Abstract
The imaging and clinical examination (ICE) algorithm used in the Benchmark Evidence from South American Trials: Treatment of Intracranial Pressure (BEST TRIP) randomized controlled trial is the only prospectively investigated clinical protocol for traumatic brain injury management without intracranial pressure (ICP) monitoring. As the default literature standard, it warrants careful evaluation. We present the ICE protocol in detail and analyze the demographics, outcome, treatment intensity, frequency of intervention usage, and related adverse events in the ICE-protocol cohort. The 167 ICE protocol patients were young (median 29 years) with a median Glasgow Coma Scale motor score of 4 but with anisocoria or abnormal pupillary reactivity in 40%. This protocol produced outcomes not significantly different from those randomized to the monitor-based protocol (favorable 6-month extended Glasgow Outcome Score in 39%; 41% mortality rate). Agents commonly employed to treat suspected intracranial hypertension included low-/moderate-dose hypertonic saline (72%) and mannitol (57%), mild hyperventilation (adjusted partial pressure of carbon dioxide 30-35 mm Hg in 73%), and pressors to maintain cerebral perfusion (62%). High-dose hyperosmotics or barbiturates were uncommonly used. Adverse event incidence was low and comparable to the BEST TRIP monitored group. Although this protocol should produce similar/acceptable results under circumstances comparable to those in the trial, influences such as longer pre-hospital times and non-specialist transport personnel, plus an intensive care unit model of aggressive physician-intensive care by small groups of neurotrauma-focused intensivists, which differs from most high-resource models, support caution in expecting the same results in dissimilar settings. Finally, this protocol's ICP-titration approach to suspected intracranial hypertension (vs. crisis management for monitored ICP) warrants further study.
Keywords: : global health, intracranial hypertension, intracranial pressure monitoring, neurocritical care, severe traumatic brain injury
Introduction
The penetrance of intracranial pressure (ICP) monitoring in severe traumatic brain injury (sTBI) management is incomplete in high-income countries1–5 and much less common in low- and middle-income countries, making it likely that the vast majority of sTBI patients are treated without it. Although many algorithms and treatment philosophies are available for managing monitored ICP, there are no such resources available for those caring for sTBI patients in the absence of monitoring. In designing the National Institutes of Health–supported Benchmark Evidence from South American Trials: Treatment of Intracranial Pressure (BEST TRIP) randomized controlled trial,6 we designed an ad hoc protocol, based on the then current practices of our initial principle investigators group amalgamated into an approach acceptable to them. This protocol directs the treatment of suspected intracranial hypertension (SICH) based on serial imaging and clinical examination (the ICE protocol). In the BEST TRIP trial, the pre-specified outcome measure, as well as all other post hoc outcomes analyses, revealed no significant difference between groups. With the publication of that study and those results, the ICE protocol becomes the de facto literature standard for the management of SICH in the setting of sTBI when ICP data is not available. We are therefore describing it here in more detail.
Methods
The parent study was a prospective trial performed at six trauma centers in Latin America wherein patients were randomized to ICP management based on monitored ICP versus treatment directed by the imaging and clinical examination (ICE) protocol.6 Patients entered were those aged ≥13 years with closed-head injury who had a Glasgow Coma Scale (GCS)7 score of 3-8 on presentation to hospital or who deteriorated to that level within 48 h of injury. Patients were excluded for GCS 3 with bilateral fixed and dilated pupils or injury otherwise considered non-survivable. All patients received standard intensive care unit (ICU)–based supportive care, including mechanical ventilation, sedation, and analgesia, and aggressive resuscitation and management of general non-neurologic intensive care issues (Table 1). Protocol for all patients included computed tomography (CT) imaging at baseline, 48 h, and 5–7 days. This study involves those patients randomized to the ICE protocol; therefore, that group and protocol will be detailed here. For details on the ICP-monitored group, please see the initial study.6
Table 1.
Basic Care Protocol for sTBI Patients
| 1. Patient monitoring measures: We strongly suggest using these interventions whenever available and/or possible. |
| a. Place continuous SaO2 and EtCO2 monitors |
| b. Insert indwelling urinary catheter to monitor urine output |
| c. Insert arterial catheter for arterial pressure monitoring |
| d. Insert central venous catheter for infusion of solution and central venous pressure monitoring |
| e. Monitor clinical neurological status each hour |
| i. Pupil size and reactivity |
| ii. GCS |
| f. Obtain brain CT |
| i. To evaluate evolution 48 h after the admission CT |
| ii. To evaluate evolution 5–7 days after the admission CT |
| iii. As needed based on patient clinical condition |
| 2. General management measures |
| a. Place patient on mechanical ventilation, goal SaO2 > 90% and PaO2 > 60 mm Hg |
| b. Use adequate sedation and analgesia |
| i. Acceptable medications include benzodiazepines, opioids, propofol, and low-dose barbiturates |
| 1. Low-dose barbiturate dosing: |
| a. Thiopental 1-2 mg/kg/h IV continuous infusion (∼1.5-3 g/day) |
| c. Maintain head of bed at 30° |
| d. Maintain head and neck aligned and in neutral position |
| e. Actively monitor body temperature and treat hyperthermia |
| f. Hyperthermia defined as central temperature ≥38°C |
| i. Non-pharmaceutical cooling measures |
| 1. Cooling blanket, ice packs |
| ii. Pharmaceutical cooling measures |
| 1. Metamizole sodium |
| g. Early enteral nutritional support |
| i. Initiate within 48 h of injury |
| ii. Give 25 kcal/kg patient weight per day |
| h. Pharmacologic prophylaxis for early post traumatic seizures |
| i. Phenytoin (IV or PO) |
| 1. Loading and maintenance doses as per individual hospital guidelines |
| 2. Continue for 7–28 days |
| i. Gastric bleeding prophylaxis |
| i. Ranitidine or Omeprazole (IV or PO) |
| 1. Administer as per individual hospital guidelines |
| j. Prevent decubitus lesions and treat as indicated |
| k. Deep venous thrombosis prophylaxis |
| l. Frequent tracheal suctioning with sterile technique to prevent pulmonary infections |
| m. Maintain Hb ≥7 mg/dL, use blood transfusions as needed |
| 3. Treatment goals for adequate cerebral perfusion and oxygenation |
| a. Avoid hypotension—SBP >90 mm Hg, MAP >70 mm Hg |
| b. Arterial blood oxygen saturation (SaO2) > 90% or PaO2 > 60 mm Hg |
| 4. CT scans |
| a. First CT: upon hospital admission |
| b. Second CT: 48 h after the first CT |
| c. Third CT: 5–7 days after the first CT |
| d. Additional CT scans as needed based on patient clinical condition |
| 5. Initial therapeutic interventions |
| a. Normal saline solution (0.9% NaCl) to obtain a CVP of 10-12 cm H2O |
| b. Vasopressors when necessary to obtain a SBP >90 mm Hg or MAP >70 mm Hg |
| c. Maintain PaCO2 35-40 mm Hg if CT is normal (correcting for altitude) |
| d. If a space-occupying lesion exists, surgical evacuation is indicated if possible |
sTBI, severe traumatic brain injury; SaO2, oxygen saturation; EtCO2, end-tidal carbon dioxide; GCS, Glasgow Coma Scale; CT, computed tomography; IV, intravenous; PO, orally; Hb, hemoglobin; CVP, central venous pressure; MAP, mean arterial pressure.
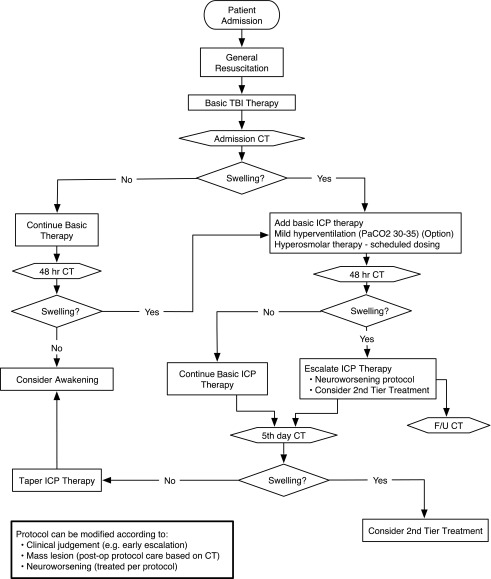
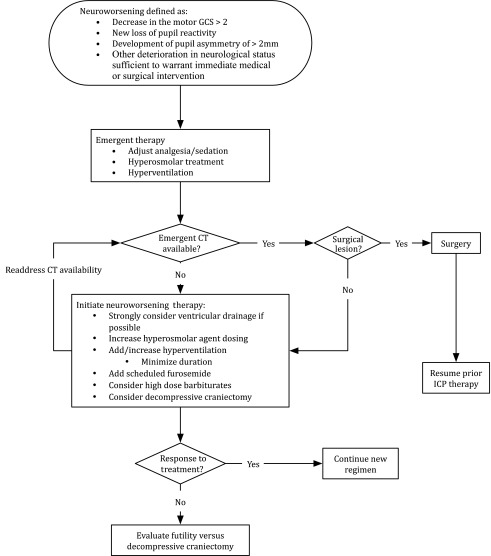
The ICE protocol was founded on pre-trial standard care at the three original hospitals (see above; Table 2 and Fig. 1). Excepting surgical mass lesions, imaging or clinical indications of SICH were treated first with scheduled hyperosmolar therapies, optional mild hyperventilation (partial pressure of carbon dioxide [PaCO2] 30-35 mm Hg), and optional ventricular drainage. Neuroworsening,8 continuing edema, or worsening clinical signs of SICH required treatment escalation, including consideration of high-dose barbiturates or decompressive craniectomy (Fig. 2).
Table 2.
ICE Protocol Treatment of Intracranial Pressure
| 1. Specific therapeutic interventions |
| a. After optimized sedation and analgesia, hyperventilation and hyperosmotic therapy should be started simultaneously if there is evidence of edema on CT, as indicated as following: |
| 1. Compressed peri-mesencephalic cisterns |
| 2. Midline shift |
| 3. Cortical sulcal compression/effacement |
| b. Mild hyperventilation |
| i. Maintain PaCO2 30-35 mm Hg (correcting for altitude) |
| c. Hyperosmolar/hypertonic therapy |
| i. Mannitol should be used first except in the following situations (HHH): |
| a. Arterial hypotension |
| b. Hypovolemia |
| c. Hyponatremia |
| 2. Hyperosmolar (mannitol) therapy guidelines and dosing |
| a. Plasma osmolarity or tonicity should be monitored at least every 12–24 h |
| i. Plasma osmolarity or tonicity should be calculated using the following formulae: |
| 1. Osmolarity = 2 * (Na) + (BUN/ 2.8) + (Glucose/18) |
| 2. Tonicity = 2 * (Na + K) + (Glucose/18) |
| ii. Hyperosmolar (mannitol) therapy should be suspended for plasma osmolarity >320 or tonicity >340 |
| b. Mannitol dosing regimen using 20% mannitol bolus: |
| i. 100 mL (20 g) IV every 3-4 h for the first 3 days, then |
| ii. 80 mL (16 g) IV every 3-4 h on Day 4, then |
| iii. 60 mL (12 g) IV every 3-4 h on Day 5, then |
| iv. 40 mL (8 g) IV every 3-4 h on Day 6 and suspend |
| 3. Hypertonic saline therapy guidelines and dosing |
| a. Hypertonic saline should only be used in cases of HHH as described above |
| b. Plasma osmolarity or tonicity and serum sodium should be monitored at least every 12-24 h |
| i. Plasma osmolarity or tonicity should be calculated using the following formulae: |
| 1. Osmolarity = 2 * (Na) + (BUN/ 2.8) + (Glucose/18) |
| 2. Tonicity = 2 * (Na + K) + (Glucose/18) |
| ii. Hypertonic saline therapy should be suspended for plasma osmolarity >360 or tonicity >380 or serum sodium >160 |
| c. Hypertonic saline dosing regimen using 5% NaCl solution bolus: |
| i. 80 mL normal saline (0.9% NaCl) +20 mL 20% NaCl = 100 mL 5% NaCl solution |
| ii. 100 mL IV every 4-12 h for 6 days then suspend |
| d. High dose IV barbiturates |
| i. Use after hyperventilation and hyperosmolar/hypertonic therapies |
| ii. Should be used if second CT shows evidence of compressed PMC |
| iii. Dosing: thiopental (pentothal) 2.5-4.0 mg/kg/h IV continuous infusion for 3 days (approximately 4-6 g/day) |
| iv. Hypotension must be avoided |
ICE, CT, computed tomography; PaCO2, partial pressure of carbon dioxide; BUN, blood urea nitrogen; IV, intravenous.
FIG. 1.
Evaluation and management algorithm for severe traumatic brain injury patients randomized to the Imaging and Clinical Examination (non-monitored) arm of the Benchmark Evidence from South American Trials: Treatment of Intracranial Pressure trial. Neurological evaluations are carried out frequently throughout the pathway and imaging studies are obtained on admission, at 48 h, and at 5–7 days post-injury at a minimum. The suspicion of intracranial hypertension and appraisal of its course is based on these evaluations. The individual steps involved in the basic care and treatment of intracranial hypertension are contained in Tables 1 and 2.
FIG. 2.
Algorithm for evaluation and management of neurological worsening occurring during the care of severe traumatic brain injury patients in either arm of the Benchmark Evidence from South American Trials: Treatment of Intracranial Pressure trial. In that study, failure to document a change in therapy within 1 h of a neuroworsening event was a protocol violation.
The primary outcome was a composite9 of 21 elements measuring survival, duration and level of consciousness, functional status and orientation measures at 3 months after injury, and functional and neuropsychological measures at 6 months after injury.6 Trained investigators blinded to the intervention assessed outcomes. We also prospectively collected hourly clinical and treatment data in the ICU. We defined brain-specific treatments as those directed at SICH, including hyperosmotics, hyperventilation, pressors, etc., but excluding ventilation, sedation, and analgesia. We defined the duration of therapy as the days from injury until the last brain-specific treatment. We analyzed patients according to whether they survived more than 1 day beyond these treatments (brain-treatment survivors group). We integrated brain-specific treatments by summing the number of treatments delivered per hour over the treatment interval.
Statistical analysis
Hypotheses were tested by blocked Wilcoxon tests,10 with blocking on stratification factors: site, severity (GCS 3-5 or Motor 1-2 if intubated vs. GCS 6-8 or Motor 3-5 if intubated) and age (< 40 vs. ≥40). Odds ratios and confidence intervals were obtained from logistic proportional odds models,11 accounting for the same factors. All odds ratios were calculated so that numbers higher than unity reflect more favorable results with the ICP protocol and numbers lower than unity reflect more favorable results with the ICE protocol.
This study was approved by the University of Washington Institutional Review Board and Federal Wide Assurance–approved ethics committees at all centers. Integra Life Sciences Corporation donated the ICP catheters and provided additional unrestricted support for this project. Integra had no role in study design or conduct, data analysis, or writing of associated manuscripts.
Results
Of the 324 study patients, 167 were randomized to the ICE protocol. Their demographics are displayed in Table 3. They were predominantly young males. The injury mechanism involved motor vehicles in 73% (19% pedestrians), with motorcycles being the most involved vehicle (36%). Thirty-nine percent reached the study institution in transfer from another hospital, which increased the median time to arrival from 1.0 h to 7.5 h versus direct admissions. Although the median GCS motor score at randomization was 4, 40% had abnormal pupillary reactivity or anisocoria at their first ICU examination. The Marshall Classification12 of their first head CT was III in 41% and a mass lesion requiring surgical evacuation was found in 35%. There was ≥5 mm of midline shift in 39% and only 13% had normal basal cisterns.
Table 3.
Demographics and Injury Characteristics
| Category | Imaging/clinical examination |
|---|---|
| N | 167 |
| Age—median (IQR1) | 29 (22, 44) |
| Sex—Male n (%1) | 140 (84%) |
| Circumstances of injury | |
| Car | 23 (14%) |
| Motorcycle | 58 (36%) |
| Bicycle | 7 (4%) |
| Pedestrian | 30 (19%) |
| Fall | 30 (19%) |
| Assault | 11 (7%) |
| Other | 2 (2%) |
| Transferred from another hospital n (%) | 101 (61%) |
| Hours to study hospital—median (IQR) | 2.9 (1.0, 6.5) |
| Direct admits—median (IQR) | 1.0 (0.5, 2.0) |
| Transfers—median (IQR) | 5.0 (2.8, 9.8) |
| Time to first hospital transfers—median (IQR) | 2.5 (1.3, 6.3) |
| Time from injury to randomization—median (IQR) | 15.66 (7.8, 21.4) |
| Randomized due to late deterioration n (%) | |
| Yes | 45 (27%) |
| Randomization Glasgow Coma Scale motor score—median (IQR) | 4 (3, 5) |
| Head Abbreviated Injury Scale—median(IQR) | 5 (4, 5) |
| Injury Severity Score—median (IQR) | 25 (19, 29) |
| 16+ | 147 (89%) |
| First pupil reactivity in ICU abnormal n (%) | |
| Abnormal (at least 1 pupil) | 57 (40%) |
| Marshall Classification first CT2n (%) | |
| Diffuse injury I | 0 (0%) |
| Diffuse injury II | 20 (12%) |
| Diffuse injury III | 68 (41%) |
| Diffuse injury IV | 12 (7%) |
| Evacuated mass lesion | 58 (35%) |
| Not evacuated mass lesion | 7 (4%) |
| Head Abbreviated Injury Scale—median(IQR) | 5 (4, 5) |
| Mesencephalic cisterns compressed or absent first CT n (%) | 143 (87%) |
| Midline shift (≥ 5 mm) first CT n (%) | 64 (39%) |
| CT signs of intracranial hypertension3n (%) | 146 (89%) |
Interquartile ranges = 25th percentile, 75th percentile. Percentages exclude unknown values.
Marshall and colleagues.12
Impression of interpreting physician.
IQR, interquartile range; ICU, intensive care unit; CT, computed tomography.
Patient outcome for the ICE group, as described in the original report,6 is shown in Table 4. The pre-specified outcome was the 21-element Composite Score, with a median score of 53. Secondary analyses of 6-month extended Glasgow Outcome Score (GOS-E) revealed favorable outcome in 39% and a 41% mortality rate.
Table 4.
Clinical Outcomes
| Measure | Imaging/clinical examination |
|---|---|
| N | 167 |
| Followed at 6 months | 153 (92%) |
| Primary outcome: 21-element composite1 median (IQR) | 53 (21, 76) |
| 6-month cumulative mortality | 41% |
| 6-month GOS-E | |
| Death (1) | 67 (44%2) |
| Unfavorable (2-4) | 26 (17%) |
| Favorable (5-8) | 60 (39%) |
Composite is average percentile over the 21 elements. Range: 0 to 100, higher is better.
Higher than estimated cumulative mortality above because participants who were lost to follow-up are excluded from the 6-month GOS-E but included as censored observations for the cumulative mortality.
IQR, interquartile range; GOS-E, extended Glasgow Outcome Score.
As shown in Table 5, although there was no difference between the ICP and ICE groups in terms of total length of ICU stay, the ICE group had significantly more (41%) ICU days involving brain-specific treatment. This was supported by post hoc comparison of the total number of brain-specific treatments, which demonstrated a significantly greater (81%) number of interventions for the ICE group. There were no differences between groups in terms of serious adverse events, the frequency of neurosurgical procedures, or the incidence of neurological worsening.
Table 5.
Comparison of ICE and ICP Protocol Variables
| All randomized cases | ||||
|---|---|---|---|---|
| Measure | ICP | Imaging/clinical examination | p value | Proportional odds ratio |
| Protocol-specified comparisons | ||||
| ICU length of stay (days) median (IQR) | 12 (6, 17) | 9 (6, 16) | 0.25 | 0.81 (0.55, 1.18) |
| ICU length of stay with brain-specific treatment4 (days) median (IQR) | 3.4 (1.1, 7.0) | 4.8 (2.3, 7.4) | 0.002 | 1.87 (1.28, 2.75) |
| Post hoc comparisons | ||||
| Integrated brain-specific treatment intensity5 median (IQR) | 69 (13, 181) | 125 (45, 233) | <0.001 | 2.36 (1.60, 3.47) |
| Neuroworsening events after randomization | ||||
| Yes | 35 (22%) | 44 (27%) | 0.44 | 1.29 (0.74, 2.25) |
| Neurosurgical procedures | ||||
| Epidural/subdural | 51 (33%) | 61 (37%) | 0.48 | 1.19 (0.75, 1.89) |
| Contusions/intracerebral | 15 (10%) | 21 (13%) | 0.48 | 1.40 (0.68, 2.88) |
| Craniectomy | 44 (28%) | 49 (30%) | 0.81 | 1.04 (0.63, 1.69) |
| Craniectomy alone | 9 (6%) | 9 (5%) | 1.00 | 0.93 (0.35, 2.42) |
| Craniectomy with other NP | 35 (22%) | 40 (24%) | 0.79 | 1.07 (0.63, 1.80) |
| Any neurosurgery | 69 (44%) | 81 (49%) | 0.44 | 1.20 (0.77, 1.87) |
| Any serious adverse event | 70 (45%) | 76 (46%) | 0.91 | |
| Infections | 13 (8%) | 10 (6%) | 0.52 | |
| Nervous system excluding infections | 19 (12%) | 29 (17%) | 0.21 | |
| Respiratory system excluding infections | 9 (6%) | 8 (5%) | 0.81 | |
| Cardiovascular system | 17 (11%) | 13 (8%) | 0.44 | |
| Death (unspecified cause) | 12 (8%) | 12 (7%) | 1.00 | |
| Other | 2 (1%) | 2 (1%) | 1.00 | |
| Barbiturates | 38 (24%) | 22 (13%) | 0.02 | 0.46 (0.25, 0.83) |
Adapted with permission from Chestnut and colleagues.
All tests of significance exclude unknown values. The p values are from Blocked Wilcoxon10 tests block on stratification factors.
Proportional odds ratio reported with 95% confidence interval. A value >1 indicates a better disposition for the ICP group.
Defined as the time between the first and last use of a brain-specific treatment (i.e., excluding ventilation, sedation, or analgesia).
Number of different intracranial hypertension treatments per hour, summed over the duration, and counting high dose as double.
ICE, imaging and clinical examination; ICP, intracranial pressure; ICU, intensive care unit; IQR, interquartile range; NP, neurosurgical procedure.
Examination of the frequency of individual interventions is shown in Table 6. Most notable is the rarity of use of high-dose hyperosmotic agents and the 62% frequency of pressor administration for presumed cerebral perfusion pressure (CPP) deficits. Hyperventilation also was commonly employed (73%), with 16% of patients being ventilated to PaCO2 values below 30 mm Hg. Interpretation of these numbers should be tempered by the lack of ready availability of co-oximeters at these hospitals, which frequently limited blood gas analyses to once or twice a day, hindering precise PaCO2 titration. The 13% rate of high-dose barbiturate use was significantly lower than that for the ICP monitored group (38/157, 24%; Table 5).
Table 6.
Treatments Received under the ICE Protocol
| Treatment | Imaging/clinical examination |
|---|---|
| N | 167 |
| Treatments for intracranial hypertension | |
| Mechanical ventilation1 | 100% (128.5) |
| Sedation1 | 99% (105.7) |
| Analgesia1 | 99% (109.8) |
| Paralytics1 | 5% (3.9) |
| Mannitol (any dose)1 | 57% (20.8) |
| Mannitol (high dose)1 | 5% (3.3) |
| Hypertonic saline (any dose)1 | 72% (21.3) |
| Hypertonic saline (high dose)1 | 3% (1.6) |
| CSF drain1 | 2% (1.3) |
| Furosemide1 | 8% (14.5) |
| Pressors1 | 62% (95.1) |
| High dose barbiturates1 | 13% (90.5) |
| Hyperventilation (any dose)1 | 73% (84.0) |
| Hyperventilation to PaCO2 < 30 mm Hg1 | 16% (10.4) |
Cells report the proportion of subjects who had each intracranial hypertension treatment, and the average number of hours per subject (among those who had the treatment).
ICE, imaging and clinical examination; CSF, cerebrospinal fluid; PaCO2, partial pressure of carbon dioxide.
Adverse events associated with application of the ICE protocol under these conditions are listed in Table 7. Comparison to the ICP protocol found no significant differences in occurrence of any serious adverse events between cohorts and the only significant adverse event distribution was a greater occurrence of decubitus ulcers in the ICP group (19/157 [19%] vs. 8/167 [8%]; p = 0.03). The high incidence of respiratory complications and non-neurological complications in the pre-specified analyses represent the broad definitions of these categories. As shown in the post hoc analyses section of Table 7, the specific incidences of pneumonia, acute respiratory distress syndrome (ARDS), and respiratory failure are acceptably low. The category of non-neurological complications included cardiac arrest, acute lung injury, ARDS, sepsis, septic shock, coagulopathy, nosocomial pneumonia, community-acquired pneumonia, wound infection, decubitus ulcers, pulmonary thromboembolism, deep vein thrombosis, acute renal failure, urinary infection, gastrointestinal hemorrhage, hyponatremia (< 135 meq/L), hypernatremia (> 145 meq/L), other water and ionic disorders, and a miscellaneous category.
Table 7.
Complications Associated with the ICE Protocol
| Event | ICE/clinical examination |
|---|---|
| Protocol-specified comparisons (all events) | |
| Respiratory complications | 108 (65%) |
| Sepsis | 12 (7%) |
| Decubitus ulcers | 8 (5%) |
| Non-neurological complications | 147 (88%) |
| Post hoc analyses (non–ICP-related serious adverse events) | 76 (46%) |
| Infections | 10 (6%) |
| Pneumonia | 4 (2%) |
| Sepsis | 4 (2%) |
| Nervous system | 2 (1%) |
| Nervous system (excluding infections) | 29 (17%) |
| Respiratory system (excluding infections) | 8 (5%) |
| ARDS | 3 (2%) |
| Respiratory failure | 5 (3%) |
| Cardiovascular system | 13 (8%) |
| Shock | 5 (3%) |
| Cardiac arrest | 7 (4%) |
| Urinary system | 2 (1%) |
| Gastrointestinal system | 2 (1%) |
| Metabolism | 1 (1%) |
| Skin and skeletal muscle | 0 (0%) |
| Death (unspecified cause) | 12 (7%) |
| Hematological | 0 (0%) |
| Other | 2 (1%) |
ICE, imaging and clinical examination; ICP, intracranial pressure; ARDS, acute respiratory distress syndrome.
Discussion
In response to many inquiries following the publication of the BEST TRIP trial,6 our purpose here is to present the ICE protocol in detail, including the management algorithms for general patient care, treatment of SICH, and response to neurological worsening. We have included the distribution of treatments delivered and the adverse events encountered consequent to the use of this management scheme.
As the only published algorithm for the management of sTBI without ICP monitoring, the ICE protocol can be viewed as the default de facto literature standard for the management of SICH when ICP data is not available. In light of its apparently satisfactory performance in the setting of the BEST TRIP randomized trial wherein it was contrasted with ICP monitor-based care, the tendency may therefore exist to simply adopt this algorithm when managing patients without ICP monitoring. As a caveat, it is important to recognize that there are several conditions that should be respected when considering employing this algorithm. Although we do not feel that these qualifications invalidate the utility of this management approach, they do support its situational modification or tempering outcome expectations.
The first of these caveats involves the conditions under which the randomized control trial was carried out. The realities surrounding the early management of sTBI in Latin America required us to widen the window of inclusion for sTBI to 12 h following trauma. This delay may be significant since most of us believe that earlier treatment may confer greater likelihood of benefit. In addition, only 41% of patients were transported to the hospital in an ambulance in the BEST TRIP trial and even in the setting of specialist transport, very little was routinely done towards resuscitation. Unfortunately, our data are insufficient to accurately describe or control for vital signs or therapeutic interventions during the pre-hospital period. We suggest that in combination with the longer transport times, there exists a potentially substantial period of uncertainty the nature and magnitude of which likely differs greatly from the situation in highly resourced medical communities. Caution is strongly suggested in assuming that the study cohort in the BEST TRIP trial generalizes directly to the incoming sTBI population in high-income countries in that this may influence the efficacy of the ICE protocol under situations different from those in the study.
Another consideration that may be germane to achieving the same outcomes as in the BEST TRIP trial is the practice patterns for ICU management that exist in Latin America. Our study patients were managed by intensivists with specific interest in neurointensive care in small ICUs where the involvement of nursing in assessment and intervention is much less developed than in most high-income countries. As such, the physicians practice a hands-on brand of intensive care medicine where all serial examinations are personally done and most treatments are directly supervised by a small group of intensivists who are directly responsible for all management decisions. Therefore, it must be considered that the enforced continuity existing under these conditions may have a role in the sensitivity of the management system to neurologic changes in patients, particularly in patients lacking physiologic monitors. It is notable that the rate of neurological worsening in the ICE group did not differ from that in the ICP group, despite the lack of a quantitative monitor.6 Such considerations suggest that the employment of the ICE protocol must be accompanied by a high degree of physician-involved close observation and do not allow that a non-aggressive stance would achieve the same results. The strong correlation in the literature between improved recovery and “aggressive” management,13–17 often benchmarked by the use of ICP monitoring, further stresses that the decision to manage sTBI patients without monitoring as part of a “less-aggressive” management philosophy is inconsistent with the ICE approach.
This study shows what might be expected if the ICE protocol is applied under similar conditions. The high average severity of injury is reflected in the admission CT evidence of frequent Marshall categories of DI III and surgical mass lesions, accompanied by the majority (87%) having abnormal mesencephalic cisterns and more than one-third having a midline shift of ≥5 mm. As noted in Table 3, the managing intensivist felt that CT evidence of SICH existed in 89% of patients. In addition, 40% of patients had abnormal pupillary reactivity or anisocoria on their ICU admission examination. Although mortality was high (44%), 39% achieved a favorable GOS-E at 6 months. Despite the young age of this population, we believe that these outcomes reflect the efficacy of the ICE protocol, as applied by highly involved neurotrauma intensivists, in mitigating the detrimental effects of severe traumatic brain injury (including intracranial hypertension), despite the lack of ICP monitoring.
The lack of real-time quantitative monitoring is reflected in the type and frequency of treatments delivered. The ICE protocol was based on scheduled administration of tapered (mannitol) or fixed-dose (hypertonic saline) hyperosmotic agents, with ancillary use of other treatments such as mild hyperventilation or furosemide. The absence of quantitative ICP information with high temporal resolution appears to have eliminated (or obscured) the indications for high-dose hyperosmotic administration, as well as the addition of paralytics, cerebrospinal fluid drainage, or high-dose barbiturates to the treatment regimen. The frequency of use of any-dose mannitol or hypertonic saline is consistent with the scheduled-dose regimen.
Administering treatments for non-confirmed SICH allows possible overtreatment or treatment for a mistaken diagnosis (e.g., neuroworsening due to seizures). Although the ICE protocol was safe and effective as tested, it also was associated with significantly longer brain-specific ICU treatment and twice as many individual interventions. One major question related to these issues is whether these differences reflect inefficiency or are integral to the success of the approach. If the former holds, it is possible that modification of the protocol's imaging or examination components could improve the efficiency by introducing mechanisms to shorten the treatment duration. The timing of the follow-up CT scans for the trial was somewhat arbitrary, and based on balancing the need for repeated imaging against the cost and availability of obtaining such studies. Outside of the specific situation of neuroworsening, there were no guidelines as to how the evolution of the clinical examination should influence the treatment regimen. Finally, although composed around the current clinical practices of our site investigators who routinely treated sTBI patients without monitoring, the duration and dosing patterns for the hyperosmotics is not evidence based and is amenable to modification. Our current prospective structured comparative effectiveness study (NIH NS080648) will hopefully address some of these issues but, at its current stage of development, the ICE protocol should be recognized as a simple practice outline and not a comprehensive treatment approach.
One potentially important aspect incidental to the ICE protocol that differs fundamentally from a monitor-based approach is the underlying treatment philosophy. Management based on monitored ICP tends to intersperse variable periods of simple observation under conditions of sub-threshold ICP with much more intense periodic reactions focused towards correcting intracranial hypertension when such obtains. When effective, such “crisis management” often drives the ICP to a relatively low level, where it either may remain or the process may repeat itself. Interventions are minimal when ICP is below a certain threshold but become relatively intense and often polyvalent when the threshold is exceeded. In contrast, the ICE protocol management philosophy is more of a “tranquility approach.” Under this philosophy, once it is suspected that ICP elevation may play a role in determining a patient's outcome, treatment is initiated that follows a fixed schedule that is not directly dependent on instantaneous ICP values. Lacking monitoring, there are no indications for crisis interventions outside of neuroworsening on exam. Smith and colleagues fortuitously investigated such a paradigm in their randomized study of the role of ICP monitoring in determining mannitol administration.18 They monitored ICP in all patients, but ignored the ICP values in the half of patients randomized to scheduled-dose mannitol. Although the power of this study was limited by its small size (N = 80), there was no difference in outcome between the two groups. The mean ICP was significantly (5.5 mm Hg) lower in the empirically treated group (p = 0.048). Similar to the BEST TRIP trial, although not the intention of the study, such a “tranquility approach” was associated with apparently acceptable efficacy.
Is it possible that the efficacy of the ICE protocol is related to the underlying treatment philosophy? Smith and colleagues felt that their results may have reflected improved ICP control (and, possibly, improved mean intracranial compliance) in the empirically treated group.18 An alternative, however, is that the “crisis response” to intermittent ICP elevations is less effective (or even toxic) with respect to a titration approach, particularly in the absence of a currently accepted “dose” of intracranial hypertension.19,20 Whether a tranquility approach triggered by documented intracranial hypertension might be preferable in some patients with elevated values of monitored ICP is worthy of consideration. Basing the initiation of such a protocol on documented intracranial hypertension (rather than suspected risk) would avoid the 19% of patients in the trial of Smith and colleagues18 and 31% in the BEST TRIP trial who received ICP-based treatment in the absence of traditional intracranial hypertension.
Although the ICE protocol is the only published algorithm for managing severe TBI patients in the absence of ICP monitoring, we feel that there are sufficient areas of uncertainty related to this treatment approach to prevent its being recommended as a true management standard. It does strongly support the idea that aggressive, diligent treatment of intracranial hypertension, suspected or monitored, is likely a critical component of successful TBI management; in other words, the aggressiveness of management should not diminish in the absence of ICP monitoring. It is likely that this protocol could be modified toward increased efficiency and this is the topic of our ongoing, prospective research. The efficacy of the ICE protocol also suggests that the philosophy underlying management of established intracranial hypertension may be a fruitful area for further investigation.
The authors wish to note that this report does not represent an endorsement of this protocol or of managing sTBI in the absence of ICP monitoring.21 We simply wish to present what information is available to guide management when monitored ICP is not available, since the evidence suggests that the absence of ICP monitoring does not preclude obtaining satisfactory recovery through aggressive management without such monitoring.
Author Disclosure Statement
No competing financial interests exist.
Integra LifeSciences donated the ICP monitoring catheters and monitors and provided additional unrestricted support for the parent study. Integra had no role in study design or conduct, data analysis, or the writing of any manuscripts.
References
- 1.Ghajar J., Hariri R.J., Narayan R.K., Iacono L.A., Firlik K., and Patterson R.H. (1995). Survey of critical care management of comatose, head-injured patients in the United States. Crit. Care Med. 23, 560–567 [DOI] [PubMed] [Google Scholar]
- 2.Hesdorffer D.C. and Ghajar J. (2007). Marked improvement in adherence to traumatic brain injury guidelines in United States trauma centers. J. Trauma 63, 841–847 [DOI] [PubMed] [Google Scholar]
- 3.Myburgh J.A., Cooper D.J., Finfer S.R., Venkatesh B., Jones D., Higgins A., Bishop N., and Higlett T. (2008). Epidemiology and 12-month outcomes from traumatic brain injury in Australia and New Zealand. J. Trauma 64, 854–862 [DOI] [PubMed] [Google Scholar]
- 4.Sahjpaul R. and Girotti M. (2000). Intracranial pressure monitoring in severe traumatic brain injury–results of a Canadian survey. Can. J. Neurol. Sci. 27, 143–147 [PubMed] [Google Scholar]
- 5.Stocchetti N., Penny K.I., Dearden M., Braakman R., Cohadon F., Iannotti F., Lapierre F., Karimi A., Maas A., Jr., Murray G.D., Ohman J., Persson L., Servadei F., Teasdale G.M., Trojanowski T., and Unterberg A. (2001). Intensive care management of head-injured patients in Europe: a survey from the European brain injury consortium. Intensive Care Med. 27, 400–406 [DOI] [PubMed] [Google Scholar]
- 6.Chesnut R.M., Temkin N., Carney N., Dikmen S., Rondina C., Videtta W., Petroni G., Lujan S., Pridgeon J., Barber J., Machamer J., Chaddock K., Celix J.M., Cherner M., and Hendrix T, (2012). A trial of intracranial-pressure monitoring in traumatic brain injury. N. Engl. J. Med. 367, 2471–2481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teasdale G. and Jennett B. (1974). Assessment of coma and impaired consciousness: a practical scale. Lancet 2, 81–84 [DOI] [PubMed] [Google Scholar]
- 8.Morris G.F., Juul N., Marshall S.B., Benedict B., and Marshall L.F. (1998). Neurological deterioration as a potential alternative endpoint in human clinical trials of experimental pharmacological agents for treatment of severe traumatic brain injuries. Executive Committee of the International Selfotel Trial. Neurosurgery 43 1369–1372 [PubMed] [Google Scholar]
- 9.O'Brien P.C. (1984). Procedures for comparing samples with multiple end points. Biometrics 40, 1079–1087 [PubMed] [Google Scholar]
- 10.van Elteren P. (1960). On the combination of independent two sample tests of Wilcoxon. Bull. Inst. Int. Stat. 37, 351–361 [Google Scholar]
- 11.McCullagh P. (1980). Regression models for ordinal data. J. R Stat. Soc. Series B Stat. Methodol. 42, 109–142 [Google Scholar]
- 12.Marshall L.F., Bowers-Marshall S., Klauber M.R., van Berkum-Clark M., Eisenberg H.M., Jane J.A., Luerssen T.G., Marmarou A., and Foulkes M.A. (1991). A new classification of head injury based on computerized tomography. J. Neurosurg. 75, S14–S20 [Google Scholar]
- 13.Arabi Y.M., Haddad S., Tamim H.M., Al-Dawood A., Al-Qahtani S., Ferayan A., Al-Abdulmughni I., Al-Oweis J., and Rugaan A. (2010). Mortality reduction after implementing a clinical practice guidelines-based management protocol for severe traumatic brain injury. J. Crit. Care 25, 190–195 [DOI] [PubMed] [Google Scholar]
- 14.Bulger E.M., Nathens A.B., Rivara F.P., Moore M., MacKenzie E.J., and Jurkovich GJ; Brain Trauma Foundation. (2002). Management of severe head injury: institutional variations in care and effect on outcome. Crit. Care Med. 30, 1870–1876 [DOI] [PubMed] [Google Scholar]
- 15.Mauritz W., Steltzer H., Bauer P., Dolanski-Aghamanoukjan L., and Metnitz P. (2008). Monitoring of intracranial pressure in patients with severe traumatic brain injury: an Austrian prospective multicenter study. Intensive Care Med. 34, 1208–1215 [DOI] [PubMed] [Google Scholar]
- 16.Stein S.C., Georgoff P., Meghan S., Mirza K.L., and El Falaky O.M. (2010). Relationship of aggressive monitoring and treatment to improved outcomes in severe traumatic brain injury. J. Neurosurg. 112, 1105–1112 [DOI] [PubMed] [Google Scholar]
- 17.Young J.S., Blow O., Turrentine F., Claridge J.A., and Schulman A. (2003). Is there an upper limit of intracranial pressure in patients with severe head injury if cerebral perfusion pressure is maintained? Neurosurgical focus 15, E2. [DOI] [PubMed] [Google Scholar]
- 18.Smith H.P., Kelly D.L., Jr., McWhorter J.M., Armstrong D., Johnson R., Transou C., and Howard G. (1986). Comparison of mannitol regimens in patients with severe head injury undergoing intracranial monitoring. J. Neurosurg. 65, 820–824 [DOI] [PubMed] [Google Scholar]
- 19.Lazaridis C., Desantis S.M., Smielewski P., Menon D.K., Hutchinson P., Pickard J.D., and Czosnyka M. (2014). Patient-specific thresholds of intracranial pressure in severe traumatic brain injury. J. Neurosurg. 120, 893–900 [DOI] [PubMed] [Google Scholar]
- 20.Vik A., Nag T., Fredriksli O.A., Skandsen T., Moen K.G., Schirmer-Mikalsen K., and Manley G.T. (2008). Relationship of “dose” of intracranial hypertension to outcome in severe traumatic brain injury. J. Neurosurg. 109, 678–684 [DOI] [PubMed] [Google Scholar]
- 21.Chesnut R.M., Bleck T.P., Citerio G., Classen J., Cooper D.J., Coplin W.M., Diringer M.N., Grande P.O., Hemphill J.C., 3rd, Hutchinson P.J., Le Roux P., Mayer S.A., Menon D.K., Myburgh J.A., Okonkwo D.O., Robertson C.S., Sahuquillo J., Stocchetti N., Sung G., Temkin N., Vespa P.M., Videtta W., and Yonas H. (2015). A consensus-based interpretation of the benchmark evidence from South American Trials: Treatment of Intracranial Pressure Trial. J. Neurotrauma 32, 1722–1724 [DOI] [PubMed] [Google Scholar]