Introduction
Permethrin [(±)-3-phenoxybenzyl 3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate] is one of the synthetic pyrethroid insecticides structurally based on the natural pyrethrins. First synthesized in 1973 and marketed in 1977, permethrin is a photo-stable ester composed of the dichloro analogue of chrysanthemic acid and the 3-phenoxybenzyl alcohol (Elliott, et al., 1973). In addition to the structural advancement for the enhanced environmental stability, permethrin exhibits excellent potency against a wide spectrum of insect pests, while retaining a large margin of mammalian safety (Clark, et al., 2012; Soderlund, 2015). Four different stereoisomers can be found in the technical grade of permethrin products, and among these isomers, one with the [1R, cis] configuration is known to be the most potent form against insects (WHO, 1990b).
The action of permethrin on its molecular targets has been extensively studied and reviewed previously (Choi, et al., 2006; Ray, et al., 2006; Soderlund, et al., 2002). Briefly, permethrin elicits a rapid functional disruption in the neuromuscular system by membrane depolarization. One of the important major target sites is located on the voltage sensitive sodium channel (VSSC) alpha-subunit, a pore forming transmembrane protein that consists of four homologous domains (I–IV) (Soderlund, et al., 2002; Vijverberg, et al., 1982). Permethrin is known to slow the inactivation of VSSCs during steady-state depolarization and produces prolonged tail currents upon repolarization of the cell membrane once the voltage-clamp is removed during the electrophysiological recording protocol (Choi, et al., 2006; Tan, et al., 2005; Yoon, et al., 2008).
Permethrin in mammals is rapidly biotransformed by ester cleavage and oxidation reactions and almost completely eliminated via urinary and fecal excretions within 12 days (WHO, 1990a). Environmental fate of permethrin varies depending on the environmental conditions. A half-life of permethrin in soil under aerobic conditions has been estimated to be 28 days or less under laboratory study conditions (WHO, 1990a). Based on these characteristics, permethrin became the first pyrethroid to be widely used, making up approximately 17% of the global insecticide market by 2013 (Soderlund, 2015; Soderlund, et al., 2002; Sparks, 2013). In the US alone, ~2.2 million lbs of permethrin have been sprayed annually to agricultural plots and approximately 63% is applied to the residential area for public health (Feo, et al., 2010). In particular, permethrin has been formulated in pet products and veterinary medications to control ectoparasitic arthropod pests, such as ticks and fleas. An over-the-counter 1% permethrin formulation for human head louse control has been available since 1994 to treat infested school aged children (Clark, et al., 2013; Durand, et al., 2012). Additionally, many biting arthropods show avoidance behaviors to permethrin. Hence many permethrin-treated materials (e.g., permethrin impregnated clothing including military uniforms, pet collars, and mosquito nets) have been developed and used to repel blood feeding arthropods (National Research Council, 1994). The widespread use of permethrin suggests that human exposure to permethrin is highly likely.
Permethrin was reported to promote adipogenesis and induce insulin resistance in cell culture models similar to other types of insecticides (Howell, et al., 2011; J. Kim, et al., 2013, 2014; Moreno-Aliaga, et al., 2002; Park, et al., 2013; Shen, et al., 2017; Sun, Peng, et al., 2017; Sun, Qi, et al., 2016; Xiao, Qi, et al., 2017; Xiao, Clark, et al. 2017). In addition, permethrin treatment promoted high fat diet-induced insulin resistance in female mice (Xiao, Kim, et al., 2017); however, the effects of permethrin on high fat diet-induced obesity and insulin resistance in male mice remain unknown. Previously, several reports of sex-dependent effects of insecticides, particularly organochlorine, organophosphorus, and neonicotinoid insecticides, on weight gain have been reported (Lassiter & Brimijoin, 2008; Lassiter, Ryde, et al., 2008; Sun, Qi, et al., 2017; Sun, Xiao, et al., 2016; Villeneuve, et al., 1977; Xiao, Kim, et al. 2017). Oral exposure to organochlorine insecticide, hexachlorobenzene, for four weeks was reported to increase weight gain only in male rats (Villeneuve, et al., 1977). Neonatal exposure (postnatal day 1–4) to organophosphorus insecticide, parathion, increased weight gain in males, but decreased weight gain in female rats (Lassiter, Ryde, et al., 2008). Others reported that developmental exposure (from gestational through weaning) to organophosphorus insecticide, chlorpyrifos, increased weight gain only in male rats, but not females (Lassiter & Brimijoin, 2008). Our group previously reported that oral exposure to a neonicotinoid insecticide, imidacloprid, at the NOAEL or lower doses, increased weight gain in both male and females (Sun, Qi, et al., 2017; Sun, Xiao, et al., 2016). These findings support the contention that there are sex-dependent effects of insecticides on weight gain in animals. Thus, the purpose of this study was to investigate the effect of permethrin exposure on development of dietary fat-induced obesity and type 2 diabetes using a male mouse model.
Material and methods
Materials
Permethrin (98% pure, mixture of 38.7% cis and 59.4% trans isomers) was from Sigma-Aldrich Co. (St. Louis, MO). Insulin (human recombinant) was obtained from Novo Nordisk Inc. (Princeton, NJ). D-glucose solution (50%) was from Hospira Inc. (Lake Forest, IL). Triglyceride (TG), cholesterol, glucose, and Pierce BCA protein assay kits were purchased from Thermo Fisher Scientific (Rockford, IL). Insulin ELISA kit was from ALPCO (Salem, NH). Leptin ELISA kit was purchased from R&D systems (Minneapolis, MN). Non-esterified fatty acid (NEFA) assay kit was from Wako Diagnostics (Richmond, VA). Rabbit antibodies of phosphorylated adenosine monophosphate-activated protein kinase α (pAMPKα), adenosine monophosphate-activated protein kinase α (AMPKα), phosphorylated acetyl-CoA carboxylase (pACC), and acetyl-CoA carboxylase (ACC) were purchased from Cell Signaling Technology (Danvers, MA). Rabbit antibodies of glyceraldehyde 3-phosphate dehydrogenase (GAPDH), goat antibodies of calcium/calmodulin-dependent protein kinase kinase 2 (CaMKKβ) and mouse antibody of β-actin were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Horseradish peroxidase-conjugated anti-rabbit, anti-goat, or anti-mouse secondary antibodies were from Cell Signaling Technology (Danvers, MA). High capacity cDNA reverse transcription kit, real-time PCR primers and TaqMan gene expression master mix were obtained from Applied Biosystem (Carlsbad, CA). Other chemicals were either from Fisher Scientific (Waltham, MA) or Sigma-Aldrich Co. (St. Louis, MO).
Animals and diet
All animal care and procedures were approved by the Institutional Animal Care and Use Committee of the University of Massachusetts Amherst (Protocol Number 2013-0014). Male C57BL/6J mice at three weeks of age from the Jackson Laboratory (Bar Harbor, ME) were housed in pairs with a 12-h light-dark cycle in a temperature and humidity controlled room. Mice were adapted to new environment with low fat, semi-purified, AIN-93-based diet in powdered form (TD94048, Harlan Laboratories, Madison, WI) for three weeks. All mice were given a baseline insulin tolerance test (ITT) in the second week of adaptation and a baseline glucose tolerance test (GTT) in the third week of the adaptation period. Then, animals were randomly divided into two dietary groups: low fat diet-fed (4 w/w % fat) and high fat diet-fed groups (20 w/w % fat, TD07518, Harlan Laboratories, Madison, WI). The diet composition for low fat diet was as follows (ingredient, g/kg): corn starch, 465.7; maltodextrin, 155; casein, 140; sucrose, 100; cellulose, 50; soybean oil, 40; mineral mix, 35; vitamin mix, 10; choline bitartrate, 2.5; L-cystine, 1.8; tert-butylhydroquinone, 0.008. The diet composition for high fat diet was as follows (ingredient, g/kg): corn starch, 288.5; maltodextrin, 132; casein, 169.1; sucrose, 100; cellulose, 50; soybean oil, 200; mineral mix, 42.8; vitamin mix, 12.4; choline bitartrate, 3; L-cystine, 2.2; tert-butylhydroquinone, 0.04. Permethrin was first dissolved in soybean oil and then mixed with other ingredients.
Within each dietary fat group, control and three different doses of permethrin-containing diet were given to mice for 12 weeks. Body weight and food intake were measured weekly. Permethrin doses used in the current study were chosen based on acceptable daily intake of permethrin is 50 μg/kg body weight (BW)/day and the chronic no observed adverse effect level (NOAEL) of permethrin is 5000 μg/kg BW/day (CEPADP, 1987; WHO, 1990b). Since calorie densities are different between low and high fat diets, doses of permethrin were adjusted accordingly to achieve comparable permethrin doses delivered. Permethrin concentrations in low fat diet were 0.43, 4.3, and 43 μg per g of diet to deliver 50, 500, and 5000 μg/kg BW/day, respectively. For high fat diet, permethrin concentrations were 0.62, 6.2, and 62 μg per g of diet to deliver 50, 500, and 5000 μg/kg BW/day, respectively. Estimated permethrin intake in low fat diet-fed animals were 58 ± 1, 594 ± 1, and 6184 ± 151 μg/kg BW/day for 50, 500, and 5000 μg/kg BW/day, respectively. Estimated permethrin intake in high fat diet-fed animals were 72 ± 1, 610 ± 47, and 6422 ± 133 μg/kg BW/day for 50, 500, and 5000 μg/kg BW/day, respectively. There were no statistical differences in three permethrin doses delivered between low vs. high fat diets.
After 12 weeks of permethrin treatment, mice were sacrificed by CO2 asphyxiation after 4 hours of fasting. Blood was collected by cardiac puncture and serum were separated by centrifugation at 3,000 g for 20 mins at 4°C. Internal organs (heart, liver, kidneys, pancreas, spleen) and adipose tissue; including epididymal, retroperitoneal, mesenteric, and subcutaneous fat from abdominal area, were weighed at sacrifice. The liver, adipose tissue, and gastrocnemius muscles were snap-frozen in liquid nitrogen and kept in −80°C for further analysis. A part of epididymal white adipose tissue was preserved in 10% neutralized formalin for histological analysis.
Determination of glucose homeostasis
Insulin tolerance test (ITT) was carried out three times (the second week of adaptation period and weeks 5 and 9) according to the method described previously (Di Gregorio, et al., 2004). Mice were fasted for 4 hours before a bolus of insulin (0.75 U/kg) was injected intraperitoneally. Then, tail vein blood samples were obtained at 0, 15, 30, 60, and 120 minutes after insulin injection and tested for glucose level using a hand-held glucometer (Advocate, Pharma Supply Inc, Wellington, FL). The areas under the curve (AUC) were calculated using SigmaPlot 11.0 (Systat Software, Inc., San Jose, CA).
Intraperitoneal glucose tolerance tests (IPGTT) were conducted at the third week of adaptation and weeks 6 and 11 according to a method described previously with slight modification (Andrikopoulos, et al., 2008). After 6 hours of fasting, a bolus of glucose solution (2 g/kg) was injected into the intraperitoneal cavity of each mouse. Blood glucose level was then measured at 0, 15, 30, 60, and 120 minutes using a glucometer as described above. Blood samples at 0, 30, 60, and 120 min were also obtained for insulin determination by lateral tail incision using a method described previously (Christensen, et al., 2009). HOMA-IR was calculated using HOMA2 calculator (Wallace, et al., 2004).
Hematoxylin & Eosin (H&E) staining
Epididymal adipose tissues were fixed with 10% neutralized formalin solution before embedding in paraffin. The 5 μm-thick sections were made for hematoxylin and eosin (H&E) staining using Shandon TissueWave 2 Microwave Specimen Processing System and Rotary Microtome HM 325 (Thermo Fisher Scientific, Waltham, MA). Photographs were captured using Olympus CK2 inverted microscope (Olympus, Tokyo, Japan) and microscope eyepiece camera (AmScope, Irvine, CA, USA). Adipocyte size was measured using a method described previously (Sun, Xiao, et al., 2016). Briefly, 50 cells for each sample slide were randomly chosen and measured by four individuals, who were blinded to the treatment groups, using ImageJ software v1.48 (National Institutes of Health, Bethesda, MD).
Western blot & reverse transcriptase quantitative PCR (RT-qPCR) analyses
Immunoblot analyses and RT-qPCR were done based on previous methods (Y. Kim, et al., 2015; Xiao, et al., 2015). For RT-qPCR, the liver, gastrocnemius muscle, and epididymal adipose tissue were homogenized using TRIzol reagent and total RNA extracted according to manufacturer’s protocol. TaqMan Gene Expression Assays for Glucose transporter 4 (GLUT4, Mm00436615_m1), Sterol regulatory element-binding protein 1 (SREBP1, Mm00550338_m1), diacylglycerol O-acyltransferase 1 (DGAT1, Mm00515643_m1), diacylglycerol O-acyltransferase 2 (DGAT2, Mm00499536_m1), cluster of differentiation 36 (CD36, Mm00432403_m1), phosphoenolpyruvate carboxykinase 2 (PEPCK, Mm00551411_m1), pyruvate dehydrogenase kinase 4 (PDK4, Mm01166879_m1), peroxisome proliferator-activated receptor alpha (PPARα, Mm00440939_m1) were performed on StepOne Plus real time PCR system (Applied Biosystems, Carlsbad, CA). The oligonucleotide primers for TNFα (NM_013693.2) were purchased from Eurofins MWG Operon (Huntsville, AL). Threshold values were determined by comparative CT (ΔΔCT) method. Relative quantities of gene expression with RT-qPCR were calculated relative to 18S ribosomal RNA.
Statistical analysis
Data were analyzed by PROC MIXED using the SAS software (Version 9.3, SAS Institute Inc., Cary, NC, USA). Body weight (Fig. 1A) data were analyzed by two-way repeated measure Analysis of Variance (ANOVA) and the slice option in the Least Square (LS) means statement. All the other results were analyzed by two-way ANOVA with LS means statement. The Tukey-Kramer’s method was used for the multiple comparisons among the experimental groups. Letters were used to present differences between each experimental group if there were significant interactions between diet and permethrin. When there were no interactions between diet and permethrin, brackets were used in the figures to represent differences between permethrin treatments and control groups. P-values less than 0.05 were reported as statistically significant.
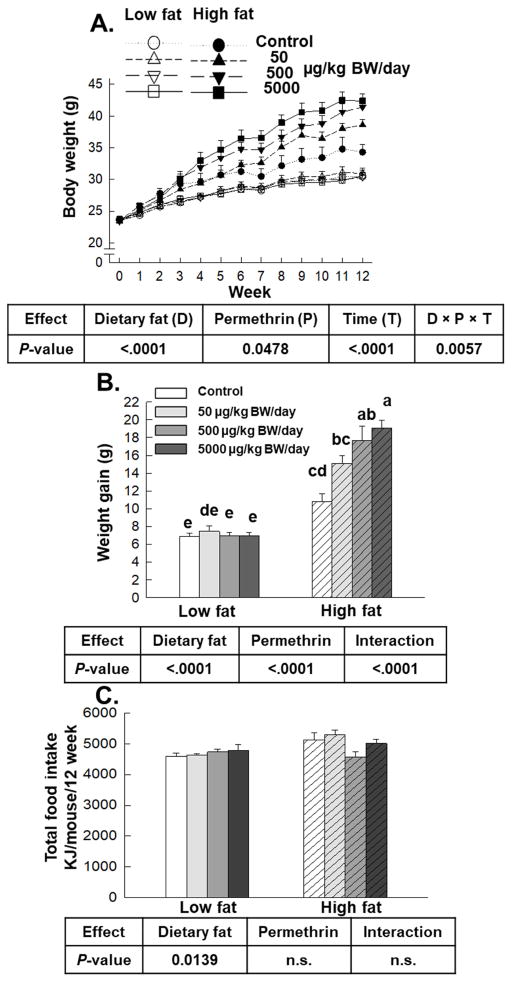
Figure 1.
Effects of permethrin treatment on body weight (A), weight gain (B) and energy intake (C). Mice were treated with either control or permethrin [50, 500, and 5000 μg/kg body weight (BW)/day] in either low fat or high fat diet for 12 weeks. (A) Open symbols, low fat diet-fed mice; Filled symbols, high fat diet-fed mice. Circles, control; Up-triangles, 50 μg/kg BW/day; Down-triangles 500 μg/kg BW/day; Squares, 5000 μg/kg BW/day. Values represent means ± S.E. (n= 5–8). Means with different letters are significantly different (P<0.05).
Results
Permethrin promoted weight gain without influencing energy intake in high fat diet-fed mice
High fat diet significantly increased body weight compared with low fat diet (22% increase, P<0.0001, Fig. 1A). Permethrin treatments significantly increased body weight versus control groups (97% increase, P=0.0478) and there was a significant three-way interaction on body weight (diet × permethrin × time) (P=0.0057). Similarly, high fat diet and permethrin treatment significantly increased body weight gain with significant interaction (97% and 35% increases, respectively; P<0.0001) for all (Fig. 1B). Permethrin treatments at 500 and 5000 μg/kg BW/day in high fat diet-fed groups significantly increased body weight gain versus the high fat diet control (56% and 76% increases, respectively). However, no effect of permethrin on body weight gain was observed in low fat diet-fed groups. There was significant effect of diet on energy intake (low fat diet 4678 ± 56 KJ; high fat diet 5007± 111 KJ), without permethrin effects or interaction (Fig. 1C). These results suggest that permethrin promoted weight gain along with high fat diet without influencing energy intake.
Effect of permethrin on organ weights and adipocyte size
Organ weights (liver, pancreas, heart, kidneys, and spleen) as well as adipose tissue weights (epididymal, subcutaneous, mesenteric, retroperitoneal, and total adipose tissue) are shown in Table 1. High fat diet treatments significantly decreased the organ weights of heart (16%, P=0.0001), kidney (11%, P=0.0007), and spleen (30%, P=0.0001) versus low fat diet-fed groups, but not liver and pancreas weights. Permethrin treatments had no effect on any of the organ weights measured (Table 1). Significant diet and permethrin interactions were observed for heart and kidney weights (P=0.0025 for heart and P=0.0125 for kidneys).
Table 1. Organ weights.
(% of Body Weight)
| Low fat | High fat | P-value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||
| Permethrin doses | Permethrin doses | ||||||||||
|
|
|
|
|||||||||
| Control | 50 μg/kg | 500 μg/kg | 5000 μg/kg | Control | 50 μg/kg | 500 μg/kg | 5000 μg/kg | Dietary fat | Perm | Interaction | |
| Liver | 3.90±0.12 | 3.60±0.23 | 3.95±0.23 | 3.59±0.17 | 3.51±0.18 | 3.33±0.21 | 3.67±0.13 | 3.64±0.07 | n.s. | n.s. | n.s. |
| Pancreas | 0.43±0.04 | 0.50±0.05 | 0.46±0.01 | 0.44±0.02 | 0.54±0.05 | 0.45±0.04 | 0.36±0.03 | 0.38±0.03 | n.s. | n.s. | n.s. |
| Heart | 0.44±0.02ab | 0.49±0.03a | 0.48±0.02a | 0.52±0.01a | 0.47±0.03ab | 0.43±0.02ab | 0.35±0.04b | 0.35±0.02b | 0.0001 | n.s. | 0.0025 |
| Kidneys | 1.06±0.05abc | 1.12±0.04a | 1.07±0.04abc | 1.11±0.01ab | 1.12±0.07a | 0.99±0.02abc | 0.88±0.05bc | 0.87±0.04c | 0.0007 | n.s. | 0.0125 |
| Spleen | 0.30±0.02 | 0.28±0.04 | 0.30±0.03 | 0.30±0.04 | 0.26±0.03 | 0.21±0.01 | 0.17±0.02 | 0.18±0.02 | 0.0001 | n.s. | n.s. |
| Adipose tissue | |||||||||||
| Epididymal | 2.19±0.20c | 2.19±0.17c | 1.74±0.17c | 1.79±0.14c | 3.57±0.42b | 4.57±0.27ab | 5.65±0.17a | 5.41±0.43a | <.0001 | 0.0209 | 0.0001 |
| Subcutaneous | 1.36±0.24d | 1.47±0.15cd | 1.05±0.08d | 1.05±0.17d | 2.65±0.45c | 3.39±0.29b | 4.63±0.50ab | 4.95±0.21a | <.0001 | 0.0093 | 0.0002 |
| Mesenteric | 1.32±0.09c | 1.52±0.13bc | 1.22±0.06c | 1.12±0.10c | 2.02±0.23ab | 2.17±0.14a | 2.74±0.22a | 2.52±0.19a | <.0001 | n.s. | 0.0149 |
| Retroperitoneal | 0.59±0.08c | 0.63±0.09c | 0.39±0.05c | 0.42±0.07c | 1.09±0.12b | 1.56±0.11a | 2.00±0.09a | 1.77±0.09a | <.0001 | 0.0016 | <.0001 |
| Total | 5.45±0.57c | 5.81±0.46c | 4.40±0.28c | 4.38±0.46c | 9.34±1.13b | 11.70±0.69ab | 15.02±0.85a | 14.65±0.62a | <.0001 | 0.0085 | <.0001 |
Mice were treated with three doses of permethrin (50, 500, and 5000 μg/kg body weight/day) for 12 weeks. Values represent means ± SE (n=5–8). Means with different superscripts within the same row are significantly different at P<0.05. Abbreviations: n.s., not significant; Perm, permethrin.
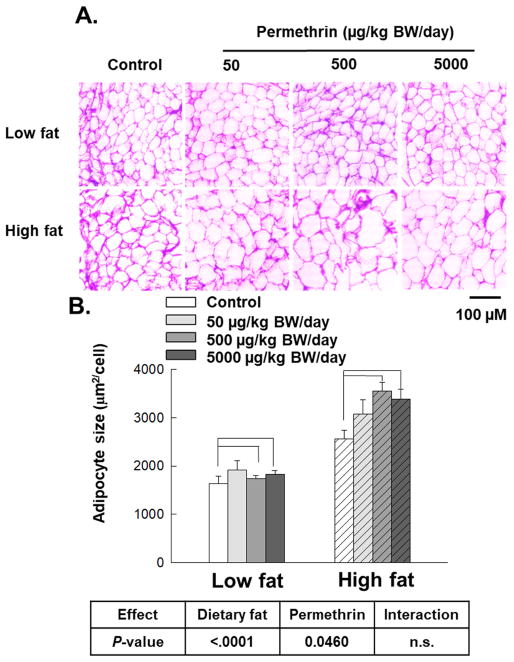
High fat diet treatments significantly increased total adipose tissue weight versus low fat diet-fed groups (202% increase, P<0.0001). Permethrin treatments significantly increased total adipose tissue weight versus the control groups (36% increase, P=0.0085). In particular, permethrin treatments (500 and 5000 μg/kg BW/day) with high fat diet significantly increased total adipose tissue mass compared with the high fat diet control (78% and 80% increases; P<0.0001 and P=0.0002, respectively), while no effects of permethrin was observed in low fat diet-fed groups. Consistently, adipocyte cell sizes were significantly increased by high fat diet (94% increase over low fat diet-fed groups, P<0.0001) and permethrin treatments (17% increase over the controls, P=0.0460), while no diet and permethrin interaction was observed (Fig. 2B).
Figure 2.
Effects of permethrin treatment on epididymal adipocyte size. Mice were treated with either control or permethrin [50, 500, and 5000 μg/kg body weight (BW)/day] in either low fat or high fat diet for 12 weeks. Values represent means ± S.E. (n= 3).
Effect of permethrin on glucose homeostasis
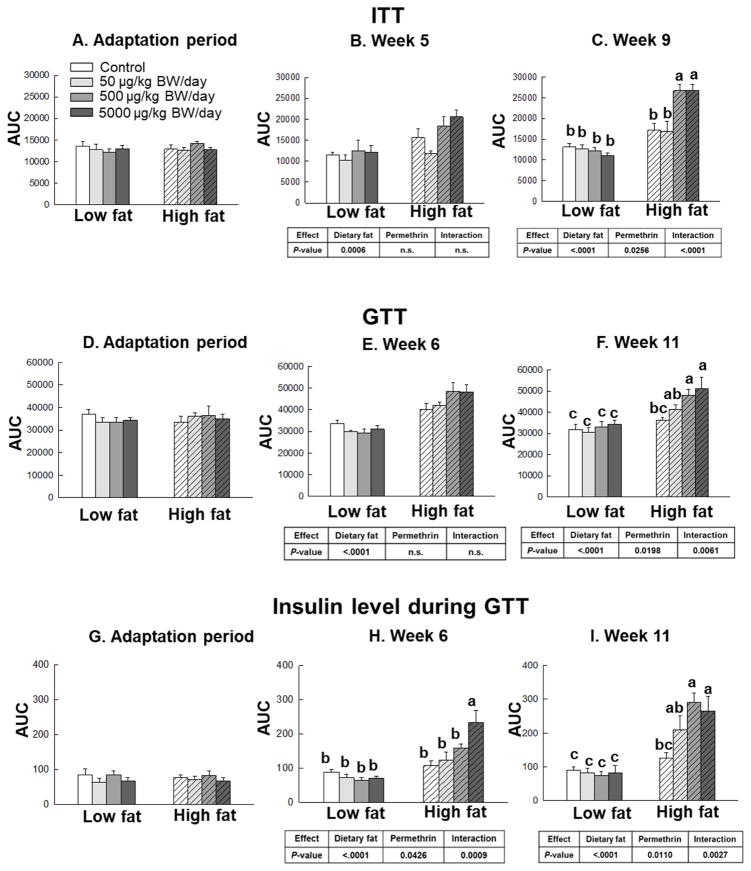
To determine the role of permethrin in dietary fat-induced insulin resistance, insulin tolerance and glucose tolerance tests were completed along with measurement of serum insulin during GTT and HOMA-IR calculations (Fig. 3 and 4, Supplementary Fig. S1, S2, and S3). There were no significant differences on insulin responsiveness measured by ITT, GTT, insulin levels, or HOMA-IR during adaptation period (Fig. 3A, 3D, 3G, and 4A). In weeks 5 and 9, the high fat diet-fed groups showed significantly increased insulin resistance as measured by ITT vesus the low fat diet-fed groups (46% and 73% increases, P=0.0006 and P<0.0001, respectively, Fig. 3B and 3C). Permethrin treatments only showed significant effect on insulin responsiveness measured by ITT in week 9 (15% increase over control groups, P=0.0256) with significant interaction (P<0.0001). In the high fat diet-fed groups, animals with the middle and highest dose of permethrin (500 μg/kg and 5000 μg/kg, respectively) showed significantly increased insulin resistance compared to the high fat diet control at week 9 (56% and 56% increases, P=0.0046 and P=0.0041, respectively, Fig. 3C). No significant effects of permethrin treatment, however, were found between low fat diet-fed groups.
Figure 3.
Effects of permethrin treatment on insulin responsiveness. Insulin tolerance test (ITT, Figure 3A–C), glucose tolerance test (GTT, Figure 3D–F), and insulin level during GTT (Figure 3G–I). Mice were treated with either control or permethrin [50, 500, and 5000 μg/kg body weight (BW)/day] in either low fat or high fat diet for 12 weeks. Value represent means ± S.E. (n= 4–8). Means with different letters are significantly different (P< 0.05).
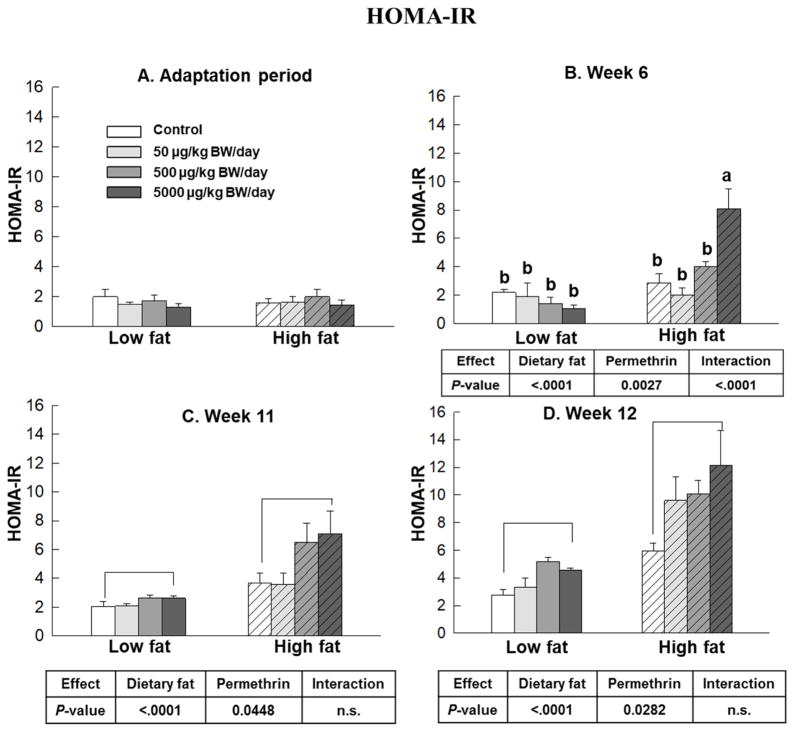
Figure 4.
Effects of permethrin on HOMA-IR score. HOMA-IR score was calculated during adaptation period, weeks 6, 11 and 12 with HOMA-IR calculator. Mice were treated with either control or permethrin [50, 500, and 5000 μg/kg body weight (BW)/day] in either low fat or high fat diet for 12 weeks. Value represent means ± S.E. (n= 4–8). Means with different letters are significantly different (P< 0.05).
For GTT, the high fat diet significantly increased glucose intolerance in weeks 6 and 11 (43% and 36% increase over the low fat diet-fed groups, P<0.0001 for both weeks, Figure 3E and 3F). Permethrin treatments significantly impaired glucose tolerance only in week 11 (17% increase over the controls, P=0.0198) with significant interaction (P = 0.0061). In the high fat diet-fed groups, animals with permethrin (500 μg/kg and 5000 μg/kg) showed significantly impaired glucose tolerance over the high fat diet control at week 11 (33% and 41% increases over the high fat diet control, respectively).
Insulin levels were measured during glucose tolerance test as a marker of glucose homeostasis (Figure 3G–3I) (Ayala, et al., 2010). The high fat diet-fed groups showed significantly increased insulin level in weeks 6 and 11 compared to the low fat diet-fed groups (103% and 156% increases, respectively, P<0.0001 for both weeks). Permethrin treatments significantly increased insulin levels during the GTT in weeks 6 and 11 (24% and 58% increases over the controls, P=0.0426 and P=0.011, respectively, Fig. 3H and 3I). There was also significant interaction effect between dietary fat and permethrin treatment for insulin level in week 6 and 11 (P=0.0009 and P=0.0027). Permethrin treatments at 500 and 5000 μg/kg BW/day with the high fat diet significantly increased insulin level versus the high fat diet control (132% increase, P =0.0030 for 500 μg/kg BW/day in week 6; 117% and 112% increase, P=0.0008 and P=0.0179, for 500 and 5000 μg/kg BW/day in week 11, respectively).
Results of HOMA-IR are shown in Figure 4. The high fat diet-fed groups showed significantly increased HOMA-IR score compared with the low fat diet-fed groups in weeks 6, 11, and 12 (162%, 218%, and 300% increases, respectively, P<0.0001 for all, Fig. 4B–4D). Permethrin treatments significantly increased HOMA-IR score versus the control groups in weeks 6, 11, and 12 (54%, 47%, and 99% increases; P=0.0027, P=0.0448, and P=0.0282, respectively). Significant interactions were observed at week 6, but not in weeks 11 and 12. In week 6, permethrin treatment at 5000 μg/kg BW/day in the high fat diet-fed groups significantly increased HOMA-IR compared to the high fat diet control (252% increase, P<0.0001). Overall, these results suggest that permethrin aggravate high fat diet-induced insulin resistance.
Effect of permethrin on serum markers
Results of serum analyses are shown in Table 2. The high fat diet significantly increased serum levels of insulin (254% increase, P< 0.0001), glucose (22% increase, P= 0.0003), leptin (779% increase, P< 0.0001) and cholesterol (27% increase, P<0.0001) when compared with the low fat diet-fed groups, but not the non-esterified fatty acid or TG levels. Overall, permethrin treatments significantly increased serum levels of insulin (104% increase, P=0.0487), glucose (8% increase, P=0.0099), leptin (131% increase, P<0.0001), TG (21% increase, P=0.0357), and cholesterol (11% increase, P=0.0132), but not the non-esterified fatty acid levels. There was a significant interaction between dietary fat and permethrin treatment on glucose (P=0.0011), leptin (P<0.0001) and cholesterol (P=0.0150). There were no significant effects of permethrin on any of the serum parameters measured in the low fat diet-fed groups.
Table 2.
Serum parameters
| Low fat | High fat | P-value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||
| Permethrin doses | Permethrin doses | ||||||||||
|
|
|
|
|||||||||
| Control | 50 μg/kg | 500 μg/kg | 5000 μg/kg | Control | 50 μg/kg | 500 μg/kg | 5000 μg/kg | Dietary fat | Perm | Interaction | |
| Insulin (ng/mL) | 0.77±0.11 | 0.86±0.29 | 1.43±0.48 | 1.31±0.08 | 2.05±0.34 | 3.76±0.47 | 2.89±0.35 | 3.01±0.64 | <.0001 | 0.0487 | n.s. |
| Glucose (mg/dL) | 162.8±7.9b | 159.6±10.7b | 168.3±15.3b | 147.6±13.0b | 178.2±17.8b | 150.4±14.3b | 207.3±5.7ab | 260.1±13.3a | 0.0003 | 0.0099 | 0.0011 |
| Leptin (ng/mL) | 4.9±1.0c | 6.7±1.1c | 3.9±0.6c | 3.2±0.5c | 14.4±3.7b | 35.5±4.4b | 62.4±11.8a | 63.0±5.9a | <.0001 | <.0001 | <.0001 |
| NEFA (mEq/L) | 1.20±0.08 | 1.21±0.14 | 1.46±0.11 | 1.10±0.02 | 1.06±0.09 | 1.20±0.14 | 1.13±0.08 | 1.28±0.04 | n.s. | n.s. | n.s. |
| TG (mmol/L) | 0.77±0.06 | 0.87±0.10 | 0.93±0.13 | 0.74±0.03 | 0.62±0.04 | 0.83±0.05 | 0.91±0.05 | 1.06±0.11 | n.s. | 0.0357 | n.s. |
| Cholesterol (mg/dL) | 162±9c | 156±14c | 169±10bc | 142±13c | 171±15bc | 167±18bc | 246±19a | 229±12ab | <.0001 | 0.0132 | 0.0150 |
Mice were treated with three doses of permethrin (50, 500, and 5000 μg/kg body weight/day) for 12 weeks. Values represent means ± SE (n=5–8). Means with different superscripts within the same row are significantly different at P<0.05. Abbreviations: n.s., not significant; TG, triglyceride; NEFA, non-esterified fatty acid. Perm, permethrin.
Effects of permethrin on markers of epididymal white adipose tissue
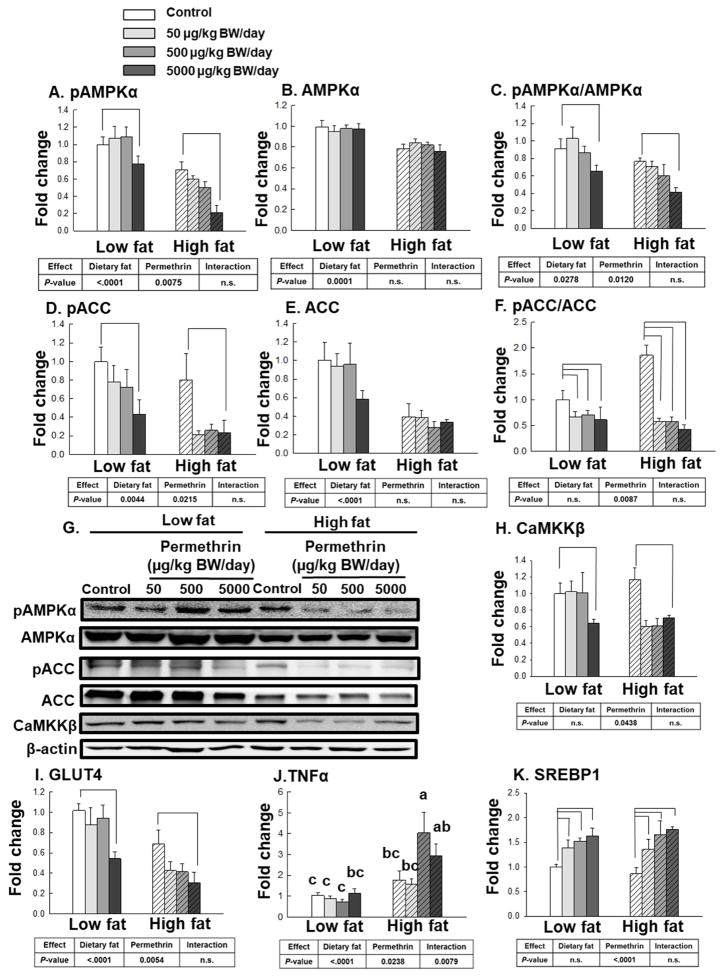
Based on the previous report that permethrin potentiate adipogenesis via inhibiting the activation of AMPK in 3T3-L1 adipocytes (J. Kim, et al., 2014), we have measured AMPKα activation in epididymal adipose tissue (Fig. 5). The high fat diet significantly decreased phosphorylated AMPKα (49%, P<0.0001), AMPKα (38%, P<0.0001), and the ratio of pAMPKα/AMPKα (25%, P=0.0278) compared with the low fat diet-fed groups (Fig. 5A–5C). Permethrin treatments significantly decreased pAMPKα (17% reduction, P=0.0075) and pAMPKα/AMPKα (18% reduction, P=0.0120), but not AMPKα, compared with the controls. No significant interaction effects were observed for pAMPKα, AMPKα, and pAMPKα/AMPKα ratio.
Figure 5.
Effects of permethrin treatment on molecular targets involved in lipid metabolism and inflammation in the epididymal white adipose tissue. A. Protein levels of phosphorylated AMPKα (pAMPKα); B. AMPKα; C. pAMPKα to AMPKα ratio; D. Phosphorylated acetyl-CoA carboxylase (pACC); E. Acetyl-CoA carboxylase (ACC); F. pACC to ACC ratio; and G. Representative pictures. H. Ca2+/calmodulin-dependent protein kinase kinase β (CaMKKβ); I, glucose transporter type 4 (GLUT4); J, tumor necrosis factor-α (TNFα); and K, sterol regulatory element-binding protein (SREBP1). Mice were treated with either control or permethrin [50, 500, and 5000 μg/kg body weight (BW)/day] in either low fat or high fat diet for 12 weeks. Value represent means ± S.E. (n= 4–5). Means with different letters are significantly different (P<0.05).
As one of down-stream targets of AMPK, ACC gets phosphorylated at Ser 79 (pACC) resulting in inactivation of ACC (Canto, et al., 2010; Ha, et al., 1994). The high fat diet significantly decreased pACC (49% reduction, P=0.0044) and ACC (60% reduction, P<0.0001), but not pACC/ACC ratio (Fig. 5D–5F). Permethrin treatments significantly decreased pACC (51% reduction, P=0.0215) and pACC/ACC ratio (59% reduction, P=0.0087), but not ACC. No significant interaction effects were found for pACC, ACC, and pACC/ACC ratio.
Calcium/calmodulin-dependent protein kinase kinase-beta (CaMKKβ) is one of the upstream regulators of AMPK (Hawley, et al., 2005). The current results showed permethrin significantly decreased the protein level of CaMKKβ (30% reduction, P=0.0438), while no effects of dietary fat or interactions were observed (Fig. 5H).
Glucose transporter-4 (GLUT4) is the major glucose transporter responsible for insulin-stimulated glucose uptake expressed in both adipose tissue and muscle (Bell, et al., 1990). High fat diet and permethrin treatments significantly decreased GLUT4 expression compared with low fat diet-fed and the control groups, respectively (46% and 31% reductions; P<0.0001 and P=0.0054, respectively, Fig. 5I). We also measured the expression of tumor necrosis factor-α (TNFα), as it is an important inflammatory cytokine that plays a key role in obesity induced insulin resistance in type 2 diabetes (Hotamisligil, 1999b). Significantly higher levels of TNFα expression were observed by both high fat diet (172% increase, P<0.0001) and permethrin (34% increase, P=0.0238) with interaction (P=0.0079) from the white adipose tissue (Fig. 5J). Sterol regulatory element-binding protein 1 (SREBP1) is another important regulator of adipogenesis (J. B. Kim, et al., 1996) and permethrin significantly increased SREBP (50% increase, P<0.0001) but no effects of dietary fat or interaction were observed (Fig. 5K). We also tested CD36 (regulates fatty acid uptake) and acyl CoA: diacylglycerol acyltransferase 1 and 2 (DGAT, catalyzes mammalian triacylglycerol synthesis and lipid droplet formation), however, there were no effects of dietary fat or permethrin for these markers (Supplementary Fig. S4) (Coburn, et al., 2000; Harris, et al., 2011).
Effects of permethrin on the liver
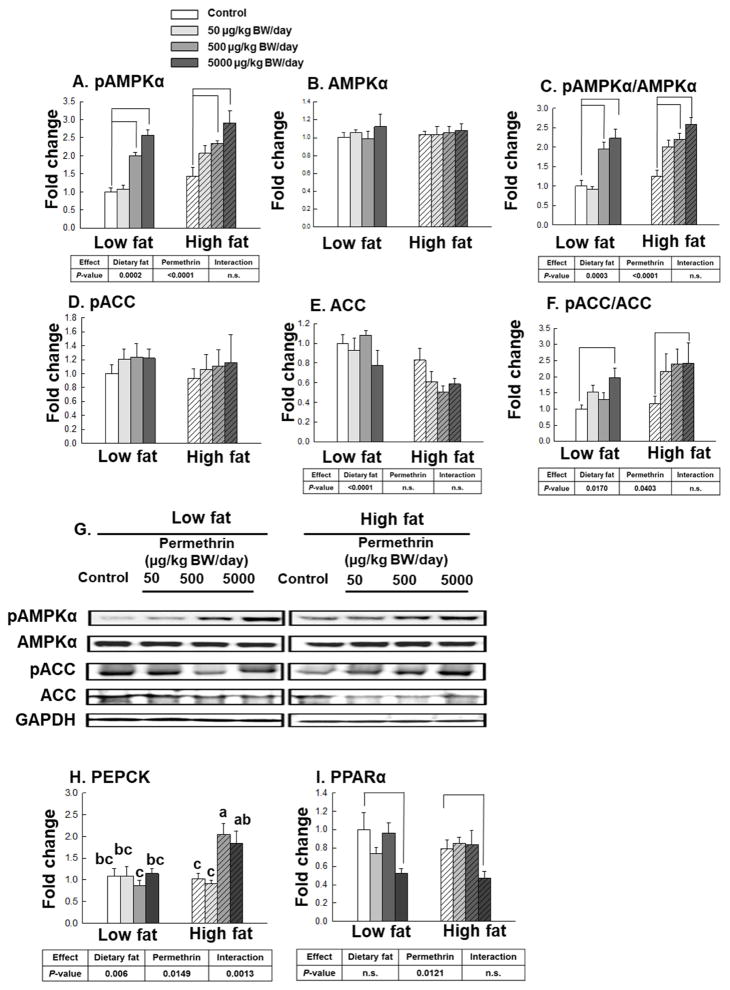
We have measured AMPKα in the liver (Fig. 6). The high fat diet significantly increased phosphorylated AMPKα (32% increase, P=0.0002) and ratio of pAMPKα/AMPKα (32% increase, P=0.0003) compared with the low fat diet-fed groups, but not AMPKα (Fig. 6A–6C). Permethrin treatments significantly increased pAMPKα (78% increase, P<0.0001) and pAMPKα/AMPKα ratio (77% increase, P<0.0001), but not AMPKα, compared with the controls. No significant interaction effects were observed for pAMPKα, AMPKα, and pAMPKα/AMPKα ratio. The high fat diet significantly decreased ACC (33% reduction, P<0.0001) but not pACC, which result in increased pACC/ACC ratio (40% increase, P=0.0170) (Fig. 6D–6F). Permethrin treatments significantly increased pACC/ACC ratio (81% increase, P=0.0403), but not pACC or ACC, compared with the controls. No significant interaction effects were found for pACC, ACC, and pACC/ACC ratio.
Figure 6.
Effects of permethrin treatment on molecular targets involved in glucose and lipid metabolism in the liver. A. Phosphorylated AMPKα (pAMPKα); B. AMPKα; C. pAMPKα to AMPKα ratio; D. Phosphorylated acetyl-CoA carboxylase (pACC); E. Acetyl-CoA carboxylase (ACC); pACC to ACC ratio; G. Representative pictures; H. phosphoenolpyruvate carboxykinase (PEPCK); I, peroxisome proliferator-activated receptor-α (PPARα). Mice were treated with either control or permethrin [50, 500, and 5000 μg/kg body weight (BW)/day] in either low fat or high fat diet for 12 weeks. Value represent means ± S.E. (n= 3–5). Means with different letters are significantly different (P< 0.05).
The increase in hepatic gluconeogenesis is believed to play an important role in the elevation of fasting blood glucose level and pathogenesis of diabetes (Beale, et al., 2007; Valera, et al., 1994). Phosphoenolpyruvate carboxykinase (PEPCK) is the key enzyme regulating gluconeogenesis, as overexpression of hepatic PEPCK gene in mice lead to the development of non-insulin-dependent diabetes mellitus (Beale, et al., 2007; Valera, et al., 1994). High fat diet and permethrin treatments significantly increased PEPCK gene expression versus low fat diet-fed groups and control groups, respectively (39% and 25% increases; P=0.006 and P=0.0149, respectively) with significant interactions (P=0.0013, Fig. 6H). Permethrin treatments at 500 and 5000 μg/kg BW/day significantly elevated PEPCK gene expression over the controls in the high fat diet-fed groups (99% and 79%; P=0.0056 and P=0.0431, respectively). No significant difference, however, was found in the low fat diet-fed groups.
PPARα is expressed principally in the liver where it plays important role in regulating fatty acid oxidation (Reddy, et al., 2001). Permethrin treatments significantly decreased PPARα versus the controls (19% reduction, P=0.0121, Figure 6I), but there were no significant effects of diet or interaction.
Effects of permethrin on glucose and lipid metabolism in gastrocnemius skeletal muscle
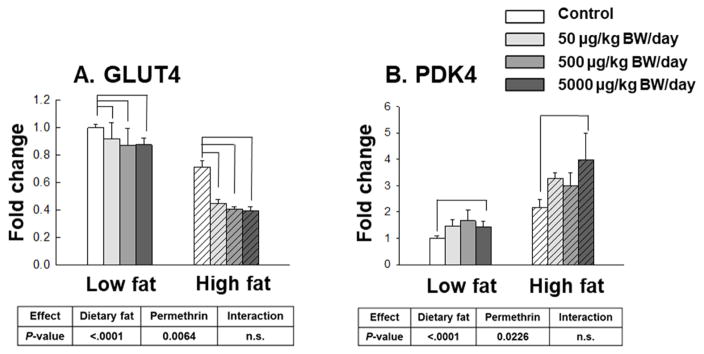
High fat diet and permethrin treatments significantly decreased GLUT4 gene expression (47% and 24% reductions, P<0.0001 and P=0.0064, respectively) compared with low fat diet-fed and the control groups without interactions (Fig. 7A). All permethrin treatments significantly decreased GLUT4 gene expression in muscle compared with control (as shown by brackets).
Figure 7.
Effects of permethrin treatment on gene expression regulating glucose metabolism in gastrocnemius skeletal muscle. A. Glucose transporter type 4 (GLUT4); B. pyruvate dehydrogenase kinase 4 (PDK4). Mice were treated with either control or permethrin [50, 500, and 5000 μg/kg body weight (BW)/day] in either low fat or high fat diet for 12 weeks. Value represent means ± S.E. (n= 4–5).
Pyruvate dehydrogenase kinase (PDK) acts to phosphorylate and inactivate pyruvate dehydrogenase complex, which facilitate a rate-limiting step in glucose oxidation by converting pyruvate to acetyl-CoA (Sugden, et al., 2003). A previous study showed that increased skeletal muscle PDK gene expression was usually found in the insulin resistance states (Y. I. Kim, et al., 2006). In this study, high fat diet and permethrin treatments significantly increased PDK4 gene expression (114% and 58% increases, P<0.0001 and P=0.0226, respectively) compared with the low fat diet-fed and control groups without interactions (Fig. 7B).
Discussion
The current study showed that daily administration of permethrin, at the NOAEL or lower doses, potentiated high fat diet-induced weight and fat mass gains as well as altered insulin resistance in male mice. Previously, permethrin was found to promote high fat diet-induced insulin resistance without effect on body weight in female mice (Xiao, Kim, et al., 2017). To our knowledge, this is the first study reporting the potential role of permethrin in dietary fat-induced weight gain and insulin resistance in male mice. The current results along with previous report (Xiao, Kim, et al., 2017) suggest that there was a sex-dependent effect of permethrin on high fat diet-induced weight gain and insulin resistance. Increase intracellular calcium and ER stress may be contributed to altered adipogenesis and insulin resistance by permethrin (Basseri, et al., 2009; Cnop, et al., 2012; Draznin, et al., 1988; Jones, et al., 1996; Ozcan, et al., 2004; Sha, et al., 2009; Xiao, Qi, et al., 2017; Zemel, et al., 1995; Zemel, et al., 2000), although this does not explain the sex-dependent and tissue specific effects of permtherin.
The current and our previous report suggest sex-dependent responses to permethrin on high fat-diet induced weight gain (Xiao, Kim, et al., 2017). In the previous study, treatment with permethrin had no effect on weight gain in females, even though permethrin decreased voluntary activities, thus reducing energy expenditure, without any influence on energy intake (Xiao, Kim, et al., 2017). Since we did not measure voluntary activities in the current study, we are unable to make any conclusion whether permethrin influenced energy expenditure by altering activity levels in male mice. In addition, it is known that the brown/beige adipose tissue plays important roles in energy expenditure by producing heat through thermogenesis (Kajimura, et al., 2015; Seale, et al., 2007), however, it is not clear whether permethrin could target brown/beige adipose tissue to influence energy expenditure, sex-dependently. Thus, it would be helpful to measure total energy expenditure, including thermogenesis, along with phenotypes of the brown/beige adipose tissues in both females and males to directly compare the sex-dependent effects of permethrin on weight gain.
The current and previous report indicate that permethrin increased insulin resistance in both males and females (Xiao, Kim, et al., 2017). This may be due in part by decreasing the activation of AKT via extracellular signal-regulated kinase-1 (ERK)-mediated, but not AMP-activated protein kinase α (AMPKα)-mediated mechanism as seen in C2C12 myotubes (J. Kim, et al., 2014; Sun, Peng, et al., 2017). In addition to AKT, permethrin treatment significantly increased TNFα gene expression in adipose tissue. In this study, we did not measure the TNFα level in the serum samples, however, it was previously established that adipose tissue gene expression level of TNFα is correlated with serum TNFα level (Winkler, et al., 2003). As TNFα is one of the major factors in obesity-induced insulin resistance (Hotamisligil, 1999b) by increasing serine phosphorylation of insulin receptor substrate 1 (IRS-1) and by inhibiting insulin receptor activities (Hotamisligil, 1999a), the effect of permethrin on increased TNFα gene expression may contribute to permethrin’s effects on insulin resistance. Lastly, increased PDK4 gene expression in the skeletal muscle and increased PEPCK gene expression in the liver by permethrin treatment would further contributed to altered glucose metabolism in these animals. Additional mechanistic studies on insulin signaling pathway including AKT signaling and insulin-stimulated GLUT4 translocation will be needed to identify the exact mechanisms of permethrin on altered insulin responsiveness.
Previously, our group reported that neonicotinoid insecticide, imidacloprid, promoted high fat diet-induced weight gain and insulin resistance in male mice (Sun, Xiao, et al., 2016). However, imidacloprid treatment promoted high fat diet-induced weight gain without causing insulin resistance in females (Sun, Qi, et al., 2017), while permethrin treatment increased high fat diet-induced insulin resistance without weight gain (Xiao, Kim, et al., 2017). Imidacloprid and permethrin belongs to different structural classes of insecticides and have different mechanisms of action; imidacloprid targets the nicotinic acetylcholine receptors (Xiao, Clark, et al., 2017), while permethrin activates the voltage sensitive sodium channels (VSSCs) (Soderlund, 2012). However, both imidacloprid and permethrin are known to cause membrane-depolarization, leading to common physiological responses in non-target tissues (J. Kim, et al., 2013, 2014; Park, et al., 2013). Moreover, even in males, imidacloprid treatment significantly decreased the activation of AMPKα both in the liver and the white adipose tissue (Sun, Xiao, et al., 2016), whereas permethrin treatment resulted in different responses in those tissues; decreased the activation of AMPKα in the white adipose tissue while increased it in the liver. In addition, exposure to imidacloprid was previously reported to increase CD36 in white adipose tissue of male mice (Sun, Xiao, et al., 2016), whereas permethrin treatment resulted no effect. Currently, it is not clear how these two insecticides elicit high fat diet-induced sex-dependent responses of weight gain and/or insulin resistance. While it seems likely that there are common underlying mechanisms leading these symptoms, there may be specific responses that are different between these
In summary, the current study reports the potential role of daily exposure to relatively low levels of permethrin in the development of high fat diet-induced obesity and insulin resistance in male mice in comparison to the previous report on females (Xiao, Kim, et al., 2017). Although further mechanistic studies are needed to explore how permethrin elicits these effects sex-dependently, it can be concluded from the current study that permethrin might target AMPK, fatty acid oxidation and energy expenditure to induce excessive weight gain and AKT signaling pathway to induce insulin resistance in male mice.
Supplementary Material
Permethrin promoted high fat diet-induced insulin resistance in male mice
Permethrin promoted high fat diet-induced weight and fat mass gain in male mice
Permethrin inhibited AMP-activated protein kinase in white adipose tissue
Acknowledgments
This project was supported by NIH R21ES023676. Authors would like to thank Renalison Farias Pereira, Jinning Liu and Jiaying Wang for help to analyze the adipocyte size data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andrikopoulos S, Blair AR, Deluca N, Fam BC, Proietto J. Evaluating the glucose tolerance test in mice. Am J Physiol Endocrinol Metab. 2008;295(6):E1323–1332. doi: 10.1152/ajpendo.90617.2008. [DOI] [PubMed] [Google Scholar]
- Ayala JE, Samuel VT, Morton GJ, Obici S, Croniger CM, Shulman GI, Wasserman DH, McGuinness OP, Consortium NIHMMPC. Standard operating procedures for describing and performing metabolic tests of glucose homeostasis in mice. Dis Model Mech. 2010;3(9–10):525–534. doi: 10.1242/dmm.006239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basseri S, Lhotak S, Sharma AM, Austin RC. The chemical chaperone 4-phenylbutyrate inhibits adipogenesis by modulating the unfolded protein response. J Lipid Res. 2009;50(12):2486–2501. doi: 10.1194/jlr.M900216-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beale EG, Harvey BJ, Forest C. PCK1 and PCK2 as candidate diabetes and obesity genes. Cell Biochem Biophys. 2007;48(2–3):89–95. doi: 10.1007/s12013-007-0025-6. [DOI] [PubMed] [Google Scholar]
- Bell GI, Kayano T, Buse JB, Burant CF, Takeda J, Lin D, Fukumoto H, Seino S. Molecular biology of mammalian glucose transporters. Diabetes Care. 1990;13(3):198–208. doi: 10.2337/diacare.13.3.198. [DOI] [PubMed] [Google Scholar]
- Canto C, Auwerx J. AMP-activated protein kinase and its downstream transcriptional pathways. Cell Mol Life Sci. 2010;67(20):3407–3423. doi: 10.1007/s00018-010-0454-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Z, Shafer TJ, Murray TF. Mechanisms of pyrethroid insecticide-induced stimulation of calcium influx in neocortical neurons. J Pharmacol Exp Ther. 2011;336(1):197–205. doi: 10.1124/jpet.110.171850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CEPADP. California enviromental protection agency department of pesticide regulation medical toxicology branch summary of toxicology data pyrethrins 1987 [Google Scholar]
- Choi JS, Soderlund DM. Structure-activity relationships for the action of 11 pyrethroid insecticides on rat Na(v)1.8 sodium channels expressed in Xenopus oocytes. Toxicol Appl Pharmacol. 2006;211(3):233–244. doi: 10.1016/j.taap.2005.06.022. [DOI] [PubMed] [Google Scholar]
- Christensen SD, Mikkelsen LF, Fels JJ, Bodvarsdottir TB, Hansen AK. Quality of plasma sampled by different methods for multiple blood sampling in mice. Lab Anim. 2009;43(1):65–71. doi: 10.1258/la.2008.007075. [DOI] [PubMed] [Google Scholar]
- Clark JM, Symington SB. Pyrethroid action on calcium channels: neurotoxicological implications. Invert Neurosci. 2007;7(1):3–16. doi: 10.1007/s10158-006-0038-7. [DOI] [PubMed] [Google Scholar]
- Clark JM, Symington SB. Advances in the mode of action of pyrethroids. Top Curr Chem. 2012;314:49–72. doi: 10.1007/128_2011_268. [DOI] [PubMed] [Google Scholar]
- Clark JM, Yoon KS, Lee SH, Pittendrigh BR. Human lice: Past, present and future control. Pestic Biochem Physiol. 2013;106(3):162–171. [Google Scholar]
- Cnop M, Foufelle F, Velloso LA. Endoplasmic reticulum stress, obesity and diabetes. Trends Mol Med. 2012;18(1):59–68. doi: 10.1016/j.molmed.2011.07.010. [DOI] [PubMed] [Google Scholar]
- Coburn CT, Knapp FF, Jr, Febbraio M, Beets AL, Silverstein RL, Abumrad NA. Defective uptake and utilization of long chain fatty acids in muscle and adipose tissues of CD36 knockout mice. J Biol Chem. 2000;275(42):32523–32529. doi: 10.1074/jbc.M003826200. [DOI] [PubMed] [Google Scholar]
- Di Gregorio GB, Hensley L, Lu T, Ranganathan G, Kern PA. Lipid and carbohydrate metabolism in mice with a targeted mutation in the IL-6 gene: absence of development of age-related obesity. Am J Physiol Endocrinol Metab. 2004;287(1):E182–187. doi: 10.1152/ajpendo.00189.2003. [DOI] [PubMed] [Google Scholar]
- Draznin B, Sussman KE, Eckel RH, Kao M, Yost T, Sherman NA. Possible role of cytosolic free calcium concentrations in mediating insulin resistance of obesity and hyperinsulinemia. J Clin Invest. 1988;82(6):1848–1852. doi: 10.1172/JCI113801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand R, Bouvresse S, Berdjane Z, Izri A, Chosidow O, Clark JM. Insecticide resistance in head lice: clinical, parasitological and genetic aspects. Clin Microbiol Infect. 2012;18(4):338–344. doi: 10.1111/j.1469-0691.2012.03806.x. [DOI] [PubMed] [Google Scholar]
- Elliott M, Farnham AW, Janes NF, Needham PH, Pulman DA, Stevenson JH. A photostable pyrethroid. Nature. 1973;246(5429):169–170. doi: 10.1038/246169a0. [DOI] [PubMed] [Google Scholar]
- Feo ML, Eljarrat E, Barcelo D. Determination of pyrethroid insecticides in environmental samples. Trac-Trends in Analytical Chemistry. 2010;29(7):692–705. [Google Scholar]
- Ha J, Daniel S, Broyles SS, Kim KH. Critical phosphorylation sites for acetyl-CoA carboxylase activity. J Biol Chem. 1994;269(35):22162–22168. [PubMed] [Google Scholar]
- Harris CA, Haas JT, Streeper RS, Stone SJ, Kumari M, Yang K, Han X, Brownell N, Gross RW, Zechner R, Farese RV., Jr DGAT enzymes are required for triacylglycerol synthesis and lipid droplets in adipocytes. J Lipid Res. 2011;52(4):657–667. doi: 10.1194/jlr.M013003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley SA, Pan DA, Mustard KJ, Ross L, Bain J, Edelman AM, Frenguelli BG, Hardie DG. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005;2(1):9–19. doi: 10.1016/j.cmet.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS. Mechanisms of TNF-alpha-induced insulin resistance. Exp Clin Endocrinol Diabetes. 1999a;107(2):119–125. doi: 10.1055/s-0029-1212086. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS. The role of TNFalpha and TNF receptors in obesity and insulin resistance. J Intern Med. 1999b;245(6):621–625. doi: 10.1046/j.1365-2796.1999.00490.x. [DOI] [PubMed] [Google Scholar]
- Howell GIII, Mangum L. Exposure to bioaccumulative organochlorine compounds alters adipogenesis, fatty acid uptake, and adipokine production in NIH3T3-L1 cells. Toxicol In Vitro. 2011;25(1):394–402. doi: 10.1016/j.tiv.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BH, Kim JH, Zemel MB, Woychik RP, Michaud EJ, Wilkison WO, Moustaid N. Upregulation of adipocyte metabolism by agouti protein: possible paracrine actions in yellow mouse obesity. Am J Physiol. 1996;270(1 Pt 1):E192–196. doi: 10.1152/ajpendo.1996.270.1.E192. [DOI] [PubMed] [Google Scholar]
- Kajimura S, Spiegelman BM, Seale P. Brown and Beige Fat: Physiological Roles beyond Heat Generation. Cell Metabolism. 2015;22(4):546–559. doi: 10.1016/j.cmet.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Park Y, Yoon KS, Clark JM, Park Y. Imidacloprid, a neonicotinoid insecticide, induces insulin resistance. J Toxicol Sci. 2013;38(5):655–660. doi: 10.2131/jts.38.655. [DOI] [PubMed] [Google Scholar]
- Kim J, Park Y, Yoon KS, Clark JM, Park Y. Permethrin alters adipogenesis in 3T3-L1 adipocytes and causes insulin resistance in C2C12 myotubes. J Biochem Mol Toxicol. 2014;28(9):418–424. doi: 10.1002/jbt.21580. [DOI] [PubMed] [Google Scholar]
- Kim JB, Spiegelman BM. ADD1/SREBP1 promotes adipocyte differentiation and gene expression linked to fatty acid metabolism. Genes Dev. 1996;10(9):1096–1107. doi: 10.1101/gad.10.9.1096. [DOI] [PubMed] [Google Scholar]
- Kim Y, Kim D, Good DJ, Park Y. Effects of postweaning administration of conjugated linoleic acid on development of obesity in nescient basic helix-loop-helix 2 knockout mice. J Agric Food Chem. 2015;63(21):5212–5223. doi: 10.1021/acs.jafc.5b00840. [DOI] [PubMed] [Google Scholar]
- Kim YI, Lee FN, Choi WS, Lee S, Youn JH. Insulin regulation of skeletal muscle PDK4 mRNA expression is impaired in acute insulin-resistant states. Diabetes. 2006;55(8):2311–2317. doi: 10.107/db05-1606. [DOI] [PubMed] [Google Scholar]
- Lassiter TL, Brimijoin S. Rats gain excess weight after developmental exposure to the organophosphorothionate pesticide, chlorpyrifos. Neurotoxicol Teratol. 2008;30(2):125–130. doi: 10.1016/j.ntt.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Lassiter TL, Ryde IT, Mackillop EA, Brown KK, Levin ED, Seidler FJ, Slotkin TA. Exposure of neonatal rats to parathion elicits sex-selective reprogramming of metabolism and alters the response to a high-fat diet in adulthood. Environ Health Perspect. 2008;116(11):1456–1462. doi: 10.1289/ehp.11673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Aliaga MJ, Matsumura F. Effects of 1,1,1-trichloro-2,2-bis (p-chlorophenyl)-ethane (p,p ‘-DDT) on 3T3-L1 and 3T3-F442A adipocyte differentiation. Biochem Pharmacol. 2002;63(5):997–1007. doi: 10.1016/s0006-2952(01)00933-9. [DOI] [PubMed] [Google Scholar]
- National Research Council. Health effects of permethrin-impregnated army battle-dress uniforms. National Academy of Sciences; Washington DC: 1994. [PubMed] [Google Scholar]
- Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Gorgun C, Glimcher LH, Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306(5695):457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- Park Y, Kim Y, Kim J, Yoon KS, Clark J, Lee J, Park Y. Imidacloprid, a neonicotinoid insecticide, potentiates adipogenesis in 3T3-L1 adipocytes. J Agric Food Chem. 2013;61(1):255–259. doi: 10.1021/jf3039814. [DOI] [PubMed] [Google Scholar]
- Ray DE, Fry JR. A reassessment of the neurotoxicity of pyrethroid insecticides. Pharmacol Ther. 2006;111(1):174–193. doi: 10.1016/j.pharmthera.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Reddy JK, Hashimoto T. Peroxisomal beta-oxidation and peroxisome proliferator-activated receptor alpha: an adaptive metabolic system. Annu Rev Nutr. 2001;21:193–230. doi: 10.1146/annurev.nutr.21.1.193. [DOI] [PubMed] [Google Scholar]
- Seale P, Kajimura S, Yang W, Chin S, Rohas LM, Uldry M, Tavernier G, Langin D, Spiegelman BM. Transcriptional control of brown fat determination by PRDM16. Cell Metab. 2007;6(1):38–54. doi: 10.1016/j.cmet.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha HB, He Y, Chen H, Wang C, Zenno A, Shi H, Yang XY, Zhang XM, Qi L. The IRE1 alpha-XBP1 pathway of the unfolded protein response is required for adipogenesis. Cell Metab. 2009;9(6):556–564. doi: 10.1016/j.cmet.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen P, Hsieh TH, Yue Y, Sun Q, Clark JM, Park Y. Deltamethrin increases the fat accumulation in 3T3-L1 adipocytes and Caenorhabditis elegans. Food Chem Toxicol. 2017;101:149–156. doi: 10.1016/j.fct.2017.01.015. [DOI] [PubMed] [Google Scholar]
- Soderlund DM. Molecular mechanisms of pyrethroid insecticide neurotoxicity: recent advances. Arch Toxicol. 2012;86(2):165–181. doi: 10.1007/s00204-011-0726-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderlund DM. Resmethrin, the first modern pyrethroid insecticide. Pest Manag Sci. 2015;71(6):801–807. doi: 10.1002/ps.3881. [DOI] [PubMed] [Google Scholar]
- Soderlund DM, Clark JM, Sheets LP, Mullin LS, Piccirillo VJ, Sargent D, Stevens JT, Weiner ML. Mechanisms of pyrethroid neurotoxicity: implications for cumulative risk assessment. Toxicology. 2002;171(1):3–59. doi: 10.1016/s0300-483x(01)00569-8. [DOI] [PubMed] [Google Scholar]
- Sparks TC. Insecticide discovery: An evaluation and analysis. Pestic Biochem Physiol. 2013;107(1):8–17. doi: 10.1016/j.pestbp.2013.05.012. [DOI] [PubMed] [Google Scholar]
- Sugden MC, Holness MJ. Recent advances in mechanisms regulating glucose oxidation at the level of the pyruvate dehydrogenase complex by PDKs. Am J Physiol-Endoc M. 2003;284(5):E855–E862. doi: 10.1152/ajpendo.00526.2002. [DOI] [PubMed] [Google Scholar]
- Sun Q, Peng Y, Qi W, Kim Y, Clark JM, Kim D, Park Y. Permethrin decreased insulin-stimulated AKT phosphorylation dependent on extracellular signal-regulated kinase-1 (ERK), but not AMP-activated protein kinase alpha (AMPKalpha), in C2C12 myotubes. Food Chem Toxicol. 2017;109:95–101. doi: 10.1016/j.fct.2017.08.046. [DOI] [PubMed] [Google Scholar]
- Sun Q, Qi W, Xiao X, Yang SH, Kim D, Yoon KS, Clark JM, Park Y. Imidacloprid promotes high fat diet-induced adiposity in female C57BL/6J mice and enhance adipogenesis in 3T3-L1 adipocytes via AMPKalpha-mediated pathway. J Agric Food Chem. 2017;65:6672–6581. doi: 10.1021/acs.jafc.7b02584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Qi W, Yang J, Yoon KS, Clark JM, Park Y. Fipronil promotes adipogenesis via AMPK alpha-mediated pathway in 3T3-L1 adipocytes. Food Chem Toxicol. 2016;92:217–223. doi: 10.1016/j.fct.2016.04.011. [DOI] [PubMed] [Google Scholar]
- Sun Q, Xiao X, Kim Y, Kim D, Yoon KS, Clark JM, Park Y. Imidacloprid promotes high fat diet-induced adiposity and insulin resistance in male C57BL/6J mice. J Agric Food Chem. 2016;64(49):9293–9306. doi: 10.1021/acs.jafc.6b04322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J, Liu Z, Wang R, Huang ZY, Chen AC, Gurevitz M, Dong K. Identification of amino acid residues in the insect sodium channel critical for pyrethroid binding. Mol Pharmacol. 2005;67(2):513–522. doi: 10.1124/mol.104.006205. [DOI] [PubMed] [Google Scholar]
- Valera A, Pujol A, Pelegrin M, Bosch F. Transgenic mice overexpressing phosphoenolpyruvate carboxykinase develop non-insulin-dependent diabetes mellitus. Proc Natl Acad Sci U S A. 1994;91(19):9151–9154. doi: 10.1073/pnas.91.19.9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijverberg HP, van den Bercken J. Annotation. Action of pyrethroid insecticides on the vertebrate nervous system. Neuropathol Appl Neurobiol. 1982;8(6):421–440. doi: 10.1111/j.1365-2990.1982.tb00311.x. [DOI] [PubMed] [Google Scholar]
- Villeneuve DC, Van Logten MJ, Den Tonkelaar EM, Greve PA, Vos JG, Speijers GJA, Van Esch GJ. Effect of food deprivation on low level hexa chloro benzene exposure in rats. Sci Total Environ. 1977;8(2):179–186. doi: 10.1016/0048-9697(77)90076-6. [DOI] [PubMed] [Google Scholar]
- Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27(6):1487–1495. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- WHO. Environmental health criteria for permethrin. Vol. 94. WHO; Geneva: 1990a. [Google Scholar]
- WHO. Permethrin (40:60 cis:trans isomer ratio) world health organization WHO specifications and evaluations for public health pesticides. 1990b. [Google Scholar]
- Winkler G, Kiss S, Keszthelyi L, Sapi Z, Ory I, Salamon F, Kovacs M, Vargha P, Szekeres O, Speer G, Karadi I, Sikter M, Kaszas E, Dworak O, Gero G, Cseh K. Expression of tumor necrosis factor (TNF)-alpha protein in the subcutaneous and visceral adipose tissue in correlation with adipocyte cell volume, serum TNF-alpha, soluble serum TNF-receptor-2 concentrations and C-peptide level. Eur J Endocrinol. 2003;149(2):129–135. doi: 10.1530/eje.0.1490129. [DOI] [PubMed] [Google Scholar]
- Xiao X, Clark JM, Park Y. Potential contribution of insecticide exposure and development of obesity and type 2 diabetes. Food Chem Toxicol. 2017;105:456–474. doi: 10.1016/j.fct.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X, Kim J, Sun Q, Kim D, Park CS, Lu TS, Park Y. Preventive effects of cranberry products on experimental colitis induced by dextran sulphate sodium in mice. Food Chem. 2015;167:438–446. doi: 10.1016/j.foodchem.2014.07.006. [DOI] [PubMed] [Google Scholar]
- Xiao X, Kim Y, Kim D, Yoon KS, Clark JM, Park Y. Permethrin alters glucose metabolism in conjunction with high fat diet by potentiating insulin resistance and decreases voluntary activities in female C57BL/6J mice. Food Chem Toxicol. 2017;108:161–170. doi: 10.1016/j.fct.2017.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X, Qi W, Clark JM, Park Y. Permethrin potentiates adipogenesis via intracellular calcium and endoplasmic reticulum stress-mediated mechanisms in 3T3-L1 adipocytes. Food Chem Toxicol. 2017;109:123–129. doi: 10.1016/j.fct.2017.08.049. [DOI] [PubMed] [Google Scholar]
- Yoon KS, Symington SB, Lee SH, Soderlund DM, Clark JM. Three mutations identified in the voltage-sensitive sodium channel alpha-subunit gene of permethrin-resistant human head lice reduce the permethrin sensitivity of house fly Vssc1 sodium channels expressed in Xenopus oocytes. Insect Biochem Mol Biol. 2008;38(3):296–306. doi: 10.1016/j.ibmb.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemel MB, Kim JH, Woychik RP, Michaud EJ, Kadwell SH, Patel IR, Wilkison WO. Agouti regulation of intracellular calcium: role in the insulin resistance of viable yellow mice. Proc Natl Acad Sci U S A. 1995;92(11):4733–4737. doi: 10.1073/pnas.92.11.4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemel MB, Shi H, Greer B, Dirienzo D, Zemel PC. Regulation of adiposity by dietary calcium. FASEB J. 2000;14(9):1132–1138. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.