Abstract
This study compared cerebrospinal fluid (CSF) levels of microtubule-associated protein 2 (MAP-2) from adult patients with severe traumatic brain injury (TBI) with uninjured controls over 10 days, and examined the relationship between MAP-2 concentrations and acute clinical and radiologic measures of injury severity along with mortality at 2 weeks and over 6 months. This prospective study, conducted at two Level 1 trauma centers, enrolled adults with severe TBI (Glasgow Coma Scale [GCS] score ≤8) requiring a ventriculostomy, as well as controls. Ventricular CSF was sampled from each patient at 6, 12, 24, 48, 72, 96, 120, 144, 168, 192, 216, and 240 h following TBI and analyzed via enzyme-linked immunosorbent assay for MAP-2 (ng/mL). Injury severity was assessed by the GCS score, Marshall Classification on computed tomography (CT), Rotterdam CT score, and mortality. There were 151 patients enrolled—130 TBI and 21 control patients. MAP-2 was detectable within 6 h of injury and was significantly elevated compared with controls (p < 0.001) at each time-point. MAP-2 was highest within 72 h of injury and decreased gradually over 10 days. The area under the receiver operating characteristic curve for deciphering TBI versus controls at the earliest time-point CSF was obtained was 0.96 (95% CI 0.93–0.99) and for the maximal 24-h level was 0.98 (95% CI 0.97–1.00). The area under the curve for initial MAP-2 levels predicting 2-week mortality was 0.80 at 6 h, 0.81 at 12 h, 0.75 at 18 h, 0.75 at 24 h, and 0.80 at 48 h. Those with Diffuse Injury III-IV had much higher initial (p = 0.033) and maximal (p = 0.003) MAP-2 levels than those with Diffuse Injury I-II. There was a graded increase in the overall levels and peaks of MAP-2 as the degree of diffuse injury increased within the first 120 h post-injury. These data suggest that early levels of MAP-2 reflect severity of diffuse brain injury and predict 2-week mortality in TBI patients. These findings have implications for counseling families and improving clinical decision making early after injury and guiding multidisciplinary care. Further studies are needed to validate these findings in a larger sample.
Keywords: : biomarkers, diffuse axonal injury, microtubule-associated protein, mortality, neuronal injury, outcome, severe traumatic brain injury, traumatic brain injury
Introduction
At least 2 million individuals sustain a traumatic brain injury (TBI) annually in the United States.1 A small proportion are severe but these injuries result in permanent damage that produce lifelong impairment. Early tools to detect and prognosticate these severe injuries, such as biofluid biomarkers, are needed.2,3 Several advances have been made in the detection of brain injury biomarkers and over the last several years, with a number of markers proving to be good indicators of brain injury magnitude.4–8 Together with clinical assessment, the quantitative evaluation of biomarkers measured from biofluids could assist in determining the type, severity, and features of injury including anatomical and cellular pathology of the injury.
A relatively unexplored neurobiomarker in human TBI is microtubule-associated protein 2 (MAP-2). Microtubule-associated proteins are components of the neuron microtubule system and act as important modulators of microtubule organization and function. MAP-2 is among the most abundant proteins in the brain and is localized mainly in neuronal cell bodies and dendrites and is vulnerable to proteolytic degradation following TBI.9,10 MAP-2 loss has been documented following ischemic damage11,12 and excitotoxic lesioning.13 Moreover, there is evidence of loss of MAP-2 immunoreactivity following TBI in animal models.10,14,15 In a study by Posmantur and colleagues, MAP-2 immunoreactivity loss was a prominent feature at TBI sites but the loss also extended beyond the focal areas of damage.15 Dramatic alterations in MAP-2 dendritic immunoreactivity were observed within and beyond the contusion sites, both ipsilateral and contralateral to the injury. Areas of decreased immunoreactivity were associated with neuronal death.
In a recent clinical TBI study, seven distinct biomarkers were measured in severe TBI patients and compared with validated clinical models to predict 6-month outcome. The biomarker that consistently improved prognostic performance in this large cohort was MAP-2, suggesting that early cerebrospinal fluid (CSF) levels of MAP-2 in combination with clinical data provide enhanced prognostic capabilities for mortality at 6 months in patients with severe TBI.7
This study examined the time course of MAP-2 in CSF after a severe TBI, compared with control patients over 10 days and assessed its relationship to important clinical and radiologic measures of injury severity and outcome after injury, including Glasgow Coma Scale (GCS) score, Rotterdam CT (computed tomography) score, Marshall Classification, and early and late mortality.
Methods
Design and population
This prospective controlled cohort study enrolled a convenience sample adult patients with severe TBI from March 2007 to August 2011 presenting to two Level 1 trauma centers: Shands Hospital at University of Florida in Gainesville Florida and Ben Taub General Hospital (Baylor College of Medicine) in Houston, Texas. Patients met inclusion criteria if they were ≥18 years old with a non-penetrating head injury and had a GCS score <8 requiring the placement of an intraventricular catheter (IVC). Patients were excluded if they had a history of pre-existing end-stage organ disease or severe psychiatric illness. All patients had a CT scan done as part of their routine evaluation per hospital protocol.
Biomarker sampling and analysis
CSF samples were obtained at 6, 12, 24, 48, 72, 96, 120, 144, 168, 192, 216, and 240 h following TBI by collection from the IVC reservoir that had been emptied 1 h prior to collection. Samples were then stored on ice for up to 12 h before being centrifuged and frozen at −80°C as 1 mL serum aliquots for future analysis at Banyan Biomarkers Inc. (Alachua, FL). Samples were measured using a standard MAP-2 sandwich enzyme-linked immunosorbent assay (ELISA) protocol reported in a previous study.7 MAP-2 sandwich ELISA was performed using 10 uL CSF for quantitative determination. Mouse MAb anti-MAP2A/2B (clone M13, Zymed #13-1500) was used as capture antibody (5 μg/well) to coat the plate. Biofluid samples (10 μL CSF or recombinant antigen as GST-fusion protein with residue 1078-1551 of MAP-2 at 0.10 – 6.67 ng/mL) were added with diluent (100 uL total) to microtiter plate wells. After 2-h incubation and washing, HRP-labeled mouse monoclonal anti-MAP-2 (clone AP20; BD Bioscience; #552320) antibody was added. After washing, plates were developed with substrate solution Ultra-TMB ELISA (Pierce# 34028), stopped with acidic solution and read at 450 nm with a spectrophotometer (Molecular Device SpectraMax 190). The interassay and intra-assay CV were <15% within the assay dynamic range. The upper limit of detection was 22.320 ng/mL, the lower limit of detection (LLoD) 0.054 ng/mL and the imputed concentration for values lower than the LLoD was 0.027 ng/mL, which was half of lowest level of the LLoD.
Outcome measures
We compared CSF levels of MAP-2 in control and TBI patients at each of the specified time-points over 10 days post-injury. The relationship between level of MAP-2 and injury severity acutely was assessed using the GCS score, the initial CT findings using the Rotterdam CT score and the Marshall classification along with early and late mortality.
Control subjects had a normal mental status at the time of enrollment and had no evidence of acute brain injury or hemodynamic instability (n = 21) and they required CSF drainage for other medical conditions such as for routine anesthetic or surgical management (e.g., endovascular aortic aneurysm stent repair, selected orthopedic procedures) performed via lumbar puncture, or for chronic hydrocephalus performed via shunt tapping. The GCS score 16,17 was assessed immediately post-resuscitation (measured after ventriculostomy placement) and within 24 h post-injury. The initial CT scan performed in the emergency department for each patient was reviewed by a single board-certified neuroradiologist blinded to the patient's clinical examination and outcome. The principal scoring system used in the CT interpretation was the Rotterdam CT score.18 The Rotterdam CT score was developed for prognostic purposes in TBI to determine the risk for mortality. It is based on CT findings of basal cistern compression, midline shift, presence of an epidural hematoma, and the presence of either intraventricular blood and/or traumatic subarachnoid hemorrhage. The secondary CT scoring system used the Marshall classification.19 This classification identifies six groups of patients with TBI based on morphological abnormalities (Diffuse Injury I-IV and mass lesions).
Because patients were unconscious upon eligibility determination, a 24-h waiver of consent was granted by the institutional review board. If informed consent could not be obtained from a patient's legally authorized representative within 24 h, samples were discarded and the patient withdrawn from the study. The study was approved by the respective institutional review boards of the University of Florida and Baylor College of Medicine, as well as the University of Houston Committee for the Protection of Human Subjects.
Statistical analysis
Data were analyzed using descriptive statistics using means, standard deviations, medians, and interquartile ranges. Data were assessed for distribution and variance before any statistical analyses were performed. Statistical comparisons of biomarker levels between various groups (post-resuscitation GCS score, Rotterdam CT score, Marshall Classification, mortality, and Glasgow Outcome Score) were performed using the Mann-Whitney U and Kruskal-Wallis tests. A receiver operating characteristic (ROC) curve was constructed to explore the diagnostic ability of the biomarker to distinguish between uninjured controls and TBI patients and also to predict mortality at 2 weeks. Significance was set at p ≤ 0.05.
Results
There were at total 151 patients enrolled: 130 were TBI patients (47 from Houston and 83 from Gainesville) and 21 were control patients. Characteristics of the TBI patients are described in Table 1. Control patients were a mean age of 73 (standard deviation [SD] 8; range 56–85), 68% were male, and 5% were Asian, 5% Black, 5% Hispanic, and 85% were white. CSF MAP-2 biomarker concentrations were available in 130 patients, 109 TBI patients and 21 controls. Characteristics of these 109 TBI patients are in Table 1.
Table 1.
Patient Characteristics
| TBI patients n = 130 | TBI patients with MAP-2 available n = 109 | |
|---|---|---|
| Mean age (years) | 38 (SD 15) | 38 (SD 15) |
| (range) | (18–83) | (18–83) |
| Gender (male) | 102 (79%) | 88 (81%) |
| Race | ||
| Asian | 1 (< 1%) | 1 (1%) |
| Black | 19 (15%) | 17 (16%) |
| Hispanic | 23 (18%) | 18 (17%) |
| White | 85 (65%) | 71 (65%) |
| Other/unknown | 2 (2%) | 2 (2%) |
| Hospital length of stay | 31 (SD 27) | 31 (SD 24) |
| Rotterdam CT score& | ||
| 1 | 1 (1%) | 1 (1%) |
| 2 | 29 (22%) | 25 (23%) |
| 3 | 43 (33%) | 36 (33%) |
| 4 | 28 (22%) | 25 (23%) |
| 5 | 25 (19%) | 18 (17%) |
| 6 | 4 (3%) | 4 (4%) |
| Marshall Classification | ||
| Diffuse Injury I | 2 (2%) | 2 (2%) |
| Diffuse Injury II | 48 (37%) | 41 (38%) |
| Diffuse Injury III | 32 (24%) | 26 (24%) |
| Diffuse Injury IV | 6 (5%) | 6 (6%) |
| Evacuated Mass Lesion | 25 (19%) | 19 (17%) |
| Non-evacuated Mass Lesion | 17 (13%) | 15 (14%) |
| Dichotomized post-resuscitation GCS Score | ||
| GCS 3–5 | 62 (48%) | 51 (47%) |
| GCS 6–8 | 68 (52%) | 58 (53%) |
| Post-resuscitation GCS Motor Score | ||
| 1 | 45 (35%) | 37 (34%) |
| 2 | 10 (8%) | 8 (7%) |
| 3 | 8 (6%) | 7 (6%) |
| 4 | 15 (12%) | 12 (11%) |
| 5 | 47 (36%) | 41 (38%) |
| 6 | 5 (4%) | 4 (4%) |
| Pupils | ||
| Both reactive | 86 (66%) | 74 (68%) |
| One reactive | 12 (9%) | 10 (9%) |
| None reactive | 32 (25%) | 25 (23%) |
| Pre-hospital hypoxia | 52 (41%) | 44 (41%) |
| Pre-hospital hypotension | 13 (10%) | 9 (8%) |
| Mortality at 6 months | 35 | 30 |
Percentages are rounded and may not add up to 100%.
The Rotterdam CT (computed tomography) score was developed for prognostic purposes in traumatic brain injury (TBI) to determine the risk for mortality. It is based on CT findings of basal cistern compression, midline shift, presence of an epidural hematoma, and the presence of either intraventricular blood and/or traumatic subarachnoid hemorrhage.
SD, standard deviation; GCS, Glasgow Coma Scale.
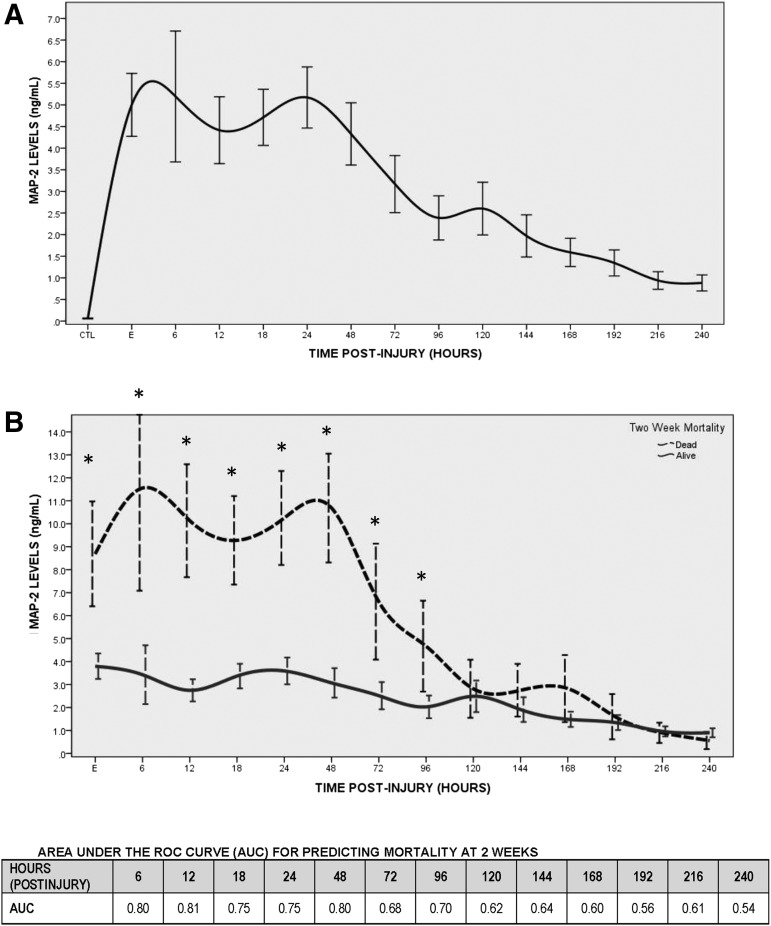
The temporal profile of MAP-2 within 10 days post-injury is shown in Figure 1A. MAP-2 was detectable at the earliest time-point measured in the CSF (i.e., within 6 h of injury) and was significantly elevated, compared with controls (p < 0.001), at each time-point. MAP-2 was highest within 72 h of injury and decreased gradually over 10 days. Overall levels of MAP-2 in TBI patients was mean = 3.32 (± SD 5.3) and median = 1.13 (interquartile range [IQR] 0.31–3.89), compared with mean = 0.06 (± SD 0.05) and median = 0.03 (IQR 0.03–0.08) in controls (p = 0.005). The area under the ROC curve for deciphering TBI versus controls at the earliest time-point CSF was obtained (i.e., initial levels within 24 h) was 0.96 (95% CI 0.93–0.99; p < 0.001). At a level of 0.25 ng/mL, initial MAP-2 levels would yield a sensitivity of 89% (81–94) and a specificity of 100% (81–100). The area under the curve (AUC) for the maximal level within 24 h was 0.98 (95% CI 0.97–1.00) and at a level of 0.25 ng/mL sensitivity would be 95% (89–98) and a specificity 100% (81–100).
FIG. 1.
(A) Temporal profile of microtubule-associated protein 2 (MAP-2) in severe traumatic brain injury patients measured over 10 days versus uninjured control patients. MAP-2 was detectable at the earliest time-point measured in the cerebrospinal fluid (CSF; i.e., within 6 h of injury) and was significantly elevated compared to controls (p < 0.001) at each time-point. Lines represent means ± standard error. The number of available samples at each time-point was 21 controls, 69 at enrollment, 23 at 6 h, 57 at 12 h, 82 at 18 h, 89 at 24 h, 87 at 48 h, 74 at 72 h, 66 at 96 h, 58 at 120 h, 53 at 144 h, 50 at 168 h, 48 at 192 h, 49 at 216 h, and 41 at 240 h. (B) Temporal profile of MAP-2 is compared in survivors versus non-survivors at 2 weeks. CSF levels are significantly higher in non-survivors compared to survivors for the first 48 h after injury (p < 0.001). Listed below the figure are the areas under the curve for predicting mortality at 2 weeks with MAP-2 at each time-point collected post-injury.
The temporal profile of MAP-2 is compared in survivors versus non-survivors at 2 weeks in Figure 1B. Levels were significantly higher in non-survivors, compared with survivors at each time-point for the first 96 h after injury (p < 0.05), with significance falling at 120 h. The AUC for initial MAP-2 levels predicting early mortality at 1 weeks with initial MAP-2 levels was 0.73 (95% CI 0.60–0.85) and for maximal levels it was 0.77 (95% CI 0.66–0.88). Additionally, AUCs for predicting early mortality at each individual time-point post-injury is shown in Figure 1B, with the earliest individual time-points demonstrating the highest AUC's with 0.80 (95% CI 0.59–1.00) at 6 h, 0.81 (95%CI 0.67–0.94) at 12 h, 0.75 (95%CI 0.62–0.88) at 18 h, 0.75 (95% CI 0.61–0.89) at 24 h, and 0.80 (95% CI 0.67–0.92) at 48 h. For predicting 2-week mortality, a cutoff level of 1.0 ng/mL7 for MAP-2 (earliest concentration within 24 h) would yield a sensitivity of 92% (72–99) and a specificity of 33% (24–45). At a level of 1.0 ng/mL the sensitivity and specificity for MAP-2 (maximal concentration within 24 h) would be 100% (83–100) and 24% (15–34), respectively.
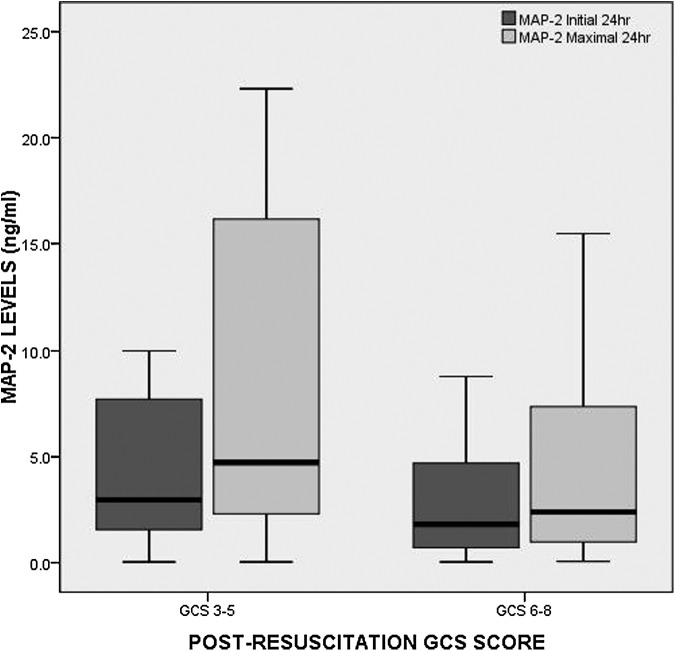
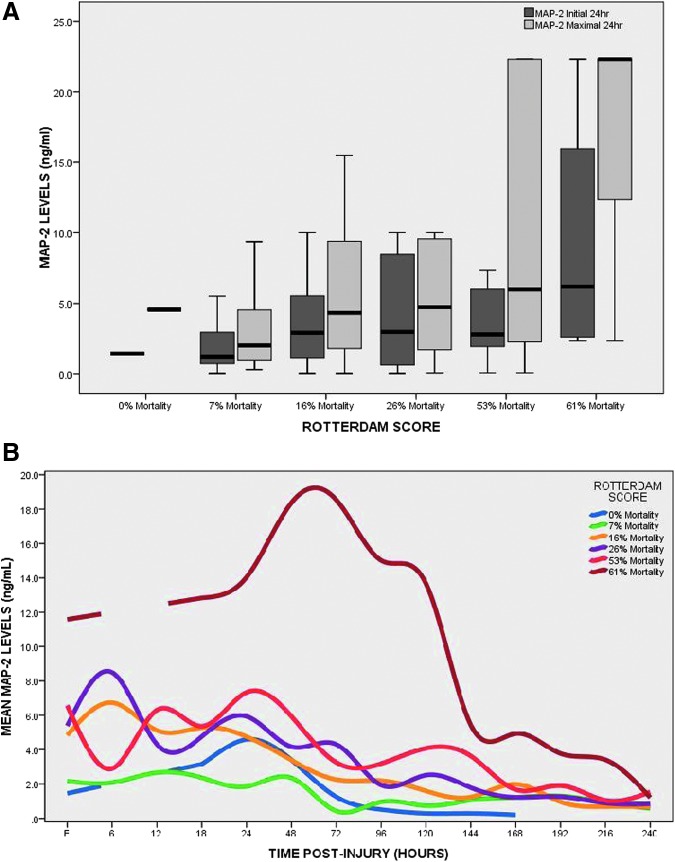
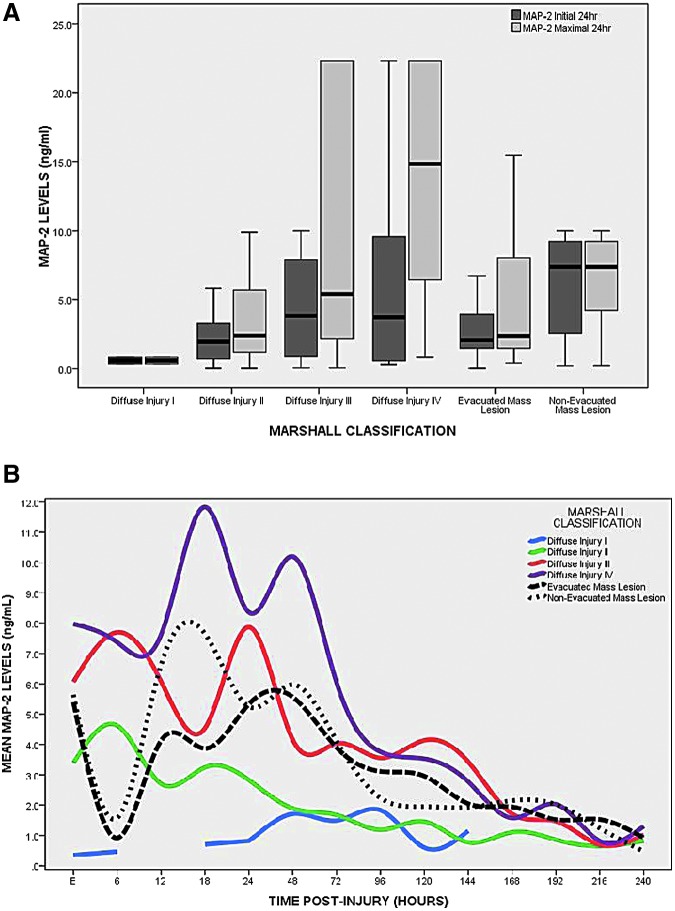
Patients with a post-resuscitation GCS score of 3–5 had significantly higher initial (obtained after ventriculostomy; p = 0.040) and maximal 24-h levels (p = 0.024) of MAP-2, compared with patients with a GCS score of 6–8 (Fig. 2). Median levels of MAP-2 were plotted in each of the categories of the Rotterdam CT score, representing increasing risk categories of mortality (Fig. 3A). Initial (p = 0.125) and maximal 24 h (p = 0.064) concentrations of MAP-2 increased with risk of mortality but did not reach statistical significance. The pattern of biomarker release in the different risk categories of the Rotterdam CT score is displayed in Figure 3B. The levels of MAP-2 increase dramatically once the risk of death is over 63%. In Figure 4A, the median levels of MAP-2 are plotted in each of the categories of the of the Marshall classification. Overall, there were significant differences between the groups for both initial MAP-2 levels (p = 0.052) and maximal 24-h levels (p = 0.008). Levels were highest among patients with diffuse injury, compared with those with mass lesions. Among the diffuse injury group, those with Diffuse Injury III-IV had much higher initial (p = 0.033) and maximal (p = 0.003) MAP-2 levels than those with Diffuse Injury I-II. There was a graded increase in the overall levels and peaks of MAP-2 as the degree of diffuse injury increased (Fig. 4B). This was mostly seen within the first 120 h post-injury. Also, MAP-2 levels appeared more elevated in non-evacuated mass lesions, compared with evacuated mass lesions.
FIG. 2.
Boxplot of initial microtubule-associated protein 2 (MAP-2) levels within 24 h versus dichotomized post-resuscitation Glasgow Coma Scale (GCS) score. Patients with a post-resuscitation GCS score of 3–5 had significantly higher initial cerebrospinal fluid (obtained after ventriculostomy; p = 0.040) and maximal 24-h levels (p = 0.024) of MAP-2, compared with patients with a GCS 6–8. Boxplots represent medians with interquartile ranges.
FIG. 3.
(A) Boxplot of levels microtubule-associated protein 2 (MAP-2) levels versus Rotterdam CT (computed tomography) score. Concentrations of MAP-2 in CSF increased with risk of mortality but did not reach statistical significance. Boxplots represent medians with interquartile ranges. (B) Pattern of biomarker release over 10 days versus Rotterdam CT score categories. The pattern of biomarker release in the different risk categories of the Rotterdam CT score is displayed. The levels of MAP-2 increase dramatically once the risk of death is over 63%. Lines represent mean values of MAP-2 over time.
FIG. 4.
(A) Boxplot of levels microtubule-associated protein 2 (MAP-2) levels versus Marshall Classification. Overall, there were significant differences between the groups for both initial MAP-2 levels (p = 0.05) and maximal 24-h levels in cerebrospinal fluid (p = 0.008). Levels were highest among patients with diffuse injury, compared with those with mass lesions. Among the diffuse injury group, those with Diffuse Injury III-IV had higher initial (p = 0.115) and maximal (p = 0.007) MAP-2 levels than those with Diffuse Injury I-II. Boxplots represent medians with interquartile ranges. (B) Pattern of biomarker release over 10 days versus Marshall Classification. There is a graded increase in the overall levels and the peaks of MAP-2 as the degree of diffuse injury increases. This is mostly seen within the first 120 h post-injury. Also, MAP-2 levels were slightly higher in non-evacuated mass lesions than evacuated mass lesions. Lines represent mean values of MAP-2 over time.
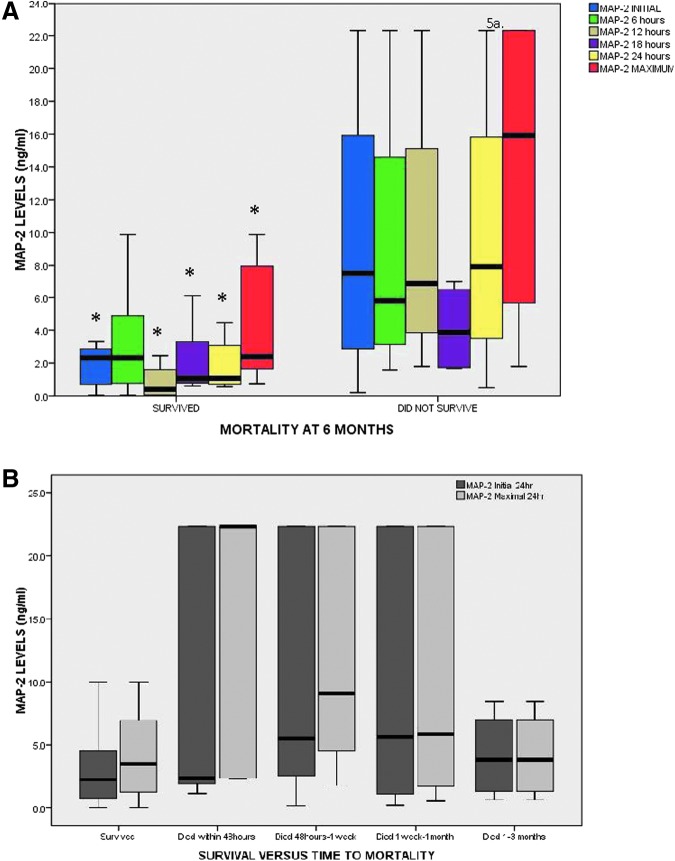
In Figure 5A, MAP-2 levels were compared in those who did and did not survive to 6 months post-injury. Initial (p = 0.015) and maximal 24-h (p = 0.006) levels were significantly higher in those in those who did not survive (Fig. 5A). All MAP-2 levels drawn within the first 24 h were significantly higher in non-survivors at 6 months. There were 79 patients who survived to 6 months; five died within 48 h, 15 died between 48 h to 1 week, seven died between 1 week to 1 month, and four died between 1 to 3 months. Levels of MAP-2 relative to time of death showed insignificant differences in initial (p = 0.073) but significant differences in maximal MAP-2 levels (p = 0.011) between groups. The highest concentrations of MAP-2 were in those who died within 48 h.
FIG. 5.
(A) Boxplot of levels microtubule-associated protein 2 (MAP-2) levels versus survival at 6 months. Cerebrospinal fluid MAP-2 levels are compared in those who did and did not survive to 6 months post-injury. Initial (p = 0.015) and maximal 24-h (p = 0.006) levels were significantly higher in those in those who did not survive. All MAP-2 levels drawn within the first 24 h were significantly higher in non-survivors at 6 months (p ≤ 0.05). (B) Boxplot of levels MAP-2 levels versus time of death. There were 79 patients who survived to 6 months, five who died within 48 h, 15 who died between 48 h to 1 week, seven who died 1 week to 1 month, and four who died between 1 to 3 months. Levels of MAP-2 relative to time of death showed insignificant differences in initial (p = 0.073) but significant differences in maximal MAP-2 levels (p = 0.011) between groups. The highest concentrations of MAP-2 were in those who died within 48 h. Boxplots represent medians with interquartile ranges.
Discussion
This study described a cohort of severe TBI patients with non-penetrating injury who underwent prospective clinical, radiologic, and biomarker evaluation over 10 days post-injury. It showed that MAP-2 was significantly elevated in human CSF following a severe TBI, and was detectable within a few hours of injury and remained significantly elevated for over 240 h. Rapid appearance of a biomarker in biologic material is essential to its clinical utility, and the AUC from the ROC curves for deciphering TBI versus controls at the earliest time-point CSF was obtained was 0.96 and for the maximal level within 24 h was 0.98. A cutoff level of 0.25 ng/mL would yield sensitivities of 89%-95% and a specificity of 100%. This is clinically important because identifying the presence of TBI when mental status is altered secondary to drugs, alcohol or sedatives will help with triaging patients to trauma centers with neurosurgical expertise. Further, this would afford TBI patients the opportunity for earlier intervention and could help prevent secondary insults. To this end, MAP-2 was able to distinguish between TBI and uninjured controls within 6 h with a very high precision and could potentially improve management.
Most notable was the finding that MAP-2 reflected the degree of diffuse damage. Diffuse injury was measured radiologically based on the Marshall Classification. There was an incremental increase in the overall mean/median levels and peaks of MAP-2 as the degree of diffuse injury increased from Diffuse Injury I to Diffuse Injury IV. This was mostly evident over the first 120 h post-injury. Although MAP-2 concentrations were elevated with mass lesions (non-evacuated being higher than evacuated), they were much higher with diffuse injuries. The degree of diffuse neuronal injury, particularly axonal injury, is an important determinant of outcome, as it is known to be associated with significant mortality and morbidity.20 Further, in a subset of patients with prominent diffuse axonal injury on their initial CT reports, MAP-2 concentrations were significantly higher than in other injury types, suggesting elevation with axonal injury. The discovery that MAP-2 is associated with degree of diffuse axonal injury is significant given that this is of great importance for the implementation of appropriate multidisciplinary care and health policies aimed at the prevention and rehabilitation of patients with such injuries.20
Previously, we reported that early MAP-2 concentrations within 24 h significantly improved the clinical prediction models of 6-month mortality in severe TBI patients and also had predictive ability independent of the clinical data.7 Accordingly, MAP-2 also was able to predict early mortality at 2 weeks post-injury. By examining the area under the ROC curve and at 14 distinct time-points over 240 h post-injury, MAP-2 measured over 48 h consistently showed good discrimination between survivors and non-survivors at 2 weeks. The predictive ability was highest from MAP-2 levels drawn from 6 to 48 h post-injury with AUCs of 0.75 to 0.81. Additionally, with sensitivities of 92% and 100% for initial and maximal concentrations within 24 h, respectively, would improve upon the clinical predictors of early mortality. In practice, this would be especially useful when certain pieces of clinical information and CT evaluation are not readily available at the time of assessment. Predicting individual patient outcome within 2 weeks is important for planning that patient's care and for counseling relatives. It allows for risk stratification and benchmarking quality of care.21
Depending on the hospital center or the patient's condition, CSF may be accessible at different times during the first 24 h after injury. Therefore, the analysis included biomarker evaluation at the earliest available sample time-point at time of ventriculostomy (usually within 6 h post-injury) and the maximal concentration within 24 h of injury. The rationale for these two different types of analyses was to see how the biomarkers could be applied clinically. The results had better performance using the “maximal concentration than the single “initial” or “earliest” time-point analysis, yet both provided important information about the severity of injury. We also assessed the performance of MAP-2 at discrete time-points. This underscores the need to evaluate biomarkers at multiple time-points, especially within the first 24 h. The study provides a glimpse into the optimal times for clinical use of the MAP-2 biomarker. The pattern of release is such that it elevates within 6 h of injury and peaks within 24 h and starts to decrease after about 48 h. The pattern is different based on the clinical course of the patient as seen in Figure 1B, where the difference in MAP-2 release differs between survivors and non-survivors.
While these data are encouraging, the authors recognize there are limitations to this study. Clinical management dictated when the ventriculostomy was placed and therefore patients had a variable number of samples available for analysis at the different time-points during the first 24 h. The most common surgically placed monitors for intracranial pressure monitoring in severe TBI patients are intraventricular catheters (ventriculostomy). However, intraparenchymal catheters are becoming an increasingly popular alternative to ventriculostomy in many countries as they are easier to use, less invasive, and can be inserted in the intensive care unit by non-neurosurgeons.22 Since the purpose of the study was to identify biomarkers that are related to outcome in CSF, intraventricular catheters were the logical choice for study purposes. More importantly, it was standard of care at the participating institutions.
Based on the current results, we cannot recommend a change in patient management, but these results provide a starting point for larger studies. If validated, MAP-2 levels could be used to determine severity of injury, particularly diffuse neuronal injury and axonal injury, and provide early prognostic information that would be most helpful in discussing management options with families and making decisions about futility of care. As an extension to this study, it would be interesting to compare MAP-2 levels with magnetic resonance imaging and to assess MAP-2 as a surrogate measure of diffuse injury in clinical trials to gauge effectiveness of therapy.
Conclusion
These data suggest that early levels of MAP-2 in CSF have the potential to determine injury severity, reflect degree of diffuse brain damage, and predict 2-week mortality in severe TBI patients. These findings have implications for counseling families and improving clinical decision-making early after injury and guiding multidisciplinary care. Further studies are needed to validate these findings in a larger sample.
Author Disclosure Statement
This study was funded by NIH RO1 NS052831, “Biochemical Markers of Severe Traumatic Brain Injury.”
Drs. Gabrielli, Hannay, Heaton, Robertson, Robicsek, and Schmalfuss have no competing financial interests.
Drs. Brophy and Papa are consultants of Banyan Biomarkers, Inc., but receive no stocks or royalties from the company and will not benefit financially from this publication.
Dr. Hayes and Wang own stock in and receive royalties from Banyan Biomarkers Inc., and as such may benefit financially as a result of the outcomes of this research or work reported in this publication.
References
- 1.Faul M., Xu L., Wald M.M., and Coronado V.G. (2010). Traumatic Brain Injury in the United States. Emergency Department Visits, Hospitalizations and Deaths 2002–2006. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention: Atlanta, GA [Google Scholar]
- 2.Papa L. (2012) Exploring the role of biomarkers for the diagnosis and management of traumatic brain injury patients, in: Proteomics—Human Diseases and Protein Functions. Man T.K. and Flores R.J. (eds). InTech Open Access Publisher: Rijeka, Croatia [Google Scholar]
- 3.Papa L., Robinson G., Oli M., Pineda J., Demery J., Brophy G., Robicsek S.A., Gabrielli A., Robertson C.S., Wang K.W., and Hayes R.L. (2008) Use of biomarkers for diagnosis and management of traumatic brain injury patients. Expert Opin. Med. Diagn. 2, 937–945 [DOI] [PubMed] [Google Scholar]
- 4.Pineda J.A., Lewis S.B., Valadka A.B., Papa L., Hannay H.J., Heaton S.C., Demery J.A., Liu M.C., Aikman J.M., Akle V., Brophy G.M., Tepas J.J., Wang K.K., Robertson C.S., and Hayes R.L. (2007) Clinical significance of alphaII-spectrin breakdown products in cerebrospinal fluid after severe traumatic brain injury. J. Neurotrauma 24, 354–366 [DOI] [PubMed] [Google Scholar]
- 5.Brophy G.M., Pineda J.A., Papa L., Lewis S.B., Valadka A.B., Hanna H.J., Heaton S.C., Demery J.A., Liu M.C., Tepas J.J., 3rd, Gabrielli A., Robicsek S., Wang K.K., Robertson C.S., Hayes R.L. (2009) alphaII-Spectrin breakdown product cerebrospinal fluid exposure metrics suggest differences in cellular injury mechanisms after severe traumatic brain injury. J. Neurotrauma 26, 471–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Papa L., Akinyi L., Liu M.C., Pineda J.A., Tepas J.J., 3rd, Oli M.W., Zheng W., Robinson G., Robicsek S.A., Gabrielli A., Heaton S.C., Hannay H.J., Demery J.A., Brophy G.M., Layon J., Robertson C.S., Hayes R.L., and Wang K.K. (2010) Ubiquitin C-terminal hydrolase is a novel biomarker in humans for severe traumatic brain injury. Crit. Care Med. 38, 138–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Papa L., Robertson C.S., Wang K.K., Brophy G.M., Hannay H.J., Heaton S, Schmalfuss I, Gabrielli A, Hayes RL, and Robicsek SA. (2015). Biomarkers improve clinical outcome predictors of mortality following non-penetrating severe traumatic brain injury. Neurocrit. Care 22, 52–64 [DOI] [PubMed] [Google Scholar]
- 8.Papa L., Brophy G.M., Welch R.D., Lewis L.M., Braga C.F., Tan C.N., Ameli N.J., Lopez M.A., Haeussler C.A., Mendez Giordano D.I., Silvestri S., Giordano P., Weber KD., Hill-Pryor C., and Hack D.C. (2016). Time course and diagnostic accuracy of glial and neuronal blood biomarkers GFAP and UCH-L1 in a large cohort of trauma patients with and without mild traumatic brain injury. JAMA Neurol. 73, 551–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Folkerts M.M., Berman R.F., Muizelaar J.P., and Rafols J.A. (1998). Disruption of MAP-2 immunostaining in rat hippocampus after traumatic brain injury. J. Neurotrauma 15, 349–363 [DOI] [PubMed] [Google Scholar]
- 10.Taft W.C., Yang K., Dixon C.E., and Hayes R.L. (1992). Microtubule-associated protein 2 levels decrease in hippocampus following traumatic brain injury. J. Neurotrauma 9, 281–290 [DOI] [PubMed] [Google Scholar]
- 11.Kitagawa K., Matsumoto M., Niinobe M., Mikoshiba K., Hata R., Ueda H., Handa N., Fukunaga R., Isaka Y., and Kimura K. (1989). Microtubule-associated protein 2 as a sensitive marker for cerebral ischemic damage–immunohistochemical investigation of dendritic damage. Neuroscience 31, 401–411 [DOI] [PubMed] [Google Scholar]
- 12.Pettigrew L.C., Holtz M.L., Craddock S.D., Minger S.L., Hall N., and Geddes J.W. (1996). Microtubular proteolysis in focal cerebral ischemia. J. Cereb. Blood Flow Metab. 16, 1189–1202 [DOI] [PubMed] [Google Scholar]
- 13.Siman R. and Noszek J.C. (1988). Excitatory amino acids activate calpain I and induce structural protein breakdown in vivo. Neuron 1, 279–287 [DOI] [PubMed] [Google Scholar]
- 14.Saatman K.E., Graham D.I., and McIntosh T.K. (1998). The neuronal cytoskeleton is at risk after mild and moderate brain injury. J, Neurotrauma 15, 1047–1058 [DOI] [PubMed] [Google Scholar]
- 15.Posmantur R.M., Kampfl A., Taft W.C., Bhattacharjee M., Dixon C.E., Bao J., and Hayes R.L. (1996) Diminished microtubule-associated protein 2 (MAP2) immunoreactivity following cortical impact brain injury. J, Neurotrauma 13, 125–137 [DOI] [PubMed] [Google Scholar]
- 16.Teasdale G, Jennett B. (1976). Assessment and prognosis of coma after head injury. Acta Neurochir, (Wien.) 34, 45–55 [DOI] [PubMed] [Google Scholar]
- 17.Teasdale G. and Jennett B. (1974). Assessment of coma and impaired consciousness. A practical scale. Lancet 2, 81–84 [DOI] [PubMed] [Google Scholar]
- 18.Steyerberg E.W., Mushkudiani N., Perel P., Butcher I., Lu J., McHugh G.S., Murray G.D., Marmarou A., Roberts I., Habbema J.D., and Maas A.I. (2008). Predicting outcome after traumatic brain injury: development and international validation of prognostic scores based on admission characteristics. PLoS Med 5, e165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marshall L.F., Marshall S.B., Klauber M.R., Van Berkum Clark M., Eisenberg H., Jane J.A., Luerssen T.G., Marmarou A., and Foulkes M.A. (1992). The diagnosis of head injury requires a classification based on computed axial tomography. J. Neurotrauma 9 Suppl 1, S287–S292 [PubMed] [Google Scholar]
- 20.Vieira R.C., Paiva W.S., de Oliveira D.V., Teixeira M.J., de Andrade A.F., and de Sousa R.M. (2016). Diffuse Axonal Injury: Epidemiology, Outcome and Associated Risk Factors. Front. Neurol 7, 178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roozenbeek B., Chiu Y.L., Lingsma H.F., Gerber L.M., Steyerberg E.W., Ghajar J., and Maas A.I. (2012). Predicting 14-day mortality after severe traumatic brain injury: application of the IMPACT models in the brain trauma foundation TBI-trac(R) New York State database. J. Neurotrauma 29, 1306–1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khan S.H., Kureshi I.U., Mulgrew T., Ho S.Y., and Onyiuke H.C. (1998). Comparison of percutaneous ventriculostomies and intraparenchymal monitor: a retrospective evaluation of 156 patients. Acta Neurochir. Suppl 71, 50–52 [DOI] [PubMed] [Google Scholar]