Abstract
Background
The standard treatment for patients with stage IV non-small cell lung cancer (NSCLC) is systemic chemotherapy. However, certain patients, such as those with oligometastasis or M1a disease undergo resection of the primary lesion.
Methods
We conducted a retrospective review of the records of 1,471 consecutive patients with NSCLC who underwent resection of the primary lesion for between June 2005 and May 2016. The present study included 38 patients with stage IV NSCLC who underwent complete resection of the primary lesion as first-line treatment.
Results
The median follow-up duration for the 38 patients (27 men) was 17.7 months (range, 1–82.3 months). The T factors were T1/T2/T3/T4 in 4/16/12/6 patients, respectively. The N factors were N0/N1/N2/N3 in 16/8/12/2 patients, respectively. The M factors were M1a/M1b/M1c in 19/13/6 patients, respectively. Of the 19 M1a patients, 11 were classified as cM0. We introduced the novel classification M-better/M-worse. M-better includes cM0 patients and M1b and M1c patients in whom all lesions have been locally controlled. M-worse includes cM1a patients and M1b and M1c patients in whom lesions cannot be locally controlled. The new M-better/M-worse statuses were 24/14 patients, respectively. The histology of NSCLC was adenocarcinoma/squamous cell carcinoma/others in 30/5/3 patients, respectively. The 5-year overall survival rate was 29%, and the median survival time was 725 days. Squamous cell carcinoma and M-worse were significant factors predicting poor outcomes (P=0.0017, P=0.0007, respectively).
Conclusions
Even for stage IV NSCLC patients, resection of the primary lesion may be beneficial, especially for those with M-better status and those not diagnosed with squamous-cell carcinoma (SCC).
Keywords: Stage IV, complete resection, non-small lung cancer (NSCLC), primary lesion, metastasis, intrathoracic metastasis, extrathoracic metastasis
Introduction
The treatment strategy for patients with advanced lung cancer is controversial, and because of this the guidelines have undergone numerous revisions (1). However, surgical treatment of stage IV non-small cell lung cancer (NSCLC) is generally not selected as the primary intervention. However, appropriately selected candidates with stage IV disease may benefit from surgery (2-4).
In the seventh edition of the TNM classification of the International Association for the Study of Lung Cancer (IASLC), the M component had two categories: intrathoracic metastasis (M1a) and extrathoracic metastasis (M1b). However, in the eighth edition of the IASLC TNM classification, enacted in January 2017, the M component has three categories: intrathoracic metastasis (M1a), single extrathoracic metastasis (M1b), and multiple extrathoracic metastases (M1c) (5). Furthermore, M1a and M1b descriptors are staged as IVA, while the M1c descriptor is staged as IVB. Stage IV NSCLC patients are a heterogeneous group, and to investigate the prognosis of stage IV NSCLC it is necessary to divide subjects into intrathoracic metastasis (M1a) and extrathoracic metastasis (M1b and M1c) groups.
In patients with intrathoracic metastasis, when malignant pleural disease (MPD) is first detected at thoracotomy, it is controversial whether the primary lesion should be resected (2). However, primary lesion resection had survival benefits for certain patients with unexpected intraoperatively proven MPD or MPD patients without pleural effusion (3). In cases of extrathoracic metastasis, Hellman and Weichselbaum proposed a clinically recognizable state termed oligometastasis, which is characterized by a limited number of metastatic tumors in a limited number of sites (6). Local therapies such as surgery or radiotherapy, given alone or combination with systemic therapy, may allow curative treatment of oligometastasis (4,7,8). In synchronous oligometastatic patients, local therapy may significantly prolong the overall survival time (4,7-10). It is therefore important to clarify the selection of appropriate candidates for radical local therapy.
In the present study, we retrospectively evaluated factors that predicted a better prognosis for patients with stage IV NSCLC who underwent complete resection of the primary lesion. We took into account the fact that the patients form a heterogeneous characteristic of the patients, such as intrathoracic or extrathoracic metastases. Our primary aim was to determine whether complete resection of the primary lesion conferred a survival benefit.
Methods
Patients
The records of 1,471 consecutive patients who underwent primary lesion resection for NSCLC between June 2005 and May 2016 at the University of Occupational and Environmental Health, Kitakyushu, Japan, were retrospectively reviewed. The subjects of the present study were 38 patients with stage IV NSCLC who underwent complete resection of the primary lesion. All 38 patients had either synchronous distant metastasis or pleural dissemination. We excluded patients who underwent systemic chemotherapy before surgery. Preoperative assessments included chest roentgenography and computed tomography (CT) of the chest, upper abdomen, and brain. Moreover, most (n=26) patients underwent positron-emission tomographic (PET) examinations. Clinical N2 status was defined as the presence of a lymph node with a short axis diameter of more than 1 cm. Clinical M1a status was defined as the diagnosis by radiologists of intrathoracic metastasis on CT or PET images. Clinical M1b/M1c status was defined as the diagnosis of extrathoracic metastasis on CT or PET images or using other modalities such as brain magnetic resonance imaging or bone scintigraphy. Bronchoscopy was routinely performed to obtain a pathological diagnosis from transbronchial lung biopsy specimens and to evaluate the endobronchial staging. Primary lesions were resected by wedge resection, segmentectomy, lobectomy, or pneumonectomy. Examples of the major reasons for performing resection of primary lesion resection are as follows: radical treatment of oligometastasis, palliation for hemoptysis or pneumonia, genetic diagnosis requiring a large specimen is required. If the patient was diagnosed with stage IV (very advanced disease, such as N2 and N3 plus M), we suggested that the patient undergo systemic chemotherapy, which is the gold standard, and that surgery was an option. When surgery was selected, we attempted complete resection of the primary lesion.
All resected specimens, including the primary tumor and resected hilar and mediastinal lymph nodes, were examined to determine both the tumor histology and the extent of lymph node metastasis. Histological diagnosis of the tumor cell type was classified according to the World Health Organization histological classification of lung tumors (11), and the IASLC TNM staging system (eighth edition) was used (5). N factor was defined using dissected lymph nodes or from imaging findings, if lymph node dissection was not performed. Genetic diagnosis was performed when the pathological diagnosis was determined, but varied depending on the time available, mainly because genetic diagnosis was not routinely in the past. Clinicopathological findings were reviewed to divide the subjects into an intrathoracic metastasis (M1a) group and an extrathoracic metastasis (M1b, M1c) group. In the extrathoracic group, subjects were further divided into a locally controlled (LC) group and a locally uncontrolled (LUC) group. The LC group contained patients who had been treated locally for all lesions by surgery or radiation therapy, whereas the LUC group contained patients with lesions that had not received local treatment. Furthermore, we defined two new metastatic categories: M-better and M-worse. The M-better group contained patients clinically free of intrathoracic metastasis (cM0) and the LC group of patients with extrathoracic metastasis. The M-worse group contained patients clinically diagnosed with intrathoracic metastasis (cM1a) and the LUC group of patients with extrathoracic metastasis.
Systemic chemotherapy
Patients did not undergo preoperative therapy. Postoperative systemic chemotherapy was administered to patients who could tolerate such treatment after surgery, unless the patients refused additional chemotherapy. For some patients with pleural dissemination, pleurodesis was done using cis-diamminedichloroplatinum (15–100 mg) followed by OK-432 (5 Klinische Einheit).
Follow-up
Moment 0 of the analysis was defined as the time when the primary lesion was resected. Follow-up information was obtained for all patients through office visits or from their primary-care physicians. Patients were evaluated every 3 months by chest roentgenography, and CT scans were performed every 6 months for 5 years. The median follow-up time was 17.7 months (range, 1–82.3 months).
Statistical analysis
Survival curves were calculated using the Kaplan-Meier method, and data were compared using the log-rank test for univariate analysis. Cox proportional hazards regression was used for survival analysis. Categorical variables were compared using the Fisher exact test. Statistical differences were considered to be significant for P values less than 0.05. Statview version 5.0 (Abacus Concepts, Inc., Berkeley, CA, USA) was used for all statistical analyses.
Results
Patient characteristics and survival in the 19 NSCLC patients with intrathoracic metastasis
There were 19 patients (14 men) with pathological diagnoses of M1a (pM1a). The mean age of these 19 patients was 70 years (range, 47–86 years). The primary NSCLC lesions were resected by pneumonectomy in 1 patient, lobectomy in 12 patients, segmentectomy in 1 patient, and wedge resection of the lung in 5 patients. Among patients diagnosed with M1a, 4, 6, 9, and 0 experienced malignant pleural effusion (MPE), malignant pleural nodules (MPNs), MPE + MPN, and contralateral lung metastasis, respectively. The histological types of NSCLC were 16 adenocarcinomas (84.2%) and 3 squamous-cell carcinomas (SCCs) (15.8%). The epidermal growth factor receptor (EGFR) mutation status was positive in 4 of 19 patients and negative or undetermined in the remaining 15. The T factor was T1 in 2 (10.5%) patients, T2 in 8 (42.1%) patients, T3 in 5 (26.3%) patients, and T4 in 4 (21.1%) patients. The lymph node metastasis status was N0 in 8 (42.1%) patients, N1 in 5 (26.3%) patients, and N2 in 6 (31.6%) patients. Eleven patients (57.9%) were cM1a. Six patients had undergone pleurodesis (Table 1).
Table 1. Clinical and pathological characteristics of stage IV NSCLC patients who underwent resection of the primary lesion.
| Variable | No. of patients in intrathoracic metastasis (n=19) | No. of patients in extrathoracic metastasis (n=19) | No. of patients in all of stage IV (n=38) |
|---|---|---|---|
| Gender | |||
| Male | 14 | 13 | 27 |
| Female | 5 | 6 | 11 |
| Age (years) | |||
| <68 | 7 | 12 | 19 |
| ≥68 | 12 | 7 | 19 |
| Surgical procedure | |||
| Sublob | 6 | 4 | 10 |
| Over lob. | 13 | 15 | 28 |
| Pleurodesis | |||
| Done | 13 | – | – |
| None | 6 | – | – |
| Histology | |||
| Non-SCC | 16 | 17 | 33 |
| SCC | 3 | 2 | 5 |
| EGFR mutation | |||
| Positive | 4 | 2 | 6 |
| Negative or unknown | 15 | 17 | 32 |
| T status | |||
| T1–2 | 10 | 10 | 20 |
| T3–4 | 9 | 9 | 18 |
| N status | |||
| Negative | 8 | 8 | 16 |
| Positive | 11 | 11 | 22 |
| cM status | |||
| cM0 | 11 | – | 19 (cM0 + cM1a) |
| cM1a | 8 | – | |
| cM1b | – | 13 | 19 (cM1b + cM1c) |
| cM1c | – | 6 | |
| Meta site | |||
| Brain | – | 5 | – |
| Not brain | – | 14 | – |
| Local control | |||
| Controlled | – | 13 | – |
| Uncontrolled | – | 6 | – |
| M category | |||
| M-better | (cM0 + controlled) | – | 24 |
| M worse | (cM1c + uncontrolled) | – | 14 |
NSCLC, non-small cell lung cancer; Sublob, sublobar resection; lob, lobectomy; SCC, squamous cell carcinoma.
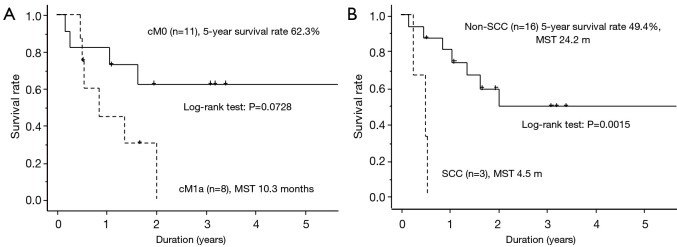
The results of univariate analysis of potential factors affecting overall survival of the intrathoracic metastasis group are shown in Table 2. Patient age, operative procedure, EGFR mutation, T factor, N factor, cM factor, and pleurodesis showed no statistically significant effect on survival (P>0.05). However, patients without SCC were likely to have better survival (P<0.05), and cM0 patients tended to have better survival (P=0.0728). The survival curves of patients with and without SCC and with different cM statuses (cM0 vs. cM1a) are shown in Figure 1A,B. The results of multivariate analysis of the potential factors affecting overall survival in the intrathoracic metastasis group are also shown in Table 2. The operative procedure, histology, EGFR mutation, N factor, and cM factor, showed no statistically significant effects on survival (P>0.05). However, cM0 was associated with tendency for better survival (P=0.0853).
Table 2. Univariate and multivariate analyses of prognostic factors using the Cox proportional hazards model in patients with intrathoracic metastasis.
| Variables | Characteristics | Univariate analysis | Multivariate analysis | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Unfavorable | Favorable | 95% CI | HR | P | 95% CI | HR | P | |||
| Age (years) | ≥68 | <68 | 0.277–3.528 | 0.989 | 0.9869 | – | – | – | ||
| Surgical procedure | Over lob. | Sublob. | 0.085–1.905 | 0.402 | 0.2348 | 0.015–2.049 | 0.173 | 0.164 | ||
| Histology | SCC | Non-SCC | 0.015–0.570 | 0.093 | 0.0015 | 0.045–2.800 | 0.354 | 0.3249 | ||
| EGFR | Negative or unknown | Positive | 0.429–5.510 | 1.538 | 0.5053 | – | – | – | ||
| Pleurodesis | Done | None | 0.122–1.862 | 0.477 | 0.2761 | 0.188–5.210 | 0.989 | 0.9892 | ||
| T status | 3–4 | 1–2 | 0.429–5.510 | 1.538 | 0.5053 | – | – | – | ||
| N status | Positive | Negative | 0.122–1.862 | 0.477 | 0.2761 | 0.188–5.210 | 0.989 | 0.9892 | ||
| cM status | cM1a | cM0 | 0.088–1.178 | 0.321 | 0.0728 | 0.018–1.297 | 0.154 | 0.0853 | ||
95% CI, 95% confidence interval; HR, hazard ratio; Sublob, sublobar resection; lob., lobectomy; SCC, squamous cell carcinoma; EGFR, epidermal growth factor receptor.
Figure 1.
Postoperative survival curves of the 19 patients with intrathoracic metastasis. (A) According to clinical M status. The heavy and dotted lines indicate cM0 and cM1a, respectively; (B) according to SCC (heavy line) or non-SCC (dotted line). SCC, squamous cell carcinoma; MST, median survival time.
Patient characteristics and survival in the 19 NSCLC patients with extrathoracic metastasis
There were 19 patients (13 men) with pM1b or pM1c. The mean age of the patients was 65 years (range, 54–86 years). The primary NSCLC lesions were resected by pneumonectomy in 1 patient, lobectomy in 14 patients, segmentectomy in 1 patient, and wedge resection in 3 patients. The histological types of NSCLC were 14 adenocarcinomas (73.7%), 2 SCCs (10.5%), and 3 others (15.8%). The EGFR mutation status was positive in 2 of 19 patients and negative or unknown in the remaining 17. The T factor was T1 in 2 (10.5%) patients, T2 in 8 (42.1%) patients, T3 in 7 (36.8%) patients, and T4 in 2 (10.5%) patients. The lymph node metastasis status was N0 in 8 (42.1%) patients, N1 in 3 (15.8%) patients, N2 in 6 (31.6%) patients, and N3 in 2 (10.5%) patients. The M factor was M1b in 13 (68.4%) patients and M1c in 6 (31.6%) patients. Metastasis was present in bone in 8 (42.1%) patients, in brain in 5 (26.3%) patients, in adrenal gland in 4 (21.1%) patients, and others in 3 (15.8%) patients (some patients had metastases in more than one tissue type). A total of 13 patients were in the LC group (68.4%) (Table 1). In the LC group, upfront resection of the primary lesion metastasis or synchronous metastasis was administered to seven, two, and four patients, respectively (Table 3). Extrathoracic metastasis to the brain included one and four patients in LUC and LC groups, respectively.
Table 3. Metastatic sites and upfront lesion.
| Metastatic sites | Upfront | N |
|---|---|---|
| Brain | 4 | |
| Primary lesion | 2 | |
| Metastatic lesion | 2 | |
| Adrenal | 4 | |
| Primary lesion | 4 | |
| Bone | 4 | |
| Synchronous | 4 | |
| Liver | 1 | |
| Primary lesion | 1 |
The results of univariate analysis of the potential factors affecting overall survival of patients with extrathoracic metastasis are shown in Table 4. Patient age, the operative procedure, histology, T factor, N factor, M factor, and metastatic site showed no statistically significant effects on survival (P>0.05). However, being in the controlled group was associated with better survival (P<0.05). Because all patients positive for EGFR mutation were alive, the effect of EGFR mutation on survival was not calculated. The survival curves of patients in the controlled and uncontrolled groups are shown in Figure 2. The results of multivariate analysis of potential factors affecting overall survival for patients with extrathoracic metastasis are shown in Table 4. The N factor, M factor, metastatic site, and controlled status showed no statistically significant effects on survival (P>0.10). However, not having SCC was associated with a tendency to better survival (P=0.0681).
Table 4. Univariate and multivariate analyses of prognostic factors using the Cox proportional hazards model in patients with extrathoracic metastasis.
| Variables | Characteristics | Univariate analysis | Multivariate analysis | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Unfavorable | Favorable | 95% CI | HR | P | 95% CI | HR | P | |||
| Age (years) | ≥68 | <68 | 0.338–3.205 | 1.041 | 0.9439 | – | – | – | ||
| Surgical procedure | Over lob. | Sublob. | 0.293–6.594 | 1.391 | 0.6762 | – | – | – | ||
| Histology | SCC | Non-SCC | 0.084–1.973 | 0.407 | 0.2488 | 0.019–1.153 | 0.147 | 0.0681 | ||
| T status | 3–4 | 1–2 | 0.206–1.876 | 0.622 | 0.3952 | – | – | – | ||
| N status | Positive | Negative | 0.179–1.748 | 0.559 | 0.3115 | 0.165–2.790 | 0.678 | 0.5904 | ||
| M status | cM1c | cM1b | 0.168–1.590 | 0.516 | 0.2409 | 0.072–2.121 | 0.39 | 0.2755 | ||
| Meta site | Not brain | Brain | 0.079–1.659 | 0.361 | 0.1731 | 0.047–2.597 | 0.349 | 0.3039 | ||
| Local control | Uncontrolled | Controlled | 0.034–0.618 | 0.144 | 0.0027 | 0.020–1.727 | 0.185 | 0.1387 | ||
95% CI, 95% confidence interval; HR, hazard ratio; Sublob, sublobar resection; lob., lobectomy; SCC, squamous cell carcinoma.
Figure 2.
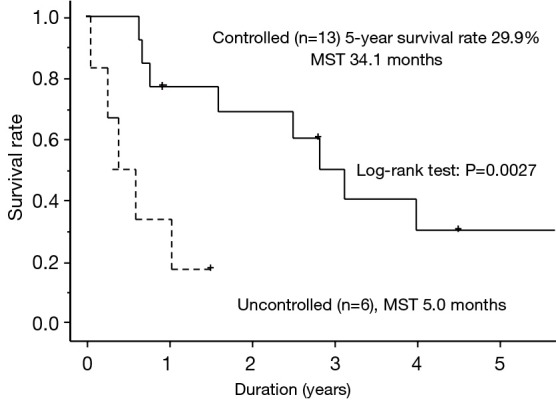
Postoperative survival curves of the 19 patients with extrathoracic metastasis for the LC group (heavy line) and the LUC group (dotted line). LC, controlled; LUC, uncontrolled; MST, median survival time.
Patient characteristics and survival in all 38 stage IV NSCLC patients
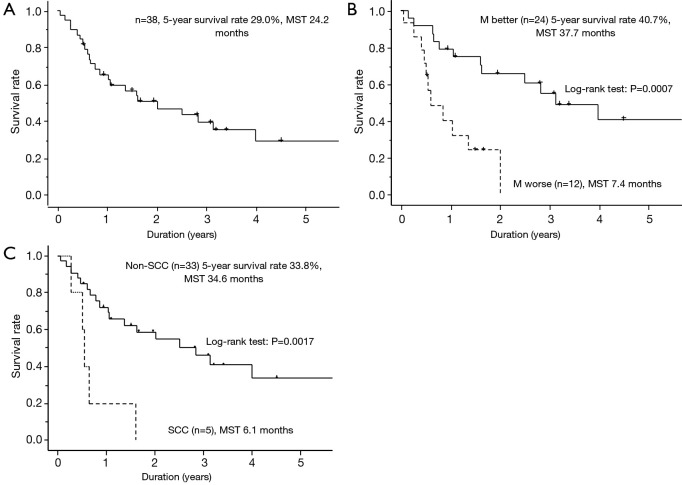
The 5-year survival rate after surgery for the 38 patients (27 men) was 29.0%, and median survival was 24.2 months (Figure 3A). The results of univariate analysis of potential factors affecting overall survival for all stage IV NSCLC patients are shown in Table 5. Patient age, the operative procedure, EGFR mutation, T factor, N factor, and M factor had no statistically significant effects on survival (P>0.05). However, being female, not having SCC, and being in the M-better group were significantly associated with improved survival (P<0.05). The survival curves for M-worse and M-better patients are shown in Figure 3B. The survival curves of patients with and without SCC are shown in Figure 3C. The results of multivariate analysis of the potential factors affecting overall survival of all stage IV NSCLC patients are shown in Table 5. Patient gender, EGFR mutation, the N factor, and the M factor showed no statistically significant effect on survival (P>0.05). However, not having SCC and being in the M-better group were significantly associated with improved survival (P<0.05). The numbers of 3-year survivors and 5-year survivors as a function of clinical factors are shown in Table 6.
Figure 3.
Postoperative survival curves of all patients (n=38) with stage IV NSCLC. (A) All stage IV NSCLC patients underwent complete resection of the primary lesion; (B) survival curves of the M-better (heavy line) and M-worse (dotted line) groups; (C) according to SCC (heavy line) or non-SCC (dotted line). NSCLC, non-small cell lung cancer; SCC, squamous cell carcinoma; MST, median survival time.
Table 5. Univariate and multivariate analyses of prognostic factors using the Cox proportional hazards model for all stage IV NSCLC patients.
| Variables | Characteristics | Univariate analysis | Multivariate analysis | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Unfavorable | Favorable | 95% CI | HR | P | 95% CI | HR | P | |||
| Gender | Male | Female | 0.044–0.800 | 0.187 | 0.0113 | 0.056–1.397 | 0.28 | 0.1206 | ||
| Age (years) | ≥68 | <68 | 0.427–2.122 | 0.952 | 0.9035 | – | – | – | ||
| Surgical procedure | Over lob. | Sublob. | 0.231–2.071 | 0.692 | 0.5084 | – | – | – | ||
| Histology | SCC | Non-SCC | 0.072–0.612 | 0.209 | 0.0017 | 0.071–0.697 | 0.223 | 0.0098 | ||
| EGFR | Negative or unknown | Positive | 0.029–1.578 | 0.212 | 0.0949 | 0.033–2.205 | 0.268 | 0.2208 | ||
| T status | 3–4 | 1–2 | 0.413–2.156 | 0.944 | 0.8905 | – | – | – | ||
| N status | Positive | Negative | 0.214–1.181 | 0.502 | 0.1077 | 0.193–1.457 | 0.53 | 0.2183 | ||
| M status | M1b + M1c | M1a | 0.408–2.046 | 0.913 | 0.3511 | 0.200–1.242 | 0.499 | 0.1351 | ||
| M category | M worse | M better | 0.087–0.573 | 0.223 | 0.0007 | 0.068–0.721 | 0.222 | 0.0123 | ||
95% CI, 95% confidence interval; HR, hazard ratio; Sublob, sublobar resection; lob., lobectomy; SCC, squamous cell carcinoma; EGFR, epidermal growth factor receptor.
Table 6. Number of 3-year survivors and 5-year survivors according to clinical factors.
| Clinical factor | No. of patients | No. of 3-year survivors | No. of 5-year survivors |
|---|---|---|---|
| Total | 38 | 10 | 4 |
| Surgical procedure | |||
| Sublob. | 10 | 1 | 0 |
| Over lob. | 28 | 9 | 4 |
| EGFR mutation | |||
| Positive | 6 | 2 | 1 |
| Negative or unknown | 32 | 8 | 3 |
| T status | |||
| T1–2 | 20 | 3 | 2 |
| T3–4 | 18 | 7 | 2 |
| N status | |||
| Negative | 16 | 6 | 3 |
| Positive | 22 | 4 | 1 |
| cM status | |||
| cM0 | 11 | 5 | 2 |
| cM1a | 8 | 0 | 0 |
| cM1b | 13 | 4 | 1 |
| cM1c | 6 | 1 | 1 |
| Meta site | |||
| With brain | 5 | 2 | 1 |
| Without brain | 14 | 3 | 1 |
| Local control | |||
| Controlled | 13 | 5 | 2 |
| Uncontrolled | 6 | 0 | 0 |
| M category | |||
| M better | 24 | 10 | 4 |
| M worse | 14 | 0 | 0 |
Sublob, sublobar resection; lob., lobectomy; EGFR, epidermal growth factor receptor.
Discussion
To our knowledge, our results are unique for the reasons as follows: (I) We reviewed our institution’s data for 38 consecutive patients with stage IV NSCLC who underwent complete resection of the primary lesion; (II) our analysis was limited to the first treatment of for NSCLC, which excluded induction therapy and salvage surgery; (III) for the first time to our knowledge, we classified the M status as M-worse and M-better. Some studies have analyzed oligometastatic disease (4,6-10) and pleural dissemination in stage IV NSCLC (2,3). However, there are few reports that deal only with stage IV NSCLC. The reason that few reports deal exclusively with stage IV NSCLC may be that stage IV NSCLC comprises heterogeneous groups, e.g., those with intrathoracic disease and with extrathoracic disease. The approach of the current study uniquely fused and reclassified these heterogeneous groups as M-worse and M-better.
The present study demonstrated five major findings. First, the M-better group was a significantly favorable prognostic factor in both univariate and multivariate analyses using the log-rank test. In our experience, patients preoperatively diagnosed as cM1a may in fact have multiple intrathoracic metastases. It can be inferred from the above results that when cM1a patients have the primary lesion resected, multiple tumor cells may remain macroscopically. In contrast, if cM0 patients are pM1a, it remains possible that very few intrathoracic tumor cells are present. We can speculate that primary lesion resection in cM0 patients with pM1a represents nearly complete resection. In the LUC group of patients with extrathoracic metastasis, macroscopic tumor cells not receiving local therapy remain. However, in the LC group, no macroscopic tumor cells remain that are not undergoing local therapy. Based on this hypothesis, investigating the novel M-better/M-worse classification seems reasonable. For patients with NSCLC stage IV, if we select complete resection of the primary lesion as the initial treatment, an important consideration is whether local therapy, such as surgical resection or radiotherapy, is possible for the metastasis. These issues have been considered in both prospective and retrospective studies on oligometastasis (4,6-9).
Second, we show here that SCC was a prognostic factor indicating poor outcome. The current most common treatment for stage IV NSCLC is chemotherapy, and new drugs have recently improved outcomes for NSCLC. However, key drugs, e.g., pemetrexed, bevacizumab, and molecularly-targeted therapy [e.g., EGFR tyrosine kinase inhibitors (TKIs) and anaplastic lymphoma kinase inhibitor], are not effective against SCC (12-15). However, immune checkpoint inhibitors are effective for SCC as well as other types of NSCLC (16) and may improve outcomes of patients with SCC in the future.
Third, the N factor and EGFR mutation status were not significant prognostic factors in the current study. However, previous studies found that the N factor and EGFR mutation are significant prognostic factors (2,4,10). Additional patient data may be required to arrive at a definitive conclusion regarding whether the N factor and EGFR mutation status may serve as prognostic factors.
Fourth, female sex was a favorable prognostic factor, although the number of female subjects was small in the present study. The postoperative prognosis of female patients with NSCLC is reportedly better than that of male patients (17-19). Moreover, the smoking rate in female patients was found to be significantly lower than that in males (19). EGFR mutation-positive status, which generally indicates a high sensitivity to EGFR TKI, tended to be more likely both in non-smokers than in smokers and in women than in men (14,20). It is reasonable to assume that EGFR mutation affects prognosis in women.
Fifth, in the present study, the 5-year survival rate after complete resection of the primary lesion of all patients was 29.0%. According to previous reports, the 5-year survival rate of clinical stage IV NSCLC is 2–5.8% (21,22). Although the difference between these and the present study may reflect the relatively small sample size of the latter, we argue that even for patients with stage IV NSCLC complete resection of the primary lesion may improve prognosis, attributed, in part, to the implicit selection bias.
There are some limitations to the present study. First, it was a retrospective study based on our institution’s data. A randomized prospective study is required to confirm the effects of surgery for patients with stage IV NSCLC. Second, the present study did not include a sufficient number of subjects required for a robust analysis, and more patients and longer follow-up are required to validate our findings.
Conclusions
In conclusion, although there are some limitations to the current study, even for stage IV NSCLC patients, resection of the primary lesion may be beneficial, especially for those with M-better status and those not diagnosed with SCC.
Acknowledgements
We thank Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Ethical Statement: The study was approved by the Ethics Committee of the University of Occupational and Environmental Health Japan (H26-15) and written informed consent was obtained from all patients.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Hanna N, Johnson D, Temin S, at al. Systemic Therapy for Stage IV Non-Small-Cell Lung Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol 2017;35:3484-515. 10.1200/JCO.2017.74.6065 [DOI] [PubMed] [Google Scholar]
- 2.Okamoto T, Iwata T, Mizobuchi T, et al. Pulmonary resection for lung cancer with malignant pleural disease first detected at thoracotomy. Eur J Cardiothorac Surg 2012;41:25-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ren YJ, She YL, Dai CY, et al. Primary tumour resection showed survival benefits for non-small-cell lung cancers with unexpected malignant pleural dissemination. Interact Cardiovasc Thorac Surg 2016;22:321-6. 10.1093/icvts/ivv353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Endo C, Hasumi T, Matsumura Y, et al. A prospective study of surgical procedures for patients with oligometastatic non-small cell lung cancer. Ann Thorac Surg 2014;98:258-64. 10.1016/j.athoracsur.2014.01.052 [DOI] [PubMed] [Google Scholar]
- 5.Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. 10.1016/j.jtho.2015.09.009 [DOI] [PubMed] [Google Scholar]
- 6.Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol 1995;13:8-10. 10.1200/JCO.1995.13.1.8 [DOI] [PubMed] [Google Scholar]
- 7.Richard PJ, Rengan R. Oligometastatic non-small-cell lung cancer: current treatment strategies. Lung Cancer (Auckl) 2016;7:129-40. 10.2147/LCTT.S101639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Ruysscher D, Wanders R, van Baardwijk A, et al. Radical treatment of non-small-cell lung cancer patients with synchronous oligometastases: long-term results of a prospective phase II trial (Nct01282450). J Thorac Oncol 2012;7:1547-55. 10.1097/JTO.0b013e318262caf6 [DOI] [PubMed] [Google Scholar]
- 9.Li D, Zhu X, Wang H, et al. Should aggressive thoracic therapy be performed in patients with synchronous oligometastatic non-small cell lung cancer? A meta-analysis. J Thorac Dis 2017;9:310-7. 10.21037/jtd.2017.02.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He J, Li Y, An J, et al. Surgical treatment in non-small cell lung cancer with pulmonary oligometastasis. World J Surg Oncol 2017;15:36. 10.1186/s12957-017-1105-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Travis WD, Brambilla E, Burke AP, et al. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart. Lyon: International Agency for Research on Cancer, 2015. [DOI] [PubMed] [Google Scholar]
- 12.Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 2008;26:3543-51. 10.1200/JCO.2007.15.0375 [DOI] [PubMed] [Google Scholar]
- 13.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 2006;355:2542-50. 10.1056/NEJMoa061884 [DOI] [PubMed] [Google Scholar]
- 14.Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380-8. 10.1056/NEJMoa0909530 [DOI] [PubMed] [Google Scholar]
- 15.Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 2013;368:2385-94. 10.1056/NEJMoa1214886 [DOI] [PubMed] [Google Scholar]
- 16.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. 10.1056/NEJMoa1504627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawai H, Saito Y, Suzuki Y. Gender differences in the correlation between prognosis and postoperative weight loss in patients with non-small cell lung cancer. Interact Cardiovasc Thorac Surg 2017;25:272-7. 10.1093/icvts/ivx092 [DOI] [PubMed] [Google Scholar]
- 18.Chatkin JM, Abreu CM, Fritscher CC, et al. Is there a gender difference in non-small cell lung cancer survival? Gend Med 2004;1:41-7. 10.1016/S1550-8579(04)80009-3 [DOI] [PubMed] [Google Scholar]
- 19.de Perrot M, Licker M, Bouchardy C, et al. Sex differences in presentation, management, and prognosis of patients with non-small cell lung carcinoma. J Thorac Cardiovasc Surg 2000;119:21-6. 10.1016/S0022-5223(00)70213-3 [DOI] [PubMed] [Google Scholar]
- 20.Sasaki H, Shimizu S, Endo K, et al. EGFR and erbB2 mutation status in Japanese lung cancer patients. Int J Cancer 2006;118:180-4. 10.1002/ijc.21301 [DOI] [PubMed] [Google Scholar]
- 21.Sawabata N, Asamura H, Goya T, et al. Japanese Lung Cancer Registry Study: first prospective enrollment of a large number of surgical and nonsurgical cases in 2002. J Thorac Oncol 2010;5:1369-75. 10.1097/JTO.0b013e3181e452b9 [DOI] [PubMed] [Google Scholar]
- 22.Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2007;2:706-14. 10.1097/JTO.0b013e31812f3c1a [DOI] [PubMed] [Google Scholar]