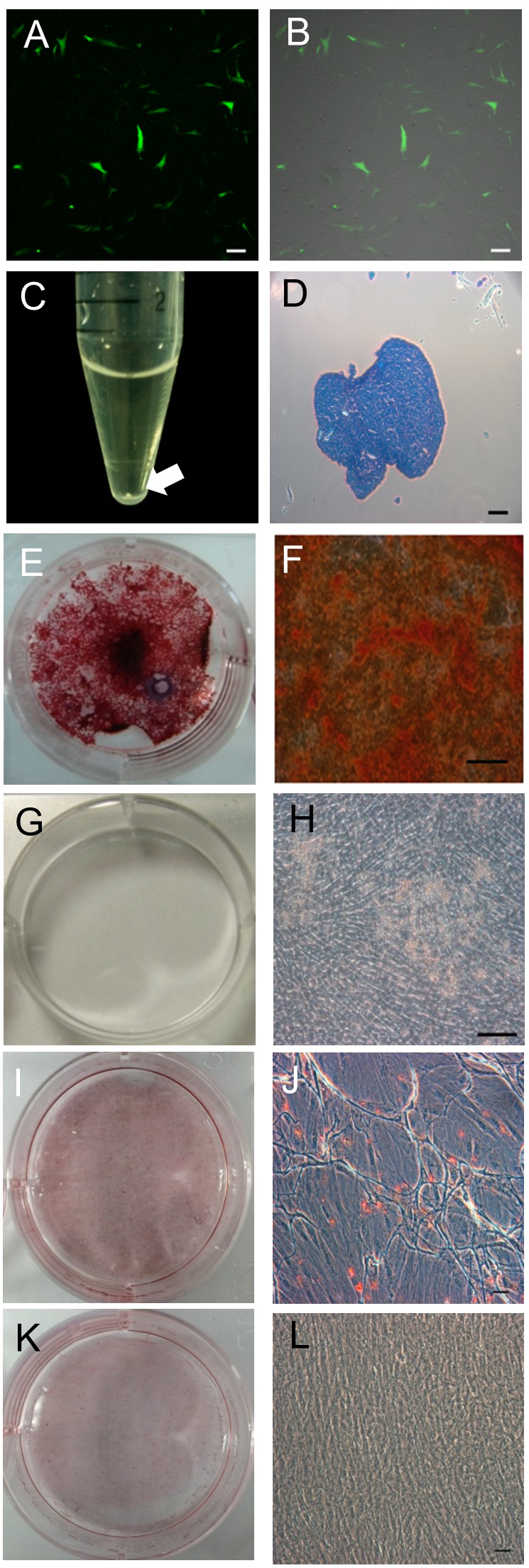
Characterization of cUCB-MSCs. cUCB-MSCs were labelled with GFP to enable tracking (Figure 3A and B). After confirming the successful preparation of GFP-positive cUCB-MSCs with fluorescence microscopy, the cells were used for cell transplantation. To characterize cUCB-MSCs, differentiation assays were performed under specific differentiation conditions. During chondrogenic differentiation induction, we confirmed pellet formation at the bottom of the polypropylene tube. The pellet was ovoid and opaque (Figure 3C). The pellet was positively stained with toluidine blue (Figure 3D). Alizarin Red S staining was used to detect osteogenic differentiation, which positively stains calcium deposits. Basal culture medium (LG-DMEM with 10% FBS) was used as the control condition. After differentiation, we observed positive Alizarin Red S staining (Figure 3E and F) under osteogenic differentiation but not under the control conditions (Figure 3G and H). Oil Red O staining, which detects fatty droplets, was used to detect adipogenic differentiation. The fatty droplets were detected under adipogenic differentiation conditions (Figure 3I and J) but not under control conditions (Figure 3K and L).
Figure 3. Characterization of canine umbilical cord blood-derived mesenchymal stem cells (cUCB-MSCs). A, B: cUCB-MSCs labelled with green fluorescent protein (GFP). A: Fluorescent microscopic image, B: merge with a light microscopic image, scale bar=100 μm. C, D: Chondrogenic induction: C: Pellet formation, aggregated to form a round shape. The white arrow indicates a pellet. D: Toluidine blue staining of chondrogenic pellets. The stained tissue showed a typical cartilaginous tissue phenotype. Scale bar=100 μm. E–H: Osteogenic induction: E, F: cUCB-MSCs grown in osteogenic induction medium. Differentiated cells stained strongly with Alizarin Red S, unlike control cells (G, H): scale bar=50 μm. I, J: Adipogenic induction. cUCB-MSCs grown in adipogenic induction medium. Differentiated cells stained strongly with Oil Red O, unlike control cells (K, L): scale bar=50 μm. I-L: cUCB-MSCs grown in maintenance medium: scale bar=50 μm.
Generation of canine AKI models. Dogs with experimentally induced AKI showed clinical symptoms such as vomiting, dehydration, and weight loss, while dogs in the non-AKI group were healthy. Serum concentrations of BUN and creatinine increased after AKI was induced in the AKI plus PBS group and the AKI plus MSC group, in contrast to the non-AKI group.
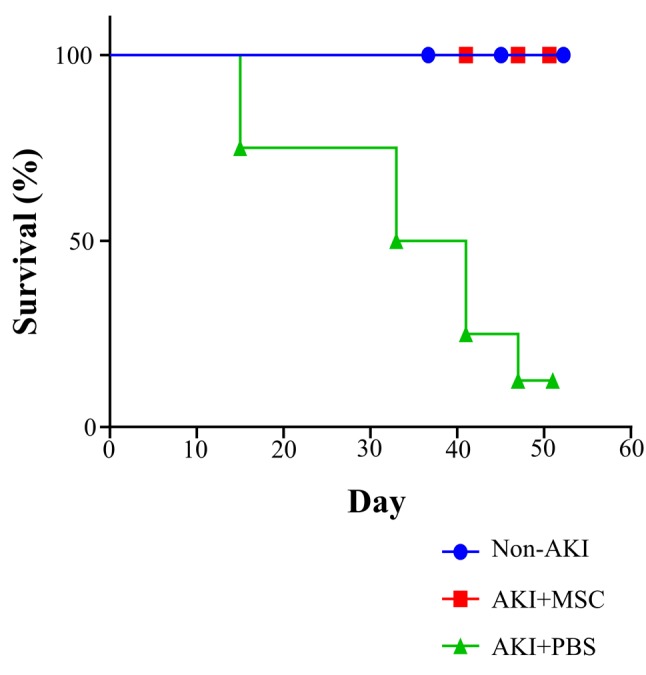
Survival rate in the AKI plus MSC group versus the AKI plus PBS control group. During the experiment, survival rate was determined to evaluate the effect of cell transplantation. At the end of the experiment (day 51), all animals in the cUCB-MSC-treated AKI group (n=3) were alive, whereas only one animal in the AKI plus PBS group (n=8) survived. In the AKI plus PBS group, a six dogs died, two each on day 15, 33, and 41; one further animal died on day 47. Thus, the survival rate increased when the dogs were treated with cUCB-MSCs (Figure 4; p=0.007, log-rank test for trend).
Figure 4. Survival rate in the acute kidney injury (AKI)+mesenchymal stem cell (MSC) group (n=3) versus the AKI+phosphate buffer solution (PBS) group (n=8) and non-AKI group (n=3). Survival rate was measured from day 0 to day 51 (the end point of the experiment). Statistical analysis of survival rate was performed by log-rank test for trend (p=0.007).
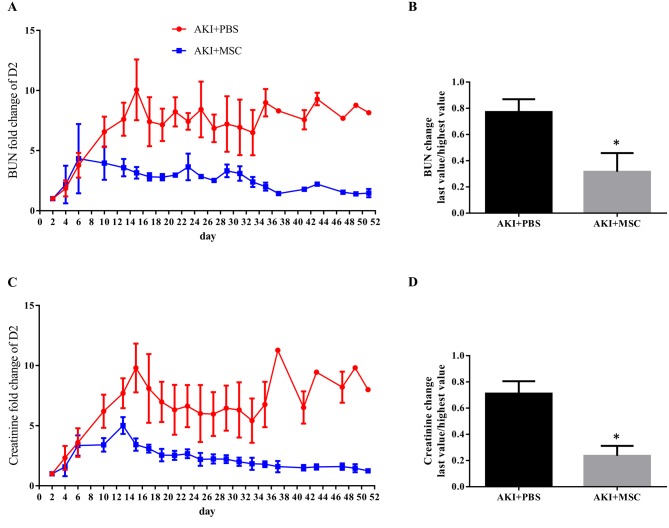
Evaluation of renal function based on BUN and creatinine levels. To determine whether cUCB-MSCs could ameliorate renal dysfunction, cell transplantation into the renal corticomedullary junction was performed in the AKI plus MSC group, and the levels of serum BUN and creatinine were evaluated (Figure 5). After administration of gentamicin and cisplatin, the levels of BUN and creatinine increased continuously. On day 6, we performed the first administration of cUCB-MSCs into the renal corticomedullary junction of the AKI plus MSC group. At the same time, the AKI plus PBS group received PBS. After the first administration of cUCB-MSCs, levels of BUN and creatinine decreased in the AKI plus MSC group but not in the AKI plus PBS group. On day 23, the cUCB-MSC-treated AKI group received the second administration of cUCB-MSCs, whereas the AKI plus PBS group received PBS; after the second injection, levels of BUN and creatinine steadily decreased until the end of the experiment.
Figure 5. Blood urea nitrogen (BUN) and creatinine levels. A: BUN levels were measured throughout the experimental period. B: The highest value after induction of acute kidney injury (AKI) and the last value of BUN were compared. C: Creatinine levels were measured throughout the experimental period. D: The highest value after inducing AKI and the last value of creatinine were compared. Data for the AKI+phosphate-buffer solution (PBS) group (n=8) and AKI+mesenchymal stem cell (MSC) group (n=3) are shown, those for the non-AKI group are not shown. *Statistical analysis was performed by the Mann-Whitney test (BUN: p=0.0485, creatinine: p=0.0121).
Comparing the highest value after induction of AKI and the last value to determine the effect of cUCB-MSC, both BUN and creatinine differed significantly (Figure 5B and D) (p=0.0485 and p=0.0121, respectively, Mann-Whitney test).
The administration of cUCB-MSCs improved renal excretory function, which was verified by the levels of serum BUN and creatinine in the canine AKI model.
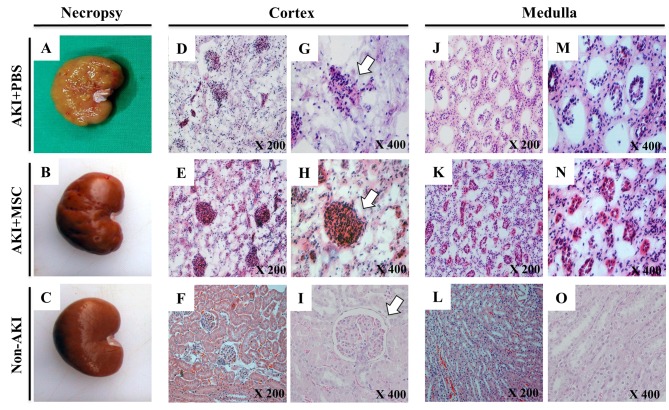
Histological examination of the kidneys. At the end of the experiment, kidneys were harvested for histological analysis. In the AKI plus PBS group, the kidneys had a pale and rough surface (Figure 6A) compared to the kidneys of the non-AKI group (Figure 6C). However, histological examination showed that in the AKI plus MSC group, the kidneys showed less damaged than those from the AKI plus PBS group (Figure 6B), which exhibited distinctive renal lesions. We observed degenerative and necrotic lesions (tubular necrosis, shedding of tubular epithelial cells, and glomerular necrosis) in both the cortex and the medulla (Figure 6D-O). For the description of these lesions, we classified their severity into four categories: null, mild, moderate, and severe. Regarding tubular necrosis, the cUCB-MSC-treated AKI group had moderate lesions whereas the AKI plus PBS group had severe ones, exhibiting global cystic change of tubular tissue and massive interstitial leukocyte infiltration. Shedding of tubular epithelial cells was mild to moderate in the AKI plus MSC group but severe in the AKI plus PBS group. Regarding glomerular necrosis, the AKI plus PBS group had moderately damaged glomeruli which kept their structural integrity more intact than the severe findings of the AKI plus PBS group. These results showed that administration of cUCB-MSCs improved the renal lesions that appeared in the canine AKI model.
Figure 6. Histological manifestation of the kidneys after transplantation of canine umbilical cord blood-derived mesenchymal stem cell (cUCBMSC) into dogs with induced acute kidney injury (AKI). At day 51, all dogs were euthanized, and their kidneys were harvested for histological examination. A-C: Gross features of the kidneys. D-I: The renal cortex at low (D, E, F) and at high (G, H, I) magnification. J-O: The renal medulla at low (J- L) and at high (M-O) magnification. White arrows indicate the renal glomerulus.
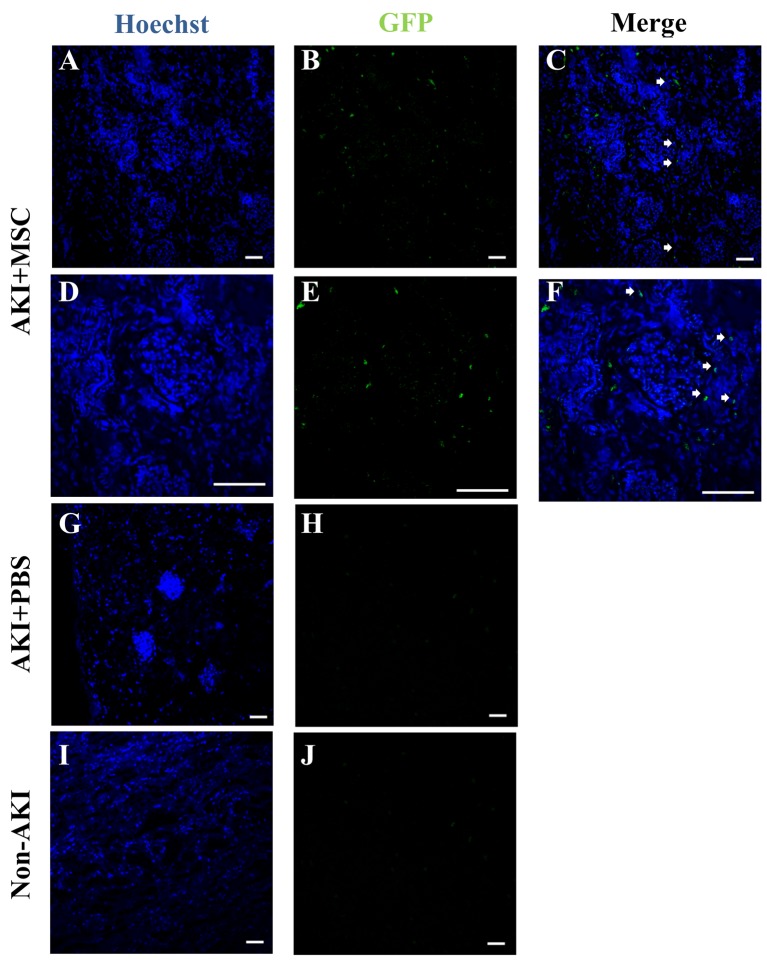
Engraftment of GFP-labelled cUCB-MSCs in the AKI plus MSC group. To determine the presence of cUCB-MSCs in the kidneys of the AKI plus MSC group, we detected the presence of GFP-labelled cells by using confocal laser microscopy (Figure 7). GFP-positive cells were found in the kidneys of the AKI plus MSC group (Figure 7B, C, E and F). Most of the GFP-positive cells were detected around the tubules of these kidneys. Such cells were only detected in the cortex of the kidneys of the AKI plus MSC group (mean ± standard error of the mean=1.4828±0.2136%). The kidneys of the AKI plus PBS and non-AKI groups did not show GFP-positive cells (Figure 7G-J).
Figure 7. Detection of transplanted mesenchymal stem cells in the kidneys of dogs with and without acute kidney injury (AKI). Cell transplantation with green fluorescent protein (GFP)-labelled canine umbilical blood-derived mesenchymal stem cells (cUCB-MSCs) was confirmed by confocal laser microscopy. Hoechst 33238 stained the nuclei in all kidneys. GFP-positive cells were detected in tubules in the AKI+MSC group (A–F, white arrows indicate GFP-positive cells, scale bar=50 μm). The kidneys of the AKI+PBS and l groups did not show the presence of GFP-positive cells (G–J, scale bar=50 μm).
Discussion
MSCs can exhibit self-renewal and multi-lineage differentiation, which are distinctive characteristics and have been reported to be present in various tissues such as bone marrow, umbilical cord blood, and adipose tissue (16). These stem cells have many advantages in cell therapy, including immune privilege and absence of associated ethical issues. In humans, it is well known that umbilical cord blood represents a major source of stem cells. Indeed, there have been several reports on the characteristics of stem cells (16,17) and clinical trials evaluating the therapeutic effects of umbilical cord blood-derived stem cells on various degenerative diseases (18-20). Our previous studies demonstrated the isolation of cUCB-MSCs (14) and reported on clinical trials evaluating the therapeutic effect of these cells on degenerative diseases (21).
In the present study, we established a canine AKI model for studying degenerative kidney diseases induced by chemical toxins. To date, the rodent model was commonly used and reported on extensively in the context of renal failure research (22,23). Nevertheless, it is necessary to establish a canine model for clinical applications. To establish the canine AKI model, artificial renal failure is induced by medical or mechanical methodology. Gentamicin-induced AKI is one of the most well-established models in dogs (24,25). The most well-known adverse effect of gentamicin is the accumulation of renal tubular toxin in proximal tubular cells; the toxin interferes with electrolyte and water reabsorption and causes cellular necrosis and detachment from basement membranes, which result in cast formation and ultimately tubular obstruction (9). However, induction of renal failure using gentamicin usually results in a high mortality rate. Another nephrotoxic chemical, cisplatin, is known to cause maximal tubular necrosis (26), although the underlying mechanism is unclear. Usually, AKI induced by cisplatin recovers spontaneously within about 2 weeks (27). The combination of gentamicin and cisplatin is expected to cause permanent damage to the kidney in addition to lowering mortality. In our model, the induction time was 2 weeks, and most of the dogs were sacrificed within 7 weeks after induction of kidney injury (at 7 weeks after induction: survival rate=12.5%). We believe that this new AKI model could contribute to veterinary nephrology research.
BUN is an indicator of renal disease. The concentration of BUN is controlled by maintaining the balance between urea formation and urinary excretion; therefore, these levels may increase owing to elevated urea reabsorption caused by prolonged renal retention due to decreased glomerular flow rate (28). Creatinine is a muscular metabolic product. As a more precise indicator of renal function than BUN, creatinine is a significant criterion of the severity of renal failure (29-31). Some retrospective studies in dogs showed that the severity of AKI is strongly related to the serum creatinine level. Serum creatinine higher than 10 mg/dl was associated with failure to recover from AKI (4). In another human retrospective report, the outcome of AKI was correlated to AKI stage, defined by serum creatinine level (32). In the present study, serum BUN and creatinine levels of the cUCB-MSC-treated AKI group decreased after MSC transplantation compared with those observed in the AKI plus PBS group. Although the expression levels of BUN and creatinine were not within the reference range, this result was remarkable considering the fact that cisplatin/gentamicin-induced renal failure led to a high mortality. We measured serum BUN and creatinine levels in the renal function test, although various other parameters indicative of renal function may be determined, e.g. inulin clearance test, creatinine clearance test, and analysis of urinary biomarkers (e.g. neutrophil gelatinase-associated lipocain, kidney injury molecule-1, N-acetyl-β-D-glucosaminidase) (33-35). For a more accurate evaluation of renal function, one of the supplementary methods mentioned above may be performed as well; however, monitoring serum BUN and creatinine levels alone is quite an accurate measure of renal function and a meaningful method in a clinical setting (6,36).
The survival rate was the major difference between the AKI plus MSC group and the AKI plus PBS group; up to the end of the experiment, no mortality was recorded in the AKI group treated with cUCB-MSCs.
The transplanted cUCB-MSCs, which were labelled with GFP, were detected by confocal laser microscopy. Most GFP-positive cells were observed in the tubules of the kidneys of the cUCB-MSC-treated AKI group. We also confirmed that the kidneys of the AKI plus PBS and non-AKI groups did not show GFP-positive cells. To investigate the mechanism underlying the therapeutic effects of cUCB-MSCs in the AKI plus MSC group, we conducted H&E staining during histopathological analysis. Damage, including necrosis of tubules and the glomerulus and shedding of tubular epithelial cells, was less in the cUCB-MSC-treated AKI group than in the AKI plus PBS group. We cautiously suggest that the transplanted MSCs might differentiate in the impaired renal tissue, regarding the effect of MSC administration and identification of renal-entrapped MSCs.
The results obtained in this study contradict those in another report that showed the non-therapeutic effects of MSC transplantation in an ovine model of renal injury (37). Considering other reports, the mechanism by which cUCB-MSCs contribute to ameliorating renal failure in the present study could be related to the MSC secretome or prevention of progressive pathology or transdifferentiation of MSCs (38,39). Contrary to the results of another report that stated that transplanted MSCs were not detected in the kidneys of AKI-induced rats (39), the present study demonstrated that MSCs were located in the kidney. This discrepancy may be attributed to the route of MSC administration; in the other report, intracarotid injection was used, whereas we directly injected MSCs into the renal corticomedullary junction. Many reports have shown that systemically administered MSCs move through the circulation and are eventually retained at the lung Biodistribution research using an immunodeficient animal model showed a high proportion of MSCs localized in the lungs (40). For this reason, we decided to use the renal corticomedullary junction as the route of injection, as it could also hold the cells.
We believe that the findings of this study could contribute to the understanding of the therapeutic effects of cUCB-MSCs in a canine AKI model. This is the first time that positive therapeutic effects of cUCB-MSC transplantation have been demonstrated in a canine AKI model.
Conflicts of Interest
The Authors declare that they have no competing interests in regard to this study.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) under Grant [MEST, 2010-0020265]. We wish to thank the Research Institute for Veterinary Science, Seoul National University, and the BK21 Plus Program for Veterinary Science.
References
- 1.Schrier RW, Wang W, Poole B, Mitra A. Acute renal failure: Definitions, diagnosis, pathogenesis, and therapy. J Clin Invest. 2004;114(1):5. doi: 10.1172/JCI22353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nielsen LK, Bracker K, Price LL. Administration of fenoldopam in critically ill small animal patients with acute kidney injury: 28 dogs and 34 cats (2008-2012) J Vet Emerg Crit Care. 2015;25(3):396–404. doi: 10.1111/vec.12303. [DOI] [PubMed] [Google Scholar]
- 3.English P. Acute renal failure in the dog and cat. Aus Vet J. 1974;50(9):384–392. doi: 10.1111/j.1751-0813.1974.tb05343.x. [DOI] [PubMed] [Google Scholar]
- 4.Vaden SL, Levine J, Breitschwerdt EB. A retrospective case-control of acute renal failure in 99 dogs. J Vet Intern Med. 1997;11(2):58–64. doi: 10.1111/j.1939-1676.1997.tb00074.x. [DOI] [PubMed] [Google Scholar]
- 5.Ross L. Acute kidney injury in dogs and cats. Veterinary Clinics of North America: J Small Anim Pract. 2011;41(1):1–14. doi: 10.1016/j.cvsm.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure–definition, outcome measures, animal models, fluid therapy and information technology needs: The second international consensus conference of the acute dialysis quality initiative (adqi) group. Crit care. 2004;8(4):R204. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harel Z, Bell CM, Dixon SN, McArthur E, James MT, Garg AX, Harel S, Silver S, Wald R. Predictors of progression to chronic dialysis in survivors of severe acute kidney injury: A competing risk study. BMC Nephrol. 2014;15:114. doi: 10.1186/1471-2369-15-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schiffl H, Lang SM, Fischer R. Daily hemodialysis and the outcome of acute renal failure. N Engl J Med. 2002;346(5):305–310. doi: 10.1056/NEJMoa010877. [DOI] [PubMed] [Google Scholar]
- 9.Mannick JA, Powers JH, Mithoefer J, Ferrebee JW. Renal transplantation in azotemic dogs. Surgery (US) 1960;47:340–345. [PubMed] [Google Scholar]
- 10.Laupacis A, Keown P, Pus N, Krueger H, Ferguson B, Wong C, Muirhead N. A study of the quality of life and cost-utility of renal transplantation. Kidney int. 1996;50(1):235–242. doi: 10.1038/ki.1996.307. [DOI] [PubMed] [Google Scholar]
- 11.Neirinckx V, Cantinieaux D, Coste C, Rogister B, Franzen R, Wislet-Gendebien S. Concise review: Spinal cord injuries: How could adult mesenchymal and neural crest stem cells take up the challenge. Stem Cells. 2014;32(4):829–843. doi: 10.1002/stem.1579. [DOI] [PubMed] [Google Scholar]
- 12.Hong SB, Seo MS, Park SB, Seo YJ, Kim JS, Kang KS. Therapeutic effects of human amniotic epithelial stem cells in niemann-pick type c1 mice. Cytotherapy. 2012;14(5):630–638. doi: 10.3109/14653249.2012.663485. [DOI] [PubMed] [Google Scholar]
- 13.Kono TM, Sims EK, Moss DR, Yamamoto W, Ahn G, Diamond J, Tong X, Day KH, Territo PR, Hanenberg H, Traktuev DO, March KL, Evans-Molina C. Human adipose-derived stromal/stem cells protect against STZSTZ-induced hyperglycemia: Analysis of HASCHASC-derived paracrine effectors. Stem Cells. 2014;32(7):1831–1842. doi: 10.1002/stem.1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seo MS, Jeong YH, Park JR, Park SB, Rho KH, Kim HS, Yu KR, Lee SH, Jung JW, Lee YS, Kang KS. Isolation and characterization of canine umbilical cord blood-derived mesenchymal stem cells. J Vet Sci. 2009;10(3):181–187. doi: 10.4142/jvs.2009.10.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun B, Roh KH, Park JR, Lee SR, Park SB, Jung JW, Kang SK, Lee YS, Kang KS. Therapeutic potential of mesenchymal stromal cells in a mouse breast cancer metastasis model. Cytotherapy. 2009;11(3):289–298. doi: 10.1080/14653240902807026. [DOI] [PubMed] [Google Scholar]
- 16.Wagner W, Wein F, Seckinger A, Frankhauser M, Wirkner U, Krause U, Blake J, Schwager C, Eckstein V, Ansorge W, Ho AD. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp Hematol. 2005;33(11):1402–1416. doi: 10.1016/j.exphem.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 17.Kang X-Q, Zang W-J, Bao L-J, Li D-L, Xu X-L, Yu X-H. Differentiating characterization of human umbilical cord blood-derived mesenchymal stem cells in vitro. Cell Biol Int. 2006;30(7):569–575. doi: 10.1016/j.cellbi.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 18.Kim S-W, Han H, Chae G-T, Lee S-H, Bo S, Yoon J-H, Lee Y-S, Lee K-S, Park H-K, Kang K-S. Successful stem cell therapy using umbilical cord blood-derived multipotent stem cells for BBuerger’s disease and ischemic limb disease animal model. Stem Cells. 2006;24(6):1620–1626. doi: 10.1634/stemcells.2005-0365. [DOI] [PubMed] [Google Scholar]
- 19.Jung KH, Uhm Y-K, Lim YJ, Yim S-V. Human umbilical cord blood-derived mesenchymal stem cells improve glucose homeostasis in rats with liver cirrhosis. Int J Oncol. 2011;39(1):137–143. doi: 10.3892/ijo.2011.1016. [DOI] [PubMed] [Google Scholar]
- 20.Kaner T, Karadag T, Cirak B, Erken HA, Karabulut A, Kiroglu Y, Akkaya S, Acar F, Coskun E, Genc O, Colakoglu N. The effects of human umbilical cord blood transplantation in rats with experimentally induced spinal cord injury. J Neurosurg Spine. 2010;13(4):543–551. doi: 10.3171/2010.4.SPINE09685. [DOI] [PubMed] [Google Scholar]
- 21.Byeon YE, Ryu HH, Park SS, Koyama Y, Kikuchi M, Kim WH, Kang KS, Kweon OK. Paracrine effect of canine allogenic umbilical cord blood-derived mesenchymal stromal cells mixed with beta-tricalcium phosphate on bone regeneration in ectopic implantations. Cytotherapy. 2010;12(5):626–636. doi: 10.3109/14653249.2010.481665. [DOI] [PubMed] [Google Scholar]
- 22.Morigi M, Introna M, Imberti B, Corna D, Abbate M, Rota C, Rottoli D, Benigni A, Perico N, Zoja C, Rambaldi A, Remuzzi A, Remuzzi G. Human bone marrow mesenchymal stem cells accelerate recovery of acute renal injury and prolong survival in mice. Stem Cells. 2008;26(8):2075–2082. doi: 10.1634/stemcells.2007-0795. [DOI] [PubMed] [Google Scholar]
- 23.Lin FM, Cordes K, Li LH, Hood L, Couser WG, Shankland SJ, Igarashi P. Hematopoietic stem cells contribute to the regeneration of renal tubules after renal ischemia-reperfusion injury in mice. J Am Soc Nephrol. 2003;14(5):1188–1199. doi: 10.1097/01.asn.0000061595.28546.a0. [DOI] [PubMed] [Google Scholar]
- 24.Davies C, Forrester SD, Troy GC, Saunders GK, Shell LG, Johnston SA. Effects of a prostaglandin E1 analogue, misoprostol, on renal function in dogs receiving nephrotoxic doses of gentamicin. Am J Vet Res. 1998;59(8):1048–1054. [PubMed] [Google Scholar]
- 25.Mendezma A, Ortizgon A. Acute renal failure due to simultaneous administration of cephalotin and gentamycin. Rev Clin Esp. 1974;133(4):365–370. [PubMed] [Google Scholar]
- 26.Yao X, Panichpisal K, Kurtzman N, Nugent K. Cisplatin nephrotoxicity: A review. Am J Med Sci. 2007;334(2):115–124. doi: 10.1097/MAJ.0b013e31812dfe1e. [DOI] [PubMed] [Google Scholar]
- 27.Miller RP, Tadagavadi RK, Ramesh G, Reeves WB. Mechanisms of cisplatin nephrotoxicity. Toxins. 2010;2(11):2490–2518. doi: 10.3390/toxins2112490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dossetor JB. Creatininemia versus uremia: The relative significance of blood urea nitrogen and serum creatinine concentrations in azotemia. Ann Intern Med. 1966;65(6):1287–1299. doi: 10.7326/0003-4819-65-6-1287. [DOI] [PubMed] [Google Scholar]
- 29.Perrone RD, Madias NE, Levey AS. Serum creatinine as an index of renal function: New insights into old concepts. Clin Chem. 1992;38(10):1933–1953. [PubMed] [Google Scholar]
- 30.Baum N, Dichoso CC, Carlton CE. Blood urea nitrogen and serum creatinine: Physiology and interpretations. Urology. 1975;5(5):583–588. doi: 10.1016/0090-4295(75)90105-3. [DOI] [PubMed] [Google Scholar]
- 31.Lee Y, Chang C, Chan J, Hsu W, Lin K, Wong M. Prognosis of acute kidney injury in dogs using rifle (risk, injury, failure, loss and end-stage renal failure)-like criteria. Vet Rec. 2011;168(10):264–264. doi: 10.1136/vr.c6234. [DOI] [PubMed] [Google Scholar]
- 32.Ostermann M, Chang R. Correlation between the aki classification and outcome. Crit Care. 2008;12(6):R144. doi: 10.1186/cc7123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou X, Ma B, Lin Z, Qu Z, Huo Y, Wang J, Li B. Evaluation of the usefulness of novel biomarkers for drug-induced acute kidney injury in beagle dogs. Toxicol Appl Pharmacol. 2014;280(1):30–35. doi: 10.1016/j.taap.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 34.Matheny ME, Peterson JF, Eden SK, Hung AM, Speroff T, Abdel-Kader K, Parr SK, Ikizler TA, Siew ED. Laboratory test surveillance following acute kidney injury. PLoS One. 2014;9(8):e103746. doi: 10.1371/journal.pone.0103746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carlos CP, Sonehara NM, Oliani SM, Burdmann EA. Predictive usefulness of urinary biomarkers for the identification of cyclosporine a-induced nephrotoxicity in a rat model. PLoS One. 2014;9(7):e103660. doi: 10.1371/journal.pone.0103660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moran SM, Myers BD. Course of acute renal failure studied by a model of creatinine kinetics. Kidney Int. 1985;27(6):928–937. doi: 10.1038/ki.1985.101. [DOI] [PubMed] [Google Scholar]
- 37.Behr L, Hekmati M, Lucchini A, Houcinet K, Faussat AM, Borenstein N, Noel LH, Lelievre-Pegorier M, Laborde K. Evaluation of the effect of autologous mesenchymal stem cell injection in a large-animal model of bilateral kidney ischaemia reperfusion injury. Cell Prolif. 2009;42(3):284–297. doi: 10.1111/j.1365-2184.2009.00591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sugandhi N, Srinivas M, Agarwala S, Gupta DK, Sharma S, Sinha A, Dinda A, Mohanty S. Effect of stem cells on renal recovery in rat model of partial unilateral upper ureteric obstruction. Pediatr Surg Int. 2014;30(2):233–238. doi: 10.1007/s00383-013-3456-8. [DOI] [PubMed] [Google Scholar]
- 39.Tögel F, Hu Z, Weiss K, Isaac J, Lange C, Westenfelder C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol. 2005;289(1):F31–F42. doi: 10.1152/ajprenal.00007.2005. [DOI] [PubMed] [Google Scholar]
- 40.Meyerrose TE, De Ugarte DA, Hofling AA, Herrbrich PE, Cordonnier TD, Shultz LD, Eagon JC, Wirthlin L, Sands MS, Hedrick MA. In vivo distribution of human adipose-derived mesenchymal stem cells in novel xenotransplantation models. Stem Cells. 2007;25(1):220–227. doi: 10.1634/stemcells.2006-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]