Abstract
Objective
Tolerance of and complications due to minimally invasive hysterectomy and staging in the older endometrial cancer population is largely unknown despite the fact that this is the most rapidly growing age group in the United States. The objective of this retrospective review is to compare operative morbidity by age in patients on the Gynecologic Oncology Group (GOG) LAP2 trial.
Methods
This is a retrospective analysis of patients from GOG LAP2, a trial that included clinically early stage uterine cancer patients randomized to laparotomy vs. laparoscopy for surgical staging. Differences in the rates and types of intraoperative and perioperative complications were compared by age. Specifically complications between patients <60 vs ≥60 years old were compared due to toxicity analysis showing a sharp increase in toxicity starting at age 60 years old in the laparotomy group.
Results
LAP2 included 1,477 patients ≥60 years old. As expected, with increasing age there is worsening performance status and disease characteristics including higher rates of serous histology, high stage disease, and lymphovascular space invasion. There is no significant difference in lymph node dissection rate by age for the entire population or within the laparotomy or laparoscopy groups. Toxicity analysis shows a sharp increase in toxicity seen in patients ≥60 years old in the laparotomy group. Further analysis shows that when comparing laparotomy to laparoscopy in patients <60 years old vs ≥60 years old and controlling for race, body mass index, stage, grade, and performance status patients <60 years old undergoing laparotomy had more hospital stays >2 days (OR 17.48; 95% CI 11.71–27.00, p<0.001) compared to patients <60 years old undergoing laparoscopy. However, when comparing laparotomy to laparoscopy in patients ≥60 years old, in addition to hospital stay >2 days (OR 12.77; 95% CI 8.74–19.32, p<0.001), there were higher rates of the following postoperative complications: antibiotic administration (OR 1.63; 95% CI 1.24–2.14, p<0.001), ileus (OR 2.16; 95% CI 1.42–3.31, p<0.001), pneumonias (OR 2.36; 95% CI 1.01–5.66, p=0.048), deep vein thromboses (OR 2.87; 95% CI 1.08–8.03, p=0.035), and arrhythmias (OR 3.21; 95% CI 1.60–6.65, p=0.001) in the laparotomy group.
Conclusion
Laparoscopic staging for uterine cancer is associated with decreased morbidity in the immediate postoperative period in patients ≥60 years old. These results allow for more accurate preoperative counseling. A minimally invasive approach to uterine cancer staging may decrease morbidity that could affect long term survival.
Keywords: endometrial, older, LAP2
Introduction
Endometrial cancer, which is predominantly a disease of post-menopausal women, is expected to increase in prevalence with an increasingly aged and obese population. In 2017, there will be an estimated 61,380 cases of endometrial cancer diagnosed in the United States and 10,920 deaths.1 Despite the increased rates of endometrial cancer mortality seen in older patients, studies show these patients receive less surgical and adjuvant therapy than their younger counterparts, which is, in part, due to the fact that treating physicians believe older patients cannot tolerate such therapy. This view is supported by literature showing that advanced age is an independent risk factor for perioperative morbidity even when controlling for medical comorbidities2.
Minimally invasive surgical (MIS) management is used for many types of cancers. There is a significant amount of data showing similar oncologic outcomes and decreased morbidity with minimally invasive techniques versus laparotomy (LAP).3,4 Most recently, The Laparoscopic Approach to Cancer of the Endometrium (LACE) trial was reported. This randomized phase 3 trial compared exploratory laparotomy to total laparoscopic hysterectomy (TLH) with or without lymph node dissection in clinical stage I endometrioid endometrial cancer. They demonstrated no difference in disease free survival. While age >65 was a prognostic factor for disease recurrence (HR 3.14(1.57–6.26)), no separate analysis of complications among study participants, including the 44% who were > 65yo was presented in this paper.5 Based on this, and other work, we know that oncologic outcomes appear equivalent regardless of surgical approach (LAP, TLH, laparoscopic assisted vaginal hysterectomy (LAVH) or robotic assisted total laparoscopic hysterectomy (RaTLH)).
How well older patients tolerate each of these surgical options remains unknown. Several retrospective studies show decreased morbidity in older patients managed with minimally invasive techniques, however, there is no prospective data comparing outcomes in older patients.6–9
In 1996, the Gynecologic Oncology Group (GOG) opened a randomized prospective clinical trial (GOG-2222 or LAP2) to compare comprehensive surgical staging by LAP versus LAVH for the treatment of women with stage I to stage IIA uterine cancer (n = 2616). In 2009 and 2012, the GOG published the results of LAP2 regarding the completeness of surgical staging, recurrence-free survival, complications, and quality of life (QOL) of LAVH versus LAP. Results show improved quality of life and decreased complication in the LAVH group with no decrement in survival in patients managed with laparoscopy compared with laparotomy. LAP2 is the largest prospective trial to date looking at minimally invasive surgical approaches in clinically early stage endometrial cancer. Our current study includes all patients from LAP2 with 1,477 patients ≥ 60yo. This allows for assessment of a large subset of older patients with clinically early stage endometrial cancer.10,11
The goal of this ancillary review is to compare intraoperative, perioperative and postoperative surgical morbidity outcomes in LAVH versus LAP by age in patients who participated in the GOG LAP2 trial. GOG LAP2 included patients with primarily early stage disease and good performance status and required complete surgical staging making this a highly selected patient population. The results from this study will allow clinicians to more accurately evaluate the benefits of surgery and its potential complications in older endometrial cancer patients.
Materials and Methods
This is an analysis of patients who were enrolled on LAP-2, a GOG clinical trial. The details of inclusion and exclusion criteria were reported in the original manuscript10. Briefly, the study was designed to compare LAVH with LAP for the purpose of complete comprehensive surgical staging of uterine cancer. The primary outcome of the study was recurrence-free survival. Other end points included perioperative adverse events, LAVH conversion to LAP, length of hospital stay after surgery, operative time, quality of life, sites of recurrence and survival. Eligibility requirements were clinical stage I to IIA uterine cancer, adequate bone marrow, renal and hepatic function and GOG performance status of less than 4. All patients gave written informed consent prior to study entry in compliance with local IRB and federal guidelines.
In our current study, surgical outcomes were compared between patients by age. While it is generally agreed upon in the literature that ‘elderly’ is defined as >65 or 70yo, our initial toxicity data showed increased toxicity starting at age 60, therefore our results show surgical outcomes compared between patients <60yo vs ≥60yo. The same analysis was performed using an age cut-off of 70yo and similar results were found. An intention to treat analysis was used for assessment of surgical complications. The perioperative time period included the first 30 days after surgery and the postoperative time period included up to 6 weeks after surgery. These parameters were set by the LAP2 protocol.
For statistical analysis, categorical variables were compared between the patient subgroups by the Pearson chi-square test12, and continuous variables by the Wilcoxon–Mann– Whitney test13 or the Kruskal–Wallis test14. A logistic regression model was used to evaluate specific operative morbidities and to estimate their covariate-adjusted odds of following LAVH or LAP. A linear regression model was used to estimate the covariate-adjusted relationships of patients’ independent baseline factors to severe toxicity. The nonlinearity of the effect of continuous variables was assessed using restricted cubic splines15. All statistical tests were two-tailed with the significance level set at alpha=0.05. Statistical analyses were performed using the R programming language and environment16.
Institutional Review Board and Institutional Biosafety Committee approvals were obtained at each institution and all eligible patients signed an informed consent before study entry in compliance with institutional, state, and federal regulations. Permission to perform this retrospective analysis was obtained from the GOG.
Results
LAP2 population demographics, pathology and outcomes
From the total LAP2 population 762 patients are 60–69yo, and 715 patients are ≥ 70yo. Demographic data, including BMI, performance status, disease characteristics, postoperative therapy, recurrence and survival by decade of age are shown in Table 1. As age increases, BMI decreases (p<0.001), performance status worsens (p<0.001) and, when looking at disease characteristics, there are increasing rates of serous histology (p<0.001), higher stage disease (p<0.001), and more LVSI (p<0.001). The majority of patients on LAP2 had endometrioid or serous histology, so only these histologies are shown in Table 1 but all histological subtypes were included in the analysis. When looking at survival by age, older patients have significantly higher rates of recurrence (p<0.001), and higher rates of death due to disease (p<0.001). With increasing age there is also a higher rate of conversion to LAP (<50yo: 23.8% vs ≥80yo 36.8%; p= 0.003 for all ages).
Table 1.
Pathology and outcomes by age (years)
| Variable | < 50 (n = 285) % (n) |
50–59 (n = 754) % (n) |
60–69 (n = 762) % (n) |
70–79 (n =555) % (n) |
≥80 (n =160) % (n) |
Test Statistic* |
|---|---|---|---|---|---|---|
|
| ||||||
| BMIa kg/m2 | 24.4
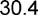 37.1 37.1 |
24.1
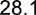 34.8 34.8 |
24.7
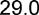 34.2 34.2 |
24.2
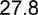 32.7 32.7 |
24.2
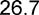 30.4 30.4 |
< 0.001 |
|
| ||||||
| Performance status | < 0.001 | |||||
| 0: normal, asymptomatic | 93.3 (266) | 93.6 (706) | 90.7 (691) | 86.1 (478) | 75 (120) | |
| 1: symptomatic, ambulatory | 6.7 (19) | 6 (45) | 8.8 (67) | 13 (72) | 21.9 (35) | |
| 2: symptomatic, in bed < 50% | 0.0 (0) | 0.3 (2) | 0.4 (3) | 0.7 (4) | 3.1 (5) | |
|
| ||||||
| Laparotomy | 39.6 (113) | 34.7 (262) | 35.4 (270) | 33.7 (187) | 33.8 (54) | 0.516 |
|
| ||||||
| Conversion to laparotomy | 23.8 (41) | 20.2 (100) | 27.3 (134) | 26.3 (97) | 36.8 (39) | 0.003 |
|
| ||||||
| # of hospitalization daysa | 2
 4 4 |
2
 4 4 |
2
 4 4 |
2
 5 5 |
3
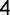 6 6 |
< 0.001 |
|
| ||||||
| Histology | < 0.001 | |||||
| endometrioid | 92.3 (263) | 88.1 (664) | 79.1 (603) | 71.4 (396) | 60.6 (97) | |
| serous | 2.8 (8) | 5.7 (43) | 11.4 (87) | 19.5 (108) | 26.9 (43) | |
|
| ||||||
| 2009 FIGO surgical stage | < 0.001 | |||||
| IA | 78.9 (224) | 75 (561) | 70.1 (531) | 59.7 (331) | 53.5 (85) | |
| IB | 6.0 (17) | 8.8 (66) | 12.4 (94) | 18.6 (103) | 21.4 (34) | |
| II | 6.3 (18) | 3.3 (25) | 3.6 (27) | 3.4 (19) | 6.3 (10) | |
| IIIA | 2.1 (6) | 2.3 (17) | 2.5 (19) | 3.8 (21) | 0.6 (1) | |
| IIIC1 | 3.2 (9) | 4.7 (35) | 4.1 (31) | 6.3 (35) | 4.4 7) | |
| IIIC2 | 2.8 (8) | 4.1 (31) | 4.1 (31) | 5.1 (28) | 6.9 (11) | |
| IVB | 0.7 (2) | 1.7 (13) | 3.2 (24) | 3.1 (17) | 6.9 (11) | |
|
| ||||||
| Tumor grade (differentiation) | < 0.001 | |||||
| well | 33.7 (96) | 26.3 (198) | 19.4 (148) | 13 (72) | 13.1 (21) | |
| moderate | 47.7 (136) | 49.5 (373) | 47.2 (360) | 40.2 (223) | 31.9 (51) | |
| poor | 17.2 (49) | 22.5 (170) | 29.3 (223) | 42.7 (237) | 50.6 (81) | |
| Not graded | 1.4 (4) | 1.7 (13) | 4.1 (31) | 4.1 (23) | 4.4 (7) | |
|
| ||||||
| Myometrial invasion | < 0.001 | |||||
| none | 14.2 (40) | 9.6 (72) | 7.9 (60) | 4.7 (26) | 3.8 (6) | |
| endometrial | 41.8 (118) | 34.1 (256) | 30.2 (228) | 24.6 (136) | 18.2 (29) | |
| < 50% myometrial | 29.8 (84) | 38.7 (291) | 39.3 (297) | 36.6 (202) | 37.7 (60) | |
| ≥ 50% myometrial | 12.4 (35) | 16.1 (121) | 20.4 (154) | 29.7 (164) | 36.5 (58) | |
| Serosal | 1.8 (5) | 1.5 (11) | 2.1 (16) | 4.3 (24) | 3.8 (6) | |
|
| ||||||
| Lymphovascular invasion | 13.5 (38) | 17.4 (129) | 19.5 (146) | 24.5 (134) | 27.2 (43) | < 0.001 |
|
| ||||||
| Nodal metastasis present | 7 (20) | 9.4 (71) | 9.3 (71) | 12.3 (68) | 13.1 (21) | 0.079 |
|
| ||||||
| Recurrence | 4.9 (14) | 6.6 (50) | 10.5 (80) | 14.6 (81) | 14.4 (23) | < 0.001 |
|
| ||||||
| PFS | < 0.001† | |||||
| progression or death | 6 (17) | 9.7 (73) | 16.5 (126) | 25.9 (144) | 44.4 (71) | |
|
| ||||||
| Overall survival status | < 0.001† | |||||
| death | 2.8 (8) | 7 (53) | 13.0 (99) | 22.9 (127) | 40.6 (65) | |
|
| ||||||
| Cause of death | ||||||
| treatment | 0 (0) | 0.5 (4) | 0.4 (3) | 1.1 (6) | 1.9 (3) | < 0.001 |
| disease | 1.8 (5) | 4.9 (37) | 8.2 (62) | 11.6 (64) | 16.5 (26) | |
| neither | 1.1 (3) | 0.5 (4) | 2.6 (20) | 6.9 (38) | 13.9 (22) | |
Variables were compared between the patient subgroups by the Pearson chi-square
The p-value is from the log-rank test of survival differences among the age subgroups.
a b c represents the lower quartile a, the median b, and the upper quartile c for continuous variables.
Although full surgical staging was required in GOG LAP2, not all patients underwent complete lymph node dissection. While a small percent of patients had only pelvic or only para-aortic lymph nodes removed, only 1.1% of the entire LAP2 population had no lymph nodes removed. The group with the largest number of patients having no lymph node dissection was the LAP group ≥80yo (4%) (Supplemental Table 1). There is no significant difference in lymph node dissection rate by age for the entire population or within the LAP or LAVH groups.
Intraoperative, postoperative, and perioperative complications by age
Complication rates were broken down into surgical approach and age. In the LAP group (n=886) there are no significant differences in intraoperative complication rates by age. Postoperatively, there are increased rates of complications by age with the increase observed starting at age 60yo (16.3% in <60yo vs 24.5% in ≥60yo, p=0.002 for all age groups). Postoperative complications that increase with age include urinary tract infections (UTIs) (2.7% vs 13% in patients <50yo vs ≥80yo, p<0.001 for all age groups) pneumonias (0.9% vs 9.3% in patients <50yo vs ≥80yo, p=0.006 for all age groups), congestive heart failure (0% vs 2.7% in patients <50yo vs ≥80yo, p=0.016 for all age groups) and arrhythmias (0% vs 2.7% in patients <50yo vs ≥80yo, p<0.001 for all age groups). Deep vein thrombosis also increased with increasing age (0% vs 5.6% in patients <50yo vs ≥80yo, p=0.05 for all age groups). Rates of readmission (3.5% vs 14.8% in patients <50yo vs ≥80yo, p=0.022 for all age groups) and treatment-related deaths increased significantly with increasing age as well with a treatment-related mortality rate of 0% in patients <50yo vs 5.6% in patients ≥80yo, p=0.005 for all age groups (Table 2).
Table 2.
Complications and Adverse Events by Age (Laparotomy n=886)
| Variable | < 50 n = 113 % (n) |
50–59 n = 262 % (n) |
60–69 n = 270 % (n) |
70–79 n = 187 % (n) |
≥ 80 n = 54 % (n) |
Test statistic* |
|---|---|---|---|---|---|---|
| Intraoperative complications (any) | 9.7 (11) | 6.5 (17) | 5.6 (15) | 8.0 (15) | 16.7 (9) | 0.056 |
|
| ||||||
| Postoperative adverse events (grade ≥ 2) | ||||||
| any | 15.9 (18) | 16.4 (43) | 22.2 (60) | 23.5 (44) | 38.9 (21) | 0.002 |
| urinary tract infection | 2.7 (3) | 1.5 (4) | 2.2 (6) | 3.2 (6) | 13.0 (7) | < 0.001 |
| DVT | 0.0 (0) | 0.8 (2) | 1.5 (4) | 1.6 (3) | 5.6 (3) | 0.050 |
| ileus | 2.7 (3) | 5.3 (14) | 9.6 (26) | 10.2 (19) | 7.4 (4) | 0.054 |
| pneumonia | 0.9 (1) | 1.9 (5) | 1.9 (5) | 1.6 (3) | 9.3 (5) | 0.006 |
| congestive heart failure | 0.0 (0) | 0.4 (1) | 0.7 (2) | 3.2 (6) | 3.7 (2) | 0.016 |
| Arrhythmia | 0.0 (0) | 0.4 (1) | 1.5 (4) | 8.0 (15) | 3.7 (2) | < 0.001 |
|
| ||||||
| Perioperative and postoperative period | ||||||
| readmission | 3.5 (4) | 4.2 (11) | 7.0 (19) | 8.6 (16) | 14.8 (8) | 0.022 |
| treatment-related deaths | 0.0 (0) | 0.8 (2) | 0.4 (1) | 1.1 (2) | 5.6 (3) | 0.005 |
| hospital stay > 2 days | 92 (104) | 92 (241) | 93 (251) | 95.2 (178) | 96.3 (52) | 0.580 |
Variables were compared between the patient subgroups by the Pearson chi-square
In the LAVH group (n=1630), there are no differences in intraoperative complication rates by age; and postoperatively, the only difference is increasing rates of hospitals stay >2 days with increasing age (43.6% vs 68.9% in patients <50yo vs ≥80yo, p<0.001 for all age groups) (Table 3).
Table 3.
Complications and Adverse Events by Age (Laparoscopy n=1630)
| Variable | < 50 n = 172 % (n) |
50–59 n = 492 % (n) |
60–69 n = 492 % (n) |
70–79 n = 368 % (n) |
≥ 80 n = 106 % (n) |
Test statistic* |
|---|---|---|---|---|---|---|
| Intraoperative complications (any) | 8.7 (15) | 9.3 (46) | 9.6 (47) | 8.7 (32) | 11.3 (12) | 0.942 |
|
| ||||||
| Postoperative adverse events (grade ≥ 2) | ||||||
| any | 15.7 (27) | 11 (54) | 14.8 (73) | 16.3 (60) | 19.8 (21) | 0.072 |
|
| ||||||
| Perioperative and postoperative period | ||||||
| treatment-related deaths | 0 (0) | 0.6 (3) | 0.4 (2) | 1.4 (5) | 0 (0) | 0.247 |
| hospital stay > 2 days | 43.6 (75) | 42.1 (207) | 53.5 (263) | 57.6 (212) | 68.9 (73) | < 0.001 |
Variables were compared between the patient subgroups by the Pearson chi-square
Overall, there is a higher rate of complication in the LAP group and this difference gets larger with increasing age starting at age 60. Patients <50yo have the same rates of postoperative complications (LAP 15.9% vs LAVH 15.7%), while older, and especially the very old (patients ≥80yo), have increasing rates of complications after LAP 38.9% vs LAVH 19.8%.
Intraoperative, postoperative, and perioperative complications by age and surgical approach
Figure 1 describes a linear model with outcome of maximum toxicity and explanatory variables including age, race, BMI, stage, grade, histology, myometrial invasion, lymphovascular space invasion and presence of nodal metastasis. As shown in Figure 1, the change in maximum toxicity before approximately age 60 is not significant, but after age 60 the toxicity appears to increase sharply, with the surgical approach by age interaction with a moderate effect (p=0.035). Therefore, as age increases, the benefit from LAVH appears to increase as well, and according to this model, the benefit occurs beginning at age 60.
Figure 1.
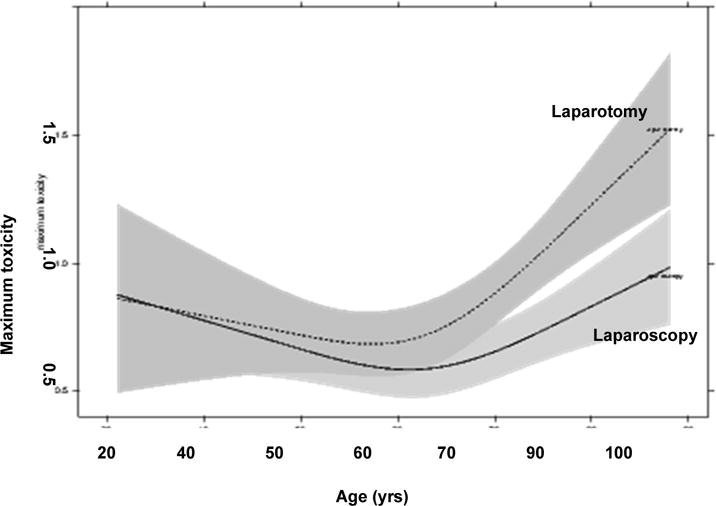
Relationship between age and maximum toxicity for each treatment group. This is a linear model with outcome maximum toxicity. The model shows a treatment × age interaction with a moderate effect (P = 0.035). The effects plot suggests that the LAP group is prone to higher maximum toxicity after about age 60.
Based on the increasing rates of complications seen starting at age 60yo in the LAP group in this study we used 60yo as our cut-off and next compared complications in LAP versus LAVH controlling for race, BMI, stage, grade, and performance status. In patients <60yo there are no differences in intraoperative complications by surgical approach. In the postoperative period, as expected, the LAP group has higher rates of hospital stay >2 days (OR 17.48; 95% CI 11.71–27.00, p<0.001) (Supplemental Table 2).
In patients ≥60yo (Table 4), the only difference in intraoperative complications is arterial injury, which occurred more frequently in the LAVH group (OR 0.31; 95% CI 0.07–0.94, p=0.037). The original LAP2 manuscript states that the majority of these were controlled without conversion to LAP. The postoperative time period reveals more differences in complications by surgical approach in older patients. In addition to hospital stay >2 days (OR 12.77; 95% CI 8.74–19.32, p<0.001), patients ≥60yo in the LAP group have higher rates of postoperative antibiotics (OR 1.63; 95% CI 1.24–2.14, p<0.001), ileus (OR 2.16; 95% CI 1.42–3.31, p<0.001), pneumonias (OR 2.36; 95% CI 1.01–5.66, p=0.048), deep vein thromboses (OR 2.87; 95% CI 1.08–8.03, p=0.035), and arrhythmias (OR 3.21; 95% CI 1.60–6.65, p=0.001) compared to patients ≥60yo in the LAVH group (Table 4).
Table 4.
| Variable | Laparoscopy n = 966 |
Laparotomy n = 511 |
Laparoscopy vs laparotomy | ||
|---|---|---|---|---|---|
| % (n) | % (n) | p value | Odds ratio* | 95% CI | |
| Intraoperative complications | |||||
| any | 9.4 (91) | 7.6 (39) | 0.202 | 0.78 | 0.52–1.14 |
| bowel | 2.3 (22) | 2 (10) | 0.541 | 0.79 | 0.35–1.66 |
| veins | 2.7 (26) | 3.1 (16) | 0.668 | 1.15 | 0.60–2.16 |
| artery | 1.9 (18) | 0.6 (3) | 0.037 | 0.31 | 0.07–0.94 |
| GI tract | 0.5 (5) | 0.6 (3) | 0.882 | 1.12 | 0.22–4.66 |
| bladder | 0.7 (7) | 0.4 (2) | 0.376 | 0.50 | 0.07–2.15 |
| ureter | 1 (10) | 0.4 (2) | 0.152 | 0.35 | 0.05–1.41 |
| Postoperative adverse events (grade ≥ 2) | |||||
| any | 15.9 (154) | 24.5 (125) | < 0.001 | 1.71 | 1.31–2.25 |
| urinary tract infection | 2.1 (20) | 3.7 (19) | 0.075 | 1.80 | 0.94–3.44 |
| fever | 2.7 (26) | 2.9 (15) | 0.853 | 1.06 | 0.54–2.01 |
| pelvic cellulitis | 0.9 (9) | 1 (5) | 0.940 | 1.04 | 0.32–3.08 |
| abscess | 0.9 (9) | 1 (5) | 0.918 | 1.06 | 0.32–3.11 |
| DVT | 0.7 (7) | 2 (10) | 0.035 | 2.87 | 1.08–8.03 |
| pulmonary embolus | 1.7 (16) | 1.6 (8) | 0.656 | 0.82 | 0.32–1.92 |
| bowel obstruction | 1 (10) | 2 (10) | 0.180 | 1.85 | 0.75–4.56 |
| ileus | 4.9 (47) | 9.6 (49) | < 0.001 | 2.16 | 1.42–3.31 |
| pneumonia | 1 (10) | 2.5 (13) | 0.048 | 2.36 | 1.01–5.66 |
| wound infection | 3.7 (36) | 2.9 (15) | 0.286 | 0.71 | 0.37–1.31 |
| congestive heart failure | 1.1 (11) | 2 (10) | 0.279 | 1.65 | 0.66–4.03 |
| arrhythmia | 1.3 (13) | 4.1 (21) | 0.001 | 3.21 | 1.60–6.65 |
| Perioperative and postoperative period | |||||
| blood transfusion | 3.4 (33) | 2.7 (14) | 0.317 | 0.72 | 0.36–1.36 |
| antibiotics | 16.3 (157) | 24.3 (124) | < 0.001 | 1.63 | 1.24–2.14 |
| readmission | 5.6 (54) | 8.4 (43) | 0.056 | 1.52 | 0.99–2.31 |
| reoperation | 3.2 (31) | 2.9 (15) | 0.828 | 0.93 | 0.48–1.73 |
| treatment-related deaths | 0.7 (7) | 1.2 (6) | 0.806 | 1.16 | 0.33–3.76 |
| hospital stay > 2 days | 56.7 (548) | 94.1 (481) | < 0.001 | 12.77 | 8.74–19.32 |
Adjusted for race, body mass index, stage, grade, and performance status
DISCUSSION
This analysis shows a clear difference in morbidity associated with surgical approach in older endometrial cancer patients. Results from this analysis show that while there are overall low rates of intraoperative and postoperative complications during complete surgical staging regardless of surgical approach, patients undergoing LAP experience more postoperative complications with increasing age.
An increased benefit to laparoscopic staging is seen beginning at age 60yo with increased toxicity seen in the LAP group starting at this age. In patients <60yo there is very little difference in complication rates between LAVH and LAP and these patients generally do well from a surgical standpoint, with higher rates of expected complications from LAP such as longer hospital stay. In contrast, patients ≥60yo who undergo LAP have higher rates of additional complications compared to LAVH such as ileus, deep vein thrombosis, pneumonia, and cardiac adverse events (arrhythmias), suggesting that these patients truly benefit from a minimally invasive approach. It is important to note that this study includes relatively healthy patients who met inclusion criteria for a surgical study and therefore these results may not be applicable to advanced-stage patients or patients with poor performance status and do not account for medical comorbidities.
A recent Surveillance, Epidemiology and End Results (SEER) analysis highlights the increased morbidity seen in older patients undergoing LAP for surgical management of endometrial cancer. This study examined over 25,000 women ≥65yo who underwent hysterectomy for endometrial cancer. Compared with women 65–69 years old, women ≥85yo were more likely to have perioperative complications (12% versus 17%), postoperative medical complications (24% versus 34%), longer hospital stay (3 versus 5 days), and require more blood transfusions (6% versus 10%). Perioperative mortality rates were significantly higher in patients ≥85yo compared to those 65–69yo (1.6% versus 0.4%). These results were the same when controlling for medical comorbidities. This study population included all stages and medical comorbidities and not all patients underwent staging, so differs from our population, but demonstrates the morbidity of LAP in elderly patients.2
Studies from the colorectal literature have looked at the potential benefits of laparoscopic colorectal surgery compared to laparotomy in the elderly with concern for worse outcomes in this population due to increase in operative time. In 727 patients, the laparoscopic patients did have longer operating times compared to laparotomy but length of stay was significantly shorter in the laparoscopic group and there was no significant difference in median recovery of bowel function and post-operative morbidity. Thirty-day mortality was significantly lower in the laparoscopic arm (1.3% vs 4.6%, p=0.03).17
In addition to the immediate effects of intraoperative and postoperative morbidity, perioperative complications are also important for survival. A large study looking at 30-day mortality and long term survival after major surgery found that a 30-day postoperative complication was more important than preoperative patient risk and intraoperative factors in determining survival after major surgery.18 This suggests that postoperative complications do not just affect immediate recovery, but also potentially survival, perhaps due to loss of reserve and the inability to start or complete additional therapy.
The requirement for complete surgical staging in LAP2 is a strength of this study, making it a true evaluation of operative morbidity in a fully staged population. This sets this group of older patients apart from other studies where a large percentage of older patients did not undergo lymph node dissection. However, the requirement for full staging may also impact some of the complications noted in the study. LAP2 took place at a time when there was a steep learning curve for laparoscopy in general and especially for lymph nodes. As full lymph node dissection was required, patients were converted from LAVH to LAP when a full dissection could not be completed. The prevalence of conversion among the entire population and especially among the older patients is much higher than would be expected in a more contemporary population and should be viewed through that lens. Weaknesses of this study include the retrospective nature of the analysis and the performance status and early clinical stage required for eligibility in LAP2, making results less applicable to patients with poor performance status and advanced stage disease. Additionally, this study uses age 60yo to define elderly patients. This age was used based on our toxicity data (Figure 1), but is generally younger than age 65 or 70 which is commonly used in the literature. Additional analysis of our data using 70yo at the cut-off age shows similar results (data not shown). Finally, peri- and post-operative care has advanced since the publication of LAP2 with increased incorporation of enhanced recovery after surgery protocols, evidence based use of anti-coagulation, increased expertise for MIS procedures among our anesthesia colleagues and most recently, incorporation of sentinel lymph node identification into LAVH or RaTLH instead of full lymphadenectomy.19 All of these impact on expected complications following MIS procedures in present day and we can assume the complication rates noted in this retrospective analysis are higher than what we would expect under current practice. Despite this, the increased benefit of LAVH as compared to LAP with age should reassure treating physicians that MIS procedures are not only safe, but safer for older patients with endometrial cancer.
With the widespread incorporation of RaTLH into the care of patients with endometrial cancer, the question arises of how these LAVH versus LAP results inform use of RaTLH. While there are no large randomized trials to compare LAVH versus RaTLH, there is a meta-analysis evaluating this question. While limited by the expected methodologic issues, this analysis did show that compared with what they termed conventional laparoscopy, RaTLH was associated with lower conversion rates (risk ratio 0.4 (0.25–0.64; p =0.0002)), less complications (risk ratio 0.72 (0.56–0.94; p =0.02), shorter hospital stay (weighted mean difference −0.37 days; 95% CI − 0.57 to −0.17; p=0.0003), and less blood loss (weighted mean difference −79.2ml; 95% CI −103.43 to −54.97; <000001).20 Recent retrospective studies have compared outcomes in elderly endometrial cancer patients undergoing staging with robotics vs LAP. These studies show that elderly patients (defined as 65 to 70yo) have overall increased rates of perioperative morbidity but that robotic staging in this population is associated with decreased perioperative morbidity and higher incidence of completion of lymphadenectomy compared to LAP.7–9 If anything, RaTLH appears as safe as LAVH and our data reinforces incorporation of MIS procedures preferentially into the care of older endometrial cancer patients.
In summary, our results demonstrate that performing laparoscopic staging for uterine cancer is not just more convenient for surgeons and patients, but is associated with decreased morbidity in patients ≥60yo in the immediate postoperative period. This includes lower cardiovascular and infectious complications. These results allow clinicians to provide more accurate counseling about risks and benefits of surgery to older uterine cancer patients and highlight the importance of offering a minimally invasive approach to decrease immediate postoperative morbidity that could potentially have impact on longer-term outcomes.
Supplementary Material
CONDENSATION.
Laparoscopic staging for endometrial cancer patients ≥60 years old results in decreased morbidity in the immediate postoperative period.
Acknowledgments
This study was supported by National Cancer Institute grants to the Gynecologic Oncology Group Administrative Office (CA 27469) and the Gynecologic Oncology Group Statistical Office (CA 37517) and NRG Oncology SDMC Grant U10CA180822 and U10CA180868 (NRG Oncology Operations).
The following Gynecologic Oncology Group member institutions participated in the primary treatment studies: Abington Memorial Hospital, Walter Reed Army Medical Center, University of Minnesota Medical School, University of Mississippi Medical Center, University of Pennsylvania Cancer Center, University of California at San Diego, University of Iowa Hospitals and Clinics, University of Texas Southwestern Medical Center at Dallas, Indiana University School of Medicine, University of California Medical Center at Irvine, Tufts-New England Medical Center, Rush-Presbyterian-St. Luke’s Medical Center, University of New Mexico, The Cleveland Clinic Foundation, State University of New York at Stony Brook, Washington University School of Medicine, Memorial Sloan-Kettering Cancer Center, Columbus Cancer Council, MD Anderson Cancer Center, University of Massachusetts Medical School, Women’s Cancer Center, University of Oklahoma, Tacoma General Hospital, Tampa Bay Cancer Consortium, Gynecologic Oncology Network, Fletcher Allen Health Care, University of Wisconsin Hospital, Women and Infants Hospital, and CCOP.
This study was supported by National Cancer Institute grants to the Gynecologic Oncology Group Administrative Office (CA 27469), the Gynecologic Oncology Group Statistical and Data Center (CA 37517), the NRG Oncology SDMC grant U10 CA180822 and the NRG Oncology Operations grant U10CA 180868.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This was presented in part at the 44th Annual Meeting of the Society of Gynecologic Oncologists, March 9–12, 2013, Los Angeles, CA.
The authors report no conflict of interest.
Clinical Trial Registration: NCT00002706; https://clinicaltrials.gov
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA: a Cancer Journal for Clinicians. 67(1):7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Wright JD, Lewin SN, Barrena Medel NI, et al. Morbidity and mortality of surgery for endometrial cancer in the oldest old. American journal of obstetrics and gynecology. 2011;205(1):66 e61–68. doi: 10.1016/j.ajog.2011.02.067. [DOI] [PubMed] [Google Scholar]
- 3.Veldkamp R, Kuhry E, Hop WC, et al. Laparoscopic surgery versus open surgery for colon cancer: short-term outcomes of a randomised trial. The Lancet. Oncology. 2005;6(7):477–484. doi: 10.1016/S1470-2045(05)70221-7. [DOI] [PubMed] [Google Scholar]
- 4.Smith JA, Jr, Chan RC, Chang SS, et al. A comparison of the incidence and location of positive surgical margins in robotic assisted laparoscopic radical prostatectomy and open retropubic radical prostatectomy. The Journal of urology. 2007;178(6):2385–2389. doi: 10.1016/j.juro.2007.08.008. discussion 2389-2390. [DOI] [PubMed] [Google Scholar]
- 5.Janda M, Gebski V, Davies LC, et al. Effect of Total Laparoscopic Hysterectomy vs Total Abdominal Hysterectomy on Disease-Free Survival Among Women With Stage I Endometrial Cancer: A Randomized Clinical Trial. Jama. 2017;317(12):1224–1233. doi: 10.1001/jama.2017.2068. [DOI] [PubMed] [Google Scholar]
- 6.Bogani G, Cromi A, Uccella S, et al. Perioperative and long-term outcomes of laparoscopic, open abdominal, and vaginal surgery for endometrial cancer in patients aged 80 years or older. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 2014;24(5):894–900. doi: 10.1097/IGC.0000000000000128. [DOI] [PubMed] [Google Scholar]
- 7.Guy MS, Sheeder J, Behbakht K, Wright JD, Guntupalli SR. Comparative outcomes in older and younger women undergoing laparotomy or robotic surgical staging for endometrial cancer. American journal of obstetrics and gynecology. 2016;214(3):350 e351, 350 e310. doi: 10.1016/j.ajog.2015.09.085. [DOI] [PubMed] [Google Scholar]
- 8.Backes FJ, ElNaggar AC, Farrell MR, et al. Perioperative Outcomes for Laparotomy Compared to Robotic Surgical Staging of Endometrial Cancer in the Elderly: A Retrospective Cohort. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 2016;26(9):1717–1721. doi: 10.1097/IGC.0000000000000822. [DOI] [PubMed] [Google Scholar]
- 9.Doo DW, Guntupalli SR, Corr BR, et al. Comparative Surgical Outcomes for Endometrial Cancer Patients 65 Years Old or Older Staged With Robotics or Laparotomy. Annals of surgical oncology. 2015;22(11):3687–3694. doi: 10.1245/s10434-015-4428-0. [DOI] [PubMed] [Google Scholar]
- 10.Walker JL, Piedmonte MR, Spirtos NM, et al. Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Study LAP2. Journal of Clinical Oncology. 2009;27(32):5331–5336. doi: 10.1200/JCO.2009.22.3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walker JL, Piedmonte MR, Spirtos NM, et al. Recurrence and survival after random assignment to laparoscopy versus laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group LAP2 Study.[Erratum appears in J Clin Oncol. 2012 May 1;30(13):1570] Journal of Clinical Oncology. 2012;30(7):695–700. doi: 10.1200/JCO.2011.38.8645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pearson K. On the criterion that a given system of deviations from the probable in the case of a correlated system of variables is such that it can be reasonably supposed to have arisen from random sampling. Philosophical Magazine. 51900:157–175. [Google Scholar]
- 13.Whitney D, Mann H. On a test of whether one of two random variables is stochastically larger than the other. Ann Math Stat. 1947;18:50–60. [Google Scholar]
- 14.Kruskal W, Wallis W. Use of ranks in one-criterion variance analysis. J Am Stat Assoc. 1952;47:583–621. [Google Scholar]
- 15.Harrell F. Regression Modeling Strategies, with Applications to Linear Models, Survival Analysis and Logistic Regression. New York: Springer; 2001. [Google Scholar]
- 16.R Core Team. A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2013. http://www.R-project.org/, 2013. [Google Scholar]
- 17.Tan WS, Chew MH, Lim IA, Ng KH, Tang CL, Eu KW. Evaluation of laparoscopic versus open colorectal surgery in elderly patients more than 70 years old: an evaluation of 727 patients. International journal of colorectal disease. 2012;27(6):773–780. doi: 10.1007/s00384-011-1375-5. [DOI] [PubMed] [Google Scholar]
- 18.Khuri SF, Henderson WG, DePalma RG, et al. Determinants of long-term survival after major surgery and the adverse effect of postoperative complications. Annals of surgery. 2005;242(3):326–341. doi: 10.1097/01.sla.0000179621.33268.83. discussion 341–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rossi EC, Kowalski LD, Scalici J, et al. A comparison of sentinel lymph node biopsy to lymphadenectomy for endometrial cancer staging (FIRES trial): a multicentre, prospective, cohort study. The Lancet. Oncology. 2017;18(3):384–392. doi: 10.1016/S1470-2045(17)30068-2. [DOI] [PubMed] [Google Scholar]
- 20.Chen SH, Li ZA, Huang R, Xue HQ. Robot-assisted versus conventional laparoscopic surgery for endometrial cancer staging: A meta-analysis. Taiwanese journal of obstetrics & gynecology. 2016;55(4):488–494. doi: 10.1016/j.tjog.2016.01.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


