Abstract
Bird species richness is mediated by local, regional, and historical factors, for example, competition, environmental heterogeneity, contemporary, and historical climate. Here, we related bird species richness with phylogenetic relatedness of bird assemblages, plant species richness, topography, contemporary climate, and glacial‐interglacial climate change to investigate the relative importance of these factors. This study was conducted in Inner Mongolia, an arid and semiarid region with diverse vegetation types and strong species richness gradients. The following associated variables were included as follows: phylogenetic relatedness of bird assemblages (Net Relatedness Index, NRI), plant species richness, altitudinal range, contemporary climate (mean annual temperature and precipitation, MAT and MAP), and contemporary‐Last Glacial Maximum (LGM) change in climate (change in MAT and change in MAP). Ordinary least squares linear, simultaneous autoregressive linear, and Random Forest models were used to assess the associations between these variables and bird species richness across this region. We found that bird species richness was correlated negatively with NRI and positively with plant species richness and altitudinal range, with no significant correlations with contemporary climate and glacial–interglacial climate change. The six best combinations of variables ranked by Random Forest models consistently included NRI, plant species richness, and contemporary‐LGM change in MAT. Our results suggest important roles of local ecological factors in shaping the distribution of bird species richness across this semiarid region. Our findings highlight the potential importance of these local ecological factors, for example, environmental heterogeneity, habitat filtering, and biotic interactions, in biodiversity maintenance.
Keywords: biotic interaction, bird species richness, contemporary climate, environmental heterogeneity, glacial–interglacial climate change
1. INTRODUCTION
Geographic distribution of species diversity and its drivers at broad scales is an important topic in ecology and biogeography (Brown, 2014; Currie, 1991; Fine, 2015). The underlying factors associated with species distribution include local (e.g., biotic interactions and habitat heterogeneity), regional (e.g., energy availability and water–energy dynamics), and historical (e.g., geological events and glacial–interglacial climate change) variables (Fine, 2015; Qu et al., 2015; Svenning, Eiserhardt, Normand, Ordonez, & Sandel, 2015).
Local ecological factors, including both biotic and abiotic factors, not only strongly constrain local community composition and structure, but could also affect species distribution at regional and global scales (Feng et al., 2016; Fine, 2015; Schemske, Mittelbach, Cornell, Sobel, & Roy, 2009). For instance, environmental heterogeneity, especially elevation range, is associated with patterns of species richness (Jetz & Rahbek, 2002; Kerr & Packer, 1997; Novillo & Ojeda, 2014). Availability of food resources and vegetation structure is also strongly associated with bird species richness at regional scales (Ferger, Schleuning, Hemp, Howell, & Böhning‐Gaese, 2014; Zhang, Kissling, & He, 2013). Both competition and facilitation among bird species could improve the prediction of bird species distribution at macro‐ecological scales (Heikkinen, Luoto, Virkkala, Pearson, & Körber, 2007; Laube, Graham, & Böhning‐Gaese, 2013; Pigot & Tobias, 2013).
In addition to those local ecological drivers, it has also been reported that regional and historical factors play important roles in shaping the geographic distribution of species richness at macro‐ecological scales, supporting the energy availability hypothesis, the water–energy dynamics hypothesis, the historical hypothesis, the refuge hypothesis, etc. (Davies et al., 2007; Fjeldså & Lovett, 1997; Li et al., 2013; Qu et al., 2015; Rahbek & Graves, 2001). Specifically, bird species richness is strongly associated with contemporary climate variables, such as precipitation, temperature, and actual evapotranspiration (Davies et al., 2007; Li et al., 2013; Rahbek & Graves, 2001). Glacial–interglacial refuge and geological events may also affect the distribution of species richness through their impact on species speciation and extinction (Fjeldså & Lovett, 1997; Qu et al., 2015).
With an area of 120 million ha (3.3 times the size of Germany and running 3,000 km from northwest to southeast), Inner Mongolia has a wide range of climate (e.g., mean annual temperature ranging from −2 to 6°C and mean annual precipitation ranging from 40 to 450 mm, Wu, Zhang, Li, & Liang, 2015). As a result, vegetation types, plant, and bird species are very diverse in Inner Mongolia, for example, there are forest, grassland, and desert, which are home to 2,447 known vascular plant species and 467 known bird species (Xu, 2007, 2015; Zhao, 2012), which provides an ideal system to investigate the geographic distribution of bird diversity. In this study, we assessed the associations between bird species richness and phylogenetic relatedness of bird assemblages, plant species richness, topography, contemporary climate, as well as glacial–interglacial climate change in Inner Mongolia (97°12′–126°04′E, 37°24′–53°23′N).
2. MATERIALS AND METHODS
2.1. Geographic data
Bird distribution data at the county scale were compiled from the third and fourth volumes of Fauna of Inner Mongolia (Xu, 2007, 2015). Plant distribution data at the county scale were collected from Chinese Vascular Plant Distribution Database, which was compiled from plant occurrence records in counties from Flora Reipublicae Popularis Sinicae (Delecti Florae Reipublicae Popularis Sinicae Agendae Academiae Sinicae, 1959), provincial and regional floras, as well as herbarium specimens. Plant species richness is interpreted as food resources and habitat diversity (Zhang et al., 2013). Eighty‐six counties were included (see Table S1 for more information), with areas ranging from 100 km2 to 90,000 km2 (area was not a factor significantly affecting bird species richness, Table 1).
Table 1.
Results of single‐variable analysis by ordinary least squares (OLS) and simultaneous autoregressive (SAR) models. MAT and MAP are mean annual temperature and precipitation. ChangeMAT and ChangeMAP are the contemporary‐Last Glacial Maximum change in MAT and MAP. SRplant is species richness of plants. ALTrange is altitudinal range. NRI is phylogenetic relatedness of bird assemblages. Coefficients (coef) and adjusted r 2 were given. All statistically significant p‐values were less than .01 and are indicated as *
| CoefOLS | r 2 OLS | CoefSAR | r 2 SAR | |
|---|---|---|---|---|
| Area | 0.06 | −.01 | 0.09 | .08 |
| MAT | −0.05 | −.01 | 0.02 | .08 |
| MAP | 0.08 | −.01 | 0.07 | .08 |
| ChangeMAT | −0.18 | .02 | −0.22 | .10 |
| ChangeMAP | 0.20 | .03 | 0.17 | .09 |
| SRplant | 0.52 | .26* | 0.49 | .30* |
| ALTrange | 0.37 | .13* | 0.34 | .17* |
| NRI | −0.68 | .45* | −0.67 | .48* |
2.2. Environmental data
Climate variables, mean annual temperature (MAT), mean annual precipitation (MAP), temperature in Last Glacial Maximum (MAT in LGM), precipitation in Last Glacial Maximum (MAP in LGM), and altitudinal range were collected from WorldClim (Hijmans, Cameron, Parra, Jones, & Jarvis, 2005). Altitudinal range is a proxy of environmental heterogeneity (Stein, Gerstner, & Kreft, 2014). MAT in LGM and MAP in LGM were extracted from the Community Climate System Model version 3 (CCSM3; Hijmans et al., 2005; Otto‐Bliesner et al., 2006) and the Model for Interdisciplinary Research on Climate version 3.2 (MIROC3.2; Hasumi & Emori, 2004). MAT in LGM and MAP in LGM were then summarized as the mean values of the two models. Change in MAT and change in MAP were calculated as contemporary values minus LGM values (Sandel et al., 2011).
2.3. Phylogeny
A distribution of 10,000 phylogenies was downloaded from the global phylogeny of birds (Jetz et al., 2014), including all 112 resident bird species in Inner Mongolia. Five thousand pseudoposterior distributions were sampled, and the maximum clade credibility tree was constructed using mean node heights by the software TreeAnnonator v1.8.2 of the BEAST package (Drummond & Rambaut, 2007; Ricklefs & Jønsson, 2014; Si et al., 2017). We used the resulting consensus phylogeny for all subsequent phylogenetic analyses. Phylogenetic relatedness of bird assemblages was represented by the Net Relatedness Index (NRI) (Webb, Ackerly, McPeek, & Donoghue, 2002). NRI is computed as
where MPDobs is the observed mean phylogenetic distance (MPD) of birds in a county, meanMPDrnd is the mean MPD of the null models (shuffle distance matrix labels 999 times), and sdMPDrnd is the standard deviation of MPD of the null models. Positive NRI means birds in a county are more closed related than expected (clustered), while negative NRI means birds in a county are more distantly related than expected (overdispersed) (Webb et al., 2002). According to the phylogenetic niche conservatism hypothesis, a clustered phylogenetic structure indicates a dominant role of environmental filtering, and an overdispersed phylogenetic structure is driven by competition or facilitation (Webb et al., 2002).
2.4. Statistical analyses
Bird species richness, plant species richness, and county area were log transformed to obtain normal distributed residuals. All variables were standardized (mean = 0 and standard deviation = 1) to make the regression coefficients comparable. Relationships between bird species richness and each associated variable were then estimated by ordinary least squares (OLS) models. To account for spatial autocorrelation of residuals, simultaneous autoregressive (SAR) models were also used for the single‐variable analyses. Because Random Forest models could effectively capture interactions (e.g., in this study correlation between MAP and change in MAP is 0.76, and correlation between MAT and change in MAT is −0.70) and nonlinear relationships, and do not require the data to follow strict assumptions, for example, homoscedasticity and normality in errors (Breiman, 2001), they were implemented for the multiple‐variable analyses, aiming to find the combination of variables most associated with bird species richness. For each combination, the Random Forest models were run 1,000 times on random splits of the data (50% training data and 50% evaluation data) and averaging the Pearson correlation between the predicted and the observed values (species richness of birds). To check which variables always occurred in the best combinations, the six combinations with highest Pearson correlations were chosen. SAR models were also conducted for the six combinations because of spatial autocorrelation of residuals. AICw and r 2 of SAR models were listed for the six combinations. AICw is interpreted as the probability of each model being the best model and indicates the relative merit of the competing models (Wagenmakers & Farrell, 2004). OLS, SAR, and Random Forest models, as well as data transformations, were performed in R 3.3.0 (R Core Team, 2016) using vegan (Oksanen et al., 2015), spdep (Bivand et al., 2015), and randomForest (Liaw & Wiener, 2002) R packages.
3. RESULTS
The single‐variable ordinary least squares models and simultaneous autoregressive models showed similar patterns about the relationships between bird species richness and the associated variables (Table 1). The three variables significantly correlated with bird species richness were phylogenetic relatedness of bird assemblages (negatively, r 2 = .45), plant species richness (positively, r 2 = .26), and altitudinal range (positively, r 2 = .13), indicating that there were more birds with more overdispersed phylogenetic structure, more plant species, and larger altitudinal range (Figure 1, Table 1). Other variables, that is, contemporary climate and contemporary‐Last Glacial Maximum (LGM) change in climate, were not significantly correlated with bird species richness (Table 1).
Figure 1.
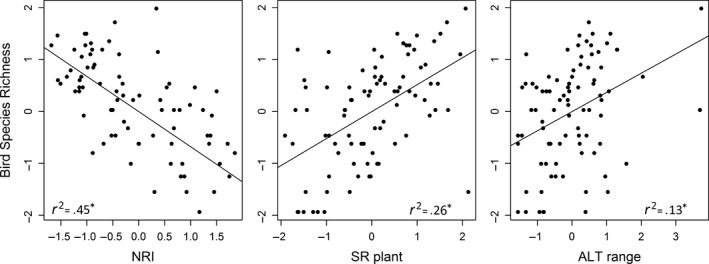
Relationships between bird species richness (log transformed) and the three most associated variables: phylogenetic relatedness of bird assemblages (Net Relatedness Index, NRI), plant species richness (SR plant, log transformed), and altitudinal range (ALT range). Single‐variable OLS linear fits (standardized) are shown, and their r 2 is given. All statistically significant p‐values were less than .01 and are indicated as *
Random Forest analyses showed that the six combinations of variables most associated with bird species richness consistently included phylogenetic relatedness of bird assemblages, plant species richness, and contemporary‐LGM change in MAT, indicating that although contemporary‐LGM change in MAT was not significantly correlated with bird species richness, it may still play a supplementary role in predicting distribution of bird species richness (Table 2).
Table 2.
The six combinations of variables most associated with bird species richness, ranked by the correlations between observed and predicted species richness, from the Random Forest models (CorRF). Each column is a different variable (NRI, phylogenetic relatedness of bird assemblages; ChangeMAT, contemporary‐Last Glacial Maximum change in temperature; SRplant, species richness of plant; MAP, mean annual precipitation; ALTrange, altitudinal range; MAT, mean annual temperature; ChangeMAP, contemporary‐Last Glacial Maximum change in precipitation). White cell indicates that the variable was not included in the particular combination (each row). AIC weights (AICw) and adjusted r 2 from simultaneous autoregressive (SAR) models of each combination of variables were also listed
| NRI | ChangeMAT | SRplant | ALTrange | MAP | MAT | ChangeMAP | CorRF | AICw_SAR | r 2 SAR |
|---|---|---|---|---|---|---|---|---|---|
| 0.620 | 0.100 | .544 | |||||||
| 0.614 | 0.049 | .547 | |||||||
| 0.610 | 0.074 | .551 | |||||||
| 0.605 | 0.049 | .557 | |||||||
| 0.604 | 0.045 | .546 | |||||||
| 0.604 | 0.020 | .548 |
4. DISCUSSION
It has been reported that the geographic distributions of bird species richness are shaped by divergent factors, including local, regional, and continental variables (Heikkinen et al., 2007; Qu et al., 2015; Rahbek & Graves, 2001). However, few studies have simultaneously tested the relative roles of a comprehensive set of the potential factors, especially in an arid and semiarid region with diverse vegetation types and species richness. Our results indicated an important role of local ecological factors, likely linked to vegetation diversity or productivity, environmental heterogeneity, and possibly, competition, in addition to the broad‐scale past and present climate factors.
4.1. Net relatedness index and bird species richness
A study of tropical humming birds communities found overdispersed phylogenetic structure (more distantly related than expected) in wet lowlands and clustered phylogenetic structure (more closely related than expected) at high altitude, and interpreted this as evidence of the strong influence of competition and environmental filtering in these two habitats (Graham, Parra, Rahbek, & McGuire, 2009). In this study, our analyses showed an increasing overdispersion of phylogenetic structure with higher species richness, potentially indicating a role of competition in shaping the build‐up of species‐rich bird assemblages and a role of environmental filtering on phylogenetically conserved traits in species‐poor assemblages (Figure 1). Being a fundamental process in ecology, competition is an important driver of both local community assembly and macro‐ecological scales species distribution (Feng et al., 2016; Fine, 2015).
4.2. Plant species richness and bird species richness
Positive correlations between plant species richness and bird species richness at regional scales have been widely reported, for example, in western Canada (Zhang et al., 2013), sub‐Saharan Africa (Kissling, Rahbek, & Böhning‐Gaese, 2007), and China (Qian & Kissling, 2010). Consistent with these studies, we also found positive correlations between bird species richness and plant species richness (Figure 1, Table 1). It is possible that higher plant species richness could provide more diverse habitats and food supplies for birds, thereby supporting more bird species (Zhang et al., 2013). It is also possible that there could be other factors (biotic and abiotic) affecting the diversity of birds and plants in similar ways (Kissling et al., 2007).
4.3. Environmental heterogeneity and bird species richness
Environmental heterogeneity is also an important driver of geographic distribution of species richness for different taxa, biomes, and spatial scales (Stein et al., 2014). The increase in environmental heterogeneity could provide more niches, refuges, and opportunities for speciation (Stein et al., 2014). Being an important and easily quantified proxy of environmental heterogeneity, altitudinal range has been widely related to bird species richness at macro‐ecological scales (Davies et al., 2007; Jetz & Rahbek, 2002; Jiménez‐Alfaro, Chytrý, Mucina, Grace, & Rejmanek, 2016). Consistent with these studies, we also found that bird species richness in Inner Mongolia increases with altitudinal range (Figure 1). Two counties with high bird species richness in our study, which are Alxa Left Banner (59 species) and Hexigten‐Banner (39 species), have high altitudinal ranges (2,243 m and 1,139 m). Most of these species (75% and 72%) prefer to live in forests. These two counties also have high species richness of plants (768 and 597 species), again emphasizing the importance of habitat heterogeneity in shaping the distribution patterns of bird species richness.
4.4. Climate and bird species richness
The refuge hypothesis assumes that stable glacial–interglacial climate change may both facilitate speciation and restrict extinction, thus regions with stable climate may have more species due to the accumulation of both relict and new species (Fjeldså & Lovett, 1997). Correlations between glacial climate fluctuation and bird species richness have been tested in Africa, Australia, and the New World (Fjeldså & Lovett, 1997; Hawkins, Diniz‐Filho, Jaramillo, & Soeller, 2006; Hawkins, Diniz‐Filho, & Soeller, 2005). Our analyses showed no significant correlations between bird species richness and historical climate variables (Table 1). However, the contemporary‐Last Glacial Maximum change in temperature occurred in all six of the most informative combinations of variables for bird species richness (Table 2), indicating that the glacial–interglacial climate change may have left a potential legacy in this region and may play a supplementary role in shaping the distribution of bird species richness.
Numerous previous studies have also examined the correlations between contemporary climate and bird species richness to test the energy availability hypothesis and the water–energy dynamics hypothesis (Jetz & Rahbek, 2002; Rahbek & Graves, 2001). However, our analyses did not show significant correlations between contemporary climate variables and bird species richness. Contemporary climate may affect the distribution of bird species richness through their effects on the distribution of vegetation and plant diversity in Inner Mongolia (Wu et al., 2015).
5. CONCLUSIONS
Through investigating the patterns and underlying drivers of the geographic distribution of bird species richness in Inner Mongolia, we found that local ecological factors, for example, environmental heterogeneity, habitat filtering, and biotic interactions, were associated with bird species richness, while regional and historical factors, that is, contemporary and historical climate, were not. Our findings highlight the importance of these local ecological factors in biodiversity maintenance.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
CL collected the data and drafted the article. GF designed the study, analyzed the data, drafted, and revised the article. XS and LM provided data and revised the article. GY, J.‐C.S., and JY revised the article.
Supporting information
ACKNOWLEDGMENTS
We thank the Starting Funding for Scientific Research from Inner Mongolia University (21400‐5165111) for the support to GF. J.‐C.S. was supported by the European Research Council (ERC‐2012‐StG‐310886‐HISTFUNC). We would also like to thank the editor and three reviewers for their comments and suggestions, which have substantially improved this paper.
Liang C, Feng G, Si X, et al. Bird species richness is associated with phylogenetic relatedness, plant species richness, and altitudinal range in Inner Mongolia. Ecol Evol. 2018;8:53–58. https://doi.org/10.1002/ece3.3606
Contributor Information
Gang Feng, Email: qaufenggang@163.com.
Jie Yang, Email: yangjie@imu.edu.cn.
REFERENCES
- Bivand, R. , Altman, M. , Anselin, L. , Assunção, R. , Berke, O. , Bernat, A. , … Yu, D. (2015). spdep: Spatial dependence: weighting schemes, statistics and models. R package version 0.5‐92. Retrieved from http://CRAN.R-project.org/package=spdep. [Google Scholar]
- Breiman, L. (2001). Random forests. Machine Learning, 45, 5–32. https://doi.org/10.1023/A:1010933404324 [Google Scholar]
- Brown, J. H. (2014). Why are there so many species in the tropics? Journal of Biogeography, 41, 8–22. https://doi.org/10.1111/jbi.12228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie, D. J. (1991). Energy and large‐scale patterns of animal‐ and plant‐species richness. The American Naturalist, 137, 27–49. https://doi.org/10.1086/285144 [Google Scholar]
- Davies, R. G. , Orme, C. D. L. , Storch, D. , Olson, V. A. , Thomas, G. H. , Ross, S. G. , … Gaston, K. J. (2007). Topography, energy and the global distribution of bird species richness. Proceedings of the Royal Society of London B: Biological Sciences, 274, 1189–1197. https://doi.org/10.1098/rspb.2006.0061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delecti Florae Reipublicae Popularis Sinicae Agendae Academiae Sinicae (1959. –2004). Flora Reipublicae Popularis Sinicae. Beijing, China: Science Press. [Google Scholar]
- Drummond, A. J. , & Rambaut, A. (2007). BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evolutionary Biology, 7, 214 https://doi.org/10.1186/1471-2148-7-214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng, G. , Mi, X. , Yan, H. , Li, F. Y. , Svenning, J.‐C. , & Ma, K. (2016). CForBio: A network monitoring Chinese forest biodiversity‐progress and perspective. Science Bulletin, 61, 1163–1170. https://doi.org/10.1007/s11434-016-1132-9 [Google Scholar]
- Ferger, S. W. , Schleuning, M. , Hemp, A. , Howell, K. M. , & Böhning‐Gaese, K. (2014). Food resources and vegetation structure mediate climatic effects on species richness of birds. Global Ecology and Biogeography, 23, 541–549. https://doi.org/10.1111/geb.2014.23.issue-5 [Google Scholar]
- Fine, P. (2015). Ecological and evolutionary drivers of geographic variation in species diversity. Annual Review of Ecology, Evolution, and Systematics, 46, 369–392. https://doi.org/10.1146/annurev-ecolsys-112414-054102 [Google Scholar]
- Fjeldså, J. , & Lovett, J. C. (1997). Geographical patterns of old and young species in African forest biota: The significance of specific montane areas as evolutionary centers. Biodiversity and Conservation, 6, 325–347. https://doi.org/10.1023/A:1018356506390 [Google Scholar]
- Graham, C. H. , Parra, J. L. , Rahbek, C. , & McGuire, J. A. (2009). Phylogenetic structure in tropical hummingbird communities. Proceedings of the National Academy of Sciences of the United States of America, 106, 19673–19678. https://doi.org/10.1073/pnas.0901649106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasumi, H. , & Emori, S. (2004). K‐1 Coupled Model (MIROC) description. K‐1 Technical Report No. 1. Center for Climate System Research, University of Tokyo, Tokyo.
- Hawkins, B. A. , Diniz‐Filho, J. A. F. , Jaramillo, C. A. , & Soeller, S. A. (2006). Post‐Eocene climate change, niche conservatism, and the latitudinal diversity gradient of New World birds. Journal of Biogeography, 33, 770–780. https://doi.org/10.1111/jbi.2006.33.issue-5 [Google Scholar]
- Hawkins, B. A. , Diniz‐Filho, J. A. F. , & Soeller, S. A. (2005). Water links the historical and contemporary components of the Australian bird diversity gradient. Journal of Biogeography, 32, 1035–1042. https://doi.org/10.1111/jbi.2005.32.issue-6 [Google Scholar]
- Heikkinen, R. K. , Luoto, M. , Virkkala, R. , Pearson, R. G. , & Körber, J. H. (2007). Biotic interactions improve prediction of boreal bird distributions at macro‐scales. Global Ecology and Biogeography, 16, 754–763. https://doi.org/10.1111/geb.2007.16.issue-6 [Google Scholar]
- Hijmans, R. J. , Cameron, S. E. , Parra, J. L. , Jones, P. G. , & Jarvis, A. (2005). Very high resolution interpolated climate surfaces for global land areas. International Journal of Climatology, 25, 1965–1978. https://doi.org/10.1002/(ISSN)1097-0088 [Google Scholar]
- Jetz, W. , & Rahbek, C. (2002). Geographic range size and determinants of avian species richness. Science, 297, 1548–1551. https://doi.org/10.1126/science.1072779 [DOI] [PubMed] [Google Scholar]
- Jetz, W. , Thomas, G. H. , Joy, J. B. , Hartmann, K. , Redding, D. , & Mooers, A. O. (2014). Distribution and conservation of global evolutionary distinctness in birds. Current Biology, 24, 919–930. https://doi.org/10.1016/j.cub.2014.03.011 [DOI] [PubMed] [Google Scholar]
- Jiménez‐Alfaro, B. , Chytrý, M. , Mucina, L. , Grace, J. B. , & Rejmanek, M. (2016). Disentangling vegetation diversity from climate–energy and habitat heterogeneity for explaining animal geographic patterns. Ecology and Evolution, 6(1515), 1526 https://doi.org/10.1002/ece3.1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr, J. T. , & Packer, L. (1997). Habitat heterogeneity as a determinant of mammal species richness in high‐energy regions. Nature, 385, 252–254. https://doi.org/10.1038/385252a0 [Google Scholar]
- Kissling, W. D. , Rahbek, C. , & Böhning‐Gaese, K. (2007). Food plant diversity as broad‐scale determinant of avian frugivore richness. Proceedings of the Royal Society London Series B, 274, 799–808. https://doi.org/10.1098/rspb.2006.0311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laube, I. , Graham, C. H. , & Böhning‐Gaese, K. (2013). Intra‐generic species richness and dispersal ability interact to determine geographic ranges of birds. Global Ecology and Biogeography, 22, 223–232. https://doi.org/10.1111/j.1466-8238.2012.00796.x [Google Scholar]
- Li, L. , Wang, Z. , Zerbe, S. , Abdusalih, N. , Tang, Z. , Ma, M. , … Fang, J. (2013). Species richness patterns and water‐energy dynamics in the drylands of northwest China. PLoS ONE, 8, e66450 https://doi.org/10.1371/journal.pone.0066450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liaw, A. , & Wiener, M. (2002). Classification and regression by random forest. R News, 2, 18–22. [Google Scholar]
- Novillo, A. , & Ojeda, R. A. (2014). Elevation patterns in rodent diversity in the dry Andes: Disentangling the role of environmental factors. Journal of Mammalogy, 95, 99–107. https://doi.org/10.1644/13-MAMM-A-086.1 [Google Scholar]
- Oksanen, J. , Blanchet, F. G. , Kindt, R. , Legendre, P. , Minchin, P. R. , O'Hara, R. B. , … Wagner, H. (2015). vegan: Community ecology package. Retrieved from http://cran.r-project.org/package=vegan. [Google Scholar]
- Otto‐Bliesner, B. , Brady, E. , Clauzet, G. , Tomas, R. , Levis, S. , & Kothavala, Z. (2006). Last Glacial Maximum and Holocene Climate in CCSM3. Journal of Climate, 19, 2526–2544. https://doi.org/10.1175/JCLI3748.1 [Google Scholar]
- Pigot, A. L. , & Tobias, J. A. (2013). Species interactions constrain geographic range expansion over evolutionary time. Ecology Letters, 16, 330–338. https://doi.org/10.1111/ele.2013.16.issue-3 [DOI] [PubMed] [Google Scholar]
- Qian, H. , & Kissling, W. D. (2010). Spatial scale and cross‐taxon congruence of terrestrial vertebrate and vascular plant species richness in China. Ecology, 91, 1172–1183. https://doi.org/10.1890/09-0620.1 [DOI] [PubMed] [Google Scholar]
- Qu, Y. , Song, G. , Gao, B. , Quan, Q. , Ericson, P. G. P. , & Lei, F. (2015). The influence of geological events on the endemism of East Asian birds studied through comparative phylogeography. Journal of Biogeography, 42(179–192), 13 https://doi.org/10.1111/jbi.12407 [Google Scholar]
- R Core Team (2016). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Rahbek, C. , & Graves, G. R. (2001). Multiscale assessment of patterns of avian species richness. Proceedings of the National Academy of Sciences of the United States of America, 98, 4534–4539. https://doi.org/10.1073/pnas.071034898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricklefs, R. E. , & Jønsson, K. A. (2014). Clade extinction appears to balance species diversification in sister lineages of Afro‐Oriental passerine birds. Proceedings of the National Academy of Sciences of the United States of America, 111, 11756–11761. https://doi.org/10.1073/pnas.1411601111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandel, B. , Arge, L. , Dalsgaard, B. , Davies, R. G. , Gaston, K. J. , Sutherland, W. J. , & Svenning, J.‐C. (2011). The influence of Late Quaternary climate‐change velocity on species endemism. Science, 334, 660–664. https://doi.org/10.1126/science.1210173 [DOI] [PubMed] [Google Scholar]
- Schemske, D. W. , Mittelbach, G. G. , Cornell, H. V. , Sobel, J. M. , & Roy, K. (2009). Is there a latitudinal gradient in the importance of biotic interactions? Annual Review of Ecology, Evolution, and Systematics, 40, 245–269. https://doi.org/10.1146/annurev.ecolsys.39.110707.173430 [Google Scholar]
- Si, X. , Cadotte, M. W. , Zeng, D. , Baselga, A. , Zhao, Y. , Li, J. , … Ding, P. (2017). Functional and phylogenetic structure of island bird communities. Journal of Animal Ecology, 86, 532–542. https://doi.org/10.1111/1365-2656.12650 [DOI] [PubMed] [Google Scholar]
- Stein, A. , Gerstner, K. , & Kreft, H. (2014). Environmental heterogeneity as a universal driver of species richness across taxa, biomes and spatial scales. Ecology Letters, 17, 866–880. https://doi.org/10.1111/ele.2014.17.issue-7 [DOI] [PubMed] [Google Scholar]
- Svenning, J.‐C. , Eiserhardt, W. L. , Normand, S. , Ordonez, A. , & Sandel, B. (2015). The Influence of Paleoclimate on present‐day patterns in biodiversity and ecosystems. Annual Review of Ecology, Evolution, and Systematics, 46, 551–572. https://doi.org/10.1146/annurev-ecolsys-112414-054314 [Google Scholar]
- Wagenmakers, E. J. , & Farrell, S. (2004). AIC model selection using Akaike weights. Psychonomic Bulletin & Review, 11, 192–196. https://doi.org/10.3758/BF03206482 [DOI] [PubMed] [Google Scholar]
- Webb, C. O. , Ackerly, D. D. , McPeek, M. A. , & Donoghue, M. J. (2002). Phylogenies and community ecology. Annual Review of Ecology and Systematics, 33, 475–505. https://doi.org/10.1146/annurev.ecolsys.33.010802.150448 [Google Scholar]
- Wu, J. , Zhang, Q. , Li, A. , & Liang, C. (2015). Historical landscape dynamics of Inner Mongolia: Patterns, drivers, and impacts. Landscape Ecology, 30, 1579–1598. https://doi.org/10.1007/s10980-015-0209-1 [Google Scholar]
- Xu, R. (2007). Fauna of inner Mongolia, Volume III. Hohhot, China: Inner Mongolia University Press. (In Chinese.) [Google Scholar]
- Xu, R. (2015). Fauna of inner Mongolia, Volume IV. Hohhot, China: Inner Mongolia University Press. (In Chinese.) [Google Scholar]
- Zhang, J. , Kissling, W. D. , & He, F. (2013). Local forest structure, climate and human disturbance determine regional distribution of boreal bird species richness in Alberta, Canada. Journal of Biogeography, 40(6), 1131–1142. https://doi.org/10.1111/jbi.12063 [Google Scholar]
- Zhao, Y. (2012). Classification and its florristic ecological geographic distributions of vascular plants in inner Mongolia. Hohhot, China: Inner Mongolia University Press. (In Chinese.) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


