Abstract
A 65‐year‐old man with chronic progressive pulmonary aspergillosis (CPPA) was admitted for the treatment of lung adenocarcinoma while receiving an immune checkpoint inhibitor, nivolumab. The tumour responded well to the therapy, but the cavity of CPPA became large in contrast to the tumour after 20 courses of therapy. He was diagnosed as having exacerbation of CPPA and successfully and concurrently treated with an antifungal agent and nivolumab. Since there was absence of obvious immunosuppression and the presence of a drastic effect on tumour remission during nivolumab therapy, this phenomenon suggested that the trigger of CPPA progression was dependent not on immunosuppression but on a hyperreaction to microorganisms, which was similar to the immune reconstitution inflammatory syndrome caused by nivolumab. This was a thought‐provoking case in which the immune checkpoint inhibitor had a paradoxical effect for the tumour and infection.
Keywords: Aspergillosis, immune checkpoint inhibitor, infectious disease, nivolumab, NSCLC
Introduction
Cancer immunotherapy using immune checkpoint inhibitors has become the mainstream in the treatment of lung cancer. In Japan, the anti‐programmed cell death (PD)‐1 antibody has been approved for treating non‐small cell lung cancer (NSCLC) in December 2015. The use of immune checkpoint inhibitors is associated with unique adverse events called immune‐related adverse events (irAEs), which are caused by the disruption of homeostasis of the immune system. However, until recently, little was known about the relationship between infectious disease and therapy with immune checkpoint inhibitors. Two remarkable cases that suggested reactivation of the immune response to microorganisms by the treatment with nivolumab have been reported. These reports suggested that immune checkpoint inhibitors have an immunomodulatory effect that enhances the immune reaction to infectious disease and often causes the reactivation of latent infections 1, 2. We experienced acute progression of pulmonary aspergillosis in a patient with lung cancer receiving nivolumab treatment. This case involved a thought‐provoking phenomenon that might support the hypothesis of reactivation of infection with immune checkpoint inhibitor treatment.
Case Report
A 65‐year‐old male patient with chronic progressive pulmonary aspergillosis (CPPA) was admitted to our hospital for the development of a pulmonary nodule in the left upper lobe. He had a history of tuberculosis and smoking (40 packs per year). The computed tomography (CT) scan showed the existence of the nodule with marginal irregularity in the left upper lobe and the cavity with marginal infiltration in the right upper lobe (Fig. 1A, B). The positron emission tomography–CT scan revealed accumulated fluorodeoxyglucose concurrently with the nodule of the left upper lobe, which suggested chest wall invasion (Fig. 1C, D). The clinical stage was determined to be cT3N0M0 (stage IIB). Bronchoscopy revealed that the nodule was an adenocarcinoma with a wild‐type driver oncogene. He received two cycles of combination chemotherapy of carboplatin and albumin‐bound paclitaxel as the first‐line therapy. Due to Common Terminology Criteria for Adverse Events grade 3 anorexia, first‐line therapy was discontinued, and he received a second‐line regimen of docetaxel monotherapy. After two cycles of docetaxel, the CT scan showed the progression of the tumour, which was considered a progressive disease. As it was approved for NSCLC as the first‐line immune checkpoint inhibitor since December 2015 in Japan, we selected nivolumab as the third‐line therapy. Immunohistochemical PD‐ligand 1 staining by 22C3 pharmDx showed 80% positive expression in the adenocarcinoma. After 20 cycles of nivolumab, the CT scan revealed remarkable remission of the tumour in the left upper lobe but acute progression of a fungus ball in the cavity with infiltration, suggesting the exacerbation of CPPA (Fig. 2A, B). Antifungal therapy with voriconazole for CPPA was started after confirming the progression of respiratory symptoms in addition to radiological images of the CT scan data. After administering voriconazole, the cavity size of the fungus ball was stable with an asymptomatic condition, and the patient could continue receiving nivolumab with stable disease.
Figure 1.
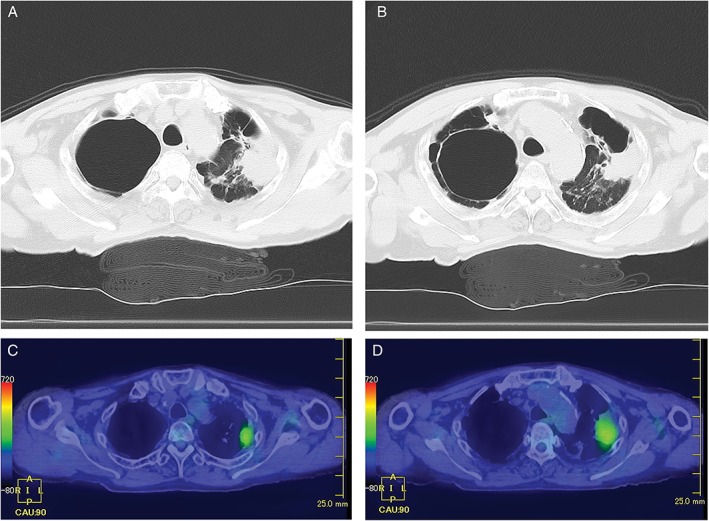
(A–D) Computed tomography (CT) scan showing the nodule with marginal irregularity in the left upper lobe and the cavity with marginal infiltration in the right upper lobe (A, B). Positron emission tomography–CT scan revealing accumulated fluorodeoxyglucose concurrently with the nodule of the left upper lobe with pleural dissemination (C, D).
Figure 2.
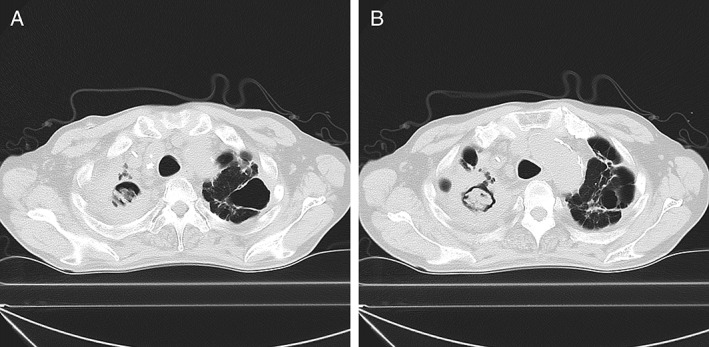
(A, B) Follow‐up computed tomography scan showing progressive enlargement of the fungus ball in the cavity of the right upper lobe with spreading marginal infiltration (A, B). The tumour of the left upper lobe is diminished after nivolumab treatment (A, B).
Discussion
Immune checkpoint inhibitors are known to enhance host cytotoxic T‐cell immunity, which can generate disruption of the homeostasis of the immune system. Although the use of immune checkpoint inhibitors is associated with irAEs, which typically involves the skin, lung, and gastrointestinal and endocrine system, there is little concern about infectious disease. A couple of recent case reports suggested that immune checkpoint inhibitors can cause upregulation of the immune response to microorganisms and provoked paradoxical reactions 1, 2. Other studies have shown that immunosuppressive therapy for irAEs also remain a risk factor for infectious disease 3, 4.
There were two interesting points in the present case. First, the progression of pulmonary aspergillosis was not detected during cytotoxic chemotherapy, which is known to cause immunosuppression; instead, it was detected only after nivolumab induction. This phenomenon suggested that the trigger of aspergillosis progression was dependent not on an immunosuppression but on a nivolumab‐induced hyperreaction to microorganisms, which was similar to the immune reconstitution inflammatory syndrome.
Second, the tumour response to nivolumab showed a near‐complete response inversely with the progression of pulmonary aspergillosis. This clinical condition supported that the immune system of this patient responded well to nivolumab. Activation of T‐cell immunity might influence not only the tumour but also the infection with Aspergillus. It is interesting to note that a recent case report showed reactivation of pulmonary granulomatosis caused by the other anti‐PD‐1 antibody, pembrolizumab 5. Considering this previous case and our case, we speculated that immune checkpoint inhibitors can reactivate T‐cell mediated underlying diseases involving infection and granulomas.
In conclusion, we experienced acute progression of pulmonary aspergillosis during nivolumab treatment. Although the effects of immune checkpoint inhibitors for chronic infection remained controversial and precise mechanisms were unknown, immune checkpoint inhibitors might raise another aspect of irAEs: reactivation of chronic infection.
Disclosure Statement
Appropriate written informed consent was obtained for publication of this case report and accompanying images.
Acknowledgment
The authors thank Dr Osamu Kanai and Dr Misato Okamura for their helpful support with the patient’s care.
Uchida, N. , Fujita, K. , Nakatani, K. and Mio, T. (2018) Acute progression of aspergillosis in a patient with lung cancer receiving nivolumab. Respirology Case Reports, 6 (2), e00289. doi: 10.1002/rcr2.289.
Associate Editor: Stephen Lam
References
- 1. Fujita K, Terashima T, and Mio T. 2016. Anti‐PD1 antibody treatment and the development of acute pulmonary tuberculosis. J. Thorac. Oncol. 11(12):2238–2240. [DOI] [PubMed] [Google Scholar]
- 2. Chu YC, Fang KC, Chen HC, et al. 2017. Pericardial tamponade caused by a hypersensitivity response to tuberculosis reactivation after anti‐PD‐1 treatment in a patient with advanced pulmonary adenocarcinoma. J. Thorac. Oncol. 12(8):e111–e114. [DOI] [PubMed] [Google Scholar]
- 3. Kyi C, Hellmann MD, Wolchok JD, et al. 2014. Opportunistic infections in patients treated with immunotherapy for cancer. J. Immunother. Cancer 2:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Del Castillo M, Romero FA, Arguello E, et al. 2016. The Spectrum of serious infections among patients receiving immune checkpoint blockade for the treatment of melanoma. Clin. Infect. Dis. 63(11):1490–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Al‐Dliw M, Megri M, Shahoub I, et al. 2017. Pembrolizumab reactivates pulmonary granulomatosis. Respir. Med. Case Rep. 22:126–129. [DOI] [PMC free article] [PubMed] [Google Scholar]


