Abstract
Background
To examine the association between myocardial strain and arterial thickness and stiffness in young adults. Increased common carotid artery intima media thickness (cIMT) and peripheral arterial stiffness are known to precede coronary artery disease and cardiovascular events such as myocardial infarction and congestive heart failure. However, subclinical cardiac dysfunction can be detected in high risk adults by myocardial strain echocardiography. We hypothesized that increased cIMT would be associated with abnormal myocardial strain in young subjects who had obesity and type 2 diabetes mellitus.
Methods
Cardiovascular (CV) risk factors were collected in 338 young adults participating in a prospective, cross-sectional study. The CV parameters collected included intima media thickness (IMT), peripheral arterial stiffness by brachial distensibility (BrachD), myocardial strain and strain rate. General linear models were constructed to determine if vascular structure and function measures were independently associated with myocardial strain and strain rate.
Results
A linear relationship was found between global longitudinal strain obtained from the 4-chamber view (GS 4-chamber) and global strain rate in systole (GSRs) and carotid IMT (GS 4-chamber beta = 3.0, CV risk factor-adjusted R2 = 0.34; GSRs beta = 0.0053, R2 =0.21, p ≤0.0001) and between GS 4-chamber and lower BrachD (beta = −0.42, R2 = 0.22, p≤ 0.001).
Conclusions
We conclude that adverse changes in vascular structure and function are simultaneously present with reduced myocardial systolic function.
Keywords: Carotid intima-media thickness, Brachial artery distensibility, Vascular stiffness, Left ventricular dysfunction, myocardial strain
Introduction
Obesity, hypertension, and diabetes mellitus are known to lead to cardiovascular (CV) events which may be preceded by subclinical cardiac dysfunction.
The importance of evaluating LV systolic mechanics in risk stratification is demonstrated in the work of Stanton et al who studied 546 subjects of whom ½ were hypertensive, ½ had dyslipidemia, 20% had diabetes and 1/3 had a previous myocardial infarction. In these high risk adults, global longitudinal strain was a better predictor of outcome after 5 years as compared to ejection fraction or wall motion score index and demonstrated the superiority of strain to traditional echo measures in predicting incident CV disease.[2]
It is hypothesized that cardiac abnormalities may be due to arterial stiffening caused by CV risk factors.[3] Lembo et al found global longitudinal strain was lower in adults in the highest tertile of pulse pressure, a rough estimate of arterial stiffness[4] and our previous study of adolescents where global arterial stiffness index was an independent correlate of LV mass in adolescents and young adults.[5] Whether subtle reduction in systolic function (less negative values for strain) related to arterial dysfunction can be demonstrated in adolescents and young adults with normal ejection fraction is not well described. In these analyses, we tested the hypothesis that CV risk factor-related changes in adverse arterial measures would be independently associated with reduced myocardial strain and strain rate in young subjects.
Material and Methods
Subjects
Data were collected from 338 subjects in a prospective longitudinal study designed to evaluate the CV effects of obesity and Type 2 diabetes mellitus (T2DM) on adolescents and young adults. Although risk factors, vascular data and limited echocardiographic parameters were obtained at baseline, cardiac strain was added at the follow-up visit where subjects had a mean age of 22 years (38% male, 63% non-Caucasian). For the initial study, all subjects with T2DM between the age of 10 and 23 years seen at Cincinnati Children’s Hospital endocrinology clinic were eligible. Diabetic subjects that were recruited were matched by age, race, and gender to both lean (L, Body Mass Index (BMI) <85th percentile) and obese (O, BMI ≥ 95th percentile) controls. The BMI percentiles were obtained from the Centers for Disease Control and Prevention (Atlanta, GA) growth charts. All obese patients were proven non-diabetic by a 2-hour oral glucose tolerance test. Pregnant females and subjects with pre-existing cardiac disease were excluded from enrollment in the original cohort. Institutional Review Board approval was obtained and consent obtained from all subjects or their guardians (if subjects were <18 years of age).
Data collection
Two measures of height and weight were taken using a stadiometer (Holtain Ltd, Great Britain) and a digital scale (SECA Inc, Hanover MD, USA), and the mean values used. Blood pressure (BP) was measured with a mercury sphygmomanometer according to the 2011 NIH CV risk reduction guidelines for adolescent[6] or according to current adult recommendations. [7] Careful attention was made to cuff size and measurement technique. The average of 3 measures of systolic BP (SBP), diastolic BP (DBP), and heart rate (HR) were used in analyses.
Blood samples were obtained after a minimum 10 hours fast. Plasma glucose was measured with a Hitachi glucose analyzer with intra-assay and inter-assay coefficients of variation of 1.2% and 1.6% respectively.[8] Plasma insulin was measured by radioimmunoassay with an anti-insulin serum raised in guinea pigs, 125I-labeled insulin (Linco, St Loius, MO), and a double antibody method to separate bound from free tracer. This assay has a sensitivity of 2 pmol and has intra-assay and inter-assay coefficients of variation of 5% and 8% respectively.[8] Lipid profile assays were performed in a lab standardized by the National Heart, lung, and Blood Institute and Centers for Disease Control and Prevention. The low-density lipoprotein cholesterol (LDL) concentration was calculated using the Friedewald equation. High-sensitivity C-reactive protein (HS-CRP) was measured using an enzyme-linked immunosorbent assay. Glycosylated hemoglobin A1c (HbA1c) was measured in red blood cells by high-performance liquid chromatography methods.
Cardiovascular testing
All measurements were obtained by certified sonographers and vascular technicians blinded to study group assignment.
Cardiac structure
Echocardiograms were performed on a GE Vivid 5 or 7 (Milwaukee, WI, USA) ultrasound system. All images were obtained with the participant in the left lateral decubitus position to acquire parasternal long-axis, parasternal short-axis and apical 4-chamber views for a total of three cardiac cycles. Absence of structural heart disease was confirmed and then the mean of 3 readings of left ventricular end-diastolic, end-diastolic septal thickness and end-diastolic posterior wall thicknesses were measured off-line by one sonographer using a Digiview Image Management and Reporting System (Digisonics, Houston, Texas). LVM was calculated by the Devereaux formula[9] and normalized to ht2.7 as recommended by De Simone.[10]
Cardiac diastolic function
Mitral inflow velocity was obtained with pulsed wave Doppler parallel to mitral inflow in the apical 4-chamber view and maximal velocity measured at the mitral valve leaflet tips. The mitral peak E (early filling) and A (inflow with atrial contraction) waves were measured off-line, and an E/A ratio was calculated. Tissue Doppler imaging of blood flow velocities was acquired in the apical 4-chamber view. The peak and late velocities of mitral annular flow were recorded at both the septal annulus (Ea-sept, Aa-sept) and lateral annulus (Ea-lat, Aa-lat). The Ea/Aa ratios were calculated in addition to E/average of Ea-lat and E/Ea sept. The E/Ea ratio corrects for myocardial relaxation in transmitral flow (E) and has been shown to correlate with LV end-diastolic pressure.7 In adults, an E/Ea <10 is normal.[11]
Cardiac systolic function
Global longitudinal strain from the 4-chamber view (GS 4-chamber, in %) and strain rate in systole (GSRs per second) were obtained with tissue velocity imaging using a tissue Doppler technique (not speckle tracking) from the apical four-chamber view using high resolution images (>80 frames/second). Tissue Doppler imaging was also obtained from the para-sternal short axis view for measurement of circumferential strain (circumferential shortening).[12] Images were analyzed with GE EchoPAC® software (GE Healthcare, Minneapolis, MN, USA). The sonographer identified the medial mitral valve annulus, the apex and the lateral mitral valve annulus and the software automatically traced the endocardial and epicardial borders. If 2 or more of the six segments averaged for global strain were missing the image was re-read until no or up to 1 segment was rejected by the software. Images with 2 or more rejected segments were excluded from the analyses. Reproducibility of global strain and strain rate in the 4-chamber view was good with coefficient of variation of 5.4 and 8.7% respectively. Reproducibility of strain obtained from the para-sternal short axis views was lower (higher coefficients of variation of 17.5 and 15.3% respectively).
Carotid intima-media thickness (cIMT)
The carotid arteries were evaluated with high-resolution B-mode ultrasonography using a Vivid 7 (GE Vingmed) ultrasound imaging system with a multi-frequency linear array transducers at 7–14 mHz. The thickest cIMT of the far wall of each carotid segment was measured using a manual trace (Vericis Merge, Emageon) technique of the common carotid, carotid bulb, and internal carotid artery. The cIMT measurement inter- observer variability in our lab has been documented as <5% and intra-observer variability as 1.9% (unpublished data).
Brachial artery distensibility (BrachD)
BrachD was measured by a DynaPulse device (PulseMetric Inc, San Diego, CA, USA) using validated and reproducible method of pulse wave form analysis that is independent of body size and baseline brachial artery diameter. [13] The average values of 3 resting measures of BrachD were used in analyses. This is a reproducible measure with coefficients of variability of less than 9%. [14]
Statistical Analysis
Statistical analyses were performed utilizing Statistical Analysis Software (SAS® Institute Inc, v9.3, Cary, NC, USA). Average values for the demographic, anthropometric and laboratory data were obtained by group. Analysis of variance (ANOVA) was performed for continuous variables and chi square for categorical variables to determine statistically significant differences by group. Variance stabilizing transformations were performed as necessary for analyses. Associations between strain variables and risk factors were assessed by correlation analysis. Variables that were significant in bivariate analyses (age, sex, race, BMI z-score, mean arterial pressure (MAP), study group, TG, HDL, LDL, HbA1c, and HS-CRP, medication use) were included in the final general linear models constructed to determine if vascular function and/or structure were independent correlates of GS 4-chamber and GSRs. Bonferroni correction was employed to adjust for multiple comparisons. To illustrate the effect of obesity on strain, subjects were stratified by tertiles of obesity and ANOVA performed to determine differences in strain by BMI tertile.
Results
The characteristics of the study population stratified into three subgroups (lean = L, n = 112; obese = O, n =121; Type 2 diabetes mellitus = T, n = 105) are presented in Table 1. There were no differences in race but there were a higher proportion of males in the lean group. By design, the differences in weight and BMI were significantly different between the lean and both the obese and T2DM groups. In general, cardiovascular risk profile (adiposity, BP, lipids, glycemic control, and inflammation) worsened from lean to obese to T2DM subjects. There was a graded increase in SBP from lean to obese to diabetic groups (112.2 vs 117 vs 121 mmHg p≤0.05). The lean group had a more favorable DBP, HDL, insulin and HS-CRP as compared to the obese and diabetic groups. Both the lean and obese groups had a lower HR, LDL, TG, glucose, and HbA1c as compared to the diabetic group (all p ≤0.05)
Table 1.
Description of the Study Population (mean ± SD)
| Variable | Lean (N=112) | Obese (N=121) | T2DM (N=105) |
|---|---|---|---|
| Age (years) | 21.8 ± 3.9 | 22.1 ± 3.3 | 22.8 ± 3.8 |
| Sex (% male)* | 54 48% | 40 33% | 36 34% |
| Race (% non-Caucasian) | 62 55% | 79 65% | 72 69% |
| Height (cm) | 170.6 ± 9.7 | 169.8 ± 10.0 | 170.3 ± 9.7 |
| Weight (kg)† | 67.9 ± 11.7 | 110.3 ± 26.7 | 106.9 ± 25.2 |
| BMI (kg/m2)† | 23.2 ± 3.3 | 38 ± 9.1 | 36.6 ± 7.3 |
| SBP (mmHg)‡ | 112.2 ± 11.0 | 117 ± 11.5 | 121 ± 14.7 |
| DBP (mmHg)† | 70 ± 8.7 | 74.2 ± 10.2 | 75.8 ± 10.4 |
| HR (beats/min)§ | 64.5 ± 10.0 | 67.4 ± 11.3 | 75.5 ± 12.7 |
| Tchol (mg/dl) | 168 ± 34.3 | 168.7 ± 32.6 | 178.6 ± 43.2 |
| LDL (mg/dl)** | 99.2 ± 28.9 | 105.8 ± 28.3 | 114.6 ± 38.1 |
| HDL (mg/dl)* | 60.4 ± 16.5 | 51.4 ± 12.3 | 47.8 ± 13.4 |
| TG (mg/dl)§ | 84.5 ± 40.4 | 102 ± 64.4 | 137.2 ± 81.3 |
| Glucose (mg/dl)§ | 91.5 ± 7.3 | 92.7 ± 10.3 | 199.7 ± 127.3 |
| Insulin(μU/mL)† | 8.5 ± 6.8 | 18.4 ± 13.1 | 21 ± 17.6 |
| HbA1c (%)§ | 5 ± 0.6 | 5 ± 0.5 | 8.5 ± 3.3 |
| HS-CRP (mg/L)† | 1.5 ± 2.3 | 4.2 ± 4.6 | 5.2 ± 4.6 |
P ≤0.05 Group differences by ANOVA and Chi-square for categorical variables with Bonferroni correction for multiple comparisons:
Lean > Obese and Type 2 Diabetic;
Lean < Obese and Type2 Diabetic;
Lean < Obese < Type 2 Diabetic;
Lean and Obese < Type 2 Diabetic;
Lean > Obese >Type 2 Diabetic,
Lean < Type 2 Diabetic
Table 2 shows the results of cardiac and vascular parameters by group. Left ventricular mass index was lower in lean versus obese and diabetic subjects (L = 29.5, O = 38.3, T = 38.7 g/m2.7). There was no difference in shortening fraction. Diastolic function worsened across groups with significant difference in mitral E/e′ (L = 5.9, O = 6.7, O = 8.0) and e′/a′ ratios (L = 11.0, O = 10.3, T = 9.8). Global longitudinal strain in the 4-chamber view (L = −18.0%, vs O = −15.7%, T = −14.9%) and strain rate in systole from the 4-chamber (L = −0.91 vs O = −0.79, T = −0.79) were worse (lower absolute magnitude of shortening) in obese and T2DM groups as compared to lean. The global strain rate in systole (GS 4-chamber) showed a trend of worsening in T2DM as compared to obese (p=0.08). Circumferential strain measured from the para-sternal short axis also demonstrated less shortening in diabetic versus lean (L = −16.1 vs T = −14.7). There was no difference in circumferential strain rate. BrachD was lower from lean to obese to T2DM (L = 6.38, O = 5.62, T = 5.23), reflecting increased arterial stiffness. In the common carotid (L = 0.469, O = 0.496, T = 0.531) and the carotid bulb (L = 0.496, O = 0.520, T = 0.573), IMT was lower in lean and obese as compared to the T2DM group. In the internal carotid (L = 0.394, O = 434, T = 0.448) lean subjects had lower cIMT than the obese and T2DM subjects (all p ≤ 0.05).
Table 2.
Cardiac and Vascular Parameters by Group (mean ± SD)
| Variable | Lean (N=112) | Obese (N=121) | T2DM (N=105) |
|---|---|---|---|
| LVM Index (g/m2.7)† | 29.5 ±8.9 | 38.3 ±11.1 | 38.7 ±12.2 |
| Shortening Fraction (%) | 33.9 ±7.0 | 34.7 ±6.8 | 34.5 ±7.1 |
| Mitral E/Aǁ | 2.2 ±0.7 | 2.0 ±0.7 | 1.7 ±0.6 |
| Mitral E/e′‡ | 5.9 ±1.2 | 6.7 ±1.3 | 7.9 2±.3 |
| Mitral e′/a′ǁ | 2.5 ±0.7 | 2.1 ±0.7 | 1.7 ±0.6 |
| GS 4-chamber (%)† | −18.0 ±2.9 | −15.7 ±3.4 | −14.9 ±3.1 |
| GSRs 4-chamber (per sec)† | −0.91 ±0.17 | −0.79 ±0.17 | −0.79 ±0.17 |
| GS short axis (%)†† | −16.1 ±3.6 | −15.5 ±3.7 | −14.7 ±4.5 |
| GSRs short axis (sec−1) | −0.92 ±0.22 | −0.97 ±0.22 | −0.96 ±0.29 |
| BrachD (%/mmHg)ǁ | 6.38 ±1.04 | 5.62 ±1.13 | 5.23 ±0.91 |
| Common cIMT (mm)§ | 0.469 ±0.080 | 0.496 ±0.091 | 0.531 ±0.104 |
| Bulb cIMT (mm)§ | 0.496 ±0.103 | 0.520 ±0.116 | 0.573 ±0.166 |
| Internal cIMT (mm)† | 0.394 ±0.088 | 0.434 ±0.114 | 0.448 ±0.103 |
P ≤0.05 for group differences by ANOVA with Bonferoni correction:
Lean > Obese and Type 2 Diabetic;
Lean < Obese and Type2 Diabetic;
Lean < Obese < Type 2 Diabetic;
Lean and Obese < Type 2 Diabetic;
Lean > Obese > Type 2 Diabetic,
Lean < Type 2 Diabetic,
Lean > Type 2 Diabetic
Bivariate analyses revealed significant associations (p ≤ 0.05) between GS 4-chamber and GSRs with BMI, BP, lipids, and HbA1c. Longitudinal global strain (4-chamber) and longitudinal global strain rate in systole (GSRs) were associated with peripheral arterial stiffness (lower BrachD) and changes in structure (thicker cIMT; Figure 1) although the overall correlation was weak (R2 between cIMT & GS 4-chamber = 0.11, p≤0.01).
Figure 1.
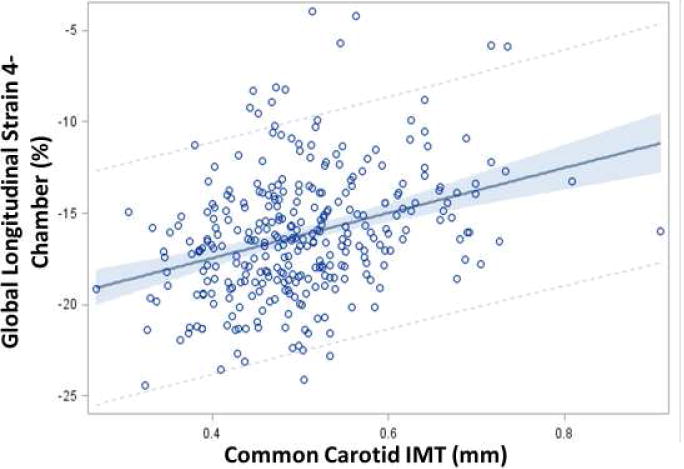
Regression of global strain on common carotid artery cIMT, (mean, 95% CI, R2 = 0.11, p ≤0.0001). A linear relationship is seen between increasing cIMT and reduced global strain indicating worsening cardiac systolic function with thicker carotid arteries.
Table 3 shows multivariable models with standardized beta coefficients for parameters associated with GS in the 4-chamber view (GS 4-chamber). Vascular parameters were not independently associated with circumferential strain measured in the short axis. Common cIMT was an independent correlate of GS 4-chamber with age, male sex, adiposity, BP and HDL also contributing (GS 4-chamber = −10.2 + 3*Common cIMT − 0.11*Age + 0.79*Male sex + 0.92* BMIz + 0.062*MAP − 2.2*HDL-C; R2 = 0.34, p<.0001). Therefore, 34% of the variability in GS 4-chamber is explained by the parameters remaining in the model. Common cIMT was also an independent correlate of GSRs (GSRs = −0.82 + 0.0053*common cIMT + 0.05*male + 0.06* BMIz; R2 = 0.21, p<.0001). BrachD was an independent correlate of GS 4-chamber with male sex, race and BP as other important determinants (GS = −15.9 − 4.2*BrachD + 1.0*Male sex + 0.098*MAP, R2 = 0.22, p<.0001). HbA1c did not enter any model. The standardized beta coefficients demonstrate that adiposity had the largest effect on GS 4-chamber such that in the common cIMT model, a 1 unit increase in BMI z-score led to a 0.18 unit reduction in GS 4-chamber. For the BrachD model, MAP was the strongest contributor such that a 1 mmHg increase in MAP lead to a 0.30 unit decrease in GS 4-chamber. Overall, only a modest amount of the variability in GS 4-chamber was explained by the models. In order to better visualize the effect of arterial stiffness, adiposity and MAP on GS 4-chamber, mean GS 4-chamber is also displayed by tertiles of common cIMT, BrachD, BMI or MAP (Figure 2). A significant reduction in strain is seen with increasing arterial stiffness (higher cIMT or lower BrachD), adiposity or BP (all p for trend ≤0.01).
Table 3.
Independent relationship of arterial structure and function to cardiac strain (standardized beta estimates & R2)
| Parameter | Common cIMT | P | BrachD | P |
|---|---|---|---|---|
| Arterial stiffness (IMT or BrachD) | 0.18 | 0.001 | −0.23 | <.0001 |
| Age | −0.12 | 0.02 | ||
| Male Sex | 0.12 | 0.03 | 0.15 | 0.01 |
| BMI z score | 0.29 | <.0001 | ||
| MAP | 0.18 | 0.001 | 0.30 | <.0001 |
| HDL | −0.20 | 0.0006 | ||
|
| ||||
| R2 | 0.34 | 0.22 | ||
All model p ≤ 0.0001.
Figure 2.
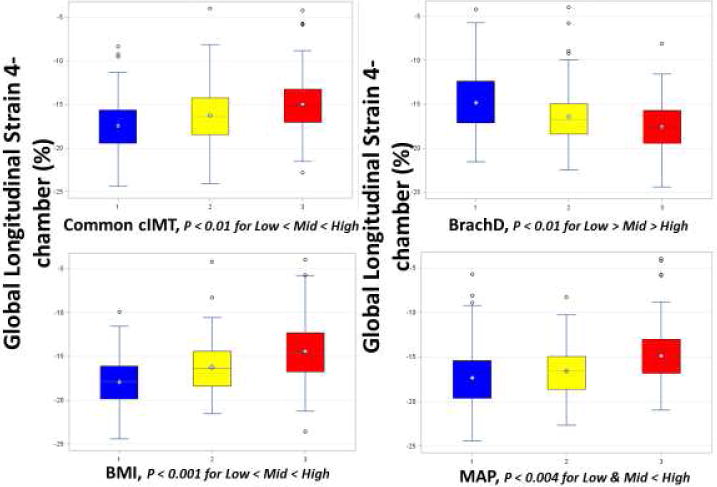
Global longitudinal strain stratified by arterial stiffness, BMI and MAP. There was a significant decrease in strain with increasing common cIMT, BMI, MAP or decreasing BrachD (p for trend ≤0.01).
Discussion
Our data demonstrate a worsening of CV risk profile from lean to obese to subjects with Type 2 diabetes mellitus. Furthermore, GS 4-chamber and GSRs were significantly worse in both obese subjects and those with T2DM as compared to lean subjects. Measures of arterial stiffness (BrachD) and structure (cIMT) were independent correlates of strain measures even after adjusting for CV risk factors. The risk factors that most consistently entered the models were adiposity and BP. Adiposity and HDL levels also contributed to the models including cIMT.
The reduction in strain in high risk subjects may be related to risk factor-related increase arterial stiffness since similar to our results, Ye et al[15] found characteristic aortic impedence, which represents LV afterload, was associated with worse (less negative) GLS and this relationship remained after adjustment for CV risk factors and history of myocardial infarction and stroke.[15] In another study of adults with type 1 diabetes mellitus a strong correlation was found between pulse wave velocity measured with MRI and left atrial strain.[16] Direct comparisons cannot be made to our study however, since the authors did not measure left ventricular strain. Only one small (N=21) study related cardiac strain to vascular structure and function in youth (14 ± 2 years).[17] The obese adolescents had lower longitudinal LV strain associated with higher cIMT, and lower arterial distension as compared to normal-weight controls. Furthermore, ventricular vascular coupling as measured by the ratio of arterial elastance to LV end-systolic elastance measured by echo, was abnormal in obese children and longitudinal deformation abnormalities were related to the vascular measures.[17] Our study extends these observations to a larger (although somewhat older) group of high risk young adults by replicating the relationship between vascular measures and reduced strain.
The effect of cardiovascular risk factors on strain has been previously evaluated in adults. Reduced strain has been demonstrated in the presence of obesity,[18, 19] hyperinsulinism,[20] diabetes,[21–23] and elevated BP.[24–26] However, few studies of strain have been conducted in children. Obert et al studied 37 asymptomatic adolescents (mean age 14 years) with severe obesity, none of whom had diabetes, hypertension, coronary artery disease, or obstructive sleep apnea (OSA). They found impaired myocardial LV longitudinal strain and strain rate compared to lean controls. [27] Ingul et al from Norway found a similar reduction in strain in less severely obese youth (mean age 14.8 years).[28] Our study confirms reduced strain related to adiposity and elevated BP can be identified in adolescents and young adults and for the first time demonstrates a relationship between arterial health and cardiac strain. Although the CARDIA study also found a relationship between HbA1c and strain in a large cohort of late middle-aged adults,[29] we did not find an independent effect of HbA1c on strain in multivariable models. It is possible that a longer exposure to glycemic dyscontrol is needed before HgA1c becomes significant (our cohort is younger) or it is possible that vascular structure and function have a more powerful effect on strain in young subjects.
Fortunately, weight loss and exercise may be useful in improving myocardial mechanics. One study compared strain in 28 adults following bariatric surgery who had significant weight loss and compared them with 35 obese patients managed conservatively (22). They found that longitudinal strain was significantly reduced in patients with obesity, but improved in the surgical group after weight loss. Ingul et al also showed that aerobic exercise intervention of 40 minutes per week resulted in resolution of impaired global strain and strain rate in the study of obese adolescents (mean age 14.8 years). [28]
Study limitations
Although an association between vascular measures and cardiac strain was found, due to the cross-sectional nature of our study, causality cannot be inferred. We also did not have access to MRI which would have allowed evaluation of radial strain. Furthermore, reproducibility of strain measures by MRI is superior to echocardiography. [30] Also, due to our study design, our population was enriched with subjects with type 2 DM, reducing its generalizability to cohorts of young subjects enriched with other CV risk factors such as hypertension or dyslipidemia.
General limitations of strain imaging include more difficulty in imaging in obese subjects. However, we only included subjects with optimal images. Strain may also differ with pre-load,[31] however, our subjects came to the laboratory fasting for their blood work. Although tissue Doppler imaging methods to measure strain have higher resolution than speckle methods, the TDI method is dependent on precise angle placement of the Doppler transducer.[32]
There may also be other factors contributing to vascular dysfunction that influence the development of myocardial strain abnormalities that we did not measure. These may include advanced glycation end-products, [33] oxidized LDL, [34] and fibroblast growth factor [35] substances associated with vascular parameters and cardiac structure and function.
Conclusions
Our data show that subclinical myocardial change is present at a young age in subjects with obesity and type 2 diabetes. We also found vascular measures were independent correlates of decreased strain. This suggests that cardiovascular risk factor-related arterial abnormalities may lead to cardiac dysfunction eventually leading to hard cardiovascular events. Further investigation to characterize correlates of and the time course for development of cardiac strain abnormalities will aid in determining appropriate interventions and their timing.
Highlights.
Reduced strain and strain rate can be identified in adolescents and young adults with obesity and T2MD.
Arterial thickness and stiffness are independently associated with cardiac strain.
CV risk factors such as demographics, BP and lipids are associated with adverse strain in young subjects.
Acknowledgments
We acknowledge with gratitude the entire ‘CV Aging’ study team. All patients and their families who participated in this study are also gratefully acknowledged. This study was funded by grant support: NIH (NHLBI) R01 HL105591 & USPHSG/NIH (CTSA) UL1 RR026314. Sponsors had no role in design, conduct, analyses, interpretation of data or approval of manuscript.
Abbreviations
- AGE
Advanced glycation end-products
- ANOVA
Analysis of variance
- BMI
Body Mass Index
- BrachD
Brachial artery distensibility
- cIMT
Carotid intima-media thickness
- CV
Cardiovascular
- DBP
Diastolic blood pressure
- FGF
Fibroblast growth factor
- GS 4 chamber
Global longitudinal strain obtained from the 4 chamber view
- GSRs
Global strain rate in systole
- HbA1c
Glycosylated hemoglobin A1c
- HDL
High-density lipoprotein cholesterol
- HR
Heart rate
- HS-CRP
High sensitivity C-reactive protein
- L
Lean subject
- LDL
Low-density lipoprotein cholesterol
- LV
Left ventricular
- MAP
Mean arterial pressure
- O
Obese subject
- SBP
Systolic blood pressure
- T
Subject with Type 2 diabetes mellitus
- T2DM
Type 2 diabetes mellitus
- TG
Triglycerides
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
There are no industry relationships to disclose.
References
- 1.Pencina MJ, D’Agostino RB, Larson MG, Massaro JM, Vasan RS. Predicting the 30-Year Risk of Cardiovascular Disease The Framingham Heart Study. Circulation. 2009;119:3078–U61. doi: 10.1161/CIRCULATIONAHA.108.816694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stanton T, Leano R, Marwick TH. Prediction of All-Cause Mortality From Global Longitudinal Speckle Strain Comparison With Ejection Fraction and Wall Motion Scoring. Circ-Cardiovasc Imag. 2009;2:356–64. doi: 10.1161/CIRCIMAGING.109.862334. [DOI] [PubMed] [Google Scholar]
- 3.Roman MJ, Pickering TG, Schwartz JE, Pini R, Devereux RB. Association of carotid atherosclerosis and left ventricular hypertrophy. J Am Coll Cardiol. 1995;25:83–90. doi: 10.1016/0735-1097(94)00316-i. [DOI] [PubMed] [Google Scholar]
- 4.Lembo M, Esposito R, Lo Iudice F, Santoro C, Izzo R, De Luca N, et al. Impact of pulse pressure on left ventricular global longitudinal strain in normotensive and newly diagnosed, untreated hypertensive patients. J Hypertens. 2016;34:1201–7. doi: 10.1097/HJH.0000000000000906. [DOI] [PubMed] [Google Scholar]
- 5.Urbina EM, Dolan LM, McCoy CE, Khoury PR, Daniels SR, Kimball TR. Relationship between Elevated Arterial Stiffness and Increased Left Ventricular Mass in Adolescents and Young Adults. J Pediatr. 2011;158:715–21. doi: 10.1016/j.jpeds.2010.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics. 2011;128(Suppl 5):S213–56. doi: 10.1542/peds.2009-2107C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. 2014 Evidence-Based Guideline for the Management of High Blood Pressure in Adults Report From the Panel Members Appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311:507–20. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 8.Goodman E, Daniels SR, Morrison JA, Huang B, Dolan LM. Contrasting prevalence of and demographic disparities in the World Health Organization and National Cholesterol Education Program Adult Treatment Panel III definitions of metabolic syndrome among adolescents. J Pediatr. 2004;145:445–51. doi: 10.1016/j.jpeds.2004.04.059. [DOI] [PubMed] [Google Scholar]
- 9.Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–8. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 10.de Simone G, Daniels SR, Devereux RB, Meyer RA, Roman MJ, de Divitiis O, et al. Left ventricular mass and body size in normotensive children and adults: assessment of allometric relations and impact of overweight. J Am Coll Cardiol. 1992;20:1251–60. doi: 10.1016/0735-1097(92)90385-z. [DOI] [PubMed] [Google Scholar]
- 11.Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, 3rd, Dokainish H, Edvardsen T, et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2016;17:1321–60. doi: 10.1093/ehjci/jew082. [DOI] [PubMed] [Google Scholar]
- 12.Dandel M, Lehmkuhl H, Knosalla C, Suramelashvili N, Hetzer R. Strain and strain rate imaging by echocardiography - basic concepts and clinical applicability. Curr Cardiol Rev. 2009;5:133–48. doi: 10.2174/157340309788166642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brinton TJ, Cotter B, Kailasam MT, Brown DL, Chio SS, OConnor DT, et al. Development and validation of a noninvasive method to determine arterial pressure and vascular compliance. Am J Cardiol. 1997;80:323–30. doi: 10.1016/s0002-9149(97)00353-6. [DOI] [PubMed] [Google Scholar]
- 14.Urbina EM, Kimball TR, Khoury PR, Daniels SR, Dolan LM. Increased arterial stiffness is found in adolescents with obesity or obesity-related type 2 diabetes mellitus. Journal of Hypertension. 2010;28:1692–8. doi: 10.1097/HJH.0b013e32833a6132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ye Z, Coutinho T, Pellikka PA, Villarraga HR, Borlaug BA, Kullo IJ. Associations of Alterations in Pulsatile Arterial Load With Left Ventricular Longitudinal Strain. Am J Hypertens. 2015;28:1325–31. doi: 10.1093/ajh/hpv039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Schinkel LD, Auger D, van Elderen SGC, Marsan NA, Delgado V, Lamb HJ, et al. Aortic stiffness is related to left ventricular diastolic function in patients with diabetes mellitus type 1: assessment with MRI and speckle tracking strain analysis. International Journal of Cardiovascular Imaging. 2013;29:633–41. doi: 10.1007/s10554-012-0125-2. [DOI] [PubMed] [Google Scholar]
- 17.Koopman LP, McCrindle BW, Slorach C, Chahal N, Hui W, Sarkola T, et al. Interaction between Myocardial and Vascular Changes in Obese Children: A Pilot Study. J Am Soc Echocardiog. 2012;25:401–U157. doi: 10.1016/j.echo.2011.12.018. [DOI] [PubMed] [Google Scholar]
- 18.Orhan AL, Uslu N, Dayi SU, Nurkalem Z, Uzun F, Erer HB, et al. Effects of isolated obesity on left and right ventricular function: a tissue Doppler and strain rate imaging study. Echocardiography. 2010;27:236–43. doi: 10.1111/j.1540-8175.2009.01024.x. [DOI] [PubMed] [Google Scholar]
- 19.Wong CY, O’Moore-Sullivan T, Leano R, Byrne N, Beller E, Marwick TH. Alterations of left ventricular myocardial characteristics associated with obesity. Circulation. 2004;110:3081–7. doi: 10.1161/01.CIR.0000147184.13872.0F. [DOI] [PubMed] [Google Scholar]
- 20.Kosmala W, Wong C, Kuliczkowska J, Leano R, Przewlocka-Kosmala M, Marwick TH. Use of body weight and insulin resistance to select obese patients for echocardiographic assessment of subclinical left ventricular dysfunction. Am J Cardiol. 2008;101:1334–40. doi: 10.1016/j.amjcard.2007.12.034. [DOI] [PubMed] [Google Scholar]
- 21.Zoroufian A, Razmi T, Taghavi-Shavazi M, Lotfi-Tokaldany M, Jalali A. Longitudinal Strain Velocities and Left Ventricular Dyssynchrony by Two-Dimensional Speckle Tracking Echocardiography Study. Echocardiogr-J Card. 2014;31:456–63. doi: 10.1111/echo.12389. [DOI] [PubMed] [Google Scholar]
- 22.Masugata H, Senda S, Goda F, Yamagami A, Okuyama H, Kohno T, et al. Influences of Hypertension and Diabetes on Normal Age-Related Changes in Left Ventricular Function as Assessed by Tissue Doppler Echocardiography. Clin Exp Hypertens. 2009;31:400–14. doi: 10.1080/10641960802668722. [DOI] [PubMed] [Google Scholar]
- 23.Zhang X, Wei X, Liang Y, Liu M, Li C, Tang H. Differential changes of left ventricular myocardial deformation in diabetic patients with controlled and uncontrolled blood glucose: a three-dimensional speckle-tracking echocardiography-based study. J Am Soc Echocardiogr. 2013;26:499–506. doi: 10.1016/j.echo.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 24.Narayanan A, Aurigemma GP, Chinali M, Hill JC, Meyer TE, Tighe DA. Cardiac Mechanics in Mild Hypertensive Heart Disease A Speckle-Strain Imaging Study. Circ-Cardiovasc Imag. 2009;2:382–90. doi: 10.1161/CIRCIMAGING.108.811620. [DOI] [PubMed] [Google Scholar]
- 25.Galderisi M, Esposito R, Schiano-Lomoriello V, Santoro A, Ippolito R, Schiattarella P, et al. Correlates of global area strain in native hypertensive patients: a three-dimensional speckle-tracking echocardiography study. Eur Heart J Card Img. 2012;13:730–8. doi: 10.1093/ehjci/jes026. [DOI] [PubMed] [Google Scholar]
- 26.Galderisi M, Lomoriello VS, Santoro A, Esposito R, Olibet M, Raia R, et al. Differences of Myocardial Systolic Deformation and Correlates of Diastolic Function in Competitive Rowers and Young Hypertensives: A Speckle-Tracking Echocardiography Study. J Am Soc Echocardiog. 2010;23:1190–8. doi: 10.1016/j.echo.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 27.Obert P, Gueugnon C, Nottin S, Vinet A, Gayrard S, Rupp T, et al. Two-Dimensional Strain and Twist by Vector Velocity Imaging in Adolescents With Severe Obesity. Obesity. 2012;20:2397–405. doi: 10.1038/oby.2012.111. [DOI] [PubMed] [Google Scholar]
- 28.Ingul CB, Tjonna AE, Stolen TO, Stoylen A, Wisloff U. Impaired Cardiac Function Among Obese Adolescents. Arch Pediat Adol Med. 2010;164:852–9. doi: 10.1001/archpediatrics.2010.158. [DOI] [PubMed] [Google Scholar]
- 29.Kishi S, Gidding SS, Reis JP, Colangelo LA, Venkatesh BA, Armstrong AC, et al. Association of Insulin Resistance and Glycemic Metabolic Abnormalities With LV Structure and Function in Middle Age. The CARDIA Study. 2017;10:105–14. doi: 10.1016/j.jcmg.2016.02.033. [DOI] [PubMed] [Google Scholar]
- 30.Cho GY, Chan J, Leano R, Marwick TH. Comparison of 2 dimensional speckle and tissue velocity based strain: Validation with harmonic phase magnetic resonance imaging. Circulation. 2005;112:U508–U. doi: 10.1016/j.amjcard.2005.12.063. [DOI] [PubMed] [Google Scholar]
- 31.Choi JO, Shin DH, Cho SW, Song YB, Kim JH, Kim YG, et al. Effect of preload on left ventricular longitudinal strain by 2D speckle tracking. Echocardiography. 2008;25:873–9. doi: 10.1111/j.1540-8175.2008.00707.x. [DOI] [PubMed] [Google Scholar]
- 32.Hoit BD. Strain and strain rate echocardiography and coronary artery disease. Circ Cardiovasc Imaging. 2011;4:179–90. doi: 10.1161/CIRCIMAGING.110.959817. [DOI] [PubMed] [Google Scholar]
- 33.Aronson D. Cross-linking of glycated collagen in the pathogenesis of arterial and myocardial stiffening of aging and diabetes. J Hypertens. 2003;21:3–12. doi: 10.1097/00004872-200301000-00002. [DOI] [PubMed] [Google Scholar]
- 34.Yang CM, Chien CS, Hsiao LD, Pan SL, Wang CC, Chiu CT, et al. Mitogenic effect of oxidized low-density lipoprotein on vascular smooth muscle cells mediated by activation of Ras/Raf/MEK/MAPK pathway. Brit J Pharmacol. 2001;132:1531–41. doi: 10.1038/sj.bjp.0703976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murakami M, Nguyen LT, Zhang ZW, Moodie KL, Carmeliet P, Stan RV, et al. The FGF system has a key role in regulating vascular integrity. J Clin Invest. 2008;118:3355–66. doi: 10.1172/JCI35298. [DOI] [PMC free article] [PubMed] [Google Scholar]


