Abstract
Background
Thymic cysts are rare benign developmental anomalies and there is no consensus management for thymic cysts. The aim of this study was to disclose the efficacy of perioperative diagnosis for thymic cysts by chest computerized tomography (CT) and to elucidate the surgical procedure by video-assisted thoracic surgery (VATS) in the management of thymic cysts.
Methods
We retrospectively reviewed 108 consecutive thymic cyst patients who underwent VATS at our institution between April 2001 and August 2015. All patients received chest CT preoperatively and underwent VATS treatment. Clinical characteristics, imaging features and surgical exploration were taken into consideration to determine the surgical extent.
Results
Multivariate logistic regression analysis showed that a diameter ≤3 cm [risk ratio (RR) =4.525; 95% confidence interval (CI), 1.027–20.000; P=0.046] and an unenhanced CT value >20 Hounsfield unit (Hu) (RR =7.043; 95% CI, 1.750–28.345; P=0.006) were independent factors of incorrect diagnosis of chest CT. Three different surgical procedures were performed, which included thymectomy (n=49), cyst resection and partial thymectomy (n=46), and extended thymectomy (n=13). No serious postoperative complications were observed. The median follow-up-time was 60.6 months (range, 12.0–168.0 months) with no late complications or recurrences.
Conclusions
A diameter ≤3 cm and an unenhanced CT value >20 Hu were independent factors of incorrect diagnosis of chest CT. VATS is a reliable approach for the surgical resection of thymic cysts. We think that local resection is adequate for simple thymic cysts. However, thymectomy is necessary when there is suspicion of a thymoma or multilocular thymic cyst, and radical thymectomy is advisable for patients with autoimmune diseases.
Keywords: Thymic cyst, mediastinal cyst, video-assisted thoracic surgery (VATS)
Introduction
Thymic cysts are a rare, benign anomaly and represent 1–3% of all mediastinal masses. They are usually asymptomatic and mostly occur in the anterior mediastinum (1). It is difficult to distinguish thymic cysts from solid neoplasms of the thymus by imaging examination in some cases, especially for protein-rich cysts (2). However, there are few large-scale studies that have analyzed the preoperative diagnosis of thymic cysts. Additionally, most surgeons advocate early surgical resection because complications, such as aggravation or rupture of the cysts, malignant transformation or compression of the surrounding organs in the mediastinum, can arise in some patients (1,3). However, there is no guidance regarding the optimal surgical approach for thymic cysts (4). Therefore, we performed a retrospective study in our institution covering over 15 years to investigate the diagnosis and surgical management of thymic cysts and to discuss selection of the optimal surgical procedure based on the clinical and imaging features of the cysts. To our knowledge, this is the largest single-center study of video-assisted thoracic surgery (VATS) for thymic cysts.
Methods
Data collection
We retrospectively reviewed 108 consecutive patients who were diagnosed with thymic cysts by pathological examination following surgical resection at Peking University People’s Hospital between April 2001 and August 2015. For the preoperative diagnoses, all patients received a chest imaging examination, which included an X-ray and a chest computerized tomography (CT) scan. The size and locations of the cysts and the relationships between the cyst and vital mediastinal structures were evaluated based on the preoperative imaging examinations. In our institution, the mass might be diagnosed as thymic cyst preoperatively by radiologists based on typical performance of chest CT which included: (I) the mass is located in thymic region or the anterior mediastinum adjacent to thymus; (II) they mostly manifest as round or oval, homogeneous attenuation, well-circumscribed, water-attenuation [CT value ≤20 Hounsfield unit (Hu)] masses with thin or imperceptible walls; (III) they may be uniloculated or multiloculated cyst; (IV) low-grade enhancement or no enhancement (≤10 Hu) of the cyst on contrast-enhanced CT scan.
All patients underwent resection by VATS, and the diagnoses were confirmed by histopathological examination. Medical records were reviewed in detail, including age, sex, symptoms at presentation, concomitant malignancy, preoperative chest X-ray and chest CT report, surgical approach, extent of surgery, operation time, intraoperative bleeding, postoperative hospital stay, postoperative complications, mortality, and recurrence. Follow-up was completed yearly by telephone or at an outpatient clinic, and the deadline for follow-up was August 2016. The protocol of the study was approved by the Institutional Review Board (IRB) of Peking University People’s Hospital (IRB No. 2016PHB156-01).
Surgical technique
We began each VATS operation under general anesthesia using one-lung ventilation by double-lumen endotracheal intubation. Surgical procedures were conducted in the 30°–45° lateral recumbent position with a three-port or two-port technique. The camera port was usually placed in the mid-axillary line of the 5th or 6th intercostal space. Under direct thoracoscopic vision, the operator port was usually placed at the 5th or 6th intercostal space of the midclavicular line and the assisted-operator port was placed at the third intercostal space of the anterior axillary line. For simple cases, the assisted-operator port was omitted.
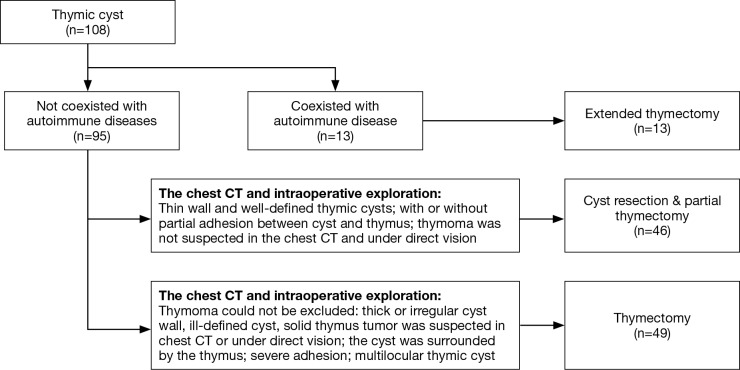
Our surgical principle is described as follows (Figure 1): (I) the cyst should be resected completely, and leakage of the cystic fluid should be avoided; (II) if a malignant tumor or thymoma cannot be excluded according to the preoperative imaging examination and intraoperative exploration, then a thymectomy should be performed and suspicious residual lesions should be resected; (III) an extended thymectomy should be performed in patients with myasthenia gravis; (IV) the surgical procedure must be performed carefully to avoid damage to the mediastinal structures. At the end of each surgery, one chest tube was inserted in the thoracic cavity. Histological examinations confirmed the diagnoses of thymic cysts containing flattened, cuboidal, or columnar stratified squamous epithelium, respiratory-type, or a mixture of these types.
Figure 1.
Flow chart for the surgical management of thymic cysts.
Statistical analysis
Differences were compared using a t-test and analysis of variance (ANOVA) for continuous variables and χ2 test for categorical variables. Univariate and multivariate logistic regression were used to select independent factors of preoperative diagnosis. Predictors (P<0.2) in the univariate analysis were incorporated into a multivariate analysis. A P value <0.05 was considered statistically significant. SPSS 20.0 software (2011; IBM, Armonk, NY, USA) was used for statistical analysis.
Results
Between April 2001 and August 2015, 108 consecutive patients (42 male and 66 female patients) received surgical treatment at our institution. The median age was 55.6±11.1 years (range, 23.0–75.0 years). Thirty-eight (35.2%) patients were symptomatic. Overall, common symptoms caused by thymic cysts included cough (10.2%), followed by dyspnea (5.6%), retrosternal chest pain (5.6%), and fever (1.9%). Thirteen patients had a concomitant disease, myasthenia gravis, and complaints of muscular weakness. One patient had concomitant thymoma, and three patients had concomitant lung cancer (Table 1).
Table 1. Clinical characteristics of patients with thymic cysts.
| Variables | Number (%) |
|---|---|
| Total | 108 (100.0) |
| Sex | |
| Male | 42 (38.9) |
| Female | 66 (61.1) |
| Clinical symptom | |
| No symptom | 70 (64.8) |
| Cough | 11 (10.2) |
| Dyspnea | 6 (5.6) |
| Chest pain | 6 (5.6) |
| Fever | 2 (1.9) |
| Muscular weakness* | 13 (12.0) |
| Cyst of other organs | 17 (15.7) |
| Hepatic cyst | 14 (13.0) |
| Renal cyst | 1 (0.9) |
| Pericardial cyst | 1 (0.9) |
| Adrenal cyst | 1 (0.9) |
| Concomitant autoimmune disease (myasthenia gravis) | 13 (12.0) |
| Concomitant cancer | 4 (3.7) |
| Lung cancer | 3 (2.8) |
| Thymoma (B1) | 1 (0.9) |
| True thymic hyperplasia** | 2 (1.9) |
*, muscular weakness was observed in 13 patients with myasthenia gravis; **, two myasthenia gravis patients had concomitant true thymic hyperplasia.
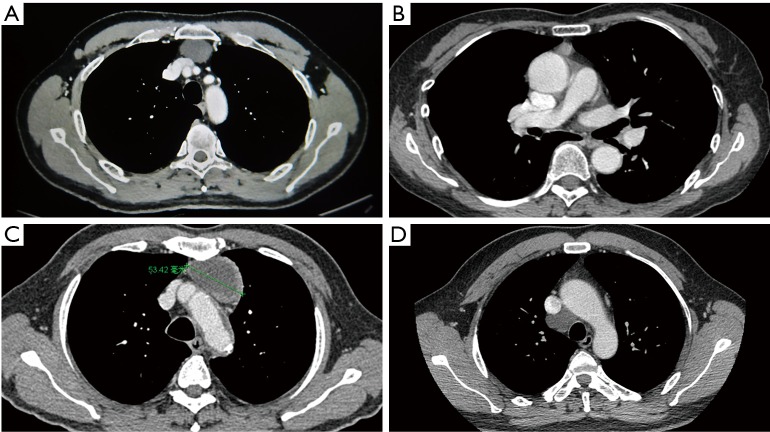
Most thymic cysts (n=106) were in the anterior mediastinum. There were two ectopic thymic cysts; one was located between the superior vena cava and trachea, and the other was in the left posterior mediastinum. In our study, the thymic cysts mostly appeared as round or oval (78.7%), well-defined (97.2%), lateral (85.2%) lesions in the mediastinum (Figure 2). The average size of the thymic cysts was 4.83±3.37 cm at the largest diameter (range, 0.5–16.0 cm). The average size of the retrosternal thymic cysts was 2.2±0.8 cm. Thymic cysts were correctly diagnosed by CT in 59 (54.6%) patients. Forty (37.0%) patients demonstrated a widened mediastinum or mediastinal mass in the chest X-ray, however, none of these were diagnosed as a thymic cyst by chest X-ray. The mean CT value was 18.9±13.8 Hu (range, 0–50.0 Hu) on unenhanced CT, and 25.3±17.6 Hu (range, 0–62.0 Hu) on contrast-enhanced CT.
Figure 2.
The imaging features of the thymic cyst. (A) Typical imaging performance of a thymic cyst: cystic density, homogeneous attenuation, and imperceptible walls; (B) atypical imaging performance of a thymic cyst: solid density mass in the thymus; (C) atypical imaging performance of a thymic cyst: thymic cyst with thick and irregular wall; (D) atypical imaging performance of a thymic cyst: thymic cyst in the middle mediastinum.
Thymic cysts demonstrated cystic density (CT value ≤20 Hu) in 62.0% patients. The diagnostic sensitivity for unenhanced CT value >20 Hu and unenhanced CT value ≤20 Hu in patients were 79.6% and 23.1%, respectively [risk ratio (RR) =12.593; 95% confidence interval (CI), 3.903–40.629; P<0.001]. The diagnostic sensitivity for patients with cysts of diameter ≤3 and >3 cm was 23.4% and 78.7%, respectively (RR=6.849; 95% CI, 2.950–15.873; P<0.001). The cyst fluids were described in detail for 94 patients within operation records, and the diagnostic sensitivity for clear cyst fluid and sticky cyst fluid were 73.0% and 22.6% (χ2=21.49,P<0.001), respectively. Multivariate logistic regression showed that cysts with a diameter ≤3 cm (RR =4.525; 95% CI, 1.027–20.000; P=0.046) and unenhanced CT value >20 Hu (RR =7.043; 95% CI, 1.750–28.345; P=0.006) were independent risk factors for incorrect preoperative diagnosis of the chest CT (Table 2).
Table 2. Univariate and multivariate logistic regression analysis for the factors of incorrect diagnosis by CT.
| Variables | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| RR | 95% CI | P value | RR | 95% CI | P value | ||
| Age (years) | 0.978 | 0.944–1.013 | 0.211 | – | – | – | |
| Sex (male vs. female) | 3.038 | 1.362–6.779 | 0.007 | 2.252 | 0.535–9.490 | 0.269 | |
| Clinical symptom (yes vs. no) | 1.021 | 0.454–2.293 | 0.960 | – | – | – | |
| Autoimmune disease (yes vs. no) | 11.317 | 1.363–93.990 | 0.025 | 11.538 | 0.795–167.520 | 0.073 | |
| Cyst in other organs (yes vs. no) | 0.687 | 0.430–3.600 | 1.244 | – | – | – | |
| X-ray positive (yes vs. no) | 0.359 | 0.157–0.822 | 0.015 | 0.788 | 0.142–4.376 | 0.786 | |
| Location (middle vs. lateral) | 11.400 | 2.443–53.188 | 0.002 | 2.458 | 0.380–15.912 | 0.345 | |
| Diameter (cm) (≤3 vs. >3 cm) | 6.849 | 2.950–15.873 | <0.001 | 4.525 | 1.027–20.000 | 0.046 | |
| Shape of the cyst (oval vs. irregular) | 1.415 | 0.562–3.564 | 0.461 | – | – | – | |
| Margin (clear vs. unclear) | 1.859 | 0.298–11.597 | 0.507 | – | – | – | |
| Unenhanced CT value (>20 vs. ≤20 Hu) | 12.593 | 3.903–40.629 | <0.001 | 7.043 | 1.750–28.345 | 0.006 | |
RR, risk ratio; CI, confidence interval; Hu, Hounsfield unit; CT, computerized tomography.
The cysts were completely removed by VATS in 107 patients. The surgery of one patient was converted to a thoracotomy due to severe pleural adhesion and difficulty of surgical exposure of the thymic cyst. The median operation time was 105.0 min (range, 40.0–250.0 min), the median intraoperative bleeding was 50.0 mL (range, 5.0–500.0 mL), the mean chest tube indwelling was 2.3 days (range, 1.0–19.0 days), and the patients were discharged after a mean of 3.6 days (range, 2.0–21.0 days) postoperatively. No intraoperative complications or operative mortalities were observed. We identified 7 (6.5%) postoperative complications: atrial fibrillation in three patients, chylothorax in two patients and pneumonia in two patients. After conservative treatment, all cases with postoperative complications were resolved. According to our charts, three patients had concomitant lung cancer and received a simultaneous lung resection, and one patient had a simultaneous type B1 thymoma (Masaoka I).
We performed three different types of surgical procedures. Forty-nine patients underwent a thymectomy, 46 patients underwent cyst resection and a partial thymectomy, and 13 patients underwent an extended thymectomy. The patients with myasthenia gravis had smaller cysts than the other two groups (F=12.192, P<0.001). The extended thymectomy prolonged the operation time (F=1.416, P=0.015), chest tube indwelling time (F=3.241, P=0.043) and length of hospital stay (F=3.173, P=0.046) more than the other two groups, but did not lead to more complications (Table 3). The postoperative follow-up was completed in 93 (86.1%) patients, and the long-term follow-up time ranged from 12 to 168 months with a median follow-up time of 60.6 months. There were no late complications or recurrences of thymic cysts in the 93 patients.
Table 3. Perioperative and surgical characteristics of 108 patients in three groups.
| Variables | Cyst resection & partial thymectomy (n=46) | Thymectomy (n=49) | Extended thymectomy (n=13) | F value | P value |
|---|---|---|---|---|---|
| Cyst size (cm) (mean ± SD) | 6.6±3.6 | 3.5±2.3 | 3.8±3.4 | 12.192 | <0.001 |
| Intraoperative bleeding (mL) | 30.0 | 50.0 | 50.0 | 2.106 | 0.049 |
| Operation time (min) | 92.5 | 105.0 | 140.0 | 1.416 | 0.015 |
| Chest tube indwelling time (days) (mean ± SD) | 2.0±1.3 | 2.2±1.5 | 3.6±3.7 | 3.241 | 0.043 |
| Length of hospital stay (days) (mean ± SD) | 3.2±2.5 | 3.3±2.3 | 6.0±4.5 | 3.173 | 0.046 |
| Complication, n (%) | 2/46 (4.3) | 3/49 (6.1) | 2/13 (15.4) | 0.804 | 0.423 |
| Atrial fibrillation | 1 (2.2) | 2 (4.0) | 0 | ||
| Chylothorax | 0 | 0 | 2 (15.4) | ||
| Pneumonia | 1 (2.2) | 1 (2.0) | 0 | ||
| Conversion of surgical approach | 1 | 0 | 0 | – | – |
| Recurrence/follow-up patients | 0/39 | 0/42 | 0/12 | – | – |
SD, standard deviation.
Discussion
A thymic cyst is a benign mediastinal disease which has been reported to be the second most common type of primary mediastinal cyst. Thymic cysts are rare, and the majority are believed to be congenital in origin. Approximately 60% of patients with a thymic cyst were asymptomatic, and the most common symptoms were cough, dyspnea, and chest pain, according to the literature (1,4). However, there were no specific symptoms for thymic cysts. There were 13 patients with simultaneous myasthenia in the present cohort. The histological examinations of these patients showed unilocular thymic cysts (including two patients with thymic hyperplasia). However, there might be no relationship between the unilocular thymic cysts and autoimmune disease. Multilocular thymic cysts occasionally involve an autoimmune disease, including Sjogren’s disease, rheumatoid arthritis, and myasthenia gravis, and were thought to be local occurrences in the thymus associated with autoimmune disease (5-8). However, the relationship between thymic cysts and autoimmune disease and the significance of these medical conditions remain to be investigated in a future study.
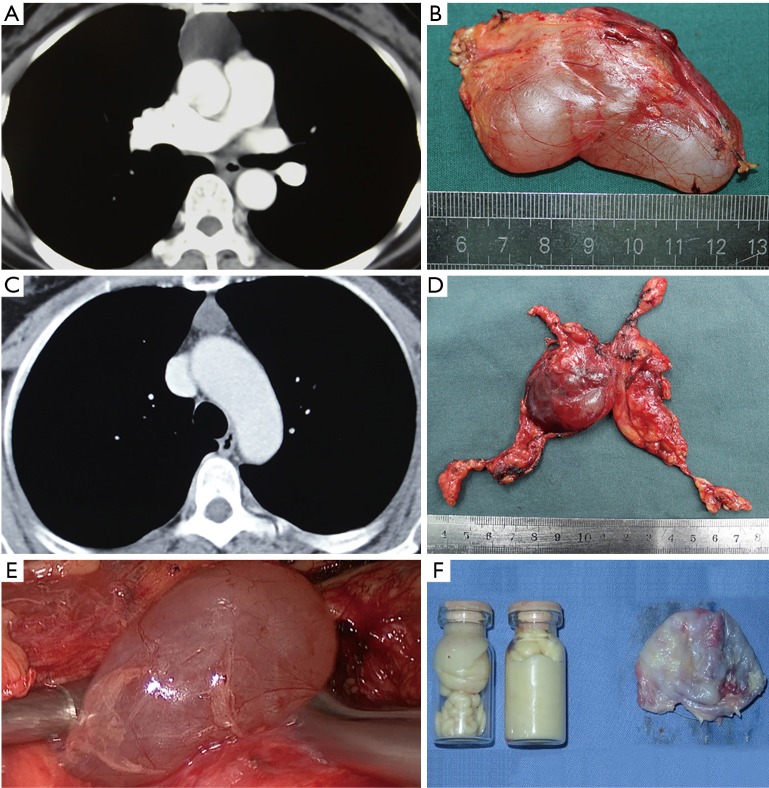
It is difficult to distinguish thymic cysts from the solid neoplasm of the thymus in some patients, and there are few large-scale studies that analyzed the preoperative diagnoses of thymic cysts. Preoperative diagnosis of a thymic cyst mainly depends on an imaging examination. In our study, only 37.0% patients presented with a widened mediastinum or mediastinal mass in the chest X-ray, and none were diagnosed with a thymic cyst. The chest X-ray can only be considered a screening examination. The chest CT is the most widely used imaging examination for mediastinal disease. For thymic cysts, the chest CT can describe the shape, contour, CT value, relationship to adjacent tissue, and the contrast-enhanced CT appearance. The typical imaging performance of the thymic cyst was oval in shape, and had a smooth contour, cystic density, homogeneous attenuation, and thin or imperceptible walls (9-11). However, hemorrhaging or inflammation of the cyst could lead to a protein-rich cyst fluid, which presents as a soft tissue density in the chest imaging examination. According to the operation records, we found that the CT values of the patients with sticky cyst fluid were higher than in the patients with clear cyst fluid (32.7±11.3 vs. 10.6±6.9 Hu, P<0.01). The cyst fluid properties largely affect the CT values. In our study, a chest CT could detect abnormities in the mediastinum in all patients, but the diagnostic sensitivity of the CT examination was 54.6%. Univariate analysis showed that a diameter ≤3 cm, a CT value >20 Hu, a negative X-ray, and a location in the midline reduced the diagnostic sensitivity of CT. The multivariate logistic regression showed that a diameter ≤3 cm and an unenhanced CT value >20 Hu were independent factors indicative of an incorrect preoperative diagnosis from the chest CT. Ackman et al. (12) reported that the CT values of thymic cysts ranged from −19.9 to 58.2 Hu, and a CT value >20 Hu was found in 62.5% (5/8) patients, which is similar to our results. It is difficult to discern cystic lesions from solid masses simply by the CT value in some thymic cyst patients. Additional radiological examinations could be taken into consideration for patients with a diameter ≤3 cm or CT value >20 Hu (Figure 3).
Figure 3.
The chest CT and resected specimen performance of the thymic cyst. (A,B) Chest CT showing a cystic density mass (CT value, 16 Hu) with a thin wall in the anterior mediastinum; the resected specimen reveals a thymic cyst containing serous fluid; (C,D) chest CT showing a solid density mass (CT value, 48 Hu); the resected specimen reveals a heterogeneous cyst in the thymus, and the histological examinations conformed the thymic cyst; (E) thymic cyst with clear cyst fluid; (F) thymic cyst with sticky fluid (protein-rich content in the thymic cyst). CT, computerized tomography.
Magnetic resonance imaging (MRI) is considered superior to CT scans for cystic lesions in the thymus. Tomiyama et al. (13) reported that correct diagnosis rates for CT and MRI for thymic cysts were 46.0% and 71.0% (P<0.05), respectively. MRI may be better in some certain circumstances, such as for a thymic cyst with hemorrhage or inflammation, which mimics a solid tumor. In the process of clinical diagnosis, the thymic cyst should be differentiated from a thymoma, lymphoma in the thymus, thymic hyperplasia, or cystic teratoma. The chest CT is equal or superior to MRI in the diagnosis of anterior mediastinal masses (13). There is a consensus that the chest CT is a reliable, cost-effective, and appropriate diagnostic tool for thymic tumors according to the European Society of Thoracic Surgeons (ESTS) (14). In our study, the chest CT was primarily performed in all patients, and most patients received VATS resection for definite pathologic diagnosis and surgical treatment. About one in ten patients received additional radiological examination at our institution, even though thymic cysts were suspected in some cases by MRI or positron emission tomography-CT (PET-CT), and the further treatment strategy was not changed. We recommend that all patients first receive a chest CT for diagnosis. For the purpose of differentiating thymic cysts from mediastinal neoplasia when in CT scans they manifest as soft-tissue-like lesions, MRI or PET-CT could be performed for more diagnostic information.
Since it is difficult to make an accurate diagnosis before surgical treatment in most cases according to the image examination, surgeons generally accept that symptomatic thymic cysts or those with progressive growth should undergo surgical resection. However, there is still controversy regarding the surgical indication for asymptomatic thymic cyst patients (1,9). Rupturing of a thymic cyst could lead to a pleural effusion, infection or hemorrhage in the thoracic cavity, and malignant transformation cannot be ruled out in some circumstances. We consider that active surgical management should be performed in patients for which thymoma or any other malignant tumor cannot be ruled out by imaging, even in asymptomatic patients, because of the unpredictability of clinical behavior.
Lewis et al. (15) performed the first VATS resection for a mediastinal cyst in 1992. Since then, most thymic cysts have been removed using the VATS approach. Compared with an open procedure, VATS has less intraoperative blood loss and postoperative pain (16). In the study from Kozu et al., the thoracoscopic procedure was used in 17 thymic cyst patients who were selected before the operation. The mean operation time was 73.0 min, the mean intraoperative blood loss was 114.0 mL, and there was no conversion to thoracotomy in this cohort (4). In our institution, we began each operation with the VATS technique, and this is the largest study of VATS surgery for thymic cysts to our knowledge. The operation time, intraoperative blood loss, and duration of hospital stay were similar to the values reported in the literature (4,16). Conversion to thoracotomy only occurred in one patient due to severely dense adhesion and pleural thickening. There was no recurrence during the long-term follow-up. This result demonstrated VATS for thymic cysts is safe and feasible, with the advantage of minimal invasiveness.
Few studies discuss the surgical management of thymic cysts. The optimal extent of surgical resection for thymic cysts is still pending. Theoretically, a thymic cyst is a congenital benign disorder, and thus, thymectomy for a thymic cyst would be considered unnecessary (8). However, in some cases, it might be difficult to exclude thymoma before histological examination. Based on the different features of preoperative imaging examinations and intraoperative exploration, we performed the following three different surgical procedures: (I) for cysts with a unilocular thin wall, smooth counters and a well-defined boundary that could be easily differentiated from a thymoma by radiological examination and intraoperative exploration, it is possible that in some cases a partial rupture of the cyst may lead to inflammatory reaction around the thymic cyst. Adhesion between the cyst and the adjacent mediastinum structure or an inhomogeneous change in the thymus would be followed by inflammation. In these situations, the cyst and the thymus, which is adhered to the cyst, should be resected so the cyst wall can be resected completely; (II) in Suster’s study (17), there were four patients with a simultaneous thymoma among 18 multilocular thymic cyst patients, in which two occurred postoperatively. In our study, there was one patient with a thymic cyst existing simultaneously with a thymoma. We consider that thymectomy should be performed in the following situations: (i) according to the preoperative imaging examination or intraoperative exploration, thymoma, cystic degradation of thymoma and other malignant tumors of the thymus, or those coexisting with a thymoma, cannot be excluded, e.g., a cyst with a thick or irregular wall, or with a boundary that is ill-defined; (ii) the intraoperative exploration is unsatisfactory, e.g., a cyst that is surrounded by the thymus completely; (iii) concerns of recurrence are present, e.g., a multilocular thymic cyst in the thymus or severe adhesion around the cyst that may lead to incomplete resection; (III) chronic inflammation in the thymus caused by the autoimmune diseases may lead to a multilocular thymic cyst. A unilocular thymic cyst could also coexist with autoimmune diseases (8,10). We think that an extended thymectomy should be performed on thymic cyst patients with autoimmune diseases as a curative treatment. Overall, the complete resection of thymic lesions should be guaranteed. The surgical procedures that we followed were safe and efficacious, and did not cause further surgical injury or complications for patients with thymic cysts.
In conclusion, the diagnostic sensitivity of chest CT was mainly affected by the size and CT value of the cyst. A diameter ≤3 cm and an unenhanced CT value >20 Hu were independent factors of incorrect diagnosis of chest CT. VATS resection should be considered the primary therapeutic option for the management of thymic cysts. We think that local resection is adequate for simple thymic cysts. However, thymectomy is necessary when there is suspicion of a thymoma or multilocular thymic cyst. For patients with autoimmune diseases, a radical extended thymectomy is advisable. However, this study has limitations that should be noticed. This is a single-institution, retrospective analysis. And the follow-up time is relatively short to assess the recurrence of thymic cyst. Large prospective and long-term follow-up studies are suggested to confirm the conclusion.
Acknowledgements
Funding: This study was supported by the National Natural Science Foundation of China (No. 81602001).
Ethical Statement: The study was approved by the Institutional Review Board of Peking University People’s Hospital (IRB No. 2016PHB156-01).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Takeda S, Miyoshi S, Minami M, et al. Clinical spectrum of mediastinal cysts. Chest 2003;124:125-32. 10.1378/chest.124.1.125 [DOI] [PubMed] [Google Scholar]
- 2.Vargas D, Suby-Long T, Restrepo CS. Cystic Lesions of the Mediastinum. Semin Ultrasound CT MR 2016;37:212-22. 10.1053/j.sult.2015.12.005 [DOI] [PubMed] [Google Scholar]
- 3.Leong AS, Brown JH. Malignant transformation in a thymic cyst. Am J Surg Pathol 1984;8:471-5. 10.1097/00000478-198406000-00009 [DOI] [PubMed] [Google Scholar]
- 4.Kozu Y, Suzuki K, Oh S, et al. Single institutional experience with primary mediastinal cysts: clinicopathological study of 108 resected cases. Ann Thorac Cardiovasc Surg 2014;20:365-9. 10.5761/atcs.oa.13-00151 [DOI] [PubMed] [Google Scholar]
- 5.Nakamura S, Tateyama H, Taniguchi T, et al. Multilocular thymic cyst associated with thymoma: a clinicopathologic study of 20 cases with an emphasis on the pathogenesis of cyst formation. Am J Surg Pathol 2012;36:1857-64. 10.1097/PAS.0b013e31826320c4 [DOI] [PubMed] [Google Scholar]
- 6.Wick MR. Cystic lesions of the mediastinum. Semin Diagn Pathol 2005;22:241-53. 10.1053/j.semdp.2006.02.008 [DOI] [PubMed] [Google Scholar]
- 7.Jennings S, Stuklis RG, Chan J, et al. Successful Giant Thymic Cyst Removal: Case Report and Review of the Literature. Heart Lung Circ 2015;24:e89-92. 10.1016/j.hlc.2015.02.013 [DOI] [PubMed] [Google Scholar]
- 8.Izumi H, Nobukawa B, Takahashi K, et al. Multilocular thymic cyst associated with follicular hyperplasia: clinicopathologic study of 4 resected cases. Hum Pathol 2005;36:841-4. 10.1016/j.humpath.2005.05.004 [DOI] [PubMed] [Google Scholar]
- 9.Kent MS, Wang T, Gangadharan SP, et al. What is the prevalence of a "nontherapeutic" thymectomy? Ann Thorac Surg 2014;97:276-82; discussion 82. 10.1016/j.athoracsur.2013.07.121 [DOI] [PubMed] [Google Scholar]
- 10.Araki T, Sholl LM, Gerbaudo VH, et al. Intrathymic cyst: clinical and radiological features in surgically resected cases. Clin Radiol 2014;69:732-8. 10.1016/j.crad.2014.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jeung MY, Gasser B, Gangi A, et al. Imaging of cystic masses of the mediastinum. Radiographics 2002;22 Spec No:S79-93. 10.1148/radiographics.22.suppl_1.g02oc09s79 [DOI] [PubMed] [Google Scholar]
- 12.Ackman JB, Verzosa S, Kovach AE, et al. High rate of unnecessary thymectomy and its cause. Can computed tomography distinguish thymoma, lymphoma, thymic hyperplasia, and thymic cysts? Eur J Radiol 2015;84:524-33. 10.1016/j.ejrad.2014.11.042 [DOI] [PubMed] [Google Scholar]
- 13.Tomiyama N, Honda O, Tsubamoto M, et al. Anterior mediastinal tumors: diagnostic accuracy of CT and MRI. Eur J Radiol 2009;69:280-8. 10.1016/j.ejrad.2007.10.002 [DOI] [PubMed] [Google Scholar]
- 14.Ruffini E, Van Raemdonck D, Detterbeck F, et al. Management of thymic tumors: a survey of current practice among members of the European Society of Thoracic Surgeons. J Thorac Oncol 2011;6:614-23. 10.1097/JTO.0b013e318207cd74 [DOI] [PubMed] [Google Scholar]
- 15.Lewis RJ, Caccavale RJ, Sisler GE. Imaged thoracoscopic surgery: a new thoracic technique for resection of mediastinal cysts. Ann Thorac Surg 1992;53:318-20. 10.1016/0003-4975(92)91340-F [DOI] [PubMed] [Google Scholar]
- 16.Augustin F, Schmid T, Sieb M, et al. Video-assisted thoracoscopic surgery versus robotic-assisted thoracoscopic surgery thymectomy. Ann Thorac Surg 2008;85:S768-71. 10.1016/j.athoracsur.2007.11.079 [DOI] [PubMed] [Google Scholar]
- 17.Suster S, Rosai J. Multilocular thymic cyst: an acquired reactive process. Study of 18 cases. Am J Surg Pathol 1991;15:388-98. 10.1097/00000478-199104000-00008 [DOI] [PubMed] [Google Scholar]