Abstract
Purpose
To investigate the feasibility of a polycaprolactone (PCL) scaffold fabricated by three-dimensional (3D) printing for tissue engineering applications for tunica albuginea.
Materials and Methods
PCL scaffolds were fabricated by use of a 3D printing system. Two scaffolds were fabricated that differed in the architecture of the lay-down pattern: a 90°PCL scaffold and a 45°PCL scaffold. Mechanical properties were measured to compare tensile strength between the two scaffold types. The scaffolds were characterized by scanning electron microscope (SEM) images. The scaffolds were seeded with fibroblast cells, and the ability of these scaffolds to support the cells was evaluated by immunofluorescence staining.
Results
The PCL scaffolds had well-structured shapes, regular arrays, and good interconnection in SEM images. The horizontal and vertical Young's modulus coefficients were 13 and 12 MPa for the 90°PCL scaffold and 19 and 21 MPa for the 45°PCL scaffold, respectively. Microscopy images revealed that human fibroblast cells covered the entire scaffold surface. Immunofluorescence staining of ER-TR7 confirmed that the fibroblast cells remained viable and proliferated throughout the time course of the culture.
Conclusions
This preliminary study provides experimental evidence for the feasibility of 3D printing of PCL scaffolds for tissue engineering applications of tunica albuginea.
Keywords: Fibroblasts; Penis; Printing, three-dimensional; Tissue engineering
INTRODUCTION
Reconstructive surgery in the urologic field is performed in patients with urinary tract dysfunction, urinary incontinence, neurogenic disorder, trauma, and pediatric/congenital malformations [1]. Many different materials are used for substitution of the urinary tract, such as oral mucosa for urethra and small or large intestine for ureter, and urinary bladder [2,3,4,5]. However, these techniques have limitations regarding wound healing, complications in the gastrointestinal tract, and the invasiveness of the surgery [3]. These limitations of autologous tissue have led urologists to investigate regenerative medicine and tissue engineering techniques [6].
Tissue engineering is an attractive technology for producing alternative grafts that require only a small tissue sample to obtain cells, resulting in minimal scarring defects at the sampling site. Penile reconstruction may be necessary in cases of trauma, penile carcinoma, and Peyronie's disease [7]. A few studies of tissue engineering for tunica albuginea replacement have been performed [8,9,10]. In these studies, autologous tunica albuginea was produced in vitro and seeded with fibroblast cells on an acellular scaffold.
Polycaprolactone (PCL) is a highly biocompatible aliphatic polyester obtained by polymerization to an open-loop structure of ε-caprolactone. PCL can support the creation of a polymer-cell complex in vitro with subsequent implantation in vivo [11]. There are some advantages of PCL. It was approved by the Food and Drug Administration (FDA) for use in humans. It is biodegradable, compatible, and has good processibility, which enables fabrication of a variety of structures and forms. It is readily suitable for melt processing due to its high thermal stability and relatively low cost [12]. Nevertheless, there have been no investigations to make scaffolds fabricated by using three-dimensional (3D) printing for tissue engineering applications for tunica albuginea.
We hypothesized that PCL scaffolds made by 3D printing and seeded with fibroblast cells would be feasible for tissue engineering of tunica albuginea replacement and that a scaffold fabricated in an oblique pattern would have greater tensile strength. In this experimental study, we evaluated the biocompatibility and strength of two types of PCL scaffolds fabricated by use of a 3D printing technique.
MATERIALS AND METHODS
1. Scaffold fabrication
The scaffolds were fabricated by using a 3D bioprinter (Korea Institute of Machinery & Materials, Daejeon, Korea) that consisted of a heating jacket, nozzle, pressure pump, x-y-z stage, and computer. The resolution of the 3D printer was ±5 µm and the scaffold pattern was designed by lab-made software. A computer system controlled the pressure, nozzle size, and plotting velocity over the stage. PCL pellets were melted at 80℃ in a cylinder by using the heating jacket of the bioprinter. By applying a computer model to control manufacturing, the bioprinter allows for layer-by-layer construction of complex 3D scaffolds. The scaffold was plotted by converting a CAD model to a CAM model. Each layer was filled with the designed scaffold pattern at 90° and 45°orientation to generate the porous structure [13]. Strand thickness and strand period were both 300 µm.
2. Mechanical characterization of the scaffolds
Two types of scaffolds were produced according to the angle between strands: 90° and 45°. A total of 8 samples, 4 of the 90° scaffolds and 4 of the 45°scaffolds, were tested with a tensile machine (Shimadzu EZ-test 500N; SHIMADZU Corp, Kyoto, Japan) to evaluate tensile stress, tensile strain, and Young's modulus.
3. Cell culture
Fibroblast cells (ATCC, Rockville, MD, USA) were cultured with Fibroblast Basal Medium containing 2% fetal bovine serum and 1% antibiotics (ATCC). The fibroblast cells were maintained up to passage 3 and were collected by trypsin-ethylenediaminetetraacetic acid treatment. PCL scaffolds were sterilized by using 70% EtOH, and washed twice with phosphate buffer saline (PBS). The scaffolds were put in culture medium overnight. Fibroblast cells were then seeded on scaffolds at a density of 5×105 cells/sample. The cell-scaffolds were incubated in 5% CO2 at 37℃ and cultured for a period of 2 weeks.
To inspect the attachment and detailed morphologies of cells on the scaffolds, scanning electron microscope (SEM, SNE-1500M; SEC Co., Ltd., Suwon, Korea) was used at 2 weeks after seeding. The scaffolds were washed twice with PBS, fixed with 2.5% glutaraldehyde (Sigma-Aldrich, St. Louis, MO,USA) for 2 hours, rinsed in PBS, and dehydrated through a graded series of ethanol (30%, 40%, 50%, 60%, 70%, 80%, 90%, and 100%) at 5-minute intervals. After air drying, the scaffolds were coated with platinum and scanned at an accelerating voltage of 15 kV.
4. Immunofluorescence analysis
Proliferation and viability of human fibroblast cells on the scaffold was evaluated after immunofluorescence staining. The fibroblast cell-seeded scaffolds were washed with PBS. After several washes, the scaffolds were fixed in 3.7% paraformaldehyde solution. The scaffolds were first blocked with 0.5% (v/v) normal chicken serum for 30 minutes at room temperature and then stained with fluorescein isothiocyanate-conjugated ER-TR7 antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 2 hours at room temperature. Immunoreactivity for ER-TR7 was detected using Alexa Fluor 594-conjugated chicken anti-rabbit- IgG (H+L) (Molecular Probes Inc., Eugene, OR, USA). Finally, 4′,6-diamino-2-phenylindole dihydrochloride (DAPI; Sigma-Aldrich) was added to stain the cell nuclei. The confocal images were acquired by use of a model LSM 510 confocal microscope (Carl Zeiss, Jena, Germany) with an excitation wavelength appropriate for Alexa Fluor (405 or 594 nm). Final images were constructed with the use of LSM Image Examiner software.
5. Statistical analysis
Data were expressed as measured and average values. The significance of differences among the groups was determined using the Mann-Whitney U-test, with differences considered significant at p<0.05.
6. Ethics statement
This study was approved by the Ethics Committee of the Chonnam National University Medical School (CNUIACUC-H-2016-41).
RESULTS
1. Morphology of three-dimensional printed scaffolds
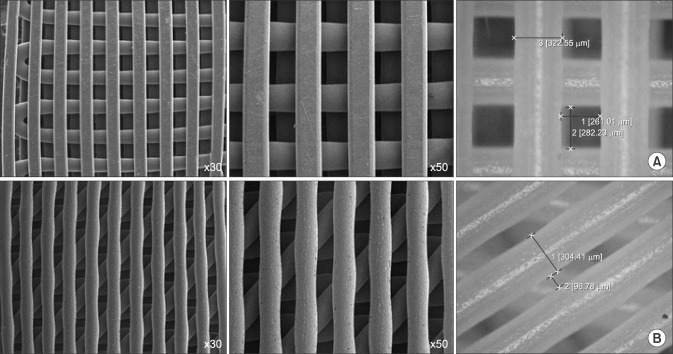
SEM was used to visualize the fabricated scaffolds. Both types of 3D printed scaffolds composed with 90° and 45° angles (strand thickness/strand period, 300/300 µm) had well-structured shapes, regular arrays, and good interconnection, resulting in highly regular pores and strands (Fig. 1).
Fig. 1. Scanning electron micrograph images of 90° plotted polycaprolactone (PCL) 300/300 scaffold (A) and 45° plotted PCL 300/300 scaffold (B).
2. Mechanical characteristics of the scaffolds
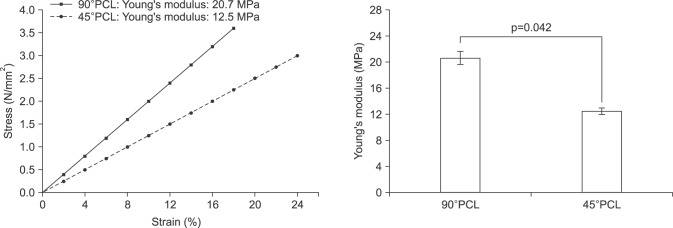
The tensile ranges of the 90° and 45°PCL scaffolds were 16% to 20% and 20% to 25%, respectively. The horizontal and vertical Young's moduli were 19 MPa and 21 MPa for the 90° scaffold and 13 MPa and 12 MPa for the 45° scaffold, respectively. Average Young's moduli were significantly lower in 45°PCL scaffolds (12.5 MPa) than in 90°PCL scaffolds (20.7 MPa) (Fig. 2).
Fig. 2. Mechanical properties of three-dimensional printed polycaprolactone (PCL) scaffolds. Mean tensile stress, strain, and Young's modulus values of two different types of PCL scaffolds.
3. Scanning electron microscope images of cultured fibroblast cells
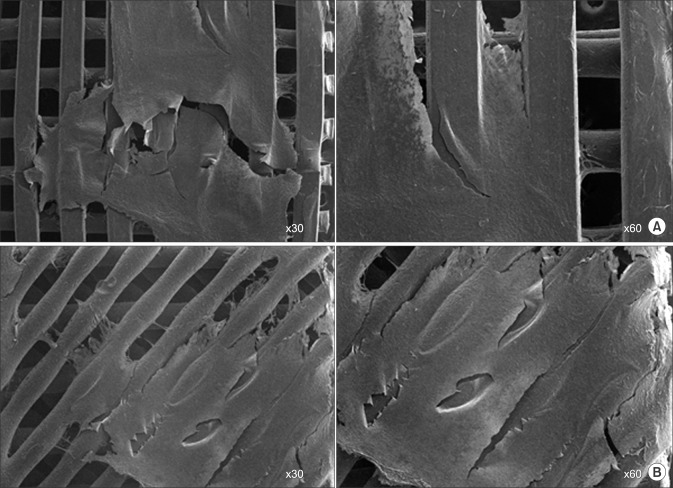
After 2 weeks of seeding and culture, SEM images showed that fibroblast cells were well attached and covered the entire surface of the 3D printed scaffolds. Cellular bridge formations were seen throughout the interconnected adjacent strands of the scaffolds. There was no significant difference in cell sheet formation between the 45°PCL scaffolds and the 90°PCL scaffolds after 2 weeks (Fig. 3).
Fig. 3. Scanning electron microscopic images of fibroblasts on 90° polycaprolactone (PCL) scaffold (A) and 45°PCL scaffold cultured for 2 weeks in vitro (B).
4. Immunofluorescence images of cultured fibroblast cells
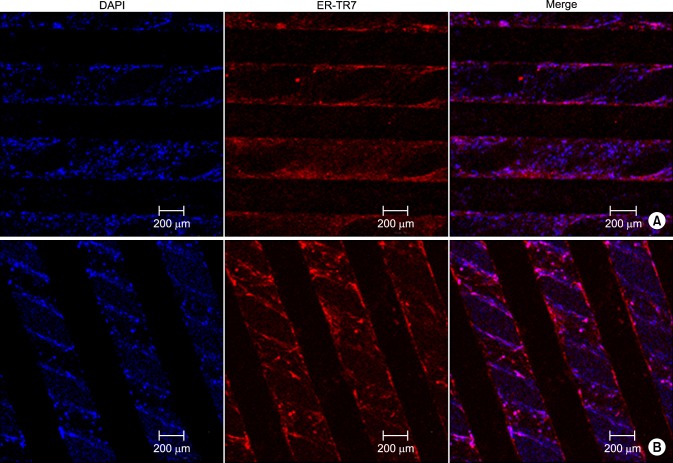
Viable and proliferating human fibroblast cells were found with immunofluorescence staining with DAPI and ER-TR7 on both types of scaffolds (Fig. 4).
Fig. 4. Immunofluorescence staining of fibroblast cells with DAPI (4',6-diamidino-2-phenylindole) and ER-TR7 on 90° (A) and 45° (A, B) polycaprolactone (PCL) scaffolds. Confocal microscopic images of 45°PCL scaffolds (B).
DISCUSSION
The human tunica albuginea is a bilayered structure with multiple collagen bundle sublayers. The finer, circularly oriented bundles that constitute the inner layer surround and penetrate the cavernous tissue, and the outer-layer bundles are coarser and are directed in a longitudinal manner [14].
The architecture of scaffolds is important and in tissue engineering should take the macro-, micro-, and nano-levels into consideration [15]. The macroarchitecture reflects organ specificity and anatomical features, whereas the microarchitecture reflects the shape, pore size, porosity, and pore interconnections. The nanoarchitecture has relevance to biomolecule attachment for cell adhesion, proliferation, and differentiation [16]. The 3D printing technology can be used to fabricate 3D scaffolds using various biomaterials for tissue engineering. The 3D printing scaffold has an interconnected structure for cell ingrowth and tissue regeneration. It also has good mechanical properties to control custom-made scaffold fabrication.
The present study was conducted to demonstrate the feasibility of using fibroblast cells seeded onto 3D printed PCL scaffolds for the creation of cell sheets in vitro. Positive immunofluorescence staining revealed well-attached fibroblast cells throughout the PCL scaffolds. These results showed that the 3D printed PCL scaffold had the proper macro-, micro-, and nano-architecture.
A few experimental studies have shown that bioengineered material can achieve adequate structure and function in substitution of tunica albuginea [9,10,17]. For example, Ferretti et al [17] compared noncellular and cell-seeded synthetic grafts for tunica albuginea replacement in a rat model. Less retraction and better erectile responses were observed in the group with a fibroblast-seeded polyglycolic acid (PGA) scaffold than in the noncellular PGA scaffold group. However, the PGA scaffold partially degraded in vitro during 2 weeks of cell culture. Some amount of acid is produced during local PGA hydrolysis, which may result in partial degradation of the scaffold. PCL hydrolysis produces less acid than does PGA hydrolysis.
In the present study, we used PCL in manufacturing scaffolds and compared the tensile strength of 2 types of 3D printed PCL scaffolds. Tunica albuginea is a bilayered structure with multiple sublayers. Inner-layer bundles are oriented circularly and contain the cavernous tissue. Outer-layer bundles are oriented longitudinally, extending from the glans penis to the proximal crura. Less abundant are oblique-oriented fibers that connect the two main layers [18]. So, we hypothesized that scaffolds with 45° angles may have more similarity to the structure of human tunica albuginea and more physiologic characteristics in common than scaffolds with 90° angles.
The 45°PCL scaffolds had a higher tensile range than the 90°PCL scaffolds. The horizontal and vertical Young's modulus values of the 45°PCL scaffolds (13 and 12 MPa, respectively) were lower than those of the 90°PCL scaffolds. Young's modulus, which is also known as the elastic modulus, is the most commonly used mechanical property of linear solid materials and is defined as the ratio of stress to strain. Our result showed that the PCL scaffold with a 45° angle between strands had less stress at the same tensile strain and greater elasticity. PCL, a synthetic biodegradable polymer, has excellent mechanical properties and has been used in many biotechnology fields since its approval by the FDA. It has a lower melting point (58℃∼63℃), lower glass transition temperature (−65℃), higher degradation time (>24 months), and lower tensile module than other biodegradable polymers, such as poly lactic-co-glycolic acid (PLGA) and PGA [9].
In this study, we evaluated the feasibility of fibroblast cells seeded onto 3D printed PCL scaffolds as tunica albuginea graft material. The PCL scaffolds manufactured by 3D printing had regular arrays with interconnected pores, resulting in highly regular pores and strands. Cultured fibroblast cells were well spread and covered the PCL scaffold surface and formed cell sheets with cellular bridge formation throughout the interconnected adjacent strands.
This preliminary study provides evidence of the feasibility of using cultured and proliferated fibroblast cells in conjunction with 3D printed PCL scaffolds. However, our study had several limitations. The sample size was small and the bioavailability of this fibroblast-seeded PCL scaffold in an in vivo animal model has not been investigated. Thus, future studies to test the 2 types of PCL scaffolds in an animal model and to evaluate the deformation or degradability of the scaffold during a longer culture period are warranted.
CONCLUSIONS
In this investigation, we fabricated 3D printed PCL scaffolds. All scaffolds showed well-defined architecture and a uniform porous structure. Fibroblast cells were well attached, proliferated, and differentiated on the PCL scaffolds. The scaffold composed with a 45° angle between strands had more tensile strength than the 90° scaffold. This is a preliminary study showing that 3D printed PCL scaffolds can be used for tissue engineering applications in the field of sexual medicine. A 45°PCL scaffold may allow for better reconstructive tissue engineering technique for tunica albuginea replacement.
ACKNOWLEDGEMENTS
This study was supported by a grant of the Korean Health Technology R&D Project (HI13C1527), Ministry of Health & Welfare, Republic of Korea.
We thank Ms. Jennifer Holmes for assistance in editing the text.
Footnotes
Disclosure: The authors have no potential conflicts of interest to disclose.
Author Contribution: Research conception & design: Park K. Data acquisition: Park J, Lee HS, Park SA, Lee DW. Data analysis and interpretation: Yu HS, Lee DW, Park SA. Statistical analysis: Yu HS. Drafting of the manuscript: Yu HS, Park K. Critical revision of the manuscript: Park K, Yu HS. Receiving grant: Park K. Approval of final manuscript: all authors.
References
- 1.Garriboli M, Radford A, Southgate J. Regenerative medicine in urology. Eur J Pediatr Surg. 2014;24:227–236. doi: 10.1055/s-0034-1382259. [DOI] [PubMed] [Google Scholar]
- 2.Osman NI, Hillary C, Bullock AJ, MacNeil S, Chapple CR. Tissue engineered buccal mucosa for urethroplasty: progress and future directions. Adv Drug Deliv Rev. 2015;82-83:69–76. doi: 10.1016/j.addr.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 3.Takeuchi M, Masumori N, Tsukamoto T. Ureteral reconstruction with bowel segments: experience with eight patients in a single institute. Korean J Urol. 2014;55:742–749. doi: 10.4111/kju.2014.55.11.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lam Van Ba O, Aharony S, Loutochin O, Corcos J. Bladder tissue engineering: a literature review. Adv Drug Deliv Rev. 2015;82-83:31–37. doi: 10.1016/j.addr.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 5.Kim JH, Lee HJ, Song YS. Treatment of bladder dysfunction using stem cell or tissue engineering technique. Korean J Urol. 2014;55:228–238. doi: 10.4111/kju.2014.55.4.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Damaser MS, Sievert KD. Tissue engineering and regenerative medicine: bench to bedside in urology. Preface. Adv Drug Deliv Rev. 2015;82-83:v–vii. doi: 10.1016/j.addr.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang C, Murphy SV, Atala A. Regenerative medicine in urology. Semin Pediatr Surg. 2014;23:106–111. doi: 10.1053/j.sempedsurg.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 8.Wefer J, Schlote N, Sekido N, Sievert KD, Wefer AE, Nunes L, et al. Tunica albuginea acellular matrix graft for penile reconstruction in the rabbit: a model for treating Peyronie's disease. BJU Int. 2002;90:326–331. doi: 10.1046/j.1464-410x.2002.02808.x. [DOI] [PubMed] [Google Scholar]
- 9.Schultheiss D, Lorenz RR, Meister R, Westphal M, Gabouev AI, Mertsching H, et al. Functional tissue engineering of autologous tunica albuginea: a possible graft for Peyronie's disease surgery. Eur Urol. 2004;45:781–786. doi: 10.1016/j.eururo.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 10.Imbeault A, Bernard G, Ouellet G, Bouhout S, Carrier S, Bolduc S. Surgical option for the correction of Peyronie's disease: an autologous tissue-engineered endothelialized graft. J Sex Med. 2011;8:3227–3235. doi: 10.1111/j.1743-6109.2011.02374.x. [DOI] [PubMed] [Google Scholar]
- 11.Ledger WJ. Current problems in antibiotic treatment in obstetrics and gynecology. Rev Infect Dis. 1985;7(Suppl 4):S679–S689. doi: 10.1093/clinids/7.supplement_4.s679. [DOI] [PubMed] [Google Scholar]
- 12.Cipitri A, Skelton A, Dargaville TR, Dalton PD, Hutmacher DW. Design, fabrication and characterization of PCL electrospun scaffolds-a review. J Mater Chem. 2011;21:9419–9453. [Google Scholar]
- 13.Park SA, Kim HJ, Lee SH, Lee JH, Kim HK, Yoon TR, et al. Fabrication of nano/microfiber scaffolds using a combination of rapid prototyping and electrospinning systems. Polym Eng Sci. 2011;51:1883–1890. [Google Scholar]
- 14.Brock G, Hsu GL, Nunes L, von Heyden B, Lue TF. The anatomy of the tunica albuginea in the normal penis and Peyronie's disease. J Urol. 1997;157:276–281. [PubMed] [Google Scholar]
- 15.Hollister SJ. Porous scaffold design for tissue engineering. Nat Mater. 2005;4:518–524. doi: 10.1038/nmat1421. [DOI] [PubMed] [Google Scholar]
- 16.Chia HN, Wu BM. Recent advances in 3D printing of biomaterials. J Biol Eng. 2015;9:4. doi: 10.1186/s13036-015-0001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferretti L, Giuliani M, Bessède T, Qiu X, Zhang H, Alsaid B, et al. Tissue engineering for penile surgery: comparative study of noncellular and cell-seeded synthetic grafts for tunica albuginea replacement. J Sex Med. 2012;9:625–631. doi: 10.1111/j.1743-6109.2011.02561.x. [DOI] [PubMed] [Google Scholar]
- 18.Lue TF. Physiology of penile erection and pathophysiology of erectile dysfunction. In: Wein AJ, Kavoussi LR, Partin AW, Peters CA, editors. Campbell-Walsh urology. 11th ed. Philadelphia: Elsevier; 2016. pp. 612–642. [Google Scholar]