Abstract
Bronchial thermoplasty (BT) is a bronchoscopic treatment for severe asthma using thermal energy to reduce smooth muscle in the bronchial wall. A 47‐year‐old man underwent BT for uncontrolled severe asthma despite maximal pharmacological treatment. After a third procedure, he experienced hypoxaemia because of complete bilateral upper lobe atelectasis. A pulmonary cyst suddenly emerged in to the right middle lobe, associated with the pneumothorax on postoperative day 6, and a chest drainage tube was inserted. As atelectasis of the right upper lung suddenly improved on postoperative day 12, pneumothorax and the cyst improved. Excess stress on the middle lobe due to upper lobe collapse, and check valve due to airway oedema and phlegm, might be related to pulmonary cyst formation. Tissue fragility related to systemic steroid usage and pressure load during pulmonary function testing might influence the occurrence of pneumothorax. Severe adverse events under complete atelectasis after BT require careful attention.
Keywords: Bronchial thermoplasty, pneumothorax, pulmonary cyst, severe asthma
Introduction
Bronchial thermoplasty (BT) is a bronchoscopic procedure for treating severe uncontrolled asthma using thermal energy to reduce airway smooth muscle. Previous studies have reported that BT improved asthma‐related quality of life (QOL) and exacerbation of severe asthmatic patients 1. However, clinicians need to pay close attention as some unexpected severe complications of BT have been reported. We describe a case of pneumothorax preceded by pulmonary cyst development after BT.
Case Report
A 47‐year‐old man was undergoing treatment for severe asthma with high‐dose inhaled corticosteroid and long‐acting β2 agonist, along with frequent systemic steroid burst twice or more a month. He was a 1.25 pack‐year ex‐smoker. As symptoms were poorly controlled despite maximal pharmacological treatment, he was admitted to our hospital for BT. Blood testing showed an elevated level of immunoglobulin E at 477 U/mL, and the white blood cell count was 9750/μL with 1.0% eosinophils under administration of 20 mg of prednisolone. Only mild thickening of the bronchial walls in the lower lobe was seen on chest computed tomography (CT).
The first two procedures were conducted at an interval of 3 weeks and contributed to the alleviation of asthmatic symptoms. Although he experienced complications of local bronchial oedema and small amounts of bloody sputum after each procedure, these resolved within 2 weeks.
The third procedure was performed with 5.4 mg of midazolam and 90 mg of propofol for intravenous anaesthesia for 82 min. The total number of activations was 110, comprising 61 in the left upper lobe bronchi and 49 in the right upper lobe bronchi. Daily pulmonary function tests and chest X‐rays were performed to detect immediate adverse events after BT treatment.
On postoperative day 2, the patient showed hypoxaemia, and bilateral upper lobes appeared completely collapsed on chest radiographs (Fig. 1). To prevent infection due to poor airway clearance in atelectasis, we initiated antibiotics and decided to continue prednisolone at 50 mg/day in order to quell the strong airway inflammation. Findings on chest radiographs did not show any changes for 4 days, but on postoperative day 6, a pulmonary cyst about 85 mm in diameter with air‐fluid level suddenly appeared in the right upper lung field. Before we noticed this drastic change, the patient underwent a routine daily pulmonary function test, in which he experienced sudden right chest pain. Chest CT after this episode revealed a pulmonary cyst in the right middle lobe and right pneumothorax, in addition to complete atelectasis of bilateral upper lobes (Fig. 2).
Figure 1.
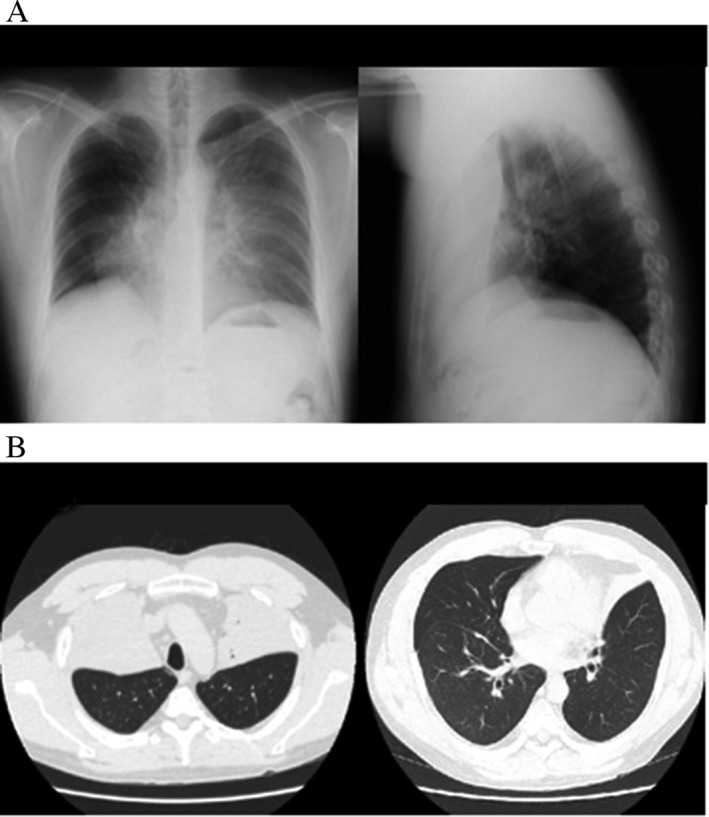
Chest X‐ray (A) and computed tomography (CT) (B) on postoperative day 2. Complete bilateral upper lobe atelectasis is apparent.
Figure 2.
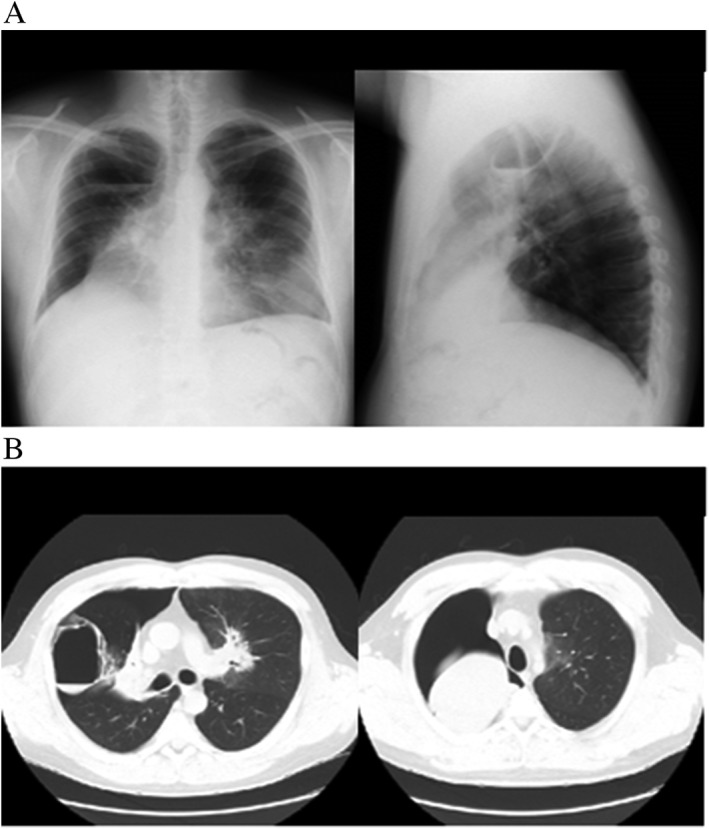
Chest X‐ray (A) and computed tomography (CT) (B) on postoperative day 6. Right pulmonary cyst in the middle lobe and right pneumothorax, in addition to atelectasis of bilateral upper lobes, are observed.
We placed a thoracic drainage tube in the right pleural space. However, pneumothorax and upper lobe atelectasis remained until postoperative day 9. When atelectasis improved on postoperative day 9, pneumothorax also improved, and the cyst diminished and shifted towards the right lower outer field, as seen on chest X‐ray. We tapered steroid dosage and removed the thoracic tube on postoperative day 12. A CT on postoperative day 16 revealed shrinkage of the cyst in the right middle lobe, and follow‐up CT after 2 months showed disappearance of the cyst.
Discussion
Previous studies have evaluated and validated the efficacy, feasibility, and safety of BT. One of the definitive studies for BT was the AIR2 study, which showed that BT improved asthma‐related QOL and reduced severe exacerbations compared to a sham‐controlled group 1. We also reported that BT improved QOL and exacerbations, as well as symptoms and obstructive lung function, in Japanese asthmatic patients with few adverse events 2.
However, as BT is becoming a familiar treatment, reports regarding complications associated with BT are increasing. Burn et al. pointed out that their BT patients were experiencing adverse events more frequently than described in previous clinical trials 3, and some unexpected complications have been presented. The present case is, to the best of our knowledge, the first case to show complications of pneumothorax due to pulmonary cyst developing after BT.
Major adverse events after BT are most likely related to transient oedema of the treated bronchial wall due to the delivery of thermal energy, resulting in worsening of asthmatic symptoms and/or atelectasis. Debray et al. analysed CT data from 13 patients who underwent BT and showed focal inflammations peripheral to the bronchus after BT, which improved in a month. However, three of the cases exhibited complete lobar collapse due to intense inflammation of the bronchus 4. In a prospective study of six patients, Zhang et al. presented a case in which upper lobe atelectasis and then pneumothorax followed a third procedure 5. They explained the pneumothorax as resulting from complete collapse of the upper lobe causing excess traction on the middle lobe.
In our case, the probable cause of pneumothorax was the rupture of a pulmonary cyst in the right middle lobe that had been generated after the third BT procedure during a pulmonary function test. However, the pathogenesis of pulmonary cyst in the middle lobe is unclear. We postulate that a shift in the lateral and medial bronchus due to complete atelectasis of the upper lobe may have imposed excess stress on the middle lobe. Besides physical traction, narrowing of the airways due to oedema and phlegm after BT might have resulted in a check valve, eventually forming a cyst. As the patient was taking systemic corticosteroids frequently to control asthmatic attacks, and was also on high‐dose corticosteroids at onset, tissue fragility might have been related to cyst formation and pneumothorax.
In conclusion, we have reported a case complicated by pneumothorax due to the rupture of a newly developed pulmonary cyst after BT.
Disclosure Statements
Appropriate written informed consent was obtained for publication of this case report and accompanying images.
Acknowledgments
We thank Shoki Ro for translating the Chinese reference into Japanese. This work was supported by a grant from the National Center for Global Health and Medicine (NCGM‐27‐6001).
Funatsu, A. , Kobayashi, K. , Iikura, M. , Ishii, S. , Izumi, S. and Sugiyama, H. (2018) A case of pulmonary cyst and pneumothorax after bronchial thermoplasty. Respirology Case Reports, 6 (2), e00286. doi: 10.1002/rcr2.286.
Associate Editor: Peter Wark
References
- 1. Castro M, Rubin AS, Laviolette M, et al. 2010. Effectiveness and safety of bronchial thermoplasty in the treatment of severe asthma. Am. J. Respir. Crit. Care Med. 181:116–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Iikura M, Hojo M, Nagano N, et al. 2017. Bronchial thermoplasty for severe uncontrolled asthma in Japan. Allergol. Int. https://doi.org/10.1016/j.alit.2017.07.006 [DOI] [PubMed] [Google Scholar]
- 3. Burn J, Sims AJ, Keltie K, et al. 2016. Procedural and short‐term safety of bronchial thermoplasty in clinical practice: evidence from a national registry and hospital episode statistics. J. Asthma 1:1–8. [DOI] [PubMed] [Google Scholar]
- 4. Debray MP, Dombret MC, Pretolani M, et al. 2017. Early computed tomography modifications following bronchial thermoplasty in patients with severe asthma. Eur. Respir. J. 49:1601565. [DOI] [PubMed] [Google Scholar]
- 5. Zhang Q, Zhang X, Xie J, et al. 2016. Bronchial thermoplasty in the treatment of severe asthma. Zhonghua Jie He He Hu Xi Za Zhi 39:183–188. [DOI] [PubMed] [Google Scholar]


