Notch signaling is an evolutionary conserved pathway regulating stem cell maintenance, cell fate decisions and proliferation in embryonic as well as adult development. In this editorial, we highlight the work of Lim et al. who investigated a regulatory mechanism in small cell lung cancer (SCLC) depending on Notch signaling which triggers neuroendocrine and non-neuroendocrine tumor cell differentiation and SCLC growth.
Lim and colleagues used a Cre-recombinase inducible SCLC mouse model. They generated a conditional triple knock-out Tp53 (p53), Rb1 (Rb) and Rbl2 (p130) mouse strain, carrying an additional GFP reporter under the control of the Hes1 promoter (1). The basic helix-loop-helix transcription factor Hes1 contributes to cellular differentiation processes and is one of the main targets of Notch signaling. The authors found intratumoral heterogeneity indicated by GFP-positive and GFP-negative tumor cells within one SCLC lesion. GFP-positive cells refer to tumor cells expressing high levels of Hes1, the Notch receptors 1, 2, and 3, and less neuroendocrine markers. Thus, GFP-positive tumor cells represent a non-neuroendocrine phenotype with active Notch signaling. GFP-negative cells indicate neuroendocrine differentiated tumor cells with low levels of Hes1, high expression of neuroendocrine markers and of Notch ligands. Both cell types cooperate in SCLC growth, maintain tumor heterogeneity and regulate the trans-differentiation from a neuroendocrine to a non-neuroendocrine phenotype and vice versa. This trans-differentiation is triggered by Notch-ligand-receptor interactions and the transcription factor Rest.
Finally, they propose a new SCLC therapy regimen in which non-neuroendocrine and neuroendocrine tumor cells will be targeted simultaneously by using a combination of chemotherapy along with a Notch blockade.
The Notch signaling
Notch signaling is a pivotal pathway in development and disease, mainly regulating cell fate decisions and trans-differentiation. Importantly, in lung development inactivated Notch signaling induced neuroendocrine cell differentiation and activated Notch signaling triggered non-neuroendocrine cell fates. In cancer, Notch signaling delivers context dependent tumor-suppressive or oncogenic signals through its receptors which bind their ligands on neighboring cells. In canonical Notch signaling the Notch intracellular domain (NICD) is cleaved off from the mature transmembrane receptor: first, by a disintegrin-metalloprotease called ‘tumor necrosis factor alpha-converting enzyme’ (TACE) and second, by gamma-secretase. NICD is liberated into the cytoplasm and translocates to the nucleus. In the nucleus, NICD forms a co-activator complex and replaces thereby a transcriptional repressor complex. As part of the repressor and the activator complex ‘CBF1/Suppressor of Hairless/LAG-1’ (CSL), also known as ‘Recombination signal binding protein for immunoglobulin kappa J region’ (RBP-J), actively regulates the transcription of Notch target genes. In the co-activator complex, CSL/RBP-J and Mastermind-like 1 (MAML1) recruit additional co-activator proteins such as CREB-binding protein (CBP) and p300 histone acetyltransferases and mediate transcription of ‘hairy enhancer of split’ (HES) and ‘hairy enhancer of split related with YRPW motif protein 1’ (HEY). HES and HEY are well described regulators of basic helix-loop-helix proteins such as ‘achaete-scute homologue 1’ (ASCL1) which plays a pivotal role in neuroendocrine cell differentiation (2,3).
In mammals, there are four Notch receptors (Notch1-4) which harbor a high sequence homology. They bind to the Notch ligands Jagged 1 (JAG1), JAG2, Delta-like 1 (DLL1), DLL3 and DLL4 either by trans-interaction (ligand and receptor are located on different cells) or by cis-interaction (ligand and receptor are located on the same cell). Classically, trans-interaction results in Notch activation and cis-interaction in Notch inhibition (4,5). However, DLL3 harbors a unique inhibitory function which blocks the trafficking of the Notch receptor in the cytoplasm (6). Hence, DLL3 binds Notch receptors only in a cell autonomous cis-interactive manner leading to Notch inhibition (7).
Non-canonical Notch signaling is frequently ligand- and CSL-independent and mainly observed in the regulation of the immune system and cancer. Furthermore, it concurs with the conserved NF-kappaB pathway and the WNT/beta-catenin pathway, respectively. These pathway cross-talks alter the activation and proliferation of immune cells and support tumorigenesis in a tissue specific fashion (2).
The origin of SCLC
Lung cancer is an epithelial cancer occurring peripherally or centrally located within the lung which is reasoned by the cell of origin. SCLC is the most aggressive type of lung cancer and accounts for approximately 15% of lung cancer case. It is frequently centrally located in the lung and present as pure or combined SCLC. SCLC tumor cells are recognized by their small round cell shape and a high proliferative index. In addition, they are characterized by a bi-allelic loss of the tumor suppressor genes RB1 and TP53 and neuroendocrine differentiation indicated by CD56, synaptophysin and chromogranin A expression (8,9). Thus, neuroendocrine epithelial cells and precursors which are located in the lining of the upper bronchioles are suggested as the major cell of origin (10). These neuroendocrine cells are embedded in neuroendocrine bodies (NEBs) within the epithelium and are indicated by calcitonin-gene related peptide (CGRP) expression. NEBs are surrounded by non-neuroendocrine “variant Club cells” which are also found in the proximity of bronchoalveolar duct junctions (BADJs) (11). In the microenvironment of NEBs and BADJs, “variant Club cells”, which express the CC10 marker of the classical Club cells, are the likely source for active Notch signaling (12,13). The function of “variant Club cells” was identified in naphthalene injured adult lungs which showed damaged bronchial epithelium with depleted classical Club cells and ciliated cells, which show a non-neuroendocrine phenotype. Surviving neuroendocrine cells as well as naphthalene-resistant “variant Club cells” harbored the capability to re-populate the epithelium by differentiating into ciliated and secretory classical Club cells (11,14). Thus, neuroendocrine cells are able to renew themselves and to differentiate into non-neuroendocrine cells, although they likely require a microenvironment which exposes them to Notch stimuli.
In addition, Swarts et al. discriminated peripheral SCLC from central SCLC and suggested non-neuroendocrine alveolar type 2 cells as potential cell of tumor origin (15). Alveolar type 2 cells are located in the alveoli of the lung periphery and indicated by surfactant protein C (SPC) expression. In line with these findings, Sutherland and colleagues investigated a cell type specific Rb1 and Tp53 knock-out in a mouse model for SCLC. They identified CGRP expressing neuroendocrine cells to be the predominant precursor for SCLC. Importantly, they also showed that alveolar type 2 cells serve as the cell of origin for a minor portion of induced SCLCs (10). Taken together, SCLC cells may harbor neuroendocrine and/or non-neuroendocrine features.
Lung cancer trans-differentiation in therapy resistance
Most SCLC patients show an extensive stage of disease (ED) at the time-point of diagnosis and tumors are therefore rarely resected. SCLC is initially sensitive to chemo-radiotherapy, but relapses occur rapidly and the 5-year survival rate for ED-SCLC is with 1–2% very low (8).
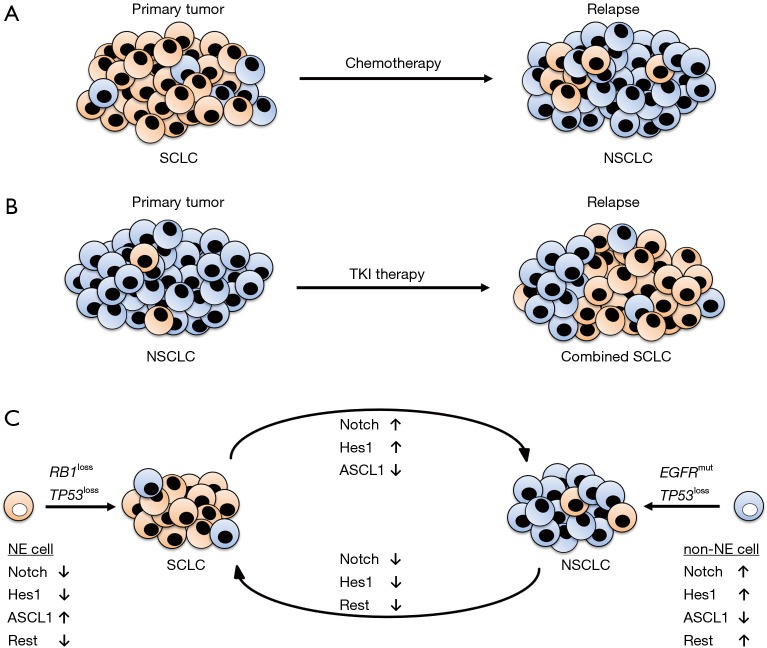
Transitions of tumor phenotypes after therapy resistance are frequently identified. Already in 1991, Brambilla et al. described a more differentiated SCLC tumor phenotype with larger tumor cells in patients with acquired resistance to chemotherapy (16). Calbó and colleagues found tumor heterogeneity in SCLC. Lesions were not only composed of neuroendocrine tumor cells but also of non-neuroendocrine tumor cells which were suggested as essential for chemotherapy resistance (17) (Figure 1A). They also showed that manipulated KRAS, the major oncogene in non-small cell lung cancer (NSCLC), was able to switch the tumor cell phenotype from neuroendocrine to non-neuroendocrine (18).
Figure 1.
Scheme of lung cancer trans-differentiation. (A) SCLC is initially sensitive to chemotherapy. Upon acquired chemotherapy resistance, the relapsed tumor frequently presents large non-neuroendocrine tumor cells referring to a NSCLC phenotype; (B) NSCLCs harboring mutated EGFR may be treated by tyrosine kinase inhibitors (TKIs). Tumors that acquired resistance to TKIs frequently relapse as combined SCLCs, comprising a neuroendocrine small cell and a non-neuroendocrine non-small cell compartment; (C) SCLCs primarily originate from neuroendocrine precursors with inactivated Notch signaling, low Hes1 and Rest levels and high ASCL1 expression. RB1 and TP53 represent the most frequently mutated genes in SCLC. For NSCLCs, non-neuroendocrine precursors are the predominant cells of origin. They show activated Notch signaling with high levels of Hes1 and Rest and low ASCL1 expression. The trans-differentiation from SCLC to NSCLC phenotypes might be triggered mainly by ASCL1, whereas Rest is suggested as the master regulator in the trans-differentiation from NSCLC to SCLC. NE, neuroendocrine; non-NE, non-neuroendocrine; SCLC, small cell lung cancer; NSCLC, non-small cell lung cancer.
In 3–14% of EGFR mutated adenocarcinomas treated with tyrosine kinase inhibitors (TKIs), the development into combined SCLC, carrying characteristics of SCLC and NSCLC, is associated with therapy resistance (19) (Figure 1B). Niederst et al. showed that an acquired bi-allelic loss of RB1 induced a trans-differentiation of TKI-treated non-neuroendocrine EGFR driven adenocarcinomas to combined SCLC. Importantly, the combined SCLC harbored the same EGFR and TP53 mutations as the primary tumor (20). In addition, inactivating genomic aberrations in NOTCH genes were identified in up to 25% of SCLC cases (21) and were associated to neuroendocrine differentiation and SCLC relapse after TKI treatment (22).
The findings of Lim et al. support the intratumoral heterogeneity of SCLC, as well. Notably, they showed that activated Notch signaling directly mediates the transition from neuroendocrine to non-neuroendocrine cells in SCLC (1). Although, Osada et al. and others demonstrated that the Notch target ASCL1 is the major player in the induction of “small-cell-ness” (3,22), Lim and colleagues found that the Notch target Rest is responsible for the transition towards “non-small-cell-ness” independent of ASCL1 (1) (Figure 1C). This finding led to the hypothesis that upon acquired resistance to therapy, transitions from NSCLC to SCLC might be triggered by ASCL1 and transitions from SCLC to NSCLC by the transcription factor Rest.
Targeting Notch signaling in SCLC
Targeting Notch signaling in SCLC in anti-cancer therapy is a double-edged sword which regard to tumor heterogeneity. On the one hand, growth of neuroendocrine tumor cells has to be blocked, because they account for the majority of tumor cells within a SCLC lesion. This might be achieved by re-activating Notch signaling. On the other hand, activation of Notch signaling reduces neuroendocrine proliferation but may trigger differentiation to non-neuroendocrine cells which boost the relapse. A combined therapy regimen, composed of classical chemotherapy targeting the rapidly proliferating majority of neuroendocrine SCLC cells and of Notch blockade depleting the tumor promoting minority of non-neuroendocrine SCLC cells, may be the targeted therapy of choice.
Lim et al. applied a combined chemotherapy with carboplatin and irinotecan together with tarextumab, a Notch2/3 targeting monoclonal antibody inhibiting Notch signaling. They observed significant SCLC shrinkage. However, the phase II PINNACLE clinical trial of tarextumab (OncoMed Pharmaceuticals) in combination with etoposide plus cis- or carboplatin compared to chemotherapy with placebo did not reveal improved median progression-free and overall survival in untreated ED-SCLC patients (NCT01859741) (1).
Re-activation of Notch signaling also induced SCLC tumor shrinkage (21) postulating a tumor suppressive effect by targeting the majority of neuroendocrine differentiated SCLC cells. Histone deacetylase (HDAC) inhibitors mediate apoptosis of tumor cells by increasing Notch1 signaling (23). A clinical phase II trial in pre-treated SCLC patients using panobinostat, a multi-HDAC inhibitor, was prematurely terminated due to lack of activity. However, de Marinis and colleagues stated at least a moderate activity of panobinostat when applied to SCLC patients because 2 of 19 patients showed a partial response (24). Hence, other Notch re-activation mechanisms might be advantageous in treating SCLC.
The Notch ligand DLL3 is expressed in up to 80% of SCLC and is known to inhibit Notch signaling. The first-in-human clinical trial using rovalpituzumab tesirine, an anti-DLL3 monoclonal antibody, provides promising anti-tumor activity in SCLC by re-activating Notch signaling. Anti-DLL3 treatment of SCLC results in 88% disease control rate and 1-year survival of 32% (25). Currently, seven clinical trials are recruiting SCLC patients for treatment with rovalpituzumab tesirine: NCT02874664, NCT02819999, NCT03086239, NCT03061812, NCT03033511, NCT03020166 and NCT03000257 (clinicaltrials.gov, assessed 11th Oct 2017).
In conclusion, targeting Notch signaling is an encouraging way in SCLC therapy and in the active battle against the SCLC tumor heterogeneity. Particularly, highly aggressive neoplasms such as SCLC are known to relapse rapidly. Thus, it is a compulsive issue to obtain routinely isolated biopsies not only for the identification of potential biomarkers of response but also to identify possible resistance mechanisms which may be targeted by further combined therapy regimens.
Acknowledgements
None.
Provenance: This is an invited Editorial commissioned by Section Editor Dr. Tianxiang Chen (Shanghai Lung Cancer Center, Shanghai Chest Hospital, Shanghai Jiao Tong University, Shanghai, China).
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Lim JS, Ibaseta A, Fischer MM, et al. Intratumoural heterogeneity generated by Notch signalling promotes small-cell lung cancer. Nature 2017;545:360-4. 10.1038/nature22323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crabtree JS, Singleton CS, Miele L. Notch Signaling in Neuroendocrine Tumors. Front Oncol 2016;6:94. 10.3389/fonc.2016.00094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Osada H, Tomida S, Yatabe Y, et al. Roles of achaete-scute homologue 1 in DKK1 and E-cadherin repression and neuroendocrine differentiation in lung cancer. Cancer Res 2008;68:1647-55. 10.1158/0008-5472.CAN-07-5039 [DOI] [PubMed] [Google Scholar]
- 4.Jacobsen TL, Brennan K, Arias AM, et al. Cis-interactions between Delta and Notch modulate neurogenic signalling in Drosophila. Development 1998;125:4531-40. [DOI] [PubMed] [Google Scholar]
- 5.Fleming RJ, Hori K, Sen A, et al. An extracellular region of Serrate is essential for ligand-induced cis-inhibition of Notch signaling. Development 2013;140:2039-49. 10.1242/dev.087916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heuss SF, Ndiaye-Lobry D, Six EM, et al. The intracellular region of Notch ligands Dll1 and Dll3 regulates their trafficking and signaling activity. Proc Natl Acad Sci U S A 2008;105:11212-7. 10.1073/pnas.0800695105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ladi E, Nichols JT, Ge W, et al. The divergent DSL ligand Dll3 does not activate Notch signaling but cell autonomously attenuates signaling induced by other DSL ligands. J Cell Biol 2005;170:983-92. 10.1083/jcb.200503113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wallace AS, Arya M, Frazier SR, et al. Combined small-cell lung carcinoma: An institutional experience. Thorac Cancer 2014;5:57-62. 10.1111/1759-7714.12059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Travis WD. Pathology and diagnosis of neuroendocrine tumors: lung neuroendocrine. Thorac Surg Clin 2014;24:257-66. 10.1016/j.thorsurg.2014.04.001 [DOI] [PubMed] [Google Scholar]
- 10.Sutherland KD, Proost N, Brouns I, et al. Cell of origin of small cell lung cancer: inactivation of Trp53 and Rb1 in distinct cell types of adult mouse lung. Cancer Cell 2011;19:754-64. 10.1016/j.ccr.2011.04.019 [DOI] [PubMed] [Google Scholar]
- 11.Giangreco A, Arwert EN, Rosewell IR, et al. Stem cells are dispensable for lung homeostasis but restore airways after injury. Proc Natl Acad Sci U S A 2009;106:9286-91. 10.1073/pnas.0900668106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guha A, Vasconcelos M, Cai Y, et al. Neuroepithelial body microenvironment is a niche for a distinct subset of Clara-like precursors in the developing airways. Proc Natl Acad Sci U S A 2012;109:12592-7. 10.1073/pnas.1204710109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morimoto M, Nishinakamura R, Saga Y, et al. Different assemblies of Notch receptors coordinate the distribution of the major bronchial Clara, ciliated and neuroendocrine cells. Development 2012;139:4365-73. 10.1242/dev.083840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song H, Yao E, Lin C, et al. Functional characterization of pulmonary neuroendocrine cells in lung development, injury, and tumorigenesis. Proc Natl Acad Sci U S A 2012;109:17531-6. 10.1073/pnas.1207238109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swarts DR, Ramaekers FC, Speel EJ. Molecular and cellular biology of neuroendocrine lung tumors: evidence for separate biological entities. Biochim Biophys Acta 2012;1826:255-71. [DOI] [PubMed] [Google Scholar]
- 16.Brambilla E, Moro D, Gazzeri S, et al. Cytotoxic chemotherapy induces cell differentiation in small-cell lung carcinoma. J Clin Oncol 1991;9:50-61. 10.1200/JCO.1991.9.1.50 [DOI] [PubMed] [Google Scholar]
- 17.Calbó J, Meuwissen R, van Montfort E, et al. Genotype-phenotype relationships in a mouse model for human small-cell lung cancer. Cold Spring Harb Symp Quant Biol 2005;70:225-32. 10.1101/sqb.2005.70.026 [DOI] [PubMed] [Google Scholar]
- 18.Calbo J, van Montfort E, Proost N, et al. A functional role for tumor cell heterogeneity in a mouse model of small cell lung cancer. Cancer Cell 2011;19:244-56. 10.1016/j.ccr.2010.12.021 [DOI] [PubMed] [Google Scholar]
- 19.Sequist LV, Waltman BA, Dias-Santagata D, P, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med 2011;3:75ra26. 10.1126/scitranslmed.3002003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niederst MJ, Sequist LV, Poirier JT, et al. RB loss in resistant EGFR mutant lung adenocarcinomas that transform to small-cell lung cancer. Nat Commun 2015;6:6377. 10.1038/ncomms7377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.George J, Lim JS, Jang SJ, et al. Comprehensive genomic profiles of small cell lung cancer. Nature 2015;524:47-53. 10.1038/nature14664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meder L, Konig K, Ozretic L, et al. NOTCH, ASCL1, p53 and RB alterations define an alternative pathway driving neuroendocrine and small cell lung carcinomas. Int J Cancer 2016;138:927-38. 10.1002/ijc.29835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takebe N, Miele L, Harris PJ, et al. Targeting Notch, Hedgehog, and Wnt pathways in cancer stem cells: clinical update. Nat Rev Clin Oncol 2015;12:445-64. 10.1038/nrclinonc.2015.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Marinis F, Atmaca A, Tiseo M, et al. A phase II study of the histone deacetylase inhibitor panobinostat (LBH589) in pretreated patients with small-cell lung cancer. J Thorac Oncol 2013;8:1091-4. 10.1097/JTO.0b013e318293d88c [DOI] [PubMed] [Google Scholar]
- 25.Rudin CM, Pietanza MC, Bauer TM, et al. Rovalpituzumab tesirine, a DLL3-targeted antibody-drug conjugate, in recurrent small-cell lung cancer: a first-in-human, first-in-class, open-label, phase 1 study. Lancet Oncol 2017;18:42-51. 10.1016/S1470-2045(16)30565-4 [DOI] [PMC free article] [PubMed] [Google Scholar]