Abstract
Objective
Due to the threat of global warming, the livestock industry is increasingly interested in exploring how feed additives may reduce anthropogenic greenhouse gas emissions, especially from ruminants. This study investigated the effect of Rhodophyta supplemented bovine diets on in vitro rumen fermentation and rumen microbial diversity.
Methods
Cannulated Holstein cows were used as rumen fluid donors. Rumen fluid:buffer (1:2; 15 mL) solution was incubated for up to 72 h in six treatments: a control (timothy hay only), along with substrates containing 5% extracts from five Rhodophyta species (Grateloupia lanceolata [Okamura] Kawaguchi, Hypnea japonica Tanaka, Pterocladia capillacea [Gmelin] Bornet, Chondria crassicaulis Harvey, or Gelidium amansii [Lam.] Lamouroux).
Results
Compared with control, Rhodophyta extracts increased cumulative gas production after 24 and 72 h (p = 0.0297 and p = 0.0047). The extracts reduced methane emission at 12 and 24 h (p<0.05). In particular, real-time polymerase chain reaction analysis indicated that at 24 h, ciliate-associated methanogens, Ruminococcus albus and Ruminococcus flavefaciens decreased at 24 h (p = 0.0002, p<0.0001, and p<0.0001), while Fibrobacter succinogenes (F. succinogenes) increased (p = 0.0004). Additionally, Rhodophyta extracts improved acetate concentration at 12 and 24 h (p = 0.0766 and p = 0.0132), as well as acetate/propionate (A/P) ratio at 6 and 12 h (p = 0.0106 and p = 0.0278).
Conclusion
Rhodophyta extracts are a viable additive that can improve ruminant growth performance (higher total gas production, lower A/P ratio) and methane abatement (less ciliate-associated methanogens, Ruminococcus albus and Ruminococcus flavefaciens and more F. succinogenes.
Keywords: Growth Rate, In vitro, Rumen Fermentation, Methane, Rhodophyta
INTRODUCTION
The past decade has seen a rapid rise in anthropogenic greenhouse gas (GHG) emissions. Notably, methane gas has a global warming potential 25 times greater than carbon dioxide [1], and its concentrations has increased twofold since the early 1800s [2]. Agricultural activities are a major source of methane emission. In particular, ruminant livestock is responsible for 25% of atmospheric methane globally [3], representing a loss of gross energy intake that could reach 15% depending on feeding intensity, diet composition, and digestibility [3]. Therefore, animal nutritionists are extremely interested in manipulating the rumen microbial ecosystem to reduce methane emission without adverse effects on rumen function. Specifically, research is needed to identify feed additives that can modify ruminal fermentation characteristics and increase feed use efficiency, thereby inhibiting ruminal methanogenesis. Existing studies focused on determining the potential of feed additives in reducing livestock GHG emissions, while also improving feed use, diet digestibility, and ultimately livestock productivity [4]. Potential feed additives include halogenated analogues, monensin, and a range of plant compounds, such as essential oils, saponins, tannins, and various secondary metabolites [5]. Successful dietary changes would be beneficial for individual livestock producers and the industry.
One promising source for feed additives is algae, already economically important due to its current applications in human foods [6] and animal feed [7]. Algae exist in a wide range of forms, broadly classified according to size (micro- versus macroalgae) and primary pigments (green, red, or brown). In particular, macroalgae are a rich source of compounds essential for metabolic function, including various minerals, vitamins, proteins, lipids, and polysaccharides; thus, macroalgae additives can increase basal feed quality [8], animal growth rates, and feed conversion efficiency [9], as well as reduce enteric methane emission [10] in ruminants. Novel food ingredients [11] and many bioactive compounds [12] have been described in reports on algal composition and properties. However, few studies have determined exactly how algae feed additives influence fermentation characteristics and methane mitigation.
Therefore, our objective here was to investigate the effect of using Rhodophyta extracts as a dietary supplement on in vitro ruminal fermentation parameters and methane emission. In vitro techniques were used to allow for rapid screening of fermentation kinetics. Five algal species were compared against a control (no-additive) basal diet, including Grateloupia lanceolata (Okamura) Kawaguchi, Hypnea japonica Tanaka, Pterocladia capillacea (Gmelin) Bornet, Chondria crassicaulis Harvey, and Gelidium amansii (Lam.) Lamouroux.
MATERIALS AND METHODS
All experimental protocols were approved by the Animal Care and Use Committee of Gyeongsang National University (Jinju, Gyeongsangnam-do, Korea).
Preparation of Rhodophyta extracts
All algae extracts were obtained from the Jeju Biodiversity Research Institute (JBRI, Jeju, Korea) (Table 1). Each plant was washed, cut into small pieces, freeze-dried, and ground into powder. The powder was than extracted with 70% or 80% methyl alcohol, using an ultrasonic cleaner (Branson Ultrasonics corporation, Danbury, CT, USA) at room temperature. After extraction, eluates were filtered through Whatman No. 1 filter paper and concentrated under vacuum. Plant extracts were re-dissolved in dimethyl sulfoxide (Sigma-Aldrich Chemical Co., St. Louis, MO, USA) and diluted using culture media before experimentation.
Table 1.
General information on Rhodophyta extracts used in the experiment1)
| Stock No. | Scientific name | Family name | Part | Solvent |
|---|---|---|---|---|
| JBRI-10356 | Grateloupia lanceolata (Okamura) Kawaguchi | Halymeniaceae | Whole plant | 80% EtoH |
| JBRI-20219 | Hypnea japonica Tanaka | Hypneaceae | Whole plant | 80% EtoH |
| JBRI-20027 | Pterocladia capillacea (Gmelin) Bornet | Gelidiaceae | Whole plant | 80% EtoH |
| JBRI-20440 | Chondria crassicaulis Harvey | Rhodomelaceae | Whole plant | 70% EtoH |
| JBRI-10244 | Gelidium amansii (Lamouroux) Lamouroux | Gelidiaceae | Whole plant | 80% EtoH |
Plant extracts were obtained from Jeju Biodiversity Research institute (JBRI, Jeju, Korea) and data were provided by JBRI.
In vitro fermentation design
One cannulated Holstein cows (450±30 kg) was used as rumen fluid donors and provided with ad libitum access to a mineral-vitamin block and water. Twice daily (09:00 and 17:00), cows were fed 2% of their body weight in timothy hay and commercial concentrate at a 60:40 (w/w) ratio. Rumen fluid was collected before morning feedings and filtered through four layers of cheesecloth. Next, it was diluted with artificial saliva and stored at 39°C.
The chemical composition (% dry matter [DM] basis) of commercial timothy hay was as follows: moisture content, 8.87%; crude protein, 13.37%; ether extracts, 2.25%; crude fiber, 21.87%; crude ash, 8.62%; neutral detergent fiber, 53.18%; and acid detergent fiber, 30.57%.
The Rumen fluid was mixed with McDougall’s buffer in a 2:1 ratio. Next, 15 mL of the mixture was dispensed anaerobically into 50-mL serum bottles containing 0.3 g of timothy substrate and one of five Rhodophyta extracts (5% of substrate). Bottles were sealed anaerobically with an aluminum-capped butyl rubber stopper in pure N2 gas, and incubated in a shaking incubator (Jeio Tech, SI-900R, Daejeon, Korea; 120×rpm) at 39°C for 72 h. The in vitro fermentation experiment was a completely randomized block design and performed in triplicate, using 126 serum bottles (6 treatments×7 incubation times×3 replicates times).
Analysis of gas profiles and ruminal fermentation characteristics
Total gas production in the samples was measured with head space gas chromatography using a detachable pressure transducer and a digital readout voltmeter (Laurel Electronics, Inc., Costa Mesa, CA, USA). The transducer was connected to the inlet of a disposable Luer-lock three-way stopcock. Gas pressure in the headspace above the culture medium was read from the LED display unit after inserting a hypodermic syringe needle. Methane and carbon dioxide content was measured using a TCD detector with a Carboxen-1006 Plot capillary column (30 mm×0.53 mm, Supelco, Bellefonte, PA, USA), after connecting another stopcock outlet to a gas chromatograph (HP 5890, Agilent Technologies, Santa Clara, CA, USA).
Next, serum bottles were uncapped and the culture medium was subsampled for pH (MP230, Mettler-Toledo, Columbus, OH, USA), ammonia-N and volatile fatty acid (VFA) analyses. Ammonia-N concentration was measured as optical density (OD) values at 630 nm using a UV/VIS spectrophotometer (Model 680, Bio-Rad laboratories, Hercules, CA, USA). For VFA measurements, sub-samples were centrifuged at 3,000×rpm for 3 min. The resultant supernatant was filtered using a 0.2 μm disposable syringe filter (Whatman Inc., Clifton, NJ, USA) high performance liquid chromatography (Agilent-1200, Waldbronn, Germany) using a UV/VIS detector with a MetaCarb 87H column (300 mm× 7.8 mm, Varian, Palo Alto, CA, USA).
In vitro DM disappearance rate was determined following a modified Ørskov’s method, using nylon-bag digestion. After incubation, the nylon bag containing serum bottles was washed twice in a water-bath equipped with a Heidolph Rotamax 120 (Heidolph Instruments, Nuremberg, Germany) at 100×rpm for 30 min and then oven dried at 60°C to a constant weight. Dry matter disappearance was the difference in serum-bottle weight before and after incubation.
Microbial growth rate
At the end of each fermentation period, samples were centrifuged at 3,000×rpm for 3 min to remove feed particles. The supernatant was then re-centrifuged at 14,000×rpm for 3 min to obtain a final supernatants for protein and glucose analysis. Some of the supernatant was dyed with Coomassie Blue G-250 for spectrophotometrically measuring protein content as OD at 595 nm (Model 680, Bio-Rad Laboratories, USA). For measuring glucose, 200 μL of supernatant was mixed with 600 μL of DNS solution and incubated for 5 min in a boiling water bath. Glucose concentration was the OD at 595 nm, determined with a microplate reader (Model 680, Bio-Rad Laboratories, USA). Pellets from the centrifugation were washed with sodium phosphate buffer (pH 6.5) four more times and then subjected to OD measurements at 550 nm (Model 680, Bio-Rad Laboratories, USA) to evaluate microorganism growth rates.
Quantitative real-time polymerase chain reaction
Samples were placed in screw-capped tubes containing silica beads for DNA extraction with a high-speed reciprocal shaker, following a modified bead-beating protocol with a Soil kit (Macherey-nagel, Düren, Germany). Briefly, a 1.0-mL aliquot of the incubated culture solution was centrifuged at 3,000×rpm, and then placed in a NanoDrop Spectrophotometer (Thermo Scientific, Wilmington, DE, USA) to determine nucleic acid concentrations.
Previous reports provided primers and thermocycling protocols used for amplification of general bacteria [13], ciliate protozoa [14], methanogenic archaea [15], Fibrobacter succinogenes (F. succinogenes) [13], Ruminococcus albus (R. albus)[16] and Ruminococcus flavefaciens (R. flavefaciens) [13].
Quantitative real-time polymerase chain reaction (qPCR) assays (CFX96 Real-Time system; Bio Rad, USA) using the SYBR Green Supermix (QPK-201, Toyobo Co., LTD., Tokyo, Japan) were performed following previously described methods (Denman and McSweeney [13] and Denman et al [15]). Microbial abundance was expressed with the following equation: relative quantification = 2−ΔCt(Target)−ΔCt(Control), where Ct represents threshold cycle. The qPCR reaction mixtures (20 μL) contained forward/ reverse primers, SYBR Green Supermix and DNA template.
Statistical analysis
All data were analyzed using the general linear model procedure of SAS [17]. Between-treatment differences were examined using Duncan’s multiple comparison tests. Orthogonal contrasts were used to test the overall effect of Rhodophyta supplementation (control [CON] vs treatment) and the effect H. japonica (control [CON] vs GLK). Data are presented as means±standard error of the mean. Significance was set at p<0.05, whereas p<0.10 was considered a tendency.
RESULTS
In vitro fermentation characteristics
Compared with CON, Rhodophyta extracts raised pH at 6, 9, 12, and 72 h (p = 0.0006, p = 0.0330, p = 0.0004, and p = 0.0136), while GLK raised pH at 6, and 12 h (p = 0.0006 and p = 0.0004)(Table 2). Rhodophyta extracts and GLK decreased cumulative gas production by ruminal microbes at 3 h (p = 0.0001 and p = 0.0016) and 6 h (p = 0.0149 and p = 0.0536), but increased production at 24 h while HJT (and p = 0.0058) and 72 h (p = 0.0047 and p = 0.0133). Except at 72 h (p = 0.0249), none of the Rhodophyta extracts significantly affected DM disappearance during the experiment (Table 2).
Table 2.
Effects of Rhodophyta extracts on in vitro rumen microbial fermentation, specifically cumulative pH, gas production, and dry matter (DM) disappearance
| Incubation times (h) | CON | Treatments1) | SEM | Contrast | |||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| GLK | HJT | PCB | CCH | GAL | CON vs Others | CON vs HJT | |||
| pH | |||||||||
| 3 | 7.35c | 7.35c | 7.42b | 7.44ab | 7.46a | 7.47a | 0.02 | 0.0003 | 0.0057 |
| 6 | 7.29c | 7.37b | 7.38b | 7.42ab | 7.46a | 7.44ab | 0.04 | 0.0006 | 0.0255 |
| 9 | 7.12c | 7.24abc | 7.17bc | 7.22abc | 7.29ab | 7.34a | 0.08 | 0.0330 | 0.5367 |
| 12 | 7.00c | 7.01c | 7.14b | 7.23ab | 7.28a | 7.29a | 0.05 | 0.0004 | 0.0139 |
| 24 | 6.46ab | 6.39b | 6.37b | 6.52ab | 6.51ab | 6.60a | 0.10 | 0.7227 | 0.3162 |
| 48 | 6.20ab | 6.15b | 6.16ab | 6.23ab | 6.26a | 6.25ab | 0.05 | 0.7995 | 0.4314 |
| 72 | 6.12b | 6.15ab | 6.16ab | 6.20a | 6.19a | 6.19a | 0.05 | 0.0136 | 0.1591 |
| Total gas production (mL/g DM) | |||||||||
| 3 | 151.20a | 148.29b | 146.97bc | 145.50c | 145.66c | 143.28d | 1.15 | 0.0001 | 0.0016 |
| 6 | 161.97a | 152.84b | 152.31b | 151.15b | 150.88b | 150.14b | 2.75 | 0.01492 | 0.0536 |
| 9 | 167.72ab | 165.03bc | 165.61abc | 169.57a | 164.61bc | 162.87c | 2.16 | 0.0962 | 0.1194 |
| 12 | 177.86 | 179.29 | 176.54 | 177.39 | 175.70 | 174.28 | 3.42 | 0.5062 | 0.5777 |
| 24 | 238.43b | 249.20ab | 252.84a | 248.19ab | 241.91ab | 240.54ab | 6.48 | 0.0297 | 0.0058 |
| 48 | 260.92 | 268.53 | 267.47 | 260.76 | 261.08 | 263.35 | 5.16 | 0.4663 | 0.2106 |
| 72 | 271.75b | 279.61a | 279.09a | 281.93a | 276.5ab | 275.65ab | 3.59 | 0.0047 | 0.0133 |
| DM disappearance (%) | |||||||||
| 3 | 21.03 | 20.54 | 21.62 | 24.39 | 21.46 | 21.39 | 2.33 | 0.5596 | 0.7541 |
| 6 | 22.25 | 22.32 | 22.42 | 22.35 | 22.83 | 22.15 | 0.85 | 0.7418 | 0.7926 |
| 9 | 24.31 | 24.80 | 24.40 | 26.53 | 24.95 | 25.71 | 1.45 | 0.3473 | 0.9440 |
| 12 | 28.08 | 27.89 | 27.39 | 26.67 | 26.68 | 27.07 | 1.34 | 0.2044 | 0.4591 |
| 24 | 38.40 | 41.02 | 40.41 | 40.67 | 41.98 | 38.56 | 2.65 | 0.2231 | 0.3639 |
| 48 | 47.57 | 48.25 | 48.24 | 46.36 | 47.35 | 44.97 | 1.88 | 0.6467 | 0.6529 |
| 72 | 51.08a | 48.69ab | 49.33ab | 49.65ab | 46.97b | 48.13ab | 1.74 | 0.0249 | 0.1872 |
SEM, standard error of the mean; DM, dry matter.
Dietary treatments were as follows: CON, basal diet (without Rhodophyta extracts); GLK, 5% Grateloupia lanceolata (Okamura) Kawaguchi; HJT, 5% Hypnea japonica Tanaka; PCB, 5% Pterocladia capillacea (Gmelin) Bornet; CCH, Chondria crassicaulis Harvey; GAL, 5% Gelidium amansii (Lam.) Lamouroux percentages are based on substrate (timothy hay) amount.
Means with different superscripts in the same row indicate significant differences (p<0.05).
Rhodophyta extracts and GLK reduced methane emissions relative to CON at 12 h (p = 0.0077 and p = 0.0306) and 24 h (p = 0.0008 and p = 0.0183) (Table 3). Furthermore, at 9 h Rhodophyta extracts were not reduced carbon dioxide, but ammonia reduce (p = 0.0440 and p = 0.0419), while GLK reduced ammonia (p = 0.0414) (Table 3).
Table 3.
Effect of Rhodophyta extracts on methane, carbon dioxide, and ammonia emissions during in vitro mixed rumen microbial fermentation
| Incubation (h) | CON | Treatments1) | SEM | Contrast | |||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| GLK | HJT | PCB | CCH | GAL | CON vs Others | CON vs HJT | |||
| Methane emission (mL/g DM) | |||||||||
| 3 | 0.51 | 0.55 | 0.60 | 0.57 | 0.55 | 0.56 | 0.20 | 0.7143 | 0.6380 |
| 6 | 1.22 | 1.14 | 0.92 | 1.02 | 1.21 | 1.31 | 0.33 | 0.6411 | 0.2810 |
| 9 | 2.21ab | 2.21ab | 2.35ab | 2.82a | 2.48ab | 2.03b | 0.55 | 0.8823 | 0.7830 |
| 12 | 4.71a | 3.36b | 2.96b | 3.35b | 2.16b | 2.76b | 0.62 | 0.0077 | 0.0306 |
| 24 | 15.16a | 7.49b | 10.09b | 9.46b | 9.12b | 9.12b | 2.57 | 0.0008 | 0.0183 |
| 48 | 29.29 | 29.51 | 28.57 | 31.27 | 32.36 | 32.05 | 5.31 | 0.9666 | 0.8938 |
| 72 | 39.23ab | 37.26ab | 34.57b | 35.24ab | 42.21a | 40.14ab | 3.30 | 0.6446 | 0.2303 |
| Carbon dioxide production (mL/g DM) | |||||||||
| 3 | 18.89 | 20.88 | 19.58 | 18.18 | 16.53 | 19.73 | 2.48 | 0.7555 | 0.4091 |
| 6 | 25.70 | 24.48 | 24.66 | 24.05 | 21.93 | 23.45 | 1.93 | 0.2795 | 0.5706 |
| 9 | 36.15b | 42.88ab | 40.82ab | 47.16a | 41.02ab | 38.06b | 3.74 | 0.0440 | 0.1970 |
| 12 | 56.77 | 52.47 | 52.38 | 51.41 | 48.28 | 55.97 | 8.65 | 0.4169 | 0.5668 |
| 24 | 116.38 | 113.77 | 131.88 | 107.76 | 124.11 | 106.26 | 14.36 | 0.5176 | 0.2312 |
| 48 | 132.16 | 155.91 | 148.45 | 132.84 | 140.13 | 150.75 | 13.16 | 0.3391 | 0.2543 |
| 72 | 170.65 | 180.96 | 169.15 | 177.34 | 215.46 | 188.69 | 21.12 | 0.5009 | 0.6047 |
| Ammonia production (mg/dL) | |||||||||
| 3 | 1.90 | 2.52 | 2.58 | 2.05 | 2.21 | 2.13 | 0.03 | 0.8463 | 0.8457 |
| 6 | 2.62b | 3.43a | 3.25ab | 3.28ab | 2.95ab | 3.03b | 0.00 | 0.3424 | 0.3071 |
| 9 | 3.28b | 3.80a | 3.77a | 3.68a | 3.72a | 3.75a | 0.02 | 0.0419 | 0.0414 |
| 12 | 4.38 | 4.53 | 4.38 | 4.15 | 4.28 | 4.10 | 0.03 | 0.4796 | 0.4246 |
| 24 | 10.43 | 10.09 | 12.30 | 12.22 | 9.36 | 11.20 | 0.22 | 0.7092 | 0.3632 |
| 48 | 24.56 | 24.96 | 24.86 | 20.94 | 20.67 | 23.27 | 0.22 | 0.1266 | 0.6478 |
| 72 | 30.78 | 30.80 | 29.01 | 31.02 | 29.58 | 29.21 | 0.21 | 0.7306 | 0.8486 |
SEM, standard error of the mean; DM, dry matter.
Dietary treatments were as follows (percent basis of timothy substrate): CON, basal diet (timothy without Rhodophyta extracts); GLK, 5% Grateloupia lanceolate (Okamura) Kawaguchi; HJT, 5% Hypnea japonica Tanake; PCB, 5% Pterocladia capillacea (Gmelin) Bornet; CCH, Chondria crassicaulis Harvey; GAL, Gelidium amansii (Lam.) Lamouroux.
Means with different superscripts in the same row indicate significant differences significantly (p<0.05).
Rhodophyta extracts improved acetate concentration over CON at 12 and 24 h (p = 0.0766 and p = 0.0132), while GLK exerted the same effect at 24 h (p = 0.0187) (Table 4). Except at 12 h (p = 0.0659), Rhodophyta extracts did not significantly differ from CON in effects on propionate concentration. Overall, Rhodophyta extracts and GLK improved A/P ratio at 6 h (p = 0.0106 and p = 0.0118) and 12 h (p = 0.0278 and p = 0.0398).
Table 4.
Effects of Rhodophyta extracts on acetic acid, propionic acid, and acetic acid/propionic acid (A/P) ratio during mixed rumen microbial fermentation
| Incubation (h) | CON | Treatments1) | SEM | Contrast | |||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| GLK | HJT | PCB | CCH | GAL | CON vs Others | CON vs HJT | |||
| Acetic acid concentration (mM/g) | |||||||||
| 3 | 25.41 | 25.70 | 25.55 | 25.48 | 25.95 | 24.60 | 1.75 | 0.9356 | 0.6172 |
| 6 | 27.37bc | 28.52ab | 28.52ab | 29.76a | 29.05ab | 26.50c | 0.89 | 0.4376 | 0.7281 |
| 9 | 28.76c | 33.40a | 30.43bc | 32.27ab | 33.31a | 31.90ab | 1.38 | 0.2816 | 0.3021 |
| 12 | 31.09c | 42.37a | 33.94bc | 34.49bc | 35.76b | 36.63b | 2.25 | 0.0766 | 0.4842 |
| 24 | 52.98b | 57.00a | 59.34a | 57.81a | 57.35a | 56.96a | 2.25 | 0.0132 | 0.0187 |
| 48 | 67.14abc | 68.43ab | 70.92a | 62.90c | 64.52bc | 62.89c | 2.82 | 0.4979 | 0.1629 |
| 72 | 75.88 | 80.48 | 76.35 | 73.04 | 79.66 | 75.06 | 3.80 | 0.7305 | 0.9382 |
| Propionic acid concentration (mM/g) | |||||||||
| 3 | 4.51 | 4.85 | 4.71 | 6.06 | 5.29 | 5.78 | 1.75 | 0.2197 | 0.4275 |
| 6 | 6.77b | 8.07ab | 9.99a | 9.86a | 9.79a | 9.44a | 1.00 | 0.7694 | 0.4424 |
| 9 | 8.95 | 12.34 | 12.33 | 10.99 | 11.95 | 12.14 | 2.76 | 0.1731 | 0.1583 |
| 12 | 11.20c | 24.20a | 19.90ab | 17.86ab | 18.93ab | 15.57bc | 3.37 | 0.0659 | 0.3819 |
| 24 | 25.11bc | 28.87a | 26.76b | 23.86c | 26.34b | 26.28b | 0.87 | 0.3991 | 0.4483 |
| 48 | 28.11ab | 29.87a | 27.85ab | 26.41b | 29.26a | 28.09ab | 1.34 | 0.9863 | 0.1338 |
| 72 | 29.41bc | 34.96a | 32.03ab | 27.21c | 33.49a | 31.26ab | 2.07 | 0.7666 | 0.9417 |
| A/P ratio | |||||||||
| 3 | 5.63 | 5.63 | 5.42 | 4.70 | 5.54 | 4.70 | 1.53 | 0.1198 | 0.5389 |
| 6 | 4.04a | 3.53a | 2.88b | 3.07b | 2.98b | 2.84b | 0.39 | 0.0106 | 0.0118 |
| 9 | 3.23 | 3.23 | 2.50 | 2.94 | 2.81 | 2.66 | 0.58 | 0.4799 | 0.7381 |
| 12 | 2.78a | 1.82b | 1.81b | 1.96b | 1.91b | 2.37ab | 0.33 | 0.0278 | 0.0398 |
| 24 | 2.11bc | 1.97c | 2.22b | 2.43a | 2.18bc | 2.17bc | 0.11 | 0.0301 | 0.0996 |
| 48 | 2.39ab | 2.39b | 2.55a | 2.38ab | 2.21b | 2.24b | 0.11 | 0.4668 | 0.5251 |
| 72 | 2.58 | 2.58 | 2.40 | 2.69 | 2.38 | 2.41 | 0.20 | 0.9440 | 0.9072 |
SEM, standard error of the mean; A/P, acetate/propionate.
Dietary treatments were as follows (percent basis of timothy substrate): CON, basal diet (timothy without Rhodophyta extracts); GLK, 5% Grateloupia lanceolata (Okamura) Kawaguchi; HJT, 5% Hypnea japonica Tanaka; PCB, 5% Pterocladia capillacea (Gmelin) Bornet; CCH, 5% Chondria crassicaulis Harvey; GAL, 5% Gelidium amansii (Lam.) Lamouroux.
Means with different superscripts in the same row differ significantly (p<0.05).
In vitro ruminal change in microbial diversity
Rhodophyta extracts reduced microbial growth rate compared with CON at 6 h (p = 0.0020 and p = 0.0072), but GLK increased growth rate at 24 h (p = 0.0247)(Table 5). Additionally, both Rhodophyta extracts and GLK increased protein concentration at 9 h expect to HJT, PCB extracts (p = 0.0298 and p = 0.0711). PCB extract, glucose concentration significantly decreased at 3 h (p = 0.0673), then increased at 24 HJT, PCB extracts and 48 h PCB extract (p = 0.0163 and p = 0.0867); glucose also increased under GLK at 24 h (p = 0.0070)(Table 5).
Table 5.
Effects of Rhodophyta extracts on rumen microbial growth rate, as well as protein and glucose concentrations
| Incubation (h) | CON | Treatments1) | SEM | Contrast | |||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| GLK | HJT | PCB | CCH | GAL | CON vs Others | CON vs HJT | |||
| Microbial growth rate (OD at 550 nm) | |||||||||
| 3 | 0.20 | 0.19 | 0.19 | 0.17 | 0.17 | 0.17 | 0.02 | 0.0822 | 0.5960 |
| 6 | 0.20a | 0.20a | 0.16b | 0.16b | 0.14b | 0.16b | 0.02 | 0.0020 | 0.0072 |
| 9 | 0.28 | 0.32 | 0.31 | 0.28 | 0.31 | 0.32 | 0.03 | 0.1542 | 0.2201 |
| 12 | 0.39 | 0.40 | 0.40 | 0.43 | 0.37 | 0.42 | 0.10 | 0.4690 | 0.6618 |
| 24 | 0.53b | 0.56ab | 0.63a | 0.56ab | 0.55ab | 0.54b | 0.05 | 0.2240 | 0.0247 |
| 48 | 0.50 | 0.51 | 0.49 | 0.42 | 0.43 | 0.42 | 0.06 | 0.2595 | 0.8321 |
| 72 | 0.47 | 0.46 | 0.41 | 0.49 | 0.42 | 0.45 | 0.05 | 0.5029 | 0.2191 |
| Protein concentration (mM/g) | |||||||||
| 3 | 0.20 | 0.20 | 0.19 | 0.20 | 0.21 | 0.20 | 0.01 | 0.5146 | 0.2318 |
| 6 | 0.20 | 0.19 | 0.20 | 0.19 | 0.19 | 0.20 | 0.01 | 0.2117 | 0.7021 |
| 9 | 0.16b | 0.23a | 0.22ab | 0.16b | 0.26a | 0.24a | 0.04 | 0.0298 | 0.0711 |
| 12 | 0.20 | 0.13 | 0.15 | 0.20 | 0.14 | 0.24 | 0.09 | 0.6423 | 0.5513 |
| 24 | 0.14 | 0.14 | 0.12 | 0.14 | 0.13 | 0.15 | 0.02 | 0.8104 | 0.2606 |
| 48 | 0.10 | 0.15 | 0.15 | 0.14 | 0.15 | 0.16 | 0.07 | 0.3134 | 0.4504 |
| 72 | 0.13 | 0.14 | 0.13 | 0.14 | 0.14 | 0.14 | 0.01 | 0.2810 | 0.6172 |
| Glucose concentration (mL/mg) | |||||||||
| 3 | 0.55a | 0.49ab | 0.48ab | 0.43b | 0.51ab | 0.47ab | 0.06 | 0.0673 | 0.1473 |
| 6 | 0.21 | 0.22 | 0.18 | 0.25 | 0.22 | 0.28 | 0.07 | 0.6734 | 0.6699 |
| 9 | 0.17 | 0.17 | 0.18 | 0.17 | 0.16 | 0.16 | 0.02 | 0.8137 | 0.3129 |
| 12 | 0.14 | 0.13 | 0.14 | 0.15 | 0.14 | 0.14 | 0.01 | 0.6933 | 0.6498 |
| 24 | 0.10b | 0.12a | 0.12a | 0.12a | 0.10b | 0.10b | 0.01 | 0.0163 | 0.0070 |
| 48 | 0.09b | 0.11ab | 0.10ab | 0.11a | 0.09ab | 0.10ab | 0.01 | 0.0867 | 0.1780 |
| 72 | 0.10 | 0.10 | 0.11 | 0.10 | 0.11 | 0.11 | 0.02 | 0.5719 | 0.4970 |
SEM, standard error of the mean; OD, optical density.
Dietary treatments were as follows (percent basis of timothy substrate): CON, basal diet (timothy without Rhodophyta extracts); GLK, 5% Grateloupia lanceolata (Okamura) Kawaguchi; HJT, 5% Hypnea japonica Tanaka; PCB, 5% Pterocladia capillacea (Gmelin) Bornet; CCH, 5% Chondria crassicaulis Harvey; GAL, 5% Gelidium amansii (Lam.) Lamouroux.
Means with different superscripts in the same row indicate significant differences (p<0.05).
Compared with CON, Rhodophyta supplementation decreased the ciliate-associated methanogen population at 24 h (p = 0.0002; Figure 1), but not the Methanogenic archaea population Rhodophyta extracts also significantly reduced R. albus at 12 and 24 h (p = 0.0860 and p<0.0001), as well as R. flavefaciens at 24 h (p<0.0001)(Figure 1). Furthermore, F. succinogenes populations increased at 12 and 24 h (p = 0.0049 and p = 0.0004) after Rhodophyta treatments (Figure 1).
Figure 1.
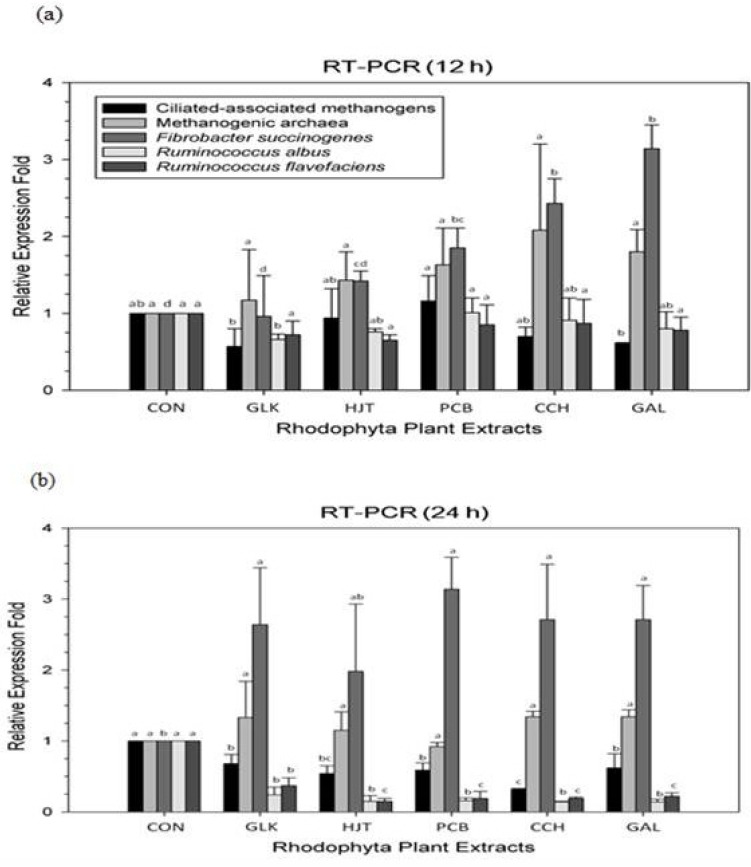
Relative quantification of rumen microbial populations under in vitro ruminal fermentation after 12 h (a) and 24 h (b) incubation with various Rhodophyta extracts. Control, no addition; GLK, Grateloupia lanceolata (Okamura) Kawaguchi; HJT, Hypnea japonica Tanaka; PCB, Pterocladia capillacea (Gmelin) Bornet; CCH, Chondria crassicaulis Harvey; GAL, Gelidium amansii (Lam.) Lamouroux. All extracts were 5% of the substrate(timothy hay) amount. abcd Means with different superscripts in the same row differ significantly (p<0.05).
DISCUSSION
This study investigated the effects of five Rhodophyta species on in vitro fermentation characteristics and changes to ruminal of microbial diversity. Below, we discussed the implications of our results in the context of previous research.
In vitro fermentation characteristics
Overall, pH remained consistently within 6.12 and 7.47 across Rhodophyta-extract treatments. Interestingly, CON had the lowest post-fermentation pH, suggesting that Rhodophyta supplementation creates a more alkaline environment during microbial fermentation. Because a pH range of 5.0 to 7.8 is ideal for ruminal microbial activity [18], the algal extracts likely had a negative effect.
Dietary fiber from Rhodophyta increased total gas production without any difference in DM disappearance rate. Overall, DM disappearance did not significantly differ across time points and Rhodophyta species, except with Chondria crassicaulis at 72 h. Additionally, total gas production of all Rhodophyta-extract treatments was significantly higher than CON only at 24 and 72 h incubation, hinting at a strategy for improving feed efficiency. Similarly interesting effects of dietary fiber in algae have been previously reported [19]. Overall, all tested Rhodophyta extracts have the potential to improve gas production and fermentation management, thus assisting in ruminant feeding.
Although the use of terrestrial plants to manipulate enteric methane emission have been extensively investigated [3], our study is the first to provide evidence that Rhodophyta extracts can effectively reduce in vitro methane emission and alter rumen microbial diversity. Thus, our data supports the hypothesis put forth by several previous reserarchers [20]. All Rhodophyta extracts significantly reduced methane emission after 12 and 24 h of incubation, with H. japonica having the strongest effect. Reduced methane emission may have been partially due to alterations in microbial diversity; protozoans (ciliate-associated methanogens) [21] and fibrolytic microbes (R. albus [22]; R. flavefaciens [23]) both decreased, while F. succinogenes increased [21]. R. albus is a very promising candidate for producing H2 from plant forage, because the bacteria can digest cellulosic and hemicellulosic biomass [22]. Likewise, R. favefaciens normally produces succinic acid as a major fermentation product together with acetic and formic acids, H2, and carbon dioxide. In contrast, F. succinogenes is a non-H2-producing species. A previous study [24] showed that when the dominant fibrolytic species was non-H2-producing, methane emission decreased significantly without impairing fiber degradation and fermentations in the rumen. Together, these results suggest that H2 is critical to the intestinal microbial ecosystem of ruminants. Thus, because hydrogen produced during ruminant enteric fermentation is the precursor of methane emission, regulating of H2 is more critical to controlling ruminant methane emission than regulating methane directly.
Ruminal methanogens primarily use hydrogen and carbon dioxide during methanogenesis, along with formate derived from acetate production. When hydrogen is removed [25], methanogen consumption of carbon dioxide and formate allows fermentation-related microbes to function optimally and support complete substrate oxidation [26]. Our study suggests that Rhodophyta extracts increase carbon dioxide emission at 9 h, as well as acetate concentration at 12 and 24 h. Unfortunately, we could not detect enough hydrogen to in this study to directly test our hypothesis regarding the removal of H2 gas.
Methane emission reduction at 12 and 24 h can be attributed to increased propionate concentration at 12 h. The increase results in more hydrogen use instead of acetate, thus lowering A/P ratios (relative to CON) and indicating a clear effect on fermentation. However, the A/P ratios were within optimal fermentation conditions [25]. Rhodophyta extracts resulted in a significant increase in acetate and propionate concentrations and a lower A/P ratio than CON at 12 h incubation, demonstrating that fermentation was significantly affected. These effects are likely due to secondary metabolites in plants that exert anti-microbial properties. In Rhodophyta specifically, secondary metabolites include terpenes [27] and halogenated compounds [28] that inhibit a wide range of microorganisms. We conclude that the extract dosage used in our study was sufficient to reduce methane emission without seriously affecting nutritionally important fermentation parameters.
Microbial growth rate
Rhodophyta extracts significantly reduced microbial growth rate at 6 h but increased at 24 h. This pattern probably occurred because rumen microorganisms were acclimating to changing environmental conditions during the first 6 h, and increased exponentially when they adapted (at around 24 h). After 48 h, however, nutrient depletion and increasing amounts of waste likely inhibited microbial growth [18]. Furthermore, we observed that Rhodophyta extract treatments with elevated microbial growth rates also had higher total gas production and lower pH than CON. This outcome agrees with previous research showing that rumen-microbe growth rate is closely correlated with total gas production and fermentation.
Although rumen ammonia concentration can vary based on feed protein proportions and degradation rate, our use of timothy hay as the sole substrate led to a lack of significant differences in ammonia concentration (except at 6 h). Optimal ammonia concentration for ruminal microbe growth is 8 mg/dL, whereas a concentration ≥140 mg/dL is inhibitory [18]. Maintaining optimal ammonia concentration can improve protein synthesis in most ruminal microorganisms but the two variables are not correlated [29]. Overall, the observed range of ammonia concentrations (1.90 to 30.80 mg/dL) is a strong indicator of proper rumen fermentation, with no negative side effects resulting from the Rhodophyta extracts.
Finally, in ruminants, rumen microbial fermentation releases VFAs as the major end products, instead of glucose. Here, we observed the propionate increased significantly at 12 h and 24 h, correlating with a later glucose increase at 24 h and 48 h. This outcome in line with the fact that propionate is the most abundant of the glucogenic acids (~15% to 40% of total ruminally released organic acids) and the predominant substrate for gluconeogenesis in ruminants [30], a characteristic that qualitatively distinguishes ruminant and non-ruminant gluconeogenesis
CONCLUSION
The results of our study indicate that Rhodophyta extracts are a viable feed additive that can improve ruminant growth performance (increased total gas production and decreased acetate/propionate ratio) and reduce methane emissions (decreased ciliate-associated methanogens, R. albus and R. flavefaciens). Although more research is necessary to clarify the exact effects of specific Rhodophyta on feed intake, feed use efficiency, and methane abatement, we found that Hypnea japonica significantly reduced methane production and had a moderate effect on total gas. These results are promising and suggest that Rhodophyta extracts could mitigate undesirable outcomes of rumen fermentation.
ACKNOWLEDGMENTS
This work was supported by the National Foundation of Korea Grant funded by the Korean Government (NRF-2015R1A6A1A 03031413). Jin Suk Jeong was supported by Postdoctoral Fellowship from the BK 21+ Program, the Ministry of Education, Science and Technology, Republic of Korea. This work was presented as a part of a doctoral dissertation by Nyeon Hak Shin.
Footnotes
CONFLICT OF INTEREST
We certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
REFERENCES
- 1.Denman K, Brasseur G, Chidthaisong A, et al. Couplings between changes in the climate system and biogeochemistry. In: Solomon S, Qin D, Manning M, et al., editors. Climate change 2007: the physical science basis, contribution of working group I to the fourth assessment report of the intergovernmental panel on climate change. Cambridge, UK: Cambridge University Press; 2007. pp. 499–587. [Google Scholar]
- 2.Wuebbles DJ, Hayhoe K. Atmospheric methane and global change. Earth Sci Rev. 2002;57:177–210. [Google Scholar]
- 3.Patra AK. Enteric methane mitigation technologies for ruminant livestock: a synthesis of current research and future directions. Environ Monit Assess. 2012;184:1929–52. doi: 10.1007/s10661-011-2090-y. [DOI] [PubMed] [Google Scholar]
- 4.Grainger C, Beauchemin KA. Can enteric methane emissions from ruminants be lowered without lowering their production. Anim Feed Sci Technol. 2011;166–67:308–20. [Google Scholar]
- 5.Kamra DN, Agarwal N, Chaudhary LC. Inhibition of ruminal methanogenesis by tropical plants containing secondary compounds. Int Congr Ser. 2006;1293:156–63. [Google Scholar]
- 6.MacArtain P, Gill CIR, Brooks M, et al. Nutritional value of edible seaweeds. Nutr Rev. 2007;65:535–43. doi: 10.1301/nr.2007.dec.535-543. [DOI] [PubMed] [Google Scholar]
- 7.Chopin T, Sawhney M. Seaweeds and their mariculture. In: Steele JH, Thorpe SA, Turekian KK, editors. The encyclopedia of ocean sciences. Oxford, UK: Elsevier; 2009. pp. 4477–87. [Google Scholar]
- 8.Paul N, Tseng CK. Seaweed. In: Lucas JS, Southgate PC, editors. Aquaculture: farming aquatic animals and plants. 2nd edition. Oxford, UK: Blackwell publishing Ltd; 2012. pp. 268–84. [Google Scholar]
- 9.Chowdhury S, Huque K, Khatun M. Algae in animal production. Agracultural Science of Biodiversity and Sustainability Workshop; Tune Landboskole, Denmark. 1995. pp. 3–7. [Google Scholar]
- 10.Bozic A, Anderson R, Carstens G, et al. Effects of the methane-inhibitors nitrate, nitroethane, lauric acid, Lauricidin® and the Hawaiian marine algae Chaetoceros on ruminal fermentation in vitro . Biore Technol. 2009;100:4017–25. doi: 10.1016/j.biortech.2008.12.061. [DOI] [PubMed] [Google Scholar]
- 11.Plaza M, Cifuentes A, Ibanez E. In the search of new functional food ingredients from algae. Trends Food Sci Technol. 2008;19:31–9. [Google Scholar]
- 12.Holdt SL, Kraan S. Bioactive compounds in seaweed: functional food applications and legislation. J Appl Phycol. 2011;23:543–97. [Google Scholar]
- 13.Denman SE, McSweeney CS. Development of a Real-Time PCR assay for monitoring anaerobic fungal and cellulolytic bacterial populations within the rumen. FEMS Microbiol Ecol. 2006;58:572–82. doi: 10.1111/j.1574-6941.2006.00190.x. [DOI] [PubMed] [Google Scholar]
- 14.Skillman LC, Toovey AF, Williams AJ, et al. Development and validation of a real-time PCR method to quantify rumen protozoa and examination of variability between Entodinium populations in sheep offered a hay-based diet. Appl Environ Microbiol. 2006;72:200–6. doi: 10.1128/AEM.72.1.200-206.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Denman SE, Tomkins NW, McSweeney CS. Quantitation and diversity analysis of ruminal methanogenic populations in response to the antimethanogenic compound bromochloromethane. FEMS Microbiol Ecol. 2007;62:313–22. doi: 10.1111/j.1574-6941.2007.00394.x. [DOI] [PubMed] [Google Scholar]
- 16.Koike S, Kobayashi Y. Development and use of competitive PCR assays for the rumen cellulolytic bacteria: Fibrobacter succinogenes, Ruminococcus albus and Ruminococcus flavefaciens . FEMS Microbiol Ecol. 2001;204:361–6. doi: 10.1111/j.1574-6968.2001.tb10911.x. [DOI] [PubMed] [Google Scholar]
- 17.SAS Institute Inc . SAS/STAT user’s guide: version 9.2 edn. Cary, NC, USA: SAS Institute Inc.; 2002. [Google Scholar]
- 18.Ha JK, Lee SS, Moon YS, et al. Ruminant nutrition and physiology. Seoul, Korea: Seoul National University Press; 2005. [Google Scholar]
- 19.Denis C, Morançais M, Li M, et al. Study of the chemical composition of edible red macroalgae Grateloupia turuturu from Brittany (France) J Food Chem. 2010;119:913–7. [Google Scholar]
- 20.Dubois B, Tomkins NW, Kinley RD, et al. Effect of tropical algae as additives on rumen in vitro gas production and fermentation characteristics. Am J Plant Sci. 2013;4:34–43. [Google Scholar]
- 21.Kim ET, Lee SJ, Guan LL, et al. Effects of flavonoid-rich plant extracts on in vitro ruminal methanogenesis, microbial populations and fermentation characteristics. Asian-Australas J Anim Sci. 2015;28:530–7. doi: 10.5713/ajas.14.0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ntaikou I, Gavala HN, Kornaros M, et al. Hydrogen production from sugars and sweet sorghum biomass using Ruminococcus albus . Int J Hydrogen Energy. 2008;33:1153–63. [Google Scholar]
- 23.Latham MJ, Wolin MJ. Fermentation of cellulose by Ruminococcus flavefaciens in the presence and absence of Methanobacterium ruminantium. Appl Environ Microbiol. 1977;34:297–301. doi: 10.1128/aem.34.3.297-301.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chaucheyras-Durand F, Masséglia S, Fonty G, et al. Influence of the composition of the cellulolytic flora on the development of hydrogenotrophic microorganisms, hydrogen utilization, and methane production in the rumens of gnotobiotically reared lambs. Appl Environ Microbiol. 2010;76:7931–7. doi: 10.1128/AEM.01784-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitsumori M, Sun W. Control of rumen microbial fermentation for mitigating methane emissions from the rumen. Asian-Australas J Anim Sci. 2008;21:144–54. [Google Scholar]
- 26.Martin C, Morgavi DP, Doreau M. Methane mitigation in ruminants: from microbe to the farm scale. Anim. 2010;4:351–65. doi: 10.1017/S1751731109990620. [DOI] [PubMed] [Google Scholar]
- 27.Davyt D, Fernandez R, Suescun L, et al. New sesquiterpene derivatives from the red alga Laurencia scoparia. Isolation, structure determination, and anthelmintic activity. J Nat Prod. 2001;64:1552–5. doi: 10.1021/np0102307. [DOI] [PubMed] [Google Scholar]
- 28.Cabrita MT, Vale C, Rauter AP. Halogenated compounds from marine algae. Mar Drugs. 2010;8:2301–17. doi: 10.3390/md8082301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mehrez AZ, Ørskov ER, Mcdonald I. Rates of rumen fermentation in relation to ammonia concentration. Br J Nutr. 1977;38:437–43. doi: 10.1079/bjn19770108. [DOI] [PubMed] [Google Scholar]
- 30.Larsen M, Kristensen NB. Effect of abomasal glucose infusion on splanchnic amino acid metabolism in periparturient dairy cows. J Dairy Sci. 2009;92:3306–18. doi: 10.3168/jds.2008-1889. [DOI] [PubMed] [Google Scholar]


