Abstract
Background
Chronic bronchitis (CB) is closely associated with the frequency and severity of chronic obstructive pulmonary disease (COPD) exacerbation. However, little is known about the impact of CB on COPD exacerbations, severe and non-severe, and on recovery from an exacerbation.
Methods
We conducted a nation-wide multicenter cross-sectional survey in China between September 2007 and December 2008. Eleven hospitals participated in this study. Patients’ demographic information, presence of CB, overall numbers of COPD exacerbation and severe exacerbation leading to emergency visit, hospitalization and intensive care unit (ICU) stay in the past year, recovery period following the last exacerbation, and well- or poor-recovery were recorded.
Results
A total of 1,101 patients with COPD were enrolled and 890 (80.8%) had CB. Patients with CB reported more history of frequent exacerbations (≥2/patient/year) (59.6% vs. 50.7%, P=0.019) and severe exacerbation (% emergency visit ≥1: 28.0% vs. 16.6%, P=0.001; % hospitalization ≥1: 51.2% vs. 28.0%, P<0.001; %ICU stay ≥1: 6.5% vs. 1.9%, P=0.009). Recovery period following the last exacerbation was longer in patients with CB (19.0±16.2 vs. 15.2±14.7 days, P=0.003) and more patients with CB reported poor recovery (85.8% vs. 78.4%, P=0.003). Multivariate analyses showed that CB was independently associated with severe exacerbation requiring emergency visit (adjusted OR, 1.512, P=0.048) and hospitalization (adjusted OR, 2.031, P<0.001) and prolonged recovery period (adjusted regression coefficient 2.861, P=0.030).
Conclusions
CB is associated with frequent exacerbations of COPD in Chinese population, especially severe exacerbations requiring emergency visit and hospitalization admission. Additionally, CB significantly prolongs recovery period following COPD exacerbation.
Keywords: Chronic obstructive pulmonary disease (COPD), chronic bronchitis (CB), exacerbation, hospitalization
Introduction
Chronic obstructive pulmonary disease (COPD) is a world-wide disease that characterized by persistent respiratory symptoms and airflow limitation. COPD exacerbation impacts patients’ health condition and quality of life negatively, accelerates disease progression and leads to hospitalization and even death (1). Among the COPD’s phenotypes, there are two main ones: emphysema phenotype or pink puffer and chronic bronchitis (CB) phenotype or blue bloated. CB, which is defined as the presence of cough and sputum production for at least 3 months in each of two consecutive years (2), has been found to be closely associated with the frequency and severity of COPD exacerbations. Chronic cough and sputum production increase the total number of COPD exacerbations per patient per year, as well as hospitalization (3,4). The COPDGene study found that patients with COPD concomitant with CB reported more exacerbations and severe exacerbations due to emergency visit or hospitalization (5). The PLATINO study also found that CB increased more respiratory symptoms and more exacerbations among patients with COPD (6). A recent study showed that COPD patients with persistent and newly developed CB had greater exacerbation frequency (7). Besides that, CB has other clinical consequences, including accelerated decline in lung function (6,8-10), worse health-related quality of life (6,7,11), and increased COPD-related or all-cause mortality (11-13). Additionally, in the patients without COPD at baseline, CB or chronic respiratory symptoms will increase the risk of airflow limitation (13) and the incidence of COPD on follow-up, independently of smoking habit which is the most common risk factor of developing COPD (14,15). Small data are available on relationship between CB and recovery following exacerbation (16,17). Therefore, COPD concomitant with CB has been considered to be an important clinical phenotype of COPD. However, studies on CB phenotype in COPD are rare in Chinese population.
The first aim of this study was to investigate the association between CB and frequency and severity of COPD exacerbations in a large Chinese cohort of patients with COPD. Second aim was to explore whether CB would lead to prolonged recovery period or poor recovery following the last exacerbation.
Methods
Population selection and study design
A nation-wide multicenter questionnaire study was conducted from September 2007 to December 2008. A total of 11 hospitals in 7 provinces (Beijing, Liaoning, Inner Mongolia, Sichuan, Shanghai, Hubei, and Guangdong) in China participated in this study. The patients who were clinically diagnosed as COPD were invited to complete a standardized questionnaire and spirometry function testing. Chest X-ray was performed to exclude the presence of other active pulmonary diseases.
COPD was diagnosed according to Chinese Thoracic Society guideline that post-bronchodilator forced expiratory volume in one second (FEV1)/forced vital capacity (FVC) was <0.7 and other airflow limitation diseases should be excluded. The exclusion criteria included: asthma, bronchiectasis, active tuberculosis, mental disease, cognitive dysfunction, communication difficulties, malignancy and severe heart dysfunction. Each patient was recruited once and data was analyzed anonymously. The study protocol was approved by the ethics committee of Peking University Third Hospital (No. M2017120), which is the primary research institution of this study. All the patients had signed the informed consent forms and the study outcomes would not affect the future management of the patients.
Questionnaires and data collection
All subjects were individually interviewed by well-trained doctors from each center, utilizing a standardized questionnaire which was strictly designed by respiratory specialists from each center (18). The questionnaire covered demographic data, tobacco exposure, COPD characteristics, presence of CB, quality of life, characteristics of COPD exacerbation, recovery period and recovery of health status following the last COPD exacerbation.
Demographic data included age, gender, height, weight and body mass index (BMI). Tobacco exposure included smoking status (current-, ex- and never) and smoking index. Current-smokers were those who smoked persistently or resumed smoking; ex-smokers were those who had quitted smoking for ≥3 months. COPD characteristics included severity of airflow limitation using the Global Initiative for Chronic Obstructive Lung Disease (GOLD) staging criteria (1) and severity of dyspnea which was assessed by the modified Medical Research Council (mMRC) dyspnea scale (19). Severity of airflow limitation was classified according to post-bronchodilator FEV1: stage I (mild), FEV1%pred ≥80%; stage II (moderate), 50%≤ FEV1%pred <80%; stage III (severe), 30%≤ FEV1%pred <50%; stage IV (very severe), FEV1%pred <30%.
CB was defined as the presence of cough and sputum production for at least 3 months in each of two consecutive years, in the absence of other causes of chronic cough (2).
Quality of life was evaluated by 36-Item Short Form Health Survey (SF-36) questionnaire. SF-36 questionnaire comprises 36 questions, which are summarized in eight health domain scores: physical functioning, role physical, general health, bodily pain, vitality, social functioning, role emotional, and mental health. These domain scores are further combined into a physical component summary (PCS) and a mental component summary (MCS) score (20). A higher SF-36 score represents better quality of life.
Exacerbation of COPD was defined as an acute worsening of respiratory symptoms (increased sputum volume, purulent sputum or dyspnea worsening) that result in additional therapy (1). Characteristics of COPD exacerbation included the overall number of exacerbation, as well as severe exacerbation in the past year. Severe exacerbation was defined as exacerbation required hospitalization or visiting the emergency room (1). The overall numbers of exacerbations and severe exacerbations (COPD-related emergency visits, COPD-related hospitalizations and ICU stay) in the prior year were self-reported by patients. Based on the exacerbation frequency, patients with COPD were further classified as frequent exacerbators (≥2 exacerbations during the past year) and infrequent or non-exacerbators (<2 exacerbations during the past year). Moreover, patients with severe exacerbations were subdivided in two subgroups according to the presence or absence of at least 1 severe exacerbation.
Recovery period following the last COPD exacerbation was self-reported by patients. Meanwhile, the patients were asked “Did your physical condition recover to pre-exacerbation state following the last exacerbation?” If the patient answered “Yes”, he or she was placed in the “well-recovery group”; if the patient answered “No”, he or she was placed in the “poor-recovery group”.
Statistical analysis
Statistical analyses were performed using SPSS software, version 21.0 (IBM, Armonk, NY, USA). Continuous variables were expressed as the mean ± standard deviation. Categorical variables were expressed as numbers (%). Unpaired t-test was used to assess the differences between groups for continuous variables. The chi-square and Fisher’s exact tests were used for categorical variables. Pearson’s correlation analysis was used to assess the relationship between recovery period following exacerbation and age, BMI and FEV1%pred. Spearman’s correlation analysis was used to assess the relationship between recovery period following exacerbation and smoking status, mMRC dyspnea score and GOLD stage. Binary logistic regression analyses were performed to assess the independent relationship of CB with the dependent variables (frequent exacerbations, COPD-related emergency visits, hospitalizations and ICU stay, and poor-recovery following the last exacerbation). General linear model analysis was performed to assess the independent relationship of CB with recovery period following exacerbation. Results were considered statistically significant at P<0.05.
Results
Patient selection
A total of 2,072 questionnaires were recruited initially. After excluding 374 questionnaires in which 337 questionnaires did not contain pulmonary function results because of quality control or patient intolerance and 37 questionnaires did not meet the diagnosis criteria of COPD, 1,698 valid questionnaires were collected. In these questionnaires, 597 patients did not report the overall number of exacerbations or overall number of COPD-related emergency visits or hospitalizations. Therefore, 1,101 patients were enrolled in the final statistical analysis (Figure 1).
Figure 1.
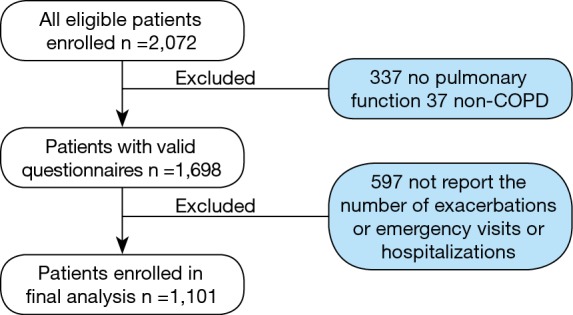
Patient selection process.
Demographic and clinical characteristics
As shown in Table 1, of the 1,101 patients, 890 (80.8%) patients had CB. The patients with CB were older and had lower BMI than those without CB. The proportion of male patients was greater in CB group. Patients with CB were more often ex-smokers and had greater smoking index, while those without CB were more often current-smokers and never-smokers. Compared with those without CB, patients with CB tended to have higher mMRC scores and have GOLD stage III and IV. Spirometry function was poorer in patients with CB. The patients with CB had lower SF-36-derived physical functioning, role physical, general health, bodily pain, vitality, social functioning, role emotional scores, and PCS and MCS than those without CB, but they had similar Mental Health score.
Table 1. Patient characteristics by chronic bronchitis.
| Clinical characteristics | CB+ (n=890) | CB− (n=211) | P |
|---|---|---|---|
| Age (year) | 67.1±11.2 | 63.2±11.3 | <0.001 |
| Male | 601 (67.5) | 125 (59.2) | 0.022 |
| Height (cm) | 165.3±8.8 | 164.3±8.0 | 0.337 |
| Weight (kg) | 61.4±11.9 | 62.6±13.0 | 0.189 |
| BMI (kg/m2) | 22.4±3.9 | 23.1±4.2 | 0.013 |
| Smoking status | 0.015 | ||
| Current- | 196 (22.0) | 54 (25.6) | |
| Ex- | 431 (48.4) | 79 (37.4) | |
| Never- | 263 (29.6) | 78 (37.0) | |
| Smoking index (pack·year) | 23.7±25.9 | 19.3±22.0 | 0.021 |
| mMRC dyspnea score | <0.001 | ||
| 0 | 64 (7.2) | 34 (16.1) | |
| 1 | 309 (34.7) | 73 (34.6) | |
| 2 | 286 (32.1) | 60 (28.4) | |
| 3 | 143 (16.1) | 36 (17.1) | |
| 4 | 88 (9.9) | 8 (3.8) | |
| GOLD stage | <0.001 | ||
| I | 41 (4.6) | 19 (9.0) | |
| II | 232 (26.1) | 94 (44.5) | |
| III | 352 (39.6) | 72 (34.1) | |
| IV | 265 (29.8) | 26 (12.3) | |
| FEV1%pred | 42.1±18.0 | 52.6±19.7 | <0.001 |
| FVC %pred | 64.7±21.2 | 75.1±24.2 | <0.001 |
| FEV1/FVC (%) | 49.8±11.1 | 55.1±10.4 | <0.001 |
| SF-36 physical functioning | 53.6±24.8 | 63.6±23.0 | <0.001 |
| SF-36 role physical | 27.7±40.9 | 46.4±47.2 | <0.001 |
| SF-36 general health | 35.4±20.9 | 43.6±23.7 | <0.001 |
| SF-36 bodily pain | 80.9±22.2 | 85.1±20.1 | 0.016 |
| SF-36 vitality | 54.0±23.9 | 59.9±24.2 | 0.001 |
| SF-36 social functioning | 56.1±26.8 | 65.7±26.7 | <0.001 |
| SF-36 role emotional | 45.3±47.1 | 55.1±48.5 | 0.008 |
| SF-36 mental health | 66.2±23.0 | 68.4±23.6 | 0.214 |
| SF-36 PCS | 49.2±19.6 | 59.9±20.2 | <0.001 |
| SF-36 MCS | 55.8±24.6 | 62.2±26.3 | 0.002 |
Data are presented as mean ± SD or number (%). CB, chronic bronchitis; BMI, body mass index; mMRC, modified Medical Research Council; GOLD, Global Initiative for Chronic Obstructive Lung Disease; FEV1%pred, forced expiratory volume in 1 second percent predicted; FVC %pred, forced vital capacity percent predicted; SF-36, 36-Item Short Form Health Survey; PCS, physical component summary score; MCS, mental component summary score.
Frequency and severity of COPD exacerbation in patients with and without CB
As shown in Table 2, the overall number of exacerbations per patient per year was slightly higher in patients with CB, but with no statistical difference. However, patients with CB reported more COPD-related emergency visits, hospitalizations and ICU stay, compared with patients without CB. Moreover, the proportion of patients with frequent exacerbations were greater in the CB+ group than in the CB− group, as were the proportions of patients who had ever experienced at least one severe exacerbation of COPD (i.e., COPD-related emergency visits, hospitalization and ICU stay) within the last year.
Table 2. Comparison of frequency and severity of exacerbation between patients with and without CB.
| Events in the past year | CB+ (n=890) | CB− (n=211) | P |
|---|---|---|---|
| Overall number of exacerbations | 2.42±2.50 | 2.13±2.83 | 0.134 |
| % Frequency of exacerbations ≥2 | 530 (59.6) | 107 (50.7) | 0.019 |
| Overall number of emergency visits | 0.69±1.78 | 0.32±0.98 | <0.001 |
| % Emergency visits ≥1 | 249 (28.0) | 35 (16.6) | 0.001 |
| Overall number of hospitalizations | 0.96±1.34 | 0.54±1.15 | <0.001 |
| % Hospitalizations ≥1 | 456 (51.2) | 59 (28.0) | <0.001 |
| Overall number of ICU stay | 0.08±0.35 | 0.02±0.14 | <0.001 |
| % ICU stay ≥1 | 58 (6.5) | 4 (1.9) | 0.009 |
Data are presented as mean ± SD or number (%). CB, chronic bronchitis; ICU, intensive care unit.
Clinical factors relevant to COPD exacerbation
As shown in Table 3, compared with those without frequent exacerbations, more patients with frequent exacerbations were ex-smokers and had higher mMRC dyspnea scores. Patients with frequent exacerbations were more likely to have CB and GOLD stage IV.
Table 3. Risk factors relevant to frequency of COPD exacerbations.
| Clinical characteristics | Frequency of COPD exacerbations | ||
|---|---|---|---|
| 0–1 (n=464) | ≥2 (n=637) | P | |
| Age (year) | 66.1±10.8 | 66.5±11.7 | 0.615 |
| Male | 294 (63.4) | 432 (67.8) | 0.123 |
| Height (cm) | 164.8±8.8 | 165.5±7.8 | 0.145 |
| Weight (kg) | 61.8±12.3 | 61.4±12.0 | 0.597 |
| BMI (kg/m2) | 22.7±3.9 | 22.4±4.0 | 0.137 |
| Smoking status | 0.049 | ||
| Current- | 112 (24.1) | 138 (21.7) | |
| Ex- | 195 (42.0) | 315 (49.5) | |
| Never- | 157 (33.8) | 184 (28.9) | |
| Smoking index (pack·year) | 21.7±24.3 | 23.8±25.8 | 0.173 |
| Chronic bronchitis | 360 (77.6) | 530 (83.2) | 0.019 |
| mMRC dyspnea score | <0.001 | ||
| 0 | 66 (14.2) | 32 (5.0) | |
| 1 | 195 (42.0) | 187 (29.4) | |
| 2 | 117 (25.2) | 229 (35.9) | |
| 3 | 59 (12.7) | 120 (18.8) | |
| 4 | 27 (5.8) | 69 (10.8) | |
| GOLD stage | <0.001 | ||
| I | 24 (5.2) | 36 (5.7) | |
| II | 163 (35.1) | 163 (25.1) | |
| III | 183 (39.4) | 241 (37.8) | |
| IV | 94 (20.3) | 197 (30.9) | |
Data are presented as mean ± SD or number (%). COPD, chronic obstructive pulmonary disease; BMI, body mass index; mMRC, modified Medical Research Council; GOLD, Global Initiative for Chronic Obstructive Lung Disease.
The probable risk factors associated with severe exacerbations were presented in Table 4. The patients who had experienced ≥1 severe exacerbations in the past year were older and had lower body weight and BMI, except the patients who had experienced ICU stay. The patients with ≥1 severe exacerbations were more likely to have CB, higher mMRC dyspnea score, and GOLD stage IV.
Table 4. Risk factors relevant to severe exacerbations of COPD (emergency visits, hospitalization and ICU stay).
| Clinical characteristics | Emergency visits | Hospitalization | ICU stay | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No (n=817) | ≥1 (n=284) | P | No (n=586) | ≥1 (n=515) | P | No (n=1,039) | ≥1 (n=62) | P | |||
| Age (year) | 65.2±11.1 | 69.6±11.5 | <0.001 | 63.4±11.2 | 69.6±10.5 | <0.001 | 66.0±11.3 | 72.5±9.2 | <0.001 | ||
| Male | 534 (65.4) | 192 (67.6) | 0.492 | 374 (63.8) | 352 (68.3) | 0.114 | 687 (66.1) | 39 (62.9) | 0.603 | ||
| Height (cm) | 165.1±8.4 | 165.4±7.9 | 0.631 | 164.9±8.3 | 165.6±8.1 | 0.145 | 165.2±8.3 | 165.9±8.0 | 0.517 | ||
| Weight (kg) | 62.1±12.1 | 60.2±12.2 | 0.023 | 62.5±12.1 | 60.5±12.1 | 0.007 | 61.7±12.2 | 60.0±12.1 | 0.255 | ||
| BMI (kg/m2) | 22.7±3.8 | 21.9±4.4 | 0.004 | 22.9±3.8 | 22.0±4.1 | <0.001 | 22.5±4.0 | 21.8±4.3 | 0.150 | ||
| Smoking status | 0.209 | <0.001 | 0.810 | ||||||||
| Current- | 193 (23.6) | 57 (20.1) | 167 (28.5) | 83 (16.1) | 238 (22.9) | 12 (19.4) | |||||
| Ex- | 366 (44.8) | 144 (50.7) | 233 (39.8) | 277 (53.8) | 480 (46.2) | 30 (48.4) | |||||
| Never- | 258 (31.6) | 83 (29.2) | 186 (31.7) | 155 (30.1) | 321 (30.9) | 20 (32.3) | |||||
| Smoking index (pack·year) | 22.9±25.7 | 22.9±23.8 | 0.966 | 22.0±24.1 | 23.9±26.4 | 0.217 | 22.6±24.7 | 26.9±33.3 | 0.329 | ||
| CB | 641 (78.5) | 249 (87.7) | 0.001 | 434 (74.1) | 456 (88.5) | <0.001 | 832 (80.1) | 59 (93.5) | 0.009 | ||
| mMRC dyspnea score | < 0.001 | <0.001 | <0.001 | ||||||||
| 0 | 86 (10.5) | 12 (4.2) | 72 (12.3) | 26 (5.0) | 97 (9.3) | 1 (1.6) | |||||
| 1 | 319 (39.0) | 63 (22.2) | 239 (40.8) | 143 (27.8) | 371 (35.7) | 11 (17.7) | |||||
| 2 | 242 (29.6) | 104 (36.6) | 177 (30.2) | 169 (32.8) | 332 (32.0) | 14 (22.6) | |||||
| 3 | 117 (14.3) | 62 (21.8) | 73 (12.5) | 106 (20.6) | 161 (15.5) | 18 (29.0) | |||||
| 4 | 53 (6.5) | 43 (15.1) | 25 (4.3) | 71 (13.8) | 78 (7.5) | 18 (29.0) | |||||
| GOLD stage | <0.001 | <0.001 | <0.001 | ||||||||
| I | 49 (6.0) | 11 (3.9) | 37 (6.3) | 23 (4.5) | 60 (5.8) | 0 (0) | |||||
| II | 262 (32.1) | 64 (22.5) | 220 (37.5) | 106 (20.6) | 316 (30.4) | 10 (16.1) | |||||
| III | 323 (39.5) | 101 (35.6) | 227 (38.7) | 197 (38.3) | 406 (39.1) | 18 (29.0) | |||||
| IV | 183 (22.4) | 108 (38.0) | 102 (17.4) | 189 (36.7) | 257 (24.7) | 34 (54.8) | |||||
Data are presented as mean ± SD or number (%). COPD, chronic obstructive pulmonary disease; ICU, intensive care unit; BMI, body mass index; CB, chronic bronchitis; mMRC, modified Medical Research Council; GOLD, Global Initiative for Chronic Obstructive Lung Disease.
In the 62 patients who experienced ICU hospitalization, the lengths of ICU stay following COPD exacerbation were compared between patients with and without CB (10.1±8.3 vs. 9.5±9.5 days, P=0.889).
Logistic regression analyses for frequency and severity of COPD exacerbation were shown in Table 5. CB was independently associated with COPD-related emergency visits and hospitalizations, but no longer related to frequent exacerbations or ICU stay. mMRC dyspnea score had independent and positive association with frequent exacerbations and all kinds of severe exacerbations of COPD. Advanced age was independently associated with emergency visit and hospitalization and ICU stay. GOLD stage was independently related to hospitalizations and ICU stay.
Table 5. Binary logistic regression analyses for frequency and severity of COPD exacerbation.
| Dependent variablesa | Independent variables in the modelb | OR (95% CI) | P |
|---|---|---|---|
| Frequency of exacerbationsa | Chronic bronchitis | 1.252 (0.915–1.715) | 0.160 |
| Current-smokers | 1.046 (0.883–1.238) | 0.604 | |
| GOLD stage | 1.084 (0.932–1.261) | 0.296 | |
| mMRC dyspnea score | 1.484 (1.310–1.681) | <0.001 | |
| Emergency visita | Chronic bronchitis | 1.512 (1.004–2.277) | 0.048 |
| Age | 1.028 (1.014–1.042) | <0.001 | |
| BMI | 0.980 (0.945–1.017) | 0.287 | |
| GOLD stage | 1.162 (0.970–1.393) | 0.103 | |
| mMRC dyspnea score | 1.443 (1.258–1.654) | <0.001 | |
| Hospitalizationa | Chronic bronchitis | 2.031 (1.423–2.897) | <0.001 |
| Age | 1.046 (1.033–1.059) | <0.001 | |
| BMI | 0.971 (0.939–1.005) | 0.092 | |
| Current-smokers | 0.681 (0.566–0.820) | <0.001 | |
| GOLD stage | 1.382 (1.173–1.629) | <0.001 | |
| mMRC dyspnea score | 1.395 (1.225–1.589) | <0.001 | |
| ICU staya | Age | 1.044 (1.016–1.073) | 0.002 |
| Chronic bronchitis | 2.231 (0.780–6.380) | 0.135 | |
| GOLD stage | 1.680 (1.149–2.455) | 0.007 | |
| mMRC dyspnea score | 1.694 (1.315–2.183) | <0.001 |
a, all of the dependent variables were two-categorical: frequency of exacerbations was classified as ≥2 and <2; emergency visit was classified as ≥1 and 0; hospitalizations and ICU stay were also classified as ≥1 and 0; b, binary logistic regression analyses included independent variables with statistically significant associations (P<0.1) in univariate analyses. COPD, chronic obstructive pulmonary disease; OR, odds ratio; CI, confidence interval; BMI, body mass index; mMRC, modified Medical Research Council; GOLD, Global Initiative for Chronic Obstructive Lung Disease
Clinical factors relevant to recovery following COPD exacerbation
Patients with CB had significantly longer recovery period following the last exacerbation than those without CB (19.0±16.2 vs. 15.2±14.7 days, P=0.003). Male patients had similar recovery period to female patients (18.0±15.0 vs. 19.0±17.7 days, P=0.364). Pearson’s correlation analyses showed that recovery period was positively associated with age (r=0.118, P<0.001), but not associated with BMI (r=–0.041, P=0.192) or FEV1%pred (r=–0.048, P=0.125). Spearman’s correlation analyses showed that recovery period was positively associated with GOLD stage (r=0.141, P<0.001) and mMRC dyspnea score (r=0.128, P<0.001), but not associated with smoking status (r=–0.043, P=0.166).
Of the 1,101 patients, 1,063 patients had answered the question “Did your physical condition recover to pre-exacerbation state following the last exacerbation”. Six hundred and fifty-four (59.4%) patients answered “Yes” and were placed in “well-recovery group”; the others were placed in “poor-recovery group”. Patients in poor-recovery group were older and more often current- and ex-smokers. Moreover, they were predominantly males and were characterized by CB, lower BMI, greater mMRC score and GOLD stage IV (Table 6).
Table 6. Comparison between patients with well-recovery and poor recovery following the last exacerbation.
| Clinical characteristics | Recovery status following the last exacerbationa | ||
|---|---|---|---|
| Well-recovery (n=654) | Poor-recovery (n=409) | P | |
| Age (year) | 64.7±11.8 | 69.4±10.0 | <0.001 |
| Male | 412 (63.0) | 284 (69.4) | 0.032 |
| Height (cm) | 165.1±8.5 | 165.3±7.8 | 0.747 |
| Weight (kg) | 62.8±11.9 | 59.3±12.2 | <0.001 |
| BMI (kg/m2) | 23.0±3.7 | 21.7±4.3 | <0.001 |
| Smoking status | 0.042 | ||
| Current- | 143 (21.9) | 93 (22.7) | |
| Ex- | 290 (44.3) | 207 (50.6) | |
| Never- | 221 (33.8) | 109 (26.7) | |
| Chronic bronchitis | 513 (78.4) | 351 (85.8) | 0.003 |
| MMRC dyspnea score | <0.001 | ||
| 0 | 65 (9.9) | 27 (6.6) | |
| 1 | 251 (38.4) | 111 (27.1) | |
| 2 | 227 (34.7) | 110 (26.9) | |
| 3 | 80 (12.2) | 96 (23.5) | |
| 4 | 31 (4.7) | 65 (15.9) | |
| GOLD stage | <0.001 | ||
| I | 49 (7.5) | 8 (2.0) | |
| II | 222 (33.9) | 84 (20.5) | |
| III | 252 (38.5) | 157 (38.4) | |
| IV | 131 (20.0) | 160 (39.1) | |
Data are presented as mean ± SD or number (%). a, in this part, 1,063 patients had answered the question “Did your physical condition recover to pre-exacerbation state following the last exacerbation?” and divided into well-recovery group and poor-recovery group. BMI, body mass index; mMRC, modified Medical Research Council; GOLD, Global Initiative for Chronic Obstructive Lung Disease.
The main results of multivariate analyses for recovery following the last exacerbation of COPD are presented in Table 7 and Table 8. The independent factors associated with recovery period included age and CB. Poor recovery following the last exacerbation was positively associated with age, mMRC dyspnea score and GOLD stage, and negatively associated with BMI. CB was no longer related to poor recovery following exacerbation.
Table 7. General linear model analysis for recovery period following the last exacerbation.
| Independent variables in the modela | Adjusted regression coefficient (95% CI) | P |
|---|---|---|
| Age | 0.133 (0.045–0.221) | 0.003 |
| GOLD stage | 0.316 (−0.884–1.516) | 0.606 |
| mMRC dyspnea score | 0.802 (−0.160–1.764) | 0.102 |
| Chronic bronchitis | 2.861 (0.282–5.439) | 0.030 |
a, including independent variables with statistically significant associations (P<0.1) in univariate analyses. CI, confidence interval; mMRC, modified Medical Research Council; GOLD, Global Initiative for Chronic Obstructive Lung Disease.
Table 8. Binary logistic regression analysis for poor recovery following exacerbation.
| Independent variables in the modela | OR (95% CI) | P |
|---|---|---|
| Age | 1.029 (1.016–1.042) | <0.001 |
| Male | 0.996 (0.731–1.359) | 0.981 |
| BMI | 0.951 (0.918–0.985) | 0.005 |
| Smoking status | 1.043 (0.852–1.276) | 0.683 |
| Chronic bronchitis | 1.172 (0.817–1.680) | 0.388 |
| mMRC dyspnea score | 1.352 (1.189–1.539) | <0.001 |
| GOLD stage | 1.491 (1.256–1.770) | <0.001 |
a, including independent variables with statistically significant associations (P<0.1) in univariate analyses. OR, odds ratio; CI, confidence interval; BMI, body mass index; mMRC, modified Medical Research Council; GOLD, Global Initiative for Chronic Obstructive Lung Disease.
Discussion
In this nation-wide, multicenter cross-sectional study, we assessed the relationship between CB and COPD exacerbation (including frequency, severity and recovery) in the prior year in a large Chinese population. Our data showed that in COPD patients, CB was associated with frequent exacerbator status (≥2/patient/year) and severe exacerbations, requiring emergency visit and COPD-related hospitalization.
Regarding the relationship between CB and COPD exacerbation, our data were consistent with the previous studies (3,5,6,9). In the Copenhagen City Heart Study, chronic mucus hypersecretion was associated with an increased risk of COPD-related hospitalization (9). A multicenter cross-sectional study found that CB was associated with frequent exacerbations and hospitalizations in COPD subjects (3). In COPDGene study, patients with COPD concomitant with CB reported more histories of exacerbation and severe exacerbation. CB phenotype was considered as a new clinical phenotype of COPD (5). Similar results could be seen in PLATINO study (6). Kim et al. further found that COPD patients with persistent and newly developed CB had greater exacerbation frequency. Even in those patients whose CB were resolved by quitting smoking, exacerbation frequency was still higher (7).
Another important result of our study was that patients with CB needed longer time to recover following the last exacerbation independently of age, GOLD stage and severity of dyspnea. A large number of patients could not reach well-recovery and most of them (>80%) had CB. To our knowledge, evidences regarding the relation between CB and recovery following COPD exacerbation were limited. Spencer et al. found that recovery period following infective exacerbation of CB was long (up to 6 months) and a further exacerbation within 6 months limited recovery markedly (16). In a large observational study, Anzueto et al. found that late recovery following exacerbation of CB/COPD were associated with aging, duration of CB >10 years, cardiac comorbidity, etc. (17). However, these two studies did not recruit patients without CB and could not illustrate the impact of CB on recovery period following COPD exacerbation adequately.
Underlying mechanism of prolonged recovery period and poor recovery in patients with CB is not clear and may be complex. In our opinion, prolonged recovery period in patients with CB could be partly explained by the following: First, advanced age. In our data, patients with CB were older. Second, worse lung function. CB is associated with airflow obstruction and accelerates decline in lung function (4,6,8,9,13,21,22). Deteriorating airflow limitation increases risk of COPD exacerbation, hospitalization and death (1). Our study also showed that FEV1%pred and FVC %pred were much lower in patients with CB. Additionally, although we did not find statistical relationship between recovery period and FEV1%pred, we found that recovery period was prolonged as severity of airflow limitation increased. Third, Mucous metaplasia and mucus overproduction are the pathologic foundation of CB (23). Therefore, we speculated that exacerbation type in patients with CB were more likely to belong to Anthonisen I/II, which was significantly associated with late recovery (17). However, Anthonisen classification was not involved in our survey.
The prevalence of CB in patients with COPD was relatively high (80.8%) in the present study. In previous studies, the prevalence of CB in patients with COPD varied from 14% to 74% (3,5,6,21,24), which are clinic-based studies. In those population-based studies, the prevalence of CB was not so high, varying from 3.4 to 22.0% (25-28). Among these studies, the race, age range, and smoking status were different. For example, patients enrolled in our study were more often ex-smokers regardless of presence of CB, while in a Finnish study, the cumulative incidence of CB was 42% in continuous smokers and only 26% in ex-smokers (12). On the other hand, CB patients had more severe dyspnea and worse physical function (lower SF-36 PCS) in our study. Worse respiratory symptoms would precipitate these patients to seek medical treatment more frequently. Therefore, the proportion of CB would be greater in this clinic-based survey.
MMRC dyspnea scale is important to assessment of symptoms of COPD. In a large United Kingdom population, mMRC score is associated with experience of severe exacerbations requiring hospitalization (29). In a prospective study of 123 Japanese patients with COPD, mMRC score ≥3 is an independent risk factor of frequency of exacerbation and hospitalization (30). Our data were consistent with these previous studies. We also found that the patients with mMRC score ≥3 reported more exacerbation frequency and more experience of severe exacerbations. Moreover, mMRC score ≥3 was independently associated with poor recovery following the last COPD exacerbation.
It is interesting that patients with the CB phenotype showed a lower BMI in our study, which was inconsistent with previous studies in which CB phenotype was associated with higher BMI and emphysematous phenotype was associated with lower BMI (31,32). The reason was not clear. First, we thought that it could not be excluded that some patients had CB and emphysematous phenotypes combination because patients enrolled in our study were mainly male and male subjects have been shown to be more likely to have a diagnosis of emphysema (33). However, not all patients underwent CT scans and it was very difficult to confirm in our study. Second, nearly 30% of the patients with CB were in GOLD stage IV. Fat free mass index had been proved to decrease as airflow limitation aggravating (34), suggesting the association between malnutrition and severe COPD and partly explaining the relation between lower BMI and CB in our study.
There are some limitations in our study: (I) our study was a cross-sectional questionnaire study based on the patients’ self-report, which is relatively subjective. In addition, memory bias cannot be excluded and some exacerbations were underdiagnosed; (II) comorbidities of COPD and treatment information which can influence mortality and hospitalizations in patients with COPD (35) were not included in our study. These two parts had been designed and included in our questionnaires. However, less than a half of patients had completed to report their concomitant chronic diseases and treatment effectively. Therefore, further follow-up study will be conducted to address these data and assess the relation between presence of CB and further exacerbation and long-term prognosis; (III) the recovery of health condition was only based on the patients’ answer and lack of objective and scientific assessment in such a questionnaire survey. The understanding of well-recovery would be various among different individuals. Peak expiratory flow rate and St. George’s Respiratory Questionnaire before and after exacerbation can be used to assess the recovery state (36). We will utilize these measurements to investigate the association between CB and recovery from COPD exacerbation in future study; (IV) CB was not independently related to ICU stay in multivariate analysis. We speculated that it was due to the small number of ICU hospitalization event, which may decrease statistical efficiency and make the confidence interval relatively wide. We noticed that the point estimate of adjusted OR of CB for ICU stay was similar to that of CB for hospitalization. CB would be independently related to ICU stay if more individuals experienced ICU stay.
Conclusions
The CB phenotype is common in Chinese COPD patients. Presence of CB in patients with COPD is associated with frequent exacerbations, especially severe exacerbations requiring emergency visit and hospitalization. Besides, CB significantly prolongs recovery period following COPD exacerbation and is associated with poor recovery of health status.
Acknowledgements
Funding: This work was supported by the Research Special Fund for Public Welfare Industry of Health (Grant Number: 201002008) and the Chinese Medical Association Chronic Respiratory Diseases Grants (Grant Number: 07010360044).
Ethical Statement: The study was approved by the ethics committee of Peking University Third Hospital (No. M2017120), which is the primary research institution for this study and written informed consent was obtained from all patients.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2017. Available online: http://www.goldcopd.org/
- 2.Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease. American Thoracic Society. Am J Respir Crit Care Med 1995;152:S77-121. [PubMed] [Google Scholar]
- 3.Burgel PR, Nesme-Meyer P, Chanez P, et al. Cough and sputum production are associated with frequent exacerbations and hospitalizations in COPD subjects. Chest 2009;135:975-82. 10.1378/chest.08-2062 [DOI] [PubMed] [Google Scholar]
- 4.Corhay JL, Vincken W, Schlesser M, et al. Chronic bronchitis in COPD patients is associated with increased risk of exacerbations: a cross-sectional multicentre study. Int J Clin Pract 2013;67:1294-301. 10.1111/ijcp.12248 [DOI] [PubMed] [Google Scholar]
- 5.Kim V, Han MK, Vance GB, et al. The chronic bronchitic phenotype of COPD: an analysis of the COPDGene Study. Chest 2011;140:626-33. 10.1378/chest.10-2948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Oca MM, Halbert RJ, Lopez MV, et al. The chronic bronchitis phenotype in subjects with and without COPD: the PLATINO study. Eur Respir J 2012;40:28-36. 10.1183/09031936.00141611 [DOI] [PubMed] [Google Scholar]
- 7.Kim V, Zhao H, Boriek AM, et al. Persistent and newly developed chronic bronchitis are associated with worse outcomes in chronic obstructive pulmonary disease. Ann Am Thorac Soc 2016;13:1016-25. 10.1513/AnnalsATS.201512-800OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sherman CB, Xu X, Speizer FE, et al. Longitudinal lung function decline in subjects with respiratory symptoms. Am Rev Respir Dis 1992;146:855-9. 10.1164/ajrccm/146.4.855 [DOI] [PubMed] [Google Scholar]
- 9.Vestbo J, Prescott E, Lange P. Association of chronic mucus hypersecretion with FEV1 decline and chronic obstructive pulmonary disease morbidity. Copenhagen City Heart Study Group. Am J Respir Crit Care Med 1996;153:1530-5. 10.1164/ajrccm.153.5.8630597 [DOI] [PubMed] [Google Scholar]
- 10.James AL, Wenzel S. Clinical relevance of airway remodelling in airway diseases. Eur Respir J 2007;30:134-55. 10.1183/09031936.00146905 [DOI] [PubMed] [Google Scholar]
- 11.Kim V, Sternberg AL, Washko G, et al. Severe chronic bronchitis in advanced emphysema increases mortality and hospitalizations. COPD 2013;10:667-78. 10.3109/15412555.2013.827166 [DOI] [PubMed] [Google Scholar]
- 12.Pelkonen M, Notkola IL, Nissinen A, et al. Thirty-year cumulative incidence of chronic bronchitis and COPD in relation to 30-year pulmonary function and 40-year mortality: a follow-up in middle-aged rural men. Chest 2006;130:1129-37. 10.1378/chest.130.4.1129 [DOI] [PubMed] [Google Scholar]
- 13.Guerra S, Sherrill DL, Venker C, et al. Chronic bronchitis before age 50 years predicts incident airflow limitation and mortality risk. Thorax 2009;64:894-900. 10.1136/thx.2008.110619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindberg A, Eriksson B, Larsson LG, et al. Seven-year cumulative incidence of COPD in an age-stratified general population sample. Chest 2006;129:879-85. 10.1378/chest.129.4.879 [DOI] [PubMed] [Google Scholar]
- 15.de Marco R, Accordini S, Cerveri I, et al. Incidence of chronic obstructive pulmonary disease in a cohort of young adults according to the presence of chronic cough and phlegm. Am J Respir Crit Care Med 2007;175:32-9. 10.1164/rccm.200603-381OC [DOI] [PubMed] [Google Scholar]
- 16.Spencer S, Jones PW. Time course of recovery of health status following an infective exacerbation of chronic bronchitis. Thorax 2003;58:589-93. 10.1136/thorax.58.7.589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anzueto A, Miravitlles M, Ewig S, et al. Identifying patients at risk of late recovery (≥ 8 days) from acute exacerbation of chronic bronchitis and COPD. Respir Med 2012;106:1258-67. 10.1016/j.rmed.2012.06.002 [DOI] [PubMed] [Google Scholar]
- 18.Chen YH, Yao WZ, Kang J, et al. Attitudes and actions of chronic obstructive pulmonary disease patients on treatment: a national multi-center investigative study. Zhonghua Jie He He Hu Xi Za Zhi 2010;33:750-3. [PubMed] [Google Scholar]
- 19.Bestall JC, Paul EA, Garrod R, et al. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax 1999;54:581-6. 10.1136/thx.54.7.581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ware JE. SF-36 health survey update. Spine (Phila Pa 1976) 2000;25:3130-9. 10.1097/00007632-200012150-00008 [DOI] [PubMed] [Google Scholar]
- 21.Lu M, Yao W, Zhong N, et al. Chronic obstructive pulmonary disease in the absence of chronic bronchitis in China. Respirology 2010;15:1072-8. 10.1111/j.1440-1843.2010.01817.x [DOI] [PubMed] [Google Scholar]
- 22.Khurana S, Ravi A, Sutula J, et al. Clinical characteristics and airway inflammation profile of COPD persistent sputum producers. Respir Med 2014;108:1761-70. 10.1016/j.rmed.2014.09.020 [DOI] [PubMed] [Google Scholar]
- 23.Kim V, Criner GJ. Chronic bronchitis and chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2013;187:228-37. 10.1164/rccm.201210-1843CI [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Agusti A, Calverley PM, Celli B, et al. Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respir Res 2010;11:122. 10.1186/1465-9921-11-122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.von HL , Reunanen A, Impivaara O, et al. Airway obstruction in relation to symptoms in chronic respiratory disease--a nationally representative population study. Respir Med 2000;94:356-63. 10.1053/rmed.1999.0715 [DOI] [PubMed] [Google Scholar]
- 26.Huchon GJ, Vergnenègre A, Neukirch F, et al. Chronic bronchitis among French adults: high prevalence and underdiagnosis. Eur Respir J 2002;20:806-12. 10.1183/09031936.02.00042002 [DOI] [PubMed] [Google Scholar]
- 27.Miravitlles M, Soriano JB, García-Río F, et al. Prevalence of COPD in Spain: impact of undiagnosed COPD on quality of life and daily life activities. Thorax 2009;64:863-8. 10.1136/thx.2009.115725 [DOI] [PubMed] [Google Scholar]
- 28.Harmsen L, Thomsen SF, Ingebrigtsen T, et al. Chronic mucus hypersecretion: prevalence and risk factors in younger individuals. Int J Tuberc Lung Dis 2010;14:1052-8. [PubMed] [Google Scholar]
- 29.McGarvey L, Lee AJ, Roberts J, et al. Characterisation of the frequent exacerbator phenotype in COPD patients in a large UK primary care population. Respir Med 2015;109:228-37. 10.1016/j.rmed.2014.12.006 [DOI] [PubMed] [Google Scholar]
- 30.Natori H, Kawayama T, Suetomo M, et al. Evaluation of the modified medical research council dyspnea scale for predicting hospitalization and exacerbation in japanese patients with chronic obstructive pulmonary disease. Intern Med 2016;55:15-24. 10.2169/internalmedicine.55.4490 [DOI] [PubMed] [Google Scholar]
- 31.Guerra S, Sherrill DL, Bobadilla A, et al. The relation of body mass index to asthma, chronic bronchitis, and emphysema. Chest 2002;122:1256-63. 10.1378/chest.122.4.1256 [DOI] [PubMed] [Google Scholar]
- 32.Voica AS, Oancea C, Tudorache E, et al. Chronic obstructive pulmonary disease phenotypes and balance impairment. Int J Chron Obstruct Pulmon Dis 2016;11:919-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dodge R, Cline MG, Burrows B. Comparisons of asthma, emphysema, and chronic bronchitis diagnoses in a general population sample. Am Rev Respir Dis 1986;133:981-6. [DOI] [PubMed] [Google Scholar]
- 34.Luo Y, Zhou L, Li Y, et al. Fat-Free Mass Index for Evaluating the Nutritional Status and Disease Severity in COPD. Respir Care 2016;61:680-8. 10.4187/respcare.04358 [DOI] [PubMed] [Google Scholar]
- 35.Mannino DM, Thorn D, Swensen A, et al. Prevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPD. Eur Respir J 2008;32:962-9. 10.1183/09031936.00012408 [DOI] [PubMed] [Google Scholar]
- 36.Seemungal TA, Donaldson GC, Paul EA, et al. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998;157:1418-22. 10.1164/ajrccm.157.5.9709032 [DOI] [PubMed] [Google Scholar]


