Abstract
Objective: Black patients are more likely than white patients to die in the hospital with intensive care and life-sustaining treatments and less likely to use hospice. Regional concentration of high end-of-life (EOL) treatment intensity practice patterns may disproportionately affect black patients. We calculated and compared race-specific hospital-level EOL treatment intensity in Pennsylvania.
Methods: We conducted a retrospective cohort analysis of Pennsylvania acute care hospital admissions, 2001–2007, among black and white admissions ≥21 years old at high probability of dying (HPD) (≥15% predicted probability of dying at admission). We calculated hospitals' race-specific observed, expected, and Bayes' shrunken observed-to-expected ratios of intensive care unit (ICU) admission, ICU length of stay (LOS), intubation/mechanical ventilation, hemodialysis, tracheostomy, and gastrostomy among HPD admissions; and an empirically weighted EOL treatment intensity index summing these ratios.
Results: There were 35,609 black HPD admissions (27,576 unique patients) and 311,896 white HPD admissions (252,662 unique patients) to 182 hospitals. Among 95 hospitals with ≥30 black HPD admissions, 80% of black admissions were concentrated in 29 hospitals, where black-specific observed and expected EOL measures were usually higher than white-specific measures (p < 0.001 for all but 5/24 measures). Hospitals' black-specific and white-specific observed-to-expected ratios of ICU and life-sustaining treatment (LST) (rho 0.52–0.90) and EOL index (rho = 0.92) were highly correlated. However, black-specific observed-to-expected ratios and overall EOL intensity index were consistently lower than white-specific ratios (p < 0.001 for all except hemodialysis).
Conclusions: In Pennsylvania, black-serving hospitals have higher standardized EOL treatment intensity than nonblack-serving hospitals, contributing to black patients' relatively higher use of intensive treatment. However, conditional on being admitted to the same high-intensity hospital and after risk adjustment, blacks are less intensively treated than whites.
Keywords: : disparity, hospital profiling, intensive care, life support, race, terminal care
“End-of-life” (EOL) treatment intensity is being used as a measure of poor quality and inefficiency.1–5 For example, claims-based measures of hospice, chemotherapy, emergency room visits, hospitalizations, and intensive care unit (ICU) admissions among patients who died of cancer have been proposed as quality indicators for advanced cancer.6,7
Since black patients are less likely to use hospice8 and more likely to die in the hospital9 with intensive care and life-sustaining treatments10,11 than white patients, this quality concern may disproportionately affect black patients. Some of these differences may be attributable to patient and family preferences,11–16 health literacy,17 and option presentation18; whereas some are attributable to black patients' concentration in hospitals with higher EOL intensity among all patients, including white patients.10,11
The purpose of our study was to explore race-specific differences in hospital EOL treatment intensity. Specifically, we sought to profile hospitals' black- and white-specific EOL treatment intensity by using a measurement approach that has theoretical advantages over the decedent follow-back approach.19 This approach involved profiling hospitals' EOL treatment intensity by using their treatment patterns among patients classified at a high probability of dying (HPD) at the time of admission.20 This distinction may be particularly important for assessing race-specific EOL treatment intensity, since black people die five years younger than their white counterparts and have a higher age-specific mortality rate across all adult years.21
We hypothesized that hospitals' black- and white-specific EOL treatment measures would be highly correlated across hospitals, but that within-hospitals' black-specific EOL treatment intensity would be systematically higher than white-specific EOL treatment intensity.
Materials and Methods
Data
This is a retrospective analysis of acute care hospital admissions that were recorded in the Pennsylvania Health Care Cost Containment Council (PHC4)'s hospital discharge dataset between April 1, 2001 and December 31, 2007 and linked to the Pennsylvania Department of Health's Vital Statistics death records through December 31, 2008. We chose these years of PHC4 data because they contained a predicted probability of in-hospital death (PPD) calculated from key clinical findings abstracted from the medical chart during the first 48 hours of admission, which allowed for “prospective” identification admissions at HPD. Between 2001 and 2003, Pennsylvania hospitals were required to submit key clinical findings for PPD calculation for each admission. Between 2003 and 2007, PHC4 limited the list of diagnosis related groups [DRGs] for which key clinical finding abstraction was mandated by law (see Appendix 1). By 2009, PHC4 no longer mandated hospital collection of key clinical findings for PPD calculation.) The PPD is a proprietary model originally developed by MediQual in the 1990s that has been used widely in hospital performance measurement.22 By adding key clinical findings, including present on admission codes, laboratory, and clinical findings, to administrative data available in hospital discharge claims, this risk-adjustment model increases discriminatory power substantially (mean c-statistic for administrative-only model = 0.79 and for administrative plus key clinical finding model = 0.86).23
Sample
As in our prior work,5,20 we use HPD as a proxy for prospectively identifying patients who may be at the EOL. The study sample included black and white admissions aged 21 and older at HPD, defined as the 95th percentile of PPD, corresponding to 15% or higher (median [interquartile range]: 26% [19–41%]). We included all adults, rather than just those >65, because black patients die approximately five years younger than their white counterparts and have a higher age-specific mortality rate across all adult years.21
Race-specific EOL intensity
For each hospital with at least 30 black admissions and/or 30 white admissions at HPD, we calculated a black- and white-specific observed rate (or mean) for each of six measures related to treatment intensity, including: ICU admission, ICU length of stay, intubation/mechanical ventilation, tracheostomy, hemodialysis, and gastrostomy. To estimate the expected (case-mixed adjusted) rate or mean for each of the measures, we applied regression coefficients derived from a patient-level prediction model (excluding race as a predictor) to all HPD admissions to the hospital and summed the admissions' predicted rates. The patient-level prediction model included age, age-squared, sex, PPD, PPD-squared, insurance status, admission type (elective, urgent, emergent), principal diagnosis, and all secondary diagnoses/comorbidities. We then calculated black- and white-specific case-mix standardized (observed-to-expected) treatment ratios for the six measures for each hospital's HPD admissions and decedents. The estimated ratios were subject to Bayes' shrinkage estimation to address instability of the measure for lower-volume hospitals. Finally, we calculated a black- and white-specific EOL intensity index, an empirically weighted factor score of the six standardized ratios. The empiric factor weights reflect the eigenvalues for each of the six ratios drawn from principal components analysis conducted on all HPD admissions. These factor weights had been scaled so that the overall (nonrace specific) EOL intensity index would have a sample mean of 0 and a standard deviation of 1. For more information, see Appendix 1.
Analysis
We summarized characteristics of black and white HPD admissions by using measures of central tendency. We classified hospitals into three groups based on the population demographics they served (predominantly white-serving, black-serving with a low concentration of black patients, black-serving with a high concentration of black patients), and we then compared EOL treatment measures within and across these three groups.
We used Pearson's correlation to explore the consistency between hospitals' case-mix adjusted race-specific measures of all seven EOL measures and a two-sided paired t-test to compare the mean within-hospital black and white case-mix adjusted EOL measures.
Human subjects and role of the sponsor
This study was reviewed and approved by the University of Pittsburgh Institutional Review Board. The funder, the National Institute of Aging, played no role in the design, analysis, and interpretation of this study or in the decision to submit it for publication.
Results
Sample characteristics
The sample included 35,609 HPD admissions among 27,576 unique black patients, and 311,896 HPD admissions among 252,662 unique white patients to 182 hospitals. Compared with white HPD admissions, black HPD admissions were younger (71.6 vs. 77.5, p < 0.001), more likely to be insured by Medicaid (46% vs. 15.5%, p < 0.001), with a higher predicted probability of dying on admission (26.7% vs. 25.5%, p < 0.001), and a different distribution of principal diagnoses (Table 1).
Table 1.
Characteristics of High Probability of Dying Admissions Used to Calculate End-of-Life Intensity Measures, Pennsylvania, 2001–2007
| Variable | Black | White | p |
|---|---|---|---|
| N | 35,609 | 311,896 | |
| Age, mean (SD) | 71.6 (15) | 77.5 (12.5) | <0.001 |
| Men, n (%) | 16,704 (47) | 151,028 (48) | <0.001 |
| Insurance status, n (%) | <0.001 | ||
| Medicare only | 9096 (26) | 81,643 (26) | |
| Medicare and Medicaid (duals) | 10,547 (30) | 36,233 (12) | |
| Medicare and commercial | 7016 (20) | 153,747 (49) | |
| Medicaid only | 5617 (16) | 10,764 (3.5) | |
| Commercial only | 3041 (8.5) | 28,011 (9) | |
| Uninsured | 285 (0.8) | 1385 (0.44) | |
| PPD, median (IQR) | 0.267 (0.19–0.437) | 0.255 (0.186–0.404) | <0.001 |
| Principal diagnosis, n (%) | |||
| Septicemia | 5793 (16) | 44,747 (14) | <0.001 |
| Respiratory failure | 4119 (12) | 35,391 (11) | 0.215 |
| AMI | 1800 (5.1) | 28,288 (9.1) | <0.001 |
| Pneumonia | 2141 (6) | 22,359 (7.2) | <0.001 |
| Acute cerebrovascular disease | 2874 (8.1) | 21,272 (6.8) | <0.001 |
| Crude treatment rates | |||
| ICU admission, n (%) | 24,642 (69) | 179,558 (58) | <0.001 |
| ICU LOS, median [IQR] | 5 (3–10) | 4 (2–8) | <0.001 |
| Intubation/MV, n (%) | 12,051 (34) | 69,550 (22) | <0.001 |
| Hemodialysis, n (%) | 3888 (11) | 14,008 (4.5) | <0.001 |
| Tracheostomy, n (%) | 1940 (5.4) | 7845 (2.5) | <0.001 |
| Gastrostomy, n (%) | 2193 (6.2) | 10,296 (3.3) | <0.001 |
HPD, high probability of dying; ICU, intensive care unit; IQR, interquartile range; LOS, length of stay; MV, mechanical ventilation; PPD, predicted probability of dying on admission
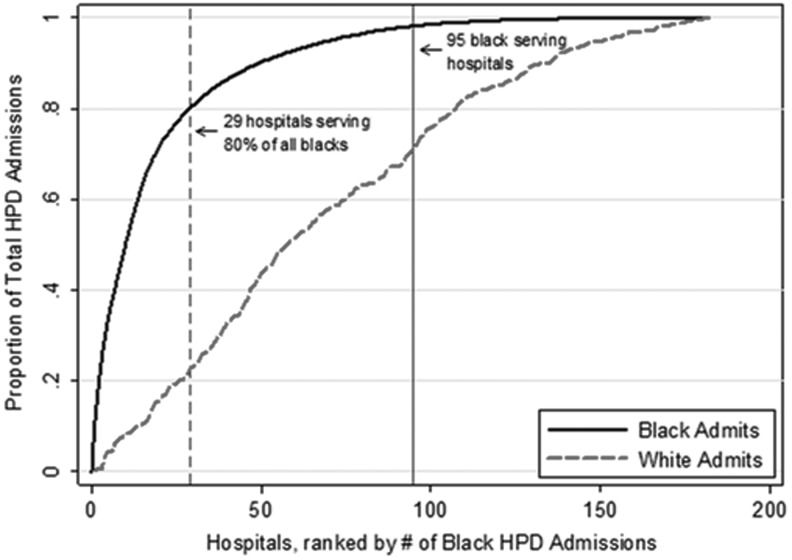
Among the 182 hospitals, 87 hospitals did not have sufficient black HPD admissions to calculate black-specific hospital EOL measures (predominantly white-serving hospitals). These predominantly white-serving hospitals accounted for 2% of all black HPD admissions and 29% of all white HPD admissions. The remaining 95 hospitals accounted for 98% of all black admissions and 71% of all white HPD admissions (Fig. 1); 29/95 (31%) accounted for 80% of black HPD admissions (black-serving, most concentrated); and 66/95 (69%) accounted for 18% of black HPD admissions (black-serving, least concentrated).
FIG. 1.
Cumulative distribution of black and white admissions in Pennsylvania hospitals. The graph depicts the cumulative distribution function for all black and white admissions at HPD for hospitals 1–182 in Pennsylvania between 2001 and 2007. Each of the hospitals in the sample is arrayed on the x-axis from highest to lowest by the total number of HPD admissions among black patients. The dashed vertical line depicts the 29 hospitals accounting for 80% of all HPD admissions among black patients (but only 23% of HPD admissions among white patients). The second vertical line depicts the 95 hospitals with sufficient HPD admissions (N = 30) to calculate black-specific EOL measures, which we term “black-serving hospitals.” EOL, end of life; HPD, high probability of dying.
EOL treatment intensity
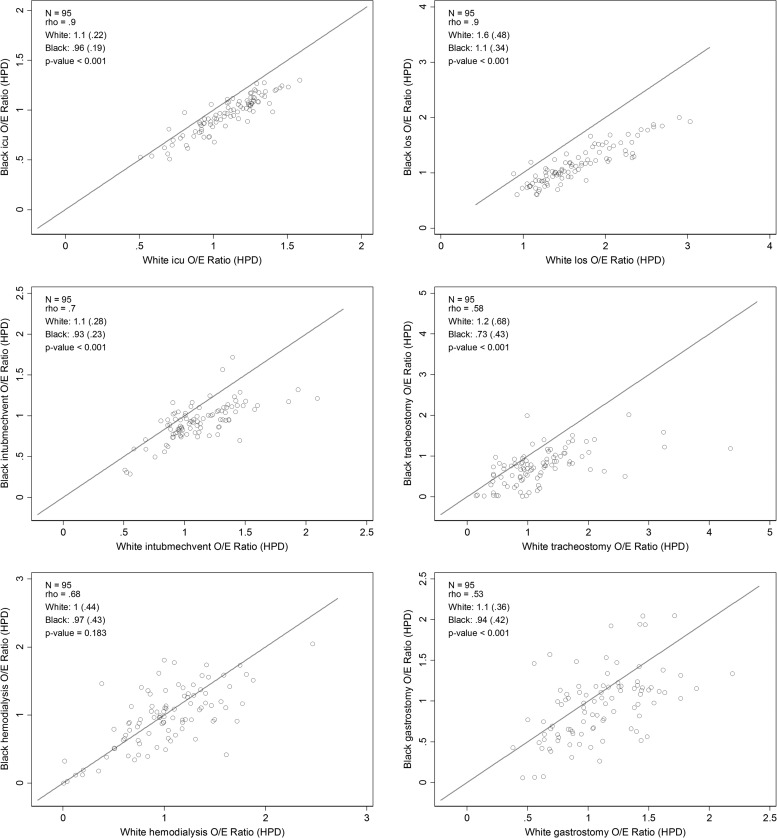
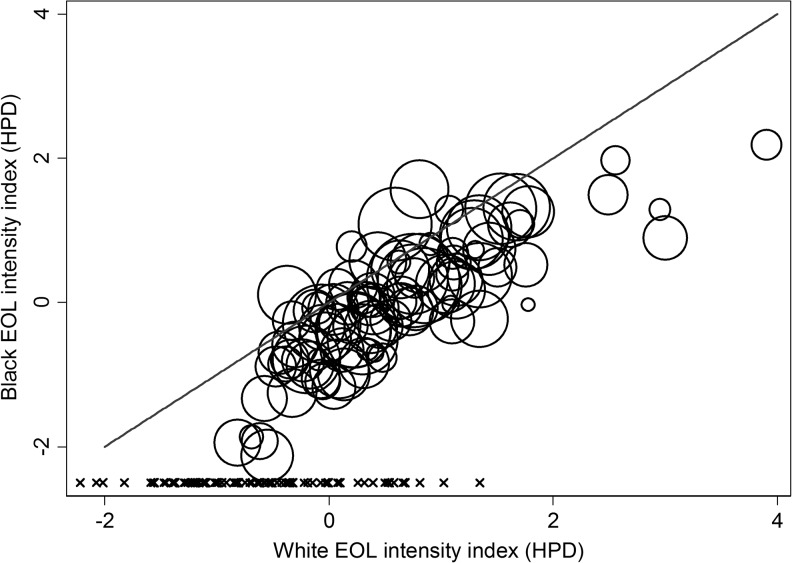
At the admission level, black HPD admissions had a higher crude use of ICU and life-sustaining treatments, compared with white HPD admissions (Table 1, unadjusted for case-mix differences). At the hospital level, EOL treatment intensity measured among white HPD admissions was higher in black-serving hospitals (Table 2; p < 0.001 for all comparisons). Within each of the three hospital strata (predominantly white-serving [n = 87]; black-serving, least concentrated [n = 66]; black-serving, most concentrated [n = 29]), the black-specific observed and expected EOL measures were consistently higher than the white-specific EOL measures (Table 2; p < 0.001 for all but 5/24 measures compared). Hospitals' black-specific and white-specific case-mix standardized (observed-to-expected) ratios of ICU and LST and the EOL index based on these measures were highly correlated (Fig. 2; rho = 0.52–0.90 and Fig. 3; rho = 0.92); however, the black-specific observed-to-expected ratios and the overall EOL-intensity index were consistently lower than the white-specific ratios and index (Table 2; Figs. 2 and 3). For an interactive version of Figure 3, or to download the hospital-specific data, visit https://public.tableau.com/views/PAhospitalEOLintensitybyrace2001-2007/Sheet1?:embed=y&:display_count=yes&:showTabs=y
Table 2.
Black- and White-Specific End-of-Life Hospital Intensity Measures among Three Mutually Exclusive Groups of Hospitals Categorized by Their Concentration of Black Admissions (From Predominantly White-Serving Hospitals to Least and Most Concentrated Black-Serving Hospitals), Pennsylvania, 2001–2007
| Predominantly white-serving hospitals (N = 87) | Black-serving; least concentrated (N = 66) | Black-serving; most concentrated (N = 29) | |||||
|---|---|---|---|---|---|---|---|
| Variable | Black | White | Black | White | Black | White | p-valuea(black/white) |
| Admissions, n (%) | 617 (0.67) | 91,040 (99) | 6513 (4.1) | 151,880 (96) | 28,779 (29) | 71,907 (71) | |
| Observed proportion | |||||||
| ICU admission | 0.46 (.37–.59) | 0.66 (0.54–0.74)*** | 0.62 (0.53–0.69) | 0.7 (0.63–0.79) | 0.72 (0.62–0.79) | 0.051/<0.001 | |
| ICU LOS, days | 4.3 (3.8–5.5) | 6.3 (5.3–7.8)* | 6.2 (5.3–7.2) | 8.5 (7.1–10) | 8.4 (7.8–11) | <0.001/<0.001 | |
| Intubation/MV | 0.13 (0.1–0.17) | 0.28 (0.25–0.34)*** | 0.22 (0.18–0.26) | 0.33 (0.28–0.38)** | 0.31 (0.24–0.36) | 0.043/<0.001 | |
| Hemodialysis | 0.016 (0–0.047) | 0.11 (0.076–0.15)*** | 0.044 (0.031–0.059) | 0.1 (0.09–0.12)*** | 0.05 (0.038–0.058) | 0.431/<0.001 | |
| Tracheostomy | 0.004 (0.002–0.010) | 0.034 (0.013–0.042)*** | 0.02 (0.012–0.028) | 0.057 (0.031–0.075) | 0.048 (0.027–0.076) | <0.001/<0.001 | |
| Gastrostomy | 0.021 (0.015–0.028) | 0.052 (0.033–0.067)*** | 0.032 (0.024–0.039) | 0.069 (0.055–0.074)*** | 0.047 (0.031–0.061) | 0.007/<0.001 | |
| Expected proportion | |||||||
| ICU admission | 0.53 (0.47–0.57) | 0.68 (0.65–0.71)*** | 0.57 (0.55–0.59) | 0.7 (0.66–0.72)*** | 0.61 (0.57–0.65) | 0.238/<0.001 | |
| ICU LOS, days | 3.9 (3.4–4.1) | 6.5 (6–6.9)*** | 4.2 (3.9–4.4) | 6.4 (6.2–7)*** | 4.4 (4.1–5.3) | 0.482/<0.001 | |
| Intubation/MV | 0.18 (0.16–0.2) | 0.32 (0.3–0.36)*** | 0.21 (0.2–0.23) | 0.34 (0.29–0.37)*** | 0.24 (0.21–0.29) | 0.544/<0.001 | |
| Hemodialysis | 0.039 (0.036–0.043) | 0.11 (0.1–0.12)*** | 0.043 (0.039–0.045) | 0.11 (0.099–0.12)*** | 0.043 (0.039–0.052) | 0.084/<0.001 | |
| Tracheostomy | 0.017 (0.014–0.019) | 0.049 (0.044–0.057)*** | 0.019 (0.018–0.022) | 0.051 (0.045–0.064)*** | 0.026 (0.02–0.043) | 0.193/<0.001 | |
| Gastrostomy | 0.032 (0.03–0.033) | 0.059 (0.055–0.064)*** | 0.032 (0.03–0.033) | 0.062 (0.059–0.065)*** | 0.035 (0.033–0.039) | 0.025/<0.001 | |
| O-to-E ratios | |||||||
| ICU admission | 0.88 (0.7–1.1) | 0.97 (0.78–1.1)*** | 1.1 (0.92–1.2) | 1 (0.91–1.1)*** | 1.2 (1–1.3) | 0.093/<0.001 | |
| ICU LOS, days | 1.2 (0.98–1.4) | 0.99 (0.8–1.2)*** | 1.4 (1.2–1.7) | 1.3 (1.1–1.5)*** | 2 (1.5–2.3) | <0.001/<0.001 | |
| Intubation/MV | 0.7 (0.56–0.92) | 0.9 (0.77–1)*** | 1 (0.91–1.3) | 1 (0.87–1.1)*** | 1.2 (1–1.4) | 0.016/<0.001 | |
| Hemodialysis | 0.42 (0.0015–1.1) | 0.95 (0.63–1.4) | 1 (0.74–1.4) | 0.96 (0.88–1.1) | 1 (0.86–1.2) | 0.974/<0.001 | |
| Tracheostomy | 0.26 (0.1–0.53) | 0.67 (0.28–0.84)*** | 0.91 (0.61–1.2) | 1 (0.69–1.2)*** | 1.6 (1.3–1.9) | <0.001/<0.001 | |
| Gastrostomy | 0.66 (0.46–0.93) | 0.85 (0.56–1.2)* | 0.98 (0.75–1.2) | 1.1 (0.87–1.2)*** | 1.3 (1–1.5) | 0.056/<0.001 | |
| EOL-I Index | −0.67 (0.73) | −0.21 (0.82)*** | 0.37 (0.72) | 0.48 (0.76)*** | 1.2 (0.9) | <0.001/<0.001 | |
Values are median (interquartile range) or mean (standard deviation).
p < 0.05; **p < 0.01; ***p < 0.001; p values reflect within-group comparisons of black- versus white-specific measures of EOL intensity using Wilcoxon signed-ranks tests, a nonparametric analog to the paired t-test.
p values reflect across-group test for trend for black-specific and white-specific measures of EOL intensity, respectively, by using an extension of the Kruskal-Wallis test that accounts for ordered categories (e.g., three-group comparison for white-specific measures and two-group comparison for black-specific measures).
EOL, end of life; EOL-I, end-of-life intensity.
FIG. 2.
Pennsylvania hospitals' black and white standardized “end of life” ICU and life-sustaining treatment ratios. This graph depicts scatterplots of 95 Pennsylvania hospitals' black versus white EOL case-mix standardized (observed-to-expected) ICU admission rate, ICU LOS, intubation and mechanical ventilation, tracheostomy, hemodialysis, and gastrostomy using 2001–2007 PHC4 data and measured among admissions at HPD. Each circle is a single hospital. The x-axis is the hospital-specific O/E ratio among white HPD patients, and the y-axis is the hospital-specific O/E ratio among black HPD patients. We present statistics regarding the Pearson correlation (rho) between the black and white measures, a summary of the black and white measure mean (SD), and the p-value for a paired t-test of the difference between the means in the upper left corner of each panel. ICU, intensive care unit; LOS, length of stay; SD, standard deviation.
FIG. 3.
Black and white hospital EOL treatment intensity indices. This figure depicts scatterplots of 95 Pennsylvania hospitals' black versus white EOL treatment intensity indices (an empirically weighted factor score of six case-mix standardized [observed-to-expected] measures of ICU and life-sustaining treatment use) using 2001–2007 PHC4 data and measured among admissions at HPD. Each circle is a single hospital; the size of the circle is proportional to the number of admissions over the eight study years. The x-axis is the hospital-specific index among white HPD patients, and the y-axis is the hospital-specific index among black HPD patients. The x marks above the x-axis represent the 86 predominantly white-serving hospitals for which a black EOL intensity index could not be calculated. For an interactive version of this figure, or to download the hospital-specific data, visit https://public.tableau.com/views/PAhospitalEOLintensitybyrace2001-2007/Sheet1?:embed=y&:display_count=yes&:showTabs=y
Discussion
In this retrospective cohort study involving all adult black and white HPD admissions to acute care hospitals in Pennsylvania over seven years, we demonstrate two key findings. In support of our first hypothesis, hospitals' black- and white-specific patterns of EOL treatment intensity are highly correlated, supporting the existence of hospital-specific norms of EOL treatment decision making. In contradiction with our second hypothesis, despite the fact that black patients at HPD receive more intensive treatment at the EOL on average, hospitals' case-mix adjusted black-specific EOL treatment intensity was lower than their white-specific treatment intensity.
Qualitative research supports the existence of hospital-specific norms of EOL treatment decision making.24,25 A key finding of this work is that provider norms—not patient preferences—appear to drive variation in the use of intensive care and life-sustaining treatments.26 If black patients do not “choose” to concentrate in higher EOL intensity hospitals, and, instead, this concentration27 reflects the geographic distribution of black people in the state, minority serving hospitals' greater EOL treatment intensity may contribute to disparities in burdensome treatment near death.
However, our finding that hospitals' black-specific EOL treatment intensity index was systematically lower than the white-specific EOL intensity raises a curious paradox in which black patients are simultaneously over- and under-treated, relative to white patients (see Supplementary Fig. S1). Within any given hospital, black patients are more likely to receive EOL, ICU, and LST (higher “observed” treatment) and such treatment is warranted due to clinical factors such as younger age and higher illness severity (higher “expected” treatment), but the ratio of the observed-to-expected treatment is lower among black patients than among white patients.
What might explain these findings? The most likely is Simpson's paradox: the mathematical phenomenon in which aggregated data reveal one pattern but the same data, when partitioned, reveal another.28 This phenomenon is relatively common in disparities research29 and, in fact, can be leveraged to identify policy-relevant disparities intervention targets.27,30 Unlike evidence-based treatments for heart attack, pneumonia, and heart failure, however, there is not a “right rate” for the use of intensive treatment near the EOL. Therefore, although the implications of the (now well documented) phenomenon that a patient's EOL care depends on the region in which he or she lives26 or the hospital to which he or she is admitted20 rather than the patient's own treatment preferences are clear (i.e., the variation is unwarranted and, therefore, should be the target of intervention), the finding that, conditional on admission to the same hospital, black patients' case-mix standardized (observed-to-expected) EOL treatment intensity is lower than white patients does not spur us to any one particular policy action. A more salient concern may be the potential over-treatment of all patients at the highest EOL intensity hospitals.
The strengths of this study include the use of a clinically robust risk-adjustment measure and “prospective” definition of the “EOL” cohort. Nevertheless, the analyses are subject to potential biases that could have resulted in spurious or unreliable findings. For example, it is possible that the modeling approach or unmeasured confounders could have inadvertently inflated black-specific “expected” treatment rates (or deflated white-specific “expected” treatment rates). The coefficients applied to calculate expected treatment rates came from a patient-level prediction model that excluded race as a predictor, but they retained variables that correlate with race (e.g., uninsurance and Medicaid insurance, which is known to decrease the likelihood of life-supporting treatment31). Our findings did not qualitatively change when we removed insurance status as a predictor from the model. Applying Bayesian shrinkage to hospitals' black HPD admissions and white HPD admissions separately (such that the estimates for smaller hospitals are shrunken toward the race-specific grand mean) may have artificially increased the expected ICU and life-sustaining treatment rates for black HPD admissions at smaller/lower black HPD admission volume hospitals. However, hospitals below the 45° line in Figures 2 and 3 include both high- and low-volume hospitals. Missing information on key confounders of the relationship between race and EOL treatment intensity could miss important, “warranted” causes for the differences. For example, white patients are more likely to have treatment-limiting advance directives.32 This could lead to a lower observed EOL treatment intensity among white patients and the opposite pattern—lower, not higher, observed-to-expected treatment ratios for white compared with black patients in higher EOL intensity hospitals.
In summary, our findings reinforce the now well-known mechanism that aggregate differences in treatment patterns among black and white patients are driven in large part by the practice patterns of the hospitals to which they are admitted. Using more robust risk-adjustment than prior studies raises the possibility that there still yet exist systematic differences in the way that black and white patients are treated at the EOL conditional on admission to the same hospital. However, it is not possible to answer with administrative data as to whether this represents a concerning healthcare disparity.
Supplementary Material
Appendix 1
Pennsylvania Health Care Cost Containment Council Predicted Probability of Dying Data
In 2003, the Pennsylvania Health Care Cost Containment Council changed its policy from requiring that hospitals abstract and report key clinical findings on all admissions to only requiring it for a restricted list of 35 Medicare Severity Diagnosis Related Groups (MS-DRGs), which we list in Appendix Table 1.
Appendix Table 1.
Cases in the Following MS-DRGs or Principal Diagnosis Are Required to Have Atlas Admission Severity Groups Scores
| 1. Heart attack | 13. Hypotension | 25. Kidney failure |
| 2. Heart failure | 14. Blood clot in extremities | 26. Kidney and urinary tract infection |
| 3. Chest pain | 15. Vascular repair | |
| 4. Abnormal heartbeat | 16. Stroke | 27. Prostatectomy |
| 5. Coronary bypass | 17. Removal of blockage in head and neck vessel | 28. Medical back |
| 6. Heart valve replacement | 18. Craniotomy | 29. Major joint repair |
| 7. Percutaneous transluminal coronary angioplasty | 19. Diabetes | 30. Neck/back repair |
| 20. Digestive diseases | 31. Breast cancer | |
| 8. Pneumonia | 21. Liver disease | 32. Hysterectomy |
| 9. Asthma (includes COPD) | 22. Colorectal repair | 33. Infectious diseases |
| 10. Respiratory failure | 23. Gallbladder removal | 34. Ventilation for respiratory diseases |
| 11. Blood clot in lung | 24. Stomach and small intestine repair | 35. Tracheostomy |
| 12. Lung repair |
Calculating Shrunken, Standardized (Observed-to-Expected) End-of-Life Treatment Ratios
The models used to standardize (case-mix) adjust the hospitals' end-of-life treatment intensity followed these steps:
Observed hospital-level treatment rates
We combined data across all six years to calculate hospital-level observed ICU and LST treatment rates for HPD admissions.
Expected hospital-level treatment rates
To calculate expected ICU and LST treatment rates for HPD admissions, we estimated six separate multivariable logistic regression models in a sample restricted to black and white patients to identify the patient-level predictors of five categorical treatments: (ICU admission, respiratory intubation and ventilation, tracheostomy, gastrostomy tube placement, hemodialysis). These models exclude race as a patient-level predictor.
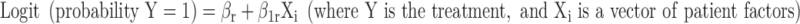 |
We estimated one linear regression model on log-transformed ICU length of stay (LOS) in a sample restricted to black and white patients to identify the patient-level predictors of LOS cancer and CHF/COPD high probability of dying (HPD) admissions. This model excluded race as a patient-level predictor.
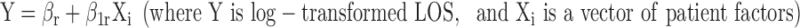 |
We then applied these coefficients to each admission to calculate a predicted probability of each of the treatments. If the patient had an ICU stay, we estimated the predicted ICU LOS by back-transforming to the original scale (days) by employing the smearing estimate developed by Duan (to allow for any non-normality in the error distribution). We then summed the predicted values across admissions for each hospital to calculate risk-adjusted, expected rates of ICU admission, intubation/mechanical ventilation, tracheostomy, gastrostomy, hemodialysis, as well as a risk-adjusted, expected ICU LOS for each hospital for HPD admissions.
Shrunken, standardized hospital treatment ratios
Finally, we divide the estimates of the hospital's observed treatment rates and LOS by the hospital's expected treatment rates and LOS, to obtain standardized treatment ratios for each of the individual measures overall and for the blacks and whites. This standardized ratio is the summary measure of hospital-level intensity for each treatment. To address instability of estimates, particularly for smaller hospitals, we then use empiric Bayes' shrinkage estimation. A Bayesian framework uses prior knowledge about a situation to produce estimates for the true mean that lie somewhere between the observed average and the expected mean based on prior knowledge. Empirical Bayes uses the data both as the basis for prior knowledge and to adjust this knowledge; generally, the expected mean used to seed the iterative analysis is the mean in the overall sample. We outline the steps that we will follow to calculate the “shrunken” estimates given later. In the equations that follow, Var = variance, O = observed, E = expected, n = hospital sample, p = proportion, N = overall sample.
- 1. Generate estimate of estimation variance for each hospital:
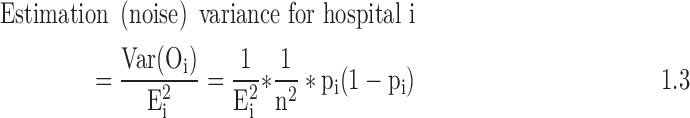
- 2. Calculate the average estimation variance in the sample:
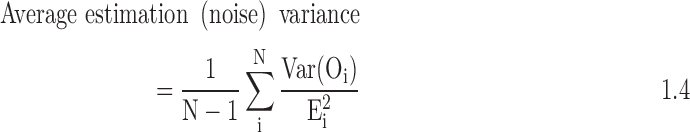
- 3. Calculate the total variance of the ratio in the sample:
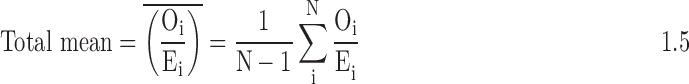
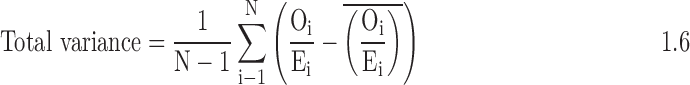
- 4. Calculate the signal variance:
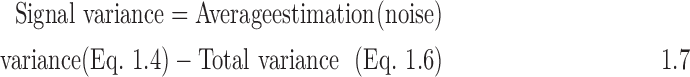
- 5. Calculate the residual for each hospital:

- 6. Generate the shrinkage factor:
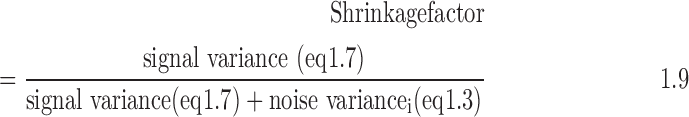
- 7. Create the shrinkage estimate:
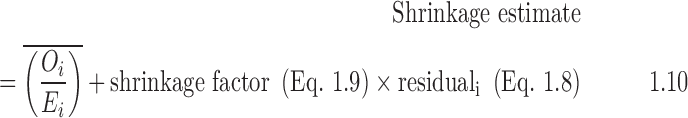
8. Repeat, applying the shrinkage estimate as the new variance estimator:
The output of these calculations will take the following form: For each hospital, there will be a “shrunken” standardized (observed-to-expected) ratio of intensive care unit (ICU) admission, ICU LOS, respiratory intubation and ventilation, tracheostomy, gastrostomy tube placement, and hemodialysis.
Empirically Weighted End-of-Life Treatment Intensity Index
We identified the empirical weights from a principal components factor analysis. Specifically, in the principal components factor analysis of the sample, hospitals' six shrunken standardized treatment ratios were calculated by using all HPD admissions (e.g., not restricted to black and white patients) loaded onto one factor. We used a scaled form of the factor loadings as empiric weights. We scaled the factor loadings to ensure our HPD end-of-life intensity score was standardized to mean 0 and variance 1. We summarize the weights in the table given next.
| Shrunken standardized treatment ratio | Factor loading (empirical weight) |
|---|---|
| ICU admission | 0.64 |
| ICU LOS | 0.78 |
| Respiratory intubation and ventilation | 0.84 |
| Tracheostomy | 0.85 |
| Gastrostomy tube placement | 0.72 |
| Hemodialysis | 0.62 |
For each hospital, we then multiplied the weight by the hospital's race-specific shrunken standardized treatment ratio and summed across the six products to obtain a race-specific EOL intensity score.
We started with the hospital's white-specific score:
ScoreWHITE = WeightICUadmit × SS_ICUadmitRatioWHITE + WeightICULOS × SS_ICULOSRatioWHITE + WeightMV × SS_MVRatioWHITE + WeightTrach × SS_TrachRatioWHITE + WeightGTube × SS_GTubeWHITE + WeightHD × SS_HDWHITE
We then calculated the cancer-specific score:
ScoreBLACK = WeightICUadmit × SS_ICUadmitRatioBLACK + WeightICULOS × SS_ICULOSRatioBLACK + WeightMV × SS_MVRatioBLACK + WeightTrach × SS_TrachRatioBLACK + WeightGTube × SS_GTubeBLACK + WeightHD × SS_HDBLACK
The reason that we used a single set of weights, rather than condition-specific weights was to ensure meaningful comparisons when testing black versus white differences. In addition, since the overall HPD EOL intensity score has mean 0 and standard deviation (SD) 1, condition-specific means and SDs represent deviations from the overall intensity score.
Acknowledgments
The authors acknowledge Max Farrell and Elan Cohen for their intellectual contributions to data management and statistical analysis. They also thank Jeremy Kahn for his comments on a previous draft of this article. This work was funded by a research grant awarded to Amber Barnato from the National Institute of Aging (R01AG035112).
Author Disclosure Statement
C.-C.H.C., J.R.L., and D.C.A. report having no actual or potential conflicts of interest.
References
- 1.Fisher ES, Wennberg DE, Stukel TA, et al. : The implications of regional variations in Medicare spending. Part 1: The content, quality, and accessibility of care. Ann Intern Med 2003;138:273–287 [DOI] [PubMed] [Google Scholar]
- 2.Fisher ES, Wennberg DE, Stukel TA, et al. : The implications of regional variations in Medicare spending. Part 2: Health outcomes and satisfaction with care. Ann Intern Med 2003;138:288–298 [DOI] [PubMed] [Google Scholar]
- 3.Wennberg JE, Fisher ES, Stukel TA, et al. : Use of hospitals, physician visits, and hospice care during last six months of life among cohorts loyal to highly respected hospitals in the United States. BMJ 2004;328:607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Teno JM, Mor V, Ward N, et al. : Bereaved family member perceptions of quality of end-of-life care in U.S. regions with high and low usage of intensive care unit care. J Am Geriatr Soc 2005;53:1905–1911 [DOI] [PubMed] [Google Scholar]
- 5.Barnato AE, Chang CC, Farrell MH, et al. : Is survival better at hospitals with higher “end-of-life” treatment intensity? Med Care 2010;48:125–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Earle CC, Neville BA, Landrum MB, et al. : Evaluating claims-based indicators of the intensity of end-of-life cancer care. Int J Qual Health Care 2005;17:505–509 [DOI] [PubMed] [Google Scholar]
- 7.Earle CC, Park ER, Lai B, et al. : Identifying potential indicators of the quality of end-of-life cancer care from administrative data. J Clin Oncol 2003;21:1133–1138 [DOI] [PubMed] [Google Scholar]
- 8.Greiner KA, Perera S, Ahluwalia JS: Hospice usage by minorities in the last year of life: Results from the National Mortality Followback Survey. J Am Geriatr Soc 2003;51:970–978 [DOI] [PubMed] [Google Scholar]
- 9.Pritchard RS, Fisher ES, Teno JM, et al. : Influence of patient preferences and local health system characteristics on the place of death. SUPPORT Investigators. Study to Understand Prognoses and Preferences for Risks and Outcomes of Treatment. J Am Geriatr Soc 1998;46:1242–1250 [DOI] [PubMed] [Google Scholar]
- 10.Barnato AE, Berhane Z, Weissfeld LA, et al. : Racial variation in end-of-life intensive care use: A race or hospital effect? Health Serv Res 2006;41:2219–2237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barnato AE, Chang CC, Saynina O, Garber AM. Influence of race on inpatient treatment intensity at the end of life. J Gen Intern Med 2007;22:338–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garrett JM, Harris RP, Norburn JK, et al. : Life-sustaining treatments during terminal illness: Who wants what? J Gen Intern Med 1993;8:361–368 [DOI] [PubMed] [Google Scholar]
- 13.O'Brien LA, Grisso JA, Maislin G, et al. : Nursing home residents' preferences for life-sustaining treatments. JAMA 1995;274:1775–1779 [PubMed] [Google Scholar]
- 14.McKinley ED, Garrett JM, Evans AT, Danis M: Differences in end-of-life decision making among black and white ambulatory cancer patients. J Gen Intern Med 1996;11:651–656 [DOI] [PubMed] [Google Scholar]
- 15.Diringer MN, Edwards DF, Aiyagari V, Hollingsworth H: Factors associated with withdrawal of mechanical ventilation in a neurology/neurosurgery intensive care unit. Crit Care Med 2001;29:1792–1797 [DOI] [PubMed] [Google Scholar]
- 16.Mebane EW, Oman RF, Kroonen LT, Goldstein MK: The influence of physician race, age, and gender on physician attitudes toward advance care directives and preferences for end-of-life decision-making. J Am Geriatr Soc 1999;47:579–591 [DOI] [PubMed] [Google Scholar]
- 17.Volandes AE, Paasche-Orlow M, Gillick MR, et al. : Health literacy not race predicts end-of-life care preferences. J Palliat Med 2008;11:754–762 [DOI] [PubMed] [Google Scholar]
- 18.Huskamp HA, Keating NL, Malin JL, et al. : Discussions with physicians about hospice among patients with metastatic lung cancer. Arch Intern Med 2009;169:954–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bach PB, Schrag D, Begg CB: Resurrecting treatment histories of dead patients: A study design that should be laid to rest. JAMA 2004;292:2765–2770 [DOI] [PubMed] [Google Scholar]
- 20.Barnato AE, Farrell MH, Chang CC, et al. : Development and validation of hospital ‘end-of-life” treatment intensity measures. Med Care 2009;47:1098–1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arias E, Heron M, Xu J: United States Life Tables, 2012. In: Centers for Disease Control and Prevention NCfHS, ed., Vol. 65 Hyattsville, MD: US Department of Health and Human Services, 2016, pp. 1–64 [PubMed] [Google Scholar]
- 22.Steen P, Cherney B: Evolution of analytical tools by MediQual Systems, Inc. Am J Med Qual 1996;11:S15–S17 [PubMed] [Google Scholar]
- 23.Pine M, Jordan HS, Elixhauser A, et al. : Enhancement of claims data to improve risk adjustment of hospital mortality. JAMA 2007;297:71–76 [DOI] [PubMed] [Google Scholar]
- 24.Barnato AE, Mohan D, Lane RK, et al. : Advance care planning norms may contribute to hospital variation in end-of-life ICU use: A simulation study. Med Decision Making 2014;34:473–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barnato AE, Tate JA, Rodriguez KL, et al. : Norms of decision making in the ICU: A case study of two academic medical centers at the extremes of end-of-life treatment intensity. Intensive Care Med 2012;38:1886–1896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barnato AE, Herndon MB, Anthony DL, et al. : Are regional variations in end-of-life care intensity explained by patient preferences?: A Study of the US Medicare Population. Med Care 2007;45:386–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jha AK, Orav EJ, Li Z, Epstein AM: Where do elderly black Americans receive hospital care? Racial concentration in hospital care and the performance of hospitals that care for blacks [abstract]. J Gen Intern Med 2006;21(Suppl. 4):16516390502 [Google Scholar]
- 28.Simpson EH: The interpretation of interaction in contingency tables. J R Stat Soc Series B 1951;13:238–241 [Google Scholar]
- 29.Asch DA, Armstrong K: Aggregating and partitioning populations in health care disparities research: Differences in perspective. J Clin Oncol 2007;25:2117–2121 [DOI] [PubMed] [Google Scholar]
- 30.Barnato AE, Lucas FL, Staiger D, et al. : Hospital-level racial disparities in acute myocardial infarction treatment and outcomes. Med Care 2005;43:308–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lyon SM, Benson NM, Cooke CR, et al. : The effect of insurance status on mortality and procedural use in critically ill patients. Am J Respir Crit Care Med 2011;184:809–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nicholas LH, Langa KM, Iwashyna TJ, Weir DR: Regional variation in the association between advance directives and end-of-life Medicare expenditures. JAMA 2011;306:1447–1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.