Abstract
Objective
Executive dysfunction is observed in a sizable number of patients with refractory temporal lobe epilepsy (TLE). The frontostriatal network has been proposed to play a significant role in executive functioning, however, due to the complex architecture of these tracts it is difficult to generate measures of fiber tract microstructure using standard diffusion tensor imaging. To examine the association between frontostriatal network compromise and executive dysfunction in TLE, we applied an advanced, multi-shell diffusion model, restriction spectrum imaging (RSI), that isolates measures of intra-axonal diffusion and may provide better estimates of fiber tract compromise in TLE.
Methods
RSI scans were obtained from 32 patients with TLE [16 right TLE (RTLE); 16 left TLE (LTLE)] and 24 healthy controls (HC). An RSI-derived measure of intra-axonal anisotropic diffusion (neurite density; ND) was calculated for the inferior frontostriatal tract (IFS) and superior frontostriatal tract (SFS) and compared between TLE patients and HC. Spearman correlations were performed to evaluate the relationships between ND of each tract and verbal (i.e., D-KEFS Category Switching Accuracy and Color Word Interference Inhibition/Switching) and visuomotor (Trail Making Test) set-shifting performances in TLE patients.
Results
Patients with TLE demonstrated reductions in ND of the left and right IFS, but not SFS, compared to HC. Reduction in ND of left and right IFS was associated with poorer performance on verbal set-shifting in TLE. Increases in extracellular diffusion (isotropic hindered; IH) were not associated with executive dysfunction in the patient group.
Significance
RSI-derived ND revealed microstructural changes within the IFS in patients with TLE, which was associated with poorer executive functioning. This suggests that axonal/myelin loss to fiber networks connecting the striatum to the inferior frontal cortex is likely contributing to executive dysfunction in TLE.
Keywords: advanced diffusion, set-shifting, inhibition, executive function, demyelination
1.1 Introduction
Up to 75% of patients with temporal lobe epilepsy (TLE) exhibit significant executive dysfunction on standard neuropsychological measures [1]. Given the impact of executive dysfunction on overall quality of life [2], there is an emerging interest in understanding the neural underpinnings of executive dysfunction in TLE. Widespread structural and functional abnormalities in TLE have been well-documented, with patients demonstrating alterations in white matter microstructure, cortical thinning, reduced regional brain activity, and glucose hypometabolism within the frontal lobe [3–5]. Despite the identification of these structural and functional changes within frontal networks, relatively few studies have directly linked these changes to impairments in executive functioning in TLE (for review see Stretton & Thompson [1]).
The frontostriatal network, consisting of parallel anatomical loops connecting frontal cortex to the striatum, has been proposed to play a critical role in executive functions [6–8]. Microstructural changes within this network have been identified in several clinical populations and linked to impairments in decision making, inhibition, set-shifting, emotion regulation, and reward processing [9–11]. Despite compelling evidence that the frontostriatal network contributes to executive dysfunction in other clinical populations, only one study has examined frontostriatal contributions to executive dysfunction in TLE [12]. Riley and colleagues applied probabilistic tractography to nine patients with left TLE (LTLE) and reported that reduced connection strength between the left caudate and the dorsolateral prefrontal cortex was associated with slower set-shifting performance. Although connection strength (i.e., the proportion of overall connectivity to a region) has been employed in previous studies as a surrogate for fiber tract integrity [13, 14], the authors acknowledge that the challenge of reliably reproducing the frontostriatal tracts prevented the use of more conventional tractography measures in their study.
Conventional diffusion tensor imaging (DTI) has provided great insight into the integrity of well-defined deep white matter tracts. However, this approach has limitations when defining pathways that fan out to connect neocortex with subcortical gray matter (e.g., frontostriatal tracts). Due to considerable fiber divergence within these pathways, which leads to low diffusion anisotropy and hence, less defined paths, Gaussian diffusion may not properly describe these paths [15]. To better model complex non-Gaussian diffusion in tissue, advanced diffusion methods, including diffusion kurtosis imaging (DKI), diffusion spectrum imaging (DSI), and restriction spectrum imaging (RSI), have been developed [16–19]. These techniques may provide better estimates of fiber tract compromise in regions with complex fiber orientation and structure, including tracts with high dispersion. In addition, multi-compartment models (e.g., DSI, RSI) provide more specific measures of disease pathology relative to standard DTI by separating the intracellular (i.e., intra-axonal) compartments associated with axonal/myelin integrity from the extracellular (i.e., extra-axonal) compartment that likely reflects water shifts in the extracellular matrix [17, 20]. To this end, we have recently shown that one advanced diffusion model, RSI, may provide a more specific measure of temporo-limbic network pathology in TLE relative to DTI due to its ability to isolate anisotropically-restricted diffusion associated with axonal/myelin loss [17]. Given our previous findings, we propose that RSI is also well-positioned to capture microstructural changes within frontostriatal tracts that may be a strong marker of cognitive dysfunction in TLE.
In this study, we investigated the association between frontostriatal network integrity and executive dysfunction in TLE using an RSI-derived measure of axonal/myelin integrity (i.e., neurite density; ND). We selected two frontostriatal tracts, the inferior frontostriatal tract (IFS) and the superior frontostriatal tract (SFS), which connect the striatum to the inferior and superior frontal cortices, respectively. We hypothesize that patients with TLE would demonstrate lower ND within frontostriatal tracts relative to healthy controls. We also hypothesize that reduced ND within the frontostriatal tracts would be associated with poorer performances on measures of executive functioning (verbal and visuomotor set-shifting) in TLE.
1.2 Methods
1.2.1 Participants
This study was approved by the Institutional Review Boards at the UC San Diego and UC San Francisco, and informed consent was collected from all participants in accordance to the Declaration of Helsinki. Thirty-two patients with medically refractory TLE and 24 healthy controls met inclusion/exclusion criteria for the study. A subset of the subjects were included in a previous study by Loi et al [17]. All TLE patients were recruited through referral from the UC San Diego or UC San Francisco Epilepsy Centers. Inclusion criteria for patients included a TLE diagnosis by a board-certified neurologist with expertise in epileptology, in accordance with the criteria defined by the International League Against Epilepsy, and unilateral seizure onset based on video-EEG telemetry, seizure semiology, and neuroimaging evaluation. Patients were excluded if there was evidence on video-EEG of multifocal seizure onset. In nine patients, MRI findings suggested the presence of ipsilateral mesial temporal sclerosis (MTS; six with left MTS and three with right MTS). Of the 32 patients, 16 patients demonstrated right temporal lobe seizure onset (RTLE) and 16 demonstrated left temporal lobe seizure onset (LTLE). Healthy controls (HC) were included if they were between the ages of 18 and 65 and had no reported history of neurological or psychiatric disease.
1.2.2 MRI acquisition
All patients were seizure-free per self-report for a minimum of 24 hours prior to the MRI scan. MRI data were collected on a General Electric Discovery MR750 3T scanner with an 8-channel phased-array head coil at the Center for Functional MRI at UC San Diego or the Surbeck Laboratory for Advanced Imaging at UC San Francisco. Image acquisitions were identical at both centers and included a conventional three-plane localizer, GE calibration scan, a T1-weighted 3D customized FSPGR structural sequence (TR = 8.08 ms, TE = 3.16 ms, TI = 600 ms, flip angle = 8°, FOV = 256 mm, matrix = 256 × 192, slice thickness = 1.2 mm), and for standard diffusion MRI, a single-shot pulse-field gradient spin-echo EPI sequence (TE/TR = 96ms/17s, FOV = 24 cm, matrix = 128×128×48, axial). Diffusion data used for RSI analyses were acquired with b = 0, 500, 1500, and 4000 s/mm2, with 1, 6, 6, and 15 unique gradient directions for each b-value, respectively (total RSI scan time = ~ 7 min). For use in nonlinear B0 distortion correction, two additional b=0 volumes were acquired with either forward or reverse phase-encode polarity.
1.2.3 RSI processing
All data were processed at the UC San Diego Center for Multimodal Imaging and Genetics (CMIG). Preprocessing of the diffusion data included corrections for distortions due to magnetic susceptibility (B0), eddy currents, and gradient nonlinearities, as well as head motion correction and registration to the T1-weighted structural image. For B0 distortion correction, a reverse gradient method was used [21]. This method provides superior accuracy and better cross-modality registration relative to the field mapping approach. A detailed description of the image processing is provided elsewhere [22]. RSI utilizes a multi-b-shell acquisition in conjunction with a linear mixture model to isolate diffusion signals from separable hindered, restricted, and free water diffusion compartments within a voxel. Technical details describing the RSI mathematical framework are described in full elsewhere [16, 23, 24]. RSI-based measures were calculated from the 2nd and 4th order spherical harmonics of the cylindrically-restricted (ND) and isotropic hindered (IH) compartments. The cylindrically-restricted compartment obtained from our model is referred to as “neurite density” throughout this paper to remain consistent with the existing biophysical literature and our previous work [17, 24]. However, it is of note that here, ND was modeled exclusively in white matter, and therefore, likely represents axonal projections rather than neurites. These measures were normalized by the square root of the sum of squares of all model coefficients, converting them into volume fractions. The RSI model was fit to the data using least-squares estimation with Tikhonov regularization [23].
1.2.4 Fiber tracts calculations
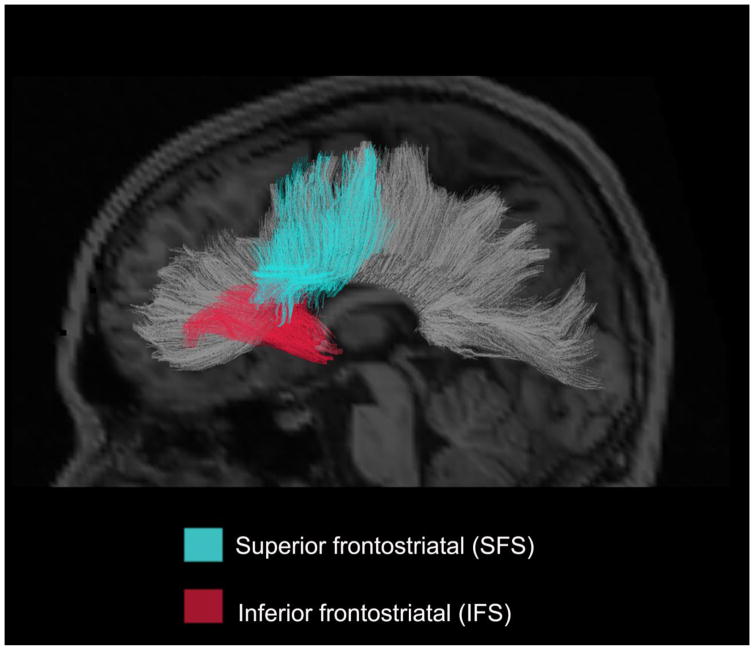
Fiber tract values for each of the RSI diffusion measures were derived using a probabilistic diffusion tensor atlas developed using in-house software written in MATLAB (i.e., AtlasTrack), which has been validated in healthy controls and TLE patients [25]. This method uses information from a single subject on the location and orientation of the tracts of interested. The diffusion measures were then derived from each participant’s atlas using T1-weighted images and the orientation estimates from diffusion tensor calculations. A full description of the atlas and the steps used to create the atlas are described elsewhere [25]. For each participant, T1-weighted images were used to nonlinearly register the brain to a common space, and diffusion tensor orientation estimates were compared to the atlas to obtain a map of the relative probability that a voxel belongs to a particular fiber given the location and similarity of diffusion orientations. Voxels identified with FreeSurfer’s automated brain segmentation as CSF or gray matter were excluded from the fiber regions of interests (ROIs). Voxels with fiber probability values less than 0.08 were also excluded. Average diffusion metrics were calculated for each fiber ROI, weighted by fiber probability, so that voxels with low probability of belonging to a given fiber contributed minimally to average values. In the current study, this probabilistic atlas-based method was used to reconstruct the following right and left hemisphere fiber tracts due to evidence of their projections to striatal structures from inferior (IFS) and superior (SFS) frontal areas involved in inhibition and set-shifting [6, 26] (see Figure 1). The IFS includes corticostriate projections that originate primarily within inferior frontal cortex, including orbitofrontal, anterior cingulate, and inferior dorsolateral regions, whereas the SFS includes corticostriate projections via the external capsule that originate primarily within superior frontal cortex, including superior dorsolateral, premotor and primary motor regions. In addition, we calculated a global measure of fiber tract ND within the left and right hemisphere by averaging the ND values for all major tracts (i.e., see Hagler et al. [25] for a description of the atlas and derived fiber tracts) within each hemisphere. For a detailed description of the IFS and SFS fiber tract’s reconstruction, see the supplementary material (Supplementary Figures 1 and 2). Information on the distribution of ND and IH values are available in Supplementary Figures 3 and 4.
Figure 1.
A) Sagittal rendering of the IFS and SFS derived from a probabilistic diffusion tensor atlas (i.e., AtlasTrack) projected onto a T1-weighted image. IFS: inferior frontostriatal tract; SFS: superior frontostriatal tract. The corpus callosum is portrayed in gray in the background in order to provide additional spatial information.
1.2.5 Neuropsychological measures
Neuropsychological data were available for all TLE patients and 17 of the 24 healthy controls. Verbal set-shifting and response inhibition was measured with the Delis-Kapan Executive Function System (D-KEFS) Color-Word Interference Inhibition/Switching condition (response inhibition) and the Verbal Fluency Category Switching Accuracy. Visuomotor set-shifting was measured with the Trail Making Test B (TMT-B). In the Verbal Fluency Test- Category Switching condition the examinee is asked to switch back and forth between overlearned semantic categories (i.e., fruits and furniture). In the Color-Word Interference Inhibition/Switching condition, examinees switch back and forth between naming dissonant ink colors and reading words, thus the test taxes both response inhibition and verbal set-shifting. In the TMT-B test, examinees switch back and forth between letters and numbers in alphanumeric order that are presented in a complex visual array. These tests are widely used and sensitive to executive dysfunction in patients with TLE [1]. In addition to measures of executive functioning, measures of verbal memory (Wechsler Memory Scale II; Logical Memory) and verbal fluency (D-KEFS Letter Fluency) were administered to determine whether any associations between fiber tract values and executive functioning were better explained by deficits in memory or fluency. Scaled scores (SS) were used for all measures. The D-KEFS Color-Word Interference test and Verbal Fluency test, and Logical Memory were corrected for age. The TMT-B test was corrected for age, gender, ethnicity, and years of formal education.
1.2.6 Statistical analysis
Independent t-tests were used to test for differences between patients with TLE and HC in demographic and neuropsychological variables and in ND and IH of the IFS and SFS tracts. To control for multiple comparisons, Bonferroni corrections were applied to the group comparisons (significance level set at P <0.05/4). Due to evidence that patients with TLE typically show bilateral frontal lobe dysfunction [3–5], patients with LTLE and RTLE were combined in the a priori analyses. Spearman’s rho correlations were used to test for associations between left and right ND and IH of the IFS and SFS and 1) D-KEFS Category Switching Accuracy SS, 2) Color-Word Interference Inhibition/Switching SS, and 3) TMT-B SS in TLE patients. Correction for false discovery rate (FDR) was used to control for multiple comparisons.
1.3 Results
1.3.1 Demographic, clinical, and neuropsychological variables
Table 1 shows the demographic and clinical variables for TLE patients and HC. There was no significant difference in age between TLE patients and HC (t (54) = −0.448, p = 0.656). However, HC attained more years of education relative to patients with TLE (t (54) = 2.88, p = 0.006). The distribution of gender across groups was comparable (χ2 (1) = 0.292, p = 0.787).
Table 1.
Demographic and clinical characteristics of the TLE and HC samples
| TLE | HC | |
|---|---|---|
| N | 32 | 24 |
| Age (years) | 38.16 (13.89) | 36.49 (13.52) |
| Education (Years) | 14.16 (2.7) | 16.04 (2.63) |
| Gender: M/F | 17//15 | 11/13 |
| MTS: Yes/No | 9/23 | |
| Side of seizure onset: Left/Right | 16/16 | |
| Age of Onset | 20.9 (14.79) | |
| Duration (years) | 16.61 (15.32) |
TLE: temporal lobe epilepsy; HC: healthy controls; F: females; M: Males; MTS: mesial temporal sclerosis; standard deviations are presented inside the parenthesis
1.3.2 Neuropsychological variables
Patients with TLE had significantly lower TMT-B SS than HC, reflecting longer completion times [TLE = 10.03 ± 2.7, HC = 12.0 ± 1.73, t (42) = 2.66, p = 0.011]. Patients with TLE also had significantly lower Category Switching Accuracy SS than HC [TLE = 10.32 ± 3.31, HC = 14.0 ± 3.08, t (43) = 3.7, p = 0.001]. However, Color-Word Interference Inhibition/Switching was not significantly different between patients and HC [TLE = 10.36 ± 3.0, HC = 10.65 ± 2.76, t (43) = 0.323, p = 0.75]. The HC’s SS were used to generate z-scores and determine the percentage of TLE patients that were impaired (> 1 standard deviation below the HC mean) across the different measures. Approximately 44 percent (n =14) of TLE patients were impaired on Category Switching Accuracy; 46.88% (n = 15) of TLE patients were impaired on TMT-B; and 12.5% (n = 4) were impaired on Color-Word Interference Inhibition/Switching.
1.3.3 Group differences in ND of the IFS and SFS
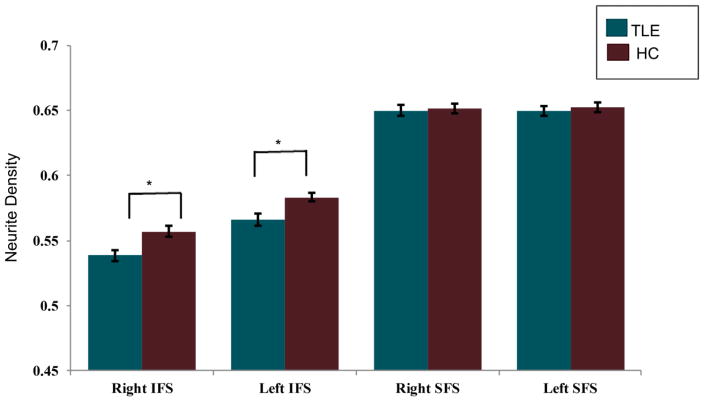
Whole brain maps are displayed in Figure 2 to illustrate the voxelwise reductions in ND in patients with TLE relative to HC within regions of the frontostriatal network. However, the main contrasts of interest were at the frontostriatal tract level. Patients with TLE showed reduced ND of the right and left IFS compared to HC [Right IFS: t (54) = 2.912, p = 0.005; Left IFS: t (54) = 2.756, p = 0.008]. No significant differences were found between patients with TLE and HC in ND of the right or left SFS [Right SFS: t (54) = 0.290, p = 0.773; Left SFS: t (54) = 0.448, p = 0.656] (See Figure 3). Effect sizes for group differences for each tract were calculated using Cohen’s d. There were medium to large effect sizes between TLE patients and HC (d = 0.800) for the right IFS and for the left IFS (d = 0.766). The effect size for group differences was small for the right SFS (d = 0.083) and for the left SFS (d = 0.124). In addition, there were only trends for higher IH values in TLE patients relative to HC after correcting for multiple comparisons (corrected p-value > 0.05) [Right IFS IH: t (54) = −2.177, p = 0.034; Left IFS IH: t (54) = −2.139, p = 0.037]; Right SFS IH: t (54) = −2.148, p = 0.036; Left SFS IH: t (54) = −2.362, p = 0.022].
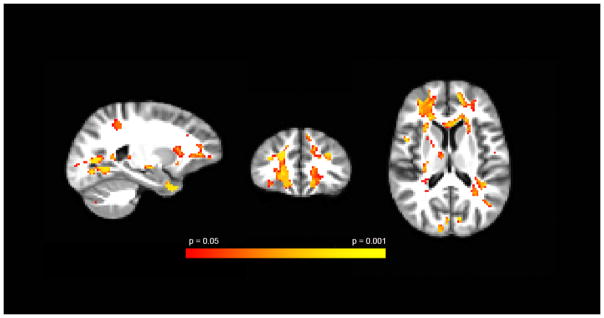
Figure 2.
Voxel-based group comparisons between patients with TLE and healthy controls using ANTS registration according to procedures described elsewhere [17]. Areas of red-yellow represent decreased ND within the vicinity of the frontostriatal networks in patients compared to controls. Sagittal, axial, and coronal views are represented respectively. All images are clustered and FDR-corrected at q <0.05.
Figure 3.
Mean neurite density (ND) in patients with TLE and HCs for the right and left inferior frontostriatal (IFS) and superior frontostriatal (SFS) tracts. Asterisk indicates p < 0.025. Error bars represent standard error.
Post-hoc independent t-test analyses were conducted to test differences in ND between LTLE and RTLE and Mann-Whitney U were conducted to test differences in ND between TLE patients with MTS and without MTS. There were no significant differences in ND of the right or left IFS between LTLE and RTLE [Right IFS: t (30) = 1.377, p = 0.179; Left IFS: t (30) = 0.169, p = 0.867] or ND of right or left SFS [Right SFS: t (30) = −0.051, p = 0.960; Left SFS: t (30) = −0.220, p = 0.827]. There were no significant differences between TLE patients with MTS and without MTS for the right or left IFS [Right IFS: U = 83, p = 0.409; Left IFS: U = 58, p = 0.058] or right or left SFS [Right SFS: U = 85, p = 0.458; Left SFS: U = 79, p = 0.321]. Left and right tracts were designated ipsilateral and contralateral according to the side of seizure onset. There were no differences between ipsilateral and contralateral IFS t (31) = −0.871, p = 0.390 or ipsilateral and contralateral SFS t (31) = −0.244, p = 0.809.
1.3.4 Frontostriatal ND and executive functioning in TLE
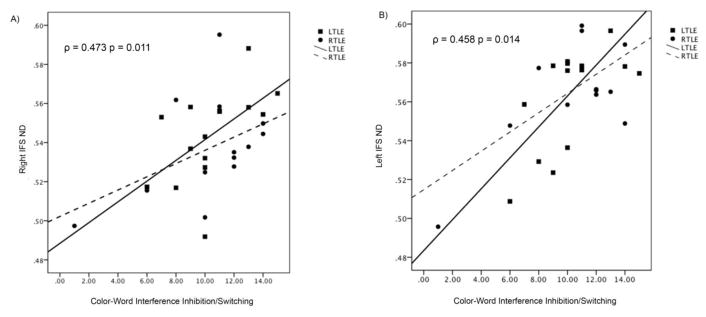
For patients with TLE, lower ND for the right and left IFS was associated with poorer Color-Word Interference Inhibition/Switching performance (See Table 2 & Figure 4). No other significant correlations were found. In order to determine whether side of seizure onset (LTLE versus RTLE) influenced associations between frontostriatal ND and executive functioning, we divided the TLE group into LTLE and RTLE. The groups did not differ in performance on Color-Word Interference Inhibition/Switching (U = 86, p = 0.618), Category Switching Accuracy (U = 79, p = 0.401), or TMT-B (U = 58, p = 0.116). In LTLE, poorer Color-Word Interference Inhibition/Switching performance was associated with decreased ND for both the right and left IFS (Right IFS: Spearman ρ = 0.622, p = 0.013; Left IFS: Spearman ρ = 0.564, p = 0.029) (See Figure 4). However, these associations were not found in RTLE (Right IFS: Spearman ρ = 0.424, p = 0.148; Left IFS: Spearman ρ = 0.230, p = 0.449). No other significant correlations were found.
Table 2.
Relationship between ND within frontostriatal tracts and executive function
| ROI | Color-Word Interference Inhibition/Switching | Category Switching | TMT-B | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Spearman Rho | p-value | Spearman Rho | p-value | Spearman Rho | p-value | |
| Right IFS | 0.473 | 0.011* | 0.239 | 0.221 | 0.086 | 0.668 |
| Left IFS | 0.458 | 0.014* | 0.097 | 0.622 | −0.046 | 0.822 |
| Right SFS | 0.357 | 0.063 | 0.120 | 0.542 | 0.024 | 0.904 |
| Left SFS | 0.307 | 0.112 | 0.102 | 0.606 | −0.012 | 0.951 |
ROI: region of interest; IFS: inferior frontostriatal tract; SFS: superior frontostriatal tract; TMT-B: trail making test- B
Indicates significance with FDR corrected significance level q* = 0.025 for Color-Word Interference Inhibition/Switching comparisons
Figure 4.
Relationship between neurite density (ND) of the right (A) and left (B) inferior frontostriatal tract (IFS) and Color Word Inhibition/Switching performance in patients with TLE.
To determine whether these associations were specific to reductions in ND (i.e., intracellular diffusion) rather than increases in IH (i.e., extracellular diffusion), we conducted post-hoc correlational analyses between the measures of executive function and IH. There were no significant correlations between IH and executive functioning in LTLE and RTLE or at the whole TLE group level. In addition, to determine whether the associations were specific to frontostriatal connections rather than overall fiber loss, we conducted post-hoc correlational analyses between measures of executive function and a global measure of ND within each hemisphere. There were no significant correlations between executive functioning and global left or right hemisphere white matter ND. Finally, verbal memory and verbal fluency were not driving the association between IFS ND and verbal set-shifting. The associations between left and right IFS and Color-Word Interference Inhibition/Switching performance were still significant when controlling for verbal memory (Logical Memory II; Left IFS: r = 0.684, p <0.001; Right IFS: r = 0.586, p = 0.001). When controlling for verbal fluency (D-KEFS Letter Fluency), the left IFS only was associated with poorer Color-Word Interference Inhibition/Switching performance (r = 0.507, p = 0.014).
1.4 Discussion
In this study, we demonstrate the ability of RSI, an advanced multi-shell diffusion model, to detect microstructural changes (i.e., reduced ND) within the frontostriatal network of patients with TLE—a network of fibers with high dispersion that may artificially reduce FA [15]. Second, we show that reductions in ND of the IFS are associated with poorer performances on neuropsychological measures of verbal set-shifting ability. Together, these findings suggest that executive dysfunction in TLE may be partially due to axonal/myelin loss within fiber tracts that project from the inferior frontal lobe to the striatum. Our study represents the first to demonstrate these structure-function associations in TLE using an advanced diffusion technique that can model multiple tissue compartments, yielding more specific measures of white matter microstructure and compromise.
In support of our first hypothesis, we found bilateral reductions in ND of fiber tracts connecting the inferior frontal cortex to the striatum (IFS) in patients with TLE compared with HC. However, we did not find ND reductions in tracts connecting the superior frontal cortex to the striatum (SFS). The projections from frontal areas are organized in a highly topographic manner (rostral-caudal) along the striatum [27]. Four frontostriatal loops have been identified: dorsolateral prefrontal cortex connecting to dorsolateral caudate, lateral orbitofrontal cortex connecting to ventromedial caudate, anterior cingulate cortex connecting to ventral striatum (i.e., nucleus accumbens), and supplementary motor area (SMA) connecting to the putamen [6, 8]. Although we are not able to specify to which region of the striatum our tracts project due to the broad placement of the striatal ROI, fibers from inferior frontal cortex are known to project predominantly to the nucleus accumbens and caudate nucleus and originate broadly from dorosolateral, orbitofrontal, anterior cingulate and lateral orbitofrontal cortices [8, 27]. Conversely, fibers projecting from superior frontal cortex (including motor regions and the SMA), project largely into the putamen. Volume loss within the caudate nucleus is commonly found in patients with chronic TLE [28] and the caudate nucleus has been implicated in both seizure generation and control [29]. Although disruptions of the putamen-frontal networks have also been reported in TLE [30], our data suggest that the pathophysiology of TLE may result in greater disruption to inferior frontal-caudate systems than to superior frontal-putamen systems.
Second, we demonstrate that reduced ND of the IFS is associated with poorer performance on a complex measure of executive functioning (i.e., set-shifting and response inhibition) in TLE, and these associations do not appear to be better accounted for by impairments in verbal memory or verbal fluency. It is noteworthy, however, that when patients with LTLE and RTLE were analyzed separately, this association was only present in LTLE. Thus, patients with LTLE may be driving this association at the whole group level. There is compelling evidence demonstrating the role of inferior frontostriatal projections in executive function [8] and the involvement of the inferior frontal lobe in response inhibition and set-shifting, specifically (27, 34). Several studies have found associations between inhibition/set-shifting and frontostriatal microstructural integrity, particularly for tracts projecting to the striatum from ventral prefrontal cortex [9–11]. These data are also in line with Riley et al. [12] who found that reduced connection strength of fibers projecting from frontal cortex to the caudate nucleus is associated with reduced set-shifting ability in left TLE. In addition, disruption within the frontostriatal network that includes orbitofrontal and ventrolateral fiber tracts has been reported in several other clinical populations with known executive dysfunction [11]. Thus, our results suggest that disruption of the frontostriatal network in TLE may be specific to inferior frontal loops projecting to the caudate and nucleus accumbens, and they support the involvement of this subnetwork in executive dysfunction in TLE.
Despite decreases in ND and marginally significant increases in IH of the IFS, we did not find increases in extracellular diffusion (IH) to be associated with executive dysfunction in TLE. Reductions in ND have been proposed to most likely reflect reductions in the density of myelinated axons [20], whereas changes in extracellular compartments are thought to reflect inflammation and/or edema [31]. Although inflammation has been proposed as one process involved in the pathophysiology of chronic TLE [32] and has been linked to cognitive dysfunction in other clinical populations [33, 34], our results suggest that executive dysfunction in TLE is more likely related to axonal/myelin loss and not increases in inflammation or other extracellular pathology.
Extratemporal pathology and executive dysfunction are common in patients with TLE [1, 3–5]. However, the mechanism(s) by which brain regions outside of the temporal lobe are affected is still a matter of debate. One possibility is that the propagation of interictal epileptiform discharges (IEDs) from the epileptogenic cortex to the frontal lobes underlies executive dysfunction in TLE [35, 36]. Thus, disruption in frontal lobe networks is a direct and potentially reversible effect of temporal lobe seizures or IED propagation. Hence, executive dysfunction should improve in patients who become seizure-free through successful surgical or medical treatment. A second hypothesis is that neurodevelopmental and/or neurodegenerative structural alterations occur within frontal lobe networks and lead to executive dysfunction in TLE. Thus, executive functioning would not be expected to significantly improve in seizure-free patients. There is currently evidence for both hypotheses, and these mechanisms may not be mutually exclusive [37]. Our results demonstrate that microstructural changes (i.e., demyelination, axonal loss) within the ventral frontostriatal network are associated with executive dysfunction in TLE, lending support to the latter hypothesis. Using DKI, Lee et al. [38] demonstrated that late-myelinated white matter tracts exhibit a higher degree of damage than early-myelinated tracts in TLE. The development of the frontostriatal network is protracted [39], thus these tracts myelinate late during brain development and may therefore be vulnerable to the effects of seizures. Age of seizure onset, lifetime seizure load and medication effects have also been shown to be associated with the extent of cognitive impairment, particularly in the domains of executive function [36]. Thus, executive dysfunction in TLE is likely multifactorial and not easily attributed to a single structural, functional, or treatment-related etiology.
1.5 Limitations
A major advantage of our study is the use of an advanced, multi-shell diffusion model that may provide more specific measures of disease pathology relative to standard DTI. However, there are several limitations to our study that should be noted. First, our study is cross-sectional and causality of executive dysfunction cannot be inferred with correlational data. Second, we did not have post-surgical data in our patients. An analysis of post-surgical changes in executive functioning would allow us to better determine the contribution of other factors (i.e., IEDs) to executive functioning performance. Third, we used measures of set-shifting and response inhibition to estimate executive functioning. Although these are among the most commonly impaired aspects of executive functioning in TLE, it is possible that incorporating other measures of executive functioning (i.e., fluency, problem solving, or working memory) would have yielded different results. In addition, we did not have functional data on our patients to determine the degree to which microstructural changes versus functional disruption within frontostriatal and associated networks account for executive dysfunction in TLE.
There are also several limitations to our diffusion method that should be noted. First, despite histological validation of the ND component derived from RSI in pre-clinical data [24], this component has yet to be validated in human brains. This is a common limitation of many advanced diffusion models, but an important one nonetheless. Second, we did not examine whether RSI-derived parameters show a stronger association with executive function than DTI-derived parameters or parameters obtained from other advanced diffusion methods. Such head-to-head comparisons in large patient samples are greatly needed to determine the value of adding these models to current clinical or research protocols. Third, although our method for extracting fiber tracts has several advantages, including the elimination of concerns about inter- or intra-operator variability that exists when streamline tractography is performed at the individual subject level, this method relies on the accuracy of registration of an individual subject to an atlas and on the accuracy of the atlas. Nevertheless, our study reveals unique associations between frontostriatal network pathology and executive dysfunction in TLE that contribute to a growing body of literature aimed at unraveling the biological underpinnings of executive dysfunction in TLE.
Supplementary Material
Highlights.
We demonstrate the ability of RSI, an advanced multi-shell diffusion model, to detect microstructural changes (reduced ND) within the frontostriatal network of patients with TLE
Reduction in ND was found in tracts connecting inferior frontal cortex to striatum, but not in tracts connecting superior frontal cortex and striatum
Reductions in ND of the IFS was associated with poorer performances on measures of verbal set-shifting
Acknowledgments
Funding
This work was supported by the National Institute of Health (R01 NS065838 to C.R.M.).
Footnotes
Disclosure of Conflicts of Interest/Ethical Publication Statement
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines. None of the authors have any conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stretton J, Thompson PJ. Frontal lobe function in temporal lobe epilepsy. Epilepsy Res. 2012;98(1):1–13. doi: 10.1016/j.eplepsyres.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sherman EM, Slick DJ, Eyrl KL. Executive dysfunction is a significant predictor of poor quality of life in children with epilepsy. Epilepsia. 2006;47(11):1936–42. doi: 10.1111/j.1528-1167.2006.00816.x. [DOI] [PubMed] [Google Scholar]
- 3.McDonald CR, Hagler DJ, Jr, Ahmadi ME, Tecoma E, Iragui V, Gharapetian L, et al. Regional neocortical thinning in mesial temporal lobe epilepsy. Epilepsia. 2008;49(5):794–803. doi: 10.1111/j.1528-1167.2008.01539.x. [DOI] [PubMed] [Google Scholar]
- 4.Reyes A, Thesen T, Wang X, Hahn D, Yoo D, Kuzniecky R, et al. Resting-state functional MRI distinguishes temporal lobe epilepsy subtypes. Epilepsia. 2016;57(9):1475–84. doi: 10.1111/epi.13456. [DOI] [PubMed] [Google Scholar]
- 5.Mueller SG, Laxer KD, Barakos J, Cheong I, Garcia P, Weiner MW. Widespread neocortical abnormalities in temporal lobe epilepsy with and without mesial sclerosis. Neuroimage. 2009;46(2):353–9. doi: 10.1016/j.neuroimage.2009.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–81. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- 7.Shepherd GM. Corticostriatal connectivity and its role in disease. Nat Rev Neurosci. 2013;14(4):278–91. doi: 10.1038/nrn3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cummings JL. Frontal-subcortical circuits and human behavior. Arch Neurol. 1993;50(8):873–80. doi: 10.1001/archneur.1993.00540080076020. [DOI] [PubMed] [Google Scholar]
- 9.Liston C, Watts R, Tottenham N, Davidson MC, Niogi S, Ulug AM, et al. Frontostriatal microstructure modulates efficient recruitment of cognitive control. Cereb Cortex. 2006;16(4):553–60. doi: 10.1093/cercor/bhj003. [DOI] [PubMed] [Google Scholar]
- 10.Chiang HL, Chen YJ, Shang CY, Tseng WY, Gau SS. Different neural substrates for executive functions in youths with ADHD: a diffusion spectrum imaging tractography study. Psychol Med. 2016;46(6):1225–38. doi: 10.1017/S0033291715002767. [DOI] [PubMed] [Google Scholar]
- 11.Shang CY, Wu YH, Gau SS, Tseng WY. Disturbed microstructural integrity of the frontostriatal fiber pathways and executive dysfunction in children with attention deficit hyperactivity disorder. Psychol Med. 2013;43(5):1093–107. doi: 10.1017/S0033291712001869. [DOI] [PubMed] [Google Scholar]
- 12.Riley JD, Moore S, Cramer SC, Lin JJ. Caudate atrophy and impaired frontostriatal connections are linked to executive dysfunction in temporal lobe epilepsy. Epilepsy Behav. 2011;21(1):80–7. doi: 10.1016/j.yebeh.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tomassini V, Jbabdi S, Klein JC, Behrens TE, Pozzilli C, Matthews PM, et al. Diffusion-weighted imaging tractography-based parcellation of the human lateral premotor cortex identifies dorsal and ventral subregions with anatomical and functional specializations. J Neurosci. 2007;27(38):10259–69. doi: 10.1523/JNEUROSCI.2144-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Croxson PL, Johansen-Berg H, Behrens TE, Robson MD, Pinsk MA, Gross CG, et al. Quantitative investigation of connections of the prefrontal cortex in the human and macaque using probabilistic diffusion tractography. J Neurosci. 2005;25(39):8854–66. doi: 10.1523/JNEUROSCI.1311-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramnani N, Behrens TE, Penny W, Matthews PM. New approaches for exploring anatomical and functional connectivity in the human brain. Biol Psychiatry. 2004;56(9):613–9. doi: 10.1016/j.biopsych.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 16.White NS, McDonald C, Farid N, Kuperman J, Karow D, Schenker-Ahmed NM, et al. Diffusion-weighted imaging in cancer: physical foundations and applications of restriction spectrum imaging. Cancer Res. 2014;74(17):4638–52. doi: 10.1158/0008-5472.CAN-13-3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loi RQ, Leyden KM, Balachandra A, Uttarwar V, Hagler DJ, Jr, Paul BM, et al. Restriction spectrum imaging reveals decreased neurite density in patients with temporal lobe epilepsy. Epilepsia. 2016;57(11):1897–1906. doi: 10.1111/epi.13570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonilha L, Lee CY, Jensen JH, Tabesh A, Spampinato MV, Edwards JC, et al. Altered microstructure in temporal lobe epilepsy: a diffusional kurtosis imaging study. AJNR Am J Neuroradiol. 2015;36(4):719–24. doi: 10.3174/ajnr.A4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lemkaddem A, Daducci A, Kunz N, Lazeyras F, Seeck M, Thiran JP, et al. Connectivity and tissue microstructural alterations in right and left temporal lobe epilepsy revealed by diffusion spectrum imaging. Neuroimage Clin. 2014;5:349–58. doi: 10.1016/j.nicl.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carper RA, Treiber JM, White NS, Kohli JS, Muller RA. Restriction Spectrum Imaging As a Potential Measure of Cortical Neurite Density in Autism. Front Neurosci. 2016;10:610. doi: 10.3389/fnins.2016.00610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holland D, Kuperman JM, Dale AM. Efficient correction of inhomogeneous static magnetic field-induced distortion in Echo Planar Imaging. Neuroimage. 2010;50(1):175–83. doi: 10.1016/j.neuroimage.2009.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McDonald CR, Leyden KM, Hagler DJ, Kucukboyaci NE, Kemmotsu N, Tecoma ES, et al. White matter microstructure complements morphometry for predicting verbal memory in epilepsy. Cortex. 2014;58:139–50. doi: 10.1016/j.cortex.2014.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.White NS, McDonald CR, Farid N, Kuperman JM, Kesari S, Dale AM. Improved conspicuity and delineation of high-grade primary and metastatic brain tumors using “restriction spectrum imaging”: quantitative comparison with high B-value DWI and ADC. AJNR Am J Neuroradiol. 2013;34(5):958–64. S1. doi: 10.3174/ajnr.A3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.White NS, Leergaard TB, D’Arceuil H, Bjaalie JG, Dale AM. Probing tissue microstructure with restriction spectrum imaging: Histological and theoretical validation. Hum Brain Mapp. 2013;34(2):327–46. doi: 10.1002/hbm.21454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hagler DJ, Jr, Ahmadi ME, Kuperman J, Holland D, McDonald CR, Halgren E, et al. Automated white-matter tractography using a probabilistic diffusion tensor atlas: Application to temporal lobe epilepsy. Hum Brain Mapp. 2009;30(5):1535–47. doi: 10.1002/hbm.20619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat Neurosci. 2003;6(2):115–6. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- 27.Verstynen TD, Badre D, Jarbo K, Schneider W. Microstructural organizational patterns in the human corticostriatal system. J Neurophysiol. 2012;107(11):2984–95. doi: 10.1152/jn.00995.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dreifuss S, Vingerhoets FJ, Lazeyras F, Andino SG, Spinelli L, Delavelle J, et al. Volumetric measurements of subcortical nuclei in patients with temporal lobe epilepsy. Neurology. 2001;57(9):1636–41. doi: 10.1212/wnl.57.9.1636. [DOI] [PubMed] [Google Scholar]
- 29.La Grutta V, Sabatino M. Focal hippocampal epilepsy: effect of caudate stimulation. Exp Neurol. 1988;99(1):38–49. doi: 10.1016/0014-4886(88)90125-2. [DOI] [PubMed] [Google Scholar]
- 30.Rektor I, Tomcik J, Mikl M, Marecek R, Brazdil M, Rektorova I. Association between the basal ganglia and large-scale brain networks in epilepsy. Brain Topogr. 2013;26(2):355–62. doi: 10.1007/s10548-012-0272-8. [DOI] [PubMed] [Google Scholar]
- 31.McDonald CR, White NS, Farid N, Lai G, Kuperman JM, Bartsch H, et al. Recovery of white matter tracts in regions of peritumoral FLAIR hyperintensity with use of restriction spectrum imaging. AJNR Am J Neuroradiol. 2013;34(6):1157–63. doi: 10.3174/ajnr.A3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crespel A, Coubes P, Rousset MC, Brana C, Rougier A, Rondouin G, et al. Inflammatory reactions in human medial temporal lobe epilepsy with hippocampal sclerosis. Brain Res. 2002;952(2):159–69. doi: 10.1016/s0006-8993(02)03050-0. [DOI] [PubMed] [Google Scholar]
- 33.Holmes C, Cunningham C, Zotova E, Woolford J, Dean C, Kerr S, et al. Systemic inflammation and disease progression in Alzheimer disease. Neurology. 2009;73(10):768–74. doi: 10.1212/WNL.0b013e3181b6bb95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harezlak J, Buchthal S, Taylor M, Schifitto G, Zhong J, Daar E, et al. Persistence of HIV-associated cognitive impairment, inflammation, and neuronal injury in era of highly active antiretroviral treatment. AIDS. 2011;25(5):625–33. doi: 10.1097/QAD.0b013e3283427da7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hermann B, Seidenberg M. Executive system dysfunction in temporal lobe epilepsy: effects of nociferous cortex versus hippocampal pathology. J Clin Exp Neuropsychol. 1995;17(6):809–19. doi: 10.1080/01688639508402430. [DOI] [PubMed] [Google Scholar]
- 36.Dinkelacker V, Xin X, Baulac M, Samson S, Dupont S. Interictal epileptic discharge correlates with global and frontal cognitive dysfunction in temporal lobe epilepsy. Epilepsy Behav. 2016;62:197–203. doi: 10.1016/j.yebeh.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 37.Helmstaedter C, Kurthen M, Lux S, Reuber M, Elger CE. Chronic epilepsy and cognition: a longitudinal study in temporal lobe epilepsy. Ann Neurol. 2003;54(4):425–32. doi: 10.1002/ana.10692. [DOI] [PubMed] [Google Scholar]
- 38.Lee CY, Tabesh A, Benitez A, Helpern JA, Jensen JH, Bonilha L. Microstructural integrity of early- versus late-myelinating white matter tracts in medial temporal lobe epilepsy. Epilepsia. 2013;54(10):1801–9. doi: 10.1111/epi.12353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huttenlocher PR. Synaptic density in human frontal cortex - developmental changes and effects of aging. Brain Res. 1979;163(2):195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.