Abstract
The use of Cannabis sativa, also known as marijuana, is believed to have dated back to thousands of years B.C. More than 200 decades later, it remains a popular recreational psychoactive substance that can be smoked through a water pipe. We report a case of marijuana smoking via a “bong” device, which has resulted in severe Pseudomonas aeruginosa necrotizing pneumonia treated with conservative medical therapy. This case highlights the importance of recognizing that life‐threatening pneumonia can potentially be linked to marijuana and “bong” usage. Complicated cases should be considered for early surgical intervention.
Keywords: Pneumonia, respiratory infection (non‐tuberculous), empyema
Introduction
The recreational use of Cannabis sativa, known as marijuana, is believed to have dated back to thousands of years B.C. Numerous reports have demonstrated links to innumerable adverse pulmonary effects ranging from infection, airflow obstruction, interstitial lung diseases, and possible risk of malignancy. While it has been linked with pulmonary aspergillosis, other pulmonary infections are less documented. Here, we describe a case we believe represents the first reported case of necrotizing pneumonia (NP) and empyema secondary to Pseudomonas aeruginosa (P. aeruginosa) infection from habitual recreational marijuana usage via a bong device.
Case Report
A 23‐year‐old‐male presented with a 3‐day history of fever, haemoptysis, and dyspnoea. He reported smoking marijuana daily for 4 years using a small water pipe or bong (Fig. 1A). He was a 10‐pack‐year cigarette smoker. On arrival, his blood pressure was 88/62 mmHg, pulse rate was 142/min, a temperature of 37°C, respiratory rate of 40/min, and oxygen saturation of 85% on room air.
Figure 1.
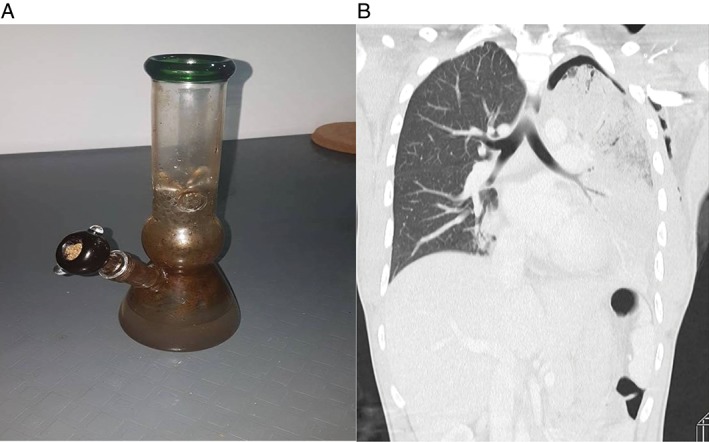
(A) A water pipe or “bong” used by the patient. (B) Computed tomography (CT) thorax (coronal) image under lung tissue setting showed area of necrotizing pneumonia with apical pneumothorax.
Examination revealed crepitations with decreased breath sounds on the left lung. A computed tomographic (CT) thorax showed left upper lobe NP with residual hydropneumothorax (Figs. 1B, 2A). Bedside ultrasound demonstrated the presence of a left‐sided pleural effusion. A left‐sided chest drain was inserted, and thoracentesis was consistent with empyema thoracis. The patient had a CURB65 score of 3. Laboratory studies demonstrated leucopenia (white blood cells (WBC): 3.6 × 109/L; absolute neutrophil count (ANC): 3.3 × 109/L), urea 8.6 mmol/L,C‐reactive protein 30 mg/dL, and procalcitonin of 19.03 ng/mL. Pleural empyema indices were exudative, and neutrophils were predominant with a protein ratio of 0.92 and glucose 0.3 mmol/L. Cultures obtained from the patient’s sputum sample, pleural fluid, and water pipe grew P. aeruginosa, which demonstrated sensitivity towards meropenem, ceftazidime, gentamicin, and amikacin.
Figure 2.
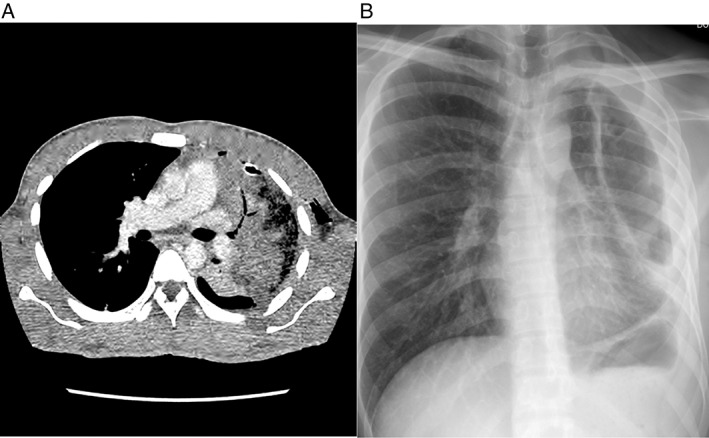
(A) Computed tomography (CT) thorax (axial) image under soft tissue setting showed area of necrotizing pneumonia involving left upper lobe with chest tube in‐situ. (B) Erect Posteroanterior (PA) view chest radiograph of patient during outpatient review showed non‐expanding “trapped” lung with thickened pleural.
The patient was treated with a total 42 days of intravenous (i.v.) ceftazidime as he declined surgical intervention (pleural decortication with or without lobectomy), which was advised on Day 10 of treatment in view of non‐resolving fever and persistent drainage of pus. However, his symptoms subsequently improved on pharmacotherapy. A repeated chest radiograph on follow up showed a left‐sided trapped lung (Fig. 2B) with possible moderate restrictive ventilatory defects demonstrated on spirometry (forced expiratory volume in 1 sec (FEV1): 59% predicted, forced vital capacity (FVC): 55% predicted, FEV1/FVC ratio: 92%). He defaulted subsequent planned full lung function testing.
Discussion
Necrotizing pneumonia is an aftermath of the vigorous inflammatory response occurring between pulmonary inflammation and pulmonary necrosis, with the formation of abscesses and gangrene. The entire process signifies a cataclysmic pulmonary infection.
As opposed to a lung abscess where anaerobic organisms predominate, both Gram‐positive and ‐negative organisms, that is, Streptococcus pneumoniae, methicillin‐sensitive or resistant Staphylococcus aureus, Klebsiella pneumoniae, Haemophilus species, and P. aeruginosa, have been linked to NP 1. Inhalation of contaminated aerosol water has been previously highlighted to cause transmission of bacteria 2. Our patient had inhaled contaminated aerosol water that resulted in pseudomonas NP through smoking marijuana through a supposedly “filtered” bong. This was confirmed by consistent cultures from both sputum and swab samples from the device.
Compared to cigarette smoking, marijuana inhalation demonstrates greater damaging effect. There is replacement of ciliated bronchial epithelial cells by predominant hyperplastic mucus‐secreting (goblet) cells and severe impairment in clearance of mucus that traps harmful particles and organisms, thus resulting in a defective protective mechanism of the lower respiratory tract. Δ9‐tetrahydrocannabinol found in marijuana inhalation demonstrates immunomodulator properties by affecting natural killer and T lymphocytes and catalyses the production of pro‐inflammatory cytokines 3. Cigarette smoking practices further weaken the host defence in mounting a bactericidal immune response.
Currently, there is a paucity of data regarding the emergence, incidence, progression, and outcome of pseudomonas lung infection. There is also no established explanation on the predilection of lung involvement, although the infection foci favours upper lobes of the lung postulated due to increased ventilation and perfusion ratio and less effective lymphatic drainage 4.
Treatment of P. aeruginosa pneumonia can be challenging, especially when the incidence of multidrug‐resistant strains is increasing. An approach using an empirical combination of antimicrobial therapy is recommended 5. Maintaining on combination therapy or subsequent streamlining of treatment to a single sensitive antimicrobial agent once susceptibility is known is controversial, and the dilemma is more pronounced when dealing with severe infection such as an underlying NP. The optimal duration of treatment is also not well established, but most clinicians will advocate treatment until resolution of clinical symptoms, biochemical markers, and radiological changes. With reference to our patient, he was started on empirical i.v. ceftriaxone and oral azithromycin as per local protocols for community‐acquired pneumonia of moderate severity (CURB3). The antimicrobial of choice was later tailored based on the culture and sensitivity results. The patient was treated with an extended duration of i.v. ceftazidime due to patient’s decision in declining surgical intervention.
Against all odds, the patient’s condition improved on pharmacotherapy, but this approach has led to a devastating long‐term sequealae of a trapped lung and impairment in his lung function.
In conclusion, marijuana smoking coupled with usage of bong results in a variety of lung disorders, including potential life‐threatening lung infection. It is paramount for clinicians to recognize and adopt the concept of “hit hard, hit fast” by considering early broad‐spectrum antimicrobial coverage as severe pseudomonas lung infection generally results in high morbidity and mortality. Complicated cases should be dealt with by early surgical intervention to achieve optimal source control.
Disclosure Statement
Appropriate written informed consent was obtained for publication of this case report and accompanying images.
Acknowledgments
The authors thank the patient for giving us the permission to publish this case report.
Kumar, A.N. , Soo, C.I. , Ng, B.H. , Hassan, T. , Ban, A.Y.‐L. and Manap, R.A. (2018) Marijuana “bong” pseudomonas lung infection: a detrimental recreational experience. Respirology Case Reports, 6 (2), e00293. doi: 10.1002/rcr2.293.
Associate Editor: Kazuhisa Takahashi
References
- 1. Reimel BA, Krishnadasen B, Cuschieri J, et al. 2006. Surgical management of acute necrotizing lung infections. Can. Respir. J. 13:369–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rose HD, Franson TR, Sheth NK, et al. 1983. Pseudomonas pneumonia associated with use of a home whirlpool spa. JAMA 250:2027–2029. [PubMed] [Google Scholar]
- 3. Roth MD, Arora A, Barsky SH, et al. 1998. Airway inflammation in young marijuana and tobacco smokers. Am. J. Respir. Crit. Care Med. 157:928–937. [DOI] [PubMed] [Google Scholar]
- 4. Maharaj S, Isache C, Seegobin K, et al. 2017. Necrotizing Pseudomonas aeruginosa community‐acquired pneumonia: a case report and review of the literature. Case Rep. Infect. Dis. 2017:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kalil AC, Metersky ML, Klompas M, et al. 2016. Management of adults with hospital‐acquired and ventilator‐associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin. Infect. Dis. 63:e61–111. [DOI] [PMC free article] [PubMed] [Google Scholar]


