Abstract
Background
Recent American Society of Echocardiography (ASE)/European Association of Cardiovascular Imaging (EACVI) guidelines for echocardiographic evaluation of left ventricular (LV) diastolic function provide a practical, simplified diagnostic algorithm for estimating LV filling pressure. The aim of this study was to test the accuracy of this algorithm against invasively measured pressures and compare it with the accuracy of the previous 2009 guidelines in the same patient cohort.
Methods
Ninety patients underwent transthoracic echocardiography immediately before left heart catheterization. Mitral inflow E/A ratio, E/e′, tricuspid regurgitation velocity, and left atrial volume index were used to estimate LV filling pressure as normal or elevated using the ASE/EACVI algorithm. Invasive LV pre-A pressure was used as a reference, with >12 mm Hg defined as elevated.
Results
Invasive LV pre-A pressure was elevated in 40 (44%) and normal in 50 (56%) patients. The 2016 algorithm resulted in classification of 9 of 90 patients (10%) as indeterminate but estimated LV filling pressures in agreement with the invasive reference in 61 of 81 patients (75%), with sensitivity of 0.69 and specificity of 0.81. The 2009 algorithm could not definitively classify 4 of 90 patients (4.4%), but estimated LV filling pressures in agreement with the invasive reference in 64 of 86 patients (74%), with sensitivity of 0.79 and specificity of 0.70.
Conclusions
The 2016 ASE/EACVI guidelines for estimation of filling pressures are more user friendly and efficient than the 2009 guidelines and provide accurate estimates of LV filling pressure in the majority of patients when compared with invasive measurements. The simplicity of the new algorithm did not compromise its accuracy and is likely to encourage its incorporation into clinical decision making.
Keywords: Left ventricular filling pressure, Left atrial pressure
The burden of heart failure continues to increase as the population ages, with commensurate increases in the financial and social costs of hospitalizations and readmissions. Projections estimate that >8 million people will have heart failure by 2030 and that health care costs could exceed ≥50 billion.1,2 Determining left ventricular (LV) filling pressure is clinically important for the management of patients with congestive heart failure, as elevated LV filling pressure results in increased risk for hospitalization and poor outcomes.2,3
Although invasive methods are considered the “gold standard” for measuring intracardiac filling pressures, echocardiography is routinely used as a noninvasive alternative. This has been done using an algorithm based on Doppler-derived parameters, described in the joint recommendations of the American Society of Echocardiography (ASE) and the European Association of Cardiovascular Imaging (EACVI) from 2009.4 These guidelines have been reported as cumbersome to use because of the number and laboriousness of measurements involved, thereby limiting application in clinical practice.5 In addition, the guidelines proposed the use of different algorithms for patients with normal versus depressed LV function, adding complexity to the diagnostic paradigm.
Accordingly, the ASE and EACVI recently developed a new set of guidelines for the evaluation of LV diastolic function,5 which includes a practical, simplified algorithm for estimating LV filling pressures that can be used in all patients irrespective of LV ejection fraction (LVEF). As stated in the revised guideline document, this recommended algorithm is based on expert consensus that stems from collective experience. The authors also state that this algorithm needs to be validated in a systematic manner against an invasive reference technique. Two recent multicenter studies compared echocardiographic estimates of LV filling pressures against invasive measurements by cardiac catheterization and reported high feasibility and good accuracy irrespective of LV function, especially when combined with clinical data.6,7 These studies were performed by investigators who constituted the core of the guidelines writing group and have extensive specific expertise in the evaluation of diastolic function by echocardiography.
Our primary goal was to assess the validity of the echocardiographic estimates of left-sided filling pressure using the latest guidelines by an independent laboratory that did not participate in the development of the ASE/EACVI guidelines. To achieve this goal, we compared echocardiographic determinations of LV filling pressures against gold-standard invasive measurements, including testing the relationship between their accuracy with LV function and also with gender. In addition, we tested the hypothesis that the accuracy of the new 2016 algorithm would not be compromised by its simplicity compared with the previous 2009 guidelines.
METHODS
Population and Study Design
We prospectively studied 90 patients (mean age, 61 ± 13; 41 men [46%]) referred for clinically indicated left heart catheterization (including for chest pain, acute coronary syndrome excluding ST-segment elevation myocardial infarction, transcatheter aortic valve replacement, preoperative evaluation, and history of ventricular arrhythmia or cardiac arrest) who also underwent transthoracic two-dimensional echocardiography just before catheterization. Hemodynamically unstable patients as well as those with atrial fibrillation, moderate or greater mitral regurgitation, moderate or greater calcification of the mitral annulus, mitral stenosis, heart transplantation, sinus tachycardia, or prosthetic valves were excluded. The study was approved by the institutional review board.
Echocardiographic measurements were performed by a panel of three board-certified echocardiographers blinded to the invasive data who finalized each measurement by consensus. These measurements were used to obtain estimates of LV filling pressure using the 2016 algorithm, resulting in classification as normal, elevated, or indeterminate. After excluding indeterminate estimates, the echocardiographic determinations of normal or elevated filling pressures were compared with invasive LV preatrial contraction (pre-A) pressure measurements, which were defined as either normal or elevated if >12 mm Hg, using the same cutoff as used by Andersen et al6 In addition, cutoffs of 15 and 18 mm Hg were also evaluated in a subanalysis to take into account interlaboratory variability. Comparisons were first performed for the entire study group to test the accuracy of the algorithm, using κ statistics of agreement. Subsequently, these comparisons were repeated for two subgroups of patients with normal (LVEF ≥ 50%) and reduced LV function, as well as male versus female patients, to determine the accuracy of this methodology in these subgroups.
In addition, to test whether the simplification of the new guidelines algorithm affected the accuracy, the same panel of three board-certified echocardiographers used the 2009 guidelines to estimate filling pressures and compare the results against the same invasive reference. Because the 2009 algorithm includes two separate flowcharts to estimate filling pressures in patients with normal and reduced LVEF, the appropriate chart was used in each patient according to LV function. All available parameters were examined in the context of the algorithm, and the determination was made on the basis of which arm of the algorithm had more parameters meeting criteria. When the numbers were similar in both arms of the algorithm, simultaneously suggesting normal and elevated left atrial (LA) pressure, these cases were classified as “indeterminate.” The readers were blinded to both the invasive data and the results of the classification using the 2016 guidelines.
Echocardiographic Imaging and Analysis
Two-dimensional echocardiographic imaging was performed using commercial equipment (iE33 imaging system with an X5 transducer; Philips Medical Systems, Andover, MA). Imaging included apical two- and four-chamber views, from which LA and LV volumes were measured using the method of disks (Xcelera; Philips Medical Systems). These volumes were used to calculate LA volume index (LAVi) and LVEF. Pulsed-wave Doppler of the mitral inflow at the level of valve leaflet tips was used to measure the peak early (E-wave) and late (A-wave) diastolic flow velocities and calculate the E/A ratio. In addition, pulsed-wave Doppler tissue imaging was performed with the sample volume at the lateral and septal mitral annulus to obtain average peak longitudinal early diastolic annular (e′) velocity, which was used to calculate the E/e′ ratio. Peak velocity of the tricuspid regurgitant jet was determined using continuous-wave Doppler. Subcostal windows were acquired to assess diameter and respiratory variation of the inferior vena cava to estimate right atrial pressure. Pulsed-wave Doppler of the pulmonary vein flow was also acquired in the apical views to allow S- and D-wave measurements.
Invasive LV Pressure Measurements
Left heart catheterization was performed according to standard procedure by an interventional cardiologist blinded to the echocardiographic data. Invasive LV pressure measurements were performed using a 6-Fr pigtail catheter (Impulse; Boston Scientific, Marlborough, MA) placed in the left ventricle via femoral or radial arterial access. A fluid-filled transducer was balanced before the measurements with the zero level at the midaxillary line. Continuous pressure tracings were acquired over three consecutive respiratory cycles. LV pre-A pressure, which best reflects the mean LA pressure that is the focus of the ASE/EACVI algorithm, was determined at end-expiration (Figure 1) and considered elevated if >12 mm Hg.
Figure 1.
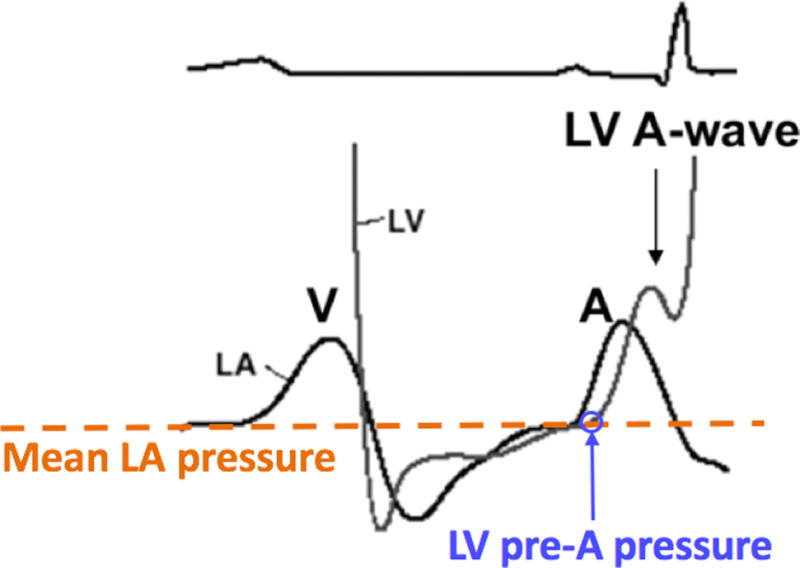
Hemodynamic tracings of LV (gray line) and LA (black line) pressures: LV pre-A pressure (blue arrow) most closely approximates mean LA pressure (dashed orange line), which is estimated by the ASE guidelines.
Statistical Analysis
Continuous variables are presented as mean ± SD and categorical variables as numbers and percentages. To determine the accuracy of the echocardiographic detection of elevated filling pressures, sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and overall accuracy were calculated from the numbers of true/false positive/negative classifications, using standard definitions. Contingency tables of normal and elevated pressure values by both echocardiographic algorithms and the invasive technique were created to depict intertechnique agreement, which was tested using κ statistics (GraphPad Software, La Jolla, CA). The calculated κ coefficients were judged as follows: 0 to 0.2, low; 0.21 to 0.4, moderate; 0.41 to 0.6, substantial; 0.61 to 0.8 good; and >0.8, excellent.
The significance of the difference in the frequency of discordant determinations between the two sets of guidelines and the invasive reference was tested using χ2 tests for ratios. Similarly, χ2 tests were used to evaluate the differences in the frequency of discordant determinations between patients with normal and reduced LVEF, as well as between male and female patients.
RESULTS
Invasive LV pre-A pressure was elevated in 40 of the 90 patients (44%) and normal in 50 (56%). Table 1 shows the characteristics of these two subgroups. Significant intergroup differences were found only in the prevalence of chronic kidney disease and aortic valve stenosis, which were more common in the patients with elevated filling pressures, and the number of urgent catheterizations and coronary interventions. Significant differences between groups were also found in two-dimensional and Doppler parameters of diastolic function. Notably, vital signs were not significantly different between the times when echocardiography and catheterization were performed.
Table 1.
Characteristics of the study patients in two groups defined by invasive LV filling pressure
| Variable | Normal invasive LV filling pressure (≤12 mm Hg) | Elevated invasive LV filling pressure (>12 mm Hg) | P |
|---|---|---|---|
| Number of patients | 50 | 40 | |
| Sex | |||
| Male | 24 | 17 | .75 |
| Female | 26 | 23 | .78 |
| Body surface area (m2) | 1.91 ± 0.39 | 1.96 ± 0.23 | .50 |
| Medical history | |||
| HTN | 37 | 37 | .48 |
| Diabetes | 23 | 29 | .19 |
| CAD | 17 | 19 | .40 |
| COPD | 7 | 5 | .86 |
| CKD (stage ≥3) or ESRD | 10 | 21 | .03 |
| AS, at least moderate | 0 | 8 | .003 |
| AI, at least moderate | 0 | 0 | – |
| Medications before catheterization* | |||
| Diuretic† | 20 | 27 | .15 |
| BB | 33 | 29 | .78 |
| ACE inhibitor | 20 | 19 | .65 |
| ARB | 7 | 11 | .20 |
| Spironolactone | 0 | 3 | .06 |
| Hydralazine | 3 | 3 | .79 |
| Nitrate‡ | 13 | 3 | .053 |
| Digoxin | 0 | 3 | .06 |
| Echocardiographic parameters | |||
| Reduced LVEF (<50%) | 15 | 19 | .26 |
| Normal LVEF (≥50%) | 35 | 21 | .41 |
| E/A ratio | 1.02 ± 0.34 | 1.43 ± 0.77 | .005 |
| E/e′ ratio | 11.1 ± 5.1 | 17.5 ± 8.1 | .001 |
| LAVi (ml/m2) | 33.2 ± 16.6 | 40.4 ± 14.7 | .05 |
| Catheterization | |||
| Inpatient | 33 | 24 | .78 |
| Outpatient | 17 | 16 | .69 |
| Urgent | 7 | 0 | .02 |
| Coronary stent placed | 30 | 8 | .01 |
| SBP at time of echocardiography (mm Hg) | 128 ± 20 | 132 ± 17 | |
| SBP at time of catheterization (mm Hg) | 133 ± 27 | 142 ± 25 | |
| P values (paired t test) | .38 | .11 | |
| HR at time of echocardiography (beats/min) | 72 ± 10 | 72 ± 15 | |
| HR at time of catheterization (beats/min) | 76 ± 13 | 80 ± 13 | |
| P values (paired t test) | .21 | .08 |
ACE, Angiotensin-converting enzyme; AI, aortic insufficiency; ARB, angiotensin II receptor blocker; AS, aortic stenosis; BB, b-blocker; CAD, coronary artery disease; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; ESRD, end-stage renal disease; HR, heart rate; HTN, hypertension; SBP, systolic blood pressure.
Data are expressed as numbers or as mean ± SD. See text for details.
Patients received these medications either prior to or during admission at some point before catheterization.
Diuretics include furosemide and hydrochlorothiazide.
Excludes short-acting sublingual nitroglycerin.
Using the 2016 guidelines, filling pressure was indeterminate in 9 of 90 patients (10%), of whom only one had elevated invasive filling pressure. Of the remaining 81 patients, the echocardiographic algorithm accurately estimated LV filling pressures in agreement with the invasive reference in 61 patients (75%; Figures 2 and 3), while in 20 (25%), the estimates were incorrect (Figures 4 and 5). Of the 20 patients in whom the echocardiographic algorithm disagreed with the reference, it underestimated elevated LV filling pressure in 12 (false negatives; Figure 4) and overestimated normal LV filling pressure in eight (false positives; Figure 5). The κ coefficient was 0.504, indicating substantial intertechnique agreement (Figure 6, top left). After excluding the nine indeterminate patients, the sensitivity of the detection of elevated LV filling pressure was 0.69, specificity was 0.81, PPV was 0.77, NPV was 0.74, and overall accuracy was 0.75 (Table 2).
Figure 2.
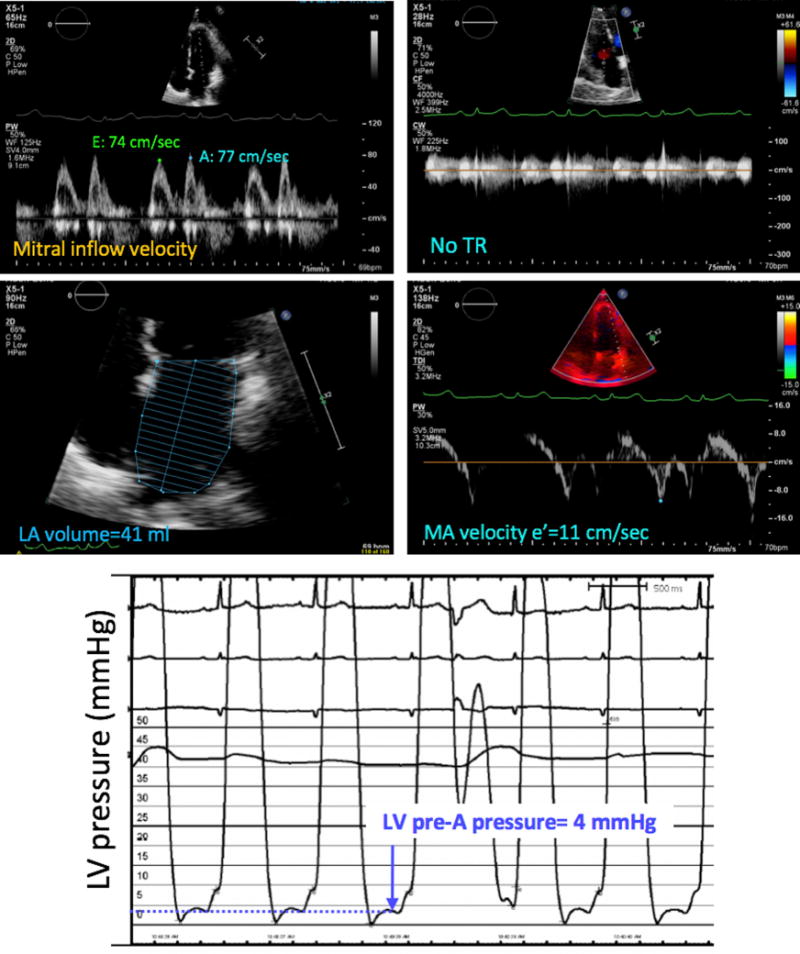
Example of a patient in whom the noninvasive estimate of LV filling pressure (top four panels) matched the invasive pressure measurement (bottom), which was normal. Measurements of individual echocardiographic parameters, including mitral inflow Doppler E and A velocities (top left), insufficient tricuspid regurgitation (TR, top right), LA volume (middle left) and tissue Doppler peak mitral annular (MA) velocity (middle right) also indicated normal LV filling pressure using the 2016 ASE algorithm.
Figure 3.
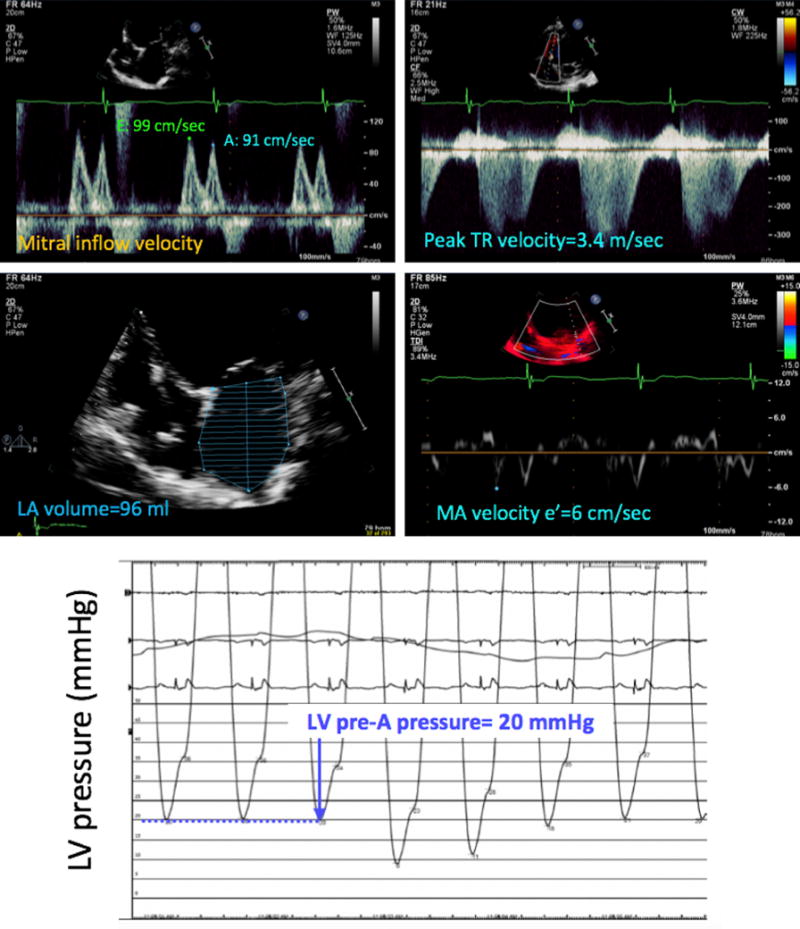
Example of a patient in whom the noninvasive estimate of LV filling pressure (top four panels) matched the invasive pressure measurement (bottom), which was elevated. Measurements of individual echocardiographic parameters, as described in Figure 2, also indicated elevated LV filling pressure using the 2016 ASE algorithm. MA, Mitral annular; Tr, tricuspid regurgitation.
Figure 4.
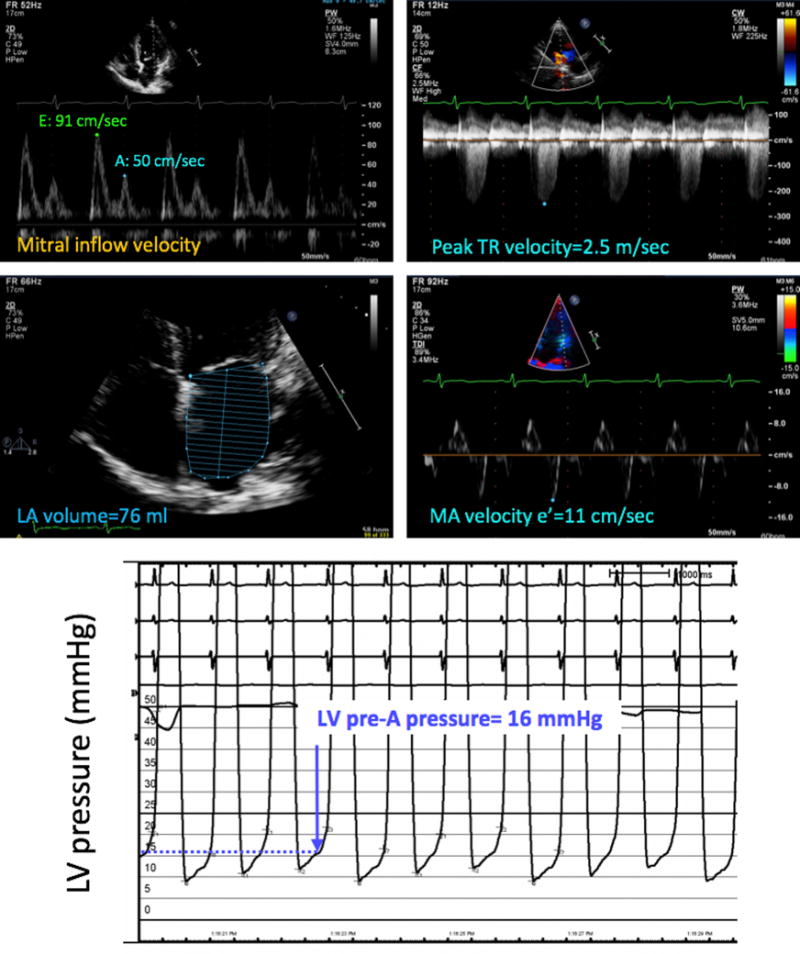
Example of a patient in whom the noninvasive estimate of LV filling pressure (top four panels) did not match the invasive pressure measurement (bottom), which was elevated. In contrast, measurements of individual echocardiographic parameters, as described in Figure 2, suggested normal LV filling pressure using the 2016 ASE algorithm. MA, Mitral annular; Tr, tricuspid regurgitation.
Figure 5.
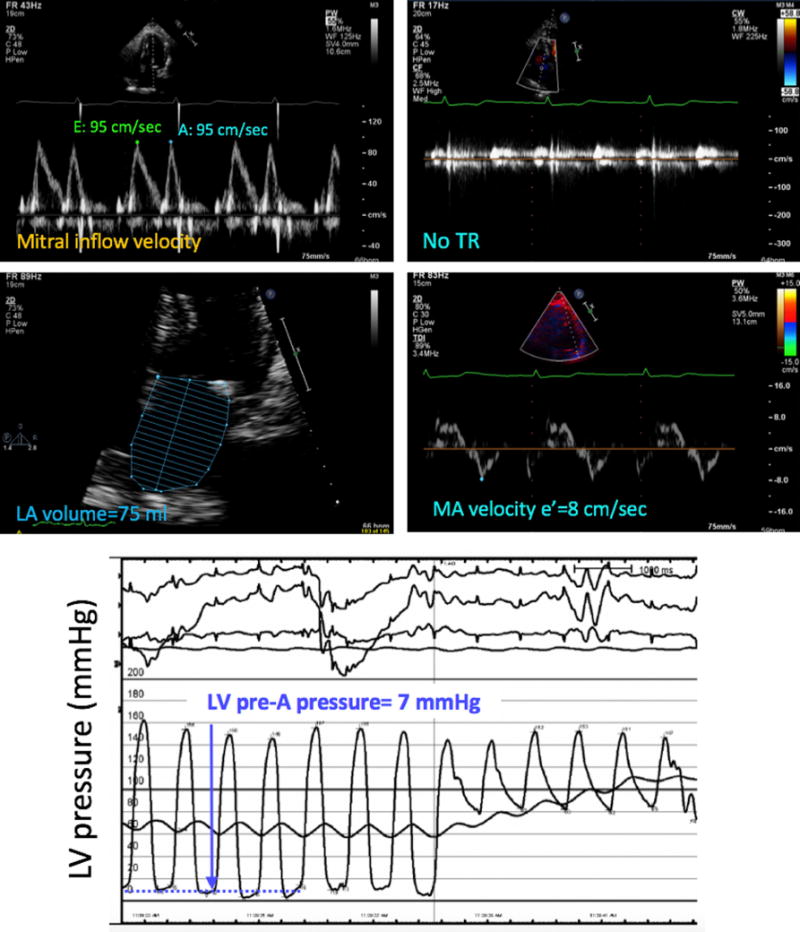
Example of a patient in whom the noninvasive estimate of LV filling pressure (top four panels) did not match the invasive pressure measurement (bottom), which was normal. In contrast, measurements of individual echocardiographic parameters, as described in Figure 2, suggested elevated LV filling pressure using the 2016 ASE algorithm. MA, Mitral annular; Tr, tricuspid regurgitation.
Figure 6.
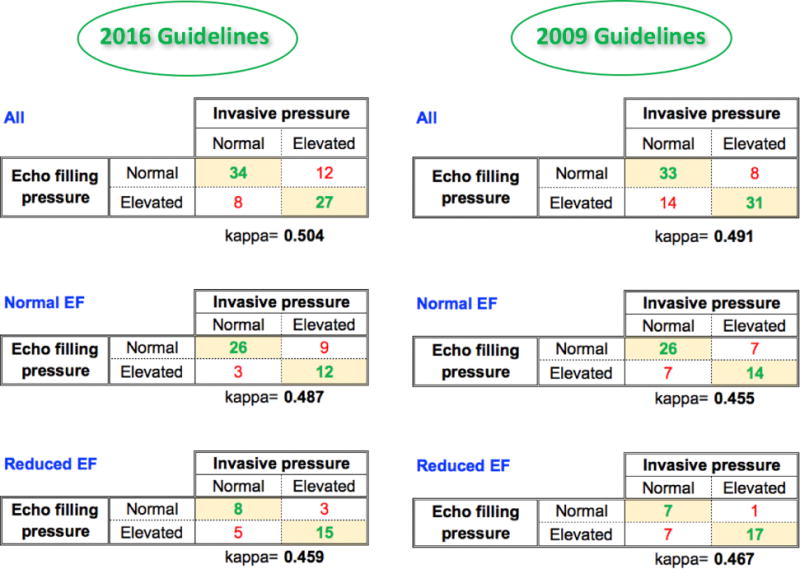
Contingency tables of agreement between noninvasive echocardiographic estimates of LV filling pressure using ASE guidelines and invasive pressure measurements, including κ coefficients. Comparisons include both the 2016 (left) and 2009 (right) guidelines for the entire study group (top), as well as for subgroups of patients with normal and reduced LVEF. See text for details. EF, Ejection fraction.
Table 2.
Sensitivity, specificity, positive and negative predictive values, and overall accuracy of noninvasive echocardiographic estimates of LV filling pressure using ASE guidelines against invasive pressure measurements
| Sensitivity | Specificity | PPV | NPV | Accuracy | |
|---|---|---|---|---|---|
| 2016 guidelines | 0.69 | 0.81 | 0.77 | 0.77 | 0.75 |
| 2009 guidelines | 0.79 | 0.70 | 0.69 | 0.80 | 0.74 |
Comparisons include both the 2016 and 2009 guidelines and showed similar overall accuracy. See text for details.
The agreement between the 2016 algorithm and invasive reference was similar in patients with normal (LVEF ≥ 50%) LV function (38 of 50 [76%]), compared with those with reduced function (23 of 31 [72%]; χ2 = 0.0047, P = .95; Figure 6, middle and bottom left). Similarly, the agreement was not significantly different in women (34 of 44 [77%]) and men (27 of 37 [73%]; χ 2 = 0.028, P = .87).
The performance of the individual parameters relative to the invasive LV filling pressure measurement was poor overall. Table 3 shows the results of a regression analysis of each parameter, revealing the highest correlation coefficient to be r = 0.40 for E/e′. Additional analysis of individual echocardiographic parameters as the sources of discordance between the 2016 guideline pressure estimates and the invasive measurements in patients who were either misclassified or indeterminate showed that the most common source of error was LAVi, followed by E/e′ ratio, tricuspid regurgitation velocity, and E/A ratio. Table 4 summarizes the distribution of the discordant measurements among the false negative, false positive, and indeterminate categories. Errors caused by LAVi were evenly distributed among these three categories, while those caused by the E/A and E/e′ ratios were mostly in the false negative category. The main reasons for the indeterminate classification were LAVi measurements and insufficient tricuspid regurgitation, resulting in an inability to measure maximum velocity. The latter was noted in 13 patients, in seven of whom having this measurement available might have swayed the results of the algorithm.
Table 3.
Relationships between the individual echocardiographic parameters used in the 2016 ASE algorithm and the invasively measured LV filling pressure
| Parameter | Invasive LV pre-A filling pressure | |
|---|---|---|
| r | P | |
| E/A ratio | 0.34 | .001 |
| E/e′ ratio | 0.40 | .0001 |
| LAVi | 0.23 | .029 |
| Peak TR velocity* | 0.29 | .030 |
TR, Tricuspid regurgitation.
See text for details.
Peak TR velocity was available in 54 of 90 patients (60%); this included similar numbers of patients with normal (29 of 54) versus elevated (25 of 54) LV filling pressure. Peak TR velocity could not be measured in 36 of 90 patients (40%), 78% of whom had normal LVEFs.
Table 4.
Distribution of the discordant results between the 2016 guideline pressure estimates and invasive pressure measurements: breakdown by false negative, false positive, and indeterminate categories
| Parameter | Total number | Number of false-negative* classifications | Number of false-positive† classifications | Number of indeterminate classifications |
|---|---|---|---|---|
| E/A ratio | 3 | 3 | 0 | 0 |
| E/e′ ratio | 18 | 10 | 6 | 2 |
| LAVi | 24 | 9 | 8 | 7 |
| Peak TR velocity | 10 | 4 | 6 | 0 |
| Insufficient TR | 13 | 7 | 1 | 5 |
TR, Tricuspid regurgitation.
See text for details.
Defined as normal filling pressure on echocardiography with elevated invasive pressure.
Defined as elevated filling pressure on echocardiography with normal invasive pressure.
When the 2009 guidelines were used to estimate filling pressures, 4 of 90 patients (4.4%) had discordant parameters that resulted in the algorithm suggesting both normal and elevated LA pressure. Accordingly, these four cases were deemed indeterminate, even though the flowchart does not specifically include such a category. Of note, only one of these four patients had elevated invasive pressure, and one was deemed indeterminate also by the 2016 algorithm. Of the remaining 86 patients, comparisons against the invasive reference resulted in agreement in 64 patients (74%), while in 22 (26%), the estimates were incorrect, including underestimated elevated LV filling pressure in eight (false negatives) and overestimated normal LV filling pressure in 14 (false positives). The κ coefficient was 0.491, indicating substantial intertechnique agreement (Figure 6, top right). After excluding the four indeterminate patients, the sensitivity of the detection of elevated LV filling pressure was 0.79, specificity was 0.70, PPV was 0.69, NPV was 0.80, and overall accuracy was 0.74 (Table 2). Thus, the overall accuracy was not different using the 2009 and 2016 guidelines.
The agreement between the 2009 algorithm and invasive reference was similar in patients with normal (LVEF ≥ 50%) LV function (40 of 54 [74%]), compared with those with reduced function (24 of 32 [75%]; χ2 = 0.001, P = .97; Figure 6, middle and bottom right).
Subanalysis with higher pre-A pressure cutoffs of 15 and 18 mm Hg showed that for both the 2009 and 2016 guidelines, the gradual increase in the pressure threshold resulted in a stepwise decrease in accuracy of classification. This loss of accuracy was more pronounced with the older guidelines.
DISCUSSION
Our study was designed to test the accuracy of the ASE/EACVI recommendations for noninvasive evaluation of LV filling pressures against the gold standard of invasive pressure measurements. The 2016 simplified algorithm was designed to address the difficulties raised by the complexity of the previous approach recommended by the 2009 guidelines. Although the 2009 algorithm included in excess of seven parameters, some of which are difficult and time-consuming to acquire, the 2016 algorithm focused on the four most feasible and reliable parameters. This new concept was based on the premise that these four parameters alone may provide as accurate an estimate of filling pressures as the previous, more comprehensive approach. Importantly, unlike the 2009 algorithm, which included two separate flowcharts to be used depending on the patient’s LVEF, the new algorithm uses a single flowchart, providing additional simplicity. Although the accuracy of both algorithms has been tested against an independent reference technique,6,8 and one study compared the two algorithms with an invasive reference of LV end-diastolic pressure,7 no study has compared side by side these two approaches for estimation of filling pressure against LV pre-A pressure in the same patient cohort to ensure that the accuracy was not compromised in favor of simplicity.
Our results confirmed the overall accuracy of the new algorithm recently reported by Andersen et al,6 when used by an independent laboratory with less specific expertise in the evaluation of LV diastolic function. We not only confirmed the algorithm’s independence from LVEF but also found no gender-dependent differences. This has not been previously reported, although there have been data suggesting that gender might influence normal values of diastolic parameters.9
We found the new algorithm to be indeed more user friendly and efficient than the previous one, both because it required fewer measurements and because these measurements were more straightforward to acquire. Importantly, this simplicity did not compromise the accuracy of the noninvasive determination of LV filling pressure, as reflected by the fact that the overall accuracy of the two approaches was similar. Interestingly, the subanalysis for different thresholds of elevated LV filling pressure revealed that the accuracy of the 2016 algorithm was less affected by the choice of the cutoff, which is an additional advantage.
Of note, the number of patients in whom filling pressure could not be determined was higher with the 2016 algorithm compared with the 2009 guidelines. However, the indeterminate classification is now an integral part of the 2016 algorithms, whereas the inability to classify a patient using 2009 algorithm could be viewed as a failure of the methodology. Analysis of individual echocardiographic parameters as the sources of discordance for the 2016 algorithm revealed that the majority of the indeterminate classifications stemmed from discrepant LAVi measurements or insufficient tricuspid regurgitation. Overall, LAVi was the most common source of discordance. Not surprisingly, individual parameters performed poorly relative to invasive LV filling pressure. This is consistent with previous studies,6-8 which demonstrated that individual variables were inaccurate for estimating LV filling pressure, but incorporating multiple parameters into algorithms resulted in better accuracy.
Additionally, the 2016 algorithm showed a different distribution of discordances with reference technique compared with the 2009 algorithm, which had a larger number of patients with overestimated filling pressures, resulting in lower specificity and PPV. However, the 2016 algorithm showed a higher number of false-negative determinations, resulting in improved specificity and PPV. These differences may have advantageous clinical implications, including increased physician confidence in echocardiographic diagnosis of elevated filling pressures.
Several details in our study design need to be clarified. First, our exclusion criteria simply mirrored those defined in the guidelines, and the reasons why these subpopulations may be difficult to assess are well characterized.4,5 Another methodologic detail is our choice of LV pressure measurement. The guidelines’ intended target of the noninvasive evaluation is mean LA pressure, because of its correlation with pulmonary capillary wedge pressures and thus with the congestive symptoms that define clinical heart failure. We used LV pre-A pressure because it best approximates mean LA pressure, and both are similarly related to early diastolic flow parameters.4,5 In fact, our study is the first to compare the diagnostic accuracy of both the 2009 and 2016 guidelines algorithms for the echocardiographic estimates of LV filling pressure in the same cohort against invasive pre-A pressure reference.
Although our use of LVEF of 50% as a cutoff for normal LV function differs slightly from the latest ASE/EACVI chamber quantification guidelines,10 this threshold was in line with the current 2016 diastolic function guidelines and the above previous studies.6,7 Similarly, in line with the report by Andersen et al,6 we used 12 mm Hg as the cutoff for elevated LV pre-A pressure.11
One of the limitations of our study is the relatively small number of patients and the single-center setting, which might have biased our findings to a certain degree. Additionally, echocardiographic imaging and invasive pressure measurements were not performed simultaneously. Theoretically, this may be a source of error. However, this was done by design to minimize interference with the work flow of the catheterization laboratory, and importantly, vital signs were not significantly different between the two time points, which minimizes the potential for this error.
CONCLUSIONS
In summary, the 2016 ASE/EACVI guidelines for estimation of left heart filling pressures are more user friendly and efficient than the 2009 guidelines and provide accurate estimates of LV filling pressure in the majority of patients compared with invasive measurements. The simplicity of the new algorithm did not compromise the diagnostic accuracy of the noninvasive evaluation of LV filling pressures and is likely to encourage its future incorporation into clinical decision making.
Abbreviations
- ASE
American Society of Echocardiography
- EACVI
European Association of Cardiovascular Imaging
- LA
Left atrial
- LAVi
Left atrial volume index
- LV
Left ventricular
- LVEF
Left ventricular ejection fraction
- NPV
Negative predictive value
- PPV
Positive predictive value
Footnotes
Alan D. Waggoner, MHS, FASE, served as guest editor for this report.
Conflicts of interest: None.
References
- 1.Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, et al. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6:606–19. doi: 10.1161/HHF.0b013e318291329a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mohan RC, Heywood JT, Small RS. Remote monitoring in heart failure: the current state. Curr Treat Options Cardiovasc Med. 2017;19:22. doi: 10.1007/s11936-017-0519-5. [DOI] [PubMed] [Google Scholar]
- 3.Hauptman PJ. Disease modification in acute decompensated heart failure. N Engl J Med. 2017;376:1987–8. doi: 10.1056/NEJMe1613708. [DOI] [PubMed] [Google Scholar]
- 4.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22:107–33. doi: 10.1016/j.echo.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 5.Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, III, Dokainish H, Edvardsen T, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29:277–314. doi: 10.1016/j.echo.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 6.Andersen OS, Smiseth OA, Dokainish H, Abudiab MM, Schutt RC, Kumar A, et al. Estimating left ventricular filling pressure by echocardiography. J Am Coll Cardiol. 2017;69:1937–48. doi: 10.1016/j.jacc.2017.01.058. [DOI] [PubMed] [Google Scholar]
- 7.Lancellotti P, Galderisi M, Edvardsen T, Donal E, Goliasch G, Cardim N, et al. Echo-Doppler estimation of left ventricular filling pressure: results of the multicentre EACVI Euro-Filling study. Eur Heart J Cardiovasc Imaging. 2017 doi: 10.1093/ehjci/jex067. In press. [DOI] [PubMed] [Google Scholar]
- 8.Dokainish H, Nguyen JS, Bobek J, Goswami R, Lakkis NM. Assessment of the American Society of Echocardiography-European Association of Echocardiography guidelines for diastolic function in patients with depressed ejection fraction: an echocardiographic and invasive haemodynamic study. Eur J Echocardiogr. 2011;12:857–64. doi: 10.1093/ejechocard/jer157. [DOI] [PubMed] [Google Scholar]
- 9.Schirmer H, Lunde P, Rasmussen K. Mitral flow derived Doppler indices of left ventricular diastolic function in a general population; the Tromso study. Eur Heart J. 2000;21:1376–86. doi: 10.1053/euhj.1999.2036. [DOI] [PubMed] [Google Scholar]
- 10.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echo-cardiogr. 2015;28:1–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 11.Paulus WJ, Tschope C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, et al. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007;28:2539–50. doi: 10.1093/eurheartj/ehm037. [DOI] [PubMed] [Google Scholar]


