Abstract
Dry eye is a common, multifactorial disease currently diagnosed by a combination of symptoms and signs. Its epidemiology and clinical presentation have many similarities with neuropathic pain outside the eye. This review highlights the similarities between dry eye and neuropathic pain, focusing on clinical features, somatosensory function, and underlying pathophysiology. Implications of these similarities on the diagnosis and treatment of dry eye are discussed.
Keywords: Dry eye, Genetics, Inflammation, Nerve sensitization, Neuropathic pain, Treatment
1. Introduction
Dry eye (DE) is a diagnosis that encompasses a wide variety of clinical manifestations that include ocular sensations of dryness, discomfort, pain, and ocular surface disturbances such as decreased tear production and increased tear evaporation.1, 2 Other symptoms that frequently accompany dryness include fluctuating and/or blurry vision and ocular dysesthesias, often described as “burning,” “tender,” and “aching.”3, 4 Prior to 1990, DE was mostly discussed in the context of Sjögren’s syndrome (SS) and other autoimmune diseases. This changed after population-based studies in various countries, including the US,5, 6 Australia,7 Japan,8 Indonesia,9 and China,10 found that DE symptoms were a frequent finding in the general population. Although some studies showed that ocular surface abnormalities, such as fast tear film breakup time, low Schirmer score, and meibomian gland abnormalities, often accompanied DE symptoms,10 subsequent studies demonstrated that for many patients, DE symptoms were not related to these DE signs.11, 12
Putting this information in context, it becomes apparent that DE is a complex, multifactorial, and heterogeneous disease that may be better understood and treated if grouped into various sub-types. For example, the presentation and underlying pathophysiology of SS and graft-versus-host-associated DE is very different from that in patients whose DE symptoms exist with minimal signs of ocular surface disease. In fact, the latter sub-type of DE has much in common with neuropathic pain (NP) conditions outside the eye (Table 1). This review highlights the similarities between NP outside the eye with some forms of DE and focus on implications with regards to the further diagnosis and treatment of these DE subtypes.
Table 1.
Common features between dry eye and neuropathic pain
| Epidemiology | Both are common conditions, affecting a large proportion of the older population. More common in females than males. |
| Shared clinical features | Frequent disconnect between symptoms and signs; spontaneous dysesthesias and evoked pain common to both entities, even in the absence of ongoing nerve damage. |
| Shared co-morbidities | Dry eye and other chronic neuropathic pain conditions often co-exist, including co-morbid conditions associated with pain amplification and psychological distress (e.g., pain, fatigue, sleep disorders, depression, anxiety, etc.). |
| Abnormal somatosensory testing | Hypoesthesia, hypo- or hyperalgesia, or allodynia commonly seen on somatosensory testing. Dynamic testing has revealed increased wind up, a surrogate metric of central sensitization in both conditions. |
| Nerve injury implicated | Anatomical abnormalities observed in corneal nerves consistent with nerve injury (confocal microscopy), similar to neuropathic pain elsewhere in the body (magnetic resonance imaging) |
| Shared pathophysiology | Somatosensory nerve sensitization, inflammation and supporting cell abnormalities are common to both entities. |
| Heritability | Both conditions appear to be heritable and shared genetic factors have been demonstrated in large twin studies. |
| Overlapping therapeutic response | Nerve modulators, such as calcium channel alpha 2 delta ligands (e.g., gabapentinoids), improve symptoms in some individuals. |
2. Overview of dry eye
2.1. Epidemiology
DE symptoms are common complaints in the general population. They are more frequently found in women than in men,5 and with increasing age.13 In the US, a population-based study out of Beaver Dam, Wisconsin, found that 14.4% of individuals between the ages of 48 and 91 years reported DE symptoms.5 A similar frequency of symptoms was found in Salisbury, Maryland, with 14.6% of the population reporting one or more DE symptom often or all the time.6 These numbers were even higher in Asia, where 34% of participants in a Taiwanese study10 and 28% of participants in an Indonesian study9 reported one or more DE symptoms often or all of the time. Ocular surface abnormalities are also common in the general population. In Melbourne, Australia, 11% of individuals had rose bengal staining scores greater than 3, 16% had Schirmer scores less than 8 mm, and 9% had tear film breakup times less than 8 seconds.7
Hospital-based studies have found even higher frequencies of both DE symptoms and signs.12, 14 In one study of an older (mean age: 69), predominantly male veteran population, 91% of individuals had at least one ocular surface abnormality on standardized testing.11 Overall, evaporative DE is more common than aqueous tear-deficient DE.11, 15 In fact, in Asia, over 60% of the population had at least one eyelid margin abnormality, including abnormal vascularity, plugging, collarettes, gland dropout, and/or abnormal meibum quality.16, 17 Beyond meibomian gland health, anatomic abnormalities such as eyelid laxity and conjuctivochalasis are commonly seen in older individuals and have been found to be associated with DE symptoms.18–22
2.2. Clinical manifestations
DE symptoms are varied and include complaints of dysesthesias (unpleasant abnormal sensations) which can be described as “dryness,” “burning,” “aching,” “tenderness,” “soreness,” etc.4 These sensations can occur spontaneously or can be evoked by wind, cold, light, air pollution, and low humidity.4, 23 DE symptoms also include visual complaints, often described as blurry vision, poor visual quality, or fluctuating vision. Some patients also report tearing. These constellations of symptoms have been found to impact quality of life, reducing both physical and mental functioning.24 In fact, utility assessment has estimated that severe DE symptoms are as debilitating as severe angina.25 Ocular signs of DE are also varied, and, therefore, a comprehensive assessment of the ocular surface is typically performed when evaluating a patient with DE symptoms. This includes assessments of eyelid function and anatomy (apposition, laxity, vascularity), meibomian gland parameters (atrophy, meibum quality), tear metrics (production, evaporation), and ocular surface disruption (with the use of various stains such as fluorescein, rose bengal, or lissamine green). More recently, point-of-care tests can measure tear osmolarity (TearLab, San Diego, CA), tear lactoferrin (Advanced Tear Diagnostics, Birmingham, AL), and ocular surface matrix metalloproteinase 9 (Inflammadry, RPS, Tampa, FL). Interestingly, just as with traditional DE signs, these newer diagnostics also often correlate poorly with patient complaints suggesting multiple subtypes of DE.26, 27
3. Overview of chronic neuropathic pain (NP) outside the eye
3.1. Definition
Pain, as defined by the International Association for the Study of Pain (IASP), is “an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage.”28 Pain disorders are broadly categorized into two groups: nociceptive pain and neuropathic pain. Nociceptive pain is defined as a pain that arises from actual or threatened damage to non-neural tissue and is due to the activation of nociceptors.28 It is usually transient in nature. Outside the eye, examples of nociceptive pain are pain in the setting of acute trauma or surgery, or tissue inflammation.
NP is defined as pain caused by a lesion or disease of the somatosensory nervous system.28 Several chronic pain entities are considered to be neuropathic in nature. Some of these have a known underlying pathology, such as diabetic neuropathy, post-herpetic neuralgia, and some cases of chronic post-operative pain that involve injury or trauma to nerves. Others have set clinical criteria, but evidence for an underlying pathophysiology is lacking. These include syndromes such as atypical facial pain (including some cases of temporomandibular joint disorders), chronic fatigue, irritable bowel, interstitial cystitis, vulvodynia, burning mouth, and fibromyalgia.29, 30 Interestingly, even though these disorders manifest in different parts of the body, they are often found to co-exist and are likely tied to each other by a unifying pathophysiology linked to neuropathic mechanisms, i.e., peripheral and/or central sensitization.
3.2. Epidemiology
Chronic pain is a common complaint in the United States, with over 100 million Americans affected at a cost of over $500 billion in health care and lost productivity.29 Chronic pain is estimated to affect 11.2% or 25.3 million adults within the United States, as defined by the National Health Interview Survey as daily pain experienced over the past 3 months.31 Within pain, the American Academy of Pain Management reports that back pain is most common (27%), followed by severe headache or migraine pain (15%), neck pain (15%), and facial ache or pain (4%).32, 33
3.3. Clinical manifestations
The diagnosis of NP is often associated with a demonstrable lesion (by history, on imaging, biopsy, or neurophysiology [nerve conduction]); or a disease that satisfies established neurological criteria (stroke, diabetes, genetic abnormality).28 In many cases, however, the term NP is used as a clinical description. Several clinical features are more common in patients with a diagnosis of neuropathic as opposed to nociceptive pain.34 These include using specific terms to describe the quality of pain, such as “burning,” “shooting,” “pins and needles,” and “itching.” The pain is typically spontaneous (no obvious inciting event) and may be continuous or episodic (the latter often being described as “stabbing,” “shooting,” or “electric shock”). Evoked pain is also a common feature in patients with neuropathic pain,34 both over the initial site of injury and also in the surrounding areas, especially with expansion of the receptive field believed to be due to central sensitization. Pain can be evoked by a stimulus (such as light touch) that normally does not cause pain (i.e., allodynia), or exaggerated pain can be evoked by a stimulus (such as noxious pin prick) that is in the painful range but is reported at a higher intensity than is normally expected (i.e., hyperalgesia). These symptoms and signs may occur in the setting of an otherwise normal clinical examination.35
4. Similarities between some subtypes of dry eye and neuropathic pain
Many patients report that their ocular dysesthesias are painful.4 Others do not consider sensations of dryness to be painful, but use words describing “discomfort.” Irrespective, many similarities exist between DE symptoms and NP outside the eye with respect to clinical characteristics, findings on somatosensory testing, and underlying biologic mechanisms.
4.1. Clinical similarities
4.1.1. Similar descriptors are used in dry eye and neuropathic pain
Most DE questionnaires combine questions about pain with other DE symptoms to arrive at a total symptom score.36 Therefore, it is often difficult to parse out the frequency of ocular pain in those with symptoms of dryness. However, several studies have focused specifically on this point. For example, we asked veterans to rate the intensity of their average and worst eye pain over a 1-week recall period using a numerical rating scale (NRS) anchored at “0” for “no pain sensation” and at “10” for “the most intense eye pain imaginable.” This type of 0–10 NRS has been validated as a measure of pain intensity across multiple populations37–41 and has been recommended for use as the primary outcome metric in clinical trials for chronic pain.42 In a cohort of 154 veterans with mild or greater DE symptoms (dry eye questionnaire 5 [DEQ5] score≥6), and based on previously defined cut-offs in diabetic peripheral neuropathy43, 11% (n = 17) of subjects reported no pain (NRS = 0) on average over a 1-week recall period, 36% (n = 56) reported mild pain (NRS 1 – 3), 34% (n = 52) reported moderate pain (NRS 4 – 6), and 19% (n = 29) reported severe pain (NRS 7 – 10).4 A similar pattern was seen for worst pain over a 1-week recall period. In a different population, Vehof et al. evaluated ocular pain in white female volunteers from the Twins UK Adult Registry at St Thomas’ Hospital, London. The group used the sum of the first three questions of the ocular surface disease index (OSDI) to investigate ocular pain (i.e., 1: eyes that are sensitive to light, 2: eyes that feel gritty, and 3: painful or sore eyes). A sum score of 3 or higher was regarded as having DE pain symptoms. Of 689 women who filled out the OSDI, 118 (17.1%) had DE pain symptoms.44
Other studies have focused on ocular pain symptoms in general (non-hospital based) populations. For example, as part of the Salisbury Eye Evaluation, a questionnaire was administered to residents of Salisbury, Maryland, that asked about the frequency (rarely, sometimes, often, or all the time) of six symptoms (dry, gritty or sandy, burn, red, crust, stick shut).6 Of 2482 volunteers, approximately 33% reported grittiness and 25% reported burning sometimes, often, or all the time. In the Blue Mountains Eye Study, Australian participants answered questions about the severity of dryness, grittiness, itchiness, and discomfort over a 12-month recall period.45 Of 1172 volunteers, mild, moderate, or severe grittiness was reported by approximately 25% and discomfort by approximately 30%. Similar to DE symptoms in general, ocular pain symptoms were more severe in women than in men.45 In another Australian study from Melborne, mild, moderate, or severe ocular discomfort was reported by 25%, and photophobia by 50%, of the approximately 890 participants.7
We have also used validated pain questionnaires to further characterize the quality and severity of the ocular pain reported by individuals with other DE symptoms. For example, the short-form McGill Pain Questionnaire (sf-MPQ)46 characterizes the severity (mild, moderate, severe) and quality of pain using 15 descriptors (throbbing, shooting, stabbing, sharp, cramping, gnawing, hot-burning, aching, heavy, tender, splitting, tiring-exhausting, sickening, fearful, and/or punishing-cruel). We found that 82% of individuals with DE symptoms endorsed one or more of these qualities when describing their eye symptoms.4 Descriptors that were most common included “tiring-exhausting” (56%), “aching” (56%), and “hot burning” (53%).4 In fact, the distribution and frequency of ocular pain descriptors used by our population with DE symptoms was similar to that of a population with central NP.47 We also applied a modified version of the Neuropathic Pain Symptom Inventory34, which we termed NPSI-Eye, to the study of ocular pain.4 The NPSI has been used in a number of pain patient populations to assess the severity of NP-related complaints, including the quality of spontaneous pain and the severity of evoked pain.34, 48 In our modified version, the spontaneous pain qualities were kept the same, but the pain-evoking stimuli were changed from brushing, pressure, or contact with cold on the skin to light, change in temperature, or wind on the eye. These environmental elements were selected because they are well-known triggers of ocular dysesthesias, appearing in other DE questionnaires, such as the OSDI.36 Spontaneous burning pain was a frequent complaint in our cohort with DE symptoms,4 as it was in individuals with chronic NP conditions.49 Similar to other studies7, 44, a subset of our patients also reported evoked pain to wind and light50, consistent with a subtype of DE with NP. Ocular pain assessment survey (OPAS) is another validated eye pain questionnaire that has been used in the clinic to detect and follow patients with neuropathic ocular pain (NOP).51 Table 2 summarizes features that suggest a neuropathic component to DE symptoms.
Table 2.
Features suggestive of a neuropathic component to dry eye symptoms
| Discordance between DE symptoms and signs, with symptoms outweighing signs145 |
| Non-response to therapies that target the ocular surface and tears57 |
| Presence of chronic overlapping pain conditions58, 191, depression, anxiety65 |
| Specific descriptors including spontaneous burning pain, sensitivity to wind and light4, 66 |
| Persistent ocular pain after topical anesthesia (e.g. proparacaine)125 |
DE=dry eye
4.1.2. The natural history of dry eye and neuropathic pain are similar
NP tends to be chronic and disconnected in time from peripheral tissue abnormalities, as maladaptive changes in peripheral and central somatosensory nerves can persist after the precipitating injury (e.g., surgery, viral infection, trauma, metabolic disease) has resolved.35 Similarly, discordance between ocular symptoms and signs is frequently seen in DE.52 For example, similar to observations on persistent post-surgical pain, chronic DE symptoms in the setting of corneal nerve injury despite a normal ocular surface exam are common after refractive surgery, particularly after laser-assisted in situ keratomileusis (LASIK).53 Like post-viral pain, chronic ocular pain and headache are common in patients with a history of herpes zoster ophthalmicus long after the eye findings have resolved.54 We found that patients with idiopathic DE symptoms who also report NOP complaints, including burning and evoked pain to wind and light, have a more severe and chronic DE symptom course52,55 and respond less well to topical therapy with artificial tears.56
4.1.3. DE symptoms are co-morbid with other chronic neuropathic pain conditions
DE symptoms are often found in those with other chronic pain conditions, both in male57 and female58 populations. DE symptoms were more severe in those with DE and at least one chronic non-ocular pain condition (mean OSDI score 46) compared to those with DE and no other chronic pain conditions (mean OSDI score 34; P-value<0.0005).59 Interestingly, ocular signs were similar or less severe in those with one or more co-morbid chronic pain condition compared to those without this association. In fact, the presence of a chronic non-ocular pain condition was the most significant predictor of discordance between DE symptoms and signs, with symptoms greatly outnumbering signs.52 This is similar to what is seen in NP outside the eye where pain complaints frequently present without peripheral tissue abnormalities.35
4.1.4. Dry eye and neuropathic pain symptoms are co-morbid with other conditions impacting pain amplification and psychological distress (e.g., depression, anxiety, fatigue and sleep disturbance)
As is common in other chronic NP conditions, several groups have found that depression and anxiety are frequently co-morbid in individuals with DE symptoms but not with DE signs.60–63 In fact, depression was more closely associated with DE symptoms in those with normal tear production.62 In a Veterans Affairs cohort, we found that DE symptoms (measured by the DEQ5 and OSDI) aligned more closely with non-ocular pain and post-traumatic stress disorder (PTSD) scores than DE signs, accounting for 36% and 40% of variability in questionnaire scores, respectively.64 In fact, this relationship was strongest in individuals with NOP complaints.65 In a similar manner, insomnia has been found to be co-morbid with both DE symptoms and NP.66,67
4.2. Similarities in somatosensory abnormalities observed for dry eye and neuropathic pain
An important feature of NP is altered somatosensory function within the area of spontaneous pain reported. Evidence of hypoesthesia (i.e., reduced sensitivity to mechanical or thermal stimuli) in a neuroanatomically plausible area based on the location of suspected neural lesion or disease, is necessary for “probable” or “definite” NP 68 and hyperalgesia (i.e., increased pain from a stimulus that normally provokes pain) is also often reported. This scenario is commonly seen in diabetes, where patients report spontaneous painful sensations in the lower extremities but have reduced sensation on exam. An analogous situation is seen in the eye after LASIK-associated nerve injury where hypoesthesia and DE symptoms are common findings after the procedure.69
Another salient feature of NP can be secondary hyperalgesia (expansion of the receptive field believed to be due to central sensitization), which is an increase in evoked pain sensitivity in the uninjured region surrounding the site of direct injury.70 In the eye, a comparable scenario may be seen by the co-existence of DE, migraines, and other pain conditions in the face or head.58,71,72
4.2.1. Patients with dry eye and neuropathic pain both have altered nerve anatomy
In vivo confocal microscopy (IVCM) allows the visualization of sub-basal corneal nerves. Morphological differences in sub-basal corneal nerves have been found in individuals with SS and non-SS-associated DE compared to controls. While most studies reported that patients with DE had reduced nerve densities73–76 compared to controls, others found no differences77–79 or increased nerve densities.80 Overall, most studies found lower nerve density in SS patients compared to non-SS DE patients and controls.81 Other morphologic abnormalities have also been described in DE, including nerve spouting and nerve growth cone-like structures,78,79 increased thickness,79 tortuosity,80,82–84 and beading.82,83 Interestingly, while some studies reported a positive correlation between nerve density and corneal sensitivity,73,82 others did not find a relationship between corneal nerve structure and function.77,79 As in NP, such discrepancies are not uncommon. In fact, diagnostic testing often yields varying, inconclusive, and even inconsistent data when patients suspected of having pain due to a neuropathic etiology are evaluated.28 More recently, IVCM technology was applied in individuals with suspected NOP. Sub-basal nerve parameters were found to be decreased in 6 patients with corneal allodynia associated with a number of conditions (dry eye, recurrent corneal erosion syndrome, exposure to ultraviolet radiation, and Accutane use) compared to 15 healthy controls.85
Individuals with other types of chronic pain thought to be neuropathic in origin also have structural abnormalities on corneal confocal microscopy examination. For example, individuals with fibromyalgia were found to have significantly decreased nerve fiber length, density and thickness, and branching of corneal nerves as compared to age- and gender-matched controls.86,87 Corneal nerve fiber density was also significantly lower in patients with migraine headache compared to controls.88 A point to consider when interpreting these data, however, is that patients with migraine and fibromyalgia frequently report DE symptoms that have neuropathic features (discordance between symptoms and signs of dry eye, non-response to therapies that target the ocular surface, descriptors of spontaneous burning pain, sensitivity to light and wind).88
Another example of nerve abnormalities seen in individuals with NP comes from brain magnetic resonance imaging (MRI) studies. In humans with painful trigeminal neuropathy, imaging revealed discrete alterations in the anatomy of primary synapses in the spinal trigeminal subnucleus caudalis and decreases in regional and higher brain center (thalamus, insula and cortex) gray matter volume.89,91 The same authors found that, in contrast to the anatomical changes at the level of the synapses, at the spinal trigeminal subnucleus the anatomy of primary afferents and efferent fibers was unaffected,89 except for changes in trigeminal nerve volume (decreased in neuralgia, increased in neuropathy). In comparison, no changes in trigeminal nerve volume were found in patients with non-NP in the face.91
4.2.2. Patients with dry eye and neuropathic pain both have abnormalities on quantitative sensory testing
Individuals with NP often have lower pain thresholds and experience pain at a greater intensity than those without NP.92,93 These alterations in pain perception are usually widespread and not limited to a specific area of the body,29 indicating a centralized somatosensory processing disorder. Patients with DE have likewise been found to have local (corneal) and systemic (forearm) sensory alterations.44,94,95 The Belmonte aesthesiometer has been used to evaluate corneal sensitivity to mechanical, chemical and thermal stimuli.96 Some studies found reduced corneal sensitivity to mechanical, chemical, and thermal stimuli in those with DE compared to controls,73,97 while others found increased sensitivity to mechanical stimuli.79,94,98,99 As above, such discrepancies are common when patients with NP are evaluated, as both hypoesthesia and hyperesthesia can be clinical features of the disease.
Individuals with DE have also been found to have increased pain sensitivity outside the eye. In a study in British women, subjects with eye pain had decreased heat pain tolerance over the forearm compared to those without eye pain.44 In a primarily male veteran population, several pain sensitivity metrics, including cold pain thresholds, ratings of pain intensity to threshold and suprathreshold thermal noxious stimuli, and temporal summation of noxious heat, correlated with measures of both ocular pain and severity of DE symptoms.95 In addition, using the Pain Sensitivity Questionnaire, Li et al. demonstrated that higher pain sensitivity scores significantly associated with increased ocular dryness and discomfort scores (quantified with the OSDI).100 Taken together, this suggests that “dry eye” is likely not a disorder limited only to the ocular surface but can also include both local and widespread somatosensory dysfunction.
5. Similarities underlying mechanisms in dry eye and neuropathic pain
5.1. Inflammation and neurogenic inflammation (interactions between somatosensory neurons and supporting cells)
5.1.1. Supporting cells
Nociceptors are in constant contact and interaction with supporting cells, which in the periphery include Schwann cells and keratinocytes. These supporting cells respond to their environment and, after injury, release a variety of molecules, including adenosine 5′-triphosphate (ATP), nerve growth factor (NGF), prostaglandin (PG) E2, and matrix metalloproteinase (MMP) 9.101 Centrally, glial cells, such as microglia, astrocytes, and oligodendrocytes, interact with and modulate central neurons. As in the periphery, microglia rapidly respond to peripheral nerve injury101 and produce a variety of pro- inflammatory molecules such as tumor necrosis factor (TNF)α, interleukin (IL)1β, and PGE2.35 Astrocytes too activate after nerve injury, with a subsequent loss in the ability to regulate levels of extracellular potassium and glutamate (an excitatory neurotransmitter) with resulting neuronal hyperexcitability.101 In the eye, corneal epithelium plays a similar role to Schwann cells and keratinocytes.102 For example, corneal epithelial cells are important in recycling nerve endings under normal conditions. As such, it is likely that injured or altered corneal epithelium may contribute to pathophysiologic changes in nerves in DE, in a fashion analogous to supporting cell alterations in non-ocular NP.
5.1.2. Inflammation
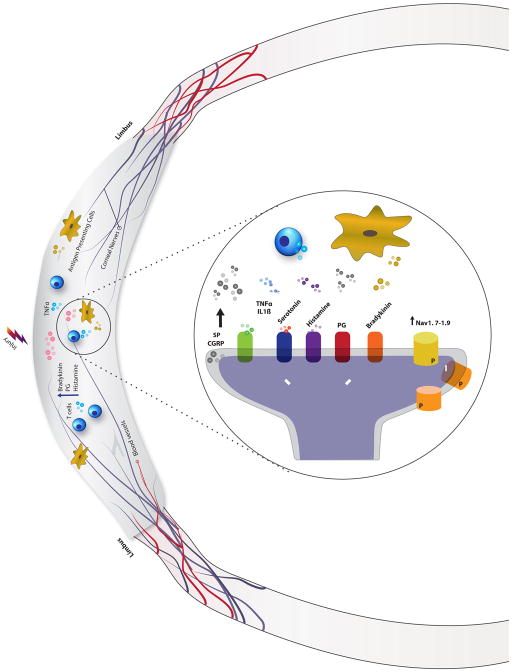
Neurogenic inflammation arises from the local release of inflammatory mediators from damaged epithelial cells (ATP, histamine, prostaglandins, bradykinin) and peripheral neurons (Substance P [SP], calcitonin gene-related peptide [CGRP], neurokinin A) as a result of injury.103 These mediators recruit inflammatory cells, which add to the pro-inflammatory environment by secreting TNFα and IL1β. Peripheral neuron terminals contain receptors for these mediators, and through protein kinase pathways, ion channels become phosphorylated, enhancing their function, and new ion channels are made via transcription and translation (Fig. 1). Electrophysiologically, these changes manifest as increased responsiveness to normal input and/or recruitment of a response to normally subthreshold inputs, both hallmarks of sensitization.28
Fig. 1.
Mechanisms of peripheral sensitization. Injury to epithelial cells results in the release of numerous inflammatory mediators such as bradykinin, prostaglandins (PG), serotonin, and histamine, to name a few. Peripheral nerve terminals have receptors that recognize these inflammatory molecules (5HT for serotonin, H1 for histamine, EP for PG, B2/B1 for bradykinin). Their activation triggers the release of substance P (SP) and calcitonin gene-related peptide (CGRP). These mediators co-activate resident antigen presenting cells and recruit additional immune system cells to the site of injury. T cells and macrophages then secrete additional inflammatory cytokines (tumor necrosis factor alpha (TNFα) and Interleukin 1 (IL1)) that changes the function of peripheral nociceptors via protein kinase cascades. Mechanistically, this is largely prompted by the changes in ion channel function (through phosphorylation) and expression of new channels. These include specific ion channels such as voltage gated sodium channels (Nav1.7–1.9) and non-specific cation channels (transient receptor potential cation channel subfamily V member 1 (TRPV1).
With regard to DE, many mediators involved in neurogenic inflammation, such as TNFα, IL1, IL6,104,105 PGE2,106,107 MMP-9,108 and NGF,109 are elevated in tears of patients with DE. Furthermore, T cells are detected in the conjunctivae of DE patients.110 A pro-inflammatory environment has been found to sensitize peripheral corneal nociceptors in a fashion similar to that seen elsewhere in the body.111
5.2. Evidence of peripheral sensitization
The cornea is innervated by the primary sensory neurons coming off the ophthalmic branch of the trigeminal nerve. Corneal nociceptors are predominantly unmyelinated C fibers, with myelinated Aδ fibers also present to a lesser extent.112 Polymodal nociceptors are the most frequent sub-type (~70%) and sense chemical, thermal, and endogenous inflammatory mediators through interactions with transient receptor potential cation channel subfamily V member 1 (TRPV1, a non-specific cation channel) on their terminal membranes.111
Aδ mechanoreceptors (~20%) sense mechanical stimuli, while C-fiber cold thermoreceptors (~10%) sense temperature changes through another non-specific cation channel, TRPM8.111,113 Corneal nociceptors’ terminal nerve endings interact with the tear film and are thus susceptible to repeated damage under conditions of inflammation or repetitive environmental injury. Recurrent injury is hypothesized to lead to maladaptive neuronal plasticity and the development of NOP. Prolonged and intense inflammation and ongoing input of nociception may lead to sensitization of the peripheral and central somatosensory pathways.
Several changes occur with peripheral sensitization, including modifications in gene expression due to alterations in translation, or post-translational modification of signaling molecules, including kinases and ion channels onto the nerve membrane.114 On a molecular level, modifications to TRPV1 lead to reduced firing threshold and enhance neuronal excitability. Neuroplastic changes include the emergence of ectopic firing of nerve fibers, spontaneous generation of action potentials, aberrantly enhanced conduction of signals, increased excitatory neurotransmitter release at presynaptic terminals, as well as nerve sprouting and rewiring, and conversion of non-nociceptive sensory afferent into fibers exhibiting nociceptive phenotype. Similar correlates have been found in the cornea. For example, corneal polymodal nociceptors were found to sensitize, with increased transduction and conduction, after injury and when exposed to an inflammatory milieu.111,115 In a similar manner, lacrimal gland transection was found to sensitize cold thermal receptors with a shift in the cooling threshold to warmer values, and with an increased peak frequency of discharge to cooling.116 Fig. 1 depicts potential peripheral mechanisms that drive chronic DE symptoms and ocular pain.
5.3. Evidence of central sensitization
The central neuronal changes that manifest in enhanced synaptic transmission of pain signals via reduced post-synaptic activation thresholds and increased post-synaptic excitability generate further abnormal signal amplification (or even spontaneous pain), and are similar to those seen in peripheral sensitization. These changes also include altered gene expression and post-translational modifications of signaling cascades (such as those mediated via cytosolic calcium and the protein kinase C), modifications in the opening of ion channels, generation of inflammatory mediators (prostaglandins, nitric oxide) and anatomic reorganization in the dorsal horns of the spinal cord or higher centers.114,117
Overstimulation and enhanced opening of N-methyl-D-aspartate (NMDA) glutamate receptor, driven by the prolonged and intense input of nociceptive signals and release of excitatory neurotransmitters such as glutamate and SP, play an important role in the generation and maintenance of central sensitization. Microglia may further activate the NMDA receptor by producing quinolinic acid (QA) and releasing pro-inflammatory cytokines. Beyond its effect on the NMDA receptor, QA also inhibits the function of astrocytes, which would normally take up excess glutamate, leading to a build-up of this excitatory mediator.
The ascending pathways that convey pain are modulated by descending pathways that can mediate analgesia when inhibitory, creating a negative feedback loop. Attenuation of descending inhibitory pathways may further enhance central sensitization and pain perception. Normally, interneurons within the central nervous system release neurotransmitters including gamma amino butyric acid (GABA) and glycine, which inhibit nociceptive signaling.118 After a noxious insult, GABA has less of an inhibitory influence on ascending pathways119 partially due to changes in chloride currents. This lack of inhibition leads to increased ascending pain pathway signals and helps maintain the chronic pain state.120,121
Various correlates to these pathophysiological findings have been found in DE. For example, lacrimal gland removal in rats led to enlarged, convergent periorbital sensory receptive fields, a finding consistent with central sensitization.122 GABA receptor activation inhibited nociceptive neuronal activity in spinal trigeminal Vi/Vc and Vc/C1 nuclei as a response to CO2 exposure on the ocular surface.123 Conversely, a loss of the inhibitory GABA-mediated chloride currents led to an upregulation of ascending pain signals and heightened painful responses.
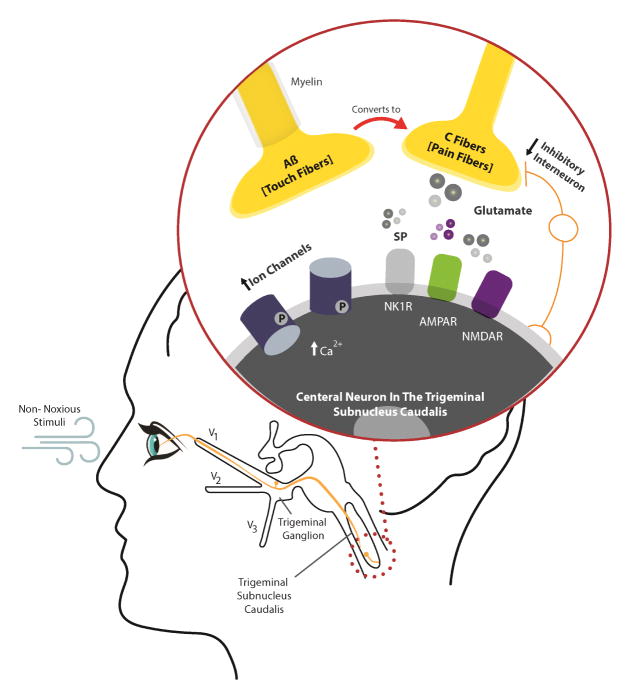
When changes occur at the level of the central nervous system, the perception of pain is disproportional to the initial peripheral stimulus and may persist after the initial peripheral pathology has resolved. Discordant DE (discrepancies between severity of signs and symptoms) is common and is associated with specific systemic profiles such as chronic pain, depression, and anxiety.52 Many affected patients have clinical correlates of sensitization, including spontaneous eye pain, hyperalgesia (evoked pain with wind), cold induced pain (cold allodynia) and photo-allodynia (evoked pain with light).124 Fig. 2 depicts potential central mechanisms that drive chronic DE symptoms and ocular pain after exposure to a non-noxious stimuli (light or wind).
Fig. 2.
Mechanisms of central sensitization that lead to ocular pain following a non-noxious stimulus (e.g., light or wind). After leaving the cornea, peripheral corneal nerves synapse with second-order neurons in the Vi/Vc and Vc/C1 regions within the trigeminal subnucleus caudalis. Sensory (touch) neurons also synapse with central neurons in this area. Increased peripheral traffic leads to the release of substance P (SP) and glutamate at the synapse junction. Several receptors respond to these mediators including the N-methyl-D-aspartate receptor (NMDAR) (ion channel that responds to glutamate), the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) (ion channel that responds to glutamate), and the neurokinin 1 receptor (NK1R) (G protein coupled receptor that responds to substance P). In a similar manner to what occurs in the periphery, the co-activation of these receptors leads to increased calcium (Ca2+) and phosphorylation of existing ion channels and production of more channels, leading to a more excitatory phenotype. Other mechanisms involved in central sensitization include decreased number and function of inhibitory interneurons (which results in pain amplification), activation of glial cells (with resulting central inflammation), and conversion of touch receptors into pain receptors. The latter has the effect of converting innocuous sensations to painful ones (hyperalgesia, allodynia). Of note, features of hyperalgesia and allodynia (in the form of sensitivity to wind and light) are often concomitant with dry eye symptoms.
5.4. Heritability – shared genetic factors
Genetic polymorphisms underlie individual differences in somatosensory processing, pain perception, and the development of persistent pain syndromes.125–128 Thus, many forms of chronic NP are heritable. Relevant to DE, polymorphisms in TRPM8, IL1, and IL6R have been implicated in both pain perception and DE.113,129–136 A large twin study utilizing the Twins UK database identified shared genetic factors common to DE, irritable bowel syndrome, fibromyalgia (chronic widespread pain), and vulvodynia. The study results show that DE and these other disorders share two latent genetic factors with an estimated overall heritability of 66%.137 There is biologic plausibility that functional alterations in inflammatory and other genes would affect the function of peripheral and central neurons common to these co-morbid conditions.138–141
6. Novel approaches in the diagnosis and treatment of neuropathic ocular pain-subtype of dry eye based on these similarities
A better understanding of the neurobiology underlying DE can improve our diagnosis and treatment algorithms. A necessary first step is the evaluation of ocular symptoms and signs for discordance suggesting an underlying NP etiology. This observation would then be followed by an assessment of somatosensory function in patients with NP subtype of DE symptoms.
6. 1. Assessing ocular pain
Pain questionnaires that are specific for eye pain can be immediately incorporated into clinical practice to help clinicians gauge the contribution of nerve activation to the DE phenotype. Pain questionnaires are commonly used by pain specialists to assess features of NP in non-ocular pain conditions. We and others have demonstrated that such questionnaires can be easily adapted to assess ocular pain.4,51 Specifically, descriptors suggestive of a neuropathic component to the pain, such as burning ocular pain and sensitivity to wind and light, have been found to correlate with other metrics of somatosensory alterations (aesthesiometry94,95, persistent pain after proparicaine124), providing criterion validity of their use.142
6.2. Assessing somatosensory function in the clinic (cursory)
A cost-effective and quick test that can be performed in the clinic to assess somatosensory function is evaluation of corneal sensation with a cotton tip or dental floss. While this is a qualitative test, the report of no sensation or the report of extreme sensitivity to light touch (tactile allodynia) points to somatosensory pathway dysfunction, such as that seen in various peripheral and central neuropathic states.68,93 Persistent pain after placement of topical anesthetic onto the eye surface also points to a central etiology,143 as peripheral topical anesthetics quiet the firing of peripheral nociceptors. Other features suggestive of somatosensory dysfunction include a disconnect between symptoms and signs of DE (more symptoms than signs)52 and a lack of symptom improvement with topical therapy.56 While none of these metrics are pathognomonic of NP (which would require advanced imaging or electrophysiological testing), they can suggest its presence.
6.3. Assessing somatosensory function in a research setting (comprehensive)
6.3.1. Afferent (sensory) arc
The Cochet-Bonnet and Belmonte aesthesiometers have been used to evaluate corneal sensation, mostly in research settings. The Cochet-Bonnet is a hand-held device that contains an adjustable fiber. The clinician touches the cornea with the fiber fully extended (6 mm) and reduces its length until the patient reports feeling a sensation. The lower the length required to elicit sensation, the lower the corneal sensitivity. A limitation of this device is that it requires contact with the patient, and it is not possible to fully sterilize the fiber between patients. Patients with DE who were tested with this device were found to have reduced mechanical sensitivity,75,144,145 implying impaired somatosensory function.
The Belmonte aesthesiometer can be used to evaluate corneal sensation in response to mechanical, chemical, and thermal stimuli. It is relatively large and requires in-house manufacturing and maintenance, as it is not commercially available. There are approximately five such devices available world-wide. With the Belmonte aesthesiometer, both increased and decreased corneal sensation have been reported in DE versus control patients.79,97,98
The confocal microscope can be used to study corneal nerve architecture in vivo.146 Commercially available confocal microscopes are produced by Heidelberg Engineering GmBH, Germany and Nidek Technologies SRL, Italy. In the Confoscan 4 (Nidek), the lens (40x) does not contact the eye directly but uses a gel interphase (such as Genteal, Novartis). In the Heidelberg Retinal Tomograph (HRT) III/Rostock Cornea Module, a disposable plastic cap (TomoCap), placed on top of the lens (63x), comes in contact with the eye. Gel is placed in between the plastic cap and lens and over the cap. The Confoscan 4 uses a bright light source to image corneal nerves, which some patients find uncomfortable, while the HRTIII uses laser technology. Both technologies require trained operators to make the imaging process easier for the patients and to acquire scans of good quality. Furthermore, there is no validated software available to automatically quantitate corneal nerve density and structural parameters, such as beading, tortuosity, and micro-neuromas. Therefore, different studies have used different tools to analyze images.147–149 In order to better apply the IVCM to evaluate patients with corneal nerve damage and NOP, commercially available software programs are needed to quickly, easily, and quantitatively assess corneal nerves. It is noteworthy that IVCM does not provide any functional assessment of corneal nerves and decreased corneal nerve density is not always equal to decreased corneal sensitivity.
6.3.2. Efferent (response) arc
Researchers have measured tear production, blink rate, and conjunctival blood flow as surrogate measures of efferent neural responses elicited by pain signaling.150–152 It is important to consider, however, that these metrics can be affected by other variables, beyond neural function, and thus represent surrogate measures of efferent function.
6.4. Therapeutics targeting somatosensory dysfunction
6.4.1. Topical therapies - treating peripheral sensitization
6.4.1.1. Anti-inflammatories
Anti-inflammatory medications are often used in the treatment of chronic pain,153 given the close relationship between inflammation and pain amplification by nerve sensitization. Several anti-inflammatories have been used in DE, including short courses of topical corticosteroids, cyclosporine, and lifitegrast.154,155 Of these, the latter two have Food and Drug Administration (FDA) approval for DE. However, not all patients with chronic pain respond to anti-inflammatories, and this is also the case for patients with DE.154 Interestingly, in one study, response to anti-inflammatories was dependent on corneal nerve status. In patients treated with loteprednol 0.5% twice daily for 4 weeks, those with low baseline corneal nerve fiber lengths did not experience improvement in DE metrics, while those with near-normal baseline lengths noted improvement in symptoms and corneal staining.156
6.4.1.2. Trophic factors
Autologous serum tears have been used off-label to treat various components of DE (symptoms and signs),157–159 and their effect is believed to be in part mediated by NGF. NGF is a neurotrophin that regulates the survival and differentiation of neurons in all vertebrate species, by activation of the tyrosine kinase A (TrkA) receptor.160 NGF may promote health and regeneration of the corneal stroma and epithelium, as well as sensory nerves, and has been shown to be therapeutic in neurotrophic keratitis and corneal ulcers, but may also result in ocular and periocular pain.161–163 Autologous serum tears, however, also contain other trophic factors, such as epidermal growth factor, platelet derived growth factor, and transforming growth factor, and their effect may be more complex.164
A concentration of 20% is most commonly used, but reported concentrations have ranged from 20% to 100%.165 The more concentrated the serum, the more blood needs to be harvested from the patient. Interestingly, in a retrospective study of 16 patients with sensitivity to light and no ocular surface disease (hallmarks of NOP), autologous serum tears led to improvements in DE symptoms and corneal nerve parameters.166 Limitations to the use of autologous serum tears include the need to harvest blood for their production, limited availability, lack of insurance coverage, and the need for storage in a freezer. Given these limitations, the effects of NGF mimetics, such as MIM-D3 (a TrkA receptor partial agonist) have been studied in patients with DE, and have demonstrated favorable responses.160,167 However, taking into consideration the controversial effects of NGF regarding pain, it is still not clear if NGF agonists will be good options for patients with NP subtype of DE. In fact, based on its pathologic role in nerve sprouting, anti-NGF therapies are currently under development to treat chronic pain.168
6.4.2. Oral medications - treating peripheral and/or central sensitization
6.4.2.1. Calcium channel alpha 2 delta ligands
Gabapentin and pregabalin are first-line therapies for the treatment of NP.169 While their mechanism of action is not entirely clear, they influence central nerve function through interactions with voltage-sensitive Ca2+ channels and inhibition of voltage gated calcium currents that mediate excitatory neurotransmitter release.170 The most recent Cochrane systematic review found that 1800 mg to 3600 mg daily gabapentin could decrease pain intensity by more than 50% in patients with diabetic neuropathy and postherpetic neuralgia.171
We have anecdotally used gabapentin as an off-label treatment for the NP subtype of DE with success in some patients. In our experience treating approximately 25 patients a year with this therapy, improvement in symptoms occurs at relatively high doses, i.e. 900 mg, or higher, three times a day. The main side effect of gabapentinoids is central nervous system depression, which can manifest as drowsiness, dizziness, headache, and/or loss of balance. In most patients, side effects are absent or subside with time. Formal studies are lacking, however, on the efficacy of alpha 2 delta ligands and other such medications in treating ocular pain and dry eye symptoms.
6.4.2.2. Anti-depressants
Anti-depressants are also used in the treatment of NP and are frequently combined with calcium channel alpha 2 delta ligands.169 In particular, serotonin-norepinephrine reuptake inhibitors (SNRIs) such as duloxetine (Cymbalta) and venlafaxine (Effexor) are used due to their mild side effect profile, which can include nausea, dizziness, and sweating. For example, seven randomized controlled trials (RCT) using duloxetine and two using venlafaxine found that both were superior to placebo in treating peripheral NP in patients with diabetes. Given their more severe side effects, tri-cyclic anti-depressants, such as amitriptyline, are generally reserved for patients who have failed newer agents given.172 Furthermore, there are less data supporting their use, as amitriptyline did not show superiority over placebo in patients with cancer-related or HIV-related NP. However, amitriptyline was more effective than placebo in patients with mixed NP, post-stroke pain, and postherpetic neuralgia.173 No data are available on their off-label use in DE.
6.4.2.3. Omega-3 fatty acids
Omega-3 fatty acids are used in the treatment of NP based on the biology that lipid mediators derived from omega-3, such as resolvins and protectins have anti-inflammatory effects. High doses of omega-3 demonstrated significant pain reduction in five patients with variable sources of NP, including cervical radiculopathy, thoracic outlet syndrome, fibromyalgia, carpal tunnel syndrome, and burn injury.174
In the eye, dietary supplementation with omega-3 decreased inflammation on the ocular surface, as demonstrated by reduced HLA-DR expression.175 In addition, the tear film of individuals taking omega-3 supplements contain a higher amount of anti-inflammatory lipids (eicosapentaenoic acid [EPA] and docosahexaenoic acid [DHA]) as compared to pro-inflammatory (arachidonic acid) mediators.107 This indicates that oral ingestion of essential fatty acids has an effect on the tear film and ocular surface. However, no formulation has been specifically approved for use in DE.
6.4.2.4. Other medications
Several other medications are used in the treatment of NP, including traditional and atypical opioids (tramadol), anticonvulsants (carbamazepine, oxcarbazepine, topiramate, lamotrigine),176,177 capsicin and lidocaine patches171, potassium channel openers,178,179 and sodium channel blockers. In fact, topical tetrodotoxin (a sodium channel blocker) suppressed corneal pain after laser keratectomy.181 However, none of the medications discussed above has been studied in ocular pain and it is not known which, if any, will help the subset of patients with DE symptoms and NOP. Furthermore, these oral medications are not routinely prescribed by ophthalmologists, so consultation with a psychiatrist or primary care physician is advised, as these medications can cause side effects that need to be monitored.
6.4.3. Ancillary therapies
Non-pharmacological approaches are important adjuvants in the treatment of NP. They may also be used as primary treatments for those whose NP is especially refractive to pharmacologic interventions. These include therapies such as exercise, massage, acupuncture, and peripheral and central stimulation.181 In particular, cognitive behavioral therapy (CBT) is an important component in treating patients with chronic pain.182 Such therapy can help decrease dysfunctional and maladaptive thoughts and behaviors, reduce anxiety and stress associated with chronic pain, and help improve coping mechanisms.
6.4.4. Nerve blocks
Injections, such as nerve blocks, are another form of therapy used to treat chronic pain. The area adjacent to a sensory nerve can be injected, typically with a combination of a sodium channel inhibitor, such as lidocaine (or other local anesthetic) and a corticosteroid, when pain arises from a defined nerve (i.e., neuralgia). Repeated neural blockade may have an effect by reducing dynamic maintenance of peripheral or central sensitization and attenuate pain.116 Blockade of nerves adjacent to the eye has been found helpful in a small case series of individuals with pain involving the periocular tissues.183,184
In patients with parasympathetically (or sympathetically) mediated pain, sphenopalatine (or superior cervical ganglion) blocks and/or stimulations have been used with some success, based on the clinical scenario.185,186 Raised parasympathetic hyperactivity via the sphenopalatine ganglion has been described in other neuropathic facial pain conditions, such as cluster headache syndromes. While not common, severe ocular pain not responsive to traditional therapies may need more aggressive treatment. In one case report, a woman with severe post-refractive surgery-associated pain was treated with a trigeminal nerve stimulator followed by intrathecal catheter placement for delivery of bupivacaine and low dose fentanyl.187
7. Limitations of the current review
Several limitations of this review should be considered. Ocular pain as a feature of DE has only recently been investigated, and many questions remain as to its epidemiology, clinical manifestations, and optimal treatments. While two groups have found similarities with regard to the epidemiology of ocular pain and somatosensory function in disparate populations (white British women44,58,59 versus predominantly male South Florida veterans57,95), the findings in these series may not be generalizable to other populations. Furthermore, only two studies have reported on genetic susceptibility in DE,129,137 and validation studies in different populations are needed to corroborate these findings. In addition, questionnaires such as NPSI-Eye have not been validated against other questionnaires for eye pain. However, their correlation with other metrics of somatosensory dysfunction provide criterion validity for their use.142 Finally, agents used to treat NP have not yet been formally evaluated in well powered controlled trials as treatments of ocular pain and dry eye symptoms, and, therefore, it is not possible to discuss which strategies, if any, would be most beneficial.
8. Conclusions
While DE was initially thought to be a “simple” disorder of tear production, a deeper understanding of the disease has revealed its complex and heterogeneous nature with diverse neuro-pathologic mechanisms.188 While ocular surface findings, including tear film status and inflammation, are important components of the disease, it is clear that somatosensory dysfunction, in a manner that resembles NP, is also involved. Developing better diagnostic techniques to evaluate nerve structure and function in DE will allow for more specific tailoring of therapy that can improve the quality of life of millions of Americans.
Acknowledgments
Authors would like to thank Sanaz Moein and Dr. Victor Sendra for their assistance with drawing graphic figures.
The contents of this study do not represent the views of the Department of Veterans Affairs or the United States Government.
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper. The intent of this policy is not to prevent authors with these relationships from publishing work, but rather to adopt transparency such that readers can make objective judgments on conclusions drawn.
Funding/Support: Supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Clinical Sciences Research EPID-006-15S (Dr. Galor), R01EY026174 (Dr. Galor), NIH Center Core Grant P30EY014801, Research to Prevent Blindness Unrestricted Grant, NIH NIDCR R01 DE022903 (Dr. Levitt), and the Department of Anesthesiology, Perioperative Medicine, and Pain Management, University of Miami Miller School of Medicine, Miami, Florida.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.The epidemiology of dry eye disease: report of the Epidemiology Subcommittee of the International Dry Eye WorkShop (2007) Ocul Surf. 2007;5(2):93–107. doi: 10.1016/s1542-0124(12)70082-4. No authors listed. [DOI] [PubMed] [Google Scholar]
- 2.Methodologies to diagnose and monitor dry eye disease: report of the Diagnostic Methodology Subcommittee of the International Dry Eye WorkShop (2007) Ocul Surf. 2007;5(2):108–52. doi: 10.1016/s1542-0124(12)70083-6. No authors listed. [DOI] [PubMed] [Google Scholar]
- 3.The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007) Ocul Surf. 2007;5(2):75–92. doi: 10.1016/s1542-0124(12)70081-2. No authors listed. [DOI] [PubMed] [Google Scholar]
- 4.Kalangara JP, Galor A, Levitt RC, Covington DB, McManus KT, Sarantopoulos CD, et al. Characteristics of ocular pain complaints in patients with idiopathic dry eye symptoms. Eye Contact Lens. 2017;43(3):192–8. doi: 10.1097/ICL.0000000000000249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moss SE, Klein R, Klein BE. Prevalence of and risk factors for dry eye syndrome. Arch Ophthalmol. 2000;118(9):1264–8. doi: 10.1001/archopht.118.9.1264. [DOI] [PubMed] [Google Scholar]
- 6.Bandeen-Roche K, Munoz B, Tielsch JM, West SK, Schein OD. Self-reported assessment of dry eye in a population-based setting. Invest Ophthalmol Vis Sci. 1997;38(12):2469–75. [PubMed] [Google Scholar]
- 7.McCarty CA, Bansal AK, Livingston PM, Stanislavsky YL, Taylor HR. The epidemiology of dry eye in Melbourne, Australia. Ophthalmology. 1998;105(6):1114–9. doi: 10.1016/S0161-6420(98)96016-X. [DOI] [PubMed] [Google Scholar]
- 8.Shimmura S, Shimazaki J, Tsubota K. Results of a population-based questionnaire on the symptoms and lifestyles associated with dry eye. Cornea. 1999;18(4):408–11. doi: 10.1097/00003226-199907000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Lee AJ, Lee J, Saw SM, Gazzard G, Koh D, Widjaja D, et al. Prevalence and risk factors associated with dry eye symptoms: a population based study in Indonesia. Br J Ophthalmol. 2002;86(12):1347–51. doi: 10.1136/bjo.86.12.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin PY, Tsai SY, Cheng CY, et al. Prevalence of dry eye among an elderly Chinese population in Taiwan: the Shihpai Eye Study. Ophthalmology. 2003;110(6):1096–101. doi: 10.1016/S0161-6420(03)00262-8. [DOI] [PubMed] [Google Scholar]
- 11.Galor A, Feuer W, Lee DJ, Liu JH, Chou P, Hsu WM. Ocular surface parameters in older male veterans. Invest Ophthalmol Vis Sci. 2013;54(2):1426–33. doi: 10.1167/iovs.12-10819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martinez JD, Galor A, Ramos-Betancourt N, Lisker-Cervantes A, Beltrán F, Ozorno-Zárate J, et al. Frequency and risk factors associated with dry eye in patients attending a tertiary care ophthalmology center in Mexico City. Clin Ophthalmol. 2016;10:1335–42. doi: 10.2147/OPTH.S106451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schaumberg DA, Sullivan DA, Buring JE, Dana MR. Prevalence of dry eye syndrome among US women. Am J Ophthalmol. 2003;136(2):318–26. doi: 10.1016/s0002-9394(03)00218-6. [DOI] [PubMed] [Google Scholar]
- 14.Begley CG, Chalmers RL, Mitchell GL, Nichols KK, Caffery B, Simpson T, et al. Characterization of ocular surface symptoms from optometric practices in North America. Cornea. 2001;20(6):610–8. doi: 10.1097/00003226-200108000-00011. [DOI] [PubMed] [Google Scholar]
- 15.Lemp MA, Crews LA, Bron AJ, Foulks GN, Sullivan BD. Distribution of aqueous-deficient and evaporative dry eye in a clinic-based patient cohort: a retrospective study. Cornea. 2012;31(5):472–8. doi: 10.1097/ICO.0b013e318225415a. [DOI] [PubMed] [Google Scholar]
- 16.Jie Y, Xu L, Wu YY, Jonas JB. Prevalence of dry eye among adult Chinese in the Beijing Eye Study. Eye (Lond) 2009;23(3):688–93. doi: 10.1038/sj.eye.6703101. [DOI] [PubMed] [Google Scholar]
- 17.Uchino M, Dogru M, Yagi Y, Goto E, Tomita M, Kon T, et al. The features of dry eye disease in a Japanese elderly population. Optom Vis Sci. 2006;83(11):797–802. doi: 10.1097/01.opx.0000232814.39651.fa. [DOI] [PubMed] [Google Scholar]
- 18.Ansari Z, Singh R, Alabiad C, Galor A. Prevalence, risk factors, and morbidity of eye lid laxity in a veteran population. Cornea. 2015;34(1):32–6. doi: 10.1097/ICO.0000000000000286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chhadva P, Alexander A, McClellan AL, McManus KT, Seiden B, Galor A. The impact of conjunctivochalasis on dry eye symptoms and signs. Invest Ophthalmol Vis Sci. 2015;56(5):2867–71. doi: 10.1167/iovs.14-16337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chhadva P, McClellan AL, Alabiad CR, Feuer WJ, Batawi H, Galor A. Impact of eyelid laxity on symptoms and signs of dry eye disease. Cornea. 2016;35(4):531–5. doi: 10.1097/ICO.0000000000000786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Le Q, Cui X, Xiang J, Ge L, Gong L, Xu J. Impact of conjunctivochalasis on visual quality of life: a community population survey. PLoS One. 2014;9(10):e110821. doi: 10.1371/journal.pone.0110821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang X, Li Q, Zou H, Peng J, Shi C, Zhou H, et al. Assessing the severity of conjunctivochalasis in a senile population: a community-based epidemiology study in Shanghai, China. BMC Public Health. 2011;11:198. doi: 10.1186/1471-2458-11-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Galor A, Kumar N, Feuer W, Lee DJ. Environmental factors affect the risk of dry eye syndrome in a United States veteran population. Ophthalmology. 2014;121(4):972–3. doi: 10.1016/j.ophtha.2013.11.036. [DOI] [PubMed] [Google Scholar]
- 24.Pouyeh B, Viteri E, Feuer W, Lee DJ, Florez H, Fabian JA, et al. Impact of ocular surface symptoms on quality of life in a United States veterans affairs population. Am J Ophthalmol. 2012;153(6):1061–66. e3. doi: 10.1016/j.ajo.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 25.Schiffman RM, Walt JG, Jacobsen G, Doyle JJ, Lebovics G, Sumner W. Utility assessment among patients with dry eye disease. Ophthalmology. 2003;110(7):1412–9. doi: 10.1016/S0161-6420(03)00462-7. [DOI] [PubMed] [Google Scholar]
- 26.Lanza NL, McClellan AL, Batawi H, Felix ER, Sarantopoulos KD, Levitt RC, et al. Dry eye profiles in patients with a positive elevated surface matrix metalloproteinase 9 point-of-care test versus negative patients. Ocul Surf. 2016;14(2):216–23. doi: 10.1016/j.jtos.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yeh TN, Graham AD, Lin MC. Relationships among tear film stability, osmolarity, and dryness symptoms. Optom Vis Sci. 2015;92(9):e264–72. doi: 10.1097/OPX.0000000000000649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. [Accessed June, 2017];IASP Taxonomy. https://www.iasp-pain.org/Taxonomy.
- 29.Impact of Chronic Overlapping Pain Conditions on Public Health and the Urgent Need for Safe and Effective Treatment. [Accessed June 2017];2015 Analysis and Policy Recommendations. 2015 May; http://www.chronicpainresearch.org/public/CPRA_WhitePaper_2015-FINAL-Digital.pdf.
- 30.Yunus MB. Editorial review: an update on central sensitivity syndromes and the issues of nosology and psychobiology. Curr Rheumatol Rev. 2015;11(2):70–85. doi: 10.2174/157339711102150702112236. [DOI] [PubMed] [Google Scholar]
- 31.Nahin RL. Estimates of pain prevalence and severity in adults: United States, 2012. J Pain. 2015;16(8):769–80. doi: 10.1016/j.jpain.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.AAPM facts and figures on pain. American Academy of Pain Medicine; http://www.painmed.org/files/facts-and-figures-on-pain.pdf. [Google Scholar]
- 33.Health UDo, Services H. Health, United States. 2005: With chartbook on trends in the health of Americans: Claitor’s Law Books and Publishing Division. 2006. [Google Scholar]
- 34.Bouhassira D, Attal N, Fermanian J, Alchaar H, Gautron M, Masquelier E, et al. Development and validation of the Neuropathic Pain Symptom Inventory. Pain. 2004;108(3):248–57. doi: 10.1016/j.pain.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 35.Woolf CJ, Mannion RJ. Neuropathic pain: aetiology, symptoms, mechanisms, and management. Lancet. 1999;353(9168):1959–64. doi: 10.1016/S0140-6736(99)01307-0. [DOI] [PubMed] [Google Scholar]
- 36.Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the Ocular Surface Disease Index. Arch Ophthalmol. 2000;118(5):615–21. doi: 10.1001/archopht.118.5.615. [DOI] [PubMed] [Google Scholar]
- 37.Caraceni A, Cherny N, Fainsinger R, Kaasa S, Poulain P, Radbruch L, et al. Pain measurement tools and methods in clinical research in palliative care: recommendations of an Expert Working Group of the European Association of Palliative Care. J Pain Symptom Manage. 2002;23(3):239–55. doi: 10.1016/s0885-3924(01)00409-2. [DOI] [PubMed] [Google Scholar]
- 38.Farrar JT, Young JP, Jr, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94(2):149–58. doi: 10.1016/S0304-3959(01)00349-9. [DOI] [PubMed] [Google Scholar]
- 39.Paice JA, Cohen FL. Validity of a verbally administered numeric rating scale to measure cancer pain intensity. Cancer Nurs. 1997;20(2):88–93. doi: 10.1097/00002820-199704000-00002. [DOI] [PubMed] [Google Scholar]
- 40.Ferreira-Valente MA, Pais-Ribeiro JL, Jensen MP. Validity of four pain intensity rating scales. Pain. 2011;152(10):2399–404. doi: 10.1016/j.pain.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 41.Jensen MP, Turner JA, Romano JM. What is the maximum number of levels needed in pain intensity measurement? Pain. 1994;58(3):387–92. doi: 10.1016/0304-3959(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 42.Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113(1–2):9–19. doi: 10.1016/j.pain.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 43.Zelman DC, Dukes E, Brandenburg N, Bostrom A, Gore M. Identification of cut-points for mild, moderate and severe pain due to diabetic peripheral neuropathy. Pain. 2005;115(1–2):29–36. doi: 10.1016/j.pain.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 44.Vehof J, Kozareva D, Hysi PG, Harris J, Nessa A, Williams FK, et al. Relationship between dry eye symptoms and pain sensitivity. JAMA Ophthalmol. 2013;131(10):1304–8. doi: 10.1001/jamaophthalmol.2013.4399. [DOI] [PubMed] [Google Scholar]
- 45.Chia EM, Mitchell P, Rochtchina E, Lee AJ, Maroun R, Wang JJ. Prevalence and associations of dry eye syndrome in an older population: the Blue Mountains Eye Study. Clin Experiment Ophthalmol. 2003;31(3):229–32. doi: 10.1046/j.1442-9071.2003.00634.x. [DOI] [PubMed] [Google Scholar]
- 46.Lovejoy TI, Turk DC, Morasco BJ. Evaluation of the psychometric properties of the revised short-form McGill Pain Questionnaire. J Pain. 2012;13(12):1250–7. doi: 10.1016/j.jpain.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Onouchi K, Koga H, Yokoyama K, Yoshiyama T. An open-label, long-term study examining the safety and tolerability of pregabalin in Japanese patients with central neuropathic pain. J Pain Res. 2014;7:439–47. doi: 10.2147/JPR.S63028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Attal N, Fermanian C, Fermanian J, Lanteri-Minet M, Alchaar H, Bouhassira D. Neuropathic pain: are there distinct subtypes depending on the aetiology or anatomical lesion? Pain. 2008;138(2):343–53. doi: 10.1016/j.pain.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 49.Sommer C, Richter H, Rogausch JP, Frettlöh J, Lungenhausen M, Maier C. A modified score to identify and discriminate neuropathic pain: a study on the German version of the Neuropathic Pain Symptom Inventory (NPSI) BMC Neurol. 2011;11:104. doi: 10.1186/1471-2377-11-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Galor A, Zlotcavitch L, Walter SD, Felix ER, Feuer W, Martin ER, et al. Dry eye symptom severity and persistence are associated with symptoms of neuropathic pain. Br J Ophthalmol. 2015;99(5):665–8. doi: 10.1136/bjophthalmol-2014-306057. [DOI] [PubMed] [Google Scholar]
- 51.Qazi Y, Hurwitz S, Khan S, Jurkunas UV, Dana R, Hamrah P. Validity and reliability of a novel ocular pain assessment survey (opas) in quantifying and monitoring corneal and ocular surface pain. Ophthalmology. 2016;123(7):1458–68. doi: 10.1016/j.ophtha.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vehof J, Sillevis Smitt-Kamminga N, Nibourg SA, Hammond CJ. Predictors of discordance between symptoms and signs in dry eye disease. Ophthalmology. 2017;124(3):280–6. doi: 10.1016/j.ophtha.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 53.Levitt AE, Galor A, Weiss JS, Felix ER, 6, Martin ER, Patin D, et al. Chronic dry eye symptoms after LASIK: parallels and lessons to be learned from other persistent post-operative pain disorders. Mol Pain. 2015;11:21. doi: 10.1186/s12990-015-0020-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pavan-Langston D. Herpes zoster antivirals and pain management. Ophthalmology. 2008;115(2 Suppl):S13–20. doi: 10.1016/j.ophtha.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 55.Ong ES, Alghamdi YA, Levitt RC, McClellan AL, Lewis G, Sarantopoulos CD, et al. Longitudinal examination of frequency of and risk factors for severe dry eye symptoms in US veterans. JAMA Ophthalmol. 2016 Dec 22; doi: 10.1001/jamaophthalmol.2016.4925. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 56.Galor A, Batawi H, Felix ER, Margolis TP, Sarantopoulos KD, Martin ER, et al. Incomplete response to artificial tears is associated with features of neuropathic ocular pain. Br J Ophthalmol. 2016;100(6):745–9. doi: 10.1136/bjophthalmol-2015-307094. [DOI] [PubMed] [Google Scholar]
- 57.Galor A, Covington D, Levitt AE, McManus KT, Seiden B, Felix ER, et al. Neuropathic ocular pain due to dry eye is associated with multiple comorbid chronic pain syndromes. J Pain. 2016;17(3):310–8. doi: 10.1016/j.jpain.2015.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vehof J, Kozareva D, Hysi PG, Hammond CJ. Prevalence and risk factors of dry eye disease in a British female cohort. Br J Ophthalmol. 2014;98(12):1712–7. doi: 10.1136/bjophthalmol-2014-305201. [DOI] [PubMed] [Google Scholar]
- 59.Vehof J, Sillevis Smitt-Kamminga N, Kozareva D, Nibourg SA, Hammond CJ. Clinical characteristics of dry eye patients with chronic pain syndromes. Am J Ophthalmol. 2016;166:203–4. doi: 10.1016/j.ajo.2016.03.023. [DOI] [PubMed] [Google Scholar]
- 60.Fernandez CA, Galor A, Arheart KL, Musselman DL, Venincasa VD, Florez HJ, et al. Dry eye syndrome, posttraumatic stress disorder, and depression in an older male veteran population. Invest Ophthalmol Vis Sci. 2013;54(5):3666–72. doi: 10.1167/iovs.13-11635. [DOI] [PubMed] [Google Scholar]
- 61.Galor A, Feuer W, Lee DJ, Florez H, Faler AL, Zann KL, et al. Depression, post-traumatic stress disorder, and dry eye syndrome: a study utilizing the national United States Veterans Affairs administrative database. Am J Ophthalmol. 2012;154(2):340–6. e2. doi: 10.1016/j.ajo.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 62.Kim KW, Han SB, Han ER, Woo SJ, Lee JJ, Yoon JC, et al. Association between depression and dry eye disease in an elderly population. Invest Ophthalmol Vis Sci. 2011;52(11):7954–8. doi: 10.1167/iovs.11-8050. [DOI] [PubMed] [Google Scholar]
- 63.Labbe A, Wang YX, Jie Y, Baudouin C, Jonas JB, Xu L. Dry eye disease, dry eye symptoms and depression: the Beijing Eye Study. Br J Ophthalmol. 2013;97(11):1399–403. doi: 10.1136/bjophthalmol-2013-303838. [DOI] [PubMed] [Google Scholar]
- 64.Galor A, Felix ER, Feuer W, Shalabi N, Martin ER, Margolis TP, et al. Dry eye symptoms align more closely to non-ocular conditions than to tear film parameters. Br J Ophthalmol. 2015;99(8):1126–9. doi: 10.1136/bjophthalmol-2014-306481. [DOI] [PubMed] [Google Scholar]
- 65.Crane AM, Levitt RC, Felix ER, Sarantopoulos KD, McClellan AL, Galor A. Patients with more severe symptoms of neuropathic ocular pain report more frequent and severe chronic overlapping pain conditions and psychiatric disease. Br J Ophthalmol. 2017;101(2):227–31. doi: 10.1136/bjophthalmol-2015-308214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Galor A, Seiden BE, Park JJ, Feuer WJ, McClellan AL, Felix ER, et al. the association of dry eye symptom severity and comorbid insomnia in US veterans. Eye Contact Lens. 2017 Jan 6; doi: 10.1097/ICL.0000000000000349. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mann R, Schaefer C, Sadosky A, Bergstrom F, Baik R, Parsons B, et al. Burden of spinal cord injury-related neuropathic pain in the United States: retrospective chart review and cross-sectional survey. Spinal Cord. 2013;51(7):564–70. doi: 10.1038/sc.2013.34. [DOI] [PubMed] [Google Scholar]
- 68.Finnerup NB, Haroutounian S, Kamerman P, Baron R, Bennett DL, Bouhassira D, et al. Neuropathic pain: an updated grading system for research and clinical practice. Pain. 2016;157(8):1599–606. doi: 10.1097/j.pain.0000000000000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mian SI, Li AY, Dutta S, Musch DC, Shtein RM. Dry eyes and corneal sensation after laser in situ keratomileusis with femtosecond laser flap creation. Effect of hinge position, hinge angle, and flap thickness. J Cataract Refract Surg. 2009;35(12):2092–8. doi: 10.1016/j.jcrs.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 70.Meyer R, Ringkamp M, Campbell J, Raja S. Neural mechanisms of hyperalgesia after tissue injury. Johns Hopkins APL technical digest. 2005;26:56–66. [Google Scholar]
- 71.Borsook D, Rosenthal P. Chronic (neuropathic) corneal pain and blepharospasm: five case reports. Pain. 2011;152(10):2427–31. doi: 10.1016/j.pain.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 72.Yang S, Kim W, Kim HS, Na KS Epidemiologic Survey Committee of the Korean Ophthalmologic Society. Association between migraine and dry eye disease: a nationwide population-based study. Curr Eye Res. 2017:1–5. doi: 10.1080/02713683.2016.1262876. [DOI] [PubMed] [Google Scholar]
- 73.Benitez-Del-Castillo JM, Acosta MC, Wassfi MA, Díaz-Valle D, Gegúndez JA, Fernandez C, García-Sánchez J. Relation between corneal innervation with confocal microscopy and corneal sensitivity with noncontact esthesiometry in patients with dry eye. Invest Ophthalmol Vis Sci. 2007;48(1):173–81. doi: 10.1167/iovs.06-0127. [DOI] [PubMed] [Google Scholar]
- 74.Benitez del Castillo JM, Wasfy MA, Fernandez C, Garcia-Sanchez J. An in vivo confocal masked study on corneal epithelium and subbasal nerves in patients with dry eye. Invest Ophthalmol Vis Sci. 2004;45(9):3030–5. doi: 10.1167/iovs.04-0251. [DOI] [PubMed] [Google Scholar]
- 75.Labbe A, Alalwani H, Van Went C, Brasnu E, Georgescu D, Baudouin C. The relationship between subbasal nerve morphology and corneal sensation in ocular surface disease. Invest Ophthalmol Vis Sci. 2012;53(8):4926–31. doi: 10.1167/iovs.11-8708. [DOI] [PubMed] [Google Scholar]
- 76.Villani E, Galimberti D, Viola F, Mapelli C, Ratiglia R. The cornea in Sjogren’s syndrome: an in vivo confocal study. Invest Ophthalmol Vis Sci. 2007;48(5):2017–22. doi: 10.1167/iovs.06-1129. [DOI] [PubMed] [Google Scholar]
- 77.Hosal BM, Ornek N, Zilelioglu G, Elhan AH. Morphology of corneal nerves and corneal sensation in dry eye: a preliminary study. Eye (Lond) 2005;19(12):1276–9. doi: 10.1038/sj.eye.6701760. [DOI] [PubMed] [Google Scholar]
- 78.Tuominen IS, Konttinen YT, Vesaluoma MH, Moilanen JA, Helintö M, Tervo TM. Corneal innervation and morphology in primary Sjogren’s syndrome. Invest Ophthalmol Vis Sci. 2003;44(6):2545–9. doi: 10.1167/iovs.02-1260. [DOI] [PubMed] [Google Scholar]
- 79.Tuisku IS, Konttinen YT, Konttinen LM, Tervo TM. Alterations in corneal sensitivity and nerve morphology in patients with primary Sjogren’s syndrome. Exp Eye Res. 2008;86(6):879–85. doi: 10.1016/j.exer.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 80.Zhang M, Chen J, Luo L, Xiao Q, Sun M, Liu Z. Altered corneal nerves in aqueous tear deficiency viewed by in vivo confocal microscopy. Cornea. 2005;24(7):818–24. doi: 10.1097/01.ico.0000154402.01710.95. [DOI] [PubMed] [Google Scholar]
- 81.Tepelus TC, Chiu GB, Huang J, Huang P, Sadda SR, Irvine J, et al. Correlation between corneal innervation and inflammation evaluated with confocal microscopy and symptomatology in patients with dry eye syndromes: a preliminary study. Graefes Arch Clin Exp Ophthalmol. 2017 May 20; doi: 10.1007/s00417-017-3680-3. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 82.Labbe A, Liang Q, Wang Z, Zhang Y, Xu L, Baudouin C, et al. Corneal nerve structure and function in patients with non-Sjogren dry eye: clinical correlations. Invest Ophthalmol Vis Sci. 2013;54(8):5144–50. doi: 10.1167/iovs.13-12370. [DOI] [PubMed] [Google Scholar]
- 83.Villani E, Magnani F, Viola F, Santaniello A, Scorza R, Nucci P, et al. In vivo confocal evaluation of the ocular surface morpho-functional unit in dry eye. Optom Vis Sci. 2013;90(6):576–86. doi: 10.1097/OPX.0b013e318294c184. [DOI] [PubMed] [Google Scholar]
- 84.Zhang X, Chen Q, Chen W, Cui L, Ma H, Lu F. Tear dynamics and corneal confocal microscopy of subjects with mild self-reported office dry eye. Ophthalmology. 2011;118(5):902–7. doi: 10.1016/j.ophtha.2010.08.033. [DOI] [PubMed] [Google Scholar]
- 85.Hamrah P, Qazi Y, Shahatit B, Dastjerdi MH, Pavan-Langston D, Jacobs DS, et al. Corneal nerve and epithelial cell alterations in corneal allodynia: an in vivo confocal microscopy case series. Ocul Surf. 2017;15(1):139–51. doi: 10.1016/j.jtos.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 86.Oudejans L, He X, Niesters M, Dahan A, Brines M, van Velzen M. Cornea nerve fiber quantification and construction of phenotypes in patients with fibromyalgia. Sci Rep. 2016;6:23573. doi: 10.1038/srep23573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ramirez M, Martinez-Martinez LA, Hernandez-Quintela E, Velazco-Casapía J, Vargas A, Martínez-Lavín M. Small fiber neuropathy in women with fibromyalgia. An in vivo assessment using corneal confocal bio-microscopy. Semin Arthritis Rheum. 2015;45(2):214–9. doi: 10.1016/j.semarthrit.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 88.Kinard KI, Smith AG, Singleton JR, Lessard MK, Katz BJ, Warner JE, et al. Chronic migraine is associated with reduced corneal nerve fiber density and symptoms of dry eye. Headache. 2015;55(4):543–9. doi: 10.1111/head.12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wilcox SL, Gustin SM, Macey PM, Peck CC, Murray GM, Henderson LA. Anatomical changes at the level of the primary synapse in neuropathic pain: evidence from the spinal trigeminal nucleus. J Neurosci. 2015;35(6):2508–15. doi: 10.1523/JNEUROSCI.3756-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gustin SM, Peck CC, Wilcox SL, Nash PG, Murray GM, Henderson LA. Different pain, different brain: thalamic anatomy in neuropathic and non-neuropathic chronic pain syndromes. J Neurosci. 2011;31(16):5956–64. doi: 10.1523/JNEUROSCI.5980-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wilcox SL, Gustin SM, Eykman EN, Fowler G, Peck CC, Murray GM, et al. Trigeminal nerve anatomy in neuropathic and non-neuropathic orofacial pain patients. J Pain. 2013;14(8):865–72. doi: 10.1016/j.jpain.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 92.Staud R, Weyl EE, Riley JL, 3rd, Fillingim RB. Slow temporal summation of pain for assessment of central pain sensitivity and clinical pain of fibromyalgia patients. PLoS One. 2014;9(2):e89086. doi: 10.1371/journal.pone.0089086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Maier C, Baron R, Tolle TR, Binder A, Birbaumer N, Birklein F, et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): somatosensory abnormalities in 1236 patients with different neuropathic pain syndromes. Pain. 2010;150(3):439–50. doi: 10.1016/j.pain.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 94.Spierer O, Felix ER, McClellan AL, Parel JM, Gonzalez A, Feuer WJ, et al. corneal mechanical thresholds negatively associate with dry eye and ocular pain symptoms. Invest Ophthalmol Vis Sci. 2016;57(2):617–25. doi: 10.1167/iovs.15-18133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Galor A, Levitt RC, McManus KT, Kalangara JP, Seiden BE, Park JJ, et al. Assessment of somatosensory function in patients with idiopathic dry eye symptoms. JAMA Ophthalmol. 2016;134(11):1290–8. doi: 10.1001/jamaophthalmol.2016.3642. [DOI] [PubMed] [Google Scholar]
- 96.Belmonte C, Acosta MC, Schmelz M, Gallar J. Measurement of corneal sensitivity to mechanical and chemical stimulation with a CO2 esthesiometer. Invest Ophthalmol Vis Sci. 1999;40(2):513–9. [PubMed] [Google Scholar]
- 97.Bourcier T, Acosta MC, Borderie V, Borrás F, Gallar J, Bury T, et al. Decreased corneal sensitivity in patients with dry eye. Invest Ophthalmol Vis Sci. 2005;46(7):2341–5. doi: 10.1167/iovs.04-1426. [DOI] [PubMed] [Google Scholar]
- 98.De Paiva CS, Pflugfelder SC. Corneal epitheliopathy of dry eye induces hyperesthesia to mechanical air jet stimulation. Am J Ophthalmol. 2004;137(1):109–15. doi: 10.1016/s0002-9394(03)00897-3. [DOI] [PubMed] [Google Scholar]
- 99.Situ P, Simpson TL, Fonn D, Jones LW. Conjunctival and corneal pneumatic sensitivity is associated with signs and symptoms of ocular dryness. Invest Ophthalmol Vis Sci. 2008;49(7):2971–6. doi: 10.1167/iovs.08-1734. [DOI] [PubMed] [Google Scholar]
- 100.Li W, Graham AD, Lin MC. Understanding ocular discomfort and dryness using the Pain Sensitivity Questionnaire. PLoS One. 2016;11(5):e0154753. doi: 10.1371/journal.pone.0154753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ji RR, Chamessian A, Zhang YQ. Pain regulation by non-neuronal cells and inflammation. Science. 2016;354(6312):572–7. doi: 10.1126/science.aaf8924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Stepp MA, Tadvalkar G, Hakh R, Pal-Ghosh S. Corneal epithelial cells function as surrogate Schwann cells for their sensory nerves. Glia. 2017;65(6):851–63. doi: 10.1002/glia.23102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Skaper SD, Facci L, Giusti P. Mast cells, glia and neuroinflammation: partners in crime? Immunology. 2014;141(3):314–27. doi: 10.1111/imm.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Enriquez-de-Salamanca A, Castellanos E, Stern ME, Fernández I, Carreño E, García-Vázquez C, et al. Tear cytokine and chemokine analysis and clinical correlations in evaporative-type dry eye disease. Mol Vis. 2010;16:862–73. [PMC free article] [PubMed] [Google Scholar]
- 105.Lam H, Bleiden L, de Paiva CS, Farley W, Stern ME, Pflugfelder SC, et al. Tear cytokine profiles in dysfunctional tear syndrome. Am J Ophthalmol. 2009;147(2):198–205. e1. doi: 10.1016/j.ajo.2008.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shim J, Park C, Lee HS, Park MS, Lim HT, Chauhan S, et al. Change in prostaglandin expression levels and synthesizing activities in dry eye disease. Ophthalmology. 2012;119(11):2211–9. doi: 10.1016/j.ophtha.2012.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Walter SD, Gronert K, McClellan AL, Levitt RC, Sarantopoulos KD, Galor A. Omega-3 tear film lipids correlate with clinical measures of dry eye. Invest Ophthalmol Vis Sci. 2016;57(6):2472–8. doi: 10.1167/iovs.16-19131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chotikavanich S, de Paiva CS, Li de Q, Chen JJ, Bian F, Farley WJ, et al. Production and activity of matrix metalloproteinase-9 on the ocular surface increase in dysfunctional tear syndrome. Invest Ophthalmol Vis Sci. 2009;50(7):3203–9. doi: 10.1167/iovs.08-2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lambiase A, Sacchetti M, Bonini S. Nerve growth factor therapy for corneal disease. Curr Opin Ophthalmol. 2012;23(4):296–302. doi: 10.1097/ICU.0b013e3283543b61. [DOI] [PubMed] [Google Scholar]
- 110.Stern ME, Gao J, Schwalb TA, Ngo M, Tieu DD, Chan CC, et al. Conjunctival T-cell subpopulations in Sjogren’s and non-Sjogren’s patients with dry eye. Invest Ophthalmol Vis Sci. 2002;43(8):2609–14. [PubMed] [Google Scholar]
- 111.Belmonte C, Acosta MC, Gallar J. Neural basis of sensation in intact and injured corneas. Exp Eye Res. 2004;78(3):513–25. doi: 10.1016/j.exer.2003.09.023. [DOI] [PubMed] [Google Scholar]
- 112.Muller LJ, Marfurt CF, Kruse F, Tervo TM. Corneal nerves: structure, contents and function. Exp Eye Res. 2003;76(5):521–42. doi: 10.1016/s0014-4835(03)00050-2. [DOI] [PubMed] [Google Scholar]
- 113.Belmonte C, Gallar J. Cold thermoreceptors, unexpected players in tear production and ocular dryness sensations. Invest Ophthalmol Vis Sci. 2011;52(6):3888–92. doi: 10.1167/iovs.09-5119. [DOI] [PubMed] [Google Scholar]
- 114.Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000;288(5472):1765–9. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- 115.Parra A, Gonzalez-Gonzalez O, Gallar J, Belmonte C. Tear fluid hyperosmolality increases nerve impulse activity of cold thermoreceptor endings of the cornea. Pain. 2014;155(8):1481–91. doi: 10.1016/j.pain.2014.04.025. [DOI] [PubMed] [Google Scholar]
- 116.Kurose M, Meng ID. Dry eye modifies the thermal and menthol responses in rat corneal primary afferent cool cells. J Neurophysiol. 2013;110(2):495–504. doi: 10.1152/jn.00222.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152(3 Suppl):S2–15. doi: 10.1016/j.pain.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Barr MS, Farzan F, Davis KD, Fitzgerald PB, Daskalakis ZJ, et al. Measuring GABAergic inhibitory activity with TMS-EEG and its potential clinical application for chronic pain. J Neuroimmune Pharmacol. 2013;8(3):535–46. doi: 10.1007/s11481-012-9383-y. [DOI] [PubMed] [Google Scholar]
- 119.von Hehn CA, Baron R, Woolf CJ. Deconstructing the neuropathic pain phenotype to reveal neural mechanisms. Neuron. 2012;73(4):638–52. doi: 10.1016/j.neuron.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Scholz J, Woolf CJ. The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci. 2007;10(11):1361–8. doi: 10.1038/nn1992. [DOI] [PubMed] [Google Scholar]
- 121.Rosenthal P, Baran I, Jacobs DS. Corneal pain without stain: is it real? Ocul Surf. 2009;7(1):28–40. doi: 10.1016/s1542-0124(12)70290-2. [DOI] [PubMed] [Google Scholar]
- 122.Rahman M, Okamoto K, Thompson R, Katagiri A, Bereiter DA. Sensitization of trigeminal brainstem pathways in a model for tear deficient dry eye. Pain. 2015;156(5):942–50. doi: 10.1097/j.pain.0000000000000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hirata H, Okamoto K, Bereiter DA. GABA(A) receptor activation modulates corneal unit activity in rostral and caudal portions of trigeminal subnucleus caudalis. J Neurophysiol. 2003;90(5):2837–49. doi: 10.1152/jn.00544.2003. [DOI] [PubMed] [Google Scholar]
- 124.Crane AM, Feuer W, Felix ER, Levitt RC, McClellan AL, Sarantopoulos KD, et al. Evidence of central sensitisation in those with dry eye symptoms and neuropathic-like ocular pain complaints: incomplete response to topical anaesthesia and generalised heightened sensitivity to evoked pain. Br J Ophthalmol. 2017 doi: 10.1136/bjophthalmol-2016-309658. [DOI] [PubMed] [Google Scholar]
- 125.Young EE, Lariviere WR, Belfer I. Genetic basis of pain variability: recent advances. J Med Genet. 2012;49(1):1–9. doi: 10.1136/jmedgenet-2011-100386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Diatchenko L, Nackley AG, Tchivileva IE, Shabalina SA, Maixner W. Genetic architecture of human pain perception. Trends Genet. 2007;23(12):605–13. doi: 10.1016/j.tig.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 127.Costigan M, Belfer I, Griffin RS, Dai F, Barrett LB, Coppola G, et al. Multiple chronic pain states are associated with a common amino acid-changing allele in KCNS1. Brain. 2010;133(9):2519–27. doi: 10.1093/brain/awq195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Katz J, Seltzer Z. Transition from acute to chronic postsurgical pain: risk factors and protective factors. Expert Rev Neurother. 2009;9(5):723–44. doi: 10.1586/ern.09.20. [DOI] [PubMed] [Google Scholar]
- 129.Na KS, Mok JW, Kim JY, Joo CK. Proinflammatory gene polymorphisms are potentially associated with Korean non-Sjogren dry eye patients. Mol Vis. 2011;17:2818–23. [PMC free article] [PubMed] [Google Scholar]
- 130.Su L, Wang C, Yu YH, Ren YY, Xie KL, Wang GL. Role of TRPM8 in dorsal root ganglion in nerve injury-induced chronic pain. BMC Neurosci. 2011;12:120. doi: 10.1186/1471-2202-12-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Xing H, Chen M, Ling J, Tan W, Gu JG. TRPM8 mechanism of cold allodynia after chronic nerve injury. J Neurosci. 2007;27(50):13680–90. doi: 10.1523/JNEUROSCI.2203-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ebbinghaus M, Uhlig B, Richter F, von Banchet GS, Gajda M, Bräuer R, et al. The role of interleukin-1beta in arthritic pain: main involvement in thermal, but not mechanical, hyperalgesia in rat antigen-induced arthritis. Arthritis Rheum. 2012;64(12):3897–907. doi: 10.1002/art.34675. [DOI] [PubMed] [Google Scholar]
- 133.Vaitla PM, Radford PM, Tighe PJ, Powell RJ, McDermott EM, Todd I, et al. Role of interleukin-6 in a patient with tumor necrosis factor receptor-associated periodic syndrome: assessment of outcomes following treatment with the anti-interleukin-6 receptor monoclonal antibody tocilizumab. Arthritis Rheum. 2011;63(4):1151–5. doi: 10.1002/art.30215. [DOI] [PubMed] [Google Scholar]
- 134.Karppinen J, Daavittila I, Noponen N, Haapea M, Taimela S, Vanharanta H, et al. Is the interleukin-6 haplotype a prognostic factor for sciatica? Eur J Pain. 2008;12(8):1018–25. doi: 10.1016/j.ejpain.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 135.Freilinger T, Anttila V, de Vries B, Malik R, Kallela M, Terwindt GM, et al. Genome-wide association analysis identifies susceptibility loci for migraine without aura. Nat Genet. 2012;44(7):777–82. doi: 10.1038/ng.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Parra A, Madrid R, Echevarria D, del Olmo S, Morenilla-Palao C, Acosta MC, et al. Ocular surface wetness is regulated by TRPM8-dependent cold thermoreceptors of the cornea. Nature Medicine. 2010;16(12):1396–9. doi: 10.1038/nm.2264. [DOI] [PubMed] [Google Scholar]
- 137.Vehof J, Wang B, Kozareva D, Hysi PG, Snieder H, Hammond CJ. The heritability of dry eye disease in a female twin cohort. Invest Ophthalmol Vis Sci. 2014;55(11):7278–83. doi: 10.1167/iovs.14-15200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Fernandez Robles CR, Degnan M, Candiotti KA. Pain and genetics. Curr Opin Anaesthesiol. 2012;25(4):444–9. doi: 10.1097/ACO.0b013e3283556228. [DOI] [PubMed] [Google Scholar]
- 139.Moen A, Schistad EI, Rygh LJ, Røe C, Gjerstad J. Role of IL1A rs1800587, IL1B rs1143627 and IL1RN rs2234677 genotype regarding development of chronic lumbar radicular pain; a prospective one-year study. PLoS One. 2014;9(9):e107301. doi: 10.1371/journal.pone.0107301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.George SZ, Parr JJ, Wallace MR, et al. Inflammatory genes and psychological factors predict induced shoulder pain phenotype. Med Sci Sports Exerc. 2014;46(10):1871–81. doi: 10.1249/MSS.0000000000000328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Schistad EI, Jacobsen LM, Roe C, Gjerstad J. The interleukin-1alpha gene C>T polymorphism rs1800587 is associated with increased pain intensity and decreased pressure pain thresholds in patients with lumbar radicular pain. Clin J Pain. 2014;30(10):869–74. doi: 10.1097/AJP.0000000000000048. [DOI] [PubMed] [Google Scholar]
- 142.Spilker B. Guide to clinical trials. Raven Press; 1991. [Google Scholar]
- 143.Rosenthal P, Borsook D. Ocular neuropathic pain. Br J Ophthalmol. 2016;100(1):128–34. doi: 10.1136/bjophthalmol-2014-306280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Adatia FA, Michaeli-Cohen A, Naor J, Caffery B, Bookman A, Slomovic A. Correlation between corneal sensitivity, subjective dry eye symptoms and corneal staining in Sjogren’s syndrome. Can J Ophthalmol. 2004;39(7):767–71. doi: 10.1016/s0008-4182(04)80071-1. [DOI] [PubMed] [Google Scholar]
- 145.Xu KP, Yagi Y, Tsubota K. Decrease in corneal sensitivity and change in tear function in dry eye. Cornea. 1996;15(3):235–9. doi: 10.1097/00003226-199605000-00002. [DOI] [PubMed] [Google Scholar]
- 146.Parissi M, Karanis G, Randjelovic S, Germundsson J, Poletti E, Ruggeri A, et al. Standardized baseline human corneal subbasal nerve density for clinical investigations with laser-scanning in vivo confocal microscopy. Invest Ophthalmol Vis Sci. 2013;54(10):7091–102. doi: 10.1167/iovs.13-12999. [DOI] [PubMed] [Google Scholar]
- 147.Midena E, Cortese M, Miotto S, Gambato C, Cavarzeran F, Ghirlando A. Confocal microscopy of corneal sub-basal nerve plexus: a quantitative and qualitative analysis in healthy and pathologic eyes. J Refract Surg. 2009;25(1 Suppl):S125–30. doi: 10.3928/1081597X-20090115-09. [DOI] [PubMed] [Google Scholar]
- 148.Scarpa F, Grisan E, Ruggeri A. Automatic recognition of corneal nerve structures in images from confocal microscopy. Invest Ophthalmol Vis Sci. 2008;49(11):4801–7. doi: 10.1167/iovs.08-2061. [DOI] [PubMed] [Google Scholar]
- 149.Zhivov A, Winter K, Hovakimyan M, Peschel S, Harder V, Schober HC, et al. Imaging and quantification of subbasal nerve plexus in healthy volunteers and diabetic patients with or without retinopathy. PLoS One. 2013;8(1):e52157. doi: 10.1371/journal.pone.0052157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Acosta MC, Peral A, Luna C, Pintor J, Belmonte C, Gallar J. Tear secretion induced by selective stimulation of corneal and conjunctival sensory nerve fibers. Invest Ophthalmol Vis Sci. 2004;45(7):2333–6. doi: 10.1167/iovs.03-1366. [DOI] [PubMed] [Google Scholar]
- 151.Situ P, Simpson TL. Interaction of corneal nociceptive stimulation and lacrimal secretion. Invest Ophthalmol Vis Sci. 2010;51(11):5640–5. doi: 10.1167/iovs.10-5502. [DOI] [PubMed] [Google Scholar]
- 152.Chen W, Batawi HI, Alava JR, Galor A, Yuan J, Sarantopoulos CD, et al. Bulbar conjunctival microvascular responses in dry eye. Ocul Surf. 2017;15(2):193–201. doi: 10.1016/j.jtos.2016.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Attal N. Neuropathic pain: mechanisms, therapeutic approach, and interpretation of clinical trials. Continuum (Minneap Minn) 2012;18(1):161–75. doi: 10.1212/01.CON.0000411564.41709.2d. [DOI] [PubMed] [Google Scholar]
- 154.Ames P, Galor A. Cyclosporine ophthalmic emulsions for the treatment of dry eye: a review of the clinical evidence. Clin Investig (Lond) 2015;5(3):267–85. doi: 10.4155/cli.14.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Holland EJ, Luchs J, Karpecki PM, Nichols KK, Jackson MA, Sall K, et al. Lifitegrast for the treatment of dry eye disease: results of a phase iii, randomized, double-masked, placebo-controlled trial (OPUS-3) Ophthalmology. 2017;124(1):53–60. doi: 10.1016/j.ophtha.2016.09.025. [DOI] [PubMed] [Google Scholar]
- 156.Kheirkhah A, Dohlman TH, Amparo F, Arnoldner MA, Jamali A, Hamrah P, et al. Effects of corneal nerve density on the response to treatment in dry eye disease. Ophthalmology. 2015;122(4):662–8. doi: 10.1016/j.ophtha.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Tsubota K, Goto E, Fujita H, Ono M, Inoue H, Saito I, et al. Treatment of dry eye by autologous serum application in Sjogren’s syndrome. Br J Ophthalmol. 1999;83(4):390–5. doi: 10.1136/bjo.83.4.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ogawa Y, Okamoto S, Mori T, Yamada M, Mashima Y, Watanabe R, et al. Autologous serum eye drops for the treatment of severe dry eye in patients with chronic graft-versus-host disease. Bone Marrow Transplant. 2003;31(7):579–83. doi: 10.1038/sj.bmt.1703862. [DOI] [PubMed] [Google Scholar]
- 159.Kojima T, Ishida R, Dogru M, Goto E, Matsumoto Y, Kaido M, et al. The effect of autologous serum eyedrops in the treatment of severe dry eye disease: a prospective randomized case-control study. Am J Ophthalmol. 2005;139(2):242–6. doi: 10.1016/j.ajo.2004.08.040. [DOI] [PubMed] [Google Scholar]
- 160.Meerovitch K, Torkildsen G, Lonsdale J, Goldfarb H, Lama T, Cumberlidge G, et al. Safety and efficacy of MIM-D3 ophthalmic solutions in a randomized, placebo-controlled Phase 2 clinical trial in patients with dry eye. Clin Ophthalmol. 2013;7:1275–85. doi: 10.2147/OPTH.S44688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lambiase A, Rama P, Bonini S, Caprioglio G, Aloe L. Topical treatment with nerve growth factor for corneal neurotrophic ulcers. N Engl J Med. 1998;338(17):1174–80. doi: 10.1056/NEJM199804233381702. [DOI] [PubMed] [Google Scholar]
- 162.Bonini S, Lambiase A, Rama P, Caprioglio G, Aloe L. Topical treatment with nerve growth factor for neurotrophic keratitis. Ophthalmology. 2000;107(7):1347–51. doi: 10.1016/s0161-6420(00)00163-9. discussion 51–2. [DOI] [PubMed] [Google Scholar]
- 163.Tan MH, Bryars J, Moore J. Use of nerve growth factor to treat congenital neurotrophic corneal ulceration. Cornea. 2006;25(3):352–5. doi: 10.1097/01.ico.0000176609.42838.df. [DOI] [PubMed] [Google Scholar]
- 164.Phasukkijwatana N, Lertrit P, Liammongkolkul S, Prabhasawat P. Stability of epitheliotrophic factors in autologous serum eye drops from chronic Stevens-Johnson syndrome dry eye compared to non-autoimmune dry eye. Curr Eye Res. 2011;36(9):775–81. doi: 10.3109/02713683.2011.587935. [DOI] [PubMed] [Google Scholar]
- 165.Ezuddin NS, Alawa KA, Galor A. Therapeutic strategies to treat dry eye in an aging population. Drugs Aging. 2015;32(7):505–13. doi: 10.1007/s40266-015-0277-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Aggarwal S, Kheirkhah A, Cavalcanti BM, Cruzat A, Colon C, Brown E, et al. Autologous serum tears for treatment of photoallodynia in patients with corneal neuropathy: efficacy and evaluation with in vivo confocal microscopy. Ocul Surf. 2015;13(3):250–62. doi: 10.1016/j.jtos.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Jain P, Li R, Lama T, Saragovi HU, Cumberlidge G, Meerovitch K. An NGF mimetic, MIM-D3, stimulates conjunctival cell glycoconjugate secretion and demonstrates therapeutic efficacy in a rat model of dry eye. Exp Eye Res. 2011;93(4):503–12. doi: 10.1016/j.exer.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 168.Gow JM, Tsuji WH, Williams GJ, Mytych D, Sciberras D, Searle S, et al. Safety, tolerability, pharmacokinetics, and efficacy of AMG 403, a human anti-nerve growth factor monoclonal antibody, in two phase I studies with healthy volunteers and knee osteoarthritis subjects. Arthritis Res Ther. 2015;17:282. doi: 10.1186/s13075-015-0797-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Finnerup NB, Attal N, Haroutounian S, McNicol E, Baron R, Dworkin RH, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14(2):162–73. doi: 10.1016/S1474-4422(14)70251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sarantopoulos C, McCallum B, Kwok WM, Hogan Q. Gabapentin decreases membrane calcium currents in injured as well as in control mammalian primary afferent neurons. Reg Anesth Pain Med. 2002;27(1):47–57. doi: 10.1053/rapm.2002.29124. [DOI] [PubMed] [Google Scholar]
- 171.Wiffen PJ, Derry S, Bell RF, Rice AS, Tölle TR, Phillips T, et al. Gabapentin for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2017;6:CD007938. doi: 10.1002/14651858.CD007938.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ghaffariyeh A, Chamacham T. Tricyclic antidepressants: potential therapeutic alternatives for treatment of dry eye symptoms after LASIK. J Refract Surg. 2008;24(8):770–1. doi: 10.3928/1081597X-20081001-01. author reply 1–2. [DOI] [PubMed] [Google Scholar]
- 173.Moore RA, Derry S, Aldington D, Cole P, Wiffen PJ. Amitriptyline for neuropathic pain in adults. Cochrane Database Syst Rev. 2015;(7):CD008242. doi: 10.1002/14651858.CD008242.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ko GD, Nowacki NB, Arseneau L, Eitel M, Hum A. Omega-3 fatty acids for neuropathic pain: case series. Clin J Pain. 2010;26(2):168–72. doi: 10.1097/AJP.0b013e3181bb8533. [DOI] [PubMed] [Google Scholar]
- 175.Brignole-Baudouin F, Baudouin C, Aragona P, Rolando M, Labetoulle M, Pisella PJ, et al. A multicentre, double-masked, randomized, controlled trial assessing the effect of oral supplementation of omega-3 and omega-6 fatty acids on a conjunctival inflammatory marker in dry eye patients. Acta Ophthalmol. 2011;89(7):e591–7. doi: 10.1111/j.1755-3768.2011.02196.x. [DOI] [PubMed] [Google Scholar]
- 176.Tremont-Lukats IW, Megeff C, Backonja MM. Anticonvulsants for neuropathic pain syndromes: mechanisms of action and place in therapy. Drugs. 2000;60(5):1029–52. doi: 10.2165/00003495-200060050-00005. [DOI] [PubMed] [Google Scholar]
- 177.Jensen TS. Anticonvulsants in neuropathic pain: rationale and clinical evidence. Eur J Pain. 2002;6(Suppl A):61–8. doi: 10.1053/eujp.2001.0324. [DOI] [PubMed] [Google Scholar]
- 178.Busserolles J, Tsantoulas C, Eschalier A, Lopez Garcia JA. Potassium channels in neuropathic pain: advances, challenges, and emerging ideas. Pain. 2016;157(Suppl 1):S7–14. doi: 10.1097/j.pain.0000000000000368. [DOI] [PubMed] [Google Scholar]
- 179.Wu Z, Li L, Xie F, Du J, Zuo Y, Frost JA, et al. Activation of KCNQ Channels suppresses spontaneous activity in dorsal root ganglion neurons and reduces chronic pain after spinal cord injury. J Neurotrauma. 2017 doi: 10.1089/neu.2016.4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Schwartz DM, Duncan KG, Duncan JL. Experimental use of tetrodotoxin for corneal pain after excimer laser keratectomy. Cornea. 1998;17(2):196–9. doi: 10.1097/00003226-199803000-00014. [DOI] [PubMed] [Google Scholar]
- 181.Abdulla A, Adams N, Bone M, Elliott AM, Gaffin J, Jones D, et al. Guidance on the management of pain in older people. Age Ageing. 2013;42(Suppl 1):i1–57. doi: 10.1093/ageing/afs200. [DOI] [PubMed] [Google Scholar]
- 182.Thomas DA, Maslin B, Legler A, Springer E, Asgerally A, Vadivelu N. Role of alternative therapies for chronic pain syndromes. Curr Pain Headache Rep. 2016;20(5):29. doi: 10.1007/s11916-016-0562-z. [DOI] [PubMed] [Google Scholar]
- 183.Villar-Quiles RN, Garcia-Moreno H, Mayo D, Gutiérrez-Viedma Á, Ramos MI, Casas-Limón J, et al. Infratrochlear neuralgia: A prospective series of seven patients treated with infratrochlear nerve blocks. Cephalalgia. 2017 doi: 10.1177/0333102417690493. 333102417690493. [DOI] [PubMed] [Google Scholar]
- 184.Cuadrado ML, Gutierrez-Viedma A, Silva-Hernandez L, Orviz A, García-Moreno H. Lacrimal nerve blocks for three new cases of lacrimal neuralgia. Headache. 2017;57(3):460–6. doi: 10.1111/head.12985. [DOI] [PubMed] [Google Scholar]
- 185.Chaturvedi A, Dash HH. Sympathetic blockade for the relief of chronic pain. J Indian Med Assoc. 2001;99(12):698–703. [PubMed] [Google Scholar]
- 186.Robbins MS, Robertson CE, Kaplan E, Ailani J, Charleston L, 4th, Kuruvilla D, et al. The sphenopalatine ganglion: anatomy, pathophysiology, and therapeutic targeting in headache. Headache. 2016;56(2):240–58. doi: 10.1111/head.12729. [DOI] [PubMed] [Google Scholar]
- 187.Hayek SM, Sweet JA, Miller JP, Sayegh RR. successful management of corneal neuropathic pain with intrathecal targeted drug delivery. Pain Med. 2016;17(7):1302–7. doi: 10.1093/pm/pnv058. [DOI] [PubMed] [Google Scholar]
- 188.Maixner W, Fillingim RB, Williams DA, Smith SB, Slade GD. Overlapping chronic pain conditions: implications for diagnosis and classification. J Pain. 2016;17(9 Suppl):T93–T107. doi: 10.1016/j.jpain.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]