Abstract
Fragile X syndrome (FXS) is associated with executive function (EF) and independent living skills (ILS) deficits. We examined the role of childhood EF in ILS during adolescence/early adulthood in females with FXS and two comparison groups in the same age range (matched for IQ [IQ/Age group] and with another genetic condition, Turner syndrome [TS group]). EF and ILS were significantly higher for the FXS group than the IQ/Age group but did not differ from the TS group. For the FXS group, age and EF were significant predictors of ILS during adolescence/early adulthood, but there were no statistically significant longitudinal associations between EF and ILS. Our findings suggest that impairments in EF may have a significant effect on ILS in FXS.
Keywords: independent living skills, fragile X syndrome, adolescents, young adult, executive function, Turner syndrome
Executive function has been found to be associated with independent living in children and adolescents with typical development and neuro-developmental disorders. Research has demonstrated that executive function (EF) difficulties contribute to lower adaptive functioning trajectories in children with high functioning Autism Spectrum Disorders (ASD; Pugliese et al., 2014; Pellicano, 2012) and attention deficit/ hyperactivity disorder (ADHD; Miller, Nevado-Montenegro, & Hinshaw, 2011). However, the influence of EF on independent living skills remains unclear in fragile X syndrome, a disorder associated with ASD and ADHD symptomatology.
Fragile X syndrome (FXS) is an X-linked genetic disorder that is the most common known genetic cause of inherited intellectual disability (Crawford, Acuña, & Sherman, 2001; Schneider, Hagerman, & Hessl, 2009). Fragile X syndrome is caused by a molecular mutation of the fragile X mental retardation type 1 gene (FMR1) that results in reduced levels of FMRP protein (Verkerk et al., 1991). Downstream effects from the mutation impact brain development and function in individuals with FXS (Lightbody & Reiss, 2009), and are associated with cognitive impairments including EF deficits (Reiss & Dant, 2003). Fragile X syndrome is associated with enlargement of the caudate nucleus (Gothelf et al., 2008; Hoeft et al., 2010) and aberrant development of the frontal lobes (Bray et al., 2011), two regions involved in EF (Alvarez & Emory, 2006).
Executive function (EF) is a term used to describe goal-oriented thoughts, actions, and behaviors that involve higher level cognitive processes such as attention, inhibition, working memory, set shifting or cognitive flexibility, and planning (Alvarez & Emory, 2006; Anderson, 2002; Best, Miller, & Jones, 2009; Miyake, Friedman, Emerson, Witzki, & Howerter, 2000; Pennington & Ozonoff, 1996; Stuss & Alexander, 2000). Attention refers to the ability to engage, sustain or shift focus and selectively divide attention between tasks (Alvarez & Emory, 2006; Best et al., 2009; Pennington & Ozonoff, 1996). Inhibition is described as the ability to suppress an automatic response, and it involves interference control, directed forgetting, and emotional control (Nigg, 2000). Working memory is the ability to temporarily store and manipulate information (Alloway, Gathercole, & Pickering, 2006; Huizinga, Dolan, & van der Molen, 2006). Set shifting or cognitive flexibility is the ability to switch between mental states, operations, or tasks (Miyake et al., 2000). Finally, planning is associated with goal-oriented behavior by helping individuals plan in advance and approach a task in an organized and efficient manner (Anderson, 2002). Planning is thought to be the most complex skill of EF as it is involved with solving problems (Zelazo, Car, Reznick, & Frye, 1997).
Executive function profiles associated with FXS vary depending on gender and intellectual ability. Research has demonstrated differences in EF ability between males and females with FXS with intellectual deficit contributing to more impairment in males. Males with FXS exhibit intellectual deficit that ranges from moderate to severe (Li & El-Mallakh, 1997) with approximately 95% of males having an IQ below 70 (Brown, 2012). The range of intellectual ability in females with FXS is quite broad, with approximately 50% of females having an IQ below 70 (Brown, 2012). In males with FXS, EF impairments include difficulties with attention, inhibition, set shifting or cognitive flexibility, problem solving, planning, goal directed behavior and organization, and working memory (Berry-Kravis et al., 2010; Hooper et al., 2008; Baker et al., 2011). Though females with FXS show less impairment in EF than males, females with FXS have also demonstrated weaknesses in EF (e.g., inhibition, set shifting or cognitive flexibility, and planning) on the Wisconsin Card Sorting Task and/or the Contingency Naming Test excluding other EF measures (Bennetto, Pennington, Porter, Taylor, & Hagerman, 2001; Kirk, Mazzocco, & Kover, 2005).
Independent living skills can involve a variety of behaviors associated with daily living. For the purpose of this study, we define independent living skills to include areas such as personal upkeep (e.g., hygiene, health, safety), management of the home and transportation, social skills (e.g., creating and maintaining relationships, social norms, social cues), and community skills (e.g., managing money, employment following community rules) consistent with our outcome measures described below. In adults with FXS, independent living is most closely associated with functional skills (e.g., hygiene, grooming, household living skills, communication skills) for males and age and interpersonal skills for females (Hartley et al., 2011). Thus, there is evidence to suggest that functional skills, interpersonal skills, and age are associated with independent living skills in FXS but additional factors (executive function) have yet to be examined.
The extant literature on outcomes in individuals with intellectual disabilities has tended to focus on adaptive functioning, a construct that overlaps with independent living. For example, Su, Chen, Wuang, Lin, and Wu (2008) state that intellectual disability is associated with limitations in adaptive behavior across the lifespan. These limitations can impact employment outcome, social competence and community integration in individuals who have an intellectual disability (Felce & Emerson, 2001). Su et al. (2008) found that general cognitive dysfunction and specific verbal memory and comprehension deficits impair daily functions in individuals with intellectual disability. Overall, there is a paucity of research on the cognitive mechanisms such as EF involved with independent living skills.
The ability to plan efficiently, organize, problem solve, initiate activities, and manage behaviors influence an individual’s ability to interact with the world and live independently. Because independent living involves a number of domains (e.g., personal upkeep, management of home and transportation, social skills, community skills), it draws upon a variety of cognitive processes, specifically EF, to approach each task. Thus, dysfunction in EF may be readily seen in the daily life of individuals with FXS with situations that require novel approaches, concentration, problem-solving, change, or making a conscious choice among alternatives as has been previously reported for individuals with ASD or ADHD (Miller et al., 2011; Pellicano, 2012; Pugliese et al., 2014). Our first hypothesis is that individuals with FXS will show a weakness in performance on measures of EF and independent living skills that cannot entirely be accounted for by intellectual level. Thus, we propose control groups matched for age and IQ. Our second hypothesis is that less impaired EF is associated with higher independent living in persons with FXS. Finally, we hypothesize that early metrics of EF may predict later ability to live independently in young adulthood in persons with FXS.
To our knowledge, this is the first study to examine the influence of the specific contribution of EF to independent living skills in individuals with FXS using both a cross-sectional and longitudinal dataset. It is imperative to understand how EF deficits impact the ability to live and function independently as individuals with FXS transition from adolescence into adulthood, a period associated with expectations of increasing independence in typical development. Additionally, identifying key cognitive mechanisms involved in independent living skills will assist in designing future, targeted cognitive interventions.
Method
Participants
We examined the association of executive function (EF) with independent living skills in 34 females with FXS, 18 females with idiopathic developmental delay (IQ/Age group), and 16 females with Turner Syndrome (TS group) at a single time point during late adolescence or early adulthood— “time 2” (Table 1). The IQ/Age group is included as a control group to account for IQ and age. The TS group is included as a genetic disorder control group because both TS and FXS are associated with executive dysfunction that is not solely attributed to IQ (Kirk et al., 2005). Participants in the FXS and IQ/Age group did not have significantly different IQs or ages (Table 1). Participants in the TS group had significantly higher IQ level compared to the FXS group but did not significantly differ in age (Table 1). Household mean and median income per zip code was used to index socioeconomic status (University of Michigan, Population Studies Center, 2013). Socioeconomic status was higher for the IQ/Age group than the FXS group, Fmean (2,61) = 3.932, p = .025, Fmedian (2,61) = 3.807, p = .028. For the TS group, there were no socioeconomic differences compared to the IQ/ Age and FXS groups.
Table 1.
Group Participant Descriptives
| Item | Group
|
FXS vs. IQ/Age
|
FXS vs. TS
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| FXS (N = 34) | IQ/Age (N = 18) | TS (N = 16) | t | df | p | T | df | p | |
| Age | 20.93 ± 3.23 | 19.84 ± 3.38 | 19.15 ± 3.13 | 1.133 | 50 | .26 | 1.828 | 48 | .07 |
| FSIQ | 81 ± 21 | 74 ± 23 | 105 ± 17 | 1.123 | 50 | .27 | −3.85** | 48 | .0001 |
| VIQ | 85 ± 21 | 76 ± 20 | 110 ± 15 | 1.54 | 50 | .13 | −4.23** | 48 | .0001 |
| PIQ | 81 ± 20 | 74 ± 23 | 98 ± 20 | .69 | 50 | .49 | −2.81* | 48 | .007 |
Note. Mean ± SD; FSIQ = full scale IQ; VIQ = verbal IQ; PIQ = performance IQ; FXS = fragile X syndrome; TS = Turner syndrome.
= p < .05
= p < .001.
Cross-sectional analyses (time 2 only) were performed with 34 females with FXS (Table 1). In our cross-sectional analyses, one participant in our TS group did not complete the Vineland Adaptive Behavior Scales. Data from previous assessments (time 1) were available for 31 of the 34 females with FXS. Thus, longitudinal analyses were performed with the 31 females with FXS for whom data were available at both time 1 and time 2 (Table 2). In our longitudinal sample, one participant did not complete measures of verbal fluency at time 1. The mean time between time points 1 and 2 was 8.3 years (SD = 2.09).
Table 2.
Longitudinal Group Descriptives for the Fragile X Syndrome (FXS) Group
| Participants | N | Age | FSIQ | VIQ | PIQ |
|---|---|---|---|---|---|
| Time 1 | 31 | 12.81 ± 2.79 | 81 ± 22 | 83 ± 21 | 82 ± 22 |
| Time 2 | 31 | 21.09 ± 3.31 | 81 ± 22 | 85 ± 22 | 81 ± 21 |
Note. Mean ± SD; FSIQ = full scale IQ; VIQ = verbal IQ; PIQ = performance IQ.
FXS diagnosis was confirmed via evidence of the full FMR1 mutation on DNA testing using the standard Southern blot analysis. Diagnosis of non-mosaic 45,X in participants with TS was confirmed from a standard karyotype assessment. Inclusion criteria for the IQ/Age group included: idiopathic developmental delay, intellectual disability or learning disability confirmed via previous diagnostic evaluations. Exclusion criteria for IQ/Age group included: any known genetic condition, prematurity (<34 weeks gestation), low birth weight (<2,000 g), or a history of severe psychiatric, neurological, or medical disorder that affected growth or development. Participants in the IQ/Age group were screened to confirm that they did not have FXS. Participants with FXS and TS were mostly drawn from a longitudinal sample participating in an ongoing study in our research center, the Center for Interdisciplinary Brain Sciences Research (CIBSR) lab at Stanford University School of Medicine, and were recruited throughout the United States. To reach recruitment goals, additional FXS and control participants were recruited through the National Fragile X Foundation and their regional chapters across the United States, advertisements in local organizations, regional centers, referrals, outreach community events, and parent groups. Participants in the IQ/Age group were only recruited from the local area, which has a high median household income contributing to group differences in socioeconomic status.
All participants were invited for participation at over the course of 2 to 3 days at time 2. Assessments at time 1 took place at our center or in the participants’ homes. Participants and/or their parents were given informed consent and assent to participate in the study. All protocols were approved by the Institutional Review Board at Stanford University, CA.
Materials
Cognitive level
Participants up to 16 years of age completed the Wechsler Intelligence Scale for Children–Third Edition (WISC-III; Wechsler, 1991), and participants aged 17 years and older received the Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler, 1999). Strong reliability and validity has been consistently demonstrated for the WISC-III and WASI (Wechsler, 1991, 1999). Both tests yield standard scores including Full Scale, Verbal, and Performance IQ scores, each with a mean of 100 and a standard deviation of 15.
Executive function
Executive function was assessed through the Contingency Naming Test (CNT; Taylor, 1988). The CNT has been frequently used to research EF deficits in children and adults with FXS (Bennetto et al., 2001; Kirk et al., 2005; Lightbody, Hall, & Reiss, 2006; Mazzocco, Hagerman, & Pennington, 1992). The CNT is a modified Stroop Color Word Test that uses colors and shapes instead of words. The CNT is sensitive to frontal lobe functioning and measures attention, verbal inhibition, set shifting or cognitive flexibility, and working memory. Participants were presented with a series of 27 colored shapes (circle, square, or triangle with a smaller embedded shape in the center) and required to follow a rule when naming each shape or the color of each shape. Subjects are required to follow a different rule in each on four trials. There are four trials. During the first trial, the rule is to name the color of each shape. During the second trial, the rule is to name the outside shapes, ignoring the smaller embedded ones. During the third trial, the rule is to name the color if two shapes of the pair are the same (e.g., a circle embedded in a circle) but to name the outside shape if the two are different (e.g., a circle embedded in a triangle). Finally, during the fourth trial, the rule is to maintain the third rule but to name the opposite (e.g., shape instead of color) if there is an arrow above the drawing. Similar to the Stroop test, the third and fourth rules assess inhibition, switching, and cognitive flexibility. Participants were given up to five practice trials prior to starting a timed trial. For each trial, time to completion (e.g., number of seconds for the participant to name all 27 items), number of correct responses, and number of errors were recorded. The dependent variable was a performance (raw) score calculated by dividing the number of correct responses by the time taken to complete the trial, and multiplying it by 60 (see Lightbody et al., 2006). A higher score reflects a more accurate and efficient performance.
Verbal fluency
Verbal fluency was assessed using the “F, A, S” test (Spreen & Benton, 1977). The Verbal Fluency test assesses the ability to generate words quickly (one minute) according to semantic (e.g., words belonging to the category ‘animals’ or ‘foods’) and phonemic (e.g., words starting with the letters F, A, or S) categories while maintaining a rule (e.g., no repetitions or proper names) in memory. The test measures attention, cognitive flexibility, self-monitoring, self-initiation, and working memory as the participant retrieves words and monitors behavior to reach a goal. The dependent variable was the number of correct responses per minute calculated by summing the total number of correct responses (raw score) for each condition and dividing by 2 (semantic fluency) or 3 (phonemic fluency). A higher score reflects increased verbal fluency skills. Similar normative data has been obtained in validation studies of the test in different countries and it has a high internal consistency and test-retest reliability (see Strauss, Sherman, & Spreen, 2006 for a review).
Independent living skills
Independent living skills were assessed with the Independent Living Scales (ILS; Loeb, 1996). The ILS has demonstrated strong reliability and content, construct, and criterion validity (Loeb, 1996). This performance-based measure assesses the ability to function independently and handle real-life situations in five domains: memory and orientation, managing money, managing home and transportation, health and safety, and social adjustment. Trained clinical research staff provided standard administration of questions that included hypothetical scenarios (e.g., What would you do if the lights and TV were to go out?) and the direct application of skills (e.g., writing a check to pay for a utilities bill). The dependent variable was a full-scale standard score with a mean of 100 and a standard deviation of 15.
Independent living skills were also assessed using the Vineland Adaptive Behavior Scales–Second Edition (VABS-II; Sparrow, Chicchetti, & Balla, 2005). The VABS-II is administered in a semi-structured interview format with an informant who is typically the primary caregiver of the participant. We focused on the Daily Living Skills (VABS-DLS) domain, which assessed personal skills (e.g., hygiene), domestic skills (e.g., maintaining a home), and community skills (e.g., understanding the function of money, employment, managing transportation). The VABS-DLS yields a standard score with a mean of 100 and a standard deviation of 15.
For our longitudinal analysis, we used the Daily Living Skills domain from Vineland Adaptive Behavior Scales–First Edition (VABS; Sparrow et al., 1984). The VABS also yields a standard score with a mean of 100 and a standard deviation of 15. The VABS-II and VABS have demonstrated strong reliability and validity (Sparrow et al., 1984; Sparrow et al., 2005).
Data Analysis
All data were manually entered into IBM SPSS Statistics software. To address our first hypothesis, we initially examined group differences between the FXS, the IQ/Age, and the TS group (all at time 2) for EF and independent living skills with a MANOVA. We entered group as the independent variable and the following dependent variables: Contingency Naming Test (CNT), Verbal Fluency phonemic (VF-P) and semantic (VF-S), Independent Living Scales (ILS), and Vineland Adaptive Behavior Scales I –Daily Living Skills (VABS-DLS). Raw scores were used for CNT, VF-P, and VF-S; and standard scores were used for IQ, ILS, and VABS-DLS. To parse out variance potentially due to differences in IQ and age, a multiple analysis of covariance (MANCOVA) was conducted as described previously but also with FSIQ and age as covariates. Effect size is depicted with partial eta square as follows: small (0.01), medium (0.06), large (0.14). Levene’s test revealed equality of variances between groups for CNT (F(2,64) = 1.19, p = .31), VF-P (F(2,64) =.98, p =.38), VF-S (F(2,64) = .73, p = .48), ILS (F(2,64) = .32, p = .72), and VABS-DLS (F(2,64) =.71, p =.49), thus justifying parametric tests. Post hoc two-group comparisons were conducted using Bonferroni adjusted pairwise comparisons (p < .05 is significant).
For our second hypothesis, we conducted separate linear regression models with either ILS or VABS-DLS as the outcome and the following predictors: age, CNT, VF-P, and VF-S (all at time 2). We repeated these analyses with our comparison groups to examine group differences.
For our third hypothesis, we conducted linear regressions to examine whether EF measures at time 1 predicted independent living skills at time 2. For the ILS, the following predictors were entered into the model: age at time 2, CNT, VF-P, and VF-S at time 1. For VABS-DLS as the outcome (at time 2), the following predictors were included in the model: VABS-DLS, CNT, VF-P, and VF-S at time 1.
Results
Group Differences
There were significant differences between FXS and control groups for the CNT and ILS (Table 3). Post hoc comparisons revealed that the FXS group performed significantly higher on the CNT (p = .02) and ILS (p =.02) than the IQ/Age group, but performed significantly lower on the ILS than the TS group (p = .001). There were no significant differences in performance between the FXS and TS group for EF though two measures approached significance suggesting better performance in the TS group (CNT: p = .09; VF-P: p = .24; VF-S: p = .07).
Table 3.
Group Differences in Executive Function and Independent Living Skills (ILS)
| Group
|
F | df | partial η2 | Main Effect
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age
|
FSIQ
|
Covarying IQ and Age
|
|||||||||||||
| FXS | IQ/Age | TS | F | df | partial η2 | F | df | partial η2 | F | df | partial η2 | ||||
| CNTa* | 34.83 ± 9.11 | 26.54 ± 12.14 | 41.72 ± 10.43 | 9.205** | 2,64 | .223 | .36 | 1,62 | .001 | 51.073** | 1,62 | .452 | 3.485* | 2,62 | .101 |
| VF-P | 8.88 ± 3.75 | 6.92 ± 4.2 | 10.9 ± 3.14 | 4.669* | 2,64 | .127 | .44 | 1,62 | .001 | 68.479** | 1,62 | .525 | .986 | 2,62 | .031 |
| VF-S | 15.07 ± 4.69 | 13.58 ± 5.14 | 18.47 ± 4.34 | 4.534* | 2,64 | .124 | .723 | 1,62 | .012 | 30.860** | 1,62 | .332 | .2 | 2,62 | .006 |
| ILSa**b** | 83.85 ± 19.53 | 69.89 ± 17.53 | 103.6 ± 9.64 | 15.629** | 2,64 | .328 | 9.062* | 1,62 | .128 | 104.46** | 1,62 | .628 | 9.248** | 2,62 | .23 |
| VABS-DLS | 76.62 ± 14.19 | 66.89 ± 13.87 | 86.4 ± 12.91 | 8.169** | 2,64 | .203 | 2.191 | 1,62 | .034 | 16.59** | 1,62 | .211 | 2.941 | 2,62 | .087 |
Note. Mean ± SD; FXS = fragile X syndrome; TS = Turner syndrome. Significant post hoc comparisons:
FXS > IQ/Age
TS > IQ/Age.
Raw scores for Contingency Naming Test (CNT) and phonemic (VF-P) and semantic (VF-S) verbal fluency; Standard scores for independent living skills (ILS) and Vineland Adaptive Behavior Scales-Daily Living Skills (VABS-DLS).
= p < .05
= p < .001.
The same pattern of results was observed after repeating the analysis with the addition of FSIQ and age as covariates. A MANCOVA demonstrated that the effect of age was significant for the ILS, and FSIQ was significant for CNT, VF-P, VF-S, ILS, and VABS-DLS (Table 3) across the study population. There was a significant effect of group on CNT and ILS after controlling for the effect of FSIQ and age (Table 3). Post hoc comparisons revealed that the FXS group performed significantly higher on the CNT (p = .04) and ILS (p = .02) compared to the IQ/Age group. There were no significant differences between the FXS and the TS group for performance on both EF and independent living skills measures after the effects of the covariates were removed.
Executive Function and Independent Living Skills in Fragile X Syndrome
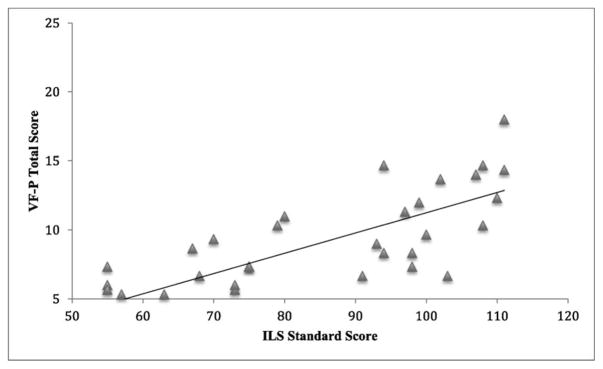
For the FXS group, age at assessment and phonemic verbal fluency (VF-P) explained a significant proportion of variance in ILS scores (Figure 1), and age at assessment explained a significant proportion of variance of VABS-DLS (Table 4). For the IQ/Age group, CNT was a significant predictor of ILS. EF measures did not predict independent living skills for the TS group.
Figure 1.
Phonemic Verbal Fluency (VF-P) as a Predictor of Independent Living Scales (ILS).
Table 4.
Predictors of Independent Living Skills (ILS)
| Group | Predictors | ILS as outcome Predictor parameters |
VABS-DLS as outcome Predictor parameters |
|||||
|---|---|---|---|---|---|---|---|---|
| B | T | P | B | t | p | |||
| FXS | Age | .264* | 2.344 | .026 | −.374* | −2.211 | .035 | |
| CNT | .227 | 1.89 | .069 | .129 | .713 | .481 | ||
| VF-P | .547** | 3.407 | .002 | .358 | 1.483 | .149 | ||
| VF-S | .024 | .171 | .865 | .1 | .469 | .642 | ||
| Model R2 = .68, F(4,29) = 15.50** | Model R2 = .28, F(4,29) = 2.81* | |||||||
| IQ/Age | Age | .121 | 1.109 | .287 | −.258 | −1.218 | .245 | |
| CNT | .873** | 4.367 | .001 | .904* | 2.322 | .037 | ||
| VF-P | .325 | 1.447 | .172 | −.124 | −.283 | .781 | ||
| VF-S | −.359 | −2.01 | .066 | −.349 | −1.003 | .334 | ||
| Model R2 = .85, F(4,13) = 18.43** | Model R2 = .43, F(4,13) = 2.46 | |||||||
| TS | Age | .466 | 1.904 | .083 | .665* | 2.41 | .037 | |
| CNT | .369 | 1.183 | .262 | −.046 | −.123 | .905 | ||
| VF-P | −.158 | −.569 | .581 | −.144 | −.506 | .624 | ||
| VF-S | −.023 | −.078 | .940 | .104 | .295 | .774 | ||
| Model R2 = .39, F(4,11) = 1.74 | Model R2 = .42, F(4,10 ) = 1.80 | |||||||
Note. FXS = fragile X syndrome; TS = Turner syndrome; VABS-DLS = Vineland Adaptive Behavior Scales-Daily Living Skills; Raw scores for Contingency Naming Test (CNT) and phonemic (VF-P) and semantic (VF-S) verbal fluency.
= p < .05;
= p < .01.
Early Predictors of Later Independent Living Skills
VABS-DLS at time 1 explained a significant proportion of variance of VABS-DLS scores at time 2 (Table 5). EF measures at time 1 did not predict a significant proportion of additional variance in ILS at time 2, though CNT at time 1 approached significance.
Table 5.
Longitudinal Regression Analysis for Independent Living Skills (ILS)
| Predictors | ILS as outcome Predictor parameters
|
VABS-DLS as outcome Predictor parameters
|
||||
|---|---|---|---|---|---|---|
| B | t | p | B | T | p | |
| Age | .247 | 1.276 | .214 | – | – | – |
| CNT T1 | .502 | 1.847 | .077 | −.273 | −.951 | .356 |
| VF-P T1 | .051 | .179 | .859 | .082 | .284 | .780 |
| VF-S T1 | −.112 | −.475 | .639 | −.173 | −.773 | .451 |
| VABS-DLS T1 | – | – | – | .862** | 5.232 | .000 |
| Model R2 = .398, F(4,24) = 3.96** | Model R2 = .638, F(4,16) = 7.061** | |||||
Note. VABS-DLS = Vineland Adaptive Behavior Scales-Daily Living Skills; Raw scores for Contingency Naming Test (CNT) and phonemic (VF-P) and semantic (VF-S) verbal fluency.
= p < .05;
= p < .01.
Discussion
The first aim of this study was to determine if there were significant differences in EF and ILS between females with FXS and control groups in young adulthood. The FXS group performed significantly higher than the IQ/Age group on one of the two measures of EF, the CNT, a test which involves specific EF skills (e.g., inhibition, set shifting, cognitive flexibility, and working memory) and one of the two measures of independent living skills, the Independent Living Scales, which examines the ability to function independently and handle real-life situations, even after controlling for the effect of IQ and age differences. We did not find significant between-group differences for phonemic and semantic verbal fluency, which assess attention, self-monitoring, self-initiation, cognitive flexibility, and working memory as the participant retrieves words and monitors behavior to reach a goal, and for the secondary measure of independent living skills, the Vineland Adaptive Behavior Scales–Daily Living Skills domain. Finding a group difference on the Independent Living Scales, a performance based measure, but not on the Vineland Adaptive Behavior Scales, a measure based on caregiver report, may be due to variances in method of measurement (e.g., direct performance measurement versus survey).
Overall, females with FXS demonstrated strength in their ability to use specific EF and independent living skills compared to individuals with a similar IQ level. A possible explanation for this observation may involve the early diagnosis of FXS because it is a known genetic condition. For ASD, there is an emerging consensus that children benefit from an early diagnosis and adaptions made to the child’s environment based on the diagnosis (Fernell, Eriksson, & Gillberg, 2013). It is possible that, with an early diagnosis of FXS, efforts to compensate and bolster associated deficits may lead to better outcomes compared to individuals with a similar IQ level but without a diagnosis. Thus, females with FXS may have had more resources and opportunities to gain skills, such as those associated with independent living. A detailed history of resources used and services received from childhood to adulthood was not available for our participants and could be included as a potential predictor of independent living skills in future studies. As previously mentioned, Hartley et al. (2011) stated that increased independence in females with FXS was significantly associated with interpersonal skills and age. Though our groups are matched for age, differences in interpersonal skills may be contributing to the higher level of independent living skills in our FXS group. However, further investigation specific to interpersonal skills is warranted to confidently state that differences in social skills are contributing to increased independence.
Contrary to prediction, we found no significant differences between the FXS and the TS groups for performance on measures of EF and independent living skills after controlling for the effect of IQ and age differences. This finding suggests that females with FXS are able to engage in the use of independent living skills at a level that is commensurate with their overall intellectual ability and age in a manner comparable to another genetic disorder associated with higher IQ. One explanation for this finding may be related to ascertainment bias in recruitment. This particular study requires a certain level of independence because of the required demands for participation such as travel from across the nation and financial resources. As a result, the FXS group may include more independent females in general. As well, the lack of predicted differences may be due to social impairments associated with TS that impact independent living skills (Amundson, Boman, Barrenas, Bryman, & Landin-Wilhelmsen, 2010).
Though difficulties with independent living skills are not specific to FXS, it is an area of weakness for the participants in this study compared to the general population. This finding sheds light on the specific developmental implications for FXS with regard to the ability to live and function independently as young adults. Females with FXS were more successful in their use of specific EF and independent living skills compared to individuals with a similar IQ level and demonstrated IQ and age commensurate skills when compared to people with another genetic disorder associated with higher IQ.
The second aim of this study was to determine if less impaired EF is associated with higher independent living skills for females with FXS. Our findings support our hypothesis that EF, in this case, phonemic verbal fluency, is associated with independent living, thus providing a potential future avenue for intervention. This finding suggests that phonemic verbal fluency, which examines attention, self-monitoring, self-initiation, cognitive flexibility, and working memory, as a cognitive mechanism, is associated and involved with the ability to live and function independently for females with FXS. In daily life, the ability to attend to the environment, adapt to unforeseen changes, initiate behavior, maintain rules, and monitor behavior is important for successfully achieving independence. Consequently, improvements and strengthening of skills associated with phonemic verbal fluency can improve the ability in females with FXS to live independently and may improve daily life skills, such as personal upkeep, management of the home and transportation, social skills, and community skills.
The third aim of this study was to determine if there are EF predictors in late childhood/ adolescence for independent living skills measured in young adulthood in females with FXS. Specific EF skills measured in our study in late childhood/adolescence did not predict later independent living. Though our findings were not statistically significant, we identified a trend for the CNT, a test that involves EF including inhibition, set shifting, cognitive flexibility, and working memory, as predicting later independent living. A possible explanation may be that EF is in the process of development and refinement in adolescence. In the general population, EF skills (e.g., attention, inhibition, shifting, planning, and working memory) have distinct developmental trajectories that continue to develop and improve in adolescence and young adulthood (Anderson, 2002; Best et al., 2009; Huizinga et al., 2006). Deficits in EF have been observed in school aged children (Hooper et al., 2008; Lightbody et al., 2006). Additionally, Bray et al. (2011) presented findings on divergent structural brain development for individuals with FXS in adolescence compared to typically developing controls. It may be a possibility that the developmental trajectory of EF within FXS is affected from an early age. As a result, EF skill development, attainment and refinement in people with FXS may be occurring at a slower rate than the general population. However, further investigation is needed to determine the distinct developmental trajectory of each EF skill within FXS.
Our study provides novel information about females with FXS, but there are limitations. We focused on two EF measures, the Contingency Naming Test and Verbal Fluency Test, which did not capture all aspects of EF such as planning and include a strong language component. Future studies could include additional EF measures including nonverbal measures to better identify the developmental trajectory of EF skills in FXS and whether they predict current or future adaptive behavioral function and independent living. Future studies could also include additional questions or measures of independent living. For example, Hartley et al. (2011) conducted a parent survey including questions about residence and employment, which were particularly relevant because the study focused on adults. Additionally, our control group sample size was relatively small compared to our FXS group. Though the IQ/Age group did not statistically differ from the FXS group in terms of intellectual ability, a larger sample may have revealed statistically significant differences between these two groups on executive function and independent living measures. Finally, our study focused solely on females with FXS. Thus, it is difficult to generalize our findings to males with FXS. Future studies may include males with FXS and corresponding male control groups (see Hustyi et al., 2015).
Our study has provided a foundation for future investigations targeting EF and independent living deficits in FXS. This study also emphasizes the importance of developing targeted interventions for individuals with this disorder. Research has demonstrated the effectiveness of cognitive remediation and the plasticity of EF in children with attention deficit and hyperactivity disorder (Kray, Karbach, Haenig, & Freitag, 2012). With task switching training, participants were able to improve their inhibition, shifting and working memory skills. Although this study examined a population that differs from FXS in terms of behavioral and genetic phenotype and features a pre/posttest study design, the mechanisms of cognitive remediation may apply to FXS. Kesler et al. (2013) studied computerized, home-based intervention programs to target EF in females with breast cancer who were treated with chemotherapy. Through a randomized controlled trial, the authors found that cognitive training led to significant improvements in EF (e.g., cognitive flexibility, verbal fluency, and processing speed). Though this particular study examined females with breast cancer and the neurocognitive late effects as a result of chemotherapy, it provides valuable information about the utility of computerized, home-based intervention programs for neurocognitive rehabilitation that might be used with people with FXS. Future studies with FXS can examine similar targeted cognitive interventions to improve EF deficits with randomized controlled trials. Because EF affects independent living, early targeted intervention may improve future outcomes for people with FXS.
Acknowledgments
We would like to thank our participants and their families. Support for this research was provided by the National Institute of Health (NIH5R01-MH50047).
Contributor Information
Arianna Martin, Center for Interdisciplinary Brain Sciences Research (CIBSR), Stanford University.
Eve-Marie Quintin, Educational and Counseling Psychology Department, McGill University.
Scott S. Hall, Center for Interdisciplinary Brain Sciences Research (CIBSR), Stanford University
Allan L. Reiss, Center for Interdisciplinary Brain Sciences Research (CIBSR), Stanford University
References
- Alloway TP, Gathercole SE, Pickering SJ. Verbal and visuospatial short-term and working memory in children: Are they separable? Child Development. 2006;77:1698–1716. doi: 10.1111/j.1467-8624.2006.00968.x. http://dx.doi.org/10.1111/j.1467-8624.2006.00968.x. [DOI] [PubMed] [Google Scholar]
- Alvarez JA, Emory E. Executive function and the frontal lobes: A meta-analytic review. Neuropsychology Review. 2006;16:17–42. doi: 10.1007/s11065-006-9002-x. http://dx.doi.org/10.1007/s11065-006-9002-x. [DOI] [PubMed] [Google Scholar]
- Amundson E, Boman UW, Barrenas M, Bryman I, Landin-Wilhelmsen K. Impact of growth hormone therapy on quality of life in adults with turner syndrome. Journal of Clinical Endocrinology and Metabolism. 2010;95:1355–1359. doi: 10.1210/jc.2009-1754. http://dx.doi.org/10.1210/jc.2009-1754. [DOI] [PubMed] [Google Scholar]
- Anderson P. Assessment and development of executive function (EF) during childhood. Child Neuropsychology. 2002;8:71–82. doi: 10.1076/chin.8.2.71.8724. http://dx.doi.org/10.1076/chin.8.2.71.8724. [DOI] [PubMed] [Google Scholar]
- Baker SB, Hooper S, Skinner M, Hatton D, Schaaf J, Ornstein P, Bailey D. Working memory subsystems and task complexity in young boys with fragile X syndrome. Journal of Intellectual Disability Research. 2011;55:19–29. doi: 10.1111/j.1365-2788.2010.01343.x. http://dx.doi.org/10.1111/j.1365-2788.2010.01343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennetto L, Pennington BF, Porter D, Taylor AK, Hagerman RJ. Profile of cognitive functioning in women with the fragile X mutation. Neuropsychology. 2001;15:290–299. http://dx.doi.org/10.1037/0894-4105.15.2.290. [PubMed] [Google Scholar]
- Berry-Kravis E, Raspa M, Loggin-Hester L, Bishop E, Holiday D, Bailey DB. Seizures in fragile X syndrome: Characteristics and comorbid diagnoses. American Journal of Intellectual Developmental Disabilities. 2010;115:461–472. doi: 10.1352/1944-7558-115.6.461. http://dx.doi.org/10.1352/1944-7558-115.6.461. [DOI] [PubMed] [Google Scholar]
- Best JR, Miller PH, Jones LL. Executive functions after age 5: Changes and correlates. Developmental Review. 2009;29:180–200. doi: 10.1016/j.dr.2009.05.002. http://dx.doi.org/10.1016/j.dr.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray S, Hirt M, Jo B, Hall SS, Lightbody AA, Walter E, … Reiss AL. Aberrant frontal lobe maturation in adolescents with fragile X syndrome is related to delayed cognitive maturation. Journal of Biological Psychiatry. 2011;70:852–858. doi: 10.1016/j.biopsych.2011.05.038. http://dx.doi.org/10.1016/j.biopsych.2011.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown WT. Clinical aspects of the fragile X syndrome. Results & Problems in Cell Differentiation. 2012;54:273–279. doi: 10.1007/978-3-642-21649-7_15. http://dx.doi.org/10.1007/978-3-642-21649-7_15. [DOI] [PubMed] [Google Scholar]
- Crawford DC, Acuña JM, Sherman SL. FMR1 and the fragile X syndrome: Human genome epidemiology review. Genetics in Medicine. 2001;3:359–371. doi: 10.1097/00125817-200109000-00006. http://dx.doi.org/10.1097/00125817-200109000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felce D, Emerson E. Living with support in a home in the community: Predictors of behavioral development and household and community activity. Mental Retardation and Developmental Disabilities Research Reviews. 2001;7:75–83. doi: 10.1002/mrdd.1011. http://dx.doi.org/10.1002/mrdd.1011. [DOI] [PubMed] [Google Scholar]
- Fernell E, Eriksson MA, Gillberg C. Early diagnosis of autism and impact on prognosis: A narrative review. Clinical Epidemiology. 2013;5:33–43. doi: 10.2147/CLEP.S41714. http://dx.doi.org/10.2147/CLEP.S41714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gothelf D, Furfaro JA, Hoeft F, Eckert MA, Hall SS, O’Hara R, … Reiss AL. Neuroanatomy of fragile X syndrome is associated with aberrant behavior and the fragile X mental retardation protein (FMRP) Annuals of Neurology. 2008;63:40–51. doi: 10.1002/ana.21243. http://dx.doi.org/10.1002/ana.21243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley SL, Seltzer MM, Raspa M, Olmstead M, Bishop E, Bailey DB. Exploring the adult life of men and women with fragile X syndrome: Results from a national survey. American Journal on Intellectual and Developmental Disabilities. 2011;116:16–35. doi: 10.1352/1944-7558-116.1.16. http://dx.doi.org/10.1352/1944-7558-116.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeft F, Carter JC, Lightbody AA, Hazlett HC, Piven J, Reiss AL. Region-specific alterations in brain development in one- to three-year-old boys with fragile X syndrome. Proceedings of the National Academy of Sciences. 2010;107:9335–9339. doi: 10.1073/pnas.1002762107. http://dx.doi.org/10.1073/pnas.1002762107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper SR, Hatton D, Sideris J, Sullivan K, Hammer J, Schaaf J, … Bailey DB. Executive functions in young males with fragile X syndrome in comparison to mental age-matched controls: Baseline findings from a longitudinal study. Neuropsychology. 2008;22:36–47. doi: 10.1037/0894-4105.22.1.36. http://dx.doi.org/10.1037/0894-4105.22.1.36. [DOI] [PubMed] [Google Scholar]
- Huizinga M, Dolan CV, van der Molen MW. Age-related change in executive function: Developmental trends and a latent variable analysis. Neuropsychologia. 2006;44:2017–2036. doi: 10.1016/j.neuropsychologia.2006.01.010. http://dx.doi.org/10.1016/j.neuropsychologia.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Hustyi KM, Hall SS, Quintin EM, Chromik LC, Lightbody AA, Reiss AL. The relationship between autistic symptomatology and independent living skills in adolescents and young adults with fragile X syndrome. Journal of Autism and Developmental Disorders. 2015;45:1836–44. doi: 10.1007/s10803-014-2342-0. http://dx.doi.org/10.1007/s10803-014-2342-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler SR, Hosseini SMH, Heckler C, Janelsins M, Palesh O, Mustian K, Morrow G. Cognitive training for improving executive function I chemotherapy-treated breast cancer survivors. Clinical Breast Cancer. 2013;13:299–306. doi: 10.1016/j.clbc.2013.02.004. http://dx.doi.org/10.1016/j.clbc.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk JW, Mazzocco MM, Kover ST. Assessing executive dysfunction in girls with fragile X or turner syndrome using the contingency naming test (CNT) Developmental Neuropsychology. 2005;28:755–777. doi: 10.1207/s15326942dn2803_2. http://dx.doi.org/10.1207/s15326942dn2803_2. [DOI] [PubMed] [Google Scholar]
- Kray J, Karbach J, Haenig S, Freitag C. Can task-switching training enhance executive control functioning in children with attention deficit/-hyperactivity disorder? Frontiers in Human Neuroscience. 2012;5:1–9. doi: 10.3389/fnhum.2011.00180. http://dx.doi.org/10.3389/fnhum.2011.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, El-Mallakh RS. Triplet repeat gene sequences in neuropsychiatric diseases. Harvard Review of Psychiatry. 1997;5:66–74. doi: 10.3109/10673229709034729. http://dx.doi.org/10.3109/10673229709034729. [DOI] [PubMed] [Google Scholar]
- Lightbody AA, Hall SS, Reiss AL. Chronological age, but not FMRP levels, predicts neuropsychological performance in girls with fragile X syndrome. American Journal of Medical Genetics. 2006;141:468–472. doi: 10.1002/ajmg.b.30307. http://dx.doi.org/10.1002/ajmg.b.30307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightbody AA, Reiss AL. Gene, brain, and behavior relationships in fragile X syndrome: Evidence from neuroimaging studies. Developmental Disabilities Research Reviews. 2009;15:343–352. doi: 10.1002/ddrr.77. http://dx.doi.org/10.1002/ddrr.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb PA. Independent Living Scales (ILS) manual. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Mazzocco MM, Hagerman RJ, Pennington BF. Problem solving limitations among cytogenetically expressing fragile X women. American Journal of Medical Genetics. 1992;43:78–86. doi: 10.1002/ajmg.1320430112. http://dx.doi.org/10.1177/026988119701100203. [DOI] [PubMed] [Google Scholar]
- Miller M, Nevado-Montenegro AJ, Hinshaw SP. Childhood executive function continues to predict outcomes in young adult females with and without childhood-diagnosed ADHD. Journal of Abnormal Child Psychology. 2012;40:657–668. doi: 10.1007/s10802-011-9599-y. http://dx.doi.org/10.1007/s10802-011-9599-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A. The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: A latent variable analysis. Cognitive Psychology. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. http://dx.doi.org/10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Nigg JT. On inhibition/disinhibition in developmental psychopathology: Views from cognitive and personality psychology and a working inhibition taxonomy. Psychological Bulletin. 2000;126:220–246. doi: 10.1037/0033-2909.126.2.220. http://dx.doi.org/10.1037/0033-2909.126.2.220. [DOI] [PubMed] [Google Scholar]
- Pellicano E. The development of executive function in autism. Autism Research and Treatment. 2012;2012:1–8. doi: 10.1155/2012/146132. http://dx.doi.org/10.1155/2012/146132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington BF, Ozonoff S. Executive functions and developmental psychopathology. Journal of Child Psychology and Psychiatry. 1996;37:51–87. doi: 10.1111/j.1469-7610.1996.tb01380.x. http://dx.doi.org/10.1111/j.1469-7610.1996.tb01380.x. [DOI] [PubMed] [Google Scholar]
- Pugliese C, Anthony L, Strang JF, Dudley K, Wallace GL, Kenworthy L. Increasing adaptive behavior skill deficits from childhood to adolescence in autism spectrum disorder: Role of executive function. Journal of Autism and Developmental Disorders. 2014;45:1579–1587. doi: 10.1007/s10803-014-2309-1. http://dx.doi.org/10.1007/s10803-014-2309-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss AL, Dant CC. The behavioral neurogenetics of fragile X syndrome: Analyzing gen-brain-behavior relationships in child developmental psychopathologies. Development and Psychopathology. 2003;15:927–968. doi: 10.1017/s0954579403000464. http://dx.doi.org/10.1017/S0954579403000464. [DOI] [PubMed] [Google Scholar]
- Schneider A, Hagerman RJ, Hessl D. Fragile X syndrome—From genes to cognition. Developmental Disabilities Research Reviews. 2009;15:333–342. doi: 10.1002/ddrr.80. http://dx.doi.org/10.1002/ddrr.80. [DOI] [PubMed] [Google Scholar]
- Sparrow SS, Balla DA, Chicchetti DV. Vineland Adaptive Behavior Scales. Circle Pines, MN: American Guidance Service; 1984. [Google Scholar]
- Sparrow SS, Chicchetti DV, Balla DA. Vineland Adaptive Behavior Scales: Second Edition. Circle Pines, MN: American Guidance Service; 2005. [Google Scholar]
- Spreen O, Benton A. Manual of instructions. Victoria, BC: University of Victoria; 1977. Neurosensory centre comprehensive examination for aphasia. [Google Scholar]
- Strauss E, Sherman EMS, Spreen O. A compendium of neuropsychological tests. 3. New York, NY: Oxford University Press; 2006. [Google Scholar]
- Stuss DT, Alexander MP. Executive functions and the frontal lobes: A conceptual view. Psychological Research. 2000;63:289–298. doi: 10.1007/s004269900007. http://dx.doi.org/10.1007/s004269900007. [DOI] [PubMed] [Google Scholar]
- Su CY, Chen CC, Wuang YP, Lin H, Wu YY. Neuropsychological predictors of everyday functioning in adults with intellectual disabilities. Journal of Intellectual Disability Research. 2008;52:18–28. doi: 10.1111/j.1365-2788.2007.00969.x. http://dx.doi.org/10.1111/j.1365-2788.2007.00969.x. [DOI] [PubMed] [Google Scholar]
- Taylor HG. Learning Disabilities. In: Mash EJ, editor. Behavioral assessments of childhood disorders. 2. New York, NY: Guilford Press; 1988. pp. 402–405. [Google Scholar]
- University of Michigan, Population Studies Center. Zip code characteristics: Mean and median household income (for 2006–2010 based on US Census data) 2013 Retrieved form http://www.psc.isr.umich.edu/dis/census/Features/tract2zip/index.html.
- Verkerk AJ, Pieretti M, Sutcliffe JS, Ying-Hui F, Kuhl DP, Pizzuti A, … Warren ST. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991;65:905–914. doi: 10.1016/0092-8674(91)90397-h. http://dx.doi.org/10.1016/0092-8674(91)90397-H. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children. 3. San Antonio, TX: The Psychological Corporation; 1991. [Google Scholar]
- Wechsler D. The Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Harcourt Assessment; 1999. [Google Scholar]
- Zelazo PD, Carter A, Reznick JS, Frye D. Early development of executive function: A problem-solving framework. Review of General Psychology. 1997;1:198–226. http://dx.doi.org/10.1037/1089-2680.1.2.198. [Google Scholar]