Abstract
Background
Primary malignant chest-wall tumors (PMCWTs) are a heterogeneous group of tumors. They require a special experience in designing resection and reconstruction. They account for less than 1% of all primary malignant tumors. This study is designed to clarify different factors contributing to the outcome of patients with PMCWTs in our institution.
Methods
A retrospective study included 98 patients with pathology proven PMCWTs, treated at the National Cancer Institute (NCI), Cairo University, Egypt, during the past 10 years. Used variables were: age, sex, forced expiratory volume in the 1st second (FEV1), site, size, multiplicity, pathologic subtype, tumor grade, safety margin (SM), excised ribs, complications, estimated blood loss (EBL), neo-adjuvant and adjuvant treatments, Overall and disease free survival (DFS) were obtained using Kaplan-Meier method and compared using Log rank test. Cox regression was used to identify DFS predictors.
Results
PMCWTs represented 10.5% of all thoracic malignancies in our institution. There were 51 females (52%). The median age was 39 years [interquartile range (IQR) =25–52.3)] years. Chondrosarcoma was the commonest tumor histology (20.4%). The median tumor size was 8 cm (IQR =5–14). Tumor multiplicity was found in 18.4% of patients. Bone resection was performed in 76 patients (78.3%), ribs resection was performed in 59 patients and the median number of resected ribs per patient was 3 (IQR =1–3) ribs. Sternal resection was done in 7 (7.1%) cases. R0 resection was achieved in 62.2% of patients. There was one operative related mortality (1.02%) and 17.3% patients suffered procedure related complications. Local recurrence developed in 35 (35.7%) patients. The overall survival (OS) at 1, 3 and 5 years was 73.9%, 45.6% and 34.6% respectively and the median OS was 33 months (95% CI, 21.8–44.2), while median DFS was 24 months (95% CI, 19.6–28.4). Predictors of better DFS were –ve SM (P<0.001), tumors <5 cm (P=0.039), low grade (P=0.033), lower EBL (P=0.003) and absence of adjuvant therapy (P=0.007); however, on multivariate analysis, only –ve SM was the only predictor (HR =0.54; 95% CI, 0.29–0.97, P=0.041).
Conclusions
In primary malignant CWTs (PMCWTs) achievement of wide resection margins is of great importance to minimize the local tumor recurrence that will have an adverse impact on long-term survival.
Keywords: Chest wall tumors, local recurrence, sternal resection, reconstruction, complications, estimated blood loss (EBL), neo-adjuvant and adjuvant treatments
Introduction
Primary malignant chest wall tumors are a heterogeneous group of tumors that originate from thoracic soft tissue and skeletal structures (1,2).
They may be asymptomatic and slowly growing with pain development during their extension (3). They need broad tumor-free margins that often lead to complex chest wall resection and reconstruction techniques, especially when the tumor is large in diameter or located posteriorly adjacent to the vertebrae or involves the sternum. Consequently, an early diagnosis of a small tumor increases the possibility of a curative therapy and decreasing the need for extended resections (4).
Extensive chest wall defects that involve soft and skeletal tissues may be the end result of chest wall resection of those tumors with 2 available method for reconstruction of such defects; prosthetic or biologic mesh and/or flaps with their blood supply (3,5-7). Recent advances in the techniques of skeletal and musculocutaneous reconstructions have facilitated the treatment of these tumors so that tumor size is not a contraindication to radical excision (8).
They require special experience in designing resection and reconstruction. This study aimed to clarify different factors contributing to the outcome of patients with PMCWTs in our institution.
Methods
Patients and data collection
This study was approved by institutional ethics committee/ethics board of National Cancer Institute (NCI)/Cairo University (No. IRB00004025). We retrospectively reviewed pathologically proven PMCWTs that were treated surgically at the NCI, Cairo University, Egypt, during the past 10 years.
Different possible prognostic variables were used as age, sex, forced expiratory volume in the 1st second (FEV1), site, size, multiplicity, pathologic subtype, tumor grade, safety margin (SM; length, whether the least SM was bone or soft tissue, and positivity), number of excised ribs, complications, intra-operative estimated blood loss (EBL), neo-adjuvant and adjuvant treatments, local and metastatic recurrence (MR).
Computed tomography (CT) of the chest and upper abdomen was the initial staging tool for all patients with magnetic resonance imaging requested only if indicated. In our institute, the commonest used biopsy methods were core needle biopsy with or without CT guidance. We reserve fine needle aspiration cytology (FNAC) for suspected recurrence. Surgical resection and reconstruction was designed according to the site and extent of the lesion resected. Adjuvant and neo-adjuvant therapy was given according to multi-disciplinary thoracic oncology team (MDT) decision.
Our cohort included 3 groups: (I) bony sarcomas as osteosarcomas, chondrosarcomas, and primitive neuro-ectodermal tumor (PNET)/Ewing’s sarcoma; (II) soft tissue sarcoma as fibromatosis/fibrosarcoma, pleomorphic undifferentiated sarcoma previously named malignant fibrous histiocytoma, dermatofibrosarcoma protuberans, synovial sarcoma, angiosarcoma, myxofibrosarcoma, rhabdomyosarcoma, myxoliposarcoma; (III) others as carcinomas, solitary plasma cell myeloma and lymphoma that were initially diagnosed as liposarcoma (9-12).
Our institutional treatment policy for PMCWT is based on MDT decision. Regarding neoadjuvant/adjuvant treatment: (I) for PNET; the primary treatment is multi-agent chemotherapy: VAC/IE (Vincristine, Doxorubicin and Cyclophosphamide alternating with Ifosfamide and Etoposide) followed by local control therapy (either surgery or radiotherapy) followed by adjuvant chemotherapy to complete 54 weeks from the beginning of treatment (13,14); (II) for other chemo- and radio-resistant tumors as chondrosarcoma and fibrosarcoma the final decision will be based on MDT meeting opinion, however; we usually give postoperative radiotherapy in case of +ve surgical margin and high grade tumors that represent a poor prognostic criteria.
Defects less than 5 cm in diameter anywhere in the chest wall did not require skeletal reconstruction and could be closed with soft tissue only. Posterior superior defects less than 10 cm in diameter did not have to be reconstructed as they are covered with shoulder blades and large back muscles that provided adequate firmness and stability and did not disturb breathing mechanics.
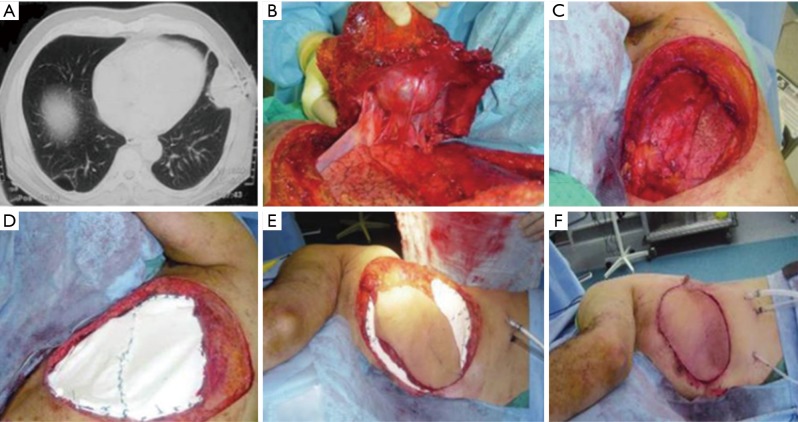
Mesh with rigid reconstruction was adopted in sternal or ≥3 anterior, lateral or posterior ribs resection. Muscle reconstruction was adopted in <2 anterior ribs resection, while mesh reconstruction was done in <2 ribs resection located laterally or posteriorly similar to prior series (8). Sandwiched bone cement i.e., methyl methacrylate enclosed with Prolene mesh (MMM) was the most common method of rigid reconstruction in our institute (Figure 1). We usually fix the MMM with sutures placed either through drill holes in adjacent ribs or through peri-costal soft tissues and fix it to the surroundings using either PDS 0 or Prolene 1 sutures with a 2–3 cm overlapping margin between the mesh and chest wall to avoid pushing the mesh inside the defect with breathing
Figure 1.
Left lateral chest wall chondrosarcoma. (A) Preoperative CT scan; (B) dissection of the tumor; (C) defect after resection, (D) defect closed with bone sandwich (MMM); (E) LD myocutaneous flap harvest; (F) final closure. CT, computed tomography; LD, latissimus dorsi.
Survival and follow-up data
Overall survival (OS) is the time from date of surgery to date of death from cancer or other causes or date of last follow-up, while disease free survival (DFS) is the time from date of surgery to date of first recurrence, death from cancer or other causes or the date of last follow-up (12). Median follow-up was 36 months [interquartile range (IQR) =11.5–60] for the whole group.
Statistical analysis
The primary outcomes were to identify OS and DFS, that were analyzed using the Kaplan-Meier survival curves. Survival curves for significant variables in the multivariate analysis were compared using Log-rank test.
The secondary outcomes were to compare the clinico-pathological and survival data between the commonest 3 pathological subtypes among our cohort (chondrosarcoma, fibromatosis/fibrosarcoma and PNET/Ewing sarcoma.
Continuous variables were presented as a median and IQR and compared using Mann-Whitney U test. Categorical variables were reported as absolute numbers (frequency percentages) and compared using Pearson’s Chi-square (χ2) test. Analysis of variance (ANOVA) test was used to compare continuous variables in >2 groups. Cox regression was used to identify factors predicting DFS in both uni- and multivariate analyses. Kaplan-Meier survival curves with P value <0.05 was considered statistically significant. Hazard ratios (HR) and 95% confidence intervals (95% CI) were calculated. Statistical analyses were conducted using SPSS version 22.0 (IBM, Armonk, NY, USA).
Results
PMCWTs represented 10.5% of all thoracic malignancies in our institution. There were 51 females (52%). The median age was 39 years (IQR =25–52.3) years (Table 1).
Table 1. Patients’ characteristics among our cohort.
| Patients’ characteristics | Median [IQR], frequency [%] |
|---|---|
| Age¶ | 39 [25–52.3] |
| Male gender | 47 [48] |
| Pathology | |
| Bony | 45 [45.9] |
| Soft tissue | 44 [44.9] |
| Others | 9 [9.2] |
| FEV1¶ | 2.29 [2.1–2.6] |
| Location (anterior only) | 35 [35.7] |
| Left side tumors | 47 [48] |
| Tumor size ≥5 cm | 82 [83.7] |
| SM ≥2 cm | 23 [23.5] |
| Least SM | 0.1 [0.2–1.5] |
| +ve margin | 37 [83.7] |
| Bony least SM | 7 [7.1] |
| High grade [2–3] | 48 [49] |
| Tumor size | 8 [5–14] |
| Multiplicity | 18 [18.4] |
| No. of excised rib | 3 [1–3] |
| Lung resection | 11 [11.2] |
| Additional lobectomy | 3 [3.1] |
| No. of dissected nodes | 12 [12.2] |
| +ve path N | 1/12 (axillary) |
| Local recurrence (LR) | 35 [35.7] |
| Time to LR (months, IQR) | 14.5 [6–34.3] |
| Metastatic recurrence (MR) | 21 [21.4] |
| Time to MR (months, IQR) | 19 [8–36] |
| EBL¶ | 775 [500–1,400] |
| Complications | 17 [17.3] |
| Neo-adjuvant treatment | 28 [28.6] |
| Adjuvant treatment | 67 [68.4] |
| Survival data; months | |
| Median OS (95% CI) | 33 (21.8–44.2) |
| Median DFS (95% CI) | 24 (19.6–28.4) |
| Median follow up (IQR) | 36 (11.5–60) |
IQR, interquartile range; FEV1, forced expiratory volume in the 1st second; SM, surgical margin; EBL, estimated blood loss; OS, overall survival; DFS, disease free survival. ∂, continuous variable.
Chondrosarcoma was the commonest tumor histology (20.4%) followed by fibromatosis/fibrosarcoma (19.4%) and PNET/Ewing sarcoma (16.3%) (Table 2). Other histopathological types were osteosarcoma in 9 (9.2%), pleomorphic undifferentiated sarcoma in 8 (8.2%), synovial sarcoma in 5 (5.1%), dermatofibrosarcoma in 3 (3.1%), 2 (2%) for each of angiosarcoma, alveolar soft part sarcoma, myxofibrosarcoma, rhabdomyosarcoma, plasma cell myeloma, lymphoma, adenocarcinoma and 1 (1%) for each of myxoliposarcoma, metaplastic carcinoma, squamous cell carcinoma and undifferentiated carcinoma.
Table 2. Patients characteristics among the commonest 3 pathological subtypes.
| Patients characteristics | Median [%], interquartile range [IQR] | |||
|---|---|---|---|---|
| Chondrosarcoma (n=20) | Fibromatosis/fibrosarcoma (n=19) | PNET (n=16) | P value | |
| Age¶ | 43.5 [30.25–56] | 35 [29–58] | 24.5 [20–30] | 0.004 |
| Male gender | 14 [70] | 7 [36.8] | 7 [43.8] | 0.093 |
| Presenting symptom | Mass [65] | Mass [60] | Pain [75] | – |
| FEV1¶ | 2.35 [2.02–2.60] | 2.45 [2.10–2.62] | 2.20 [1.90–2.65] | 0.850 |
| Anterior only location | 6 [30] | 10 [52.6] | 3 [18.8] | 0.193 |
| Tumor size ≥5 cm | 18 [90] | 15 [78.9] | 13 [81.3] | 0.618 |
| SM ≥2 cm¶ | 7 [35] | 1 [5.3] | 5 [31.3] | 0.064 |
| Least SM¶ | 0.75 [0.10–3] | 0.40 [0.10–1] | 1.25 (0.28–2.38) | 0.100 |
| +ve margin | 8 [40] | 10 [52.6] | 3 [18.8] | 0.118 |
| Bony least SM | 3 [15] | 1 [5.3] | 0 [0] | 0.208 |
| High grade [2–3] | 8 [40] | 1 [5.3] | 15 [93.8] | <0.001 |
| Tumor size | 8 [6.63–15.75] | 8 [6–13] | 7 [5–14.75] | 0.608 |
| Multiplicity | 3 [15] | 3 [15.8] | 3 [18.8] | 0.952 |
| No. of excised rib¶ | 3 [0–3] | 3 [1.5–3.75] | 1 [0–2] | 0.039 |
| Lung resection | 1 [5] | 1 [5.3] | 1 [6.3] | 0.986 |
| Metastatic recurrence (MR) | 1 [5] | 1 [5.3] | 6 [37.5] | 0.008 |
| Lung MR* | 1[5] | 1 [5.3] | 5 [31.3] | 0.042 |
| EBL¶ | 875 [500–1,650] | 750 (500–1,612.5) | 1,000 [500–1,200] | 0.393 |
| Complications | 4 [20] | 3 [15.8] | 2 [12.5] | 0.830 |
| Neoadj treatment | 1 [5] | 1 [5.3] | 13 [81.3] | <0.001 |
| Adjuv. treatment | 8 [40] | 10 [52.6] | 14 [87.5] | 0.013 |
| Survival data; months | ||||
| Median OS (95% CI) | 57 (17.16–96.84) | 58 (29.68–86.52) | 10 (13.4–52.60) | C:F =0.561 |
| C:P =0.535 | ||||
| F:P =0.121 | ||||
| Median DFS (95% CI) | 24 (15.73–32.27) | 36 (22.01–50) | 24 (18.27–29.73) | C:F =0.057 |
| C:P =0.427 | ||||
| F:P =0.173 | ||||
| Median follow up (IQR) | 18 (2.73–52.50) | 28.50 (12.80–58.78) | 52.5 (39.75–68.40) | – |
∂, P value obtained by ANOVA; C:F, chondrosarcoma vs. fibromatosis/fibrosarcoma P value; FEV1, forced expiratory volume in the 1st second; SM, surgical margin; EBL, estimated blood loss; OS, overall survival; CI, confidence interval; DFS, disease free survival.
Pain was the most common symptom in PNET/Ewing’s sarcoma (75%), osteosarcoma (57.1%), while mass was the most common symptom in chondrosarcoma (65%) and fibromatosis (60%).
The median tumor size was 8 cm (IQR =5–14). Tumor multiplicity was found in 18.4% of patients.
R0 resection was achieved in 62.2% of patients. Histopathologically, the least surgical margin was bone in 7 cases with 1 of them (14.3%) had +ve margin, while in the remaining 91 cases, 36 (39.6%) cases had positive margin (P=0.184).
Subgroup analysis regarding the clinico-pathological data between the commonest 3 pathological subtypes revealed higher prevalence of PNET in young age [24.5 years (20–30 years); P=0.004], higher prevalence of males in chondrosarcoma (70%; P=0.093) (Table 2). Higher SM ≥2 cm was more achievable in chondrosarcoma (35%) vs. 5.3% and 31.3% in fibromatosis/fibrosarcoma and PNET respectively (P=0.064). PNETs were likely to be of high grades (93.8%; P<0.001), develop metastatic potentials (M+ =37.5; P=0.008), especially lung metastasis (31.3%; P=0.042), receive neo-adjuvant (81.3%; P<0.001) and adjuvant treatments (87.5%, P=0.013) (Table 2).
Sternal and rib resection
Chest wall tumors were located anteriorly (35.7%), posteriorly (42.9%), laterally (5.1%) and in more than 1 location in the remaining.
Bone resection was performed in 76 patients (77.6%), ribs resection was performed in 59 patients and the median number of resected ribs per patient was 3 (IQR =1–3) ribs.
Sternal resection was done in 7 (7.1%) cases and their pathological diagnoses were 2 cases of chondrosarcoma, 2 rhabdomyosarcomata, 1 adenocarcinoma, 1 metaplastic carcinoma and 1 solitary plasma cell myeloma.
Among the 59 patients who had rib resection; 4 had sternal resection and 4 had vertebral resection as well. Among the remaining sternal resection cohort; 2 had sternal and clavicular resection and 1 had total sternectomy. Ten patients had scapulectomy, 2 had scapulectomy and clavicle resection, and 2 had clavicle resection
Neoadjuvant/adjuvant therapy
Thirty-one (31.6%) patients had no adjuvant therapy. Adjuvant radiotherapy was given in 20 (20.4%) patients; 8 (40%) were fibromatosis/fibrosarcoma, 5 (25%) were chondrosarcoma, 3 (15%) were synovial sarcoma and 3 (15%) were dermatofibrosarcoma and 1 (1%) was osteosarcoma. Adjuvant chemotherapy was given in 26 (26.5%) patients; 10 (38.5%) were PNET, 6 (23.1%) were osteosarcoma, 2 (7.7%) were synovial sarcoma and 1 (3.8%) of each of the followings: rhabdomyosarcoma, pleomorphic undifferentiated sarcoma, chondrosarcoma, fibrosarcoma, alveolar soft part sarcoma, metaplastic carcinoma, adenocarcinoma and squamous cell carcinoma. Adjuvant chemoradiotherapy was given in 21 (21.4%) patients; 7 (33.3%) were pleomorphic undifferentiated sarcoma, 4 (19%) were PNET, 2 (9.5%) of each of the followings: chondrosarcoma, lymphoma and plasma cell myeloma and 1 (4.8%) of each of the followings fibromatosis, osteosarcoma, rhabdomyosarcoma and undifferentiated carcinoma.
Reconstruction
Primary closure was achieved in 52%of the cases. Forty-seven patients underwent reconstruction. Among them, MMM & pectoralis flap was achieved in 38.3%, Prolene mesh in 17%, MMM in 14.9%, split skin graft in 14.9%, attachment of the humeral head to the rest of clavicle in 6.4%, MMM & latissimus dorsi (LD) flap in 4.3% (Figure 1) and similarly Prolene mesh & pectoralis in 4.3%.
Morbidity, mortality and follow-up
Complications occurred in 17 (17.3%) patients; arrhythmia in 3 cases, bleeding in 3, both bleeding and arrhythmia in 1, chest infection in 6, effusion in 1 and wound infection in 3 cases.
There was one perioperative mortality (1.02%). The median follow-up was 36 months (IQR =11.5–60) for the whole group while it was 18 months (IQR =2.73–52.50) in Chondrosarcoma, 28.50 months (IQR =12.80–58.78) in fibromatosis/fibrosarcoma and 52.5 months (IQR =39.75–68.40) in PNET. Local recurrence (LR) developed in 35.7% of patients with median time to LR of 14.50 (6–34.25), while MR developed in 21.4% of patients (metastasis to the lungs in 13 cases, bone in 3, chest wall in 2, liver in 1, >1 site in 2 cases) with median time to MR of 19 (IQR =8–36) (Table 1).
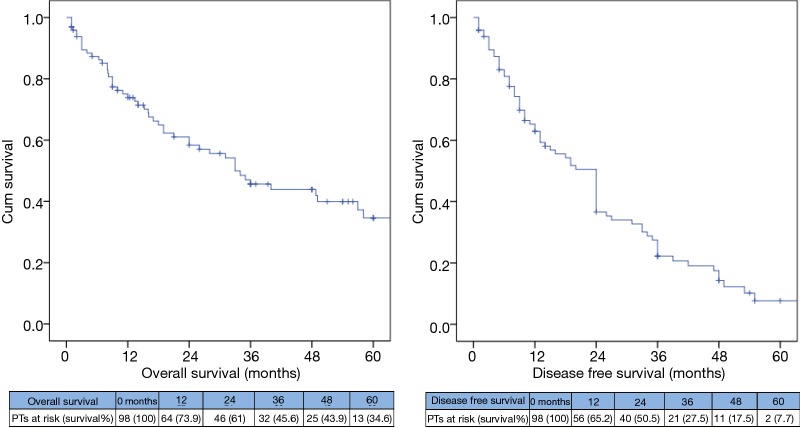
The OS for the whole group at 1, 3 and 5 years was 73.9%, 45.6% and 34.6% respectively and the median OS time was 33 months (95% CI, 21.8–44.2), while the median DFS was 24 months (95% CI, 19.6–28.4) (Figure 2).
Figure 2.
Kaplan Meier survival curves showing OS and DFS among the whole group. OS, overall survival; DFS, disease free survival.
Predictors of DFS in univariate Cox regression analysis were –ve SM (HR =0.64, 95% CI, 0.50–0.82, P<0.001), tumor size ≥5 cm (HR =2.03; 95% CI, 1.04–3.98, P=0.039), high grade tumor (HR =1.66; 95% CI, 1.04–2.63, P=0.033), higher EBL (HR =1.0001; CI, 1.0001–1.001, P=0.003) and adjuvant treatment (HR =2.16; CI, 1.24–3.76, P=0.007); however, on multivariate analysis, the only independent predictor of DFS was the –ve SM (HR =0.54; 95% CI, 0.29–0.97, P=0.041) (Table 3).
Table 3. DFS predictors among our cohort (n=98).
| Independents variables | Univariate predictors | Multivariate predictors | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age (continuous variable) | 01.01 (0.99–1.02) | 0.260 | |||
| Gender | |||||
| Female (n=51) | Reference | ||||
| Male (n=47) | 1.05 (0.66–1.66) | 0.837 | |||
| SM status | |||||
| Positive SM (n=37) | Reference | Reference | |||
| Negative SM (n=61) | 0.64 (0.50–0.82) | <0.001 | 0.54 (0.29–0.97) | 0.041 | |
| Tumor size | |||||
| <5 cm (n=16) | Reference | Reference | |||
| ≥5 cm (n=82) | 2.03 (1.04–3.98) | 0.039 | 1.45 (0.67–3.18) | 0.349 | |
| Tumor multiplicity | |||||
| Single (n=80) | Reference | ||||
| Multiple (n=18) | 1.18 (0.89–1.57) | 0.256 | |||
| FEV% (continuous variable) | 0.80 (0.40–1.59) | 0.521 | |||
| Lesion laterality | |||||
| Rt side (n=45) | Reference | ||||
| Lt side/midline (n=53) | 0.99 (0.62–1.57) | 0.948 | |||
| Grade | |||||
| Low (NA/G1; n=50) | Reference | Reference | |||
| High (G2/G3; n=48) | 1.66 (1.04–2.63) | 0.033 | 1.56 (0.87–2.78) | 0.135 | |
| Pathology | |||||
| Soft tissue (n=44) | Reference | ||||
| Bony (n=45) | 1.12 (0.69–1.83) | 0.645 | |||
| Others (n=9) | 1.63 (0.77–3.43) | 0.199 | |||
| Complications | |||||
| No (n=81) | Reference | ||||
| Yes (n=17) | 0.94 (0.50–1.80) | 0.860 | |||
| Lung resection | |||||
| No (n=87) | Reference | ||||
| Yes (n=11) | 1.04 (0.50–2.17) | 0.923 | |||
| EBL (continuous variable) | 1.0001 (1.0001–1.001) | 0.003 | 1.0002 (0.9998–1.001) | 0.201 | |
| Adjuvant treatment | |||||
| No (n=31) | Reference | Reference | |||
| Yes (n=67) | 2.16 (1.24–3.76) | 0.007 | 1.30 (0.63–2.68) | 0.475 | |
Note: variables with P value less or equal to 0.10 in univariate analysis were involved in MVA. DFS, disease free survival; SM, safety margin; FEV, forced expiratory volume; EBL, estimated blood loss; NA, not applicable grading as fibromatosis.
The median DFS for +ve SM was 15 (10.5–19.5) vs. 27 (17.9–36.1) months for –ve SM (P=0.001; Figure 3).
Figure 3.
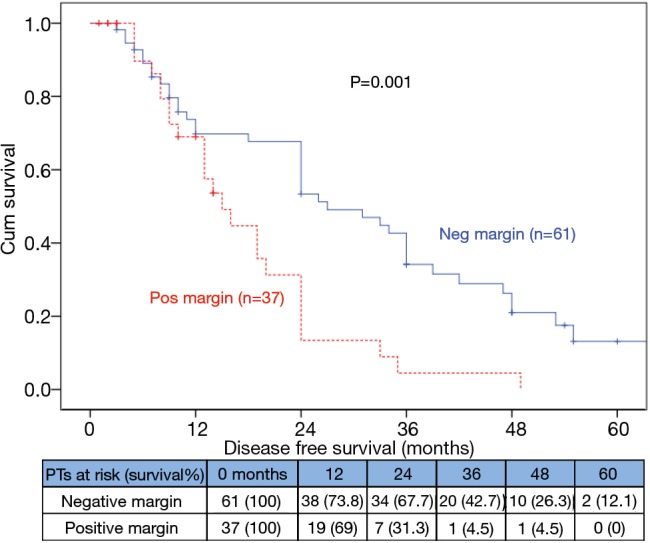
Kaplan Meier survival curves showing DFS among negative and positive surgical margin. DFS, disease free survival.
For Subgroup analysis, fibromatosis/fibrosarcoma had better median DFS of 36 (22.01–50) vs. 24 (15.73–32.27) months in chondrosarcoma (P=0.05, Figure 4), but no difference in OS (P=0.561) (Table 2).
Figure 4.
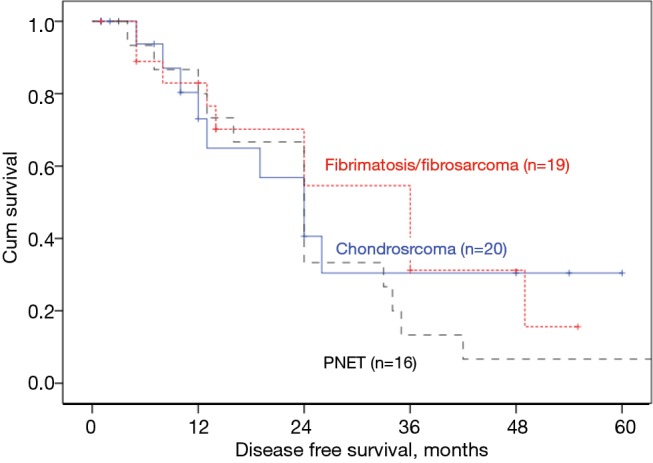
Kaplan Meier survival curves showing DFS among the commonest pathological subtypes in our cohort [the only nearly significant P value (P=0.057) between chondrosarcoma (n=20) and fibromatosis/ fibrosarcoma (n=19)]. DFS, disease free survival. PNET, primitive neuro-ectodermal tumor.
Discussion
Chest wall tumors represent 5–20% of all thoracic malignancies in adult and children respectively. In children, Ewing’s sarcoma is the most common chest wall tumor; while in adults chondrosarcoma predominates (2,15,16).In our study the figure for adult was confirmed and chondrosarcoma was found to be the most predominant pathological subtype.
There were 51 (52%) females among our series that is similar to that reported by Maeda et al. (17), but are slightly different than those reported by Hemmati et al. (18) who reported 41.77% were females, however his series main point was on resection of the chest wall metastasis.
Our cohort’s median age of 39 (IQR =25–52.3) years was younger than most reported series that ranged from 49–64.8 years (17,19-22). The median age in Ewing’s sarcoma was younger [24.5 years (20–30 years)] in comparison to the whole cohort to the prior series (15,23,24).
Pain was the most common symptom in PNET/Ewing’s sarcoma (75%), osteosarcoma (57.1%), while mass was the most common symptom in chondrosarcoma (65%) and fibromatosis (60%). Similar to our results, Anderson and Burt found that the most common symptom for chondrosarcoma was mass, PNET/Ewing’s sarcoma was pain, but they found that the most common symptoms for osteosarcoma were both mass and pain (3).
It is widely accepted that all tumors of the sternum should be considered as malignant until proved otherwise (25,26). In our series, seven cases underwent sternal resection; 2 were chondrosarcoma, 2 rhabdomyosarcomata, 1 adenocarcinoma, 1 metaplastic carcinoma and 1 solitary plasma cell myeloma.
Chest wall reconstruction is essential if there is resection of three ribs or more aiming for adequate stability and water and airtight closure of the chest cavity with acceptable cosmetic appearance. Bony reconstruction should be tailored to the extent of resection. Stabilization of the chest wall obviates the need for prolonged ventilation as patients are able to maintain their pulmonary function postoperatively (6,27). Muscle and musculocutaneous flaps are the tissues of choice to cover the wound, avoid or decrease the risk of infection, obliterate spaces and cover the synthetic mesh (28). In our study, chest wall reconstruction was done by the use of double layer Prolene mesh with or without bone cement that is covered either by rotational pedicle muscle flap (LD or serratus anterior), that were chosen by proximity, knowing the arc of rotation and calculating the area of possible coverage, or covered by the local muscle flap (pectoralis major). In most cases, only a single muscle was transferred (27,29).
CWT resection is associated with a low major morbidity as long as reconstruction was done when indicated (1,8). Among our series, complication rate was 17.3% compared to 11–27% in the previous studies (1,30).
Resection of CWT has an acceptable risk of perioperative mortality from 0% to 7% (1,28,31-34), that was confirmed in our series with a perioperative mortality of 1.02%.
Prior reported series and meta-analysis in different cardiothoracic operations reported adverse survival with higher blood loss and intra-operative blood transfusion. In our series higher intra-operative EBL was associated with adverse DFS in univariate analysis but this was not sustained in the multivariate model (35-37).
Margin positivity (R1) was the most trivial and independent factor impacting survival in multivariate analysis. This runs in parallel to prior reported series in CWTs or other thoracic malignancies (30,38).
Conclusions
In primary malignant CWTs achievement of wide resection margins is of great importance to minimize the local tumor recurrence that will have an adverse impact on long-term survival.
Acknowledgements
Authors would like to thank Eng. Mostafa Rahouma for technical support in finalizing this work.
Ethical Statement: This study was approved by institutional ethics committee/ethics board of National Cancer Institute (NCI)/Cairo University (No. IRB00004025). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Mansour KA, Thourani VH, Losken A, et al. Chest wall resections and reconstruction: a 25-year experience. Ann Thorac Surg 2002;73:1720-5; discussion 1725-6. [DOI] [PubMed]
- 2.Ito T, Suzuki H, Yoshino I. Mini review: surgical management of primary chest wall tumors. Gen Thorac Cardiovasc Surg 2016;64:707-14. 10.1007/s11748-016-0719-z [DOI] [PubMed] [Google Scholar]
- 3.Anderson BO, Burt ME. Chest wall neoplasms and their management. Ann Thorac Surg 1994;58:1774-81. 10.1016/0003-4975(94)91691-8 [DOI] [PubMed] [Google Scholar]
- 4.Marulli G, Duranti L, Cardillo G, et al. Primary chest wall chondrosarcomas: results of surgical resection and analysis of prognostic factors. Eur J Cardiothorac Surg 2014;45:e194-201. 10.1093/ejcts/ezu095 [DOI] [PubMed] [Google Scholar]
- 5.Berthet JP, Wihlm JM, Canaud L, et al. The combination of polytetrafluoroethylene mesh and titanium rib implants: an innovative process for reconstructing large full thickness chest wall defects. Eur J Cardiothorac Surg 2012;42:444-53. 10.1093/ejcts/ezs028 [DOI] [PubMed] [Google Scholar]
- 6.Kamel M, Port J, Altorki NK. Sternal resections: new materials for reconstruction. Curr Surg Rep 2015;3:16 10.1007/s40137-015-0094-1 [DOI] [Google Scholar]
- 7.Bosc R, Lepage C, Hamou C, et al. Management of chest wall reconstruction after resection for cancer: a retrospective study of 22 consecutive patients. Ann Plast Surg 2011;67:263-8. 10.1097/SAP.0b013e3181f9b292 [DOI] [PubMed] [Google Scholar]
- 8.Puvvala S, Subramanyam GM, Suraparaju SS, et al. Primary chest wall neoplasms—resection and reconstruction. Indian J Thorac Cardiovasc Surg 2016;32:184-8. 10.1007/s12055-016-0435-4 [DOI] [Google Scholar]
- 9.Tsukushi S, Nishida Y, Sugiura H, et al. Soft tissue sarcomas of the chest wall. J Thorac Oncol 2009;4:834-7. 10.1097/JTO.0b013e3181a97da3 [DOI] [PubMed] [Google Scholar]
- 10.Kim JY, Hofstetter WL. Tumors of the mediastinum and chest wall. Surg Clin North Am 2010;90:1019-40. 10.1016/j.suc.2010.06.005 [DOI] [PubMed] [Google Scholar]
- 11.Sultan I, Ortiz R, Ferrari A. Soft Tissue Sarcomas. In: Stefan DC, Rodriguez-Galindo C, eds. Pediatric Hematology-Oncology in Countries with Limited Resources. New York: Springer, 2014:303-22. [Google Scholar]
- 12.Altorki NK, Kamel MK, Narula N, et al. Anatomical segmentectomy and wedge resections are associated with comparable outcomes for patients with small ct1n0 non-small cell lung cancer. J Thorac Oncol 2016;11:1984-92. 10.1016/j.jtho.2016.06.031 [DOI] [PubMed] [Google Scholar]
- 13.Krasin MJ, Davidoff AM, Rodriguez-Galindo C, et al. Definitive surgery and multiagent systemic therapy for patients with localized Ewing sarcoma family of tumors: local outcome and prognostic factors. Cancer 2005;104:367-73. 10.1002/cncr.21160 [DOI] [PubMed] [Google Scholar]
- 14.Grier HE, Krailo MD, Tarbell NJ, et al. Addition of ifosfamide and etoposide to standard chemotherapy for Ewing’s sarcoma and primitive neuroectodermal tumor of bone. N Engl J Med 2003;348:694-701. 10.1056/NEJMoa020890 [DOI] [PubMed] [Google Scholar]
- 15.Fathalla AE, Ahmed BE. Askin tumor in Egyptian patients; 5 years experience at the National Cancer Institute, Cairo University. J Cancer Ther 2016;07:216 10.4236/jct.2016.73022 [DOI] [Google Scholar]
- 16.van den Berg H, van Rijn RR, Merks JH. Management of tumors of the chest wall in childhood: a review. J Pediatr Hematol Oncol 2008;30:214-1. 10.1097/MPH.0b013e318162bd54 [DOI] [PubMed] [Google Scholar]
- 17.Maeda S, Yamada T, Watanabe T, et al. Contribution of surgical margin for surgical outcome of the chest wall tumors. Kyobu Geka 2014;67:15-20. [PubMed] [Google Scholar]
- 18.Hemmati SH, Correa AM, Walsh GL, et al. The prognostic factors of chest wall metastasis resection. Eur J Cardiothorac Surg 2011;40:328-33. [DOI] [PubMed] [Google Scholar]
- 19.Tsushima T, Kowatari R, Kimura D, et al. Results of non-rigid prosthetic reconstruction with expanded polytetrafluoro-ethylene (ePTFE) soft tissue patch following chest wall resection for malignant tumors. Kyobu Geka 2014;67:49-53. [PubMed] [Google Scholar]
- 20.Hsu PK, Hsu HS, Lee HC, et al. Management of primary chest wall tumors: 14 years’ clinical experience. J Chin Med Assoc 2006;69:377-82. 10.1016/S1726-4901(09)70276-X [DOI] [PubMed] [Google Scholar]
- 21.Athanassiadi K, Kalavrouziotis G, Rondogianni D, et al. Primary chest wall tumors: early and long-term results of surgical treatment. Eur J Cardiothorac Surg 2001;19:589-93. 10.1016/S1010-7940(01)00655-8 [DOI] [PubMed] [Google Scholar]
- 22.Sabanathan S, Shah R, Mearns AJ. Surgical treatment of primary malignant chest wall tumours. Eur J Cardiothorac Surg 1997;11:1011-6. 10.1016/S1010-7940(97)00090-0 [DOI] [PubMed] [Google Scholar]
- 23.Womer RB, West DC, Krailo MD, et al. Randomized controlled trial of interval-compressed chemotherapy for the treatment of localized Ewing sarcoma: a report from the children’s oncology group. J Clin Oncol 2012;30:4148-54. 10.1200/JCO.2011.41.5703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Randall L, Calvert G, Sparker H, et al. Ewing’s Sarcoma Family of Tumors (ESFT). Liddy Shriver Sarcoma Initiat 2011. Available online: http://pharexmedics.com/wp-content/uploads/2016/01/ewingspdf.pdf
- 25.Martini N, Huvos AG, Burt ME, et al. Predictors of survival in malignant tumors of the sternum. J Thorac Cardiovasc Surg 1996;111:96-105; discussion 105-6. 10.1016/S0022-5223(96)70405-1 [DOI] [PubMed] [Google Scholar]
- 26.Park BJ, Flores RM. Chest wall tumors. In: Shields TW, Locicero J, Reed CE, editors. General Thoracic Surgery. Philadelphia: Lippincott, 2009:669-78. [Google Scholar]
- 27.Khalil HH, Malahias MN, Balasubramanian B, et al. Multidisciplinary Oncoplastic Approach Reduces Infection in Chest Wall Resection and Reconstruction for Malignant Chest Wall Tumors. Plast Reconstr Surg Glob Open 2016;4:e809. 10.1097/GOX.0000000000000751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arnold PG, Pairolero PC. Chest-wall reconstruction: an account of 500 consecutive patients. Plast Reconstr Surg 1996;98:804-10. 10.1097/00006534-199610000-00008 [DOI] [PubMed] [Google Scholar]
- 29.Tukiainen E. Chest wall reconstruction after oncological resections. Scand J Surg 2013;102:9-13. 10.1177/145749691310200103 [DOI] [PubMed] [Google Scholar]
- 30.Abbas AE, Deschamps C, Cassivi SD, et al. Chest-wall desmoid tumors: results of surgical intervention. Ann Thorac Surg 2004;78:1219-23. 10.1016/j.athoracsur.2004.03.015 [DOI] [PubMed] [Google Scholar]
- 31.Mentens Y, Schrijvers D, Van den Brande J, et al. Thoracic wall prosthesis prevents deep invasion by non-small-cell lung cancer. Am J Clin Oncol 2000;23:32-3. 10.1097/00000421-200002000-00008 [DOI] [PubMed] [Google Scholar]
- 32.Nash AG, Tuson JR, Andrews SM, et al. Chest wall reconstruction after resection of recurrent breast tumours. Ann R Coll Surg Engl 1991;73:105-9; discussion 109-10. [PMC free article] [PubMed] [Google Scholar]
- 33.David EA, Marshall MB. Review of Chest Wall Tumors: A Diagnostic, Therapeutic, and Reconstructive Challenge. Semin Plast Surg 2011;25:16-24. 10.1055/s-0031-1275167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Committee for Scientific Affairs , The Japanese Association for Thoracic Surgery, Masuda M, et al. Thoracic and cardiovascular surgery in Japan during 2012: annual report by The Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg 2014;62:734-64. 10.1007/s11748-014-0464-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Engoren MC, Habib RH, Zacharias A, et al. Effect of blood transfusion on long-term survival after cardiac operation. Ann Thorac Surg 2002;74:1180-86. 10.1016/S0003-4975(02)03766-9 [DOI] [PubMed] [Google Scholar]
- 36.Luan H, Ye F, Wu L, et al. Perioperative blood transfusion adversely affects prognosis after resection of lung cancer: a systematic review and a meta-analysis. BMC Surg 2014;14:34. 10.1186/1471-2482-14-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rahouma M, Kamel M, Ghaly G, et al. PS01.45: Intraoperative Blood Loss is an Independent Predictor of Poor Disease Free Survival for Patients Undergoing VATS Lobectomy for Lung Cancer: Topic: Surgery. J Thorac Oncol 2016;11:S297. 10.1016/j.jtho.2016.09.08027969513 [DOI] [Google Scholar]
- 38.Kamel M, Lee PC, Rahouma M, et al. PS01.42: Predictors of Incomplete Esophageal Cancer Resection: Questionable Role of Preoperative Therapy: Topic: Surgery. J Thorac Oncol 2016;11:S295.27969509 [Google Scholar]