Abstract
Experiences of migratory species in one habitat may affect their survival in the next habitat, in what is known as carryover effects. These effects are especially relevant for understanding how freshwater experience affects survival in anadromous fishes. Here, we study the carryover effects of juvenile salmon passage through a hydropower system (Snake and Columbia rivers, northwestern United States). To reduce the direct effect of hydrosystem passage on juveniles, some fishes are transported through the hydrosystem in barges, while the others are allowed to migrate in‐river. Although hydrosystem survival of transported fishes is greater than that of their run‐of‐river counterparts, their relative juvenile‐to‐adult survival (hereafter survival) can be less. We tested for carryover effects using generalized linear mixed effects models of survival with over 1 million tagged Chinook salmon, Oncorhynchus tshawytscha (Walbaum) (Salmonidae), migrating in 1999–2013. Carryover effects were identified with rear‐type (wild vs. hatchery), passage‐type (run‐of‐river vs. transported), and freshwater and marine covariates. Importantly, the Pacific Decadal Oscillation (PDO) index characterizing cool/warm (i.e., productive/nonproductive) ocean phases had a strong influence on the relative survival of rear‐ and passage‐types. Specifically, transportation benefited wild Chinook salmon more in cool PDO years, while hatchery counterparts benefited more in warm PDO years. Transportation was detrimental for wild Chinook salmon migrating early in the season, but beneficial for later season migrants. Hatchery counterparts benefited from transportation throughout the season. Altogether, wild fish could benefit from transportation approximately 2 weeks earlier during cool PDO years, with still a benefit to hatchery counterparts. Furthermore, we found some support for hypotheses related to higher survival with increased river flow, high predation in the estuary and plume areas, and faster migration and development‐related increased survival with temperature. Thus, pre‐ and within‐season information on local‐ and broad‐scale conditions across habitats can be useful for planning and implementing real‐time conservation programs.
Keywords: cross‐life stage and cumulative effects, delayed mortality, hydropower dams, real‐time monitoring, translocation
1. INTRODUCTION
Carryover effects are often overlooked in management and conservation of migratory species (O'Connor & Cooke, 2015; O'Connor, Norris, Crossin, & Cooke, 2014). These effects are how experiences in one habitat change performance in the next habitat. For example, the amount and quality of food available affect an individual's fat reserves and can in turn affect its later survival and reproductive success. Thus, conservation can be improved if the effect of one habitat on the next is considered. These indirect effects have been documented across wide‐ranging taxa including birds (Duriez, Ens, Choquet, Pradel, & Klaassen, 2012; Studds & Marra, 2005), mammals (Davy et al., 2016), amphibians (Chelgren, Rosenberg, Heppell, & Gitelman, 2006), reptiles (Ceriani et al., 2015), invertebrates (Hettinger et al., 2012), and fishes (Brosnan, Welch, Rechisky, & Porter, 2014; Russell et al., 2012).
To date, most carryover effects documented have been related to early‐life effects on reproductive success (Harrison, Blount, Inger, Norris, & Bearhop, 2011). Survival effects have received less attention because of the challenges in tracking individuals and recording conditions across habitats. Recent technological advances have diminished some of these challenges with increased accessibility to environmental data, usage of biologging, and availability of sufficiently long time series (Bograd, Block, Costa, & Godley, 2010; Drenner et al., 2012). It is now possible for preseason and within‐season management decisions to include these types of information more effectively. In this study, we take advantage of an extensive dataset of tagged fish to examine carryover effects. Nonetheless, the general patterns and concept of carryover effects can be applicable to other migratory species.
Conservation of anadromous fish species is complex because, by definition, their life cycle spans both freshwater and marine environments. While the marine environment exerts broad and strong effects on survival (Mantua, Hare, Zhang, Wallace, & Francis, 1997; Rupp, Wainwright, Lawson, & Peterson, 2012), carryover effects from the river environment are also important (Russell et al., 2012). In the highly human‐modified river system of the Snake and Columbia rivers (Idaho, Washington and Oregon, USA), several evolutionary significant units of salmon and steelhead, Oncorhynchus species (Walbaum) (Salmonidae), are listed under the U.S. Endangered Species Act (NMFS 2010). Hundreds of millions of U.S. dollars are spent annually in conservation efforts to reduce direct mortality during passage through multiple hydropower dams. The Juvenile Fish Transportation Program (USACE 2016) is one of the major conservation efforts designed to mitigate the effects of dam passage (Figure 1). However, the program has mixed success (Dietrich et al., 2016; Holsman, Scheuerell, Buhle, & Emmett, 2012). The direct survival of Chinook salmon through the hydropower system can be increased from 40–60% (DeHart et al., 2015; Faulkner, Widener, Smith, Marsh, & Zabel, 2016) to nearly 100% (McMichael, Skalski, & Deters, 2011). However, transported fish can suffer higher rates of posthydrosystem mortality than their run‐of‐river counterparts (DeHart et al., 2015; Smith, Marsh, Emmett, Muir, & Zabel, 2013). This can result in beneficial and detrimental net effects on adult salmon returns (reviewed in Anderson, Ham, & Gosselin, 2012). The juvenile‐to‐adult survival of transported fish also depends on rear‐types, with generally greater advantages to hatchery fish relative to wild fish. In essence, variation in survival can be attributed to three major factors: rear‐type (wild vs. hatchery), passage‐type (run‐of‐river vs. transported), and conditions experienced.
Figure 1.

Chinook salmon (a) tagged as a juvenile, (b) transported in a barge at Lower Granite Dam, and (c) returned as an adult to Lower Granite Dam. Photograph credit: Benjamin P. Sandford
Linking these factors of salmonid survival involves riverine, estuarine, coastal, and oceanic conditions (Brosnan et al., 2014; Holsman et al., 2012; Miller, Teel, Peterson, & Baptista, 2014; Scheuerell, Zabel, & Sandford, 2009). At the crux of carryover processes is ocean arrival timing of juveniles (or smolts) as they transition from freshwater to marine environments. The timing of the transition is an important predictor of Snake River Chinook salmon survival (Petrosky & Schaller, 2010; Scheuerell et al., 2009). This migration timing is dependent on river temperatures and flows the juveniles experienced. Additionally, temperature affects growth, metabolic rates, development, behavior, and predator–prey interactions (McCullough et al., 2009). Thus, transportation that reduces juvenile river passage from weeks to days can strongly affect their timing of ocean entry, fish condition, and survival.
While mortality is high in the coastal ocean (Brosnan et al., 2014), the greater prey resources also afford higher growth than in the river (Burke et al., 2013; Weitkamp et al., 2015). Growth and survival have been related to indices of local marine conditions, such as an upwelling index (Logerwell, Mantua, Lawson, Francis, & Agostini, 2003; Scheuerell & Williams, 2005) and sea surface temperatures (Drenner et al., 2012; Miller et al., 2014). These processes involve shifts in the abundance and quality of the copepod and ichthyoplankton forage base (Daly, Auth, Brodeur, & Peterson, 2013; Peterson et al., 2014). These occur through variations in the horizontal advection of oceanic surface water to the near habitat, and upwelling within the habitat; both of which vary with the PDO index (Bi, Peterson, & Strub, 2011). Beyond the local environment, broad‐scale variations of the PDO (Mantua et al., 1997), North Pacific Gyre Oscillation (NPGO) (Di Lorenzo et al., 2008), and Multivariate El Niño‐Southern Oscillation (Wolter & Timlin, 1993) indices correlate to measures of adult salmon abundances, survival, productivity, and growth (Burke et al., 2013; Peterson et al., 2014; Rupp et al., 2012; Wells, Grimes, Field, & Reiss, 2006). Thus, understanding and predicting salmonid survival in the early ocean environment require information at local and basin scales, and at seasonal and annual scales (Brosnan et al., 2014; Duffy & Beauchamp, 2011; Weitkamp et al., 2015; Wells et al., 2016).
The goal of this study was to investigate the carryover effects of salmon experience during hydrosystem passage on their juvenile‐to‐adult survival. This study demonstrates that the largest influences on survival involve seasonal river migration timing and the phase of the PDO index. The local freshwater and marine covariates have weaker effects on survival and differ among rear‐types and passage‐types. Most notably, the analysis demonstrates that benefits of the juvenile transportation for wild and hatchery salmon differ and depend on the phase of the PDO index.
2. MATERIALS AND METHODS
We examined Chinook salmon survival with generalized linear mixed effects models (Zuur, Ieno, Walker, Saveliev, & Smith, 2009). We grouped covariates in a cumulative manner that reflected their life cycle from the freshwater to the marine environment: the migration–timing models (MT) incorporated day of year (DOY) of passage at Bonneville Dam (BON) as an index; the freshwater models (MT–FW) added local, seasonal river conditions; the marine models (MT–FW–M) added local, estuarine, plume, and coastal ocean conditions; and the climate models (MT–FW–M–C) added a categorical index of large‐scale, climate‐influenced, marine conditions. Notably, the local covariates tested are collected in real time, and the large‐scale, climate covariate can be predicted at a coarse scale several months in the future (Newman et al., 2016). Therefore, the covariates tested can be used in real time for management of juveniles migrating through the hydropower system, and the marine and climate conditions are not considered without freshwater conditions. Also, our analysis builds on the results from Holsman et al. (2012), Scheuerell et al. (2009), and Satterthwaite et al. (2014) that found migration timing to be an important predictor of survival.
2.1. Data
2.1.1. Fish samples and treatment groups
We analyzed spring/summer runs of Chinook salmon from the Snake River system. These individuals originated above Lower Granite Dam (LGR) and migrated to the ocean in years 1999–2013. We tested different treatment groups by combinations of rear‐type (wild or hatchery) and passage‐type (run‐of‐river or transported). For each treatment group, we modeled its survival from its own dataset. The fish were tagged with passive‐integrated transponder (PIT) tags, and these data are publically available through the Columbia Basin PIT Tag Information System (www.ptagis.org). All run‐of‐river fish in the dataset were detected at BON, the last dam encountered during their outmigration. Transported fish were loaded onto barges at LGR and then transported to a release site downstream of BON. Survival was calculated from juvenile passage at BON or release below BON to adult returns at LGR. LGR was chosen as the adult detection site to account for any increased probability of straying during upstream migration in transported fishes (Bond et al., 2017; Keefer & Caudill, 2014). Thus, juvenile‐to‐adult survival in this study includes successful return of adults to LGR. Furthermore, only juveniles passing BON between DOY 100 and 180 were included in the analysis because of insufficient numbers of fish early and late in the season. See Figures S1 and S2, for yearly sample sizes of juveniles and adults.
2.1.2. Fish and environmental covariates
The fish and freshwater covariates from the first habitat were tested for carryover effects, while marine and climate covariates from the second habitat were tested for direct effects and their moderation of first habitat carryover effects (Table 1).
Table 1.
Model covariates related to each juvenile by day of passage at BON were grouped as migration timing (MT), freshwater (F), marine (M), or climate (C) covariates
| Name | Symbol | Description | Units | Source of data | Covariate group |
|---|---|---|---|---|---|
| Migration–timing index | d | DOY when passage at BON occurred | day | www.ptagis.org/ | MT |
| River temperature | t | Residual effect of river temperature WQM at BON, after controlling for d | °C | http://www.cbr.washington.edu/dart/river.html | F |
| River flow | f | Flow at BON when passage occurred | kcfs | http://www.cbr.washington.edu/dart/river.html | F |
| Sea surface temperature | T | Residual effect of 7‐day rolling mean of sea surface temperature from NDBC buoys (stations lapw1, 46211, 46041, 46029, and 46050), after controlling for d | °C | www.ndbc.noaa.gov or http://www.cbr.washington.edu/dart/buoy_com.html | M |
| Coastal upwelling index | U | 7‐day rolling mean of coastal upwelling index at 45°N 125°W | m3 per second per 100 m of coastline | http://www.pfeg.noaa.gov/ or http://www.cbr.washington.edu/dart/upwell_com.html | M |
| Estuary salt intrusion length | E | Residual effect of 7‐day rolling mean of maximum along channel distance upstream of the river mouth where salinity ≥1 practical salinity unit, after accounting for flow f | km | http://www.stccmop.org/datamart/virtualcolumbiariver/simulationdatabases/climatologicalatlas_db33 | M |
| Plume volume | V | Residual effect of 7‐day rolling mean of plume volume, after accounting for flow f | m3 | http://www.stccmop.org/datamart/virtualcolumbiariver/simulationdatabases/climatologicalatlas_db33 | M |
| Categorical PDO index | I | 1 for favorable ocean conditions with PDO < 0, and 0 for unfavorable ocean conditions with PDO > 0. | unitless | http://research.jisao.washington.edu/pdo/PDO.latest | C |
Migration timing
The covariate DOY of BON passage was tested for linear (i.e., d) and nonlinear (i.e., d 2) patterns (PIT Tag Information System, ptagis.org).
Freshwater covariates
The freshwater covariates were river flow (f) and the residual effect of river temperature (t) measured on the day of passage at BON (U.S. Army Corps of Engineers, accessed via www.cbr.washington.edu/dart/river.html). Because of high correlation between river temperature and d (Table S1), a residual effect t was calculated as residuals from the linear regression between river temperature and d.
Marine covariates
The estuarine and marine environmental covariates were the coastal upwelling index (U) at 45°N 125°W (Pacific Fisheries Environmental Laboratory, www.pfeg.noaa.gov) and the residual effect of mean sea surface temperature (T) across five stations in the coastal ocean near Columbia River (National Data Buoy Center, www.ndbc.noaa.gov) after accounting for the migration timing index d. We also tested the salt intrusion length (E) as a covariate of the Columbia River estuary, and the volume (V) as a covariate of the Columbia River plume (Climatological Atlas for DB33, www.stccmop.org). Covariates E and V are products of the Virtual Columbia River (Baptista et al., 2015) that were computed from numerical simulations of 3D baroclinic circulation (Kärnä & Baptista, 2016). Because the migration timing through the estuary and coastal ocean was not observed, we used a 7‐day rolling mean right‐aligned to d for these marine covariates based on estimates reviewed in Dietrich et al. (2016).
Climate covariate
The PDO index exhibits oscillatory patterns that represent large‐scale marine and climate conditions (Mantua et al., 1997; Newman et al., 2016; Peterson et al., 2014). A negative PDO index represents relatively cool sea surface temperatures along the Pacific Coast. Conversely, a positive PDO index represents relatively warm coastal sea surface temperatures. The cool/warm phases of the PDO index can be predictive of the prey resources and predators (Emmett & Krutzikowsky, 2008; Peterson et al., 2014).
Although we do not know the value of the PDO index that the salmon will experience months into the future, we can at least reasonably predict whether the index will be positive or negative based on recent trends. Newman et al. (2016) showed an autocorrelation of at least 0.5, with a lag of up to 6 months. Such a binary index is reasonable given the strong and divergent effects from opposing climate phases and the presence of thresholds or tipping points (Hunsicker et al., 2016; Samhouri et al., 2017).
We determined a binary PDO index based on the mean PDO index May through September in the year of outmigration. In the model, the negative values of the mean PDO index was scored as I = 1 to represent cool and favorable conditions. Conversely, positive values of the mean PDO index was I = 0 to represent warm and unfavorable conditions.
2.2. Analysis
2.2.1. Survival
The survival predicted from the models, expressed as a probability of a juvenile at BON returning as an adult to LGR (i.e., Bernoulli trial), was estimated with a generalized linear mixed effects model (GLMM; Zuur et al., 2009):
| (1) |
| (2) |
where we have the binary outcome y ij of whether or not individual i (i = 1, …, n) returns as an adult with probability p ij in a Bernoulli distribution, fixed effect intercept β0, random effect intercept b 0j for year j, fixed slope β1 and random slope b 1j in year j for covariate d, fixed slope β2 and random slope b 2j in year j for covariate d 2, and fixed slope βk for covariate k (i.e., all covariates in Table 1, except for d), in which x k is the measured value of covariate k for individual i. Random effects were assumed independent and normally distributed with zero means and constant respective variances σ. Fixed effect covariates, except I, were standardized to a mean of 0 and standard deviation 1 for improved model fitting and convergence.
All possible combinations of covariates in Equation (2) were tested, with the exception that models with a quadratic term (d 2) also included a linear term (d). We tested random intercept effects of year and random slope effects of year for the migration timing covariates (d and d 2) but not for other covariates. Model averaging was determined using the bias‐corrected Akaike information criterion for small sample sizes (AICc; Burnham & Anderson, 2002), and by the weighted average of the predictions , conditional on the covariate being present in model m, , where M is the total number of models (i.e., M = 768), and ωm is the ∆AICc–based weight proportional to 1: . The associated weights were used to determine the 99% confidence set of models for each “rear‐type × passage‐type” treatment group. To assess the relative importance of models at each cumulative grouping of covariates, we determined the weights for models associated with each grouping while excluding models from lower‐level groupings (e.g., MT–FW–M grouping excluded models in MT and MT–FW groupings). The model‐averaged parameters and relative importance of covariates across models in the 99% confidence set were determined. The analysis was conducted in R© 2016 The R Foundation for Statistical Computing (version 3.3.2) with the glmer function from the lme4 package (version 1.1‐12) and the model.avg function from the MuMIn package (version 1.15.6).
2.2.2. Effectiveness of transportation program
A ratio of survival (S) for transported to run‐of‐river fish (i.e., differential delayed mortality, D = S transport /S run‐of‐river), characterizes the effectiveness of the juvenile fish transportation program after fish have passed BON (reviewed in Anderson et al., 2012). Thus, D > 1 indicates an advantage from transportation on posthydrosystem survival, while D = 1 indicates no effect, and D < 1 indicates a detrimental effect. To determine the effect of transportation from LGR on survival, relative to that of run‐of‐river counterparts, we would need to incorporate the hydropower system survival of transported fish approximating 100% (McMichael et al., 2011) and the hydropower system survival of run‐of‐river fish approximating 50% (DeHart et al., 2015; Faulkner et al., 2016). D was thus compared to a threshold of 0.5 instead of 1 (reviewed in Anderson et al., 2012).
Patterns of D were determined from simulations based on our model‐averaged GLMMs of survival. We simulated parameters from the sampling distributions of the maximum likelihood estimates from our GLMMs, where the sampling distributions were assumed to be multivariate normal with means equal to the estimated regression parameters and covariances equal to the associated estimated covariance matrices for the parameters. These simulations were run in R© 2016 The R Foundation for Statistical Computing (version 3.3.2) with the sim function in the arm package (version 1.9‐3). Furthermore, to obtain model‐averaged survival estimates, a simulation was run for each candidate model of the confidence set, and the predicted survival probabilities were weighted by ωm accordingly. To determine patterns of D with fixed effects only, we first generated a set of 1,000 model‐averaged simulations for each passage‐type and rear‐type combination based on draws of fixed effect parameters only. Then, we also included the random effects from the simulated sets estimated for the 15 years of data. Finally, to visualize patterns of D in cool and warm PDO years separately, we applied the simulated parameters to the average observed values of covariates across years with I = 1 or 0, respectively.
3. RESULTS
Across both rear‐types and both passage‐types, the PDO index Ι and migration timing indices d and/or d 2 were the most influential predictors (Figures 2 and 3; Table 2). Interannual differences in survival were thus explained by Ι, in which the cool phase implies favorable ocean conditions. The seasonal survival patterns were captured by d 2 for all rear‐type and passage‐type combinations, except wild run‐of‐river Chinook salmon. The model‐averaged fits thus generally showed a dome‐shaped pattern but also other nonlinear patterns (Figures 2 and 4; Figures S3–S5). Starting in mid‐April, survival generally increased until about mid‐May and decreased thereafter through June. In contrast, wild run‐of‐river Chinook survival declined through the season. Overall, Ι characterizes the interannual survival variation, and d and d 2 characterize seasonal survival variations.
Figure 2.
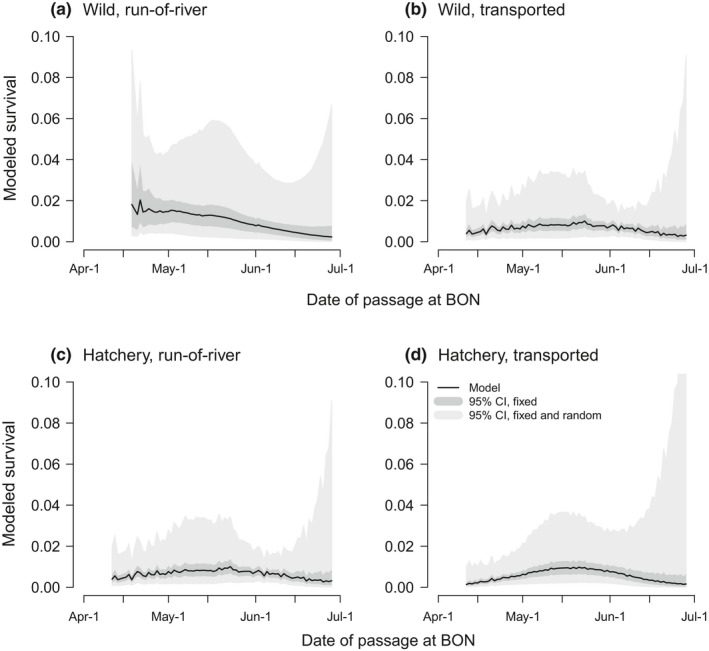
Modeled survival through outmigration seasons 1999–2013 for wild/hatchery, run‐of‐river/transported Chinook salmon. CI represents confidence interval. See Figure 3 for model parameters and relative importance of covariates
Figure 3.
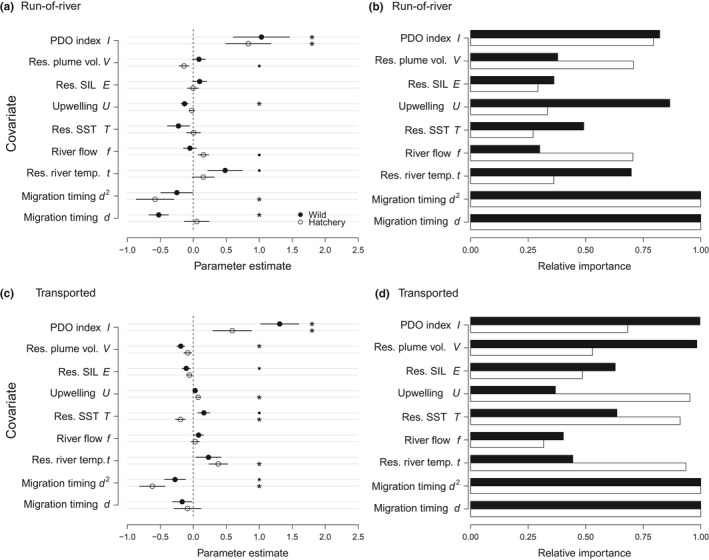
Standardized parameter estimates and relative importance of covariates in model‐averaged generalized linear mixed effects modeling of survival for run‐of‐river and transported Chinook salmon. Covariates are described in Table 1. Error bars represent standard deviation. Statistical significance denoted as * for p < .05 and • for p < .1
Table 2.
Number of models, minimum and maximum ∆AICc, and weight for the 99% confidence set, and all models tested in parentheses. Results reported are for models at each cumulative grouping of covariates (i.e., MT, MT–FW, MT–FW–M, and MT–FW–M–C), excluding models in lower‐level groupings. For each rear‐type and passage‐type combination, the cumulative grouping of covariates with greatest weight is bolded
| Cumulative grouping of covariates | Number of models | Minimum ΔAICc | Maximum ΔAICc | Weight |
|---|---|---|---|---|
| (a) Wild, run‐of‐river Chinook | ||||
| MT | 1 (6) | 7.255 (7.255) | 7.255 (65.529) | 0.0022 |
| MT‐FW | 3 (18) | 8.118 (8.118) | 9.918 (65.226) | 0.0028 |
| MT‐FW‐M | 56 (360) | 2.632 (2.632) | 12.260 (69.448) | 0.1693 |
| MT‐FW‐M‐C | 78 (384) | 0.000 (0.000) | 12.389 (67.506) | 0.8158 |
| (b) Wild, transported Chinook | ||||
| MT | 0 (6) | – (25.207) | – (97.816) | 0.0000 |
| MT‐FW | 0 (18) | – (19.219) | – (98.612) | 0.0000 |
| MT‐FW‐M | 0 (360) | – (11.365) | – (104.495) | 0.0000 |
| MT‐FW‐M‐C | 40 (384) | 0.000 (0.000) | 9.575 (100.776) | 0.9903 |
| (c) Hatchery, run‐of‐river Chinook | ||||
| MT | 1 (6) | 10.850 (10.850) | 10.850 (80.327) | 0.0004 |
| MT‐FW | 3 (18) | 3.987 (3.987) | 10.387 (61.156) | 0.0176 |
| MT‐FW‐M | 53 (360) | 2.808 (2.808) | 10.964 (81.404) | 0.1831 |
| MT‐FW‐M‐C | 75 (384) | 0.000 (0.000) | 11.068 (80.534) | 0.7890 |
| (d) Hatchery, transported Chinook | ||||
| MT | 0 (6) | – (20.079) | – (577.703) | 0.000 |
| MT‐FW | 0 (18) | – (16.441) | – (577.987) | 0.000 |
| MT‐FW‐M | 26 (360) | 1.317 (1.317) | 9.464 (581.162) | 0.3085 |
| MT‐FW‐M‐C | 40 (384) | 0.000 (0.000) | 9.2401 (578.800) | 0.6816 |
Figure 4.
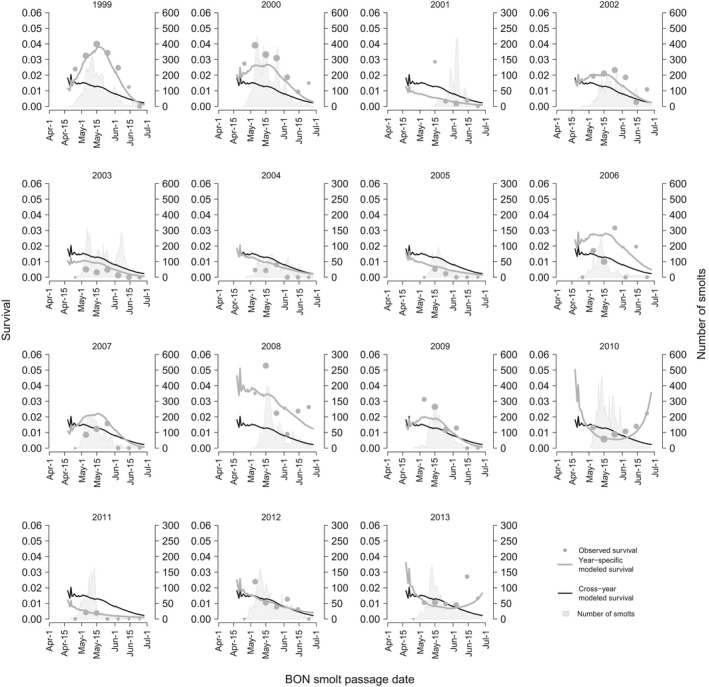
Wild, run‐of‐river Chinook salmon survival observed (passive‐integrated transponder‐tagged) and modeled (model‐averaged generalized linear mixed effects model, GLMM) estimates for each outmigration season 1999–2013. Gray points represent weekly observed estimates of survival. The size of points is representative of weekly juvenile sample sizes, as denoted numerically by light gray shading of daily smolt run. See Figures S3–S5 for other rear‐types and passage‐types of Chinook salmon
While the interannual and seasonal variations (Figures 4, S3–S5) were largely captured by Ι and d, local riverine and marine seasonal covariates also contributed to the variations (Figure 3). The wild run‐of‐river Chinook salmon survival (Figure 3a,b) was influenced positively by residual river temperature t and negatively by upwelling index U. Thus, wild fish appeared to benefit from both relatively warmer temperatures in the river and sea surface as weak upwelling can result in warmer coastal sea surface temperatures. Note that the temperature index is a residual after accounting for the negative effects from later migration timing and correlated warmer temperatures. The hatchery run‐of‐river survival was influenced positively by flow f and negatively by residual plume volume V after accounting for flow. These patterns suggest that wild Chinook salmon are responding to temperature‐related covariates, while the hatchery run‐of‐river fish are responding to flow‐related covariates.
For transported Chinook salmon (Figure 3c,d), wild fish survival was influenced by local marine covariates. In contrast, hatchery fish survival was influenced by temperature‐related covariates in both river and marine environments. Specifically, wild fish benefited from a positive residual sea surface temperature T after accounting for migration timing. The positive effect of T may involve faster migration rates and development. Also, the negative effect from the residual plume volume V suggests that the plume is an area of relatively high mortality. For the hatchery counterparts, survival improved with a positive residual river temperature t, a negative residual sea surface temperature T, and a positive upwelling index U. Together, these patterns show differing effects on survival across rear‐types and passage‐types.
The benefits and detriments of transportation represented by D were different for wild and hatchery Chinook salmon (Figure 5a,b). The value of transportation for wild Chinook went from detrimental to beneficial over the migration season. In contrast, transportation generally benefited hatchery Chinook across the season. The advantages of transportation were clearer when including the hydropower system survival and comparing D to a threshold of 0.5. Yet, detrimental effects from transportation to wild Chinook salmon were still apparent in April. When including the simulated random effects, a seasonal increase in D remained in wild fish (Figure 5c), whereas hatchery fish showed seasonally decreasing, increasing, and flat patterns (Figure 5d). The range of uncertainty in D from the simulations including random effects spanned across 1 and 0.5 (i.e., no clear benefit or detriment of transportation) throughout the season for both wild and hatchery rear‐types (Figure 5c,d). Thus, other influences on survival that were not explicitly tested in this study were captured by the random effects.
Figure 5.
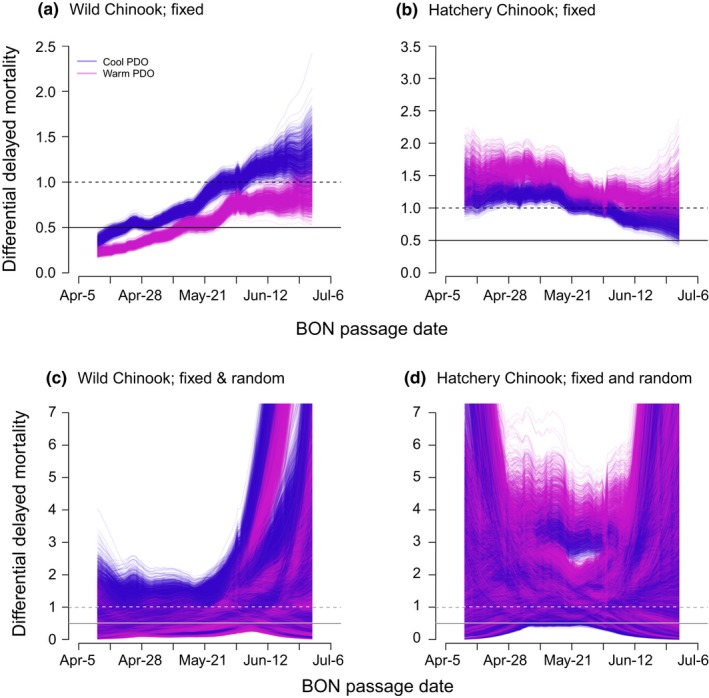
Differential delayed mortality (D = S transport /S run‐of‐river) across cool/warm PDO phases simulated from the model‐averaged GLMM of Chinook salmon survival (S) with (a and b) fixed effects parameters only, and (c and d) fixed effects and random effects parameters, for wild and hatchery rear‐types. Horizontal lines represent thresholds for which an advantage or disadvantage of transportation occurs in survival after the hydropower system (i.e., D = 1), or inclusive of the hydropower system (D ≈ 0.5)
4. DISCUSSION
Conservation efforts across wide‐ranging taxa can be improved by considering ecological carryover effects across life stages (O'Connor & Cooke, 2015). For example, experiences during rearing, overwintering, and migration in the first habitat can result in changes in performance and survival in later life stages. The effects could involve physiological processes (Davy et al., 2016; McKinnon, Stanley, & Stutchbury, 2015; Midwood, Larsen, Boel, Aarestrup, & Cooke, 2015), genetic influences (Ceriani et al., 2015), and conservation practices (Holsman et al., 2012). Figure 6 demonstrates the complexity of interactions.
Figure 6.
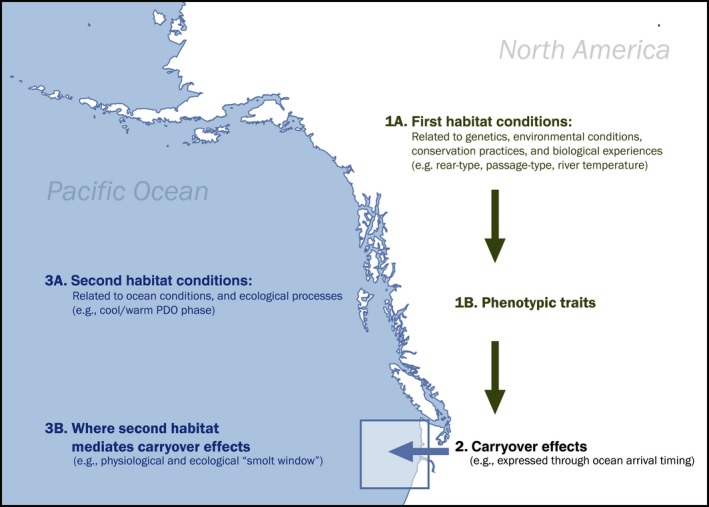
Conceptual diagram of freshwater carryover effects into the ocean with examples related to salmonids migrating through a hydropower system: (1a) First habitat experiences (1b) can be expressed as phenotypic traits (2) that can carry over into the next habitat. (3a) The strength of selection on the pool of traits will then depend on the conditions in the new habitat, (3b) particularly when juveniles leave the freshwater environment and enter the ocean
The concept of carryover effects is particularly powerful for the restoration of Columbia River salmonids. While many studies from the Columbia River Basin tested ocean conditions as a covariate to Chinook salmon productivity and abundances (Burke et al., 2013; Petrosky & Schaller, 2010), few have looked at interactions among freshwater and ocean covariates on survival linked to migration timing (Holsman et al., 2012). Our current study identified that the relative benefit of transportation on survival depends on the cool/warm PDO phase (Figure 5).
4.1. Transportation decisions
From a carryover effects perspective, we demonstrated that the effectiveness of transporting juvenile Chinook salmon on subsequent life stage survival differed among rear‐types. Transporting wild juveniles had a greater positive impact on their survival in cool than warm PDO years. Given general favorable conditions during cool years, earlier ocean arrival timing can result in greater opportunity to reach higher growth rates than that conferred in the river environment (Weitkamp et al., 2015). As well, conditions experienced during barge transportation may be more stressful to wild fish in warm than cool years. Effects can include disease (Dietrich et al., 2011) and stress from cotransportation with juvenile steelhead (Sandford, Zabel, Gilbreath, & Smith, 2012).
In contrast, transportation was more beneficial to hatchery Chinook salmon in warm than cool years. It is possible that transportation helped to minimize stressful exposure of hatchery juveniles to lower flow and warmer river conditions that generally occur in years with a positive (warm) PDO index (Mote, 2003; current study). This pattern supports the hypothesis that reducing stressful conditions experienced by juveniles in one life stage can result in higher survival in later life stages. The opposing pattern between rear‐types may occur because hatchery fish do not survive as well as wild fish in the early marine environment during years of poor ocean conditions (Beamish et al., 2012). Hatchery fish may need additional relief from stressful hydrosystem conditions that then carry over to benefit survival in subsequent life stages. Additionally, harvest is more intense on hatchery fish. Although we are not aware of differential harvest between transported and run‐of‐river fish, this may contribute to the rear‐type differences observed.
Differing patterns among rear‐types, populations, and species can make decisions about conservation strategies challenging. Generally, wild fish are the focus of conservation, and hatchery fish are produced to help enhance fishery production (Naish et al., 2007). Thus, the decision to transport in cool years could be more heavily weighted in favor of the positive effects on wild fish, but at some cost to the survival of hatchery fish. The effects of transportation on survival in hatchery Chinook salmon are generally positive or neutral (i.e., D ≥ 1) across warm and cool PDO years. Thus, transporting hatchery Chinook salmon in cool years is not necessarily a counterproductive mitigation strategy for hatchery fish, but rather a lost opportunity to increase their posthydrosystem survival. Overall, the PDO index can serve as an annual baseline to help predict whether transportation will be a beneficial or disadvantageous conservation strategy.
4.2. Annual patterns
The basis for large‐scale effects of the PDO index stems from relationships of climate indices with oceanographic and ecosystem processes. Local ecosystem dynamics (e.g., high‐lipid copepods at lower trophic levels relate to higher salmon survival) are linked to large‐scale oceanographic forcings as indexed by the PDO (Bi et al., 2011). As well, salmon recruitment links to NPGO through food web processes and feeding ecology (Hertz et al., 2016). Multiple ecological pathways link large‐scale climate indices to salmon recruitment, but the PDO appears to be more influential than the NPGO or Oceanic Niño indices (Malick, Cox, Peterman, Wainwright, & Peterson, 2015). Our study extends the importance of the PDO index as a mediator of carryover effects. Although specific ocean conditions are difficult to forecast, our study shows that even a categorical climate index provides practical and actionable information for decision makers.
Forecasting ocean conditions undoubtedly provides information for decisions on salmonid conservation. For example, Chittenden et al. (2010) suggested that upwelling forecasts are useful for timing of hatchery releases to improve marine survival. However, the timeliness, accuracy, and certainty of forecasts are essential for effective conservation. Implementation of the information from the current study will require real‐time or forecasted fish, river, and ocean data. Also of value is forecasting a simple categorical index of a positive or negative PDO phase. This is possible given the high degree of autocorrelation within a 6‐month window and ongoing advances in oceanography (Di Lorenzo et al., 2013; Newman et al., 2016).
4.3. Seasonal patterns
In addition to the annual, large‐scale conditions of the ocean, there are within‐season effects. Anadromous fishes have evolved to enter the ocean at a time of favorable conditions (i.e., physiological and ecological “smolt window”; McCormick, Hansen, Quinn, & Saunders, 1998; Thorstad et al., 2012). Correspondingly, migration timing is a major determinant of survival for salmonids (Jonsson & Jonsson, 2014; McCormick et al., 1998; Scheuerell et al., 2009). In the river, migration timing can be related to temperature and flow (McCormick et al., 1998; Petrosky & Schaller, 2010; Scheuerell et al., 2009; Thorstad et al., 2012). More specifically, increased river temperatures can result in faster physical and physiological changes and earlier migration (Russell et al., 2012; Sykes, Johnson, & Shrimpton, 2009; Zydlewski, Haro, & McCormick, 2005). At ocean entrance, timing can be an index of both the environmental conditions and the predator and prey communities the juveniles encounter (Emmett, Krutzikowsky, & Bentley, 2006; Hvidsten et al., 2009; Logerwell et al., 2003; Wells et al., 2016). Although a detailed study of biological and ecological processes that affect juvenile‐to‐adult survival was beyond the scope of the study, we identified that migration timing continues to be a covariate of significant importance.
Further research on processes underlying migration timing and survival will arm decision makers with information to improve conservation strategies. Numerous studies revealed that freshwater–marine carryover effects on salmon survival generally involve physiological development (Drenner et al., 2012; Russell et al., 2012), fish size (Jonsson & Jonsson, 2014; Zabel & Achord, 2004), and growth‐ and size‐selective mortality (Miller et al., 2014; Woodson et al., 2013). Altogether, these and other studies emphasize the importance of juveniles entering the ocean in optimal physiological and ecological conditions (Hvidsten et al., 2009; McCormick et al., 1998; Wells et al., 2016). One common underlying factor related to these physiological and ecological processes is temperature. Increasing temperatures over the last several decades correspond to juveniles migrating earlier and at smaller sizes and younger ages, particularly at northern latitudes (Kovach, Joyce, Echave, Lindberg, & Tallmon, 2013; Otero et al., 2014; Russell et al., 2012). Thus, understanding mechanisms of migration timing will be especially important with river and ocean environments warming at different rates as climate changes (IPCC, 2014). Notably, correlations between river migration timing and ocean survival will likely change (Kennedy & Crozier, 2010).
The seasonal covariates in this study have been highlighted in other studies: higher survival with increased river flow, faster migration and development with increased temperature, and high predation in the estuary and plume areas (Brosnan et al., 2014; McCullough et al., 2009; Petrosky & Schaller, 2010). Yet, much uncertainty remains with the random effects of year and their interactions with migration timing. Other modeling approaches that capture more complex ecosystem dynamics may improve forecasting carryover effects. These include dynamic linear modeling (Scheuerell & Williams, 2005), nonlinear models with generalizable thresholds for management (Hunsicker et al., 2016), and models of intermediate complexity that incorporate data closely reflecting bottom‐up and top‐down processes (Wells et al., 2017).
In addition, the patterns of carryover effects captured in random effects can be further clarified by separating the adult upstream migration life stage from the ocean life stage. Increased rates of straying are known to occur for transported fishes (Bond et al., 2017; Keefer & Caudill, 2014). We used a detection site of adult returns past known locations of straying to account for this behavior. However, river conditions during upstream migration can stimulate straying (e.g., for thermal refuge). The lack of covariates during upstream migration could explain some of the patterns expressed as random effects in our study. There are thus direct effects of conditions experienced in each habitat and carryover effects from a previous habitat that are mediated by conditions in the current habitat.
4.4. Final thoughts
A growing concern in conservation is larger and more frequent mismatches between migration timing and timing of resources in subsequent habitats (Both, Bouwhuis, Lessells, & Visser, 2006; O'Connor et al., 2014). To address this concern effectively, our study showed that the effects of migration timing on survival need to be interpreted in context of carryover effects. Furthermore, migration timing can be an index of underlying processes involving the timing, quantity, and quality of resources, competitors, and predators across habitats. Thus, data on migration timing and the underlying processes can be particularly important information for deciding when and how to release juveniles in coastal fishery practices (e.g., restocking, stock enhancement, and sea ranching; Bartley & Bell, 2008). Explicitly considering carryover effects with pre‐ and within‐season data can help target conservation efforts more effectively. As shown in our study, considering large‐scale marine conditions helped to identify which years and when in the season it is more effective to transport juveniles. Applying large‐scale ocean forecasts with knowledge of stock‐ and passage‐specific carryover effects may provide a useful and practical strategy for buffering the effects of a changing climate on anadromous fishes.
DATA ACCESSIBILITY
The datasets used in this manuscript are available at Columbia River Basin, School of Aquatic and Fishery Sciences, University of Washington (www.cbr.washington.edu).
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
JG, JF, and RZ conceived the ideas and designed methodology. BS queried the PIT‐tagged fish data. AB modeled the estuary and plume data. JG queried the other environmental covariates and analyzed the data. JG, JA, and RZ led the writing of the manuscript. All authors contributed critically to the drafts and gave final approval for publication.
Supporting information
ACKNOWLEDGEMENTS
We are grateful for the financial support from the Bonneville Power Administration, Department of Energy (00058850, 00062996, 00070642 and 00077308), and the Northwest Fisheries Science Center, NOAA Fisheries (RA133F11SE1714). We thank Eric Buhle for his advice on statistical modeling, and Bror Jonsson, Mark Scheuerell, Brian Wells, and an anonymous reviewer for their valuable comments that helped strengthen the manuscript and impact of ideas. We thank Susannah Iltis and Chris Van Holmes for additional help with the PIT tag datasets. Last but not least, we thank Kevin Gosselin for producing Figure 6.
Gosselin JL, Zabel RW, Anderson JJ, Faulkner JR, Baptista AM, Sandford BP. Conservation planning for freshwater–marine carryover effects on Chinook salmon survival. Ecol Evol. 2018;8:319–332. https://doi.org/10.1002/ece3.3663
REFERENCES
- Anderson, J. , Ham, K. , & Gosselin, J. (2012), Snake River Basin Differential Delayed Mortality Synthesis. Report No. PNWD‐4283. Battelle, Pacific Northwest Division, Richland, WA. pp. 126.
- Baptista, A. M. , Seaton, C. , Wilkin, M. P. , Riseman, S. F. , Needoba, J. A. , Maier, D. , … Simon, H. M. (2015). Infrastructure for collaborative science and societal applications in the Columbia River estuary. Frontiers of Earth Science, 9, 659–682. https://doi.org/10.1007/s11707-015-0540-5 [Google Scholar]
- Bartley, D. M. , & Bell, J. D. (2008). Restocking, stock enhancement, and sea ranching: Arenas of progress. Reviews in Fish Biology and Fisheries, 16, 357–365. https://doi.org/10.1080/10641260701678058 [Google Scholar]
- Beamish, R. J. , Sweeting, R. M. , Neville, C. M. , Lange, K. L. , Beacham, T. D. , & Preikshot, D. (2012). Wild chinook salmon survive better than hatchery salmon in a period of poor production. Environmental Biology of Fishes, 94, 135–148. https://doi.org/10.1007/s10641-011-9783-5 [Google Scholar]
- Bi, H. , Peterson, W. T. , & Strub, P. T. (2011). Transport and coastal zooplankton communities in the northern California Current system. Geophysical Research Letters, 38, L12607. [Google Scholar]
- Bograd, S. J. , Block, B. A. , Costa, D. P. , & Godley, B. J. (2010). Biologging technologies: New tools for conservation. Introduction. Endangered Species Research, 10, 1–7. https://doi.org/10.3354/esr00269 [Google Scholar]
- Bond, M. H. , Westley, P. A. H. , Dittman, A. H. , Holecek, D. , Marsh, T. , & Quinn, T. P. (2017). Combined effects of barge transportation, river environment, and rearing location on straying and migration of adult Snake River fall‐run Chinook salmon. Transactions of the American Fisheries Society, 146, 60–73. https://doi.org/10.1080/00028487.2016.1235614 [Google Scholar]
- Both, C. , Bouwhuis, S. , Lessells, C. M. , & Visser, M. E. (2006). Climate change and population declines in a long‐distance migratory bird. Nature, 441, 81–83. https://doi.org/10.1038/nature04539 [DOI] [PubMed] [Google Scholar]
- Brosnan, I. G. , Welch, D. W. , Rechisky, E. L. , & Porter, A. D. (2014). Evaluating the influence of environmental factors on yearling Chinook salmon survival in the Columbia River plume (USA). Marine Ecology Progress Series, 496, 181–196. https://doi.org/10.3354/meps10550 [Google Scholar]
- Burke, B. J. , Peterson, W. T. , Beckman, B. R. , Morgan, C. A. , Daly, E. A. , & Litz, M. (2013). Multivariate models of adult Pacific salmon returns. PLoS One, 8, e54134 https://doi.org/10.1371/journal.pone.0054134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnham, K. P. , & Anderson, D. R. (2002). Model selection and multimodel inference: A practical Information‐theoretic Approach. New York, NY: Springer‐Verlag. [Google Scholar]
- Ceriani, S. A. , Roth, J. D. , Tucker, A. D. , Evans, D. R. , Addison, D. S. , Sasso, C. R. , … Weishampel, J. F. (2015). Carry‐over effects and foraging ground dynamics of a major loggerhead breeding aggregation. Marine Biology, 162, 1955–1968. https://doi.org/10.1007/s00227-015-2721-x [Google Scholar]
- Chelgren, N. D. , Rosenberg, D. K. , Heppell, S. S. , & Gitelman, A. I. (2006). Carryover aquatic effects on survival of metamorphic frogs during pond emigration. Ecological Applications, 16, 250–261. https://doi.org/10.1890/04-0329 [DOI] [PubMed] [Google Scholar]
- Chittenden, C. M. , Jensen, J. L. A. , Ewart, D. , Anderson, S. , Balfry, S. , Downey, E. , … McKinley, R. S. (2010). Recent salmon declines: A result of lost feeding opportunities due to bad timing? PLoS One, 5, e12423 https://doi.org/10.1371/journal.pone.0012423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly, E. A. , Auth, T. D. , Brodeur, R. D. , & Peterson, W. T. (2013). Winter ichthyoplankton biomass as a predictor of early summer prey fields and survival of juvenile salmon in the northern California Current. Marine Ecology Progress Series, 484, 203–217. https://doi.org/10.3354/meps10320 [Google Scholar]
- Davy, C. M. , Mastromonaco, G. F. , Riley, J. L. , Baxter‐Gilbert, J. H. , Mayberry, H. , & Willis, C. K. R. (2016). Conservation implications of physiological carry‐over effects in bats recovering from white‐nose syndrome. Conservation Biology, 31, 615–624. [DOI] [PubMed] [Google Scholar]
- DeHart, M. , McCann, J. , Chockley, B. , Cooper, E. , Schaller, H. , Haeseker, S. , … Ehlke, R. (2015). Comparative Survival Study of PIT‐tagged Spring/Summer/Fall Chinook, Summer Steelhead, and Sockeye; 2015 Annual Report. Comparative Survival Study Oversight Committee and Fish Passage Center, Portland, Oregon. pp. 496.
- Di Lorenzo, E. , Combes, V. , Keister, J. E. , Strub, P. T. , Thomas, A. C. , Franks, P. J. S. , … Carolina, P. (2013). Synthesis of Pacific Ocean climate and ecosystem dynamics. Oceanography, 26, 68–81. https://doi.org/10.5670/oceanog [Google Scholar]
- Di Lorenzo, E. , Schneider, N. , Cobb, K. M. , Franks, P. J. S. , Chhak, K. , Miller, A. J. , … Rivère, P. (2008). North Pacific Gyre Oscillation links ocean climate and ecosystem change. Geophysical Research Letters, 35, 1–6. [Google Scholar]
- Dietrich, J. P. , Boylen, D. A. , Thompson, D. E. , Loboschefsky, E. J. , Bravo, C. F. , Spangenberg, D. K. , … Loge, F. J. (2011). An evaluation of the influence of stock origin and out‐migration history on the disease susceptibility and survival of juvenile Chinook salmon. Journal of Aquatic Animal Health, 23, 35–47. https://doi.org/10.1080/08997659.2011.568859 [DOI] [PubMed] [Google Scholar]
- Dietrich, J. , Eder, K. , Thompson, D. , Buchanan, R. , Skalski, J. , McMichael, G. , … Loge, F. (2016). Survival and transit of in‐river and transported yearling Chinook salmon in the lower Columbia River and estuary. Fisheries Research, 183, 435–446. https://doi.org/10.1016/j.fishres.2016.07.005 [Google Scholar]
- Drenner, S. M. , Clark, T. D. , Whitney, C. K. , Martins, E. G. , Cooke, S. J. , & Hinch, S. G. (2012). A synthesis of tagging studies examining the behaviour and survival of anadromous salmonids in marine environments. PLoS One, 7, e31311 https://doi.org/10.1371/journal.pone.0031311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy, E. J. , & Beauchamp, D. A. (2011). Rapid growth in the early marine period improves the marine survival of Chinook salmon (Oncorhynchus tshawytscha) in Puget Sound, Washington. Canadian Journal of Fisheries and Aquatic Sciences, 68, 232–240. https://doi.org/10.1139/F10-144 [Google Scholar]
- Duriez, O. , Ens, B. J. , Choquet, R. , Pradel, R. , & Klaassen, M. (2012). Comparing the seasonal survival of resident and migratory oystercatchers: Carry‐over effects of habitat quality and weather conditions. Oikos, 121, 862–873. https://doi.org/10.1111/j.1600-0706.2012.20326.x [Google Scholar]
- Emmett, R. L. , & Krutzikowsky, G. K. (2008). Nocturnal feeding of Pacific hake and jack mackerel off the mouth of the Columbia River, 1998–2004: Implications for juvenile salmon predation. Transactions of the American Fisheries Society, 137, 657–676. https://doi.org/10.1577/T06-058.1 [Google Scholar]
- Emmett, R. L. , Krutzikowsky, G. K. , & Bentley, P. (2006). Abundance and distribution of pelagic piscivorous fishes in the Columbia River plume during spring/early summer 1998–2003: Relationship to oceanographic conditions, forage fishes, and juvenile salmonids. Progress in Oceanography, 68, 1–26. https://doi.org/10.1016/j.pocean.2005.08.001 [Google Scholar]
- Faulkner, J. R. , Widener, D. L. , Smith, S. G. , Marsh, T. M. , & Zabel, R. W. (2016). Survival Estimates for the Passage of Spring‐Migrating Juvenile Salmonids through Snake and Columbia River Dams and Reservoirs, 2015. Contract 40735, Project 199302900. pp. 111.
- Harrison, X. A. , Blount, J. D. , Inger, R. , Norris, D. R. , & Bearhop, S. (2011). Carry‐over effects as drivers of fitness differences in animals. Journal of Animal Ecology, 80, 4–18. https://doi.org/10.1111/j.1365-2656.2010.01740.x [DOI] [PubMed] [Google Scholar]
- Hertz, E. , Trudel, M. , Tucker, S. , Beacham, T. D. , Parken, C. , Mackas, D. , & Mazumder, A. (2016). Influences of ocean conditions and feeding ecology on the survival of juvenile Chinook Salmon (Oncorhynchus tshawytscha). Fisheries Oceanography, 25, 407–419. https://doi.org/10.1111/fog.12161 [Google Scholar]
- Hettinger, A. , Sanford, E. , Hill, T. M. , Russell, A. D. , Sato, K. N. S. , Hoey, J. , … Gaylord, B. (2012). Persistent carry‐over effects of planktonic exposure to ocean acidification in the Olympia oyster. Ecology, 93, 2758–2768. https://doi.org/10.1890/12-0567.1 [DOI] [PubMed] [Google Scholar]
- Holsman, K. K. , Scheuerell, M. D. , Buhle, E. , & Emmett, R. (2012). Interacting effects of translocation, artificial propagation, and environmental conditions on the marine survival of Chinook salmon from the Columbia River, Washington, U.S.A. Conservation Biology, 26, 912–922. https://doi.org/10.1111/j.1523-1739.2012.01895.x [DOI] [PubMed] [Google Scholar]
- Hunsicker, M. E. , Kappel, C. V. , Selkoe, K. A. , Halpern, B. S. , Scarborough, C. , Mease, L. , & Amrhein, A. (2016). Characterizing driver‐response relationships in marine pelagic ecosystems for improved ocean management. Ecological Applications, 26, 651–663. https://doi.org/10.1890/14-2200 [DOI] [PubMed] [Google Scholar]
- Hvidsten, N. A. , Jensen, A. J. , Rikardsen, A. H. , Finstad, B. , Aure, J. , Stefanson, S. , & Fiske, P. (2009). Influence of sea temperature and initial marine feeding on survival of Atlantic salmon Salmo salar post‐smolts from the Rivers Orkla and Hals, Norway. Journal of Fish Biology, 74, 1532–1548. https://doi.org/10.1111/j.1095-8649.2009.02219.x [DOI] [PubMed] [Google Scholar]
- Intergovernmental Panel on Climate Change (IPCC) (2014). Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. (eds C. W. Team, R. K. Pachauri & L. A. Meyer). Geneva, Switzerland. pp. 151.
- Jonsson, N. , & Jonsson, B. (2014). Time and size at seaward migration influence the sea survival of Salmo salar . Journal of Fish Biology, 84, 1457–1473. https://doi.org/10.1111/jfb.12370 [DOI] [PubMed] [Google Scholar]
- Kärnä, T. , & Baptista, A. M. (2016). Evaluation of a long‐term hindcast simulation for the Columbia River estuary. Ocean Modelling, 99, 1–14. https://doi.org/10.1016/j.ocemod.2015.12.007 [Google Scholar]
- Keefer, M. L. , & Caudill, C. C. (2014). Homing and straying by anadromous salmonids: A review of mechanisms and rates. Reviews in Fish Biology and Fisheries, 24, 333–368. https://doi.org/10.1007/s11160-013-9334-6 [Google Scholar]
- Kennedy, R. J. , & Crozier, W. W. (2010). Evidence of changing migratory patterns of wild Atlantic salmon Salmo salar smolts in the River Bush, Northern Ireland, and possible associations with climate change. Journal of Fish Biology, 76, 1786–1805. https://doi.org/10.1111/j.1095-8649.2010.02617.x [DOI] [PubMed] [Google Scholar]
- Kovach, R. P. , Joyce, J. E. , Echave, J. D. , Lindberg, M. S. , & Tallmon, D. A. (2013). Earlier migration timing, decreasing phenotypic variation, and biocomplexity in multiple salmonid species. PLoS One, 8, e53807 https://doi.org/10.1371/journal.pone.0053807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logerwell, E. A. , Mantua, N. , Lawson, P. W. , Francis, R. C. , & Agostini, V. N. (2003). Tracking environmental processes in the coastal zone for understanding and predicting Oregon coho (Oncorhynchus kisutch) marine survival. Fisheries Oceanography, 12, 554–568. https://doi.org/10.1046/j.1365-2419.2003.00238.x [Google Scholar]
- Malick, M. J. , Cox, S. P. , Peterman, R. M. , Wainwright, T. C. , & Peterson, W. T. (2015). Accounting for multiple pathways in the connections among climate variability, ocean processes, and coho salmon recruitment in the Northern California Current. Canadian Journal of Fisheries and Aquatic Sciences, 72, 1552–1564. https://doi.org/10.1139/cjfas-2014-0509 [Google Scholar]
- Mantua, N. J. , Hare, S. R. , Zhang, Y. , Wallace, J. M. , & Francis, R. C. (1997). A Pacific Interdecadal Climate Oscillation with Impacts on Salmon Production. Bulletin of the American Meteorological Society, 78, 1069–1079. https://doi.org/10.1175/1520-0477(1997)078<1069:APICOW>2.0.CO;2 [Google Scholar]
- McCormick, S. D. , Hansen, L. P. , Quinn, T. P. , & Saunders, R. L. (1998). Movement, migration, and smolting of Atlantic salmon (Salmo salar). Canadian Journal of Fisheries and Aquatic Sciences, 55, 77–92. https://doi.org/10.1139/d98-011 [Google Scholar]
- McCullough, D. A. , Bartholow, J. M. , Jager, H. I. , Beschta, R. L. , Cheslak, E. F. , Deas, M. L. , … Wurtsbaugh, W. A. (2009). Research in thermal biology: Burning questions for coldwater stream fishes. Reviews in Fisheries Science, 17, 90–115. https://doi.org/10.1080/10641260802590152 [Google Scholar]
- McKinnon, E. A. , Stanley, C. Q. , & Stutchbury, B. J. M. (2015). Carry‐over effects of nonbreeding habitat on start‐to‐finish spring migration performance of a songbird. PLoS One, 10, e0141580 https://doi.org/10.1371/journal.pone.0141580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMichael, G. A. , Skalski, J. R. , & Deters, K. A. (2011). Survival of juvenile Chinook salmon during barge transport. North American Journal of Fisheries Management, 31, 1187–1196. https://doi.org/10.1080/02755947.2011.646455 [Google Scholar]
- Midwood, J. D. , Larsen, M. H. , Boel, M. , Aarestrup, K. , & Cooke, S. J. (2015). An experimental field evaluation of winter carryover effects in semi‐anadromous brown trout (Salmo trutta). Journal of Experimental Zoology, 323, 645–654. https://doi.org/10.1002/jez.1955 [DOI] [PubMed] [Google Scholar]
- Miller, J. A. , Teel, D. J. , Peterson, W. T. , & Baptista, A. M. (2014). Assessing the relative importance of local and regional processes on the survival of a threatened salmon population. PLoS One, 9, e99814 https://doi.org/10.1371/journal.pone.0099814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mote, P. W. (2003). Trends in snow water equivalent in the Pacific Northwest and their climatic causes. Geophysical Research Letters, 30, 1601. [Google Scholar]
- Naish, K. A. , Taylor, J. E. III , Levin, P. S. , Quinn, T. P. , Winton, J. R. , Huppert, D. , & Hilborn, R. (2007). An evaluation of the effects of conservation and fishery enhancement hatcheries on wild populations of salmon. Advances in Marine Biology, 53, 61–194. https://doi.org/10.1016/S0065-2881(07)53002-6 [DOI] [PubMed] [Google Scholar]
- National Marine Fisheries Service (NMFS) (2010). Endangered Species Act Section 7(a)(2) Consultation Supplemental Biological Opinion Supplemental Consultation on Remand for Operation of the Federal Columbia River Power System, 11 Bureau of Reclamation Projects in the Columbia Basin and ESA Section 10(a)(I)(A) Permit for Juvenile Fish Transportation Program. NMFS, Seattle, WA. pp. 246. [Google Scholar]
- Newman, M. , Alexander, M. A. , Ault, T. B. , Cobb, K. M. , Deser, C. , Di Lorenzo, E. , … Smith, C. A. (2016). The Pacific Decadal Oscillation, revisited. Journal of Climate, 29, 4399–4427. https://doi.org/10.1175/JCLI-D-15-0508.1 [Google Scholar]
- O'Connor, C. M. , & Cooke, S. J. (2015). Ecological carryover effects complicate conservation. Ambio, 44, 582–591. https://doi.org/10.1007/s13280-015-0630-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor, C. M. , Norris, D. R. , Crossin, G. T. , & Cooke, S. J. (2014). Biological carryover effects: Linking common concepts and mechanisms in ecology and evolution. Ecosphere, 5, 1–11. Article 28. [Google Scholar]
- Otero, J. , L'Abée‐Lund, J. H. , Castro‐Santos, T. , Leonardsson, K. , Storvik, G. O. , Jonsson, B. , … Vøllestad, L. A. (2014). Basin‐scale phenology and effects of climate variability on global timing of initial seaward migration of Atlantic salmon (Salmo salar). Global Change Biology, 20, 61–75. https://doi.org/10.1111/gcb.12363 [DOI] [PubMed] [Google Scholar]
- Peterson, W. T. , Fisher, J. L. , Peterson, J. O. , Morgan, C. A. , Burke, B. J. , & Fresh, K. L. (2014). Applied fisheries oceanography: Ecosystem indicators of ocean conditions inform fisheries management in the California Current. Oceanography, 27, 80–89. https://doi.org/10.5670/oceanog [Google Scholar]
- Petrosky, C. E. , & Schaller, H. A. (2010). Influence of river conditions during seaward migration and ocean conditions on survival rates of Snake River Chinook salmon and steelhead. Ecology of Freshwater Fish, 19, 520–536. https://doi.org/10.1111/j.1600-0633.2010.00425.x [Google Scholar]
- Rupp, D. E. , Wainwright, T. C. , Lawson, P. W. , & Peterson, W. T. (2012). Marine environment‐based forecasting of coho salmon (Oncorhynchus kisutch) adult recruitment. Fisheries Oceanography, 21, 1–19. https://doi.org/10.1111/j.1365-2419.2011.00605.x [Google Scholar]
- Russell, I. C. , Aprahamian, M. W. , Barry, J. , Davidson, I. C. , Fiske, P. , Ibbotson, A. T. , … Todd, C. D. (2012). The influence of the freshwater environment and the biological characteristics of Atlantic salmon smolts on their subsequent marine survival. ICES Journal of Marine Science, 69, 1563–1573. https://doi.org/10.1093/icesjms/fsr208 [Google Scholar]
- Samhouri, J. F. , Andrews, K. S. , Fay, G. , Harvey, C. J. , Hazen, E. L. , Hennessey, S. M. , … Zador, S. G. (2017). Defining ecosystem thresholds for human activities and environmental pressures in the California Current. Ecosphere, 8, e01860 https://doi.org/10.1002/ecs2.1860 [Google Scholar]
- Sandford, B. P. , Zabel, R. W. , Gilbreath, L. G. , & Smith, S. G. (2012). Exploring Latent Mortality of Juvenile Salmonids Related to Migration through the Columbia River Hydropower System. Transactions of the American Fisheries Society, 141, 343–352. https://doi.org/10.1080/00028487.2012.664601 [Google Scholar]
- Satterthwaite, W. H. , Carlson, S. M. , Allen‐Moran, S. D. , Vincenzi, S. , Bograd, S. J. , & Wells, B. K. (2014). Match‐mismatch dynamics and the relationship between ocean‐entry timing and relative ocean recoveries of Central Valley fall run Chinook salmon. Marine Ecology Progress Series, 511, 237–248. https://doi.org/10.3354/meps10934 [Google Scholar]
- Scheuerell, M. D. , & Williams, J. G. (2005). Forecasting climate‐induced changes in the survival of Snake River spring/summer Chinook salmon (Oncorhynchus tshawytscha). Fisheries Oceanography, 14, 448–457. https://doi.org/10.1111/j.1365-2419.2005.00346.x [Google Scholar]
- Scheuerell, M. D. , Zabel, R. W. , & Sandford, B. P. (2009). Relating juvenile migration timing and survival to adulthood in two species of threatened Pacific salmon (Oncorhynchus spp.). Journal of Applied Ecology, 46, 983–990. https://doi.org/10.1111/j.1365-2664.2009.01693.x [Google Scholar]
- Smith, S. G. , Marsh, D. M. , Emmett, R. L. , Muir, W. D. , & Zabel, R. W. (2013) A Study to Determine Seasonal Effects of Transporting Fish from the Snake River to Optimize a Transportation Strategy. MIPR Number W68SBV10698480. Fish Ecology Division, Northwest Fisheries Science Center, National Marine Fisheries Service, National Oceanic and Atmospheric Administration, Seattle, Washington. pp. 210.
- Studds, C. E. , & Marra, P. P. (2005). Nonbreeding Habitat Occupancy and Population Processes: An Upgrade Experiment with a Migratory Bird. Ecology, 86, 2380–2385. https://doi.org/10.1890/04-1145 [Google Scholar]
- Sykes, G. E. , Johnson, C. J. , & Shrimpton, J. M. (2009). Temperature and Flow Effects on Migration Timing of Chinook Salmon Smolts. Transactions of the American Fisheries Society, 138, 1252–1265. https://doi.org/10.1577/T08-180.1 [Google Scholar]
- Thorstad, E. B. , Whoriskey, F. , Uglem, I. , Moore, A. , Rikardsen, A. H. , & Finstad, B. (2012). A critical life stage of the Atlantic salmon Salmo salar: Behaviour and survival during the smolt and initial post‐smolt migration. Journal of Fish Biology, 81, 500–542. https://doi.org/10.1111/j.1095-8649.2012.03370.x [DOI] [PubMed] [Google Scholar]
- U.S. Army Corps of Engineers (USACE) (2016). 2016 Fish Passage Plan. Lower Columbia & Lower Snake River Hydropower Projects. CENWD‐PDW‐R. Appendix B: Corps of Engineers Juvenile Fish Transportation Plan (JFTP). Portland, Oregon. pp. 459.
- Weitkamp, L. A. , Teel, D. J. , Liermann, M. , Hinton, S. A. , Van Doornik, D. M. , & Bentley, P. J. (2015). Stock‐specific size and timing at ocean entry of Columbia River juvenile Chinook salmon and steelhead: Implications for early ocean growth. Marine and Coastal Fisheries: Dynamics, Management, and Ecosystem Science, 7, 370–392. https://doi.org/10.1080/19425120.2015.1047476 [Google Scholar]
- Wells, B. K. , Grimes, C. B. , Field, J. C. , & Reiss, C. S. (2006). Covariation between the average lengths of mature coho (Oncorhynchus kisutch) and Chinook salmon (O. tshawytscha) and the ocean environment. Fisheries Oceanography, 15, 67–79. https://doi.org/10.1111/j.1365-2419.2005.00361.x [Google Scholar]
- Wells, B. K. , Santora, J. A. , Henderson, M. J. , Warzybok, P. , Jahncke, J. , Bradley, R. W. , Huff, D. D. , Schroeder, I. D. , Nelson, P. , Field, J. C. , & Ainley, D. G. (2017). Environmental conditions and prey‐switching by a seabird predator impact juvenile salmon survival. Journal of Marine Systems, 174, 54–63. https://doi.org/10.1016/j.jmarsys.2017.05.008 [Google Scholar]
- Wells, B. K. , Santora, J. A. , Schroeder, I. D. , Mantua, N. , Sydeman, W. J. , Huff, D. D. , & Field, J. C. (2016). Marine ecosystem perspectives on Chinook salmon recruitment: A synthesis of empirical and modeling studies from a California upwelling system. Marine Ecology Progress Series, 552, 271–284. https://doi.org/10.3354/meps11757 [Google Scholar]
- Wolter, K. , & Timlin, M. S. (1993). Monitoring ENSO in COADS with seasonally adjusted principal component index. Proceedings of the 17th Climate Diagnostics Workshop, Norman, Oklahoma, pp. 52–57.
- Woodson, L. E. , Wells, B. K. , Weber, P. K. , MacFarlane, R. B. , Whitman, G. E. , & Johnson, R. C. (2013). Size, growth, and origin‐dependent mortality of juvenile Chinook salmon Oncorhynchus tshawytscha during early ocean residence. Marine Ecology Progress Series, 487, 163–175. https://doi.org/10.3354/meps10353 [Google Scholar]
- Zabel, R. W. , & Achord, S. (2004). Relating size of juveniles to survival within and among populations of Chinook salmon. Ecology, 85, 795–806. https://doi.org/10.1890/02-0719 [Google Scholar]
- Zuur, A. F. , Ieno, E. N. , Walker, N. J. , Saveliev, A. A. , & Smith, G. M. (2009). Mixed effects models and extensions in ecology with R. New York, NY: Springer; https://doi.org/10.1007/978-0-387-87458-6 [Google Scholar]
- Zydlewski, G. B. , Haro, A. , & McCormick, S. D. (2005). Evidence for cumulative temperature as an initiating and terminating factor in downstream migratory behavior of Atlantic salmon (Salmo salar) smolts. Canadian Journal of Fisheries and Aquatic Sciences, 62, 68–78. https://doi.org/10.1139/f04-179 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used in this manuscript are available at Columbia River Basin, School of Aquatic and Fishery Sciences, University of Washington (www.cbr.washington.edu).


