Abstract
Background
The characteristic and outcomes of lung cancer patients with combined pulmonary fibrosis and emphysema (CPFE) have long been assessed, but results were controversial. Therefore, we performed a meta-analysis to assess the clinical features and prognosis of lung cancer patients with CPFE.
Methods
The databases PubMed, Embase, and Web of Science (updated to October 1, 2017) were searched for eligible studies. Pooled odds ratios (ORs), weighted mean differences (WMD) or hazard ratios (HRs) with 95% confidence intervals (95% CIs) were used to evaluate the clinicopathological characteristics, the short-term outcome after operation and long-term survival of lung cancer patients with CPFE compared with lung cancer patients without CPFE (fibrosis, emphysema, and normal).
Results
Thirty original studies with 8,050 patients were included in this meta-analysis. The pooled results indicated that lung cancer patients with CPFE were associated with higher age (MD =3.39; 95% CI: 2.12–4.67, P<0.001), male (OR =8.46; 95% CI: 6.36–11.26, P<0.001), ex- or current smoker (OR =39.65; 95% CI: 15.64–100.5, P<0.001), longer smoking history (MD =15.56; 95% CI: 3.73–27.39, P=0.01), lower DLCO% (MD =−13.82; 95% CI: −21.4 to −6.24, P<0.001), squamous cell carcinoma histology (OR =3.55; 95% CI: 2.49-5.05, P<0.001), the lower lobes (OR =1.92; 95% CI: 1.52–2.43, P<0.001), advanced pathological stage (OR =1.55; 95% CI: 1.22–1.96, P<0.001). Lung cancer patients with CPFE had higher 30-day mortality (OR =4.72, 95% CI: 2.06–10.85, P<0.001), 90-day mortality (OR =5.33; 95% CI: 1.39–20.42, P=0.01), and incidence of postoperative complications (OR =5.25, 95% CI: 2.38–11.57, P<0.001). In addition, the lung cancer patients with CPFE had a poorer OS (HR =2.006, 95% CI: 1.347–2.986, P=0.001) than lung cancer patients without CPFE.
Conclusions
This meta-analysis demonstrated that lung cancer patients with CPFE have more aggressive clinical characteristic and a poor prognosis, suggesting that lung cancer patients with CPFE should be early detected, treated reasonably and be taken good care of.
Keywords: Lung cancer, combined pulmonary fibrosis and emphysema (CPFE), prognosis
Introduction
According to GLOBOCAN worldwide estimation of cancer incidence and mortality produced by the International Agency for Research on Cancer (IARC) for 2012, Lung cancer was the most frequently diagnosed cancer and the leading cause of cancer death among males in 2012 (1). Therefore, studies on prognosis of lung cancer patients and prognostic factors were much needed.
More recently, combined pulmonary fibrosis and emphysema (CPFE) with upper lobe emphysema and lower lobe fibrosis of the lung had been recognized as an unique entity (2). CPFE was increasingly acknowledged as a separate syndrome with distinct clinical, physiological and radiological characteristics (3). CPFE was most often observed in males with a mean age of 65–70 years (4). Clinical features included severe dyspnea on exertion, subnormal spirometer findings, severely impaired gas exchange, hypoxemia on exercise, and characteristic findings on imaging (5). Several previous studies had suggested that patients with CPFE could present distinct clinical characteristics that were associated with different outcomes (6,7).
Patients with CPFE had a significant increased risk of lung cancer (8). Lung cancer in patients with CPFE was most common in elderly heavy smokers with a male predominance (9). In three previous studies of lung cancer patients with CPFE adenocarcinoma was the most common type of cancer, followed by squamous cell carcinoma (10-12). However, other studies had shown that squamous cell carcinoma was the most common type of cancer (13-22). Several previous studies showed lung cancer in patients with CPFE was advanced stage (10,14), while other studies indicated that more lung cancer patients with CPFE were early stage (11-13,15-22). Pulmonary function test was conducted in several studies, and the results have varied (11-12,14-22). CPFE was an independent factor for a poor prognosis in lung cancer patient with CPFE (11,18,21). It remained unclear why CPFE was an independent prognostic factor for a poor outcome in lung cancer patients (11). Four studies clarified risk factors for long-term survival, while which factors were independent risk factors was controversial (11,12,18,21,22). The resected lung cancer patients with CPFE showed quite high postoperative mortality rates and frequent complications and the rate of lung cancer-associated mortality was high, while some studies held different views (11-12,14-16,18-22). Therefore, it is necessary to carry out a comprehensive analysis by pooling published data.
The clinical characteristics, prognostic factor and treatment of lung cancer patients with CPFE had not been fully evaluated; therefore, we performed a systematic review and meta-analysis of all available studies to provide more help for lung cancer patients with CPFE.
Methods
Literature-search strategy
A literature search was performed in October 2017 by searching multiple literature databases, including PubMed, Embase, and Web of Science. We performed our search using the keywords as follows: “CPFE” or “combined pulmonary fibrosis and emphysema” and “lung carcinoma” or “lung cancer” or “lung neoplasm”. The computer search was supplemented with manual search of the reference lists of all retrieved studies and reviews for potential eligible studies. Language was restricted to English and Chinese.
Inclusion and exclusion criteria
Study selection inclusion criteria were as follows: (I) studies compared the clinical characteristics and prognosis between lung cancer patients with CPFE and non-CPFE (fibrosis, emphysema and normal); (II) odds ratios (ORs), mean differences (MDs) or hazard ratios (HRs) with 95% confidence intervals (95% CIs) were applied to measure the strength of role of CPFE on clinicopathological or survival of lung cancer; (III) data was available for further meta-analysis. Exclusion criteria were as follows: (I) meeting abstracts, comments, case reports, reviews, and meta-analyses; (II) data couldn’t be extracted or estimated; (III) duplicate studies. When multiple reports describing the same population were published, the most recent or complete report were used. Two reviewers independently assessed publications for inclusion in the review. Discrepancies were resolved through discussion by the review team.
Quality assessment of the studies
The methodological quality of the original studies was assessed by the Newcastle-Ottawa Scale (NOS), which consisted of three factors: selection, comparability of subjects, and outcome. Each study received a score from 0 to 9 (allocated as stars), and scores higher than 6 were considered high quality. Two authors independently performed this assessment and discrepancies were resolved by discussion.
Data extraction
Two reviewers independently extracted relevant information from each eligible study using a standard form. Any disagreement was resolved by the adjudicating senior authors. The following data extracted independently from qualified studies from three aspects: clinicopathological (authors, publication year, country, ethnicity, source, number of participants, patient’s age, patient’s gender, smoking history of patients, smoking status of patients, the histology of lung cancer, the location of lung cancer, the clinical stage of lung cancer, the pathological stage of lung cancer, pulmonary function test, KL-6 of patients, BMI of patients), the short term outcome after operation (complications after operation, cause of death, 30-day mortality, 90-day mortality), and long-term survival data.
Statistical analysis
HR and its 95% CI were used to evaluate the correlation between CPFE and patient survival. If the HR with 95% CI were reported in the original study, we extracted the data directly, If not, we estimated HR from survival rates with P values from log-rank test or Kaplan-Meier survival curves using the method reported by Parmar et al. (23), The weighted mean difference (WMD) and OR were used to compare continuous and dichotomous variables, respectively. All results were reported with 95% CIs. Statistical heterogeneity between studies was assessed using the chi-square test with significance set at P<0.10 and heterogeneity was quantified using the I2 statistic. The random-effects model was used if there was heterogeneity between studies; otherwise, the fixed-effects model was used. Subgroup analyses were performed to compare lung cancer patient with CPFE, fibrosis, emphysema, and normal. Sensitivity analyses were further performed by drop out each study. Begg’s funnel plots and Egger’s test were used to screen for potential publication bias. The software stataSE12.0 and review manager 5.3 were used to perform data analysis. All P values were two-sided and considered significant if <0.05.
Results
Study identification and selection
Using the outlined searching strategy, a total of 268 citations were obtained for review of title and abstract. One hundred and seven duplicates were removed, 138 publications were excluded because the studies were animal experiment, literature reviews, meta-analysis, comments, letters, or unrelated studies based on the titles and abstract screening. Full texts of remained 23 studies were retrieved for review, of these, 10 publications were excluded due to irrelevant publications or lack of sufficient data for analysis. Finally, 13 studies with lung cancer patients with 809 CPFE and 7,228 non-CPFE (fibrosis, emphysema, normal) were included in this meta-analysis (Figure 1).
Figure 1.
The flow chart of study selection.
Characteristics of the studies
The characteristics of the included studies were shown in Table 1. We identified 13 retrospective studies published between 2011 and 2017, with 8,050 lung cancer patients from Asian (1 study from China, 12 studies from Japan). In seven studies, lung cancer patients were divided into four groups: CPFE, fibrosis, normal, emphysema. Four studies had two groups: CPFE, non-CPFE. One study divided into two groups: CPFE, Fibrosis. One study had three groups: CPFE, fibrosis, emphysema. Chest computed tomography (CT) was a significant way to diagnose lung disease among the seven studies, one study used thin-section computed tomography (TSCT), and the other five studies used high resolution computed tomography (HRCT) to diagnoses lung disease. The OS was investigated in 11 studies, 6 of these directly provided HR while survival data of other 5 studies were extracted from survival curves. The patients of nine studies were treated with surgical treatment while patients of three studies used multiple treatments.
Table 1. Characteristics of studies included in the meta-analysis.
| Author | Year | Country | Ethnicity | Na | Groupb | Diagnosis method | Treatmentc | Survival analysis | Analysis | NOS score |
|---|---|---|---|---|---|---|---|---|---|---|
| Fujiwara | 2013 | Japan | Asian | 274 | 4 | CT | NA | NA | NA | 9 |
| Fukui | 2016 | Japan | Asian | 1,368 | 4 | CT | Surgery | NA | NA | 9 |
| Gao | 2016 | China | Asian | 60 | 2 | HRCT | Mixed | OS | U | 9 |
| Hashimoto | 2016 | Japan | Asian | 685 | 4 | TSCT | Surgery | OS | M | 9 |
| Hata | 2016 | Japan | Asian | 250 | 4 | CT | Surgery | OS | M | 9 |
| Kumagai | 2014 | Japan | Asian | 365 | 4 | HRCT | Surgery | OS | M | 9 |
| Mimae | 2015 | Japan | Asian | 2,333 | 2 | CT | Surgery | OS | M | 9 |
| Minegishi | 2014 | Japan | Asian | 151 | 2 | CT | Mixed | OS | M | 9 |
| Otsuka | 2016 | Japan | Asian | 67 | 3 | HRCT | Surgery | OS | U | 9 |
| Sato | 2016 | Japan | Asian | 100 | 2 | CT | Surgery | OS | U | 9 |
| Takenaka | 2017 | Japan | Asian | 274 | 4 | CT | Surgery | OS | M | 8 |
| Usui | 2011 | Japan | Asian | 1,143 | 4 | HRCT | Mixed | OS | U | 8 |
| Zhang | 2016 | Japan | Asian | 985 | 2 | HRCT | Surgery | OS | M | 9 |
a, number of included patients; b, Group 4 includes CPFE group, fibrosis group, emphysema group, and normal group. Group 3 includes CPFE group, fibrosis group, and emphysema group. Group 2 includes CPFE group and non-CPFE group or includes CPFE group and fibrosis group; c, mixed includes patients undergoing surgery, chemotherapy, chemoradiotherapy, or other treatment. Surgery only includes patients getting surgery. NA, not available; OS, overall survival; M, multivariate analysis; U, univariate analysis; NOS, Newcastle-Ottawa Quality Assessment Scale; CT, chest computed tomography; HRCT, high resolution computed tomography; TSCT, thin-section computed tomography.
Meta-analysis of clinicopathological parameters
We disclosed the clinical characteristic of lung cancer patients with CPFE and explored the difference between lung cancer patients with CPFE and lung cancer patients without CPFE. The pooled results indicated that lung cancer patients with CPFE were associated with higher age (MD =3.39; 95% CI: 2.12–4.67, P<0.001), male (OR =8.46; 95% CI: 6.36–11.26, P<0.001), ex- or current smoker (OR =39.65; 95% CI: 15.64–100.5, P<0.001), longer smoking history (MD =15.56; 95% CI: 3.73–27.39, P=0.01), higher KL-6 (MD =200.18, 95% CI: 101.52–298.84, P<0.001), lower DLCO% (MD =−13.82; 95% CI: −21.4 to −6.24, P<0.001), squamous carcinoma (OR =3.55; 95% CI: 2.49–5.05, P<0.001), advanced clinical stage (OR =1.16; 95% CI: 1.06–2.43, P=0.02) and advanced pathological stage (OR =1.55, 95% CI: 1.22–1.96, P<0.001) (Figure S1).
Figure S1.
The clinical characteristics of age (A), sex (B), smoking status (C), DLCO% (D), pathological stages (E), histology (F) comparing lung cancer patients with CPFE with lung cancer patients without CPFE. DLCO, diffusing capacity of the lung for car-bon monoxide; CPFE, combined pulmonary fibrosis and emphysema.
Subgroup analysis comparing lung cancer patients with CPFE to lung cancer patients without CPFE which were divided into fibrosis, emphysema, and normal sub-groups further proved that patients with CPFE had higher age, more male, lower DLCO%, more squamous carcinoma, longer smoking history, and advanced pathological stage. The detailed results were presented in Table 2.
Table 2. Meta-analysis and subgroup analysis of clinical characteristics comparison of lung cancer patients with and without CPFE.
| Variables | N (study) a | N (case)b | N (control)c | Pooled data | Heterogeneity | |||
|---|---|---|---|---|---|---|---|---|
| WMD/OR (95% CI) | P | I2 (%) | Ph | |||||
| Age | 13 | 809 | 7,228 | 3.39 (2.12, 4.67) | <0.001 | 82 | <0.001 | |
| CPFE vs. fibrosis | 9 | 457 | 233 | −0.31 (−1.62, 1) | 0.64 | 0 | 0.76 | |
| CPFE vs. emphysema | 8 | 402 | 1,184 | 2.64 (0.93, 4.34) | 0.002 | 68 | 0.002 | |
| CPFE vs. normal | 7 | 379 | 2,662 | 5.38 (4.58, 6.17) | <0.001 | 23 | 0.25 | |
| Sex (male vs. female) | 13 | 809 | 7,246 | 8.46 (6.36, 11.26) | <0.001 | 0 | 0.92 | |
| CPFE vs. fibrosis | 9 | 457 | 223 | 5.46 (3.3, 9.03) | <0.001 | 0 | 0.66 | |
| CPFE vs. emphysema | 8 | 402 | 1,184 | 2.16 (1.4, 3.35) | <0.001 | 0 | 0.69 | |
| CPFE vs. normal | 7 | 379 | 2,662 | 14.87 (9.94, 22.24) | <0.001 | 0 | 0.92 | |
| Smoking status (ex or current vs. never) | 7 | 344 | 3,235 | 39.65 (15.64, 100.5) | <0.001 | 12 | 0.34 | |
| CPFE vs. fibrosis | 6 | 237 | 79 | 8.51 (2.11, 34.29) | 0.003 | 0 | 0.93 | |
| CPFE vs. emphysema | 5 | 214 | 817 | 3.48 (1.14, 10.64) | 0.03 | 0 | 0.63 | |
| CPFE vs. normal | 4 | 214 | 1,401 | 62.97 (20.44, 193.97) | <0.001 | 3 | 0.38 | |
| Smoking history | 8 | 488 | 3,492 | 15.56 (3.73, 27.39) | 0.01 | 85 | <0.001 | |
| CPFE vs. fibrosis | 6 | 365 | 148 | 15.25 (2.96, 27.55) | 0.02 | 60 | 0.03 | |
| CPFE vs. emphysema | 6 | 365 | 1,044 | 0.9 (−3.92, 5.72) | 0.71 | 0 | 0.8 | |
| CPFE vs. normal | 5 | 342 | 2,230 | 39.55 (35.11, 43.99) | <0.001 | 22 | 0.27 | |
| Histology (Sq vs. others) | 13 | 809 | 7,246 | 3.55 (2.49, 5.05) | <0.001 | 74 | <0.001 | |
| CPFE vs. fibrosis | 9 | 457 | 223 | 2.12 (1.49, 3.03) | <0.001 | 21 | 0.26 | |
| CPFE vs. emphysema | 8 | 402 | 1,186 | 1.45 (1.14, 1.84) | 0.003 | 0 | 0.58 | |
| CPFE vs. normal | 7 | 379 | 2,662 | 6.95 (5.45, 8.87) | <0.001 | 29 | 0.21 | |
| Tumor location (low vs. others) | 5 | 378 | 2,600 | 1.92 (1.52, 2.43) | <0.001 | 0 | 0.43 | |
| CPFE vs. fibrosis | 3 | 255 | 107 | 1.35 (0.84, 2.17) | 0.21 | 0 | 0.49 | |
| CPFE vs. emphysema | 3 | 255 | 663 | 2.19 (1.62, 2.96) | <0.001 | 0 | 0.46 | |
| CPFE vs. normal | 3 | 255 | 1,760 | 2.02 (1.55, 2.63) | <0.001 | 0 | 0.47 | |
| Clinical stage (III–IV vs. I–II) | 6 | 584 | 5,428 | 1.16 (1.06, 2.43) | 0.02 | 63 | 0.02 | |
| CPFE vs. fibrosis | 2 | 238 | 99 | 1.40 (0.71, 2.74) | 0.33 | 0 | 0.69 | |
| CPFE vs. emphysema | 2 | 238 | 601 | 1.44 (1.00, 2.07) | 0.32 | 0 | 0.05 | |
| CPFE vs. normal | 2 | 238 | 1,573 | 2.21 (1.56, 3.13) | <0.001 | 0 | 0.51 | |
| Pathological stage (III–IV vs. I–II) | 8 | 477 | 4,965 | 1.55 (1.22, 1.96) | <0.001 | 15 | 0.31 | |
| CPFE vs. fibrosis | 7 | 320 | 194 | 1.09 (0.70, 1.70) | 0.69 | 0 | 0.8 | |
| CPFE vs. emphysema | 6 | 265 | 702 | 1.25 (0.87, 1.80) | 0.23 | 0 | 0.55 | |
| CPFE vs. normal | 5 | 242 | 1,893 | 2.00 (1.43, 2.80) | <0.001 | 18 | 0.3 | |
| KL-6 | 3 | 320 | 1,605 | 200.18 (101.52, 298.84) | <0.001 | 77 | 0.002 | |
| CPFE vs. fibrosis | 4 | 232 | 146 | −1.76 (−75.3, 71.78) | 0.96 | 0 | 0.45 | |
| CPFE vs. emphysema | 2 | 154 | 259 | 270.24 (240.96, 299.53) | <0.001 | 0 | 0.84 | |
| CPFE vs. normal | 2 | 154 | 1,137 | 302.31 (273.49, 331.12) | <0.001 | 0 | 0.77 | |
| FVC% | 3 | 101 | 309 | 5.64 (−11.17, 22.44) | 0.51 | 88 | 0.002 | |
| CPFE vs. fibrosis | 2 | 66 | 52 | 4.92 (−2.28, 12.13) | 0.18 | 0 | 0.76 | |
| CPFE vs. emphysema | 1 | 11 | 108 | −10.1 (−21.39, 1.19) | 0.08 | NA | NA | |
| CPFE vs. normal | 1 | 11 | 124 | −11.8 (−23.21, −0.39) | 0.04 | NA | NA | |
| VC% | 7 | 499 | 4,786 | 6.17 (−2.66, 15) | 0.17 | 98 | <0.001 | |
| CPFE vs. fibrosis | 5 | 254 | 147 | 13.87 (0.42, 27.33) | 0.04 | 82 | <0.001 | |
| CPFE vs. emphysema | 5 | 254 | 596 | −4.92 (−16.19, 6.36) | 0.39 | 97 | <0.001 | |
| CPFE vs. normal | 4 | 231 | 1,788 | −4.3 (−12.21, 3.61) | 0.29 | 93 | <0.001 | |
| FEV1 | 4 | 253 | 1,814 | 0.01 (−0.05, 0.07) | 0.69 | 36 | 0.2 | |
| CPFE vs. fibrosis | 3 | 165 | 103 | 0.27 (−0.11, 0.64) | 0.16 | 92 | <0.001 | |
| CPFE vs. emphysema | 3 | 165 | 369 | −0.05 (−0.16, 0.06) | 0.4 | 23 | 0.27 | |
| CPFE vs. normal | 3 | 165 | 1,280 | 0 (−0.07, 0.06) | 0.98 | 0 | 0.48 | |
| FEV1% | 10 | 600 | 5,077 | −2.29 (−4.69, 0.12) | <0.001 | 52 | 0.03 | |
| CPFE vs. fibrosis | 7 | 320 | 197 | −5.24 (−7.01, −3.47) | <0.001 | 0 | 0.53 | |
| CPFE vs. emphysema | 6 | 265 | 704 | 5.83 (1.39, 10.27) | 0.03 | 80 | <0.001 | |
| CPFE vs. normal | 5 | 242 | 1,912 | −9.14 (−14.08, −4.2) | <0.001 | 67 | 0.05 | |
| FEV1/FVC% | 5 | 177 | 716 | 8.79 (−5.75, 23.34) | 0.24 | 98 | <0.001 | |
| CPFE vs. fibrosis | 3 | 54 | 38 | −8.37 (−12.98, −3.75) | 0.0004 | 0 | 0.59 | |
| CPFE vs. emphysema | 3 | 54 | 221 | 6.96 (−0.13, 14.05) | 0.05 | 80 | 0.006 | |
| CPFE vs. normal | 2 | 31 | 369 | −5.07 (−9.08, −1.07) | 0.01 | 0 | 0.44 | |
| DLCO% | 8 | 557 | 4,443 | −13.82 (−21.4, −6.24) | <0.001 | 96 | <0.001 | |
| CPFE vs. fibrosis | 5 | 283 | 167 | −7.23 (−14.15, −0.31) | 0.04 | 69 | 0.01 | |
| CPFE vs. emphysema | 4 | 228 | 564 | −13.41 (−16.54, −10.27) | <0.001 | 0 | 0.97 | |
| CPFE vs. normal | 3 | 205 | 1,480 | −28.5 (−36.53, −20.47) | <0.001 | 77 | 0.01 | |
a, numbers of studies included in the meta-analysis; b, number of patients of CPFE group; c, number of patients of non-CPFE group. WMD, weighted Mean Difference; OR, odds ratio; 95% CI, confidence interval; P, p value of pooled HR; I2, value of χ2 based I-squared statistics; NA, not available; Ph, P value of Heterogeneity test; CPFE, combined pulmonary fibrosis and emphysema; FVC, forced vital capacity; VC, vital capacity; FEV1, forced expiratory volume in 1 s; DLCO, diffusing capacity of the lung for car-bon monoxide; Lob, lobectomy or bilobectomy; Sq, squamous cell carcinoma; low, lower lobe; KL-6, sialylated carbohydrate antigen; BMI, body mass index.
Meta-analysis of short-term post-operation outcomes
We further estimated the short term outcome after operation of lung cancer patients with CPFE and compared it with lung cancer patients without CPFE. Lung cancer patients with CPFE had higher 30-day mortality (OR =4.72; 95% CI: 2.06–10.85, P<0.001), 90-day mortality (OR =5.33; 95% CI: 1.39–20.42, P=0.01), and the incidence of postoperative complications (OR =5.25; 95% CI: 2.38–11.57, P<0.001) (Figure S2). The CPFE patients had a significant risk for pneumonia (OR =4.49; 95% CI: 2.53–7.59, P<0.001) and pulmonary air leakage (OR =4.55; 95% CI: 2.68–7.73, P<0.001). The main cause of death was lung cancer (OR =2.99; 95% CI: 1.45–6.20, P=0.047). We further performed subgroup analysis of 30-day mortality (OR =21.44; 95% CI: 9.63–21.93, P=0.008) and 90-day mortality (OR =35.57; 95% CI: 20.44–193.97), then we found that there was difference between CPFE group and normal sub-group. In the subgroup analysis of complications (OR =2.47, 95% CI: 1.72–3.54, P<0.001; OR =7.21, 95% CI: 1.26–41.27, P=0.03) and cause of death (OR =2.38, 95% CI: 1.39–4.07, P=0.002; OR =5.17, 95% CI: 3.88–6.02, P<0.001), CPFE group was different from emphysema and normal sub-groups. The results were presented in Table 3.
Figure S2.
Short-term post-operation analyses for 30-day mortality (A), 90-day mortality (B), cause of death (C), complications (D) comparing lung cancer patients with CPFE with lung cancer patients without CPFE. CPFE, combined pulmonary fibrosis and emphysema.
Table 3. Meta-analysis and subgroup analysis of short-term post-operation comparison of lung cancer patients with and without CPFE.
| Variables | N (study) | N (case) | N (control) | Pooled data | Heterogeneity | |||
|---|---|---|---|---|---|---|---|---|
| WMD/OR (95% CI) | P | I2 (%) | Ph | |||||
| Cause of death (LC vs. others) | 4 | 143 | 1,257 | 2.99 (1.45, 6.20) | 0.003 | 62 | 0.05 | |
| CPFE vs. fibrosis | 4 | 143 | 93 | 1.48 (−1.64, 1.02) | 0.18 | 0 | 0.41 | |
| CPFE vs. emphysema | 3 | 88 | 408 | 2.38 (1.19, 3.42) | 0.002 | 31 | 0.24 | |
| CPFE vs. normal | 3 | 88 | 756 | 5.17 (3.88, 6.02) | <0.001 | 55 | 0.11 | |
| 30-day mortality | 5 | 417 | 4,343 | 4.72 (2.06, 10.85) | <0.001 | 48 | 0.12 | |
| CPFE vs. fibrosis | 4 | 362 | 158 | 2.21 (2.83, 8.08) | 0.3 | 0 | 0.75 | |
| CPFE vs. emphysema | 3 | 205 | 529 | 3.61 (1.3, 3.19) | 0.22 | 55 | 0.14 | |
| CPFE vs. normal | 2 | 68 | 511 | 21.44 (9.63, 21.93) | 0.008 | NA | NA | |
| 90-day mortality | 4 | 360 | 3,715 | 5.33 (1.39, 20.42) | 0.01 | 74 | 0.009 | |
| CPFE vs. fibrosis | 3 | 305 | 139 | 2.64 (2.11, 34.29) | 0.07 | 31 | 0.24 | |
| CPFE vs. emphysema | 2 | 148 | 307 | 2.63 (1.14, 10.64) | 0.05 | 0 | 0.8 | |
| CPFE vs. normal | 1 | 11 | 124 | 35.57 (20.44, 193.97) | 0.03 | NA | NA | |
| Complications | 6 | 434 | 4,599 | 5.25 (2.38, 11.57) | <0.001 | 87 | <0.001 | |
| CPFE vs. fibrosis | 4 | 260 | 158 | 2.27 (0.91, 5.7) | 0.08 | 66 | 0.03 | |
| CPFE vs. emphysema | 3 | 205 | 529 | 2.47 (1.72, 3.54) | <0.001 | 10 | 0.33 | |
| CPFE vs. normal | 2 | 68 | 511 | 7.21 (1.26, 41.27) | 0.03 | 69 | 0.07 | |
| AE | 5 | 375 | 1,379 | 1.54 (0.34, 6.87) | 0.57 | 78 | 0.001 | |
| Pneumonia | 4 | 260 | 2,163 | 4.49 (2.53, 7.95) | <0.001 | 22 | 0.28 | |
| CPFE vs. fibrosis | 4 | 260 | 158 | 2.63 (0.95, 0.95) | 0.06 | 0 | 0.52 | |
| CPFE vs. emphysema | 3 | 205 | 529 | 2.11 (1.14, 3.91) | 0.02 | 0 | 0.54 | |
| CPFE vs. normal | 2 | 68 | 511 | 7.67 (3.38, 17.42) | <0.001 | 0 | 0.34 | |
| Pulmonary air leakage | 3 | 249 | 1,925 | 4.55 (2.68, 7.73) | <0.001 | 0 | 0.57 | |
| CPFE vs. fibrosis | 3 | 249 | 151 | 2.52 (1.07, 5.9) | 0.03 | 0 | 0.47 | |
| CPFE vs. emphysema | 2 | 194 | 421 | 1.47 (0.5, 4.32) | 0.48 | 66 | 0.09 | |
| CPFE vs. normal | 1 | 57 | 387 | 5.57 (1.86, 16.71) | 0.002 | NA | NA | |
a, numbers of studies included in the meta-analysis; b, number of patients of CPFE group; c, number of patients of non-CPFE group. LC, lung cancer; OR, odds ratio; 95% CI, confidence interval; P, P value of pooled HR; I2, value of χ2 based I-squared statistics; Ph, P value of Heterogeneity test; CPFE, combined pulmonary fibrosis and emphysema; NA, not available.
Meta-analysis of overall survival
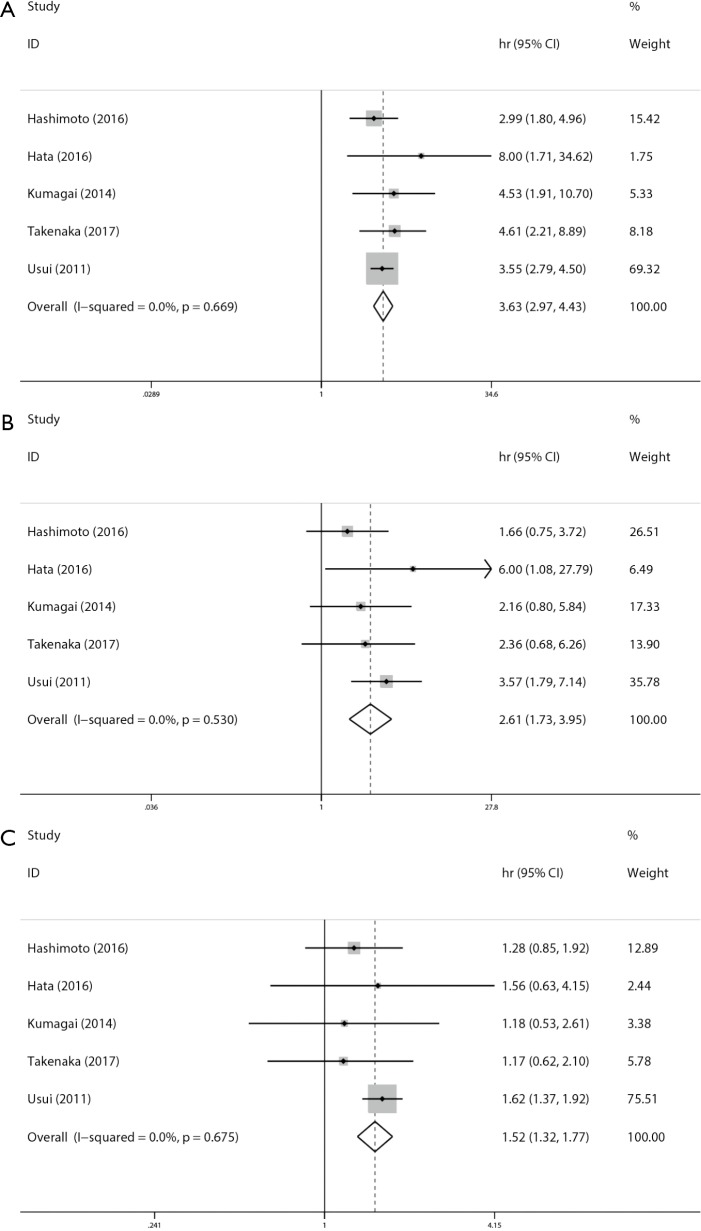
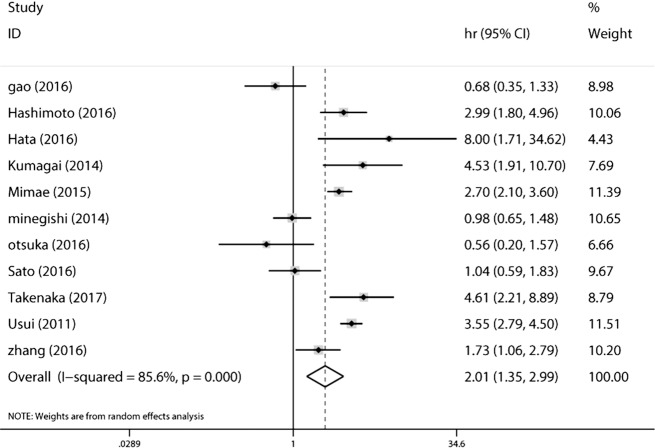
The overall analysis of 11 studies revealed that lung cancer patients with CPFE had a poorer overall survival (HR =2.006; 95% CI: 1.347–2.986, P=0.001) than lung cancer patients without CPFE (Figure 2). We further performed subgroup analysis by sample size, analysis method, and type of treatment, and patients in the mixed group (HR =2.615; 95% CI: 1.672–4.091, P=0.001) and resected group (HR =2.334; 95% CI: 1.532–3.555, P<0.001) had a poorer OS. Lung cancer patients with CPFE were associated with poorer OS (HR =3.629; 95% CI: 2.975–4.429, P<0.001) than normal patients according to pooled data of five studies (Figure 3). Lung cancer patients with emphysema (HR =1.524; 95% CI: 1.316–1.765, P<0.001) or fibrosis (HR =2.61; 95% CI: 1.725–3.947, P<0.001) had poorer OS than normal patients according to pooled data of four studies (Figure 3). More detailed subgroup analyses were shown in Table 4.
Figure 2.
Results of prognosis analysis for OS comparison of lung cancer patients with CPFE (A), fibrosis (B), and emphysema (C) and normal patients. OS, overall survival; CPFE, combined pulmonary fibrosis and emphysema.
Figure 3.
Results of prognosis analysis for OS comparison of lung cancer patients with CPFE and without CPFE. OS, overall survival; CPFE, combined pulmonary fibrosis and emphysema.
Table 4. Result of meta-analysis and subgroup analysis of overall survival.
| Variables | N (study)a | N (case)b | Pooled data | Heterogeneity | |||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | P | I2 (%) | Ph | ||||
| Overall (CPFE vs. non-CPFE) | 11 | 6,413 | 2.006 (1.347, 2.986) | 0.001 | 85.6 | 0.001 | |
| By analysis method | |||||||
| Univariate | 4 | 1,370 | 1.148 (0.42, 3.173) | 0.788 | 92.5 | <0.001 | |
| Multivariate | 7 | 5,043 | 2.615 (1.672, 4.091) | 0.001 | 79.4 | <0.001 | |
| By sample size | |||||||
| <500 | 7 | 3,449 | 1.976 (1.059, 3.69) | 0.032 | 84 | <0.001 | |
| ≥500 | 4 | 2,964 | 2.773 (2.112, 3.641) | 0.023 | 90.3 | <0.001 | |
| By type | |||||||
| Resected | 8 | 5,059 | 2.334 (1.532, 3.555) | <0.001 | 74.2 | <0.001 | |
| Mixed | 3 | 1,354 | 1.37 (0.465, 4.039) | 0.568 | 95.2 | <0.001 | |
| Overall (CPFE vs. normal) | 5 | 2,717 | 3.629 (2.975, 4.429) | <0.001 | 0 | 0.669 | |
| By analysis method | |||||||
| Univariate | 1 | 1,143 | 3.55 (2.795, 4.509) | <0.001 | NA | NA | |
| Multivariate | 4 | 1,574 | 3.816 (2.664, 5.465) | <0.001 | 0 | 0.521 | |
| By sample size | |||||||
| <500 | 3 | 889 | 4.881 (2.933, 8.124) | <0.001 | 0 | 0.79 | |
| ≥500 | 2 | 1,828 | 3.441 (2.772, 4.271) | <0.001 | 0 | 0.548 | |
| By type | |||||||
| Resected | 4 | 1,574 | 3.816 (2.664, 5.465) | <0.001 | 0 | 0.521 | |
| Mixed | 1 | 1,143 | 3.55 (2.795, 4.509) | <0.001 | NA | NA | |
| Overall (fibrosis vs. normal) | 5 | 2,717 | 2.61 (1.725, 3.947) | <0.001 | 0 | 0.53 | |
| By analysis method | |||||||
| Univariate | 1 | 1,143 | 3.57 (1.787, 7.13) | <0.001 | NA | NA | |
| Multivariate | 4 | 1,574 | 2.192 (1.308, 3.673) | 0.003 | 0 | 0.584 | |
| By sample size | |||||||
| <500 | 3 | 889 | 2.66 (1.356, 5.218) | 0.004 | 9.4 | 0.556 | |
| ≥500 | 2 | 1,828 | 2.58 (1.527, 4.358) | <0.001 | 49.8 | 0.158 | |
| By type | |||||||
| Resected | 4 | 1,574 | 2.192 (1.308, 3.673) | 0.003 | 0 | 0.584 | |
| Mixed | 1 | 1,143 | 3.57 (1.787, 7.13) | <0.001 | NA | NA | |
| Overall (emphysema vs. normal) | 5 | 2,717 | 1.524 (1.316, 1.765) | <0.001 | 0 | 0.675 | |
| By analysis method | |||||||
| Univariate | 1 | 1,143 | 1.62 (1.368, 1.918) | <0.001 | NA | NA | |
| Multivariate | 4 | 1,574 | 1.263 (0.939, 1.699) | 0.122 | 0 | 0.962 | |
| By sample size | |||||||
| <500 | 3 | 889 | 1.246 (0.81, 1.917) | 0.361 | 0 | 0.869 | |
| ≥500 | 2 | 1,828 | 1.565 (1.339, 1.829) | <0.001 | 9 | 0.295 | |
| By treatment | |||||||
| Resected | 4 | 1,574 | 1.263 (0.939, 1.699) | 0.122 | 0 | 0.962 | |
| Mixed | 1 | 1,143 | 1.62 (1.368, 1.918) | <0.001 | NA | NA | |
a, numbers of studies included in the meta-analysis; b, number of patients of included studies. HR, hazard ratio; 95% CI, confidence interval; P, P value of pooled HR; I2, value of Higgins I-squared statistics; Ph, P value of Heterogeneity test; CPFE, combined pulmonary fibrosis and emphysema; NA, not available.
Sensitivity analysis and publication bias
We performed a sensitivity analysis to assess the stability of our results and the plots illustrated that our results were robust because pooled HRs or ORs were not significantly influenced by excluding any single study. Egger’s test and Begg’s funnel plots were used to assess publication bias in this meta-analysis. No significant publication bias was detected.
Discussion
Our meta-analysis of 13 individual studies involving 8,050 patients demonstrated the clinical characteristics and prognosis of primary lung cancer patients with CPFE and compared these with normal findings, emphysematous or fibrous changes on chest CT. Our findings might be significant in the evaluation and treatment of lung cancer patients with CPFE.
Our study found that the major histological type of lung cancer patients with CPFE was squamous cell carcinoma and the location of primary lung cancers were mainly lower lobes, which meant lung cancer predominantly occur in fibrotic lesions. This finding was consistent with previous studies (10,14,16,17). We also found that the lung cancer patients with CPFE were ex-current smokers with heavy smoking history. Emphysema and fibrosis were tobacco-related disease and squamous carcinoma had been reported to be more significantly associated with tobacco compared with adenocarcinoma (24,25). Jankowich et al. said that gene alterations were associated with the histology and smoking status in patients with squamous cell carcinoma (26). Gao et al. found that lung cancer in CPFE were inside the fibrotic area (P<0.001) and most of them showed subpleural preference, suggesting a direct relationship between the fibrosis and carcinogenesis (17). Zhang et al. also found the relationship (3). Our study supported the viewpoint, as the subgroup analysis found that location of lung cancer was similar between patients with CPFE and patients with fibrosis, furthermore, the 90-day mortality of lung cancer patients with CPFE was similar to lung cancer patients with fibrosis and was different from emphysema and normal group. Therefore, we suspected that the genetic mutation associated with chronic smoking-induced inflammation occur in fibrotic lung fields of the lung cancer patients with CPFE and lung cancer in patients with CPFE has a similar developmental process to that of lung cancer in patients with fibrosis. Then we suggested that whole-genome sequencing should be conducted to investigate the gene mutation in the fibrotic fields, which could be important for the molecularly targeted therapies in lung cancer patients with CPFE.
The composite physiological index (CPI) represents a combination of pulmonary ventilation and diffusing capacity, we calculated the CPI as follows: CPI = 91.0 – [0.65 × percent predicted diffusion capacity for carbon monoxide (DLCO)] – [0.53 × percent predicted forced vital capacity (FVC)] + [0.34 × percent predicted forced expiratory volume in 1 s (FEV1)] (27). Similar to previous studies, our study showed CPFE group had lower DLCO%, FEV1% and similar FVC%, VC%, FEV1/FVC%, FEV1 in respect to other groups (11,12,14,16-20,22). Assessment of respiratory function (HR =1.9, P=0.017, 95% CI: 1.1–3.2) is useful for the prediction of lung cancer patients with CPFE (27). Only fever studies explored the association of preoperative pulmonary function parameters with prognosis in lung cancer patients with CPFE who have undergone surgery (15,16,28). Mimae et al. reported that both lower VC% (HR =1.7, P=0.013, 95% CI: 1.1–2.5) and higher FEV1% (HR =1.5, P=0.05, 95% CI: 1.0–2.3) were associated with a poorer prognosis (15). However, Ueno et al. found that a higher preoperative CPI not the individual preoperative pulmonary function parameters was associated with a high risk of death (HR =1.03, P=0.017, 95% CI: 1–1.06), which was consistent with our study because FEV1% and DLCO% were included in the formula for calculating the CPI (28). Both lung function values and radiological CT findings were important for the decision of surgical approach. However, to quantify lung emphysema and fibrosis on CT was difficult and thus, other indicators were necessary (4,26). The CPI might provide more prognostic information than the individual function parameters in lung cancer patients with CPFE who have undergone surgery. Therefore, we indicated that preoperative CPI could be important for surgeons to select surgical approaches.
In our study, we found lung cancer patients with CPFE have a poorer prognosis, a worse postoperative mortality and a significantly increased rate of lung cancer-associated mortality, which was consistent with previous studies (11,12,14-16,18-22). Mimae et al. showed that cancer related death (P<0.001) was affected by clinical stage (15). The chronic lung injury induced by smoking occurring in CPFE patients may influence the development and progression of lung cancer, which may be related to the “triple hit” effects of smoking, emphysema, and pulmonary fibrosis (13). Lung cancer patients with CPFE had more complications and respiratory-related complications were frequent and severe in our study. Hata et al. pointed out that lung cancer patients with lung cancer had a high risk of death (P<0.001) due to respiratory failure caused by bacterial infection or AE (18). Mimae et al. indicated that the prognosis of lung cancer in patients with CPFE was affected by the background lung function being comprised by fibrosis and emphysema (15). It is still unclear which treatment in lung cancer patients with CPFE is effective and safe. Patients with CPFE had a worse overall survival after lung cancer resection. Fukui et al. found that blood loss (P=0.038) during surgery is another risk factor for the surgical death of CPFE patients (16). Miyamoto et al. reported that chemotherapy could be a treatment option for advanced NSCLC patients with CPFE because the response rate was relatively good and the occurrence of acute exacerbation of interstitial pneumonia was tolerable (29). We divided studies into two groups based on treatment method and found that lung cancer patients with CPFE and without CPFE had similar prognosis in mixed group, however, in the resected group, lung cancer patients with CPFE had poorer prognosis. We assume that chemotherapy, best supported care and radiotherapy might benefit lung cancer patients with CPFE, but the validity of such outcome was questionable because of confounding factors and only three articles were included in the subgroup analysis. Therefore, we suggested that more studies on selecting optimal treatment for lung cancer patients with CPFE should be conducted to explore the most effective and safe treatment.
Our study had several limitations. First, the studies included in our meta-analysis were all retrospective collection of data and the results may have been subject to selection bias. Second, there was no uniform standard for the selection of treatment for all patients, limiting evaluation of the treatment. Although we did subgroup analysis of treatment, too few studies of mixed group could not explore the effect of difference on result. Third, although we collected all eligible studies for evaluating the clinical characteristic and prognosis of lung cancer patients with CPFE, the sample size was not large enough, which in turn weakened the statistical power of the results. Finally, in this present analysis, there were unmeasured and unknown confounders, of which the influence could not be completely excluded.
In conclusion, this meta-analysis revealed that lung cancer patients with CPFE have a different clinical characteristic and a poor short-term after surgery and a poor long-term prognosis. More investigations were needed to explore which way is appropriate for the surgical treatment of lung cancer patients with CPFE and great care should be taken after treatment.
Acknowledgements
Funding: This work was supported by National Natural Science Foundation of China (No. 81172236 and No. 81372505) and Key Science and Technology Program of Sichuan Province, China (2014SZ0148, 2016FZ0118) (to Dr. L Liu).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. 10.3322/caac.21262 [DOI] [PubMed] [Google Scholar]
- 2.Cottin V, Cordier JF. Combined pulmonary fibrosis and emphysema: an experimental and clinically relevant phenotype. Am J Respir Crit Care Med 2005;172:1605. 10.1164/ajrccm.172.12.1605a [DOI] [PubMed] [Google Scholar]
- 3.Zhang L, Zhang C, Dong F, et al. Combined pulmonary fibrosis and emphysema: a retrospective analysis of clinical characteristics, treatment and prognosis. BMC Pulm Med 2016;16:137. 10.1186/s12890-016-0300-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cottin V. The impact of emphysema in pulmonary fibrosis. Eur Respir Rev 2013;22:153-7. 10.1183/09059180.00000813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cottin V, Nunes H, Brillet P, et al. Combined pulmonary fibrosis and emphysema: A distinct under-recognized entity. Eur Respir J 2005;26:586-93. 10.1183/09031936.05.00021005 [DOI] [PubMed] [Google Scholar]
- 6.Kitaguchi Y, Fujimoto K, Hanaoka M, et al. Clinical characteristics of combined pulmonary fibrosis and emphysema. Respirology 2010;15:265-71. 10.1111/j.1440-1843.2009.01676.x [DOI] [PubMed] [Google Scholar]
- 7.Ryerson CJ, Hartman T, Elicker BM, et al. Clinical features and outcomes in combined pulmonary fibrosis and emphysema in idiopathic pulmonary fibrosis. Chest 2013;144:234-40. 10.1378/chest.12-2403 [DOI] [PubMed] [Google Scholar]
- 8.Odani K, Yoriko M, Shoji Y. Computed tomographic evaluation in the cases of coexistence of pulmonary emphysema with idiopathic pulmonary fibrosis. J Tomogr 2004;31:25-9. [Google Scholar]
- 9.Koo HJ, Do KH, Lee JB, et al. Lung Cancer in Combined Pulmonary Fibrosis and Emphysema: A Systematic Review and Meta-Analysis. PloS One 2016;11:e0161437. 10.1371/journal.pone.0161437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Usui K, Tanai C, Tanaka Y, et al. The prevalence of pulmonary fibrosis combined with emphysema in patients with lung cancer. Respirology 2011;16:326-31. 10.1111/j.1440-1843.2010.01907.x [DOI] [PubMed] [Google Scholar]
- 11.Kumagai S, Marumo S, Yamanashi K, et al. Prognostic significance of combined pulmonary fibrosis and emphysema in patients with resected non-small-cell lung cancer: a retrospective cohort study. Eur J Cardiothorac Surg 2014;46:e113-119. 10.1093/ejcts/ezu384 [DOI] [PubMed] [Google Scholar]
- 12.Hashimoto N, Iwano S, Kawaguchi K, et al. Impact of Thin-Section Computed Tomography-Determined Combined Pulmonary Fibrosis and Emphysema on Outcomes Among Patients With Resected Lung Cancer. Ann Thorac Surg 2016;102:440-7. 10.1016/j.athoracsur.2016.03.014 [DOI] [PubMed] [Google Scholar]
- 13.Fujiwara A, Tsushima K, Sugiyama S, et al. Histological types and localizations of lung cancers in patients with combined pulmonary fibrosis and emphysema. Thorac Cancer 2013;4:354-60. 10.1111/1759-7714.12023 [DOI] [PubMed] [Google Scholar]
- 14.Minegishi Y, Kokuho N, Miura Y, et al. Clinical features, anti-cancer treatments and outcomes of lung cancer patients with combined pulmonary fibrosis and emphysema. Lung Cancer 2014;85:258-63. 10.1016/j.lungcan.2014.05.016 [DOI] [PubMed] [Google Scholar]
- 15.Mimae T, Suzuki K, Tsuboi M, et al. Surgical Outcomes of Lung Cancer in Patients with Combined Pulmonary Fibrosis and Emphysema. Ann Surg Oncol 2015;22:S1371-1379. 10.1245/s10434-015-4577-1 [DOI] [PubMed] [Google Scholar]
- 16.Fukui M, Suzuki K, Matsunaga T, et al. Outcomes of lung cancer resection for patients with combined pulmonary fibrosis and emphysema. Surg Today 2016;46:341-7. 10.1007/s00595-015-1234-z [DOI] [PubMed] [Google Scholar]
- 17.Gao L, Xie S, Liu H, et al. Lung cancer in patients with combined pulmonary fibrosis and emphysema revisited with the 2015 World Health Organization classification of lung tumors. Clin Respir J 2016. [Epub ahead of print]. 10.1111/crj.12575 [DOI] [PubMed] [Google Scholar]
- 18.Hata A, Sekine Y, Kota O, et al. Impact of combined pulmonary fibrosis and emphysema on surgical complications and long-term survival in patients undergoing surgery for non-small-cell lung cancer. Int J Chron Obstruct Pulmon Dis 2016;11:1261-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Otsuka H, Sugino K, Hata Y, et al. Clinical features and outcomes of patients with lung cancer as well as combined pulmonary fibrosis and emphysema. Mol Clin Oncol 2016;5:273-8. 10.3892/mco.2016.954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sato S, Koike T, Hashimoto T, et al. Surgical Outcomes of Lung Cancer Patients with Combined Pulmonary Fibrosis and Emphysema and those with Idiopathic Pulmonary Fibrosis without Emphysema. Ann Thorac Cardiovasc Surg 2016;22:216-23. 10.5761/atcs.oa.15-00315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang M, Yoshizawa A, Kawakami S, et al. The histological characteristics and clinical outcomes of lung cancer in patients with combined pulmonary fibrosis and emphysema. Cancer Med 2016;5:2721-30. 10.1002/cam4.858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takenaka T, Furuya K, Yamazaki K, et al. The prognostic impact of combined pulmonary fibrosis and emphysema in patients with clinical stage IA non-small cell lung cancer. Surg Today 2017. [Epub ahead of print]. 10.1007/s00595-017-1577-8 [DOI] [PubMed] [Google Scholar]
- 23.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 1998;17:2815-34. [DOI] [PubMed] [Google Scholar]
- 24.Portillo K, Morera J. Combined Pulmonary Fibrosis and Emphysema Syndrome: A New Phenotype within the Spectrum of Smoking-Related Interstitial Lung Disease. Pulm Med 2012;2012:867870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khuder SA. Effect of cigarette smoking on major histological types of lung cancer: a meta-analysis. Lung Cancer 2001;31:139-48. 10.1016/S0169-5002(00)00181-1 [DOI] [PubMed] [Google Scholar]
- 26.Jankowich MD, Rounds SIS. Combined pulmonary fibrosis and emphysema syndrome: a review. Chest 2012;141:222-31. 10.1378/chest.11-1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmidt SL, Nambiar AM, Tayob N, et al. Pulmonary function measures predict mortality differently in IPF versus combined pulmonary fibrosis and emphysema. Eur Respir J 2011;38:176-83. 10.1183/09031936.00114010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ueno F, Kitaguchi Y, Shiina T, et al. The Preoperative Composite Physiologic Index May Predict Mortality in Lung Cancer Patients with Combined Pulmonary Fibrosis and Emphysema. Respiration 2017;94:198-206. 10.1159/000477587 [DOI] [PubMed] [Google Scholar]
- 29.Miyamoto A, Moriguchi S, Takahashi Y, et al. Chemotherapy for patients with advanced non-small cell lung cancer associated with combined pulmonary emphysema and fibrosis. Am J Respir Crit Care Med 2017; Conference: American Thoracic Society International Conference, ATS, 195 (no pagination). [Google Scholar]