Abstract
Background
Patients with pathological stage I (p I) lung adenocarcinoma show variabilities in prognosis even after complete resection. The factors resulting in heterogeneities of prognosis remain controversy. The aim of this study was to identify the risk factors affecting recurrence/metastasis and survival in patients with curatively resected p I lung adenocarcinoma.
Methods
A total of 252 patients with p I lung adenocarcinoma underwent curative resection between January 1st, 2009 to September 30th, 2011 were retrospectively reviewed to analyze the associations of recurrence and survival with the following clinicopathological variables: gender, age, cigarette smoking, family cancer history, tumor size, TNM stage, tumor differentiation, visceral pleural invasion, bronchial involvement, lymphovascular invasion, postoperative adjuvant treatment, pathological subtypes and micropapillary pattern.
Results
Among those 252 patients, 48 had local recurrence or distant metastasis, the rest 204 patients had no relapse until the last follow-up. Cox univariate survival analysis revealed that tumor size (P<0.001), TNM stage [disease-free survival (DFS), P<0.001; overall survival (OS), P=0.004], tumor differentiation (P<0.001), bronchial involvement (P<0.001), lymphovascular invasion (DFS, P=0.021; OS, P=0.001) and micropapillary pattern (DFS, P<0.001; OS, P=0.003) were significantly associated with DFS and OS, while cigarette smoking (P=0.029) and pathological subtypes (P=0.041) were found to be risk factors for DFS either. In multivariate analysis, tumor differentiation (P<0.001) was an independent risk factor for both DFS and OS, TNM stage (P=0.007), bronchial involvement (P=0.004) and micropapillary pattern (P=0.001) only for DFS, while tumor size (P=0.009) and lymphovascular invasion (P=0.010) were found to be independent risk factors only for OS.
Conclusions
Tumor size, TNM stage, tumor differentiation, bronchial involvement, lymphovascular invasion and micropapillary pattern could be considered as risk factors for predicting local recurrence or distant metastasis and survival in curatively resected p I lung adenocarcinoma patients.
Keywords: Lung adenocarcinoma, prognosis, recurrence, metastasis, survival
Introduction
Lung cancer is increasing rapidly in recent years and has become the leading cause of cancer-related death in China (1). Currently, the standard treatment for stage I non-small cell lung cancer (NSCLC) is still surgery, however, 20% of patients with pathological stage I (p I) NSCLC still have local recurrence or distant metastasis (2). Up to now, the risk factors resulting in treatment failure after surgery in those with p I NSCLC have not been well elucidated, and the clinicopathological features placing patients at particularly high risks of tumor recurrence have not been definitely studied. The aim of this study was to identify the risk factors of local recurrence and distant metastasis in patients with curatively resected p I lung adenocarcinoma.
Methods
Study design and patients
From January 1st, 2009 to September 30th, 2011, 265 consecutive patients underwent complete resections for p I lung adenocarcinoma at our hospital, 13 of those lost follow-up and were excluded. Eventually, 252 patients were eligible for analysis, including gender, age, smoking cigarette, family cancer history, tumor size, TNM stage, tumor differentiation, visceral invasion, bronchial involvement, lymphovascular invasion, postoperative adjuvant chemotherapy, pathological subtype and micropapillary pattern. The survival statuses were confirmed through telephone call. The association of the above parameters with recurrence and survival was retrospectively analyzed. This study was approved by the ethical committee of Cancer Hospital, Chinese Academy of Medical Sciences (No. NCC2014ST-07). Last follow-up was conducted in December 7th, 2015. The follow-up interval was 49 to 78 months.
We defined “p I” lung adenocarcinoma cases as those patients who underwent anatomical lobectomy and had p IA to IB adenocarcinoma including stage T1aN0M0, T1bN0M0 and T2aN0M0 and final cancer staging was confirmed by reviewing the official pathological reports according to 7th edition of TNM classification. All patients with pathologically reported N1 or M1 were excluded, regardless of their T stage. The patients receiving preoperative radiation or chemotherapy, or underwent incomplete resections (R1–R2), or died of postoperative complications were also excluded in this study. All patients received precise preoperative evaluation including computed tomographic scans, head magnetic resonance imaging (MRI) and bone scan prior to surgical resection.
The H & E staining slides of all the 252 patients were reviewed again by two experienced pathologists separately and the subtypes were determined according to the 8th American Joint Committee on Cancer (AJCC)/International Union for Cancer Control (UICC) lung cancer staging system. Elastic stainings were added in suspicious visceral invasion cases.
Methods
The demographic features including gender, age, smoking cigarette, family cancer history were reviewed. The correlation of prognosis with pathological factors such as the tumor size, TNM stage, grade of differentiation, invasion of visceral pleura, bronchial involvement, the lymphovascular invasion and pathological subtype as well as micropapillary pattern were analyzed. Micropapillary components were assessed and calculated in percentage, only 0% micropapillary components were considered as micropapillary pattern negative, more than 0% components were considered as micropapillary pattern positive. Surgery-related factor as postoperative adjuvant chemotherapy was also reviewed. DFS period was calculated from the date of surgery to the date of surgical treatment failure (defined as local recurrence or distant metastasis were confirmed by images or pathology of biopsy). OS period was defined as the time from date of surgery to the date of death or the date of last follow-up (2015-12-7).
Statistical analysis
SPSS (V22.0, SPSS) was used for statistical analysis. Chi-square test was used to analyze the relationship between groups. DFS and OS curves were estimated using the Kaplan-Meier method. Significance was assessed using the log rank test. A P value of <0.05 was considered statistically significant. Possible prognostic predictors of DFS and OS were analyzed using Cox univariate and multivariate proportional hazards regression.
Results
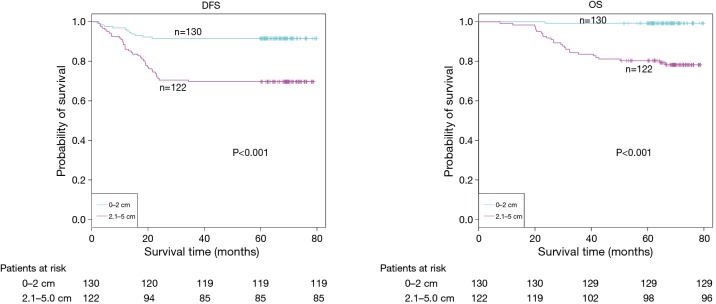
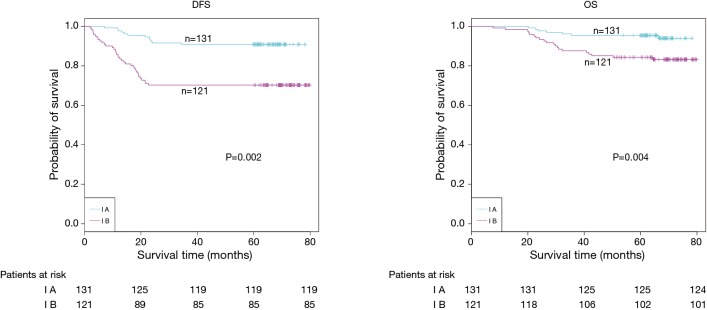
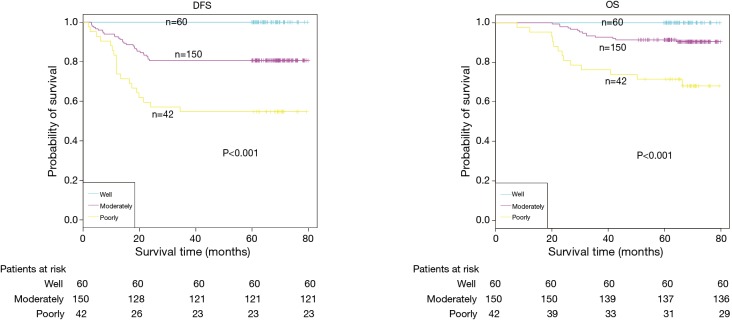
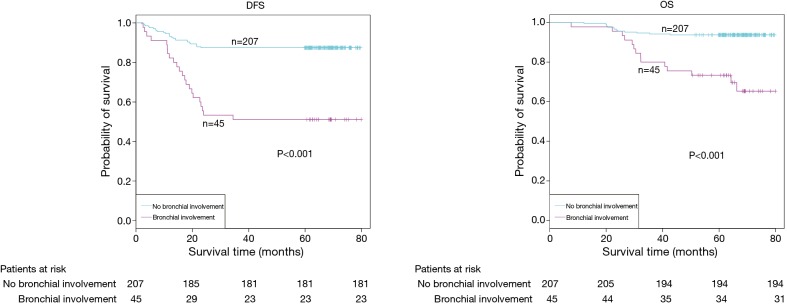
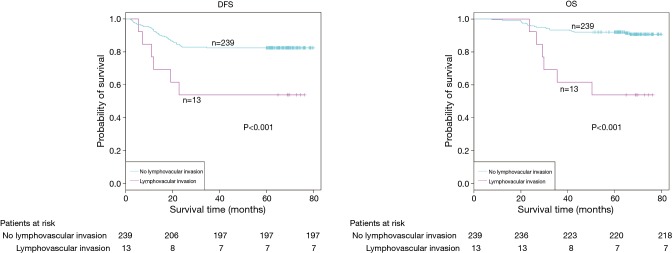
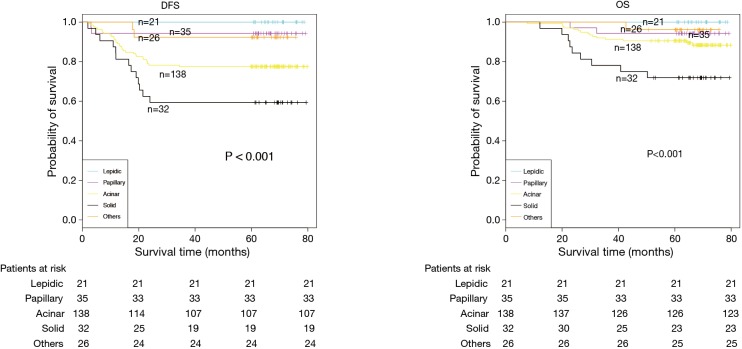
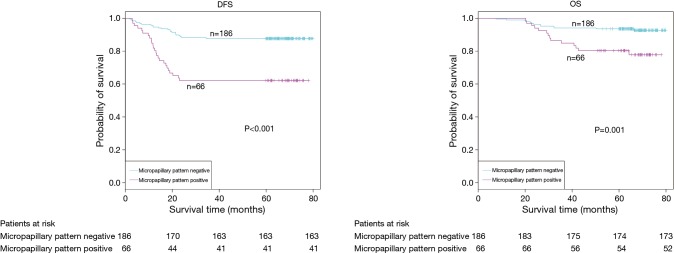
The demographic features and recurrence of the patients (n=252) were listed in Table 1, with 48 recurrence cases and 27 death cases. Recurrence and metastasis were found to be significant differences with smoking cigarette (P=0.026), tumor size (P<0.001), TNM stage (P<0.001), tumor differentiation (P<0.001), bronchial involvement (P<0.001), lymphovascular invasion (P=0.011), pathological subtype (P<0.001) and micropapillary pattern (P<0.001). The 1-, 3- and 5-year DFS and OS of this series were 91.3%, 81.0%, 77.0%, and 99.2%, 91.7%, 81.0% respectively. Kaplan-Meier analysis revealed that tumor size (Figure 1; P<0.001), TNM stage (Figure 2; P=0.002 for DFS, P=0.004 for OS), tumor differentiation (Figure 3; P<0.001), bronchial involvement (Figure 4; P<0.001), lymphovascular invasion (Figure 5; P<0.001), pathological subtype (Figure 6; P<0.001) and micropapillary pattern (Figure 7; P<0.001 for DFS, P=0.001 for OS) were significant factors affecting DFS and OS.
Table 1. Demographic features and recurrence of 252 patients with complete resected p I stage NSCLC.
| Variables | Patient number | Recurrence | 5-year DFS (%) | ||
|---|---|---|---|---|---|
| Yes (%) | No (%) | P value | |||
| Gender | 0.088 | ||||
| Male | 114 | 27 (23.7) | 87 (76.3) | 71.9 | |
| Female | 138 | 21 (15.2) | 117 (84.8) | 77.5 | |
| Age (years) | 0.239 | ||||
| ≤65 | 185 | 32 (17.3) | 153 (82.7) | 75.7 | |
| >65 | 67 | 16 (23.9) | 51 (76.1) | 73.1 | |
| Smoking cigarette | 0.026 | ||||
| smoker | 91 | 24 (26.4) | 67 (73.6) | 68.1 | |
| Non-smoker | 161 | 24 (14.9) | 137 (85.1) | 78.9 | |
| Family cancer history | 0.765 | ||||
| Yes | 64 | 13 (20.3) | 51 (79.7) | 67.2 | |
| No | 188 | 35 (18.6) | 153 (81.4) | 77.7 | |
| Tumor size (cm) | <0.001 | ||||
| ≤2 | 130 | 11 (8.5) | 119 (91.5) | 83.1 | |
| 2.1–5.0 | 122 | 37 (30.3) | 85 (69.7) | 66.4 | |
| TNM stage | <0.001 | ||||
| IA | 131 | 12 (9.2) | 119 (90.8) | 80.9 | |
| IB | 121 | 36 (29.8) | 85 (70.2) | 68.6 | |
| Tumor differentiation | <0.001 | ||||
| Well | 60 | 0 (0) | 60 (100.0) | 86.7 | |
| Moderately | 150 | 29 (19.3) | 121 (80.7) | 76.7 | |
| Poorly | 42 | 19 (45.2) | 23 (54.8) | 52.4 | |
| Visceral pleural invasion | 0.184 | ||||
| Yes | 177 | 38 (21.5) | 139 (78.5) | 73.4 | |
| No | 75 | 10 (13.3) | 65 (86.7) | 78.7 | |
| Bronchial involvement | <0.001 | ||||
| Yes | 45 | 22 (48.9) | 23 (51.1) | 48.9 | |
| No | 207 | 26 (12.6) | 181 (87.4) | 80.7 | |
| Lymphovascular invasion | 0.011 | ||||
| Yes | 13 | 6 (46.2) | 7 (53.8) | 53.8 | |
| No | 239 | 42 (17.6) | 197 (82.4) | 76.2 | |
| Postoperative adjuvant chemotherapy | 0.366 | ||||
| Yes | 101 | 22 (21.8) | 79 (78.2) | 76.2 | |
| No | 151 | 26 (17.2) | 125 (82.8) | 74.2 | |
| Pathological subtype | <0.001 | ||||
| Lepidic | 21 | 0 (0) | 21 (100.0) | 95.2 | |
| Papillary | 35 | 2 (5.7) | 33 (94.3) | 88.6 | |
| Acinar | 138 | 31 (22.5) | 107 (77.5) | 71.7 | |
| Solid | 32 | 13 (40.6) | 19 (59.4) | 59.4 | |
| Others | 26 | 2 (7.7) | 24 (92.3) | 76.9 | |
| Micropapillary pattern | <0.001 | ||||
| Negative | 186 | 23 (12.4) | 163 (87.6) | 80.6 | |
| Positive | 66 | 25 (71.4) | 41 (28.6) | 43.9 | |
p I, pathological stage I; NSCLC, non-small cell lung cancer; DFS, disease-free survival.
Figure 1.
DFS and OS estimated by Kaplan-Meier in patients with tumor ≤2 cm and 2.1–5.0 cm (P<0.001, log-rank test). DFS, disease-free survival; OS, overall survival.
Figure 2.
DFS (P=0.002, log-rank test) and OS (P=0.004, log-rank test) estimated by Kaplan-Meier in stage IA and IB patients. DFS, disease-free survival; OS, overall survival.
Figure 3.
DFS and OS estimated by Kaplan-Meier in patients with different grades of tumor differentiation (P<0.001, log-rank test). DFS, disease-free survival; OS, overall survival.
Figure 4.
DFS and OS estimated by Kaplan-Meier in patients with or without bronchial involvement (P<0.001, log-rank test). DFS, disease-free survival; OS, overall survival.
Figure 5.
DFS and OS estimated by Kaplan-Meier in patients with or without lymphovascular invasion (P<0.001, log-rank test). DFS, disease-free survival; OS, overall survival.
Figure 6.
DFS and OS estimated by Kaplan-Meier in patients with different pathological subtypes (P<0.001, log-rank test). DFS, disease-free survival; OS, overall survival.
Figure 7.
DFS (P<0.001, log-rank test) and OS (P=0.001, log-rank test) estimated by Kaplan-Meier in patients with or without micropapillary pattern. DFS, disease-free survival; OS, overall survival.
Of the 252 patients, 48 patients had local recurrences or distant metastases (Table 2), which were confirmed by repeated images or by pathological/cytological results of biopsies. Six (12.5%) patients had only local recurrences, while the other 42 (87.5%) patients had distant metastases and 9 of those (18.8%) had ≥ two cites of metastases. The common sites of metastases were contralateral lung (18.8%), brain (16.7%) and bone (12.5%).
Table 2. Locations of 48 patients with local recurrence or distant metastasis.
| Recurrence location | Patient number | % |
|---|---|---|
| Local | 6 | 12.5 |
| Recurrence | 2 | 4.2 |
| Regional lymph nodes | 4 | 8.3 |
| Distant | 42 | 87.5 |
| Pleura | 3 | 6.3 |
| Contralateral lung | 9 | 18.8 |
| Distant lymph nodes | 5 | 10.4 |
| Chest wall | 1 | 2.1 |
| Bone | 6 | 12.5 |
| Brain | 8 | 16.7 |
| Adrenal gland | 1 | 2.1 |
| ≥ two lesions | 9 | 18.8 |
Univariate analysis of the factors affecting survival was shown in Table 3. The results revealed that cigarette smoking (P=0.029), tumor size (P<0.001), TNM stage (P<0.001), tumor differentiation (P<0.001), bronchial involvement (P<0.001), lymphovascular invasion (P=0.021), pathological subtype (P=0.041) and micropapillary pattern (P<0.001) were the statistically significant predictors for DFS, while tumor size (P<0.001), TNM stage (P=0.004), tumor differentiation (P<0.001), bronchial involvement (P<0.001), lymphovascular invasion (P=0.001) and micropapillary pattern (P=0.003) were statistically significant predictors for OS.
Table 3. Univariate analysis of the factors affecting survival.
| Variables | DFS | OS | |||
|---|---|---|---|---|---|
| P value | 95% CI | P value | 95% CI | ||
| Gender | 0.085 | 0.34–1.07 | 0.121 | 0.25–1.18 | |
| Age | 0.284 | 0.99–1.05 | 0.161 | 0.99–1.08 | |
| Smoking cigarette | 0.029 | 0.30–0.94 | 0.241 | 0.37–1.27 | |
| Family cancer history | 0.860 | 0.50–1.79 | 0.606 | 0.59–2.45 | |
| Tumor size | <0.001 | 2.03–7.82 | <0.001 | 4.09–222 | |
| TNM stage | <0.001 | 1.98–7.32 | 0.004 | 1.37–7.65 | |
| Tumor differentiation | <0.001 | 2.43–6.33 | <0.001 | 2.51–9.45 | |
| Visceral invasion | 0.091 | 0.28–1.13 | 0.639 | 0.35–1.93 | |
| Bronchial involvement | <0.001 | 0.12–0.38 | <0.001 | 0.09–0.39 | |
| Lymphovascular invasion | 0.021 | 0.13–0.74 | 0.001 | 0.07–0.41 | |
| Postoperative adjuvant chemotherapy | 0.365 | 0.43–1.35 | 0.969 | 0.46–2.12 | |
| Pathological subtype | 0.041 | 1.01–1.74 | 0.100 | 0.94–1.96 | |
| Micropapillary pattern | <0.001 | 2.07–6.45 | 0.003 | 1.52–6.87 | |
DFS, disease-free survival; OS, overall survival; CI, confidence interval.
Tumor size, TNM stage, tumor differentiation, bronchial involvement, lymphovascular invasion and micropapillary pattern were significantly associated with DFS and OS. Multivariate analysis of the 6 factors affecting survival was shown in Table 4. The results showed that TNM stage (P=0.007), tumor differentiation (P<0.001), bronchial involvement (P=0.004) and micropapillary pattern (P=0.001) were found to be statistically significant factors for DFS. While, tumor size (P=0.009), tumor differentiation (P<0.001) and lymphovascular invasion (P=0.010) were statistically significant predictors for OS.
Table 4. Multivariate analysis of the factors affecting survival.
| Variables | DFS | OS | |||
|---|---|---|---|---|---|
| P value | 95% CI | P value | 95% CI | ||
| Tumor size | 0.336 | 0.68–3.12 | 0.009 | 1.93–11.5 | |
| TNM stage | 0.007 | 1.31–5.39 | 0.415 | 0.59–3.56 | |
| Tumor differentiation | <0.001 | 2.34–7.10 | <0.001 | 2.28–10.7 | |
| Bronchial involvement | 0.004 | 0.22–0.75 | 0.178 | 0.26–1.29 | |
| Lymphovascular invasion | 0.091 | 0.20–1.12 | 0.010 | 0.12–0.75 | |
| Micropapillary pattern | 0.001 | 1.49–4.91 | 0.056 | 0.98–4.84 | |
DFS, disease-free survival; OS, overall survival; CI, confidence interval.
Discussion
Although the TNM staging system has been the gold standard parameter used for predicting the survival and determining whether adjuvant treatment should be given or not after curative resection of advanced stage NSCLC for the past several decades. For p I NSCLC, TNM staging seems to be a weak predictor not only for recurrence but also for long term survival (3). However, some other clinicopathological factors were found to be also predictive for prognosis in addition to the TNM staging (4). These clinicopathological risk factors associated with the development of local recurrence or distant metastasis after curatively surgical resection could also be used for pick out the patients at high risks who may need close follow-up and aggressive postoperative adjuvant therapy (5). Although these clinicopathological factors may be helpful, actually, they are not well definitely identified and usually used subjectively in our routine clinical practice (6). Therefore, a retrospective analysis was conducted in order to elucidate the correlation between various clinicopathological factors and outcomes in 252 p I lung adenocarcinoma patients who received curative lung resection. The results of this study indicated that tumor size, TNM stage, tumor differentiation, bronchial involvement, lymphovascular invasion and micropapillary pattern not only significantly correlated with recurrence but also with survival, especially the DFS.
Tumor differentiation was useful in defining the aggressiveness of malignant tumors and was reported to be significantly associated with disease recurrence and short DFS according to the results reported by Choi et al. (7). This study also showed the similar results that poor tumor differentiation significantly affected the DFS and OS.
The presence of lymphovascular invasion was generally regarded as an unfavorable prognostic predictor. Kiankhooy et al. (8) reported that the presence of lymphovascular invasion was a significant risk factor for early recurrence (<2 years) in early-stage lung cancers (T1a to T2b) despite a R0 resection and absence of nodal involvement. Higgins et al. (9) reported that lymphovascular invasion was associated with the presence of regional lymph node (LN) involvement and was strongly associated with increased risk of developing distant metastases and death in adenocarcinoma. Hamanaka et al. (10) pointed that pleural invasion, blood vessel invasion and lymphatic vessel invasion were all independent factors for recurrence. In this study, univariate analysis also showed that lymphovascular invasion was an independent predictor for survival.
In Huang’s meta-analysis, visceral pleural invasion was reported as an independent predictor and associated with increased risk of recurrence and metastases in stage I NSCLC (11). Lakha et al. and Schuchert et al. reported similar results (12,13). But in this study, visceral pleural invasion was not associated with recurrence or survival. Hung’s report showed that visceral pleural invasion did not influence OS and DFS in patients with resected stage I NSCLC with a diameter of 3 cm or less (14). In our data, 213 patients (84.5%) with 3 cm or less tumor size, that might explain our results.
The heterogeneity of stage I lung adenocarcinoma might be correlated with complicated pathological morphology. Lung adenocarcinoma consisted of several pathological subtypes, which contributed to clinical, radiological, pathological and molecular level of heterogeneity (15,16). In 2011, new histopathological classification of lung adenocarcinoma was proposed by IASLC/ATS/ERS and divided into three groups according to the prognosis. The prognosis of the lepidic predominant adenocarcinoma was good, the acinar and papillary predominant adenocarcinoma were moderate, and solid and micropapillary adenocarcinoma were poor (17). The results of this series showed that pathological subtypes likely affected recurrence and metastasis, but no statistically significant difference was revealed in univariate and multivariate analysis, which may be caused by this limited example and it could not deny the prognosis-predictive value of new adenocarcinoma classification system and large enough examples are still needed to demonstrate its significance.
Micropapillary component was reported as one of the risk factors of poor prognosis in most of the articles. Warth et al. (18) analyzed 487 lung adenocarcinoma patients and found that OS differed significantly among five subtypes. The OS, DFS and disease-specific survival (DSS) of solid and micropapillary predominant were much worse than that of other adenocarcinomas. Kamiya et al. (19) reported that components, 383 patients with lung adenocarcinoma were classified as none (0%), focal (<10%), moderate (<50%), or extensive (≥50%) based on the proportion of micropapillary pattern area in the tumors. The 5-year and 10-year OS rates of the micropapillary pattern-positive group were significantly poor than that of micropapillary pattern-negative group, and as the micropapillary pattern proportion increased, the prognosis became worse and worse. Zhang et al. (20) reported similar result as Kamiya et al. reported based of analysis of 886 adenocarcinomas. All the above studies demonstrated that micropapillary pattern was a risk factor for lung adenocarcinoma, but no comparison between early stage and advanced stage lung adenocarcinoma was reported. Up to now, few literatures reported the influence of micropapillary pattern on the prognosis in early lung adenocarcinoma.
The prognosis of the patients with positive micropapillary pattern was compared with that of negative micropapillary pattern in this series, regardless of its proportion. We found that micropapillary pattern was a statistically significant predictor of recurrence, metastasis and prognosis. This was consistent with the results reported in several literatures (20-22). Lee et al. (23) reported that 525 lung adenocarcinoma patients were classified into three subgroups according to the presence and proportion of micropapillary subtype: (I) ≥5% of the micropapillary pattern (n=114); (II) <5% of the micropapillary pattern (n=115); and (III) absence (<1%) of the micropapillary pattern (n=296). He found that even a small proportion of micropapillary pattern (<5%) had a significant prognostic impact on OS. Therefore, the p I lung adenocarcinoma patients with micropapillary pattern might need a closer follow-up and more aggressive adjuvant therapy in order to improve the prognosis.
This was a small cohort retrospective study in a single center and some limitations might be existed due to the flaws made during the operations which were completed by different surgeons and the pathological findings which were reported by different pathologists. Therefore, in order to clarify all above discussed factors that might affecting the recurrence and survival, further research using molecular biomarkers to stratify the subgroups are still needed.
In conclusion, tumor size, TNM stage, tumor differentiation, bronchial involvement, lymphovascular invasion and micropapillary pattern could be considered as risk factors for predicting local recurrence or distant metastasis and survival in curatively resected p I lung adenocarcinoma patients. The new pathological classification system in lung adenocarcinoma had an important clinical significance for prediction of prognosis and adjuvant therapy.
Acknowledgements
None.
Ethical Statement: This study was approved by the ethical committee of Cancer Hospital, Chinese Academy of Medical Sciences (No. NCC2014ST-07).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. 10.3322/caac.21338 [DOI] [PubMed] [Google Scholar]
- 2.Shimada Y, Saji H, Yoshida K, et al. Prognostic factors and the significance of treatment after recurrence in completely resected stage I non-small cell lung cancer. Chest 2013;143:1626-34. 10.1378/chest.12-1717 [DOI] [PubMed] [Google Scholar]
- 3.Fujimoto T, Cassivi SD, Yang P, et al. Completely resected N1 non-small cell lung cancer: factors affecting recurrence and long-term survival. J Thorac Cardiovasc Surg 2006;132:499-506. 10.1016/j.jtcvs.2006.04.019 [DOI] [PubMed] [Google Scholar]
- 4.Koo HK, Jin SM, Lee CH, et al. Factors associated with recurrence in patients with curatively resected stage I-II lung cancer. Lung Cancer 2011;73:222-9. 10.1016/j.lungcan.2010.11.013 [DOI] [PubMed] [Google Scholar]
- 5.Kim IH, Lee IH, Lee JE, et al. Prognostic Impact of Multiple Clinicopathologic Risk Factors and c-MET Overexpression in Patients Who Have Undergone Resection of Stage IB Non-Small-Cell Lung Cancer. Clin Lung Cancer 2016;17:e31-43. 10.1016/j.cllc.2016.01.005 [DOI] [PubMed] [Google Scholar]
- 6.Bunn PA, Jr, Kim ES. Improving the Care of Patients With Stage IB Non-Small-Cell Lung Cancer: Role of Prognostic Signatures and Use of Cell Cycle Progression Biomarkers. Clin Lung Cancer 2015;16:245-51. 10.1016/j.cllc.2015.02.008 [DOI] [PubMed] [Google Scholar]
- 7.Choi PJ, Jeong SS, Yoon SS. Prediction and prognostic factors of post-recurrence survival in recurred patients with early-stage NSCLC who underwent complete resection. J Thorac Dis 2016;8:152-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kiankhooy A, Taylor MD, LaPar DJ, et al. Predictors of early recurrence for node-negative t1 to t2b non-small cell lung cancer. Ann Thorac Surg 2014;98:1175-83. 10.1016/j.athoracsur.2014.05.061 [DOI] [PubMed] [Google Scholar]
- 9.Higgins KA, Chino JP, Ready N, et al. Lymphovascular invasion in non-small-cell lung cancer: implications for staging and adjuvant therapy. J Thorac Oncol 2012;7:1141-7. 10.1097/JTO.0b013e3182519a42 [DOI] [PubMed] [Google Scholar]
- 10.Hamanaka R, Yokose T, Sakuma Y, et al. Prognostic impact of vascular invasion and standardization of its evaluation in stage I non-small cell lung cancer. Diagn Pathol 2015;10:17. 10.1186/s13000-015-0249-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang H, Wang T, Hu B, et al. Visceral pleural invasion remains a size-independent prognostic factor in stage I non-small cell lung cancer. Ann Thorac Surg 2015;99:1130-9. 10.1016/j.athoracsur.2014.11.052 [DOI] [PubMed] [Google Scholar]
- 12.Lakha S, Gomez JE, Flores RM, et al. Prognostic significance of visceral pleural involvement in early-stage lung cancer. Chest 2014;146:1619-26. 10.1378/chest.14-0204 [DOI] [PubMed] [Google Scholar]
- 13.Schuchert MJ, Awais O, Abbas G, et al. Influence of age and IB status after resection of node-negative non-small cell lung cancer. Ann Thorac Surg 2012;93:929-35; discussion 935-6. 10.1016/j.athoracsur.2011.09.047 [DOI] [PubMed] [Google Scholar]
- 14.Hung JJ, Wang CY, Huang MH, et al. Prognostic factors in resected stage I non-small cell lung cancer with a diameter of 3 cm or less: visceral pleural invasion did not influence overall and disease-free survival. J Thorac Cardiovasc Surg 2007;134:638-43. 10.1016/j.jtcvs.2007.04.059 [DOI] [PubMed] [Google Scholar]
- 15.Travis WD, Garg K, Franklin WA, et al. Evolving concepts in the pathology and computed tomography imaging of lung adenocarcinoma and bronchioloalveolar carcinoma. J Clin Oncol 2005;23:3279-87. 10.1200/JCO.2005.15.776 [DOI] [PubMed] [Google Scholar]
- 16.Motoi N, Szoke J, Riely GJ, et al. Lung adenocarcinoma: modification of the 2004 WHO mixed subtype to include the major histologic subtype suggests correlations between papillary and micropapillary adenocarcinoma subtypes, EGFR mutations and gene expression analysis. Am J Surg Pathol 2008;32:810-27. 10.1097/PAS.0b013e31815cb162 [DOI] [PubMed] [Google Scholar]
- 17.Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. 10.1097/JTO.0b013e318206a221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Warth A, Muley T, Meister M, et al. The novel histologic International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification system of lung adenocarcinoma is a stage-independent predictor of survival. J Clin Oncol 2012;30:1438-46. 10.1200/JCO.2011.37.2185 [DOI] [PubMed] [Google Scholar]
- 19.Kamiya K, Hayashi Y, Douguchi J, et al. Histopathological features and prognostic significance of the micropapillary pattern in lung adenocarcinoma. Mod Pathol 2008;21:992-1001. 10.1038/modpathol.2008.79 [DOI] [PubMed] [Google Scholar]
- 20.Zhang J, Liang Z, Gao J, et al. Pulmonary adenocarcinoma with a micropapillary pattern: a clinicopathological, immunophenotypic and molecular analysis. Histopathology 2011;59:1204. 10.1111/j.1365-2559.2011.04050.x [DOI] [PubMed] [Google Scholar]
- 21.Truini A, Santos Pereira P, Cavazza A, et al. Classification of different patterns of pulmonary adenocarcinomas. Expert Rev Respir Med 2015;9:571-86. 10.1586/17476348.2015.1083428 [DOI] [PubMed] [Google Scholar]
- 22.Tsutsumida H, Nomoto M, Goto M, et al. A micropapillary pattern is predictive of a poor prognosis in lung adenocarcinoma, and reduced surfactant apoprotein A expression in the micropapillary pattern is an excellent indicator of a poor prognosis. Mod Pathol 2007;20:638-47. 10.1038/modpathol.3800780 [DOI] [PubMed] [Google Scholar]
- 23.Lee G, Lee HY, Jeong JY, et al. Clinical Impact of Minimal Micropapillary Pattern in Invasive Lung Adenocarcinoma: Prognostic Significance and Survival Outcomes. Am J Surg Pathol 2015;39:660-6. 10.1097/PAS.0000000000000399 [DOI] [PubMed] [Google Scholar]