Abstract
The disruptive selection theory of the evolution of anisogamy posits that the evolution of a larger body or greater organismal complexity selects for a larger zygote, which in turn selects for larger gametes. This may provide the opportunity for one mating type to produce more numerous, small gametes, forcing the other mating type to produce fewer, large gametes. Predictions common to this and related theories have been partially upheld. Here, a prediction specific to the disruptive selection theory is derived from a previously published game‐theoretic model that represents the most complete description of the theory. The prediction, that the ratio of macrogamete to microgamete size should be above three for anisogamous species, is supported for the volvocine algae. A fully population genetic implementation of the model, involving mutation, genetic drift, and selection, is used to verify the game‐theoretic approach and accurately simulates the evolution of gamete sizes in anisogamous species. This model was extended to include a locus for gamete motility and shows that oogamy should evolve whenever there is costly motility. The classic twofold cost of sex may be derived from the fitness functions of these models, showing that this cost is ultimately due to genetic conflict.
Keywords: anisogamy, disruptive selection theory, gamete competition theory, isogamy, oogamy, sexes
1. INTRODUCTION
Gamete dimorphism, or anisogamy, in the extreme form of small motile sperm and large nonmotile eggs, or oogamy, defines sexes and is characteristic of plants and animals. An elegant and enduring theory for the evolution of anisogamy from gamete monomorphism, or isogamy, is that as body size increases there is selection for a larger zygote to store nutrients used in early development, which in turn selects for larger gametes. This may provide the opportunity for one mating type to produce more numerous, smaller gametes, thereby increasing its fertility via competition with other gametes of the same mating type, and thus forcing the other mating type to produce fewer, larger gametes (Bell, 1978; Bulmer, 1994; Charlesworth, 1978; Maynard Smith, 1978, 1982; Parker, Baker, & Smith, 1972). Therefore, this theory proposes competition among gamete size alleles within mating types and genetic conflict between mating types. As a result, models based on this theory are known as disruptive selection, or gamete competition, models to distinguish them from models based on gamete limitation or intracellular conflicts (see reviews by Randerson & Hurst, 2001b; Lessells, Snook, & Hosken, 2009; Togashi & Cox, 2011).
Two predictions from the disruptive selection theory have been partially upheld: that with an increase in structural complexity or adult body size there is an increase in both zygote size and gamete dimorphism. These predictions have been tested in the algae, which exhibit considerable interspecific variation in structural complexity, body size, and gamete dimorphism (Bell, 1978, 1985; Knowlton, 1974; Madsen & Waller, 1983; Randerson & Hurst, 2001a). However, there are many exceptions to these associations, such as unicellular species that are oogamous and multicellular species that are isogamous, and this has raised doubts about the completeness of the theory (Bell, 1978; Madsen & Waller, 1983). In addition, these predictions may not be specific to the disruptive selection theory. A positive correlation between zygote size and adult size or complexity may be required by the theory to explain the increase in zygote size that leads to the conditions for disruptive selection. However, an increase in zygote size, necessitating fewer, larger gametes, may also result in gamete limitation. If one mating type producing a large nonmotile gamete and the other producing a small motile gamete increase the gamete encounter rate, as proposed in some models of gamete limitation (Cox & Sethian, 1985; Dusenbery, 2000, 2006; Lehtonen & Kokko, 2011), then gamete dimorphism will increase with adult size if zygote size increases with adult size. Therefore, the disruptive selection theory has yet to be rigorously tested. Here, a novel specific prediction is derived from the most thoroughly developed model of the disruptive selection theory available and tested with data from the volvocine algae.
The volvocine algae (order Chlamydomonadales; formerly Volvocales) (Figure 1) have been particularly useful in testing the disruptive selection theory (Bell, 1978, 1985; Knowlton, 1974; Madsen & Waller, 1983; Randerson & Hurst, 2001a). This group ranges in complexity from unicellular to colonial to multicellular with complete separation between soma and germ, and all three structural grades exhibit both isogamy and some form of anisogamy (Hallmann, 2011). The intensely studied unicellular, isogametic Chlamydomonas reinhardtii, is often used to represent the ancestral condition. Colony development in this species is easily selected in the laboratory (Ratcliff et al., 2013) and may have a simple genetic basis (Hanschen et al., 2016; Nedelcu & Michod, 2006). And gamete dimorphism in C. reinhardtii may be simulated in the laboratory by allowing gametogenesis to occur via either intracellular transformation, producing a single large gamete, or via one or two mitotic divisions, producing two or four small gametes (Wiese, Wiese, & Edwards, 1979). This mirrors gametogenesis in the multicellular, oogametic Volvox carteri, in which eggs develop directly from large reproductive cells and sperm are the product of seven sequential mitotic divisions, resulting in 128 sperm per reproductive cell (Hallmann, Godl, Wenzl, & Sumper, 1998). Interestingly, the genetic loci specifying mating type and gamete size in V. carteri have evolved close physical linkage, as inferred by comparison with the homologous loci in C. reinhardtii, which are not closely linked (Ferris et al., 2010), and are coregulated (Geng, de Hoff, & Umen, 2014). The evolution of tight linkage between mating type and gamete size loci is predicted by a population genetic formulation of the disruptive selection theory (Charlesworth, 1978).
Figure 1.
Gonium pectorale, a colonial isogamous volvocine alga. Photograph credit: Environmental Protection Agency—http://www.epa.gov/glnpo/image/viz_nat6.html
Here, a novel prediction specific to the disruptive selection theory is derived from an existing game‐theoretic model that represents the most complete model of the theory available. This prediction is tested with data from the volvocine algae, including recently described species. Bulmer and Parker (2002) extended the game‐theoretic model of the disruptive selection theory of Maynard Smith (1978, 1982) to include explicit functions for gamete and zygote survival or viability. Making these functions explicit allows predictions of the conditions leading to evolutionarily unstable isogamy and ensuing evolutionarily stable anisogamy. Furthermore, the parameterization of this model makes it easy to estimate the parameters using few assumptions and thus test these predictions. It is shown here that anisogamous species are predicted to always have an anisogamy ratio greater than 3. This is upheld for the species analyzed. The model and predictions are then confirmed using a fully population genetic implementation. Extending the population genetic model to include a locus for motility predicts the evolution of oogamy in anisogamous species whenever there is costly motility. Finally, using the fitness functions from the model to calculate the fitness “cost of sex” for anisogamous species shows that the cost of sex increases with the degree of anisogamy, reaching the classic twofold cost with oogamy.
2. GAME‐THEORETIC MODELS AND PREDICTIONS
In the game‐theoretic model of Bulmer and Parker (2002), the fitness of a new haploid mutant producing gametes of size m 1 in a population of individuals producing gametes of size m 2 is
where M is the fixed budget for gamete production and g and f are functions for the probability of gamete and zygote survival, respectively. Predictions are derived from this model using two forms of survival function used by Bulmer and Parker.
With what Bulmer and Parker term the “Vance survival function,” which is actually a modification of Vance's function (Vance, 1973) proposed by Levitan (2000)
where S = m 1 + m 2, the zygote size, and α and β are positive scaling parameters. This function is sigmoidal, accelerating from the origin until m = α/2 (S = β/2) and then decelerating and reaching an asymptotic value of one. For an anisogamous species, the ancestral condition is likely to have been α ≈ β and then become β > α, selecting for a larger zygote (Bulmer & Parker, 2002). Under this model, with isogamy, the optimal isogamete size is , and isogamy is continuously evolutionarily stable when β < 4α. When β > 4α, isogamy becomes evolutionarily unstable and anisogamy becomes continuously evolutionarily stable. With anisogamy, if gamete sizes are a and b and a < b, a = α + ε and b = β – α − ε, where ε is a small deviation that tends to zero as β increases relative to α. Therefore, with large β, α ≈ a and β ≈ a + b. Then, because anisogamy is evolutionary stable when β > 4α, the anisogamy ratio for anisogamous species is predicted to be b/a > 3.
In a second model, Levitan's survival function is used for zygotes, but gametes do not survive below threshold size δ and have a probability of survival of one above that size. This is the implicit gamete survival function used by Maynard Smith (1978, 1982). With this model, and isogamy are continuously evolutionarily stable when . When , isogamy becomes evolutionarily unstable, and anisogamy becomes continuously evolutionarily stable. Then, with anisogamy, δ = a because microgametes always evolve to the smallest viable size, and β ≈ δ + b, for large β. Therefore, as in the case with Levitan's survival functions, with large β, the anisogamy ratio for anisogamous species is predicted to be b/a > 3.
Symbols used for variables, functions, and parameters in these models are defined in Table 1.
Table 1.
Symbol definitions for the Bulmer and Parker game‐theoretic models
| Symbol | Definition |
|---|---|
| Variables | |
| w | Fitness of a haplotype |
| m | Gamete size |
| a | Microgamete size |
| b | Macrogamete size |
| S | Zygote size |
| Functions | |
| g(m) | Gamete survival function |
| f(S) | Zygote survival function |
| Parameters | |
| M | Fixed budget for gamete production |
| α | Positive scaling parameter of g(m) |
| β | Positive scaling parameter of f(S) |
| δ | Threshold size for gamete survival |
3. METHODS
3.1. Data
Gamete volumes, a and b, were obtained from the literature for each species of the volvocine algae for which the relevant data are available. The volume of a reproductive cell was used as an estimate of the gamete budget, M. A reproductive cell refers to a cell that produces gametes, either through direct transformation or through mitotic cell divisions. This may be a zoospore (vegetative cell) of a unicellular or colonial species or a specialized (germ) cell in multicellular species.
Cell volumes were either reported in the literature or calculated from the cell radius if cells were approximately spherical. Most cells are prolate spheroids or spindle‐shaped ellipsoids, whose volume is calculated as (4/3)πr 1 r 2 r 3, where r 1, r 2, and r 3 are the three radii. Typically, the length and width of a cell are reported; the third dimension was assumed to be equal to the width. Often, a range of values is given for each dimension; in these cases, the largest values were used to ensure that mature cells were used, following Randerson and Hurst (2001a). In some cases, cell dimensions were measured from high‐quality drawings or micrographs. Because of inherent errors in estimating volume, especially of gametes (because they are often smaller than reproductive cells) and because gametes are the result of 0 or more cell divisions, macrogamete, or isogamete, volume was adjusted so that the ratio M/b is the nearest power of 2. In some anisogamous and oogamous species, the macrogamete or egg develops directly from a reproductive cell, and microgametes or spermatozoids are produced by several sequential mitotic divisions of a reproductive cell. For these species, microgamete volume was calculated as the volume of a reproductive cell divided by the number of microgametes. The protoplasmic volume was used as a measure of adult size; this is the sum of the volumes of the individual's cells making up an adult (Randerson & Hurst, 2001a). Data are from Randerson and Hurst (2001a) and references therein, and from references in Harris (2009). In addition, each genus in each family of the Chlamydomonadales, as listed in the online database AlgaeBase (Guiry & Guiry, 2017), was used as an online bibliographic search term, providing additional species for which the required data were available (Demchenko & Mikhailiuk, 2012; Hayama, Nakada, Hamaji, & Nozaki, 2010; Hoham et al., 2006; Ivan & Katya, 2014; Kaštovský, 2008; Kugrens, Clay, & Aguiar, 2000; Ling, 2001; Nováková, 2005; Nozaki & Itoh, 1994; Nozaki, Yamada, Takahashi, Matsuzaki, & Nakada, 2014; Suda, Nozaki, & Watanabe, 2005). The required data were available for a total of 45 species of the Chlamydomonadales (Table 2).
Table 2.
Structural grade, gamete dimorphism, and size data for volvocine algae
| Species | No. of cells | Structural grade | Gamete dimorphisma | Protoplasmic volume (μm3) | Reproductive cell volume (μm3), M | Macro‐ or isogamete volume (μm3), b | Micro‐ or isogamete volume (μm3), a | Inferred zygote volume (μm3), S | b/a |
|---|---|---|---|---|---|---|---|---|---|
| Carteria palmata | 1 | Unicellular | Isogamy | 5,080 | 5,080 | 5,080 | 5,080 | 10,160 | 1 |
| Chlamydomonas allensworthii | 1 | Unicellular | Anisogamy | 950 | 950 | 950 | 59 | 1,010 | 16 |
| Chlamydomonas chlamydogama | 1 | Unicellular | Isogamy | 1,120 | 1,120 | 560 | 560 | 1,120 | 1 |
| Chlamydomonas indica | 1 | Unicellular | Isogamy | 733 | 733 | 183 | 183 | 367 | 1 |
| Chlamydomonas microhalophila | 1 | Unicellular | Isogamy | 1,508 | 1,508 | 754 | 754 | 1,508 | 1 |
| Chlamydomonas moewusii | 1 | Unicellular | isogamy | 8,370 | 8,370 | 2,093 | 2,093 | 4,185 | 1 |
| Chlamydomonas reinhardtii | 1 | Unicellular | Isogamy | 11,000 | 11,000 | 5,500 | 5,500 | 11,000 | 1 |
| Chlamydomonas sphagnicola | 1 | Unicellular | Oogamy | 3,591 | 3,591 | 3,591 | 225 | 3,816 | 16 |
| Chlamydomonas suboogama | 1 | Unicellular | Anisogamy | 3,054 | 3,054 | 3,054 | 191 | 3,245 | 16 |
| Chlorogonium mariae | 1 | Unicellular | Isogamy | 524 | 524 | 131 | 131 | 262 | 1 |
| Chloromonas chenangoensis | 1 | Unicellular | Isogamy | 4,009 | 4,009 | 4,009 | 4,009 | 8,018 | 1 |
| Lobocharacium coloradoense | 1 | Unicellular | Isogamy | 1,734,159 | 1,734,159 | 524 | 524 | 1,047 | 1 |
| Lobochlamys segnis | 1 | Unicellular | Isogamy | 700 | 700 | 22 | 22 | 44 | 1 |
| Oogamochlamys ettlii | 1 | Unicellular | Oogamy | 6,842 | 6,842 | 6,842 | 428 | 7,270 | 16 |
| Oogamochlamys gigantea | 1 | Unicellular | Oogamy | 8,181 | 8,181 | 8,181 | 128 | 8,309 | 64 |
| Oogamochlamys zimbabwiensis | 1 | Unicellular | Oogamy | 4,189 | 4,189 | 4,189 | 262 | 4,451 | 16 |
| Pseudocarteria corcontica | 1 | Unicellular | Isogamy | 13,360 | 13,360 | 13,360 | 13,360 | 26,719 | 1 |
| Tetrabaena socialis | 4 | Colonial | Isogamy | 11,800 | 2,950 | 738 | 738 | 1,475 | 1 |
| Stephanosphaera pluvialis | 8 | Colonial | Isogamy | 10,648 | 1,331 | 83 | 83 | 166 | 1 |
| Desmotetra antarctica | 16 | Colonial | Isogamy | 147,244 | 9,203 | 575 | 575 | 1,150 | 1 |
| Desmotetra aureospora | 16 | Colonial | Isogamy | 77,584 | 4,849 | 303 | 303 | 606 | 1 |
| Gonium maiaprilis | 16 | colonial | Isogamy | 28,272 | 1,767 | 221 | 221 | 442 | 1 |
| Gonium pectorale | 16 | Colonial | Isogamy | 75,600 | 4,725 | 2,363 | 2,363 | 4,725 | 1 |
| Gonium quadratum | 16 | Colonial | Isogamy | 11,200 | 700 | 697 | 697 | 1,394 | 1 |
| Gonium viridistellatum | 16 | Colonial | Isogamy | 28,300 | 1,769 | 884 | 884 | 1,769 | 1 |
| Volvulina pringsheimii | 16 | Colonial | Isogamy | 28,300 | 1,769 | 1,767 | 1,767 | 3,534 | 1 |
| Colemanosphaera charkowiensis | 32 | Colonial | Anisogamy | 82,318 | 2,572 | 2,572 | 80 | 2,653 | 32 |
| Eudorina elegans | 32 | Colonial | Anisogamy/internal | 232,000 | 7,250 | 3,625 | 57 | 3,682 | 64 |
| Gonium multicoccum | 32 | Colonial | Isogamy | 97,700 | 3,053 | 3,053 | 3,053 | 6,106 | 1 |
| Pandorina morum | 32 | Colonial | Isogamy | 294,000 | 9,188 | 574 | 574 | 1,148 | 1 |
| Platydorina caudata | 32 | Colonial | Anisogamy | 262,000 | 8,188 | 4,094 | 128 | 4,222 | 32 |
| Volvulina steinii | 32 | Colonial | Isogamy | 70,600 | 2,206 | 2,206 | 2,206 | 4,413 | 1 |
| Pleodorina indica | 64 | Multicellular | Anisogamy/internal | 357,000 | 9,200 | 1,150 | 18 | 1,168 | 64 |
| Astrephomene gubernaculifera | 128 | Multicellular | Isogamy | 6,100,000 | 48,800 | 3,050 | 3,050 | 6,100 | 1 |
| Pleodorina japonica | 128 | Multicellular | Anisogamy/internal | 1,040,000 | 14,100 | 14,100 | 110 | 14,210 | 128 |
| Volvox pocockiae | 1,500 | Multicellular | Oogamy | 2,650,000 | 113,000 | 113,000 | 1,766 | 114,766 | 64 |
| Volvox tertius | 2,000 | Multicellular | Oogamy | 763,000 | 655,000 | 655,000 | 20,469 | 675,469 | 32 |
| Volvox carteri | 3,000 | Multicellular | Oogamy | 1,570,000 | 10,300 | 10,300 | 80 | 10,380 | 128 |
| Volvox aureus | 3,200 | Multicellular | Oogamy | 362,000 | 7,230 | 7,230 | 226 | 7,456 | 32 |
| Volvox obversus | 4,000 | Multicellular | Oogamy | 2,090,000 | 38,800 | 38,800 | 303 | 39,103 | 128 |
| Volvox africanus | 6,000 | Multicellular | Oogamy | 963,000 | 47,700 | 47,700 | 373 | 48,073 | 128 |
| Volvox capensis | 20,000 | Multicellular | Oogamy | 42,100,000 | 125,000 | 125,000 | 244 | 125,244 | 512 |
| Volvox globator | 22,000 | Multicellular | Oogamy | 737,000 | 11,000 | 11,000 | 43 | 11,043 | 256 |
| Volvox dissipatrix | 31,800 | Multicellular | Oogamy | 133,000,000 | 33,500 | 33,500 | 131 | 33,631 | 256 |
| Volvox rousseletii | 50,000 | Multicellular | Oogamy | 105,000,000 | 14,100 | 14,100 | 28 | 14,128 | 512 |
Anisogamy/internal refers to anisogamy with internal fertilization.
3.2. Phylogenetic analysis
Origins of structural grades and gamete dimorphisms were mapped on to a composite phylogeny constructed to include as many of the species in Table 2 as possible (27 species) (Figure 2). Multicellularity is defined as the structural grade in which some cells are somatic. The composite phylogeny was constructed from an exhaustive 18S rRNA Bayesian phylogenetic analysis of the Chlamydomonadales (Volvocales) (Nakada, Misawa, & Nozaki, 2008), with the “Reinhardtinia” of this large phylogeny replaced with a higher resolution phylogeny of the three families Tetrabaenaceae, Goniaceae, and Volvocaceae. This latter phylogeny, which includes most of the colonial and multicellular species in Table 2, is based on a Bayesian analysis of five chloroplast genes (Nozaki et al., 2014). A third phylogeny of the volvocine algae, also based on a Bayesian analysis of the same five chloroplast genes (Herron & Michod, 2008), and which is consistent with the phylogeny of Nozaki et al. (2014), was used to place Astrephomene gubernaculifera as a sister taxon to the Gonium clade. The composite phylogeny contains 27 species from Table 2, and all remaining species from the Nozaki et al. (2014) phylogeny for which structural grade and gamete dimorphism have been reported (Goldstein, 1964; Nozaki & Kuroiwa, 1990, 1991, 1992; Nozaki, Ott, & Coleman, 2006; Pocock, 1955; Smith, 1944; Stein, 1959; Vande Berg & Starr, 1971). The additional 15 taxa from the Nozaki et al. (2014) phylogeny were included to provide additional resolution to the intensely studied Volvocaceae, which are all colonial or multicellular and vary substantially in gamete dimorphism.
Figure 2.
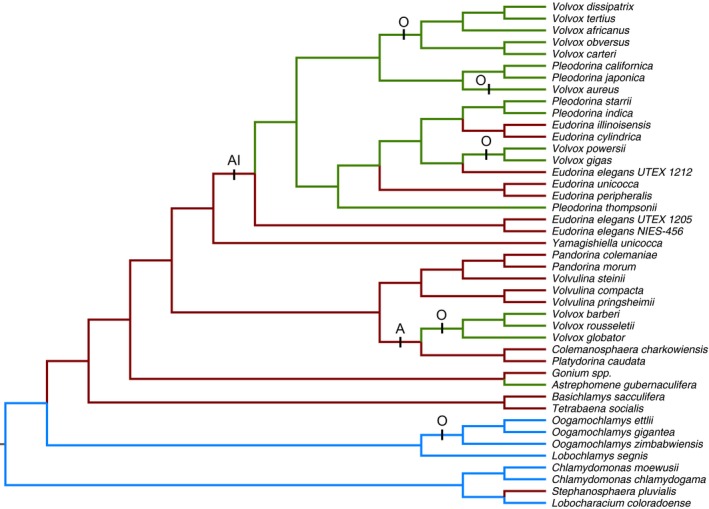
Origins of structural grades and gamete dimorphisms mapped onto a composite phylogeny of the volvocine algae. Blue branches represent unicellular lineages, red branches represent colonial lineages, and green branches represent multicellular lineages. Bars indicate gamete dimorphism: A, anisogamy; AI, anisogamy with internal fertilization; O, oogamy. Isogamy is assumed to be ancestral
The most parsimonious origins of structural grades and gamete dimorphisms were mapped on to the phylogeny, assuming that unicellular isogamy is ancestral (Figure 2). These mappings are mostly unambiguous. One of two exceptions is the origin of multicellularity from colonial ancestors in the clade defined by Eudorina spp. and allied taxa in the genera Volvox and Pleodorina, for which either four independent origins or one origin followed by three losses is equally parsimonious. A single origin is used to maintain consistency with the more detailed study of the evolution of multicellularity by Herron, Hackett, Aylward, and Michod (2009). The three origins of multicellularity in the entire tree are supported by each multicellular clade exhibiting a distinct form of development (Hallmann, 2006; Yamashita et al., 2016). The other exception to unambiguous mappings is the origin of oogamy in the clade containing Volvox tertius and Volvox aureus. Two independent origins of oogamy within the clade, or one origin in the ancestor and a subsequent loss, are equally parsimonious. Two independent origins of oogamy in this clade are assumed on the basis that reversions from oogamy appear to be rare, given that all extant plants and animals are oogamous.
3.3. Population genetic model
A population genetic implementation of the Bulmer and Parker (2002) game‐theoretic model described above was developed to numerically simulate the evolution of gamete size in the volvocine algae with realistic mutation patterns, genetic drift, and selection. The model was used to verify the game‐theoretic model and the use of the large‐β approximation for estimating α and β. A gamete size locus is physically linked to a mating type locus and determines the number of gametogenic mitoses. Allele i at this locus produces gametes of size m i = M/2i, where i is the number of gametogenic mitoses, ranging from 0 to an assigned maximum value, d. The fitness of an allele depends on the gamete's mating type because the mean viability of zygotes to which the gamete contributes depends on gamete size allele frequencies in the opposite mating type. For example, using the Levitan equation for gamete survival, the fitness of allele i in mating type plus (+) is
where is the frequency of gamete size allele j in mating type minus (−). Mutation is symmetrical and stepwise within a mating type. For example, gamete size allele 0 mutates with probability μ/2 per generation to allele 1, and allele 1 mutates with probability μ/2 to allele 0 and with probability μ/2 to allele 2.
Changes in allele frequencies after selection, mutation, and genetic drift were tracked using recursion equations. Selection occurs within mating types, as gametes within a mating type compete for fusion with gametes of the opposite mating type. Therefore, the frequency of a gamete size allele on a mating type plus chromosome after selection is
where is the current frequency and is the mean fitness of mating type plus. Genetic drift was simulated by the random sampling of allele counts from a multinomial distribution, with probabilities of success equal to allele frequencies and the number of trials equal to the population size N. Genetic drift was necessary for the evolution of the disassortative fusion of dimorphic gametes because one mating type must become statistically linked to one gamete size (Charlesworth, 1978). Genetic drift was applied to each mating type separately to maintain a “sex ratio” of one. Simulations were initialized with macrogamete isogamy, that is, with both gametes being produced by the direct transformation of a reproductive cell, and with μ = 10−3 and N = 104, and were run for 104 generations. Other values for these parameters gave the same results. The remaining parameters, M, α, and β, were estimated from each anisogamous species of volvocine algae, with α = a and β = S. The maximum number of gametogenic mitoses was set to 9, the maximum observed for the volvocine algae in this dataset. The primary output from a simulation was the gamete size for each mating type.
3.4. Modeling oogamy
The population genetic model was extended to include a locus for gamete motility to allow the evolution of oogamy, in which the microgamete is motile and the macrogamete is nonmotile, as distinct from anisogamy, in which both gametes are motile. Motility was assumed to require a proportion of a gamete's volume, p, so that its facultative size, which affects its viability and contributes to the viability of a zygote, is m(1 − p). This cost of motility is assumed to be proportional to gamete size because the amount of energy and materials required to move a gamete are expected to increase with its size. Nonmotile gametes were assumed to fuse only with motile gametes because of negligible encounter rates between nonmotile gametes in the volvocine algae. Zygote size was calculated from the sum of the facultative sizes of fusing gametes. The fitness of a haplotype (mating type, gamete size, and motility) therefore depended not only on gamete size, but also on whether it was motile; the zygote viability component of fitness for a nonmotile gamete depends on the sizes of motile gametes of the opposite mating type. As before, mutation was symmetrical and stepwise within mating types: Mutation occurred among gamete size alleles within motility types (motile, nonmotile), and between motility types for each gamete size. Simulations were initialized with the largest, motile gametes possible for the species. Initializing simulations with isogametes of other sizes gave the same results. The parameters α and β were estimated from gamete sizes, as before, but were adjusted to account for the reduced facultative size of the microgamete due to motility.
4. RESULTS
4.1. Zygote volume
A general prediction of the disruptive selection theory previously confirmed for the volvocine algae, that zygote (or macrogamete) size increases with adult size (Bell, 1985; Randerson & Hurst, 2001a), is upheld for the 45 species analyzed here (Figure 3). Only 27 of the 45 species are represented in the phylogeny (Figure 2), and therefore, no attempt was made to control for phylogeny. However, it is clear that zygote volume increases with protoplasmic volume, as previously shown (Randerson & Hurst, 2001a). Zygote volume also increases with structural grade, going from colonial to multicellular forms, but this obviously because multicellular forms are larger. The relationship for unicellular forms appears distinct from that of the other structural grades. This may be simply because the protoplasmic volume is also the reproductive cell volume for unicellular species, whereas the reproductive cell volume for colonial and multicellular species is necessarily smaller than the protoplasmic volume. An apparent departure from the zygote‐protoplasmic volume relationship for unicellular species is due to the very large multinucleate unicellular Lobocharacium coloradoense, which is effectively colonial.
Figure 3.
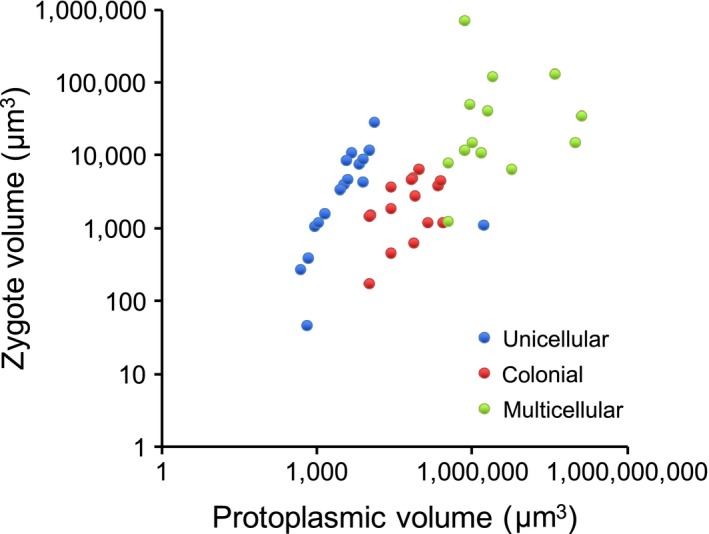
Estimated zygote volume as a function of protoplasmic volume for volvocine algae
4.2. Gamete dimorphism
Another general prediction of the disruptive selection theory previously upheld is that gamete dimorphism increases with structural complexity or adult size (Bell, 1978, 1985; Knowlton, 1974; Madsen & Waller, 1983; Randerson & Hurst, 2001a). Species in all three structural grades exhibit both isogamy and some form of anisogamy, although multicellular species are predominantly oogamous (Table 2). Exceptions to the pattern of unicellular species being isogamous are Chlamydomonas allensworthii and Chlamydomonas suboogama, which are anisogamous, and Chlamydomonas sphagnicola and members of Oogamochlamys, which are oogamous. Colonial species, with colonies containing 4–32 cells, exhibit isogamy and anisogamy. Multicellular species, containing 64–50,000 cells, are anisogamous or oogamous, except for A. gubernaculifera, which is isogamous.
Mapping the origins of coloniality and multicellularity and the origins of anisogamy and oogamy on to a phylogeny of the volvocine algae emphasizes the lack of a simple relationship between structural complexity and gamete dimorphism. Using a composite phylogeny for the species analyzed (Table 2) and additional species in the Tetrabaenaceae, Goniaceae, and Volvocaceae (TGV clade) indicates that coloniality evolved at least twice among these taxa, once in the Stephanosphaera pluvialis lineage and once in the ancestor of the TGV clade (Figure 2). These latter taxa include most of the colonial and multicellular species analyzed (Table 2). Multicellularity evolved at least three times in the TGV clade from a colonial ancestor: once in the Astrephomene lineage, once in the clade containing Volvox globator, and once in the clade containing the remaining Volvox species plus Pleodorina and Eudorina. These origins of multicellularity are consistent with a previous study (Herron et al., 2009) and are supported by having different developmental patterns (Hallmann, 2006; Yamashita et al., 2016). Anisogamy evolved at least twice in this phylogeny, once in the lineage leading to clade including the colonial Platydorina caudata and the related multicellular Volvox species, and once in the lineage leading to the colonial Eudorina and the related multicellular Pleodorina and Volvox. In the latter case, anisogamy is coupled with internal fertilization. Interestingly, in both cases, the phylogeny suggests that anisogamy evolved before multicellularity. Oogamy is estimated to have evolved at least five times in this phylogeny, once in the unicellular Oogamochlamys, apparently from an isogamous ancestor, once from anisogamy in the multicellular V. globator clade, and three times from anisogamous ancestors with internal fertilization in the clades containing the remaining Volvox species. This phylogeny suggests only a weak association between gamete dimorphism and structural complexity. While both origins of anisogamy occurred in colonial lineages, other colonial lineages remained isogamous. Similarly, while oogamy originated independently in the four multicellular Volvox clades, multicellular Pleodorina remained anisogamous, as did multicellular Astrephomene. And finally, the unicellular Oogamochlamys evolved oogamy.
4.3. Anisogamy ratio
Isogamy exists across all three structural grades and across a wide range of protoplasmic volumes (Figure 4). However, a positive correlation between the anisogamy ratio and protoplasmic volume and structural grade is apparent for anisogamous species. Therefore, what is missing is an understanding of why some species are anisogamous, while others with the same structural complexity and size are isogamous. An explanation may come partly from the model of Bulmer and Parker (2002), which shows that for sigmoidal gamete and zygote survival functions, anisogamy is continuously evolutionarily stable when . Thus, ecological conditions that increase β or decrease α may lead to anisogamy independently of adult size or complexity (Bulmer & Parker, 2002) (see Section 5). This prediction is shown here to be equivalent to an anisogamy ratio b/a > 3, which is confirmed for all 21 anisogamous (including oogamous) species; for these species, the anisogamy ratio clusters well above 3 (Figure 4).
Figure 4.
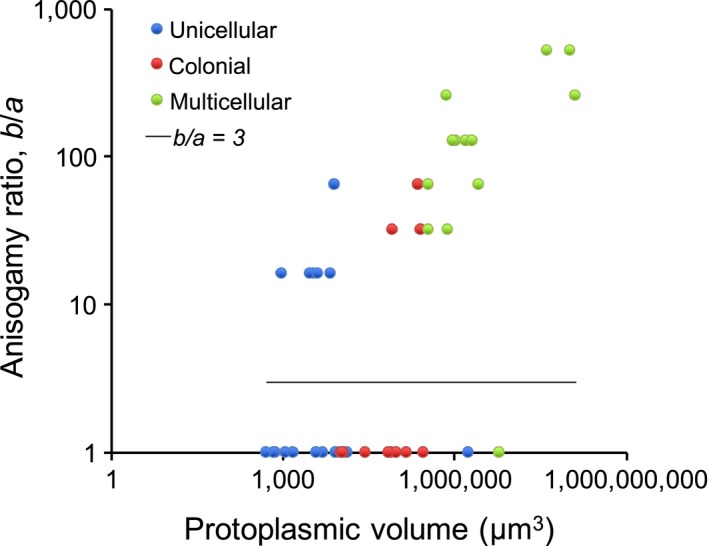
Anisogamy ratio plotted against the protoplasmic volume for volvocine algae. The horizontal line indicates the predicted “anisogamy threshold”
4.4. Population genetic verification and oogamy
For the population genetic model, parameters (M, β, α) were estimated for each anisogamous species (β and α could be estimated only for anisogamous species) and used to simulate gamete size evolution, starting from isogamy. The model accurately predicts the evolution of gamete sizes, and thus anisogamy ratios. It should be noted, however, that this is not an independent test of the Bulmer and Parker (2002) model because β and α are estimated from the data, namely the gamete volumes, a and b. Nevertheless, the population genetic implementation supports the game‐theoretic model and shows that the large‐β approximations of β and α are good. The population genetic implementation also shows that anisogamy could evolve from isogamy by mutation generating variation in gamete size and then genetic drift and selection linking a gamete size to a mating type, resulting in the disassortative fusion of dimorphic gametes.
The population genetic model extended to include gamete motility and with parameters estimated for each anisogamous species in Table 2 predicts the evolution of anisogamy, with both gametes motile, from isogamy for a low cost of motility (a low proportion of gamete size allocated to motility; p = .01). With a moderate cost of motility (p = .1), pseudooogamy, in which the microgamte is motile and ~80% of macrogamtes are nonmotile, evolves. With a high cost of motility (p = .5), oogamy evolves. Therefore, if there is a high proportional cost of motility, the macrogamete, which will bear a greater cost in absolute terms because it is larger, will evolve to be nonmotile, while the microgamete will retain motility to enable gamete contact.
4.5. Cost of sex
Estimation of model parameters for anisogamous species allows calculation of the cost of sex due to anisogamy (Maynard Smith, 1978). Using the Levitan survival function for gamete survival, the fitness for an individual of an anisogamous species at equilibrium is the fitness of the macrogamete producer because the number of zygotes produced is limited by the number of macrogametes:
With hypothetical isogamy for the same species, with isogamete size m = (a + b)/2, gamete fitness is
The fold cost of sex is then
or, with a gamete survival threshold, C = b/m. With a gamete survival threshold, the cost of sex may also be expressed as a function of the anisogamy ratio:
The cost of sex approaches two as the anisogamy ratio increases (Figure 5). The cost is more moderate for more moderate forms of anisogamy because the cost of sex is due mainly to the higher fertility with hypothetical isogamy (M/m > M/b), and this difference is less extreme with moderate anisogamy (m ≈ b). The cost is also ameliorated if gamete survival is a continuous function of gamete size, as represented by the Levitan survival function (Figure 5). This is because with the Levitan function macrogametes experience higher survival than isogametes. Thus, the classic twofold cost of sex may be derived from the model's fitness functions. In addition, it is shown here that the cost increases with the degree of anisogamy and that it is greater if gamete viability is a continuous function of gamete size, rather than dependent on a threshold size.
Figure 5.
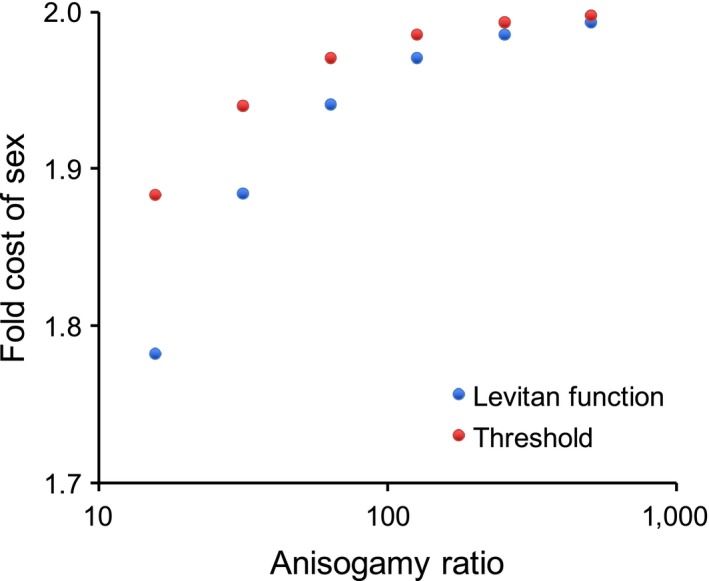
Fold fitness “cost of sex” plotted against the anisogamy ratio for the volvocine algae analyzed. Costs are shown for the Levitan gamete survival function and a gamete survival threshold
5. DISCUSSION
The Bulmer and Parker (2002) game‐theoretic model provides the most complete description of the disruptive selection theory of the evolution of anisogamy available. With sigmoidal survival functions for gametes and zygotes, this model predicts that isogamy becomes evolutionarily unstable, and anisogamy continuously evolutionarily stable, when the survival function scaling parameters have the relation β > 4α. It is shown here that with large β this is equivalent to an anisogamy ratio of b/a > 3, regardless of whether both gamete and zygote survival are described by the Levitan survival function, or whether there is a size threshold for gamete survival. This prediction is borne out for all 21 anisogamous species of volvocine algae analyzed.
The game‐theoretic approach and the use of the large‐β approximation for estimating β and α were verified using a fully population genetic version of the model, which accurately simulates the evolution of gamete sizes for anisogamous species. This model differs from the population genetic models of Charlesworth (1978) mainly in the forms of the zygote and gamete survival functions. The model developed here uses the sigmoidal (Levitan) or threshold survival functions of Bulmer and Parker (2002), whereas Charlesworth assumed that zygote survival is a monotonically increasing function of zygote size and that gamete survival is independent of size. Consideration of the shape of the survival function suggests that a monotonically increasing function is unrealistic and that a sigmoidal function is plausible (Bulmer, Luttikhuizen, & Parker, 2002; Bulmer & Parker, 2002; Randerson & Hurst, 2001b, 2002). Using the parameterization of Bulmer and Parker (2002) also facilitated parameter estimation, as for large β, α and β can be estimated from gamete sizes.
The explanation for the evolution of anisogamy via disruptive selection has been that larger or more complex organisms require more resources for timely development and growth, which selects for a larger zygote, which in turn selects for larger gametes. As gamete size increases, however, one mating type may increase its fitness by producing more numerous, smaller gametes, even if they have a lower probability of survival than larger gametes. Two predictions from this theory, that both zygote size and gamete dimorphism increase with adult size, have previously been partially supported for the volvocine algae (Bell, 1985; Randerson & Hurst, 2001a). The prediction that zygote size increases with adult size is also supported by the present study. However, with additional data on unicellular species incorporated into the present study, a simple relationship between the anisogamy ratio and adult size is no longer clear. Instead, the anisogamy ratio for anisogamous species clusters well above 3, as predicted from the Bulmer and Parker (2002) model, and for these species the anisogamy ratio increases with adult size.
The disruptive selection theory has in the past been interpreted to predict that unicellular species are isogamous and that multicellular species, because of their greater complexity and larger size, are anisogamous. The exceptions to this pattern have cast doubt on the theory's completeness (Bell, 1978; Madsen & Waller, 1983). Therefore, it is useful to examine the exceptions in this dataset. Of the 17 unicellular species studied here, six exhibit gamete dimorphism. These six species are not larger than the isogamous species (Table 2). However, all six species produce the macrogamete directly from the parent (reproductive) cell, without cell division, by intracellular transformation, while producing microgametes by several gametogenic cell divisions. For the isogamous species, both gametes are often produced by one or more gametogenic mitoses. Clearly, intracellular transformation produces the largest possible macrogamete and thus produces a large zygote. For unicellular species, the benefit of a large zygote may be increased survival of the dormant zygospore (Madsen & Waller, 1983) or rapid postzygotic growth (Bell, 1978). Whether these arguments apply to the species under consideration are not known.
Bulmer and Parker (2002) suggest that unicellular species may evolve anisogamy via a reduction in α, rather than an increase in β. That is, under conditions of rapid fertilization, one of the gametes may be small and need to survive only until fertilization. However, this explanation does not appear to fit the unicellular species analyzed here as anisogamy appears to have evolved by an increase in the size of the macrogamete, rather than a reduction in the size of the microgamete.
All of the multicellular species exhibit anisogamy with internal fertilization or oogamy, except A. gubernaculifera, which is isogamous. All the oogamous species produce macrogametes by the intracellular transformation of large reproductive cells, thereby producing large zygotes (Table 2). In contrast, A. gubernaculifera gametes are the product of four sequential gametogenic cell divisions. As a result, A. gubernaculifera produces one of the smallest zygotes of all the multicellular species analyzed, even though its reproductive cells are larger than those of many of the other multicellular species. Therefore, A. gubernaculifera either does not require a large zygote for timely development or requires both gametes to be small and motile, as has been proposed for some marine green algae (Togashi, Bartelt, Yoshimura, Tainaka, & Cox, 2012) (see below). It should be noted that A. gubernaculifera did not evolve multicellularity recently, as sterile soma evolved between 134 and 227 million years ago in the lineage leading to Astrephomene, thus preceding the evolution of multicellularity in the other lineages studied here (Herron et al., 2009). This implies that there would have been ample time for oogamy to evolve in this lineage if it was closely tied to multicellularity.
The phylogenetic analysis confirms that there is only a weak association between oogamy and multicellularity. Oogamy has evolved in the primitively unicellular Oogamochlamys lineage (as well as in the unicellular C. sphagnicola, although the phylogenetic position of this species has not been determined), multicellularity has evolved in the primitively isogamous A. gubernaculifera, and the multicellular Pleodorina have not evolved oogamy. These analyses also show that oogamy is not always preceded by anisogamy, as in the case of the Oogamochlamys lineage, although the steps in character evolution may change as species are added to this poorly studied part of the phylogeny. In contrast, oogamy may have evolved independently three times in Volvox lineages closely related to Pleodorina, having been preceded by the evolution of anisogamy with internal fertilization. This form of anisogamy has been proposed as a route to oogamy in Volvox (Nozaki et al., 2014). However, the independent evolution of oogamy in Volvox closely allied to Platydorina from anisogamy with external fertilization, suggests that this route is not necessary.
The disruptive selection theory has been criticized for ignoring the effects of gamete size on gamete encounter rates under low gamete density or gamete limitation (Cox & Sethian, 1985; Dusenbery, 2000, 2006; Iyer & Roughgarden, 2008). Although gamete limitation may select for anisogamy, its importance is not clear (Lessells et al., 2009; Parker & Lehtonen, 2014). Togashi et al. (2012) describe an individual‐based population genetic model of disruptive selection that also considers gamete collision kinetics. They use this model to explain the evolution of gamete dimorphism in marine green algae living in various environments. For example, they argue that a deep water niche should select for extreme anisogamy, from an ancestral condition of moderate anisogamy, because diminished photosynthesis in deep water selects for a zygote large enough to store sufficient resources for rapid early development. The results presented here suggest that the simpler model of Bulmer and Parker (2002) may be sufficient to explain patterns of gamete dimorphism in the volvocine algae, which have simpler life cycles and inhabit shallow freshwater or moist soil. The parameters of this model could, however, be set to reflect the different environmental conditions specified by Togashi et al. (2012). For example, conditions favoring a large zygote may be reflected in large β. Or, conditions favoring large gametes may be represented by large δ and are expected to result in the “large isogamy” of Togashi et al. (2012) if the optimal gamete size is less than the threshold for gamete viability (m* < δ) (Bulmer & Parker, 2002).
Among the marine green algae listed by Togashi et al. (2012) (their Table S1), 17 of the 18 species in the order Bryopsidales, which generally produce large zygotes (with presumably large β), have anisogamy ratios that are >2.5, and generally much higher, consistent with the prediction derived from the Bulmer and Parker (2002) model. However, some members of other orders exhibit slight anisogamy with anisogamy ratios ≤2. Togashi et al. (2012) explain slight anisogamy as the result of microgametes evolving a phototactic organ (eyespot), which limits their minimum size. It is argued that in these species eyespots evolved in both gametes because these allowed them to move to the two‐dimensional water surface, where they increase their encounter rates compared to the three‐dimensional water column. However, if it can be assumed that the volume occupied by the phototactic organ does not contribute to gamete or zygote survival, then subtracting this volume from both gametes would increase the anisogamy ratio with respect to the facultative volumes of the gametes, because the organ represents a larger proportion of the volume of the smaller gamete. Thus, the evolution of a phototactic organ in microgametes appears to explain slight anisogamy in a manner consistent with the Bulmer and Parker (2002) model. Interestingly, the isogametes of the multicellular A. gubernaculifera possess eyespots (Stein, 1958).
Bulmer and Parker (2002) also consider the case of a minimum volume for a gamete, required, for example, for chromosomes or a phototactic organ, but which does not contribute to the facultative volume affecting viability. In this case, they show that isogamy may be continuously evolutionarily stable for β ≫ 4α, perhaps explaining isogamy in large and complex species of volvocine algae (Figure 4).
Extending the population genetic version of the Bulmer and Parker (2002) model developed here to include a locus for gamete motility shows that oogamy is expected to evolve from isogamy when there is a high cost of motility. The explanation is simply that the proportional gain in facultative size to large gametes from a loss of motility is greater than the gain by small gametes, but one gamete type must remain motile to ensure fusion. This is similar to the explanation offered by Parker et al. (1972). Other hypotheses for the evolution of oogamy, reviewed by Parker (2011) and Lessells et al. (2009), have focused on a presumed increase in swimming speed of smaller gametes and an increase in target area of larger gametes. The inclusion of motility in the population genetic model developed here shows that a simple consideration of the effects of gamete sizes on viabilities may be sufficient to explain the evolution of oogamy. However, nonoogamous anisogamy may be maintained even with costly motility if motility is necessary in both gametes, for example, to allow both gametes to reach the water surface and thereby increase encounter rates (Togashi et al., 2012).
When the disruptive selection theory was first proposed, anisogamy was interpreted as parasitism of macrogamete producers (females) by microgamete producers (males), or as the original conflict between the sexes (Parker et al., 1972). The theory lends itself well to such game theory analysis, in which the evolution of anisogamy is the outcome of the susceptibility of isogamy to invasion by microgametes (Bulmer, 1994; Bulmer & Parker, 2002; Matsuda & Abrams, 1999; Maynard Smith, 1978, 1982). In this context, the cost of sex due to anisogamy (Maynard Smith, 1978), shown here to be consistent with the theory, may be viewed as ultimately the result of genetic conflict. In addition, the cost of sex is shown to depend on the degree of anisogamy and the relationship between gamete viability and gamete size.
Previous studies have generally upheld the common predictions of gamete competition and gamete limitation models, that zygote size and gamete dimorphism increase with adult size or complexity, although noting many exceptions. Here, the prediction that zygote size increases with adult size is confirmed for the volvocine algae, but gamete dimorphism is found to have more complex relationships with adult size and structural complexity. The pattern of gamete dimorphism is partially explained by a prediction specific to the disruptive selection theory that the anisogamy ratio is greater than three for anisogamous species. Consideration of restrictions to the minimal gamete size due to obligate components that do not affect viability may help explain isogamy in large and structurally complex species.
CONFLICT OF INTEREST
None declared.
ACKNOWLEDGMENTS
This work was supported by the School of Biological Sciences, University of Adelaide. Computing resources were provided by eRSA.
da Silva J. The evolution of sexes: A specific test of the disruptive selection theory. Ecol Evol. 2018;8:207–219. https://doi.org/10.1002/ece3.3656
REFERENCES
- Bell, G. (1978). The evolution of anisogamy. Journal of Theoretical Biology, 73, 247–270. https://doi.org/10.1016/0022-5193(78)90189-3 [DOI] [PubMed] [Google Scholar]
- Bell, G. (1985). The origin and early evolution of germ cells as illustrated by the Volvocales In Halvarson H. O. & Monroy A. (Eds.), The origin and evolution of sex (pp. 221–256). New York, NY: Alan R. Liss Inc. [Google Scholar]
- Bulmer, M. (1994). Theoretical evolutionary ecology. Sunderland, MA: Sinauer Associates. [Google Scholar]
- Bulmer, M. G. , Luttikhuizen, P. C. , & Parker, G. A. (2002). Survival and anisogamy. Trends in Ecology & Evolution, 17, 357–358. https://doi.org/10.1016/S0169-5347(02)02537-5 [Google Scholar]
- Bulmer, M. G. , & Parker, G. A. (2002). The evolution of anisogamy: A game‐theoretic approach. Proceedings of the Royal Society of London. Series B: Biological Sciences, 269, 2381–2388. https://doi.org/10.1098/rspb.2002.2161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlesworth, B. (1978). The population genetics of anisogamy. Journal of Theoretical Biology, 73, 347–357. https://doi.org/10.1016/0022-5193(78)90195-9 [DOI] [PubMed] [Google Scholar]
- Cox, P. A. , & Sethian, J. A. (1985). Gamete motion, search, and the evolution of anisogamy, oogamy, and chemotaxis. The American Naturalist, 125, 74–101. https://doi.org/10.1086/284329 [Google Scholar]
- Demchenko, E. , & Mikhailiuk, T. (2012). Peculiarities of sexual reproduction of some new for the flora of Ukraine and rare species of Volvocales (Chlorophyta). International Journal on Algae, 14, 120–135. https://doi.org/10.1615/InterJAlgae.v14.i2.20 [Google Scholar]
- Dusenbery, D. B. (2000). Selection for high gamete encounter rates explains the success of male and female mating types. Journal of Theoretical Biology, 202, 1–10. https://doi.org/10.1006/jtbi.1999.1017 [DOI] [PubMed] [Google Scholar]
- Dusenbery, D. B. (2006). Selection for high gamete encounter rates explains the evolution of anisogamy using plausible assumptions about size relationships of swimming speed and duration. Journal of Theoretical Biology, 241, 33–38. https://doi.org/10.1016/j.jtbi.2005.11.006 [DOI] [PubMed] [Google Scholar]
- Ferris, P. , Olson, B. J. , de Hoff, P. L. , Douglass, S. , Casero, D. , Prochnik, S. , … Umen, J. G. (2010). Evolution of an expanded sex‐determining locus in Volvox . Science, 328, 351–354. https://doi.org/10.1126/science.1186222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng, S. , de Hoff, P. , & Umen, J. G. (2014). Evolution of sexes from an ancestral mating‐type specification pathway. PLoS Biology, 12, e1001904 https://doi.org/10.1371/journal.pbio.1001904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein, M. (1964). Speciation and mating behavior in Eudorina*. The Journal of Protozoology, 11, 317–344. https://doi.org/10.1111/j.1550-7408.1964.tb01762.x [Google Scholar]
- Guiry, M. D. , & Guiry, G. M. (2017). AlgaeBase. Galway, Ireland: National University of Ireland. [Google Scholar]
- Hallmann, A. (2006). Morphogenesis in the family Volvocaceae: Different tactics for turning an embryo right‐side out. Protist, 157, 445–461. https://doi.org/10.1016/j.protis.2006.05.010 [DOI] [PubMed] [Google Scholar]
- Hallmann, A. (2011). Evolution of reproductive development in the volvocine algae. Sexual Plant Reproduction, 24, 97–112. https://doi.org/10.1007/s00497-010-0158-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallmann, A. , Godl, K. , Wenzl, S. , & Sumper, M. (1998). The highly efficient sex‐inducing pheromone system of Volvox . Trends in Microbiology, 6, 185–189. https://doi.org/10.1016/S0966-842X(98)01234-7 [DOI] [PubMed] [Google Scholar]
- Hanschen, E. R. , Marriage, T. N. , Ferris, P. J. , Hamaji, T. , Toyoda, A. , Fujiyama, A. , … Olson, B. J. S. C. (2016). The Gonium pectorale genome demonstrates co‐option of cell cycle regulation during the evolution of multicellularity. Nature Communications, 7, 11370 https://doi.org/10.1038/ncomms11370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, E. H. (2009). The sexual cycle In Harris E. H., Stern D. B. & Witman G. B. (Eds.), The Chlamydomonas sourcebook (2nd ed., pp. 119–157). London, UK: Academic Press. [Google Scholar]
- Hayama, M. , Nakada, T. , Hamaji, T. , & Nozaki, H. (2010). Morphology, molecular phylogeny and taxonomy of Gonium maiaprilis sp nov (Goniaceae, Chlorophyta) from Japan. Phycologia, 49, 221–234. https://doi.org/10.2216/PH09-56.1 [Google Scholar]
- Herron, M. D. , Hackett, J. D. , Aylward, F. O. , & Michod, R. E. (2009). Triassic origin and early radiation of multicellular volvocine algae. Proceedings of the National Academy of Sciences, 106, 3254–3258. https://doi.org/10.1073/pnas.0811205106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herron, M. D. , & Michod, R. E. (2008). Evolution of complexity in the volvocine algae: Transitions in individuality through Darwin's eye. Evolution, 62, 436–451. https://doi.org/10.1111/j.1558-5646.2007.00304.x [DOI] [PubMed] [Google Scholar]
- Hoham, R. W. , Berman, J. D. , Rogers, H. S. , Felio, J. H. , Ryba, J. B. , & Miller, P. R. (2006). Two new species of green snow algae from Upstate New York, Chloromonas chenangoensis sp nov and Chloromonas tughillensis sp nov (Volvocales, Chlorophyceae) and the effects of light on their life cycle development. Phycologia, 45, 319–330. https://doi.org/10.2216/04-103.1 [Google Scholar]
- Ivan, K. , & Katya, V. (2014). A new species Chlorogonium ehrenberg (Haematococcaceae, Chlorophyta) from Bulgaria. Journal of Biological & Scientific Opinion, 2, 298–299. https://doi.org/10.7897/2321-6328 [Google Scholar]
- Iyer, P. , & Roughgarden, J. (2008). Gametic conflict versus contact in the evolution of anisogamy. Theoretical Population Biology, 73, 461–472. https://doi.org/10.1016/j.tpb.2008.02.002 [DOI] [PubMed] [Google Scholar]
- Kaštovský, J. (2008). A report of Stephanosphaera pluvialis COHN 1852 (Chlorophyta, Chlamydophyceae). Fottea, 8, 109–110. https://doi.org/10.5507/fot.2008.006 [Google Scholar]
- Knowlton, N. (1974). A note on the evolution of gamete dimorphism. Journal of Theoretical Biology, 46, 283–285. https://doi.org/10.1016/0022-5193(74)90153-2 [DOI] [PubMed] [Google Scholar]
- Kugrens, P. , Clay, B. L. , & Aguiar, R. (2000). Ultrastructure of Lobocharacium coloradoense, gen. et sp nov (Chlorophyta, Charciciosiphonaceae), an unusual coenocyte from Colorado. Journal of Phycology, 36, 421–432. https://doi.org/10.1046/j.1529-8817.2000.99089.x [Google Scholar]
- Lehtonen, J. , & Kokko, H. (2011). Two roads to two sexes: Unifying gamete competition and gamete limitation in a single model of anisogamy evolution. Behavioral Ecology and Sociobiology, 65, 445–459. https://doi.org/10.1007/s00265-010-1116-8 [Google Scholar]
- Lessells, C. M. , Snook, R. R. , & Hosken, D. J. (2009). The evolutionary origin and maintenance of sperm: Selection for a small, motile gamete mating type In Birkhead T. R., Hosken D. J. & Pitnick S. (Eds.), Sperm biology. London, UK: Academic Press. [Google Scholar]
- Levitan, D. R. (2000). Optimal egg size in marine invertebrates: Theory and phylogenetic analysis of the critical relationship between egg size and development time in echinoids. The American Naturalist, 156, 175–192. https://doi.org/10.1086/303376 [DOI] [PubMed] [Google Scholar]
- Ling, H. U. (2001). Snow algae of the Windmill Islands, continental Antarctica: Desmotetra aureospora, sp nov and D. antarctica, comb. nov (Chlorophyta). Journal of Phycology, 37, 160–174. https://doi.org/10.1046/j.1529-8817.2001.037001160.x [Google Scholar]
- Madsen, J. D. , & Waller, D. M. (1983). A note on the evolution of gamete dimorphism in algae. The American Naturalist, 121, 443–447. https://doi.org/10.1086/284073 [Google Scholar]
- Matsuda, H. , & Abrams, P. A. (1999). Why are equally sized gametes so rare? The instability of isogamy and the cost of anisogamy. Evolutionary Ecology Research, 1, 769–784. [Google Scholar]
- Maynard Smith, J. (1978). The evolution of sex. Cambridge, UK; New York, NY: Cambridge University Press. [Google Scholar]
- Maynard Smith, J. (1982). Evolution and the theory of games. Cambridge, UK; New York, NY: Cambridge University Press; https://doi.org/10.1017/CBO9780511806292 [Google Scholar]
- Nakada, T. , Misawa, K. , & Nozaki, H. (2008). Molecular systematics of Volvocales (Chlorophyceae, Chlorophyta) based on exhaustive 18S rRNA phylogenetic analyses. Molecular Phylogenetics and Evolution, 48, 281–291. https://doi.org/10.1016/j.ympev.2008.03.016 [DOI] [PubMed] [Google Scholar]
- Nedelcu, A. M. , & Michod, R. E. (2006). The evolutionary origin of an altruistic gene. Molecular Biology and Evolution, 23, 1460–1464. https://doi.org/10.1093/molbev/msl016 [DOI] [PubMed] [Google Scholar]
- Nováková, S. (2005). Pseudocarteria corcontica, a new quadriflagellate species (Volvocales). Preslia, 77, 453–457. [Google Scholar]
- Nozaki, H. , & Itoh, M. (1994). Phylogenetic relationships within the colonial Volvocales (Chlorophyta) inferred from cladistic analysis based on morphological data. Journal of Phycology, 30, 353–365. https://doi.org/10.1111/j.0022-3646.1994.00353.x [Google Scholar]
- Nozaki, H. , & Kuroiwa, T. (1990). Volvulina compacta sp. nov. (Volvocaceae, Chlorophyta) from Nepal. Phycologia, 29, 410–417. https://doi.org/10.2216/i0031-8884-29-4-410.1 [Google Scholar]
- Nozaki, H. , & Kuroiwa, T. (1991). Pandorina colemaniae sp. nov. (Volvocaceae, Chlorophyta) from Japan. Phycologia, 30, 449–457. https://doi.org/10.2216/i0031-8884-30-5-449.1 [Google Scholar]
- Nozaki, H. , & Kuroiwa, T. (1992). Ultrastructure of the extracellular matrix and taxonomy of Eudorina, Pleodorina and Yamagishiella gen. nov. (Volvocaceae, Chlorophyta). Phycologia, 31, 529–541. https://doi.org/10.2216/i0031-8884-31-6-529.1 [Google Scholar]
- Nozaki, H. , Ott, F. D. , & Coleman, A. W. (2006). Morphology, molecular phylogeny and taxonomy of two new species of Pleodorina (Volvoceae, Chlorophyceae). Journal of Phycology, 42, 1072–1080. https://doi.org/10.1111/j.1529-8817.2006.00255.x [Google Scholar]
- Nozaki, H. , Yamada, T. , Takahashi, F. , Matsuzaki, R. , & Nakada, T. (2014). New “missing link” genus of the colonial volvocine green algae gives insights into the evolution of oogamy. BMC Evolutionary Biology, 14, 37 https://doi.org/10.1186/1471-2148-14-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker, G. A. (2011). The origin and maintenance of two sexes (anisogamy), and their gamete sizes by gamete competition In Togashi T. & Cox P. A. (Eds.), The evolution of anisogamy: A fundamental phenomenon underlying sexual selection. Cambridge, UK: Cambridge University Press. [Google Scholar]
- Parker, G. A. , Baker, R. R. , & Smith, V. G. (1972). The origin and evolution of gamete dimorphism and the male‐female phenomenon. Journal of Theoretical Biology, 36, 529–553. https://doi.org/10.1016/0022-5193(72)90007-0 [DOI] [PubMed] [Google Scholar]
- Parker, G. A. , & Lehtonen, J. (2014). Gamete evolution and sperm numbers: Sperm competition versus sperm limitation. Proceedings of the Royal Society B: Biological Sciences, 281, 20140836 https://doi.org/10.1098/rspb.2014.0836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocock, M. A. (1955). Studies in North American Volvocales. I. The genus Gonium . Madrono, 13, 49–64. [Google Scholar]
- Randerson, J. P. , & Hurst, L. D. (2001a). A comparative test of a theory for the evolution of anisogamy. Proceedings of the Royal Society of London. Series B: Biological Sciences, 268, 879–884. https://doi.org/10.1098/rspb.2000.1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randerson, J. P. , & Hurst, L. D. (2001b). The uncertain evolution of the sexes. Trends in Ecology & Evolution, 16, 571–579. https://doi.org/10.1016/S0169-5347(01)02270-4 [Google Scholar]
- Randerson, J. P. , & Hurst, L. D. (2002). Survival and anisogamy. Trends in Ecology & Evolution, 17, 358 https://doi.org/10.1016/S0169-5347(02)02538-7 [Google Scholar]
- Ratcliff, W. C. , Herron, M. D. , Howell, K. , Pentz, J. T. , Rosenzweig, F. , & Travisano, M. (2013). Experimental evolution of an alternating uni‐ and multicellular life cycle in Chlamydomonas reinhardtii . Nature Communications, 4, 2742 https://doi.org/10.1038/ncomms3742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, G. M. (1944). A comparative study of the species of Volvox . Transactions of the American Microscopical Society, 63, 265–310. https://doi.org/10.2307/3223302 [Google Scholar]
- Stein, J. R. (1958). A morphological study of Astrephomene gubernaculifera and Volvulina steinii . American Journal of Botany, 45, 388–397. https://doi.org/10.2307/2439639 [Google Scholar]
- Stein, J. R. (1959). The four‐celled species of Gonium . American Journal of Botany, 46, 366–371. https://doi.org/10.2307/2439197 [Google Scholar]
- Suda, S. , Nozaki, H. , & Watanabe, M. M. (2005). Morphology and sexual reproduction of Carteria palmata sp nov belonging to the Carteria group I sensu Lembi (Chlorophyceae, Volvocales). Phycologia, 44, 596–607. https://doi.org/10.2216/0031-8884(2005)44[596:MASROC]2.0.CO;2 [Google Scholar]
- Togashi, T. , Bartelt, J. L. , Yoshimura, J. , Tainaka, K.‐I. , & Cox, P. A. (2012). Evolutionary trajectories explain the diversified evolution of isogamy and anisogamy in marine green algae. Proceedings of the National Academy of Sciences, 109, 13692–13697. https://doi.org/10.1073/pnas.1203495109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togashi T., & Cox P. A. (Eds.) (2011). The evolution of anisogamy: A fundamental phenomenon underlying sexual selection. New York, NY: Cambridge University Press. [Google Scholar]
- Vance, R. R. (1973). On reproductive strategies in marine benthic invertebrates. The American Naturalist, 107, 339–352. https://doi.org/10.1086/282838 [DOI] [PubMed] [Google Scholar]
- Vande Berg, W. J. , & Starr, R. C. (1971). Structure, reproduction and differentiation in Volvox gigas and Volvox powersii . Archiv für Protistenkunde, 113, 195–219. [Google Scholar]
- Wiese, L. , Wiese, W. , & Edwards, D. A. (1979). Inducible anisogamy and the evolution of oogamy from isogamy. Annals of Botany, 44, 131–139. https://doi.org/10.1093/oxfordjournals.aob.a085712 [Google Scholar]
- Yamashita, S. , Arakaki, Y. , Kawai‐Toyooka, H. , Noga, A. , Hirono, M. , & Nozaki, H. (2016). Alternative evolution of a spheroidal colony in volvocine algae: Developmental analysis of embryogenesis in Astrephomene (Volvocales, Chlorophyta). BMC Evolutionary Biology, 16, 243 https://doi.org/10.1186/s12862-016-0794-x [DOI] [PMC free article] [PubMed] [Google Scholar]


