Abstract
Purpose
The pathophysiological role of detrusor overactivity (DO) in the bladder, which is commonly observed in various bladder diseases, is not well understood. DO appears in bladder outlet obstruction (BOO), and may continue even after subsequent deobstruction. DO therefore provides an excellent opportunity to observe molecular biological changes.
Methods
In this study, to understand the molecular effects of persistent DO after BOO induction and deobstruction, we performed awake cystometry on female Sprague-Dawley rats divided into 4 groups: a sham group, a BOO group, a deobstructed group with DO after BOO (DDO), and a deobstructed group without DO after BOO (non-DDO). Total RNA was extracted from the bladder samples, and gene expression profiles were compared between the sham and model groups.
Results
DO was observed in 5 of the 6 rats (83%) in the BOO group, and in 6 of the 13 rats (46%) in the deobstructed group. The non-DDO group showed a significantly greater residual volume than the DDO group. Through a clustering analysis of gene expression profiles, we identified 7,532 common upregulated and downregulated genes, the expression of which changed by more than 2 fold. In the BOO group, 898 upregulated and 2,911 downregulated genes were identified. The non-DDO group showed 3,472 upregulated and 4,025 downregulated genes, whereas in the DDO group, only 145 and 72 genes were upregulated and downregulated, respectively.
Conclusions
Abnormal function and gene expression profiles in bladders after BOO were normalized in the BOO rats with DO after deobstruction, whereas in those without DO, abnormal function persisted and the gene expression profile became more abnormal. DO may play a protective role against the stress to the bladder induced by BOO and deobstruction as a form of adaptive neuroplasticity.
Keywords: Urethral Obstruction; Urinary Bladder, Overactive; DNA; Microarray Analysis; Gene Expression Profiling
HIGHLIGHTS
- Detrusor overactivity seems to play the role of adaptive neuroplasticity in the direction of evacuating the toxin-bearing urine, in the bladder of BOO/deobstruction.
- The presence or absence of detrusor overactivity in the bladder with BOO/deobstruction appears to indicate whether the bladder is capable of protecting the bladder appropriately against stress.
INTRODUCTION
Our body is designed to defend against external stress and to respond in a way that minimizes the damages caused by stressors. Each organ constituting our body also undergoes a variety of functional changes under neural control to minimize damage within its own environment. In the brain, this phenomenon is known as neuroplasticity, which refers to the capacity of the brain to re-establish itself through new neuronal connections throughout life, compensating for injuries and illnesses [1]. In the bladder, detrusor overactivity (DO) is frequently observed in most bladder diseases, such as conditions associated with aging, bladder outlet obstruction (BOO), diabetes, interstitial cystitis, and neurogenic bladder [2-6], and DO may be a common defensive or compensatory mechanism for bladder damage or disease. Therefore, to understand neuroplasticity related to the bladder, it is necessary to determine the role that DO plays in bladder diseases on the level of molecular biology.
Animal models used in overactive bladder (OAB) research include partial BOO, spontaneous hypertensive rat, bladder stimulation models using various drugs, nerve damage models, and some gene knock-outs; nonetheless, these models all have individual strengths and weaknesses [7]. Considering the difficulty of defining DO objectively, the BOO model is one of the most reproducible DO models [8]. However, when in vivo urodynamic tests are conducted to observe the relationship between pressure and flow from the bladder, it is impossible to observe the functional changes of the bladder alone, because some residual urine is usually present in the BOO model due to the mechanically fixed obstruction of the urethra. Therefore, in order to observe changes in bladder function after BOO has been present for a certain period of time, it is important to observe bladders after BOO has been eliminated.
The aim of this study was, therefore, to advance our understanding of the role and underlying pathogenesis of DO as a key element of various bladder diseases, with a special focus on gene expression in the bladder tissue of obstructed/deobstructed rats, classified according to urodynamic results. To minimize potential confounding factors, we used a modified method of obstruction/deobstruction [9] and simultaneous recordings of intravesical pressure (IVP) and intra-abdominal pressure (IAP) [9,10].
MATERIALS AND METHODS
Experimental Animals
Adult female Sprague-Dawley Rats (n=25; Orient Bio Inc., Seongnam, Korea), weighing 215–280 g, were housed in a vivarium with free access to food and water with light/dark cycling of 12 hours. All procedures for animal handling and treatment were performed in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and were approved by the Ethics Committee of the Inha University College of Medicine (INHA 140731-321-1).
Twenty-five rats were randomly separated into 3 groups. Six control rats underwent a sham operation, and a second group of 6 rats underwent a modified method of urethral constriction, as described in our previous paper [9], to produce partial BOO (the BOO group). The third group (n=13) underwent the same method of BOO induction as the second group, and deobstruction was performed through the vaginal route without an additional abdominal incision after 2 weeks. According to cystometric results, this group was subdivided into 2 groups, depending on whether DO was present during the filling phase (DDO group; n=6) or not (non-DDO group; n=7). In this study, DO was defined as the occurrence of nonvoiding contractions in IVP readings that exceeded 2 cm H2O from baseline, without simultaneous changes in the IAP or fluid expulsion from the bladder.
Surgical Methods
Obstruction and deobstruction
Briefly, rats were anesthetized intraperitoneally with ketamine (75 mg/kg; Ketamine 50, Yuhan Corp., Seoul, Korea) and xylazine (15 mg/kg; Rompun, Bayer Korea Ltd., Seoul, Korea). Through a lower midline abdominal incision, the para-urethral space was exposed, and a silk stay suture (usually 4-0) was placed about 2–3 mm lateral to the urethra to pull up the vaginal epithelium, allowing an easy incision to be made. Through a small incision in the vaginal epithelium, a needle with nylon ligature (3-0) was inserted into the other side of the urethra via the vaginal space. The ligature was tied tightly around the urethra with a 1-mm steel rod. After the knot was made, the rod was removed, and the knot was rolled down and positioned in the vagina, allowing the knot to be easily removed through the vaginal approach, without another abdominal incision, 2 weeks after the obstruction procedure [9].
Intravesical and intra-abdominal catheter implantation
Catheters to monitor the IVP and IAP were inserted 3 days before cystometry. To record the IVP, a polyethylene catheter with a cuff (PE-50, Becton Dickinson, Franklin Lakes, NJ, USA) was inserted into the dome of the bladder and held in place by fixation with a purse-string suture, through a low midline abdominal incision. To record the IAP, a 0.05-mL balloon (Latex, Dawoo Medical, Incheon, Korea) was placed at the tip cuff of a PE-50 polyethylene catheter for each experiment, as described previously [10]. The balloon was placed directly above the bladder, just after the IVP catheter was inserted into the bladder.
Both catheters were subcutaneously tunneled and fixed to the back skin with a silk ligature. The free end of the catheter was finally sealed. Each rat was individually housed after surgery, and water and food were given ad libitum. After surgery, the animals were left to rest for at least 24 hours under controlled conditions.
In vivo cystometric investigations
Cystometric investigations were performed 2 weeks after the sham operation and BOO surgery in the sham and BOO groups and 1 week after obstruction surgery in the deobstructed group. Awake rats were put in metabolic cages (Nalgene metabolic cage, Nalge, Rochester, NY, USA). The catheter from the bladder was connected to a 2-way valve that was linked to an infusion pump (PHD 22/2000 programmable syringe pump, Harvard Apparatus, Holliston, MA, USA) and a pressure transducer (Research Grade Blood Pressure Transducer, Harvard Apparatus, Holliston, MA, USA). The balloon catheter for IAP measurement was connected to another pressure transducer. Micturition volume (MV) was simultaneously recorded using a fluid collector connected to a force displacement transducer (Research Isometric Transducer, Harvard Apparatus). Bladder infusion was performed with normal saline at a rate of 20 mL/hr in all groups. Data were stored digitally and analyzed using Acq Knowledge 3.8.1 software (Biopac Systems, Goleta, CA, USA) at a sampling rate of 100 Hz and an MP150 data acquisition system (Biopac Systems). For each animal, the mean values from 3 reproducible micturition cycles were analyzed. IAP was defined as the balloon pressure corrected by subtracting the lowest balloon pressure in each entire voiding cycle for zeroing. The detrusor pressure (DP) was defined as the IVP minus the IAP. The DOs were defined as increments of the IVP during the filling phase that exceeded 2 cm H2O from baseline without simultaneous changes in the IAP or fluid expulsion from the bladder (Fig. 1). Values are expressed as mean±standard error (SE). All pressure parameters are expressed as the DP, not the IVP. After performing the urodynamic tests, the rats were sacrificed by cervical dislocation. The bladder and urethra were removed together and their weight was measured. The bladder and urethra were separated and a microarray analysis of the bladder was performed.
Fig. 1.
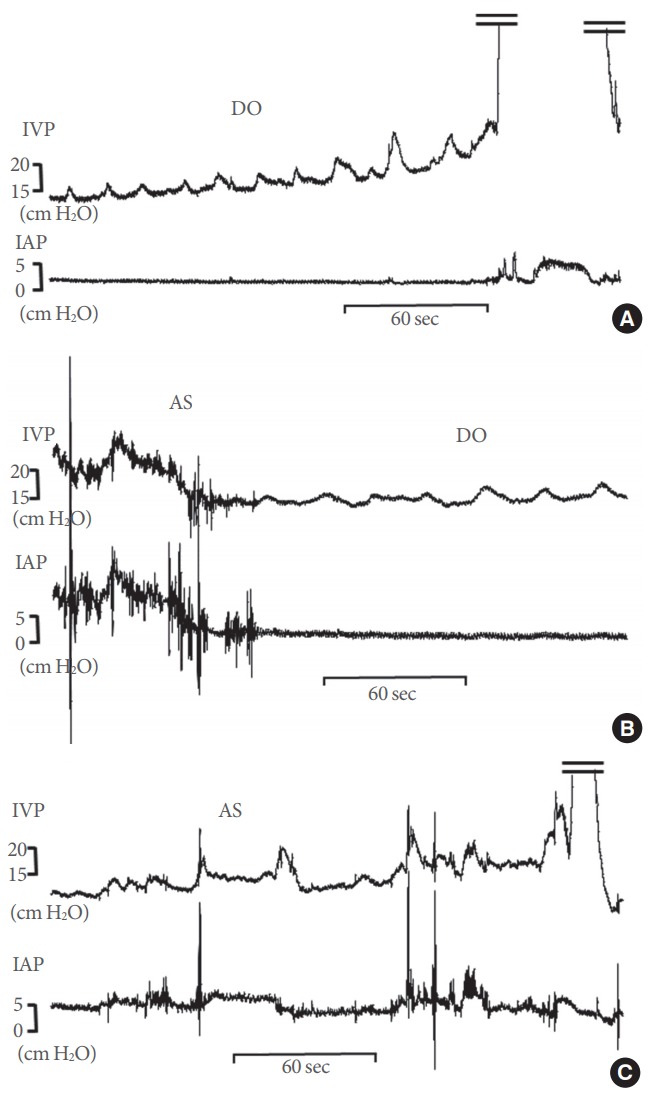
Representative tracings of detrusor overactivity (DO) and abdominal straining (AS) in the 3 experimental groups. (A) Partial bladder outlet obstruction (BOO) group. (B) The group with DO after BOO/deobstruction (DDO). (C) The group without DO after BOO/deobstruction (non-DDO). DO was defined as the occurrence of nonvoiding contractions in IVP readings that exceeded 2 cm H2O from baseline, without simultaneous changes in IAP or fluid expulsion from the bladder. AS was defined as IVP fluctuations with simultaneous fluctuations in IAP. IVP, intravesical pressure; IAP, intra-abdominal pressure.
The following cystometric parameters were quantified: basal pressure (BP), threshold pressure (TP), maximal pressure (MP), MV, residual volume (RV), bladder capacity (BC; MV+RV), and micturition interval (MI). The cystometric parameters used to evaluate DO during the filling phase were the time of the filling phase (interval between the initiation of saline infusion and micturition), the total number of instances of DO during the filling phase, the frequency of DO instances (per minute), and the average pressure difference between the peak and the base of the DO (DO pressure). Compliance was calculated by the formula (P2−P1)/(V2−V1), where P2 is the pressure of the stable curve just before the bladder contracts, and V2 is the infused volume at that time. P1 is the baseline pressure, and V1 refers to the corresponding infused volume.
Microarray
Total RNA preparation
Bladder tissue was removed from the rats in all groups, RNAlater (RNA stabilization solution, Ambion Inc., Foster City, CA, USA) was added, and the samples were then stored at 4°C for 1 day. Then, the RNAlater was removed, the tissue was disrupted using liquid nitrogen and a mortar and pestle, and total RNA was isolated using TRI REAGENT (Molecular Research Center Inc., Cincinnati, OH, USA).
Hybridization, scanning and image analysis, and normalization
Synthesized Cy3 or Cy5 fluorescent cDNA was mixed at the same concentration as that of the single-stand DNA, followed by the addition of 0.05 mg/mL of yeast tRNA and denaturation at 95°C. The hybridization slide glass was scanned using a GenePix 4000B scanner (Axon Instrument, Union City, CA, USA) and the scan image was analyzed using GenePix Pro 4.0 software (Axon Instrument). Analysis related to expression profiling, clustering, and ontology was also performed using Gene-Spring (Silicon Genetics, Redwood City, CA, USA) software. Using GeneSpring GX 7.3.1, global normalization, intensity normalization, and block scale normalization were performed to assess the accuracy and reliability of the experiments.
Expression profiling
Scatter plots, intensity values, and ratio plots of RI and M versus A plots were created using the intensity values of the normalized genes in the control and experimental groups. Variation in overall gene expression was analyzed. Ontology classification, hierarchical clustering, and linkage analysis for each experiment were performed using GeneSpring GX 7.3.1. In our study, differences of gene expression that were greater than 2 fold in the BOO, DDO, and non-DDO groups compared to the sham group were analyzed, and the number of genes showing such between-group differences was identified and compared for each group.
Statistical Analysis
The results are given as mean±SE. The normality of the distributions was checked using the Shapiro-Wilks W test. The statistical significance of the differences was calculated with the unpaired Student t-test or 1-way analysis of variance followed by the Tukey post hoc test for multiple comparisons. All analyses were performed with GraphPad Prism ver. 7.00 (Graph Pad Software, San Diego, CA, USA) and SigmaStat for Windows version 3.5 (Systat Software Inc., San Jose, CA, USA), at a significance level of 0.05 and a confidence interval of 95%.
RESULTS
Bladder Weight and Compliance
The ratio of bladder weight (mg) to body weight (g) was significantly higher in the BOO group (0.13±0.01, P<0.01), and the deobstructed group (0.15±0.01, P<0.0001), including both the DDO group (0.19±0.03, P<0.01) and the non-DDO group (0.11±0.01, P<0.01), than in the sham group (0.06±0.00, n=6). This ratio was also higher in the DDO group (P<0.01) than in the non-DDO group (Table 1).
Table 1.
Bladder weight, pressure, and volume parameters in conscious rats that had undergone a sham operation, partial BOO, or deobstruction in rats with BOO
| Variable | Sham | BOO | DEO | DDO | Non-DDO |
|---|---|---|---|---|---|
| Bladder ratio | 0.06 ± 0.00 | 0.13 ± 0.01** | 0.15 ± 0.02*** | 0.19 ± 0.03** | 0.11 ± 0.01** |
| BP (cm H2O) | 7.81 ± 0.67 | 11.72 ± 1.77* | 11.82 ± 1.24* | 14.25 ± 1.81** | 9.73 ± 1.34 |
| TP (cm H2O) | 18.64 ± 2.40 | 30.74 ± 5.06* | 23.83 ± 2.06 | 24.89 ± 4.08 | 22.92 ± 1.88 |
| MP (cm H2O) | 56.05 ± 4.31 | 86.53 ± 19.07 | 87.96 ± 9.18** | 104.20 ± 15.82** | 74.04 ± 8.02 |
| BC (mL) | 1.58 ± 0.14 | 2.99 ± 0.36** | 2.56 ± 0.29* | 2.67 ± 0.52 | 2.48 ± 0.34 |
| MV (mL) | 1.53 ± 0.14 | 1.84 ± 0.49 | 2.43 ± 0.28* | 2.59 ± 0.53 | 2.30 ± 0.31 |
| RV (mL) | 0.05 ± 0.03 | 1.15 ± 0.45* | 0.13 ± 0.04†† | 0.08 ± 0.08† | 0.17 ± 0.04*,†,‡ |
| MI (min) | 4.70 ± 0.42 | 8.86 ± 0.94** | 6.91 ± 0.71 | 7.34 ± 1.19* | 6.54 ± 0.90 |
| Compliance (mL/cm H2O) | 0.23 ± 0.01 | 0.43 ± 0.01** | 0.56 ± 0.02***,††† | 0.54 ± 0.02**,†† | 0.59 ± 0.02**,†† |
| DO frequency (/min) | 0±0 | 2.00 ± 0.35** | 0.67 ± 0.25† | 1.44 ± 0.33** | 0± 0††,‡‡‡ |
| DO pressure (cm H2O) | 0±0 | 9.51 ± 4.50** | 2.98 ± 0.99 | 6.46 ± 0.76** | 0± 0††,‡‡‡ |
Values are presented as mean±standard error.
BOO, bladder outlet obstruction; DEO, total deobstructed group in rats with BOO; DDO, DEO group showing DO during the storage phase; Non-DDO, DEO group showing no DO; Bladder ratio, ratio between bladder and body weight; BP, basal pressure; TP, threshold pressure; MP, micturition pressure; BC, bladder capacity; MV, micturition volume; RV, residual volume; MI, micturition interval; DO, detrusor overactivity.
P<0.05,
P<0.01,
P<0.0001 (unpaired Student t -test) versus sham.
P<0.05,
P<0.01,
P<0.0001 (unpaired Student t -test) versus BOO.
P<0.05,
P<0.01,
P<0.0001 (unpaired Student t -test) versus DDO.
Compliance was significantly greater in the BOO group (0.43±0.01 mL/cm H2O, P<0.01) and the deobstructed group (0.56±0.03 mL/cm H2O, P<0.0001), including the DDO (0.54 ±0.03 mL/cm H2O, P<0.01) and non-DDO (0.59±0.02 mL/cm H2O, P<0.01) groups, than in the sham group (0.23±0.01 mL/cm H2O). The deobstructed group showed greater compliance than the BOO group. However, there was no significant difference between the DDO and non-DDO groups (Table 1).
DO Measurements by In Vivo Urodynamic Tests
DO was not observed in any rats in the sham group. DO was observed in 5 of the 6 rats (83%) in the BOO group and 6 of the 13 rats (46%) in the deobstructed group. DO frequency decreased significantly in the deobstructed group as a whole (0.67±0.25/min, P<0.05) compared to the BOO group (2.00± 0.35/min), but not in the DDO group (1.44±0.33/min). The DO pressure in the BOO group (9.51±4.50 cm H2O) was not significantly different from that of the deobstructed group as a whole (2.98±0.99 cm H2O, P>0.05) or the DDO group (6.46± 0.76 cm H2O, P>0.05) (Table 1).
Pressure Parameters
BP increased in the BOO (11.72±1.77 cm H2O, P<0.05), deobstructed (11.82±1.24 cm H2O, P<0.05) and DDO groups (14.25±1.81 cm H2O, P<0.01) compared to the sham group (7.81±0.67 cm H2O). There was no significant difference in BP between the DDO (14.25±1.81 cm H2O) and non-DDO (9.73 ±1.34 cm H2O) groups. TP, which occurs at the point of initiation of voiding, increased in the BOO group (30.74±5.06 cm H2O, P<0.05) compared to the sham group (18.64±2.40 cm H2O). MP increased in the deobstructed group as a whole (87.96±9.18 cm H2O, P<0.01) and in the DDO group (104.20 ±15.82 cm H2O, P<0.01) compared to the sham group (56.05 ±4.31 cm H2O) (Fig. 2).
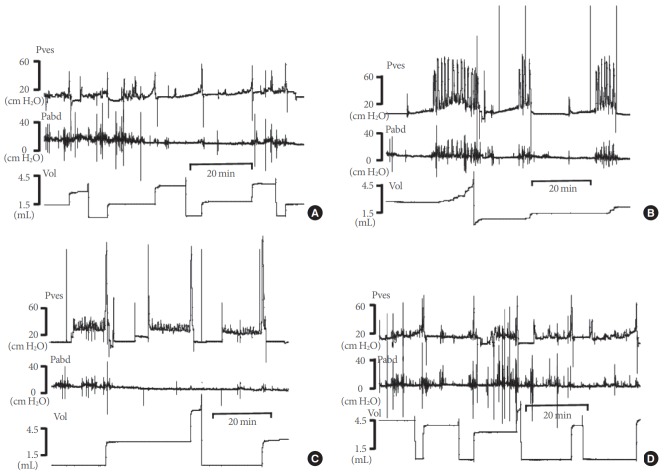
Fig. 2.
Representative tracings of rats in the 3 experimental groups and the control group. (A) Sham group. (B) Partial bladder outlet obstruction (BOO) group. (C) Group with detrusor overactivity (DO) after BOO/deobstruction (DDO). (D) Group without DO after BOO/deobstruction (non-DDO). Pves, intravesical pressure (IVP); Pabd, intra-abdominal pressure (IAP); Vol, volume.
There was no significant difference in any pressure parameters between the DDO and non-DDO groups (Table 1).
Volume Parameters
BC increased in the BOO group (2.99±0.36 mL, P<0.01) and the deobstructed group (2.56±0.29 mL, P<0.05) compared to the sham group (1.58±0.14 mL). MV increased in the deobstructed group (2.43±0.28 mL, P<0.05) compared to the sham group (1.53±0.14 mL). RV increased in the BOO group (1.15±0.45 mL, P<0.05) and non-DDO group (0.17±0.04 mL, P<.05) compared to the sham group (0.05±0.03 mL). RV decreased in the deobstructed group (0.13±0.04 mL, P<0.01) compared to the BOO group. Importantly, the non-DDO group showed a significantly greater RV than the DDO group. MI increased in the BOO group (8.86±0.94 mL, P<0.01) and the DDO group (7.34±1.19 mL, P<0.05) compared to the sham group (4.70±0.42 mL) (Fig. 2).
There was no significant difference in volume or pressure parameters except RV between the DDO and non-DDO groups, as described above (Table 1).
Total Gene Expression Profiles Measured In Bladder Tissue
The total gene expression patterns were analyzed using an Operon Rat Whole 27K Oligo chip in the sham, BOO, DDO, and non-DDO groups. The results showed a very uniform degree of fluorescence. In the gene expression results, 14,522 genes, including duplicated genes, were analyzed using the 27K Oligo chip, and a total of 7,532 genes showed an expression level with a 2-fold difference in an experimental group compared to the sham group. The number of genes in the experimental groups that showed a greater than 2-fold change in the expression level compared to the control group is shown in Fig. 3. Our results suggest that the deobstructed group with persistent DO after deobstruction had a more normalized pattern of gene expression changes than the group in which DO disappeared. Hierarchical clustering analysis showed that the gene expression profile in the DDO group was more similar than that of the non-DDO group to the sham group, and the non-DDO group showed a greater difference from the BOO group than the sham group (Fig. 4).
Fig. 3.
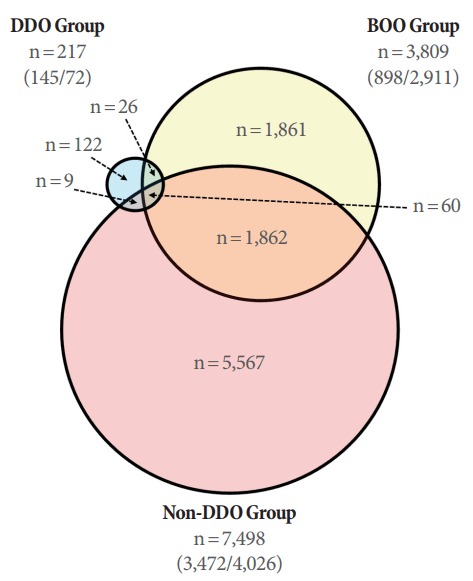
Venn diagram showing the number of genes that showed a greater than 2-fold change in expression in the bladder outlet obstruction (BOO) and deobstructed groups compared to the sham group. In the BOO group, 3,809 genes showed greater than 2-fold changes in gene expression compared to the sham group, while the group without detrusor overactivity (DO) after BOO/deobstruction (non-DDO) had significantly more such genes (n=7,498) and the group with DO after BOO/deobstruction (DDO) had significantly fewer such genes (n=217). The numbers in parentheses in each group represent (number of upregulated genes/number of downregulated genes).
Fig. 4.
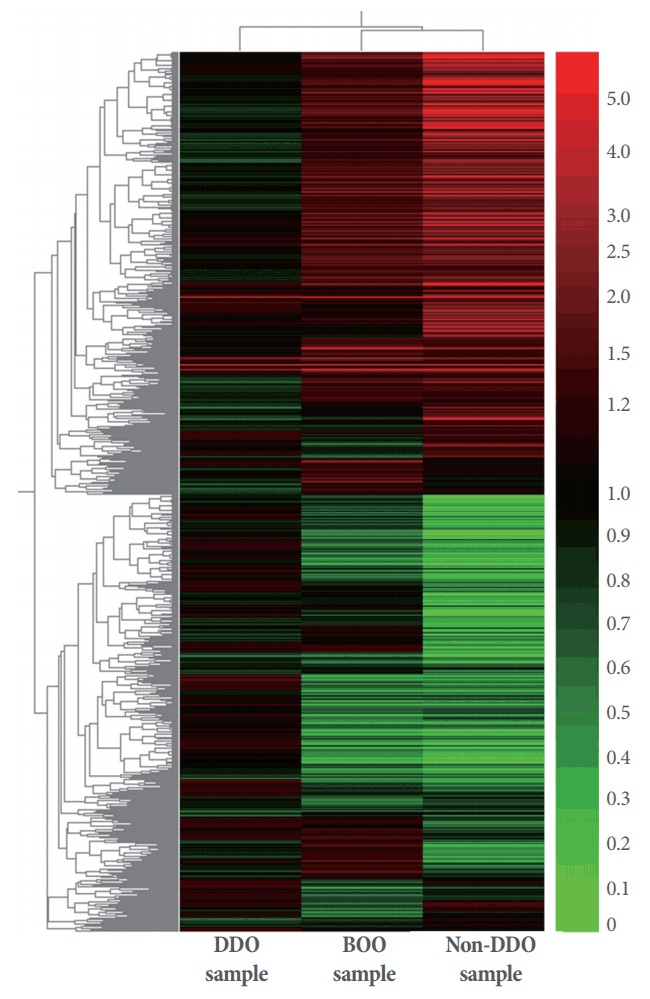
Hierarchical clustering of the total gene expression profiles analyzed on a microarray in samples from bladders from the partial bladder outlet obstruction (BOO) group and the groups with and without detrusor overactivity after BOO/deobstruction (DDO and non-DDO). Red color represents increased expression level and green color represents decreased expression levels.
Gene Expression Patterns by Functional Category
Apoptosis pathways
Among the genes that showed greater than 2-fold changes in gene expression in the experimental groups, 220 were associated with apoptosis pathways. In particular, the expression levels of inhibin alpha (INHA) and interleukin 1-alpha (IL1A) increased much more in the non-DDO group than in the BOO group. Furthermore, the expression of cyclin-dependent kinase inhibitor 1A (CDKN1A), zinc finger protein 162 (ZFP162), and vascular endothelial growth factor A (VEGFA) decreased in the BOO group, but decreased to an even greater extent in the non-DDO group. In contrast, the expression levels of these genes were not significantly different in the DDO group, or they showed a slight increase (Table 2).
Table 2.
Selected results showing the upregulation and downregulation of gene expression in functional pathway-related gene expression profiles
| Functional category | No. | Gene name | Symbol | Fold change |
||
|---|---|---|---|---|---|---|
| DDO | Non-DDO | BOO | ||||
| Apoptosis | 1 | Inhibin alpha | INHA | 0.825 | 8.932 | 2.177 |
| 2 | Interleukin 1 alpha | IL1A | 4.222 | 70.443 | 8.100 | |
| 3 | Cyclin-dependent kinase inhibitor 1A | CDKN1A | 1.994 | 0.298 | 0.500 | |
| Zinc finger protein | ZFP162 | 1.221 | 0.105 | 0.407 | ||
| V-ets erythroblastosis virus E26 oncogene homolog 1 (avian) | ETS1 | 1.291 | 0.127 | 0.399 | ||
| Vascular endothelial growth factor A | VEGFA | 1.169 | 0.419 | 0.623 | ||
| Cell adhesion | 1 | Catenin (cadherin associated protein), beta 1 | CTNNB1 | 1.200 | 0.027 | 0.448 |
| Adhesion regulating molecule 1 | ADRM1 | 1.054 | 0.086 | 0.617 | ||
| Procollagen, type 1, alpha 1 | COL1A1 | 0.986 | 0.025 | 0.504 | ||
| 2 | Cadherin 3, type 1, P-cadherin (placental) | CDH3 | 1.390 | 0.289 | 0.480 | |
| Integrin, alpha 6 | ITGA6 | 1.371 | 0.137 | 0.258 | ||
| Integrin, alpha 6 | ITGA6 | 1.379 | 0.170 | 0.265 | ||
| A disintegrin and metalloprotease domain 10 | ADAM10 | 1.982 | 0.162 | 0.141 | ||
| Thrombospondin 4 | THBS4 | 0.938 | 1.723 | 3.422 | ||
| Chemokine and receptors | 1 | Chemokine (C-X-C motif) ligand 2 | CXCL2 | 1.676 | 26.387 | 5.688 |
| Chemokine (C-C motif) ligand 4 | CCL4 | 1.285 | 25.409 | 4.399 | ||
| Chemokine (C-C motif) ligand 3 | CCL3 | 1.454 | 25.222 | 2.741 | ||
| Paired-Ig-like receptor B | PIRB | 1.365 | 13.638 | 3.319 | ||
| Chemokine (C-C motif) ligand 5 | CCL5 | 1.605 | 7.253 | 2.049 | ||
| Chemokine (C-X-X motif) ligand 9 | CXCL9 | 1.391 | 5.630 | 2.220 | ||
| Chemokine (C-X-C motif) ligand 10 | CXCL10 | 1.163 | 4.343 | 23.849 | ||
| Chemokine (C-C motif) ligand 2 | CCL2 | 0.998 | 3.678 | 2.081 | ||
| Chemokine (C-C motif) receptor 5 | CCR5 | 0.890 | 0.799 | 0.438 | ||
| Chemokine (C-X-C motif) ligand 14 | CXCL14 | 0.959 | 0.490 | 0.495 | ||
| Colony stimulating factor 2 receptor, beta 1, low-affinity (granulocyte-macrophage) | CSF2RB1 | 1.022 | 0.257 | 0.486 | ||
| Signal transducer and activator of transcription 5B | STAT5B | 1.027 | 0.208 | 0.456 | ||
| Extracellular matrix | 1 | Matrix extracellular phosphoglycoprotein with ASARM motif (bone) | MEPE | 1.327 | 0.048 | 0.551 |
| Procollagen, type 1, alpha 1 | COL1A1 | 0.986 | 0.025 | 0.504 | ||
| 2 | Biglycan | BGN | 0.939 | 0.020 | 0.282 | |
| Fibronectin 1 | FN1 | 1.587 | 0.023 | 0.353 | ||
| Procollagen, type XII, alpha 1 | COL12A1 | 1.147 | 0.042 | 0.332 | ||
| Transforming growth factor, beta 1 | TGFB1 | 1.128 | 0.075 | 0.735 | ||
| Integrin beta 4 | ITGB4 | 1.151 | 0.107 | 0.439 | ||
| 3 | Matrix metallopeptidase 12 | MMP12 | 2.895 | 31.046 | 6.216 | |
| Matrix metallopeptidase 7 | MMP7 | 8.358 | 163.101 | 12.037 | ||
| Matrix metallopeptidase 13 | MMP13 | 2.007 | 3.756 | 2.422 | ||
| Matrix metallopeptidase 8 | MMP8 | 2.710 | 7.228 | 3.778 | ||
| Inflammatory response | 1 | Chemokine (C-C motif) ligand 4 | CCL4 | 1.285 | 25.409 | 4.399 |
| Mast cell protease 6 | MCPT6 | 1.341 | 8.346 | 2.247 | ||
| Interleukin 1 alpha | IL1A | 4.222 | 70.443 | 8.100 | ||
| Ischemia-reperfusion | 1 | Lipocalin 2 | LCN2 | 1.987 | 5.604 | 5.292 |
| Midkine | MDK | 1.011 | 3.303 | 2.075 | ||
| PTK2 protein tyrosine kinase 2 | PTK2 | 1.101 | 0.181 | 0.245 | ||
| Neutrophil cytosolic factor 1 | NCF1 | 1.232 | 0.401 | 0.370 | ||
| Endothelin 1 | EDN1 | 0.966 | 12.292 | 2.462 | ||
| Angiopoietin 1 | ANGPT1 | 1.034 | 0.655 | 0.458 | ||
| Caspase 3, apoptosis related cysteine protease | CASP3 | 1.320 | 0.299 | 0.393 | ||
| Coagulation factor II (thrombin) receptor | F2R | 1.122 | 0.300 | 0.159 | ||
| Ectonucleoside triphosphate diphosphohydrolase 1 | ENTPD1 | 1.178 | 0.105 | 0.135 | ||
The numbers (No.) indicate the grouping of the differentially expressed patterns in each category.
DEO, total deobstructed group in rats with bladder outlet obstruction; DDO, DEO group showing DO during the storage phase; Non-DDO, DEO group showing no DO.
Cell adhesion pathways
The results of this functional analysis showed that the expression of genes related to cell adhesion pathways mostly decreased in the BOO group and non-DDO group. In the DDO group, there was no change in the expression of genes such as catenin-beta1 (CTNNB1), adhesion regulating molecule 1 (ADRM1), and procollagen type I alpha 1 (COL1ALPHA1). In the DDO-group, the expression levels were significantly reduced to a level more than 10 times lower than baseline. In addition, the expression of genes such as cadherin 3 (CAH3) and integrin-alpha 6 (ITGA6) was decreased by 2-5 times in the BOO and non-DDO groups. However, the degree of expression of thrombospondin 4 (THBS4) increased in the BOO group, but differentially decreased in both the DDO and non-DDO groups (Table 2).
Chemokine and receptor pathways
A total of 30 genes related to this functional pathway were overexpressed (approximately 70% of these genes), mainly in the BOO and non-DDO groups. In particular, the degree of expression of genes such as chemokine ligand 2 (CXCL2), chemokine ligand 4 (CCL4), CCL3, CCL5, and CXCL10 increased to 4.3–26.4 times their baseline levels in the non-DDO group, but in the DDO group had normalized to 1.1–1.7 times the baseline values. This tendency was similar to the above results for other chemokine ligands, except for the CXCL10 gene (Table 2).
Extracellular matrix pathway
The degree of expression of genes confirmed to be part of the pathway involving the extracellular matrix was generally decreased, but the expression of matrix metallopeptidase (MMP) increased in all 3 experimental groups (Table 2).
Inflammatory response pathway
Eighty-nine genes involved in the inflammatory response pathway showed expression changes. The genes belonging to this group are almost identical to those found in the chemokine and receptor and immune response pathways (Table 2).
Ischemia-reperfusion injury pathway
The degree of gene expression associated with the mechanism of ischemia-reperfusion injury may be highly relevant for the patterns of obstruction and deobstruction in the present experimental model. This is because when BOO occurs, the bladder expands and the blood vessels of the bladder wall are compressed and become ischemic, after which reperfusion takes place in some components of the vascular system when the bladder is deobstructed. As shown in Table 2, in these functional categories, almost all gene expression levels were normalized in the DDO group, similarly to the results of other categories, and there was little change in comparison with the sham group. It was also found that the expression of these genes was normalized in the DDO group, rather than the non-DDO group.
Comparison of 3-dimensional principal component analysis of gene expression patterns among experimental groups
Through our investigation, the results of 3-dimensional principal component analysis (PCA) of the whole and functional categories of the genotypes analyzed were confirmed. When all genes were examined, the differences in the gene expression patterns of the BOO, DDO, and non-DDO groups were clearly visualized in the 3-dimensional PCA comparison space (Fig. 5).
Fig. 5.
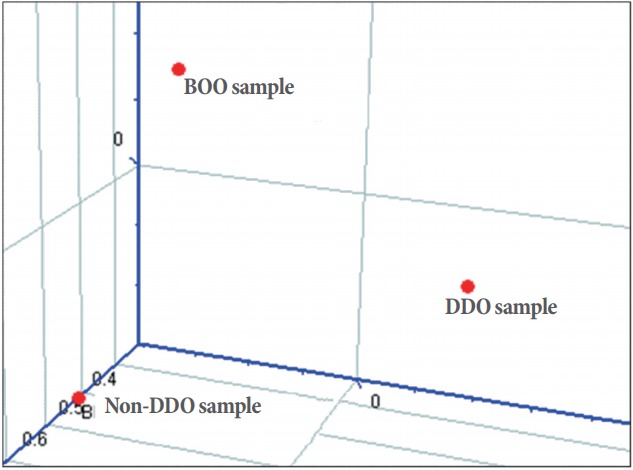
Principal component analysis for total gene expression profiles of the partial bladder outlet obstruction (BOO) group and the groups with and without detrusor overactivity after BOO/deobstruction (DDO and non-DDO).
Distinctions among experimental groups by correlation plot analysis
In the present study, gene expression patterns were clearly distinguished in all 3 experimental groups when all genes and functional categories were examined. Those of DDO (0.066) than of non-DDO (0.694) in the 2 deobstructed groups were distinguished clearly observed in the bladder of the BOO group, and the difference between the DDO and non-DDO groups was also 0.027, and these results were easily confirmed by the correlation plot.
DISCUSSION
OAB is defined by urgency with or without urinary frequency [11,12]. In other words, urgency is a key symptom of OAB, which is associated with the appearance of DO in urodynamic studies [11]. This has serious implications for patients’ daily life and quality of life, so it is known that treatment to suppress DO is clinically necessary [13-15]. However, interestingly, the present study using direct gene expression microarray technology found that the bladders in which DO persisted after BOO and deobstruction showed near-normal patterns of gene expression changes. In contrast, the bladders without DO after BOO and deobstruction showed much poorer changes in gene alteration, and continued to have abnormal bladder function. They showed much more dramatically changed gene expression patterns than were found in the BOO group, and a meaningful RV, indicating functional deterioration, even though they showed a significantly reduced RV compared to the BOO group.
The above findings imply that DO does not always play a negative role in the bladder, as has been reported [14,15]. Although DO certainly plays a negative role in patients’ lives, it seems to play a beneficial role from the point of view of an organ in the body. At least, bladders with DO associated with obstruction/ deobstruction were found to show conditions close to those of normal bladders. This suggests that the development of DO can be interpreted as a normalization process of bladder function in response to external stress, based on gene expression changes. The environment of the bladder is inherently stressful because urine, a highly toxic aqueous solution, may damage the bladder [16,17]. Under normal conditions, the bladder is protected by a barrier between the bladder and the inner space containing urine, which prevents the toxins in urine from entering the bladder wall. However, damage to this barrier by an external impact allows these toxins to enter the bladder wall, causing serious inflammation and functional problems in the bladder wall. Thus, DO serves to evacuate urine as quickly as possible to reduce the damage caused by the urine. This is consistent with the logic of bladder protection and can be seen as a step in adaptive neuroplasticity.
The bladder is composed of the urothelium, suburothelium, and a smooth muscle layer [18]. Compared to the sham group, the bladder weight significantly increased in the BOO and deobstructed groups, and increased bladder weight is characterized by an increase in the number of myofibroblasts and greater spontaneous detrusor activity [19,20]. Among the genes related to fibroblast growth factor (FGF), FGF9 expression increased by 4.0 times in the BOO and deobstructed groups. The expression level of growth arrest specific 6 (GAS6), a gene that inhibits cell proliferation, decreased by 0.65 times in the BOO group, 0.91 times in the DDO group, and more than 0.45 times in the non-DDO group. These results suggest that the increase of bladder weight by BOO is primarily mediated by the activation of cell proliferation, including smooth muscle cells. The expression level of transforming growth factor beta 1 (TGFB1) decreased by 0.74 times in the BOO group, as part of the profile of extracellular matrix-related gene expression in the functional classification of bladder cell proliferation described above. In the DDO group, it was almost normal, at an expression level of 1.1 times that of baseline, but it decreased to 0.07 times the baseline value in the non-DDO group (Table 2). Since the TGFB1 gene is generally known to inhibit cell proliferation [21], a decrease in the expression of this gene results in a further increase in cell proliferation, and the marked decrease found in the non-DDO group is expected to further promote cell proliferation. However, the significance of these results is unclear.
The expression level of most chemokine ligands increased by 2.0–26.4 times in the non-DDO group, as the result of changes in the expression patterns of genes related to chemokine and receptor functions. In contrast, in the DDO group, all chemokine genes were normalized, as other experiments conducted by Stephan et al. [22]. In addition, the degree of expression of genes involved in the cell deposition pathway, such as Catenin (CTNN), Filamin (FLN), and Adhesion regulating molecule-1 (ADRN1), decreased in the BOO group and decreased to an even further extent in the non-DDO group, whereas the expression of these genes was almost normal in the DDO group, as has been reported in human disease-related expression profiles [23]. Therefore, in these functional gene families, it was found that the persistence of OAB was associated with normalized patterns after deobstruction of BOO. In a comparison of the gene families acting on the ischemic and reperfusion injury pathways in other functional categories, the expression of antioxidant enzymes was found to be distinct. In particular, the expression of lipocalin (LCN2), which is part of both the cell proliferation pathway and the antioxidant pathway, increased to a level that was more than 5 times greater than baseline in the BOO and non-DDO groups. These gene expression results were compared in the 3-dimensional PCA analysis, as shown in Fig. 5. The correlation plot analysis also showed differences between the non-DDO group and the other groups.
The most important finding of this study, based on a comparative analysis of gene expression, is that the number of genes that showed significant reductions or increases in expression compared to the sham group was much greater in the bladders in which DO disappeared after BOO and deobstruction rather than in those in which DO persisted. In the context of this study, it may be unwarranted to conclude that the bladders with DO had a status similar to that of the normal bladders without a statistical comparison. However, this conclusion becomes more reasonable given the tremendous discrepancy in the number of genes that showed a greater than 2-fold change compared to normal bladder between the non-DDO group (7,498 such genes) and the DDO group (only 217 such genes).
These findings are confined to bladders from DO animal models that underwent BOO induction and deobstruction, and further studies are needed in bladders with DO from other diseases than BOO, such as aging-related bladder changes, interstitial cystitis, and neurogenic bladder. Our findings may also enhance further research into whether DO is beneficial in bladders with diseases other than BOO/deobstruction.
In conclusion, in the rats in which BOO was induced, the extent of altered gene expression after deobstruction was much more marked in the non-DDO group, in which DO disappeared, than in the rats with normal bladders (the sham group). The extent of changes in gene expression in the DDO group, in which DOs persisted after deobstruction, was further normalized.
Therefore, DO in the bladder is presumed to be a combined effort of the bladder and the nervous system that transforms the bladder into a state close to normal in times of stress. This can be considered as a kind of adaptive neuroplasticity of the bladder.
Footnotes
Grant/Fund Support
This work was financially supported by Medical Research Center (2014009392) through the National Research Foundation of Korea (NRF) funded by Ministry of Science, ICT and Future Planning for Chang-Shin Park, and Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2016R1D1A1B03932278) for Tack Lee.
Research Ethics
All procedures for animal handling and treatment were performed in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and were approved by the Ethics Committee of the Inha University College of Medicine (INHA 140731-321-1).
Conflict of Interest
TL, Editor-in-Chief of INJ, is the co-first author of this article. However, he played no role whatsoever in the editorial evaluation of this article or the decision to publish it. No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.May A, Hajak G, Ganßbauer S, Steffens T, Langguth B, Kleinjung T, et al. Structural brain alterations following 5 days of intervention: dynamic aspects of neuroplasticity. Cereb Cortex. 2007;17:205–10. doi: 10.1093/cercor/bhj138. [DOI] [PubMed] [Google Scholar]
- 2.Elbadawi A, Diokno AC, Millard RJ. The aging bladder: morphology and urodynamics. World J Urol. 1998;16:S10–34. doi: 10.1007/pl00014134. [DOI] [PubMed] [Google Scholar]
- 3.Kirschner-Hermanns R, Daneshgari F, Vahabi B, Birder L, Oelke M, Chacko S. Does diabetes mellitus-induced bladder remodeling affect lower urinary tract function?: ICI-RS 2011. Neurourol Urodyn. 2012;31:359–64. doi: 10.1002/nau.22228. [DOI] [PubMed] [Google Scholar]
- 4.Minaglia S, Ozel B, Bizhang R, Mishell DR., Jr Increased prevalence of interstitial cystitis in women with detrusor overactivity refractory to anticholinergic therapy. Urology. 2005;66:702–6. doi: 10.1016/j.urology.2005.04.042. [DOI] [PubMed] [Google Scholar]
- 5.Ethans K, Casey A, Bard R, Namka M. Neurogenic overactive bladder in spinal cord injury and multiple sclerosis: role of onabotulinumtoxinA. Degener Neurol Neuromuscul Dis. 2014;4:65–73. doi: 10.2147/DNND.S40349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manack A, Motsko SP, Haag-Molkenteller C, Dmochowski RR, Goehring EL, Nguyen-Khoa B, et al. Epidemiology and healthcare utilization of neurogenic bladder patients in a US claims database. Neurourol Urodyn. 2011;30:395–401. doi: 10.1002/nau.21003. [DOI] [PubMed] [Google Scholar]
- 7.Parsons BA, Drake MJ. Animal models in overactive bladder research. Handb Exp Pharmacol. 2011;202:15–43. doi: 10.1007/978-3-642-16499-6_2. [DOI] [PubMed] [Google Scholar]
- 8.Kim WH, Bae WJ, Park JW, Choi JB, Kim SJ, Cho HJ, et al. Development of an improved animal model of overactive bladder: transperineal ligation versus transperitoneal ligation in male rats. World J Mens Health. 2016;34:137–44. doi: 10.5534/wjmh.2016.34.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jin LH, Andersson KE, Han JU, Kwon YH, Park CS, Shin HY, et al. Persistent detrusor overactivity in rats after relief of partial urethral obstruction. Am J Physiol Regul Integr Comp Physiol. 2011;301:R896–904. doi: 10.1152/ajpregu.00046.2011. [DOI] [PubMed] [Google Scholar]
- 10.Lee T, Yoon SM. The role of intra-abdominal pressure measurement in awake rat cystometry. Int Neurourol J. 2013;17:44–7. doi: 10.5213/inj.2013.17.2.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Neurourol Urodyn. 2002;21:167–78. doi: 10.1002/nau.10052. [DOI] [PubMed] [Google Scholar]
- 12.Drake MJ. Do we need a new definition of the overactive bladder syndrome? ICI-RS 2013. Neurourol Urodyn. 2014;33:622–4. doi: 10.1002/nau.22609. [DOI] [PubMed] [Google Scholar]
- 13.Benner JS, Becker R, Fanning K, Jumadilova Z, Bavendam T, Brubaker L, et al. Bother related to bladder control and health care seeking behavior in adults in the United States. J Urol. 2009;181:2591–8. doi: 10.1016/j.juro.2009.02.018. [DOI] [PubMed] [Google Scholar]
- 14.Kannan H, Radican L, Turpin RS, Bolge SC. Burden of illness associated with lower urinary tract symptoms including overactive bladder/urinary incontinence. Urology. 2009;74:34–8. doi: 10.1016/j.urology.2008.12.077. [DOI] [PubMed] [Google Scholar]
- 15.Coyne KS, Sexton CC, Kopp ZS, Ebel-Bitoun C, Milsom I, Chapple C. The impact of overactive bladder on mental health, work productivity and health-related quality of life in the UK and Sweden: results from EpiLUTS. BJU Int. 2011;108:1459–71. doi: 10.1111/j.1464-410X.2010.10013.x. [DOI] [PubMed] [Google Scholar]
- 16.Hohlbrugger G. Leaky urothelium and/or vesical ischemia enable urinary potassium to cause idiopathic urgency/frequency syndrome and urge incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 1996;7:242–55. doi: 10.1007/BF01901246. [DOI] [PubMed] [Google Scholar]
- 17.Parsons CL. The role of a leaky epithelium and potassium in the generation of bladder symptoms in interstitial cystitis/overactive bladder, urethral syndrome, prostatitis and gynaecological chronic pelvic pain. BJU Int. 2011;107:370–5. doi: 10.1111/j.1464-410X.2010.09843.x. [DOI] [PubMed] [Google Scholar]
- 18.Fry CH, Vahabi B. The role of the mucosa in normal and abnormal bladder function. Basic Clin Pharmacol Toxicol. 2016;119:57–62. doi: 10.1111/bcpt.12626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ganitkevich VY, Isenberg G. Contribution of Ca(2+)-induced Ca2+ release to the [Ca2+]i transients in myocytes from guineapig urinary bladder. J Physiol. 1992;458:119–37. doi: 10.1113/jphysiol.1992.sp019409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Monson FC, Wein AJ, Eika B, Murphy M, Levin RM. Stimulation of DNA synthesis in rabbit bladder wall after partial outlet obstruction and acute overdistension. Neurourol Urodyn. 1994;13:51–61. doi: 10.1002/nau.1930130108. [DOI] [PubMed] [Google Scholar]
- 21.Moses HL. TGF-beta regulation of epithelial cell proliferation. Mol Reprod Dev. 1992;32:179–84. doi: 10.1002/mrd.1080320215. [DOI] [PubMed] [Google Scholar]
- 22.Stephan M, Conrad S, Eggert T, Heuer R, Fernandez S, Huland H. Urinary concentration and tissue messenger RNA expression of monocyte chemoattractant protein-1 as an indicator of the degree of hydronephrotic atrophy in partial ureteral obstruction. J Urol. 2002;167:1497–502. [PubMed] [Google Scholar]
- 23.Wu C. Migfilin and its binding partners: from cell biology to human diseases. J Cell Sci. 2005;118(Pt 4):659–64. doi: 10.1242/jcs.01639. [DOI] [PubMed] [Google Scholar]