Abstract
Dermatomyositis (DM) complicated with non-small cell lung cancer (NSCLC) is not rare, and could rapidly develop into severe lung cancer [performance-status score (PS) between 2 and 4]. Moreover, tumor has remarkable heterogeneity, and it is not possible to properly target treatments in cases of relapse without knowing pathological diagnosis. We retrospectively analyzed the diagnosis and treatment of a patient with DM complicated with NSCLC, which developed into severe lung cancer with heterogeneity of the tumor during chemotherapy. In this report, we addressed that in patients with severe lung cancer, both the cancer and factors associated with exacerbation should be simultaneously managed to reduce the PS score and avoid unnecessary delay. A second biopsy is important for proper management of the tumor with heterogeneity.
Keywords: Dermatomyositis (DM), non-small cell lung cancer (NSCLC), severe lung cancer, clinical management, respiratory symptoms, second biopsy
Introduction
Dermatomyositis (DM) is an idiopathic inflammatory connective tissue disease characterized by muscular and cutaneous inflammation (1). Since the delineation of the association between DM and malignancy by Stertz in 1916, there have been increasing research interests on DM with co-morbid carcinoma. DM complicated with non-small cell lung cancer (NSCLC) is not rare (2), and could rapidly develop into severe lung cancer [performance-status score (PS) between 2 and 4]. Previous literature reports mainly focused on the comorbidity, pathogenesis, clinical manifestations and diagnostic criteria. However, the principles of management of symptom exacerbation during the course of chemotherapy remain poorly elucidated.
We retrospectively analyzed the diagnosis and treatment of a patient with DM complicated with NSCLC. The profiles consisted of symptoms onset, treatment courses, physician consultation, final diagnosis, complications, exacerbation and alleviation of symptoms. Furthermore, we have first proposed the concept of “severe lung cancer”, which was referred to a state in advanced NSCLC patients with PS score between 2 and 4. There may be reversibility and fluctuation of the PS score. We addressed that in patients with severe lung cancer, both the cancer and factors associated with exacerbation should be simultaneously managed to reduce the PS score and avoid unnecessary delay. More importantly, a second biopsy is important for proper management of the tumor with heterogeneity, especially when there is a deterioration of the disease after chemotherapy. These will lead to greater insights into the management of DM complicated with lung cancer.
Case presentation
A 51-year-old female never-smoker, presenting with productive cough, chest tightness and shortness of breath upon exertion, was referred from several hospitals. She was diagnosed as having pulmonary infection and interstitial pulmonary fibrosis. Following treatment with intravenous moxifloxacin and oral corticosteroids, she was admitted to our hospital in Nov 26, 2013 because of having low-grade fever (37.5 °C < T <38.0 °C) for 4 days which was accompanied by aggravated symptoms. The patient was diagnosed as DM complicated with NSCLC, according to the results of the following physical, lab examinations and biopsies. Chest auscultation indicated coarse respiratory sounds with crepitant rale in the right middle-lower lobe. Punctuate red rashes scattering on the extremities were noted. Pulse oxygen saturation (SPO2) was 85% (breathing air). Erythrocyte sedimentation rate (ESR) 45 mm/h; C-reactive protein (CRP) 1.07 mg/dL; creatinine kinase (CK): 860 U/L; blood gas analysis (O2 4 L/min) showing type I respiratory failure with an oxygen index (OI) of 253. Pulmonary function tests showed mild restrictive ventilatory dysfunction and moderately decreased diffusing capacity. Findings of positron emission tomography-computed tomography (PET-CT) are shown in Figure 1. Muscle biopsy of the right thigh is shown in Figure 2A. Bronchoscopic biopsies from the lower right lung suggested the diagnosis of interstitial inflammation. Biopsies from subcarinal lymph nodes showed visible massive cancer cells in the lymphoid tissue (Figure 2B). The immunohistochemistry assay of subcarinal lymph node results were as follows: EMA (focus), P63(+), CK5/6(+), CgA(−), TTF1(−), Syn(−), CD56(−), LCA(−), which collectively suggested metastatic squamous cell carcinoma (Figure 2C).
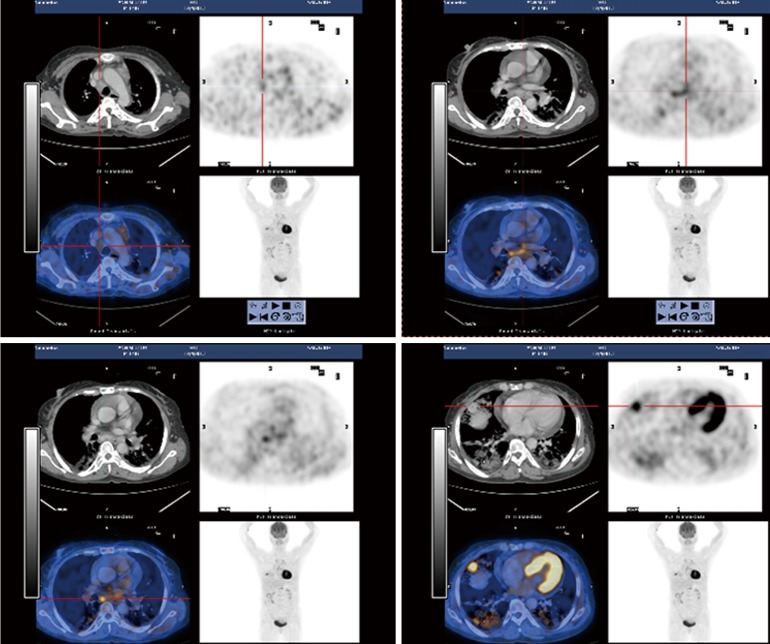
Figure 1.
PET-CT (2013.12.4). Under the pleural of the middle lobe in the right lung was a 2.1 cm × 1.5 cm round-like uneven density nodule, edges, with the maximum SUV 6.1. There were multiple sheets, patchy ground-glass opacities and grid-like shadow mainly distributed in the middle lobe of the right and lower lobes of both sides of the lungs, with the borders of the lesion unclear. Swelling lymph nodes were seen aside the right hilar, before tracheal, in the spaces before vascular, arch of aorta side, aortic pulmonic window, retrocaval, sub-eminence, and on both sides of esophagus; left pleural and pericardial effusion. PET-CT, positron emission tomography-computed tomography.
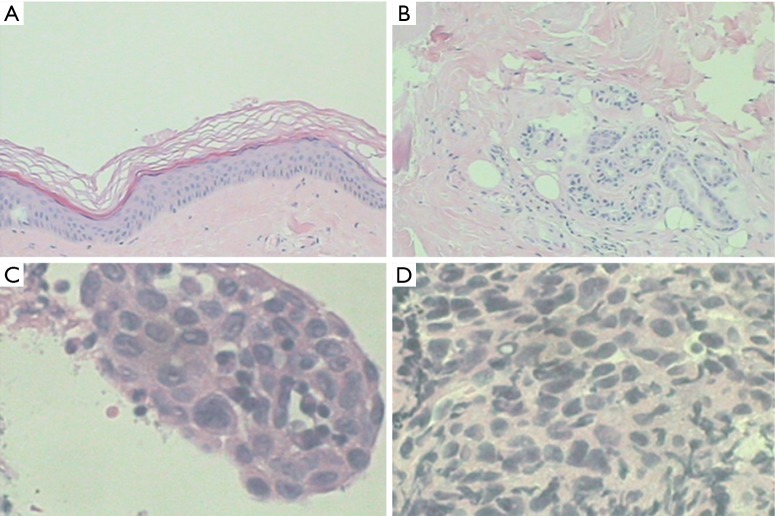
Figure 2.
Muscle biopsy of the right thigh (A,B) and the lymph nodes under the eminence (C,D) (2013.12.9). (A,B) Muscle biopsy of the right thigh indicated of epidermal atrophy, basal cell degeneration and liquefaction necrosis, cutaneous minor perivascular lymphocytic infiltration, collagen fiber hyperplasia and cutaneous appendices atrophy. This was accompanied by striated muscle fibers thickening with necrosis, regeneration, atrophy and degeneration; interstitial edema; vascular fibrosis; sparse lymphocytic infiltration, and tissue atrophy surrounding muscle fiber bundles. These features were consistent with the diagnosis of DM [hematoxylin and eosin staining; (A), 10×; (B), 20×]; (C) visible massive of cancer in the broken lymphoid tissue (hematoxylin and eosin staining, 40×); (D) immunohistochemistry: EMA(focus), P63(+), CK5/6(+), CgA(−), TTF1(−), Syn(−), CD56(−), LCA(−), metastatic squamous cell carcinoma (40×). DM, dermatomyositis.
Methylprednisolone was prescribed for DM; chemotherapy with TP-based protocol [nanoparticle albumin bound-paclitaxel: 175 mg/m2, carboplatin area under the curve (AUC): 5.0 mg/mL/min] was prescribed for NSCLC. The area of pulmonary patchy shadows was reduced after a course of TP compared with baseline, accompanied by a reduction in PS score (by 1–2 points). This was followed by the second treatment course with TP-based protocol after a 21-day interval. Ten days after the second course of TP-based chemotherapy protocol, the patient was readmitted because of developed dyspnea with a PS score of 3–4 and emerging hypoxemia, suggesting the complication of infection or “severe lung cancer”. Chest radiography showed slightly increased multiple patchy lesions with increased density (Figure 3A). Meropenem and voriconazole were empirically prescribed, along with oxygen non-invasive ventilation for 4 days. Chest radiography showed reduced multiple patchy nodules compared with those in the previous 4 days (Figure 3B). But CT showed increased patchy ground-glass opacity and grid-like shades in the lower bilateral lobes compared with those before chemotherapy (Figure 4A). Two weeks later, her symptoms were ameliorated and the prescription was switched to INP-based protocol (vinorelbine: 25 mg/m2, ifosfamide: 3,500 mg/m2 and cisplatin: 75 mg/m2) after multidisciplinary consultation, we speculated the follow interpretations: (I) following infection controlled by effective antibiotics, symptoms of DM or tumor per se predominated; (II) ifosfamide and vincristine are effective anti-neoplastic and anti-DM agents. Navelbine belongs to vincristine family and may be more suitable for lung cancer with DM than paclitaxel; (III) cisplatinum was included for advanced NSCLC though its damage to kidney. In this case, the elevated PS score was mainly due to pneumonia, precluding complications of gastrointestinal or urinary tracts and neural function remained intact. After two courses of INP-based therapy, chest CT showed reduced lesions (Figure 4B). After six courses of INP-based chemotherapy, she remained clinically stable during follow-up (PS score 0–1) and her pulmonary function has been improved. Her CT scan showed reduced sizes of mediastinal lymph nodes, multiple pulmonary patchy shadows (Figure 5A).
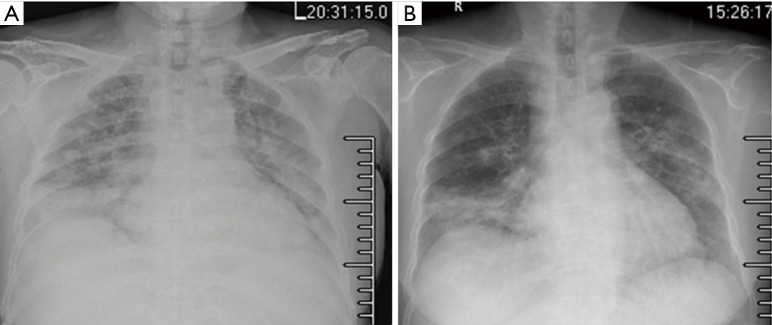
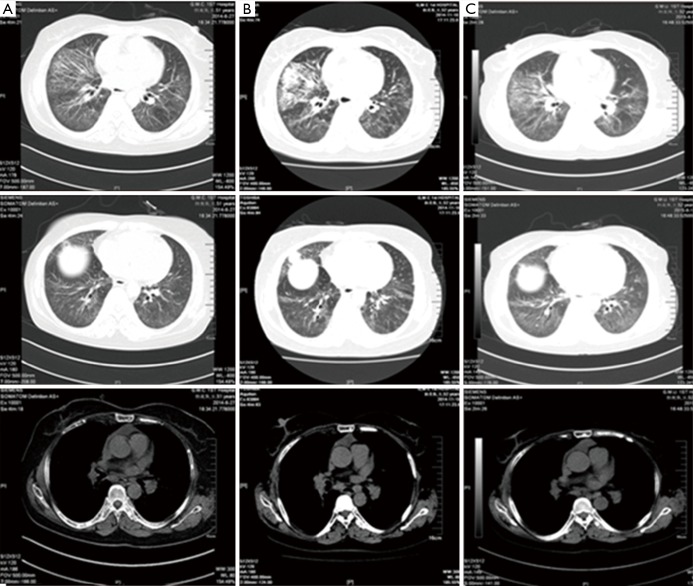
Figure 3.
Chest X-ray after two courses of TP treatment and 4 days later. (A) 2014.1.16. Slightly increased multiple patchy lesions with higher density than previous (2014.1.3); slightly fuzzy hila without widening of mediastinum; (B) 2014.1.20. The multiple patches and nodules in the lungs were reduced compared with that in (A). TP, nanoparticle albumin bound-paclitaxel: 175 mg/m2, carboplatin AUC: 5.0 mg/mL/min; AUC, area under the curve.
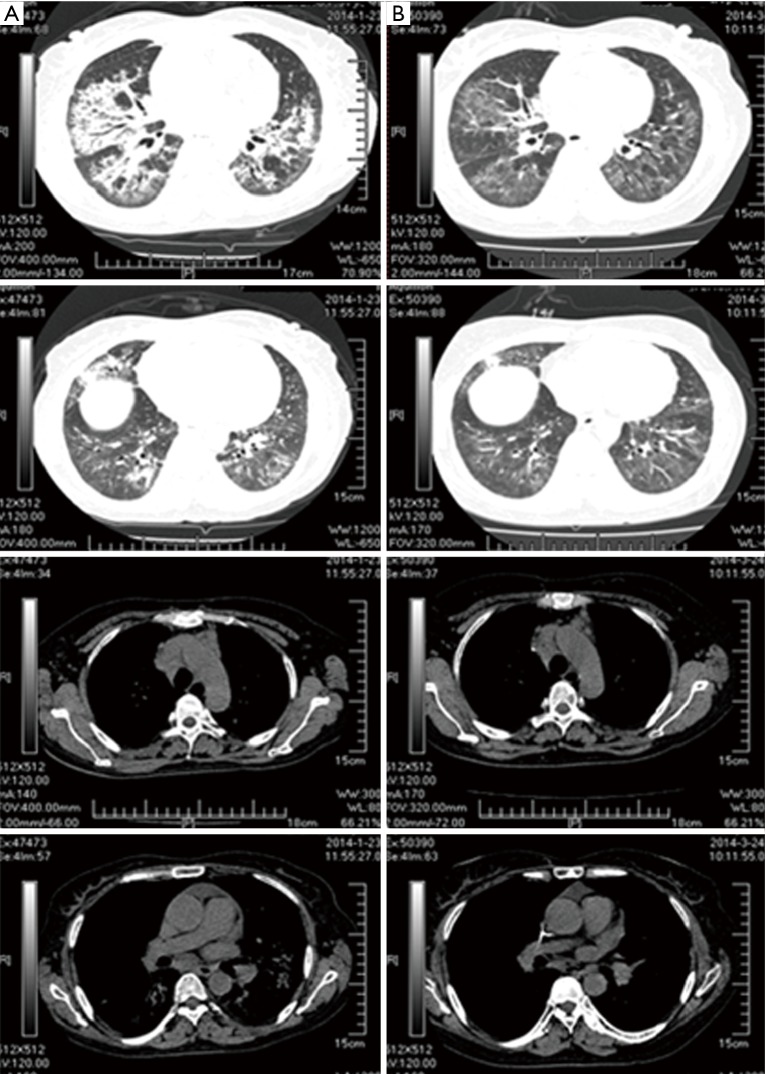
Figure 4.
Comparison of the chest CT. (A) 2014.1.23, after the treatment of two courses of TP. Multiple patchy ground-glass opacities and grid-like shades which were mainly distributed in left lingual lobe, right middle lobe, and lower bilateral lobes, were reduced than those before chemotherapy; (B) 2014.3.24, after the treatment of two courses of INP. Some lymph nodes in the mediastinum became smaller than before, multiple patchy shadows in the lung reduced than previous. CT, computed tomography; TP, nanoparticle albumin bound-paclitaxel: 175 mg/m2, carboplatin AUC: 5.0 mg/mL/min; INP, vinorelbine: 25 mg/m2, ifosfamide: 3,500 mg/m2 and cisplatin: 75 mg/m2; AUC, area under the curve.
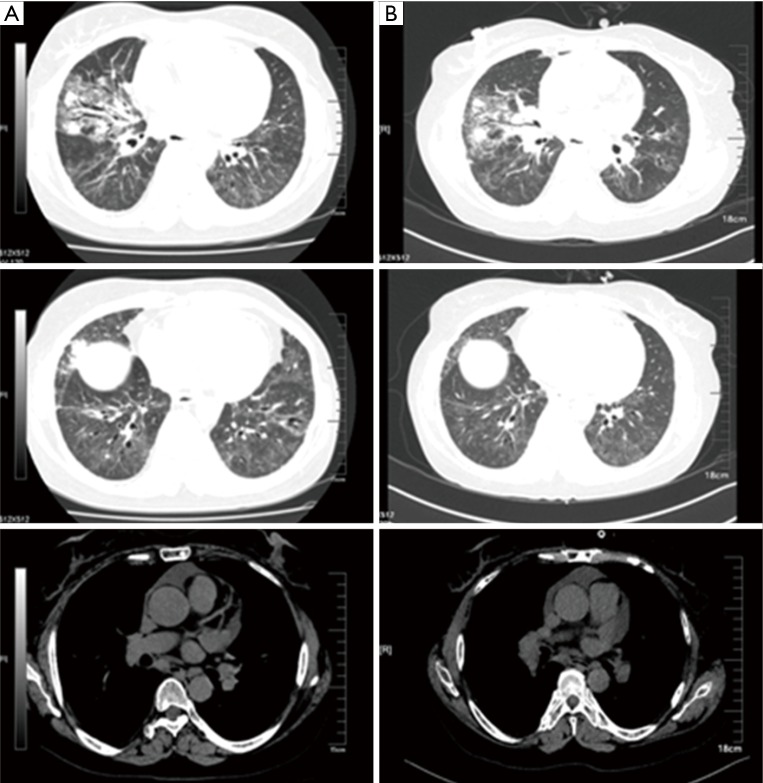
Figure 5.
Comparison of the chest CT. (A) 2014.6.27, after the treatment of six courses of INP. Nodules in the outer segment of right lung and multiple metastasis in lung hilars, mediastinal lymph nodes and thorax bone; multiple patchy shadows in the lungs; pericardial effusion absorbed; (B) 2014.11.10, 6 months after the treatment of six courses of INP. Progressed in the right lung lesions compared with the previous (2.1 cm × 1.9 cm), metastasis in lung hilars, mediastinal lymph nodes and thorax bone almost the same as before. Multiple patchy shadows in the lungs slightly increased; (C) 2015.6.12, after the treatment of S-1. Right lung cancer and right hilar and mediastinal lymph node metastasis; multiple interstitial and substance inflammation in the lungs decreased; multiple small nodules in the front left lung not clearly displayed, consider absorption. Little pericardial effusion absorbed. CT, computed tomography; INP, vinorelbine: 25 mg/m2, ifosfamide: 3,500 mg/m2 and cisplatin: 75 mg/m2.
Six months later, the lesions increased (Figure 5B), she was discharged home but failed to adhere to maintenance therapy. Two courses of INP and one course of GD-based protocols (gemcitabine + docetaxel) were administered; however, the improvement was unremarkable. The prescription was switched to tegafur/gimeracil/oteracil (S-1) 40 mg twice daily. The patient became clinically stable and the CT scan upon reassessment showed reduction of interstitial inflammation (Figure 5C). Having received S-1 triple therapy for approximately 1 year, there were signs of slightly increased lesions (Figure 6A).
Figure 6.
Comparison of the chest CT. (A) After the treatment of 11 months of S-1. Cancer in the right middle lung lesions increased compared with the previous, metastasis in right lung hilars, mediastinal lymph nodes and thorax bone almost the same as before. Multiple interstitial and substance inflammation in the lungs increased; (B) after the treatment of three courses of AP. Cancer in the right middle lung lesions decreased compared with the previous, metastasis in right lung hilars, mediastinal lymph nodes and thorax bone almost the same as before. Multiple interstitial and substance inflammation in the lungs decreased. CT, computed tomography; AP, Avastin (bevacizumab: 7.5 mg/kg) + pemetrexed disodium: 500 mg/m2.
To assess the progression and guide further treatment, the patient underwent the secondary percutaneous biopsy of the right lower lung. Results were as follows: P40(+), P63(+), CK(+), EMA(+), Vim(part +), CgA(−), Syn(−), CD56(−), TTF-1(−), NapsinA(−); in situ hybridization: EBER(+), which was consistent with pulmonary lymphoid-like carcinoma [ALK(−), C-Met(+), ROS1(−), PI3K(−); EGFR(−)]. In light of the new pathological type, chemotherapy was switched to AP-based protocol [Avastin (bevacizumab: 7.5 mg/kg) + pemetrexed disodium: 500 mg/m2]. After three courses, the CT scan showed reduced lesions (Figure 6B).
Discussion
DM associated with lung cancer has been described in various publications. It was believed to occur in 7–15% of all patients with cancer (2-5). Lung cancer could be confirmed prior to, concurrently, or following the diagnosis of DM. In this case, the age interval, increased levels of ESR and CK, and concomitant interstitial abnormalities, should prompt the suspicion of tumor-related DM.
Our experience for treatment
The concept of and treatment for “severe lung cancer”
Based on our experiences and literature review, we have first proposed the concept of “severe lung cancer” which has not been mentioned in major lung cancer guidelines (ASCO, ESMO and NCCN). Severe lung cancer refers patients who are in more advanced stage of lung cancer per se or, patients present with more co-morbidity such as cardio-vascular diseases, chronic obstructive pulmonary disease (COPD) or respiratory infection.
In this case, the patient developed dyspnea after two courses of TP, with a PS score of 3–4 and emerging hypoxemia, suggesting the complication of infection or “severe lung cancer”. In our opinion, treatments of cancer and causes underlying exacerbations are equally important, including ventilation support and appropriate chemotherapy. It is important to resolve the major contradiction for the management of patients with “severe lung cancer”, especially when patient’s status was deteriorating. Priority should be given to improve the PS score before chemotherapy.
There may be reversibility and fluctuation of the PS score. Reversibility is more significant in certain subtypes of severe lung cancer, for instance, those with target genes susceptible to chemotherapy, treatment-naïve co-morbid pericardial effusion or pleural effusion, or major airway obstruction sensitive to initial local treatment. These patients may demonstrate significant reduction in PS score after appropriate therapy and may prompt further anti-cancer treatment. The fluctuations may be present in patients with co-morbidities such as COPD or cardiovascular disease, retreated pericardial effusion or pleural effusion, major airway obstruction resistant to initial local treatment. Treatment of this subtype may be complicated, requiring sufficient duration for controlling complications.
Heterogeneity of the tumor and necessity of performing rebiopsy
Dating back to 1976, Nowell and colleagues (6) proposed a landmark concept that the incidence of malignant tumors development is an evolutionary process due to somatic mutation and clonal selection. It is likely that malignant cells share common ancestor-cancer stem cells. The gene variants that caused by genomic instability progressively accumulated, which caused the malignant cells to differentiate into different subsets with varying genetic footprints. These clones of cancer cells coexist and continue to differentiate. Tumor growth is inseparable from its micro-environment. The relation of tumor heterogeneity with systemic chemotherapy drugs has not been systematically studied; however, after systemic chemotherapy the genetic profiles of cancer cells changed and the tumor heterogeneity increased (7-9). The drug resistance was reversible when a second biopsy was performed, which may confirm the nature of the lesions and prompt better treatment (10,11). In this case, having received S-1 triple therapy for approximately 1 year, there were signs of slightly increased lesions. Heterogeneity of the tumor pathology was shown in the two biopsies, with squamous cell carcinoma in the first time and pulmonary lymphoid-like carcinoma in the second time. Therefore, the treatment regimen has been changed accordingly. A clear pathological classification is of great value in choosing suitable and more effective medication.
Conclusions
Clinical characteristics of DM with co-morbid lung cancer may be non-specific, and missed diagnosis readily occurred. In case of severe lung cancer, both lung cancer and factors contributing to exacerbation should be simultaneously treated. There may be reversibility and fluctuation of the PS score, depending on the patient’s status. A second biopsy is necessary when tumor behavior changes.
Acknowledgements
Funding: This study was supported by Science and Technology Planning Project of Guangdong Province, China (No. 2014A020212562); National Natural Science Foundation of China (No. 81670036).
Informed Consent: Informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med 1975;292:344-7. 10.1056/NEJM197502132920706 [DOI] [PubMed] [Google Scholar]
- 2.Hill CL, Zhang Y, Sigurgeirsson B, et al. Frequency of specific cancer types in dermatomyositis and polymyositis: a population-based study. Lancet 2001;357:96-100. 10.1016/S0140-6736(00)03540-6 [DOI] [PubMed] [Google Scholar]
- 3.Gabrilovich M, Raza M, Dolan S, et al. Paraneoplastic polymyositis associated with squamous cell carcinoma of the lung. Chest 2006;129:1721-3. 10.1378/chest.129.6.1721 [DOI] [PubMed] [Google Scholar]
- 4.Chen D, Yuan S, Wu X, et al. Incidence and predictive factors for malignancies with dermatomyositis: a cohort from southern China. Clin Exp Rheumatol 2014;32:615-21. [PubMed] [Google Scholar]
- 5.Yang Z, Lin F, Qin B, et al. Polymyositis/dermatomyositis and malignancy risk: a metaanalysis study. J Rheumatol 2015;42:282-91. 10.3899/jrheum.140566 [DOI] [PubMed] [Google Scholar]
- 6.Nowell PC. The clonal evolution of tumor cell populations. Science 1976;194:23-8. 10.1126/science.959840 [DOI] [PubMed] [Google Scholar]
- 7.Diaz LA, Jr, Williams RT, Wu J, et al. The molecular evolution of acquired resistance to targeted EGFR blockade in colorectal cancers. Nature 2012;486:537-40. 10.1038/nature11219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liang Y, Zhong Z, Huang Y, et al. Stem-like cancer cells are inducible by increasing genomic instability in cancer cells. J Biol Chem 2010;285:4931-40. 10.1074/jbc.M109.048397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keats JJ, Chesi M, Egan JB, et al. Clonal competition with alternating dominance in multiple myeloma. Blood 2012;120:1067-76. 10.1182/blood-2012-01-405985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sequist LV, Waltman BA, Dias-Santagata D, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med 2011;3:75ra26. 10.1126/scitranslmed.3002003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jekunen AP. Role of rebiopsy in relapsed non-small cell lung cancer for directing oncology treatments. J Oncol 2015;2015:809835. [DOI] [PMC free article] [PubMed] [Google Scholar]