Abstract
Background
Patients with small pneumothoraces are usually treated with oxygen therapy. However, evidence that oxygen therapy increases resolution rate is based on small populations with secondary spontaneous pneumothorax. Therefore, this study aimed to confirm whether oxygen therapy increases the resolution rate of primary spontaneous pneumothorax (PSP).
Methods
We retrospectively reviewed records of patients with PSP who had undergone outpatient observation (room air group) and those who were admitted for oxygen therapy (O2 group) between March 2005 and February 2016. The initial chest posteroanterior (PA) radiograph was compared with the last chest PA radiograph before the pneumothorax disappeared. The size of the pneumothorax was measured using the Collins’ method.
Results
A total of 175 episodes were identified in 160 patients. Of these, 128 episodes (73.1%) occurred in patients in the O2 group. The mean age was 19.24±4.74 years. The mean initial size of the pneumothorax was smaller in the room air group (23.32%±7.00% vs. 20.26%±6.78%, P=0.011). The resolution rate was higher in the O2 group [(4.27%±1.97%) vs. (2.06%±0.97%)/day, P<0.001]. The initial size of the pneumothorax, time interval between radiographs, and use of oxygen therapy were significantly associated with the resolution rate in multivariate analysis.
Conclusions
Oxygen therapy increases the resolution rate of PSP. However, routine use of oxygen therapy in patients with small pneumothoraces should be considered more carefully. Well-controlled prospective studies are required to confirm the indication of oxygen therapy.
Keywords: Primary pneumothorax, spontaneous pneumothorax, pleural disease, oxygen therapy
Introduction
Oxygen therapy is one of the conservative treatments for spontaneous pneumothorax. It is widely accepted that oxygen therapy increases the resolution rate of spontaneous pneumothorax (1,2). The effects of oxygen therapy on pneumothorax have been demonstrated on theoretical grounds and in experimental studies (3,4). In two clinical studies, the resolution rate increased by three or four times during periods of oxygen therapy (5,6). However, these two studies were based on small populations with secondary pneumothorax. There is a lack of clinical evidence for the effectiveness of oxygen therapy for primary spontaneous pneumothorax (PSP), which usually occurs in young adults or adolescents. Recent studies reported no association between oxygen therapy and the resolution rate of neonatal pneumothorax (7,8). We conducted a retrospective study to evaluate the effect of oxygen therapy on the resolution rate of PSP.
Methods
After institutional review board approval from Uijeongbu St. Mary’s Hospital (UC17RESI0116) had been obtained, a retrospective review was conducted of all consecutive patients with PSP under 40 years of age who had undergone outpatient observation (room air group) or had received oxygen therapy (O2 group) between March 2005 and February 2016. Informed consent was waived according to the institutional review board standards.
The criteria assigned to the group could not be strictly applied because this was a retrospective study. Most of the cases were decided by the physician who examined the chest posteroanterior (PA) radiograph; however, some cases were observed in the outpatient clinic because the patient refused admission.
Physical activity was not strictly limited for patients in the room air group. Patients were advised to avoid strenuous exercise, such as playing soccer or basketball, but were permitted mild activities of everyday life, such as class participation. In the O2 group, continuous oxygen was administered via a nasal cannula at 2–4 L/min, and bed rest was recommended on admission.
Exclusion criteria were secondary pneumothorax, neonatal pneumothorax, previous surgery for ipsilateral pneumothorax, presence of pleural adhesion on chest PA radiograph, comparison not possible (because the initial or last chest PA radiograph was not obtained with the patient in an upright position or the pneumothorax was completely healed on the next chest PA radiograph), increased pneumothorax on follow-up chest PA radiograph, and previous intervention such as aspiration or catheter drainage.
The initial chest PA radiograph was compared with the last chest PA radiograph taken when the pneumothorax was not completely absorbed. The chest radiographs were reviewed independently by two researchers. The size of the pneumothorax was measured using the Collins’ method (9). The formula requires measurements of the interpleural distance at the apex (A) and to the lateral wall at the midpoint of the upper and lower halves of the collapsed lung (B and C) (Figure 1). Estimated pneumothorax size (%) = 4.2+4.7× (A + B + C). The time at which the radiograph was taken was entered in Microsoft Excel 2010 to the minute. The time interval (days) between two radiographs was automatically calculated using Microsoft Excel 2010. The resolution rate was calculated as resolution rate (%/day) = [initial size (%) − last size (%)]/time interval (days). Descriptive data were expressed as frequencies and means ± standard deviation. Frequencies were compared using the χ2 test or Fisher’s exact test for categorical variables; continuous variables were compared using the independent two-sample t-test or Mann-Whitney U test.
Figure 1.
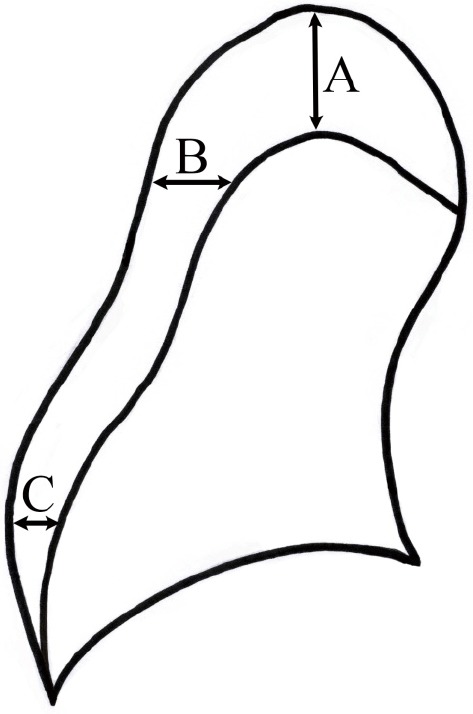
Location of interpleural distance measurements. A, maximum apical interpleural distance; B, interpleural distance at the midpoint of the upper half of the lung; C, interpleural distance at the midpoint of the lower half of the lung.
Multiple linear regression was performed to predict the resolution rate of PSP from sex, age, laterality, initial pneumothorax size, treatment method (room air or oxygen therapy), and time interval between radiographs. Statistical analysis was performed using SPSS version 24.0 (SPSS, Chicago, IL, USA). Differences were considered to be statistically significant when P<0.05.
Results
A total of 189 episodes were identified in 174 patients. Fourteen patients with increased pneumothorax size during treatment were excluded. All these patients were from O2 group. Fifteen patients had two episodes during the study period: six had repeated O2 episodes, two had repeated room air episodes, and seven had one O2 episode and one room air episode. Of the 175 episodes, 128 (73.1%) occurred in the O2 group.
The mean age was 19.24±4.74 years (range, 12–38 years), and the patients were predominantly male. The mean initial size of the pneumothorax was smaller in the room air group (23.32%±7.00% vs. 20.26%±6.78%, P=0.011). The resolution rate was higher in the O2 group [(4.27%±1.97%) vs. (2.06%±0.97%)/day, P<0.001) (Table 1). The initial size of the pneumothorax, the time interval between radiographs, and use of oxygen therapy were significantly associated with the resolution rate of PSP in multivariate analysis (Table 2). However, the use of oxygen was the least influential factor among these three variables (Partial R2 =0.070225).
Table 1. Demographic comparison of study groups.
| Characteristic | Room air group (n=47) | O2 group (n=128) | P value |
|---|---|---|---|
| Age, year | 19.19±4.24 | 19.26±4.93 | 0.740 |
| Male sex, n (%) | 41 (87.2) | 118 (92.2) | 0.375 |
| Right side, n (%) | 17 (36.2) | 42 (32.8) | 0.720 |
| Interval between radiographs (days) | 4.23±2.66 | 2.56±1.15 | <0.001 |
| Initial pneumothorax size (%) | 20.26±6.78 | 23.32±7.00 | 0.011 |
| Last pneumothorax size (%) | 12.11±4.94 | 12.98±5.27 | 0.250 |
| Resolution rate (%/day) | 2.06±0.97 | 4.27±1.97 | <0.001 |
Table 2. Association of resolution rate with variables by multiple linear regression analysis (R2 =0.407).
| Variables | β ± SE | Partial R2 | P value |
|---|---|---|---|
| Treatment (room air =0, O2 =1) | 1.129±0.314 | 0.070225 | <0.001 |
| Initial pneumothorax size (%) | 0.120±0.019 | 0.189225 | <0.001 |
| Interval between radiographs (days) | −0.426±0.078 | 0.148225 | <0.001 |
Discussion
Our study suggested that the resolution rate of PSP was related to initial pneumothorax size, the time interval between radiographs, and oxygen therapy.
The purposes of oxygen therapy for PSP are to maintain oxygen saturation and to increase the resolution rate. It is generally accepted that oxygen therapy increases the resolution rate of pneumothorax (1,2). The theoretical basis is that oxygen therapy reduces the partial pressure of nitrogen in the alveolus compared with the pleural cavity, and a diffusion gradient for nitrogen accelerates resolution (3,10). In addition, some animal studies have shown that increasing the fraction of inspired oxygen accelerates the resolution rate (4,11). However, recent neonatal studies reported no association between oxygen therapy and resolution rate (7,8).
In 1954, Kircher et al. suggested that the resolution rate of pneumothorax was 1.25%/day in room air (12). The authors used a method to indirectly compare lung areas by plotting virtual rectangles in calculating pneumothorax size (Figure 2). Two later clinical studies suggested that the resolution rate was increased three or four times by oxygen therapy (5,6). These two studies had the following limitations. First, the studies had only 6 and 10 participants. Second, most patients were older, with secondary pneumothorax. Third, the more recent study was published in 1983. The measurement method was the same as Kircher’s method, which is rarely used at present (Figure 2).
Figure 2.
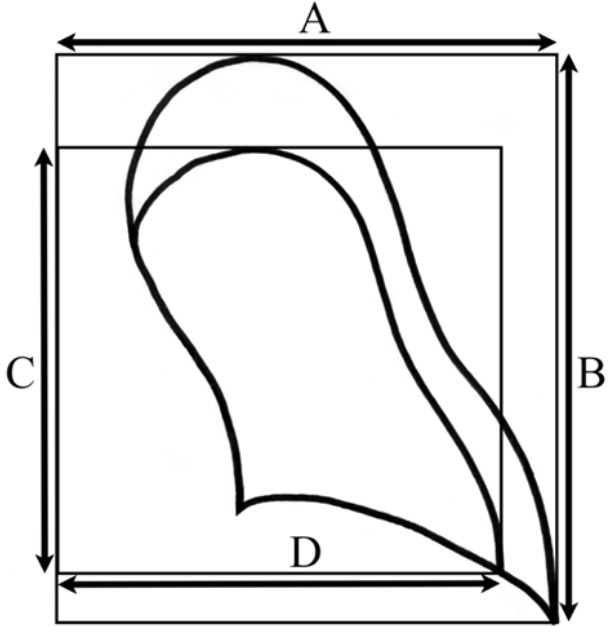
Method for calculating pneumothorax size in previous articles (5,6,12). Pneumothorax size (%) = [(A × B − C × D)/A × B] ×100.
In this study, 14 cases were excluded due to increased pneumothorax size during treatment. Bullae are predisposing factors of pneumothorax; however, the etiology of bullae rupture is unclear. Therefore, cases with an increased pneumothorax size during treatment were regarded as recurrences during treatment and were excluded because they were considered inappropriate for evaluating the treatment effect.
Pneumothorax size is a very important determinant of therapy (13,14). However, the accuracies of the commonly used methods of estimating pneumothorax size, such as the Rhea’s method, light index method, and Collins’ method, have not yet been clearly validated. Our study was conducted using Collins’ method, which is a helical computed tomography (CT)-derived formula using interpleural distance on an erect chest PA radiograph to estimate pneumothorax size (9). This method can predict the three-dimensional volume of a pneumothorax through the two-dimensional area on chest PA radiography.
A recent study using Collins’ method found that the resolution rate in room air was 2.2%/day and was positively correlated with initial pneumothorax size (15). These findings are nearly similar to our results for the room air group (Tables 1,2). The authors suggested the faster resolution rate in the early period of oxygen therapy and the higher transpulmonary pressures in large pneumothoraces as potential mechanisms for the relation between initial pneumothorax size and resolution rate (15). Chadha et al. suggested the resolution rate during oxygen supply was decreased after first 72 hours because less surface area is available for resolution (5). Our study also showed a negative correlation between the resolution rate and the time interval between radiographs. Eventually, the effect of initial size and time interval on resolution rate is explained by a similar hypothesis. In our study, the O2 group showed larger initial pneumothorax size (P=0.11) and shorter interval between radiographs (P<0.001). These two variables have a greater effect on the resolution rate than the use of oxygen (Table 2).
Current studies of the clinical use of hyperoxic management have found various adverse outcomes (16). Oxygen supplementation induces the release of reactive oxygen species, which inhibit the generation of vasodilators such as prostaglandins and nitric oxide. Vasoconstriction caused by hyperoxia decreases perfusion, and regional oxygen delivery is decreased. The tracheobronchial tree is exposed to the highest oxygen partial pressure, and the airway lining fluid has high antioxidant activity. Continued exposure to hyperoxia can lead to chest pain, cough, headache, visual disturbances, and convulsions (17). To avoid these adverse effects, careful administration of oxygen is recommended.
The adverse effects of oxygen therapy in patients with PSP have not been previously investigated. Our study demonstrated the effect of oxygen therapy in the resolution of PSP. The resolution rate was (4.27%±1.97%)/day in the O2 group vs. (2.06%±0.97%)/day in the room air group (Table 1). Although the difference between these rates is statistically significant, its clinical usefulness is uncertain for the following reasons. First, the absolute difference in resolution rate is not great. Second, PSP is also resolved in room air. Third, oxygen therapy has potential adverse effects. Fourth, patients with small pneumothoraces usually maintain adequate oxygenation without oxygen therapy.
Our study has the following limitations. First, the initial pneumothorax size and the interval between radiographs were significantly different between the two groups. These variables are significantly related to the resolution rate. Second, it is difficult to clarify the effect of differences of physical activity during treatment between the two groups. Third, we could not investigate adverse effects associated with oxygen therapy. Well-controlled prospective studies are required to investigate the effectiveness of oxygen therapy for PSP.
Conclusions
The resolution rate of PSP was increased with oxygen supplementation. However, taking into consideration current concerns about adverse outcomes of hyperoxia, the routine use of oxygen therapy in patients with small pneumothoraces should be considered carefully.
Acknowledgements
None.
Footnotes
Conflicts of Interest: Presented at the 25th European Conference on General Thoracic Surgery of the European Society of Thoracic Surgeons, Innsbruck, Austria, 28–31 May 2017.
References
- 1.Currie GP, Alluri R, Christie GL, et al. Pneumothorax: an update. Postgrad Med J 2007;83:461-5. 10.1136/pgmj.2007.056978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sahn SA, Heffner JE. Spontaneous pneumothorax. N Engl J Med 2000;342:868-74. 10.1056/NEJM200003233421207 [DOI] [PubMed] [Google Scholar]
- 3.Henderson Y, Henderson MC. The absorption of gas from any closed space within the body: Particularly in the production of atelectasis and after pneumothorax. Archives of Internal Medicine 1932;49:88-93. 10.1001/archinte.1932.00150080091006 [DOI] [Google Scholar]
- 4.Hill RC, DeCarlo DP, Jr, Hill JF, et al. Resolution of experimental pneumothorax in rabbits by oxygen therapy. Ann Thorac Surg 1995;59:825-7;discussion 7-8. 10.1016/0003-4975(95)00007-8 [DOI] [PubMed] [Google Scholar]
- 5.Chadha TS, Cohn MA. Noninvasive treatment of pneumothorax with oxygen inhalation. Respiration 1983;44:147-52. 10.1159/000194541 [DOI] [PubMed] [Google Scholar]
- 6.Northfield TC. Oxygen therapy for spontaneous pneumothorax. Br Med J 1971;4:86-8. 10.1136/bmj.4.5779.86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clark SD, Saker F, Schneeberger MT, et al. Administration of 100% oxygen does not hasten resolution of symptomatic spontaneous pneumothorax in neonates. J Perinatol 2014;34:528-31. 10.1038/jp.2014.55 [DOI] [PubMed] [Google Scholar]
- 8.Shaireen H, Rabi Y, Metcalfe A, et al. Impact of oxygen concentration on time to resolution of spontaneous pneumothorax in term infants: a population based cohort study. BMC Pediatr 2014;14:208. 10.1186/1471-2431-14-208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collins CD, Lopez A, Mathie A, et al. Quantification of pneumothorax size on chest radiographs using interpleural distances: regression analysis based on volume measurements from helical CT. AJR Am J Roentgenol 1995;165:1127-30. 10.2214/ajr.165.5.7572489 [DOI] [PubMed] [Google Scholar]
- 10.Butler DA, Orlowski JP. Nitrogen washout therapy for pneumothorax. Cleve Clin Q 1983;50:311-5. 10.3949/ccjm.50.3.311 [DOI] [PubMed] [Google Scholar]
- 11.Zierold D, Lee SL, Subramanian S, et al. Supplemental oxygen improves resolution of injury-induced pneumothorax. J Pediatr Surg 2000;35:998-1001. 10.1053/jpsu.2000.6952 [DOI] [PubMed] [Google Scholar]
- 12.Kircher LT, Jr, Swartzel RL. SPontaneous pneumothorax and its treatment. Journal of the American Medical Association 1954;155:24-9. 10.1001/jama.1954.03690190030009 [DOI] [PubMed] [Google Scholar]
- 13.Henry M, Arnold T, Harvey J. BTS guidelines for the management of spontaneous pneumothorax. Thorax 2003;58 Suppl 2:ii39-52. 10.1136/thx.58.suppl_2.ii39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MacDuff A, Arnold A, Harvey J. Management of spontaneous pneumothorax: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65 Suppl 2:ii18-31. 10.1136/thx.2010.136986 [DOI] [PubMed] [Google Scholar]
- 15.Kelly AM, Loy J, Tsang AY, et al. Estimating the rate of re-expansion of spontaneous pneumothorax by a formula derived from computed tomography volumetry studies. Emerg Med J 2006;23:780-2. 10.1136/emj.2006.037143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lumb AB, Walton LJ. Perioperative oxygen toxicity. Anesthesiol Clin 2012;30:591-605. 10.1016/j.anclin.2012.07.009 [DOI] [PubMed] [Google Scholar]
- 17.Iscoe S, Beasley R, Fisher JA. Supplementary oxygen for nonhypoxemic patients: O2 much of a good thing? Crit Care 2011;15:305. 10.1186/cc10229 [DOI] [PMC free article] [PubMed] [Google Scholar]


