Abstract
Objective
To evaluate the effects of environmental factors and microRNAs (miRNAs) (miR-126, miR-143, and miR-145) on the risk of coronary heart disease (CHD).
Methods
A frequency-matched case-control study (450 patients, 450 controls) was conducted from April 2014 to December 2016 in Fuzhou City, China. Environmental factors were investigated using a self-administered questionnaire, and the expression levels of miR-126, miR-143, and miR-145 were determined by quantitative real-time Polymerase Chain Reaction (PCR) in peripheral blood mononuclear cells. Unconditional logistic regression models were used for statistical evaluation.
Results
Alcohol consumption, high-salt diets, high-intensity work, and lack of physical activity were significantly associated with increased CHD risk, whereas light diet was significantly associated with decreased risk. MiR-126, miR-143, and miR-145 were highly expressed in the CHD group compared with the control group. After adjustment for other environmental factors, unconditional logistic regression results revealed that miR-126, miR-143, and depression were the independent risk factors of CHD, and light diet was the independent protective factor of CHD.
Conclusions
Our data suggest that a family history of CHD, anxiety, and alcohol consumption was significantly associated with increased CHD risk, whereas light diet was significantly associated with decreased risk. Furthermore, miR-126 and miR-143 in combination with several risk factors, could play a joint role in the development of CHD. Therefore, it is necessary to manage patients with CHD in all directions and multiple level.
Keywords: Case-control study, Coronary heart disease, Environmental factors, Gene-environment interaction, MiR-126, MiR-143, MiR-145
1. Introduction
Coronary heart disease (CHD) remains to be the leading cause of death worldwide, including China; in particular, the elderly population shows an increased rate of mortality and morbidity.[1],[2] CHD is caused by the formation of atherosclerotic plaques and can progress to myocardial ischemia as a result of thrombosis or atherosclerosis.[3],[4] The pathogenesis of CHD may involve a consequence of environmental, genetic, and epigenetic interactions. It is well known that environmental factors included lifestyle factor, family history, psychologic status and so on in epidemiology. Furthermore, smoking, alcohol consumption, working strength, dietary habits, and physical activity are all belong to lifestyle factors. At present, besides environmental factors, novel risk factors such as microRNAs (miRNAs) are accepted as decisive factors to fully understand the development and progression of CHD. Recent evidence and previous microarray analysis show that as novel biomarkers for cardiovascular diseases, miRNAs may be the risk factors of CHD.[5]
MiRNAs represent a class of 20 to 25 nucleotide-long, highly conserved, endogenous, non-protein-coding RNAs; MiRNAs mainly target the 3′-UTR of an mRNA to regulate gene expression at the post-transcriptional level by inhibiting the translation of a protein or by promoting mRNA degradation.[6],[7] MiRNAs have now emerged as master regulators in various biological processes, including cardiac growth, vascular development, and angiogenesis.[8],[9] Thus, the evaluation of miRNAs may be used to identify patients at risk of CHD.[4],[10]
The endothelial-specific miR-126 is the most abundant miRNA found in adult endothelial cells (ECs) and is involved in endothelial dysfunction and inflammation.[11] Studies have illustrated that miR-126 can increase the EC response to vascular endothelial growth factor (VEGF) and partly improve angiogenesis to directly repress negative regulators via the pathway of VEGF through mechanism by sprouty-related protein 1 and phosphoinositol-3-kinase regulatory subunit 2.[12],[13] Zinc finger transcription factor, klf2, was a possible candidate gene responsible for VEGF signaling. MiR-126 acts downstream of klf2 to induce flow-stimulated angiogenesis in endothelial cells.[14] MiR-143 and miR-145 are considered the key regulators of contractile phenotypes and are abundantly expressed in smooth muscle cells (SMCs).[15],[16] MiR-145 may trigger the reprogramming of various non-SMC cell types into SMC-like cells characterized in part by increasing myocd protein and functioning in a feed-forward reinforcement of its own expression by the SRF–myocd complex, whereas miR-143 represses myocd's competitor, Elk-1.[17] The antiatherosclerotic effects between ECs and SMCs were commanded by miRNAs.[14]
After hunting for cardiovascular disease in bioinformatics database, several miRNAs, associated with the pathogeny of CHD, interested us.[18],[19] Moreover in our preliminary study and microarray analysis, miR-126, miR-143, and miR-145 were revealed to be differentially expressed between CHD and control group. In particular, these three miRNAs are all play a major role in cardiac growth, vascular development, and angiogenesis. However, there are no final verdict on whether these miRNAs are associated with CHD or not and the relationship among environmental factors, these three miRNAs, and CHD is not completely confirmed in humans. Our present work aims to explore the effects of environmental factors and miRNAs (miR-126, miR-143, and miR-145) in peripheral blood mononuclear cells (PBMCs) and their interactions on the risk of patients with CHD.
2. Methods
2.1. Informed consent
Our study was approved by the institutional ethical committees of Fujian Medical University according to the requirements of the Declaration of Helsinki, and written informed consent was obtained from each participant.
2.2. Study population
A frequency-matched case control study included data from two institutions, including the First Affiliated Hospital of Fujian Medical University and Affiliated People's Hospital to Fujian University of TCM in Fujian, China, between April 2014 and December 2016. The included CHD met the following criteria: (1) present cardiac catheterization-confirmed significant stenosis (≥ 50%) of > 1 major coronary artery; (2) documented history of prior myocardial infarction (MI); (3) documented history of a prior coronary revascularization procedure (percutaneous coronary intervention or coronary artery bypass graft); (4) patients in the stable stage after acute MI; and (5) patients with ST-segment elevation/depression on ECG. Subjects with congenital heart disease, valvular disease, cardiomyopathy, somatization disorder, renal or hepatic disease, and insufficient knowledge of the Mandarin language were excluded from the study.
Healthy subjects without medical history of cardiovascular diseases were matched with CHD group in age and sex and selected as the control group after physical health examination at the hospital.
2.3. Questionnaire survey
A standard questionnaire was given to CHD and control groups by specially trained interviewers. Questions covered demographic characteristics (sex, age, occupation, education level, and marital status), lifestyle factors (smoking, alcohol consumption, working strength, and physical activity, dietary habits), family history of cardiovascular diseases, and social psychological factors. In addition, demographic characteristics including sex, age, education level, and marital status act as the balanced factors in our study. Smokers (current and previous) and nonsmokers (never) were classified based on self-report. Previous smokers were categorized as having smoked more than 100 cigarettes in a lifetime. Alcohol consumption was classified as standard drinks per day based on intake of beer, wine, and liquor combined (one standard drink containing 12 g ethanol).[20] Even tea drinking was defined as drinking a minimum of 1 cup of green tea per week for more than 6 months. Active exercise participants were defined as those who exercised at least 20 min once a week, whereas passive exercise was excluded. The recorded waist–hip ratio (WHR) and body mass index (BMI) were used to measure the presence of being overweight and obese. BMI = weight (kg) / [height (m)]2 (Underweight: BMI of less than 18.5, normal weight: BMI of 18.5 to 24.9, overweight: BMI of 25 to 29.9, obese: BMI of 30 or more). WHR = waist/hip. (Women: normal weight: WHR of less than 0.85, obesity: WHR of 0.85 or more; Men: normal weight: WHR of less than 0.90, obesity: WHR of 0.90 or more). Self-rating depression scale (SDS) and self-rating anxiety scale (SAS) were used to evaluate the psychologic status of all participants. SDS and SAS scores were obtained by summing up the 20 questions according to how they felt during the last seven days. Item responses are ranked from 1 to 4. A cut-off value of 53 or more implies clinically relevant depression, and 50 or more suggests anxiety.[21]
2.4. Blood samples
Approximately 2 mL of venous blood samples was collected from each participant for plasma removal. According to manufacturer instructions, the blood sample was gently overlaid on lymphocyte separation medium[22] (Hao Yang Biological Manufacture CO., Ltd., Tianjin, China) for a second centrifugation to obtain the peripheral blood mononuclear cells (PBMCs) and then stored at −80 °C prior to RNA extraction. All blood samples were subjected to only one freeze–thaw cycle.
2.5. RNA extraction
According to a modification of manufacturer instructions, the total RNA was extracted from PBMCs[22] by using the TRIzol reagent (Invitrogen, California, USA) and was subsequently eluted in 30 µL of nuclease-free water. In brief, the total RNA samples were quantified by an ND-2000 NanoDrop ultraviolet spectrophotometer. The OD260/OD280 readings were between 1.8 and 2.0, indicating sufficient purity of the RNA samples, and the concentration of RNA was obtained. The use of agarose gel electrophoresis to identify RNA showed three bands (28S, 18S, and 5S). The brightness of the 28S band was approximately twice as much as the 18S band, implying that RNA was not degraded.
2.6. The cDNA synthesis and quantitative real-time PCR
A total of 1 µg RNA was placed into a total volume of 20 µL reverse transcribed reaction to generate cDNA by using PrimeScript RT reagent Kit (miRNAs) (Takara Bio Inc., Shiga, Japan). Quantitative real-time PCR was used to measure the expression levels of miR-126, miR-143, and miR-145 previously demonstrated to be associated with CHD, which was performed on the Light Cycler 480 real-time PCR system. The SYBR® Premix Ex Taq™ II kit (Takara Bio Inc., Shiga, Japan) and specific PCR primers of miRNAs (Sangon Biotech Co., Ltd., Shanghai, China) were used for the quantification of miRNAs at 95 °C for 30 s, 96 °C for 5 s, 60 °C for 34 s, 40 cycles; 95 °C for 15 s and 60 °C for 1 min; dissolution curve, 95 °C for 15 s, one cycle. The primer sequences used are listed in Table 1. Experiments were performed in triplicate samples. In our experiment, the detection limit of the Ct value was defined as 40. All samples were normalized to internal controls (U6), and the relative expression level was calculated through 2−ΔΔCt analysis method.[23],[24]
Table 1. Primer sequences.
| Primer | Orientation | Sequence (5′ to 3′) |
| U6 | Forward | GCTTCGGCAGCACATATACTAA |
| Reverse | AACGCTTCACGAATTTGCGT | |
| MiR- 126 | RT | GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACCGCATT |
| Forward | GCGGCGGTCGTACCGTGAGTAA | |
| MiR-143 | RT | GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACGAGCTA |
| Forward | GCGGCGGTGAGATGAAGCACTG | |
| MiR-145 | RT | GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACAGGGAT |
| Forward | GCGGCGGGTCCAGTTTTCCCAG | |
| Universal | Reverse | ATCCAGTGCAGGGTCCGAGG |
MiR: microRNA; RT: reverse transcription.
2.7. Statistical analyses
All statistical analyses were performed using SPSS 21.0 software (IBM, Tokyo, Japan). The continuous variables were expressed as mean ± SD or median (25th–75th quartile) and discrete variables as percentages. Distribution differences were examined by the chi-square test for categorical variables or two-tailed Student's t-test for normally distributed data as well as the Mann–Whitney U test for skewed data. The odds ratios (ORs) and 95% confidence intervals (95% CIs) were calculated by unconditional binary logistic regression analyses (Forward Selection: Likelihood Ratio) to assess the association between CHD and risk factors. A P-value < 0.05 (two-tailed) was considered significant.
3. Results
3.1. Demographic characteristics of the study population
Among 900 subjects, 50.0% were CHD patients, 50.1% were women, and the mean age was 65.23 ± 9.83 years (range, 40–100 years). Associations between demographic characteristics and CHD are shown in Table 2. No significant differences were found in terms of gender, age, marital status, and education background between CHD and control groups (all P > 0.05), indicating that the frequency matching was adequate.
Table 2. Baseline characteristics of patients with and without CHD.
| Characteristics | Cases | Controls | χ2 | P value |
| Sex | 1.604 | 0.205 | ||
| Male | 234 (52.0%) | 215 (47.8%) | ||
| Female | 216 (48.0%) | 235 (52.2%) | ||
| Age group | 0.783 | 0.676 | ||
| < 60 | 241 (26.8%) | 230 (25.6%) | ||
| 60–80 | 612 (68.0%) | 610 (67.8%) | ||
| ≥ 80 | 47 (5.2%) | 60 (6.7%) | ||
| Education level | 3.466 | 0.325 | ||
| Below primary school | 173 (38.4%) | 166 (37.1%) | ||
| Middle school | 118 (26.2%) | 126 (28.2%) | ||
| High school | 97 (21.6%) | 109 (24.4%) | ||
| University or higher | 62 (13.8%) | 46 (10.3%) | ||
| Marital status | 0.925 | 0.336 | ||
| Marriage | 406 (90.8%) | 400 (88.9%) | ||
| Single/divorce | 41 (9.2%) | 50 (11.1%) |
CHD: coronary heart disease.
3.2. Associations between environmental factors and CHD risk
Table 3 shows associations between lifestyle factors and risk of CHD. Increased CHD risk was associated with alcohol consumption, high-salt diets, heavy working strength, and lack of physical activity. Decreased risk of CHD was associated with light diets. In addition, statistically significant differences in smoking, tea drinking, oiliness diets, and sweet diets were found between two groups (P > 0.05). Differences in family history of hypertension, diabetes, CHD, and stroke between two groups were not statistically significant (all P > 0.05; Table 4). Persons with current anxiety or depression disorders showed an increased prevalence of CHD (Table 5), and similar trends were observed with BMI and WHR (Table 6), both presenting statistically significant difference (P < 0.05). Unconditional logistic regression analysis was used to evaluate associations between the environmental factors and risk of CHD. After adjustment for age and sex, significant increased risk effects for CHD were found to be associated with family history of CHD [OR = 1.735; 95% CI: 1.000–3.012; P = 0.050], anxiety status (OR = 3.656; 95% CI: 2.289–5.839; P < 0.001), and alcohol consumption (OR = 1.944; 95% CI: 1.265–2.986; P = 0.002). Specifically, light diet (OR = 0.580; 95% CI: 0.420–0.803; P = 0.001) presented a significantly decreased risk of CHD. Thus, a total of four environmental factors were selected for the evaluation of their interactions with miRNAs in the risk of CHD.
Table 3. Associations between lifestyle factors and CHD risk.
| Lifestyle factors | Cases | Controls | χ2 | P value |
| Smoking | 3.699 | 0.0544 | ||
| Yes | 415 (92.2%) | 428 (95.3%) | ||
| No | 35 (7.8%) | 21 (4.7%) | ||
| Alcohol consumption | 7.796 | 0.005 | ||
| Yes | 83 (18.4%) | 53 (11.8%) | ||
| No | 367 (81.6%) | 397 (88.2%) | ||
| Tea drinking | 0.473 | 0.492 | ||
| Yes | 285 (36.7%) | 175 (38.9%) | ||
| No | 165 (63.3%) | 275 (61.1%) | ||
| High-salt diets | 20.564 | < 0.001 | ||
| Yes | 149 (33.1%) | 89 (19.8%) | ||
| No | 301 (66.9%) | 361 (80.2%) | ||
| Sweet diets | 0.013 | 0.909 | ||
| Yes | 43 (9.6%) | 42 (9.3%) | ||
| No | 407 (90.4%) | 408 (90.7%) | ||
| Oiliness diets | 0.0620 | 0.803 | ||
| Yes | 36 (8.0%) | 34 (7.6%) | ||
| No | 414 (92.9%) | 416 (92.4%) | ||
| Light diets | 21.501 | < 0.001 | ||
| Yes | 263 (58.4%) | 329 (73.1%) | ||
| No | 187 (41.6%) | 121 (26.9%) | ||
| Working strength | 9.667 | 0.008 | ||
| Mild | 253 (56.2%) | 218 (48.6%) | ||
| Moderate | 134 (29.8%) | 178 (39.6%) | ||
| Severe | 63 (14.0%) | 53 (11.8%) | ||
| Physical activity | 11.365 | 0.001 | ||
| Never | 193 (49.2%) | 149 (33.1%) | ||
| 1–2/week | 75 (16.7%) | 105 (23.3%) | ||
| 3–4/week | 25 (5.6%) | 24 (5.3%) | ||
| ≥ 5/week | 157 (34.9%) | 172 (38.2%) |
CHD: coronary heart disease.
Table 4. Associations between family history and CHD risk.
| Family history | Cases | Controls | χ2 | P value |
| Hypertension | 1.258 | 0.262 | ||
| Yes | 139 (31.0%) | 124 (27.6%) | ||
| No | 309 (69.0%) | 325 (72.4%) | ||
| Diabetes | 0.180 | 0.671 | ||
| Yes | 48 (10.7%) | 52 (11.6%) | ||
| No | 401 (89.3%) | 397 (88.4%) | ||
| CHD | 3.532 | 0.060 | ||
| Yes | 42 (9.4%) | 27 (6.0%) | ||
| No | 407 (90.6%) | 422 (94.0%) | ||
| Stroke | 0.0187 | 0.891 | ||
| Yes | 28 (6.2%) | 29 (6.5%) | ||
| No | 421 (93.8%) | 420 (93.5%) |
CHD: coronary heart disease.
Table 5. Associations between anxiety and depression and CHD risk.
| Psychological factors | Cases | Controls | χ2 | P value |
| Anxiety | 37.746 | < 0.001 | ||
| Yes | 297 (66.0%) | 257 (57.1%) | ||
| NO | 153 (34.0%) | 193 (42.9%) | ||
| Depression | 7.512 | 0.006 | ||
| Yes | 72 (33.0%) | 47 (21.6%) | ||
| NO | 37 (17.0%) | 54 (24.8%) |
CHD: coronary heart disease.
Table 6. Associations between obesity factors and CHD risk.
| Obesity Factors | Cases | Controls | χ2 | P value |
| BMI | 5.652 | 0.017 | ||
| Normal | 390 (89.9%) | 395 (94.3%) | ||
| Overweight | 44 (10.1%) | 24 (5.7%) | ||
| WHR | 6.384 | 0.012 | ||
| Normal | 30 (16.0%) | 60 (26.3%) | ||
| Overweight | 157 (84.0%) | 168 (73.7%) |
BMI: body mass index; CHD: coronary heart disease; WHR: waist–hip ratio.
Table 7. Multiple logistic regression analysis of environmental factors and CHD risk.
| Variable | B | SE | Wald χ2 | P | OR | OR (95% CI) |
| Family history of CHD | 0.551 | 0.281 | 3.838 | 0.050 | 1.735 | 1.000–3.012 |
| Alcohol consumption | 0.665 | 0.219 | 9.197 | 0.002 | 1.944 | 1.265–2.986 |
| Light diet | −0.544 | 0.165 | 10.812 | 0.001 | 0.580 | 0.420–0.803 |
| Anxiety status | 1.296 | 0.239 | 29.456 | 0.000 | 3.656 | 2.289–5.839 |
CI: confidence intervals; CHD: coronary heart disease; OR: odds ratios; SE: standard error.
3.3. Possible interactions between miRNAs and environmental factors in CHD risk
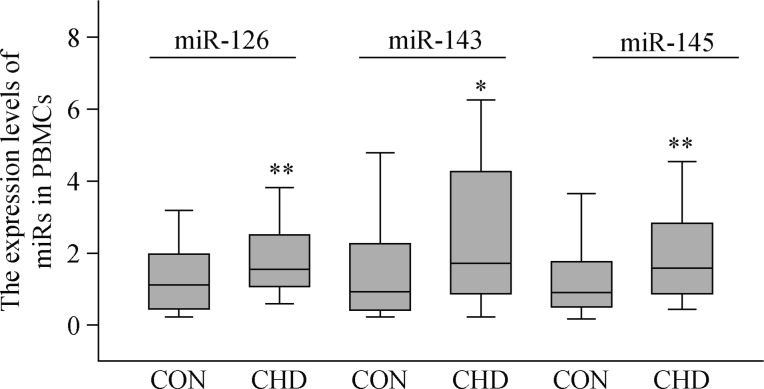
To gain certain insight into the direction of the association between miRNAs and CHD, we respectively selected 70 patients (the capacity of sample had been calculated in advance) both have blood sample and questionnaire from CHD and control groups as RT-PCR research subjects. The data were expressed by median (25th–75th quartile) and showed that the expression levels of miR-126 [1.553 (1.067, 2.511) versus 1.113 (0.444, 1.970); P = 0.004], miR-143 [1.712 (0.870, 4.258) versus 0.933 (0.416, 2.263); P = 0.011], and miR-145 [1.585 (0.872, 2.824) versus 0.903 (0.501, 1.763; P = 0.006)] in PBMCs were significantly elevated compared with the control group (Figure 1). After adjustment for age and sex, logistic regression analysis suggested that the rising expression levels of miR-126 (OR = 1.386; 95% CI: 1.009–1.904; P = 0.044), miR-143 (OR = 1.357; 95% CI: 1.1–1.673; P = 0.004), and depression (OR = 4.598; 95% CI: 1.993–10.608; P < 0.001) were the independent risk factors of CHD, while the light diet (OR = 0.295; 95% CI: 0.128–0.677; P = 0.004) was the independent protective factor of CHD. Moreover, those four factors simultaneously entered the logistic regression model, could play a joint role in the development of CHD (Table 8).
Figure 1. Expression levels of miR-126, miR-143, and miR-145 in PBMCs between CHD and control groups (*P < 0.05, **P < 0.01 compared with control groups).
CHD: coronary heart disease; PBMC: peripheral blood mononuclear cell.
Table 8. Multiple logistic regression analysis of miRNAs in PBMC and CHD risk.
| Variable | B | SE | Wald χ2 | P | OR | OR (95% CI) |
| MiR-126 | 0.326 | 0.162 | 4.053 | 0.044 | 1.386 | 1.009–1.904 |
| MiR-143 | 0.305 | 0.107 | 8.14 | 0.004 | 1.357 | 1.1–1.673 |
| Light diet | −1.222 | 0.425 | 8.28 | 0.004 | 0.295 | 0.128–0.677 |
| Depression | 1.526 | 0.426 | 12.798 | < 0.001 | 4.598 | 1.993–10.608 |
CI: confidence intervals; CHD: coronary heart disease; OR: odds ratios; PBMC: peripheral blood mononuclear cell; SE: standard error.
4. Discussion
As one of the leading causes of hospitalization and death worldwide, CHD is also prevalent among the Chinese population. Although the pathogenesis of CHD is not fully elucidated, accumulative epidemiologic evidence has shown that genetic and environmental factors play crucial roles in it etiology. In general, in case control studies, a potential gene–environment interaction is assessed. Our results show that the association between the miR-126 and miRNA-143 and CHD was modified by light diet and depression. In addition, light diet is the independent protective factor of CHD, and depression, miR-126, and miRNA-143 were the independent risk factors of CHD.
Diet is an important environmental factor in determining serum cholesterol and glucose. Our current study presents that light diet is an independent risk factor for CHD. Seven countries noted that Japan, which consumed a low-sugar, low-fat diet, presented the lowest serum cholesterol and CHD mortality, whereas the United States and Finland, with diets including large amounts of refined carbohydrates and fat, presented the highest serum cholesterol and the highest CHD mortality.[25] Therefore, light diet is considered an obtainable defense against CHD and contributed to long-term health. In addition, depression as a mental disease has been previously shown to influence CHD.
Depression is accompanied by a number of pathophysiological processes, including inflammation, over-activation of norepinephrine and hypothalamic-pituitary-adrenal axis, and elevated sympathetic nervous system activity, which can cause hypertension, coronary artery spasm, endothelial injury, and hemodynamic change. This state could also enhance the risk of thrombosis by platelet excessive activation and promote or aggravate the symptoms of CHD.[26] In the present study, we found that compared with control group, the depression population had a 4.60-fold increased risk of CHD. These findings are consistent with a recent report by Watkins L, et al.[27] who found that depression was independently associated with a twofold increased risk of all-cause mortality. To prevent CHD occurrence and development effectively, residents should refer to the social-environment-psychological-biological medical model for management and guidance of the risk factors of CHD.
Recent works on miRNAs alter our understanding on the regulation of CHD. In our present study, miR-126 was upregulated in the PBMCs of CHD patients compared with control groups, whereas several studies reported that this miRNA is downmodulated in serum or in plasma of CHD patients. Among the three miRNAs selected for current analysis, miR-126 promotes angiogenesis by inhibiting suppressors of the PI-3 kinase pathway, namely, Spred-1 and PI-3 kinase regulatory subunit two, resulting in VEGF- and FGF-induced endothelial cell proliferation and tube formation.[11],[13],[28] In addition, with the increase in miR-126, VCAM-1, the target gene of miR-126, was reduced through TNF-α, which can control vascular inflammation and resist atherosclerosis progression. No significant difference in plasma miR-126 was observed between the two groups, and the approach was unsuitable for discriminating CHD patients from patients without CHD.[29] Based on the observed changes in miR-126 level in association with low density lipoprotein cholesterol (LDL-C), the circulating miRNA levels may reflect a compensatory response to inflammation under hyperlipidemic background. Their study provides insight into the potential role of miR-126 in cholesterol metabolism, which can be used to distinguish between high LDL-C patients with or without CHD. However, other studies show that decreased expression of circulating miR-126 helps lessen atherosclerotic plaque and promotes angiogenesis.[30]
Although the expression levels of miR-143 and miR-145 in PBMCs were both significantly elevated compared with that of the control group, only miRNA-143 was the independent risk factors of CHD demonstrated by multiple logistic regression analysis. MiR-143 may be involved in the occurrence of obesity and other related disorders of lipid metabolism, which can promote the differentiation of adipocytes and lipid accumulation. Moreover, increased miR-143 reportedly will promote the proliferation of cardiac fibroblast; after miR-143, the proliferation of fibroblasts was significantly inhibited, suggesting that miR-143 was involved in the regulation of cell proliferation.[31],[32] We presume that the expression of miR-143 indirectly increased due to the independent risk factors of CHD, dyslipidemia, and myocardial fibrosis. This finding is inconsistent with a recent report that miR-143 is downregulated in injured or atherosclerotic vessels and associated with the phenotypic switch from a contractile/quiescent to asynthetic/proliferative phenotype. The same results are also observed in the plasma of patients with coronary artery disease and animal models of atherosclerosis.[33] Inhibition of miR-143 caused a doubling of the proliferative rate of VSMCs, demonstrating the function of miR-143 in negatively regulating VSMC proliferation. In addition, the increased expression levels of miR-126 and miR-143, depression, and light diet entered the logistic regression model simultaneously, which could play a potential gene-environment interaction in the development of CHD.
Detected the expression of circulating miRNAs has many advantages such as high stability, being relatively inexpensive, short analysis time, possibility of repeated sampling and minimal invasiveness, which is ideal for development into diagnostic tests. Circulating miRNAs as early detection, prognostic, and predictive biomarkers have been many studies reported in cancer.[34] Moreover, RT-PCR and microarray technology could allow the multiplexing of several miRNAs in a single experiment, thus improving efficiency. However, several limitations exist which must be taken into consideration. The current commercially available miRNA technology systems fail to show good inter-platform concordance, probably due to a lack of an adequate normalization method and unified standard.[35] Moreover, drug therapy (e.g. antiplatelet treatment) may affect the level of circulating miRNAs (e.g. miR-126 is highly enriched in endothelial cells, also present in platelet at much lower levels) and may act as a confounding factor in case-control studies.[36] Furthermore, inhibition of a specific miRNA as a treatment may be avail to preventing or alleviating the atherosclerosis progression but may adversely affect other organ systems causing immunosuppression, liver damage or even oncogenesis.[37] Therefore, careful monitoring and researching of these interactions among miRNAs, risk factors and disease are essential in order to guarantee a safe application in humans.
Based on the above-described results, our findings suggest that miR-126 and miR-143 may serve as clinical markers for the early prevention and diagnosis of CHD. However, several limitations in our findings may require future research to unravel the mechanisms underlying in different cell or animal experiments. However, the present study determined that the potential prognostic value of these miRNAs may help physicians to enhance the accuracy of clinical diagnosis and prognosis for CHD patients. Residents should limit their alcohol, consume a healthy diet, get regular exercise, and maintain a healthy weight to help reduce their risk for CHD.
In conclusion, our study shows that CHD is the result of combined consequence between environment and heredity. Bad lifestyle, psychological factors and genetic factors are main risk factors of CHD. Furthermore, miR-126 and miR-143 in combination with several risk factors, could play a joint role in the development of CHD. Therefore, it is necessary to manage patients with CHD in all directions and multiple level. Studies with large longitudinal cohort study and functional evaluation are warranted to confirm our findings.
Acknowledgments
This study was supported by the Young and Middle-aged Talent-training Project in Fujian province health system (Grant No. 2014-ZQN-ZD-24), the Local Colleges and Universities Development Foundation of Central Government (Grant No. 1003–03900130–15), and the Natural Science Foundation of Fujian Province (Grant No. 2015J01674).
References
- 1.Rodriguez-Rodero S, Fernandez-Morera JL, Fernandez AF, et al. Epigenetic regulation of aging. Discov Med. 2010;10:225–233. [PubMed] [Google Scholar]
- 2.D'Aquila P, Rose G, Bellizzi D, et al. Epigenetics and aging. Maturitas. 2013;74:130–136. doi: 10.1016/j.maturitas.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 3.Saito Y, Liang G, Egger G, et al. Specific activation of microRNA-127 with downregulation of the proto-oncogene BCL6 by chromatin-modifying drugs in human cancer cells. Cancer Cell. 2006;9:435–443. doi: 10.1016/j.ccr.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 4.Fichtlscherer S, Zeiher AM, Dimmeler S, et al. Circulating microRNAs: biomarkers or mediators of cardiovascular diseases? Arterioscler Thromb Vasc Biol. 2011;31:2383–2390. doi: 10.1161/ATVBAHA.111.226696. [DOI] [PubMed] [Google Scholar]
- 5.Bronze-da-Rocha E. MicroRNAs expression profiles in cardiovascular diseases. Biomed Res Int. 2014;2014:985408. doi: 10.1155/2014/985408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kloosterman WP, Plasterk RH. The diverse functions of microRNAs in animal development and disease. Dev Cell. 2006;11:441–450. doi: 10.1016/j.devcel.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 7.Zhao Y, Srivastava D. A developmental view of microRNA function. Trends Biochem Sci. 2007;32:189–197. doi: 10.1016/j.tibs.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 8.Zampetaki A, Mayr M. MicroRNAs in vascular and metabolic disease. Circ Res. 2012;110:508–522. doi: 10.1161/CIRCRESAHA.111.247445. [DOI] [PubMed] [Google Scholar]
- 9.Latronico MV, Catalucci D, Condorelli G, et al. Emerging role of microRNAs in cardiovascular biology. Circ Res. 2007;101:1225–1236. doi: 10.1161/CIRCRESAHA.107.163147. [DOI] [PubMed] [Google Scholar]
- 10.Tijsen AJ, Pinto YM, Creemers EE, et al. Circulating microRNAs as diagnostic biomarkers for cardiovascular diseases. Am J Physiol Heart Circ Physiol. 2012;303:H1085–H1095. doi: 10.1152/ajpheart.00191.2012. [DOI] [PubMed] [Google Scholar]
- 11.Fish JE, Santoro MM, Morton SU, et al. MiR-126 regulates angiogenic signaling and vascular integrity. Dev Cell. 2008;15:272–284. doi: 10.1016/j.devcel.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wei Y, Nazari-Jahantigh M, Neth P, et al. MicroRNA-126, -145, and -155 A therapeutic triad in atherosclerosis? Arterioscler Thromb Vasc Biol. 2013;33:449–454. doi: 10.1161/ATVBAHA.112.300279. [DOI] [PubMed] [Google Scholar]
- 13.Wang S, Aurora AB, Johnson BA, et al. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev Cell. 2008;15:261–271. doi: 10.1016/j.devcel.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nicoli S, Standley C, Walker P, et al. MicroRNA-mediated integration of haemodynamics and Vegf signalling during angiogenesis. Nature. 2010;464:1196–1200. doi: 10.1038/nature08889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elia L, Quintavalle M, Zhang J, et al. The knockout of mir-143 and -145 alters smooth muscle cell maintenance and vascular homeostasis in mice: correlates with human disease. Cell Death Differ. 2009;16:1590–1598. doi: 10.1038/cdd.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boettger T, Beetz N, Kostin S, et al. Acquisition of the contractile phenotype by murine arterial smooth muscle cells depends on the mir143/145 gene cluster. J Clin Invest. 2009;119:2634–2647. doi: 10.1172/JCI38864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cordes K R, Sheehy N T, White M, et al. MiR-145 and miR-143 regulate smooth muscle cell fate decisions. Nature. 2009;460:705–710. doi: 10.1038/nature08195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen C, Wang Y, Yang S, et al. MiR-320a contributes to atherogenesis by augmenting multiple risk factors and down-regulating SRF. J Cell Mol Med. 2015;19:970–985. doi: 10.1111/jcmm.12483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fichtlscherer S, De Rosa S, Fox H, et al. Circulating microRNAs in patients with coronary artery disease. Circ Res. 2010;107:677–684. doi: 10.1161/CIRCRESAHA.109.215566. [DOI] [PubMed] [Google Scholar]
- 20.Tolstrup J, Jensen MK, Tjonneland A, et al. Prospective study of alcohol drinking patterns and coronary heart disease in women and men. BMJ. 2006;332:1244–1248. doi: 10.1136/bmj.38831.503113.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seidel S, Frantal S, Salhofer-Polanyi S, et al. Do nocturnal headaches matter? A prospective diary study on subjective sleep parameters in snorers and their bed partners. Cephalalgia. 2014;34:533–539. doi: 10.1177/0333102413515347. [DOI] [PubMed] [Google Scholar]
- 22.Voellenkle C, van Rooij J, Cappuzzello C, et al. MicroRNA signatures in peripheral blood mononuclear cells of chronic heart failure patients. Physiol Genomics. 2010;42:420–426. doi: 10.1152/physiolgenomics.00211.2009. [DOI] [PubMed] [Google Scholar]
- 23.De Rosa S, Fichtlscherer S, Lehmann R, et al. Transcoronary concentration gradients of circulating microRNAs. Circulation. 2011;124:1936–1944. doi: 10.1161/CIRCULATIONAHA.111.037572. [DOI] [PubMed] [Google Scholar]
- 24.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 25.Franklin BA, Durstine JL, Roberts CK, et al. Impact of diet and exercise on lipid management in the modern era. Best Pract Res Clin Endocrinol Metab. 2014;28:405–421. doi: 10.1016/j.beem.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 26.Doering LV, Moser DK, Riegel B, et al. Persistent comorbid symptoms of depression and anxiety predict mortality in heart disease. Int J Cardiol. 2010;145:188–192. doi: 10.1016/j.ijcard.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watkins LL, Koch GG, Sherwood A, et al. Association of anxiety and depression with all-cause mortality in individuals with coronary heart disease. J Am Heart Assoc. 2013;2:e68. doi: 10.1161/JAHA.112.000068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Q, Kandic I, Kutryk M, et al. Dysregulation of angiogenesis-related micrornas in endothelial progenitor cells from patients with coronary artery disease. Biochem Biophys Res Commun. 2011;405:42–46. doi: 10.1016/j.bbrc.2010.12.119. [DOI] [PubMed] [Google Scholar]
- 29.Sun C, Alkhoury K, Wang YI, et al. IRF-1 and miRNA126 modulate VCAM-1 expression in response to a high-fat meal. Circ Res. 2012;111:1054–1064. doi: 10.1161/CIRCRESAHA.112.270314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zampetaki A, Kiechl S, Drozdov I, et al. Plasma microRNA profiling reveals loss of endothelial miR-126 and other microRNAs in type 2 diabetes. Circ Res. 2010;107:810–817. doi: 10.1161/CIRCRESAHA.110.226357. [DOI] [PubMed] [Google Scholar]
- 31.Hsu J, Hanna P, Van Wagoner DR, et al. Whole genome expression differences in human left and right atria ascertained by RNA sequencing. Circ Cardiovasc Genet. 2012;5:327–335. doi: 10.1161/CIRCGENETICS.111.961631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deacon DC, Nevis KR, Cashman TJ, et al. The miR-143-adducin3 pathway is essential for cardiac chamber morphogenesis. Development. 2010;137:1887–1896. doi: 10.1242/dev.050526. [DOI] [PubMed] [Google Scholar]
- 33.Cordes KR, Sheehy NT, White MP, et al. MiR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature. 2009;460:705–710. doi: 10.1038/nature08195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Madhavan D, Cuk K, Burwinkel B, et al. Cancer diagnosis and prognosis decoded by blood-based circulating microRNA signatures. Front Genet. 2013;4:116. doi: 10.3389/fgene.2013.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sato F, Tsuchiya S, Terasawa K, et al. Intra-platform repeatability and inter-platform comparability of microRNA microarray technology. PLoS One. 2009;4:e5540. doi: 10.1371/journal.pone.0005540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Economou EK, Oikonomou E, Siasos G, et al. The role of microRNAs in coronary artery disease: from pathophysiology to diagnosis and treatment. Atherosclerosis. 2015;241:624–633. doi: 10.1016/j.atherosclerosis.2015.06.037. [DOI] [PubMed] [Google Scholar]
- 37.Mayr M, Zampetaki A, Willeit P, et al. MicroRNAs within the continuum of postgenomics biomarker discovery. Arterioscler Thromb Vasc Biol. 2013;33:206–214. doi: 10.1161/ATVBAHA.112.300141. [DOI] [PubMed] [Google Scholar]