Abstract
AIM
To evaluate the short-term and long-term outcomes following laparoscopic vs open surgery for pathological T4 (pT4) colorectal cancer.
METHODS
We retrospectively analyzed the short- and long-term outcomes of proven pT4 colorectal cancer patients who underwent complete resection by laparoscopic or open surgery from 2006 to 2015 at Guangdong General Hospital.
RESULTS
A total of 211 pT4 colorectal cancer patients were included in this analysis, including 101 cases in the laparoscopy (LAP) group and 110 cases in the open surgery (OPEN) group [including 15 (12.9%) cases of conversion to open surgery]. Clinical information (age, gender, body mass index, comorbidities, American Society of Anesthesiologists score, etc.) did not differ between the two groups. In terms of blood loss, postoperative complications and rate of recovery, the LAP group performed significantly more favorably (P < 0.05). With regard to pT4a/b and combined organ resection, there were significantly more cases in the OPEN group (P < 0.05). The 3- and 5-year overall survival rates were 74.9% and 60.5%, respectively, for the LAP group and 62.4% and 46.5%, respectively, for the OPEN group (P = 0.060). The 3- and 5-year disease-free survival rates were 68.0% and 57.3%, respectively, for the LAP group and 55.8% and 39.8%, respectively, for the OPEN group (P = 0.053). Multivariate analysis showed that IIIB/IIIC stage, lymph node status, and CA19-9 were significant predictors of overall survival. PT4a/b, IIIC stage, histological subtypes, CA19-9, and adjuvant chemotherapy were independent factors affecting disease-free survival.
CONCLUSION
Laparoscopy is safely used in the treatment of pT4 colorectal cancer while offering advantages of minimal invasiveness and faster recovery. Laparoscopy is able to achieve good oncologic outcomes similar to those of open surgery. We recommend that laparoscopy be carried out in experienced centers. It is still required to screen the appropriate cases for laparoscopic surgery, optimize the preoperative diagnosis process, and reduce the conversion rate. Multi-center, prospective, and large-sample studies are required to assess these issues.
Keywords: pT4 colorectal cancer, Laparoscopy, Open surgery
Core tip: Laparoscopy has been widely used in the treatment of colorectal cancer and has achieved a good radical effect in oncology. However, current clinical association guidelines do not recommend laparoscopic surgery for T4 colorectal cancer. This study retrospectively collected the data of pathological T4 (pT4) colorectal cancer patients at Guangdong General Hospital from 2006 to 2015, aiming to compare outcomes of laparoscopic vs open surgery. The conclusion is that laparoscopy is safely used in the treatment of pT4 colorectal cancer while offering advantages of faster recovery. Laparoscopy is able to achieve good oncologic outcomes similar to those of open surgery.
INTRODUCTION
Colorectal cancer is a common malignant tumor. It is the third most diagnosed cancer and the fourth leading cause of cancer-related deaths worldwide[1]. In China, the incidence and mortality of colorectal cancer are ranked among the top five of all cancers; thus, colorectal cancer is a very serious public health problem[2]. In promoting comprehensive, individualized, and precise treatments to date, surgical treatment is still the only way to cure colorectal cancer. Since 1991, when Jacobs first reported the technical feasibility of laparoscopic colectomy[3], a number of successful randomized controlled studies have been conducted around the world to compare laparoscopy and laparotomy, with encouraging results achieved. The laparoscopic treatment of colorectal cancer can not only achieve similar short- and long-term outcomes comparable to laparotomy, but its advantage of minimal invasiveness has gradually been recognized and promoted[4-7]. The American Joint Committee on Cancer (AJCC) classifies T4 colorectal cancers as those that invade into other organs and structures and/or perforate the visceral peritoneum; laparoscopic surgery in colorectal cancer at this stage is difficult as it is hard to reach and violates the “no touch” principle. Therefore, the AJCC and European Association of Endoscopic Surgery do not recommend laparoscopic treatment of pathological T4 (pT4) colorectal cancer[8]. This study retrospectively collected the data of pT4 colorectal cancer patients at Guangdong General Hospital from 2006 to 2015, aiming to compare the outcomes of laparoscopic vs open surgery.
MATERIALS AND METHODS
Patients
All pT4 colorectal cancer patients treated at Guangdong General Hospital from 2006 to 2015 were enrolled in this study. All patients were staged according to the AJCC 7th edition manual for colorectal cancer. The inclusion criteria included the following: (1) age of 18-75 years; (2) proven T4 pathology; and (3) radical surgery (D3 lymph node dissection). The exclusion criteria included the following: (1) low rectal cancer (peritoneal reflection as the boundary); (2) preoperative neoadjuvant treatment; (3) non-neoplastic deaths; and (4) palliative resection.
Surgical procedure
Preoperative computed tomography (CT) and magnetic resonance imaging were used to determine the preoperative clinical stage. The decision to proceed with laparoscopy or open surgery was made for all subjects on a patient-by-patient basis following multidisciplinary discussions and meetings. All cases entailed surgical resection according to the Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines[9], which have the following requirements: D3 lymph node dissection (Figure 1) to ensure the appropriate resection length, and ensuring that the integrity of the mesorectum and intraoperative operations follow the principle of “no touch” (sharp separation, blood vessels first, tumor isolation, etc.). According to tumor location, the method of resection included the following: total colectomy, right colectomy, extended right colectomy, transverse colectomy, left colectomy, sigmoid colectomy, mid/upper anterior resection, and combined organ resection. Laparoscopic incision should not exceed 6 cm. The conversion cases were analyzed in the open surgery (OPEN) group.
Figure 1.
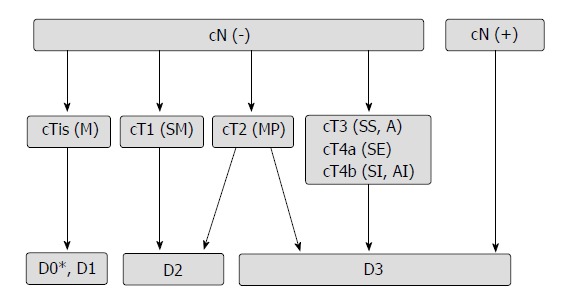
Flowchart for selection of the extent of lymph node dissection (from reference 9).
Observation indexes
The preoperative indexes included age, gender, body mass index (BMI; kg/m2), comorbidity, American Society of Anesthesiologists (ASA) score, tumor location, hemoglobin, and tumor markers (CA19-9 and CEA). The intraoperative indexes included surgical and pathological outcomes. Surgical outcomes included the conversion rate (conversion was defined as an open surgery performed during the laparoscopic procedure in order to ensure complete resection, reconstruction, or hemostasis and not just for the extraction of specimens), tumor size, resection length, operative time, blood loss, intraoperative complications, combined organ resection, postoperative complications and mortality. Pathological outcomes included the number of lymph nodes dissected, lymph node status, margin, pT stage, pN stage, pTNM stage, Dukes stage, histological subtype, and differentiation. The postoperative recovery indexes included time to flatus, diet, and ambulation and hospital stays.
Follow-up
All patients were postoperatively referred to the 7th AJCC/UICC TNM stage for adjuvant chemotherapy. All patients were followed through outpatient visits. According to the NCCN guidelines, patients were subjected to a 5-year surveillance program consisting of physical examinations and tumor marker (CEA and CA19-9) analysis every 3 mo up to 2 years. Every 6 mo, patients had complete colonoscopies at one and three years after surgery. Thoracic and abdominal CT scans were planned every year for five years of surveillance.
Statistical analysis
Statistical analyses were performed using SPSS 19.0. Quantitative data are reported as the mean ± SD or median. Categorical data were compared by χ2 tests or Fisher’s exact test. Survival curves [overall survival (OS) and disease-free survival (DFS)] were derived from Kaplan-Meier estimates, and the curves were compared by the log-rank test. Prognostic factors were identified by univariate analysis and further tested by multivariate analysis. The results are reported as a hazard ratio (HR) with 95%CI. A P-value < 0.05 was considered statistically significant.
RESULTS
During the period from 2006 to 2015, we collected a total of 211 pT4a/bN0-2M0 cases according to enrollment criteria from 2308 cases of colorectal cancer at the Department of General Surgery of Guangdong General Hospital. There were 101 cases in the laparoscopy (LAP) group and 111 cases in the OPEN group (Figure 2).
Figure 2.
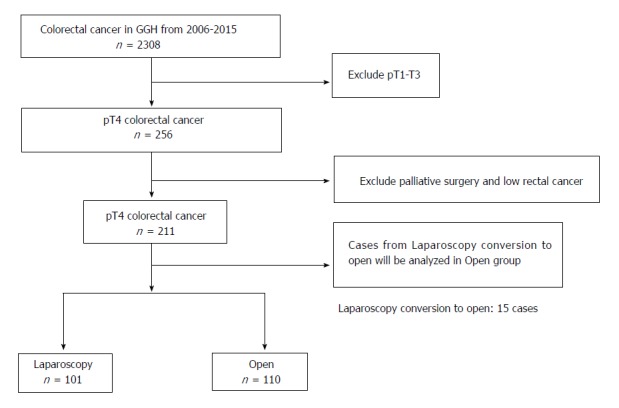
Study flowchart showing patient selection. GGH: Guangdong general hospital; pT4: Pathological proven T4.
There were no significant differences in age, gender, BMI, ASA score, tumor location, hemoglobin, CA19-9, or CEA between the two groups (P > 0.05) (Table 1).
Table 1.
Clinical information of 211 colorectal cancer cases
| Clinical information | LAP | OPEN | P value | |
| n = 101 | n = 110 | |||
| Age | > 60 yr | 55 | 58 | 0.270 |
| ≤ 60 yr | 46 | 52 | ||
| Gender | Male | 67 | 66 | 0.392 |
| Female | 34 | 44 | ||
| BMI (kg/m2) | < 24 | 67 | 73 | 0.348 |
| ≥ 24 | 34 | 37 | ||
| Comorbidities | Yes | 39 | 42 | 1.000 |
| No | 62 | 68 | ||
| ASA score | I | 8 | 9 | 0.715 |
| II | 63 | 72 | ||
| III | 30 | 29 | ||
| Tumor location | Mid/upper Rectum | 33 | 35 | 0.989 |
| Left colon | 43 | 47 | ||
| Right colon | 25 | 28 | ||
| HBG (g/L) | mean ± SD | 124.0 ± 27.1 | 120.7 ± 22.9 | 0.263 |
| CA19-9 (U/mL) | < 27 | 78 | 75 | 0.163 |
| ≥ 27 | 23 | 35 | ||
| CEA (ng/mL) | < 5 | 60 | 64 | 0.666 |
| ≥ 5 | 41 | 46 | ||
| Postoperative adjuvant chemotherapy | Yes | 49 | 46 | 0.332 |
| No | 52 | 64 | ||
| Recurrence | Yes | 22 | 25 | 0.711 |
| No | 79 | 85 | ||
CRC: Colorectal cancer; LAP: Laparoscopic surgery group; OPEN: Open surgery group; BMI: Body mass index; ASA: American Society of Anesthesiology; HGB: Hemoglobin; CA19-9: Carbohydrate antigen 19-9; CEA: Caicinoembryonic antigen; SD: Standard deviation.
For surgical outcome, conversion to open surgery occurred in 15 (12.9%) patients, and all conversion cases were analyzed in the OPEN group. There was no significant difference between the two groups in terms of intraoperative complications and postoperative complications within 30 d (P > 0.05). Laparoscopic surgery was slightly slower than open surgery (210.8 ± 88.9 min vs 173.5 ± 72.7 min, P = 0.028); there was less blood loss (155.0 ± 75.9 mL vs 235.1 ± 120.5 mL, P = 0.033) in laparoscopic surgery, whereas open surgery showed better resection lengths (15.5 ± 7.3 cm vs 19.5 ± 10.4 cm, P = 0.046). In the case of combined organ resection, there were 21 (19.1%) patients in the OPEN group, including three cases of abdominal wall resection, five cases of small bowel (except duodenum) resection, three cases of duodenum resection, two cases of urinary organ resection, one case of stomach resection, four cases of gynecologic organ resection, and three cases of liver resection; in contrast, there were only five cases in the LAP group (P = 0.001). For postoperative complications within 30 d, there were 12 (12.9%) cases in the LAP group, and there was a higher incidence in the OPEN group (31.8%, P = 0.006) (Table 2).
Table 2.
Surgical outcomes of 211 colorectal cancer cases
| Surgical outcome | LAP | OPEN | P value | |
| n = 101 | n = 110 | |||
| Conversion to open (n/%) | 15 (12.9) | NR | / | |
| Tumor size (cm) | mean ± SD | 5.4 ± 1.9 | 5.2 ± 2.5 | 0.765 |
| Resection length (cm) | mean ± SD | 15.5 ± 7.3 | 19.5 ± 10.4 | 0.046 |
| Operative time (min) | mean ± SD | 210.8 ± 88.9 | 173.5 ± 72.7 | 0.028 |
| Blood loss (mL) | mean ± SD | 155.0 ± 75.9 | 235.1 ± 120.5 | 0.033 |
| Intraoperative complications | 3 | 8 | 0.117 | |
| Combined organ resection | Total (%) | 5 (5.0) | 21 (19.1) | 0.001 |
| Abdominal wall | 2 | 3 | ||
| Small bowel (except duodenum) | 1 | 5 | ||
| Duodenum | 0 | 3 | ||
| Urinary organs | 0 | 2 | ||
| Stomach | 1 | 1 | ||
| Gynecologic organs | 1 | 4 | ||
| Liver | 0 | 3 | ||
| Postoperative complications within 30 d | Total (%) | 12 (12.9) | 35 (31.8) | 0.006 |
| Anastomotic Hemorrhage | 1 | 2 | ||
| Urinary injury | 0 | 1 | ||
| Intraabdominal bleeding | 1 | 2 | ||
| Leakage | 1 | 4 | ||
| Gastroplegia | 2 | 4 | ||
| Infection | 6 | 15 | ||
| (incision and abdomen) | ||||
| Disruption of incision | 0 | 5 | ||
| Obstruction | 1 | 2 | ||
| Postoperative morbidity within 30 d | 0 | 1 | 0.667 |
CRC: Colorectal cancer; LAP: Laparoscopic surgery group; OPEN: Open surgery group.
Regarding pathologic outcomes, no significant differences in the number of lymph nodes dissected, lymph node status, margin, pN stage, pTNM stage, Dukes stage, histological subtype, differentiation, or HER2 status were detected when comparing the two groups (P > 0.05). There were 21 pT4b cases in the OPEN group but only five cases in the LAP group; a comparison between the two groups in the pT stage revealed a statistically significant difference (P = 0.021) (Table 3).
Table 3.
Pathologic outcomes of 211 colorectal cancer cases
| Pathologic outcome | LAP | OPEN | P value | |
| n = 101 | n = 110 | |||
| Number of lymph nodes dissected | < 12 | 25 | 38 | 0.134 |
| ≥ 12 | 76 | 72 | ||
| Lymph node status | + | 67 | 74 | 1.000 |
| - | 34 | 36 | ||
| Margin | R1 | 2 | 3 | 0.779 |
| R0 | 99 | 107 | ||
| pT stage | T4a | 96 | 89 | 0.021 |
| T4b | 5 | 21 | ||
| pN stage | N0 | 36 | 35 | 0.841 |
| N1 | 28 | 32 | ||
| N2 | 37 | 43 | ||
| pTNM stage | IIB + IIC | 34 | 35 | 0.282 |
| IIIB | 30 | 24 | ||
| IIIC | 37 | 51 | ||
| Dukes | B | 34 | 35 | 0.883 |
| C | 67 | 75 | ||
| Histological subtype | Adenocarcinoma | 87 | 94 | 1.000 |
| Myxoadenocarcinoma | 14 | 16 | ||
| Differentiation | Poor | 20 | 25 | 0.719 |
| Median/high | 81 | 85 | ||
| HER2 | -/+ | 86 | 96 | 0.871 |
| ++ | 10 | 10 | ||
| +++ | 5 | 4 | ||
CRC: Colorectal cancer; LAP: Laparoscopic surgery group; OPEN: Open surgery group; SD: Standard deviation; p: Pathological.
With regard to postoperative recovery indexes, the LAP group was significantly better than the OPEN group (P < 0.05) in time to flatus, diet, and ambulation. The median hospital stay was 7 (5-21) d for the LAP group and 15 (7-31) d for the OPEN group, which showed a statistically significant difference between the two groups (P = 0.004) (Table 4).
Table 4.
Postoperative recovery outcomes of 211 colorectal cancer cases
| Recovery outcome | LAP | OPEN | P value | |
| n = 101 | n = 110 | |||
| Time to flatus (d) | Median (range) | 2 (1-9) | 4 (3-15) | 0.037 |
| Time to diet (d) | Median (range) | 3 (2-18) | 7 (5-27) | 0.003 |
| Time to ambulation (d) | Median (range) | 2 (1-5) | 5 (3-9) | 0.027 |
| Hospital stays | Median (range) | 7 (5-21) | 15 (7-31) | 0.004 |
LAP: Laparoscopic surgery group; OPEN: Open surgery group.
The mean overall follow-up time was 36 mo (range, 2-24 mo); there was no difference between the LAP and OPEN groups in terms of OS and DFS. The 3- and 5-year OS rates were 74.9% and 60.5%, respectively, for the LAP group and 62.4% and 46.5%, respectively, for the OPEN group (P = 0.60) (Figure 3). The 3- and 5-year DFS rates were 68.0% and 57.3%, respectively, for the LAP group and 55.8 and 39.8%, respectively, for the OPEN group (P = 0.053) (Figure 4). Disease recurrence over the entire follow-up period was observed in 21.8% (n = 22) of patients in the LAP group and 22.7% (n = 25) of patients in the OPEN group (P = 0.711) (Table 1), without significant differences between the LAP and OPEN groups (P = 0.711). In the multivariate regression analysis, TNM stage (IIIB, IIIC), lymph node status (pN+), and CA19-9 were significant predictors of OS. TNM stage (IIIC), histological subtype, CA19-9, and chemotherapy were predictive of DFS (Tables 5 and 6).
Figure 3.
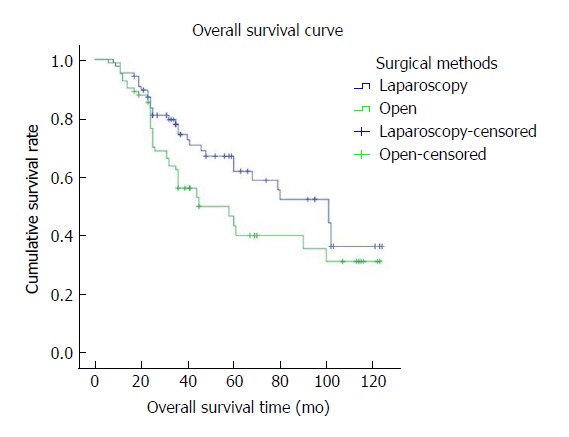
The overall survival curve shows that 3- and 5-year overall survival rates were 74.9% and 60.5%, respectively, in the LAP group and 62.4% and 46.5%, respectively, in the OPEN group. There was no significant difference between the LAP and OPEN groups (P = 0.060).
Figure 4.
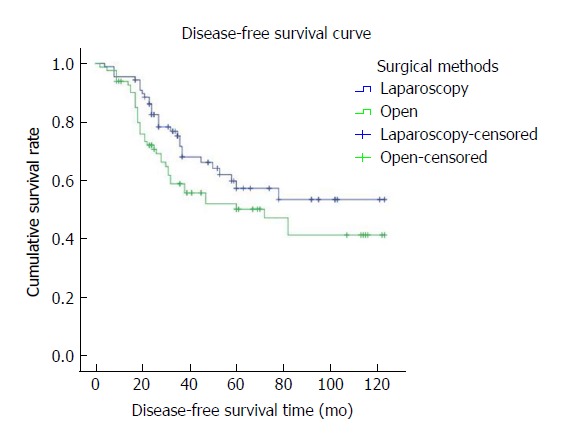
The disease-free survival curve shows that the 3- and 5-year disease-free survival rates were 68.0% and 57.3%, respectively, in the LAP group and 55.8% and 39.8%, respectively, in the OPEN group. There was no significant difference between the LAP and OPEN groups (P = 0.053).
Table 5.
Univariate and multivariate analyses of 211 pathological T4 colorectal cancer patients for overall survival
|
Univariate analysis |
Multivariate analysis |
|||
| Variable | HR (95%CI) | P value | HR (95%CI) | P value |
| Age | 1.217 (0.784-1.888) | 0.381 | ||
| Gender | 0.807 (0.508-1.281) | 0.363 | ||
| Surgical method (LAP and OPEN) | 1.528 (0.982-2.377) | 0.060 | ||
| Tumor location | ||||
| Mid/upper rectum | Reference group | - | ||
| Left colon | 1.303 (0.813-2.091) | 0.272 | ||
| Right colon | 0.792 (0.409-1.533) | 0.489 | ||
| Comorbidities | 1.603 (1.007-2.552) | 0.047 | 2.519 (1.436-4.419) | 0.142 |
| pT4a/b | 0.790 (0.692-1.236) | 0.445 | ||
| N stage | ||||
| N0 | Reference group | - | ||
| N1 | 1.328 (0.701-2.517) | 0.384 | ||
| N2 | 2.079 (1.170-3.697) | 0.013 | ||
| TNM stage | ||||
| IIB + IIC | Reference group | - | Reference group | - |
| IIIB | 1.229 (0.564-2.679) | 0.604 | 1.324 (0.785-1.753) | 0.019 |
| IIIC | 3.092 (1.617-5.913) | 0.001 | 1.104 (0.333-3.662) | 0.001 |
| Lymph node status | 0.560 (0.324-0.968) | 0.038 | 0.307 (0.103-0.919) | 0.035 |
| No. of lymph nodes resected | 0.593 (0.385-0.915) | 0.018 | 0.432 (0.264-0.708) | 0.123 |
| Histological subtype | 0.369 (0.212-0.640) | 0.000 | 0.433 (0.218-0.859) | 0.247 |
| Differentiation | 0.326 (0.204-0.519) | 0.000 | 0.460 (0.273-0.775) | 0.087 |
| CA19-9 | 1.868 (1.195-2.922) | 0.006 | 1.662 (1.212-2.280) | 0.002 |
| CEA | 1.089 (0.706-1.680) | 0.013 | 0.608 (0.356-1.038) | 0.068 |
| Chemotherapy | 1.611 (1.040-2.494) | 0.033 | 2.225 (1.394-3.552) | 0.181 |
CRC: Colorectal cancer; LAP: Laparoscopy surgery; OPEN: Open surgery; p: Pathological; CA19-9: Carbohydrate antigen 19-9; CEA: Carcinoembryonic antigen; HR: Hazard ratio.
Table 6.
Univariate and multivariate analyses of 211 pathological T4 colorectal cancer patients for disease-free survival
|
Univariate analysis |
Multivariate analysis |
|||
| Variable | HR (95%CI) | P value | HR (95%CI) | P value |
| Age | 1.621 (1.010-2.601) | 0.045 | 1.892 (1.111-3.223) | 0.419 |
| Gender | 1.328 (0.824-2.141) | 0.243 | ||
| Surgical method (LAP and OPEN) | 1.503 (0.933-2.422) | 0.094 | ||
| Tumor location | ||||
| Mid/upper rectum | Reference group | - | ||
| Left colon | 1.010 (0.601-10697) | 0.969 | ||
| Right colon | 0.818 (0.411-1.629) | 0.568 | ||
| Comorbidities | 1.787 (1.058-3.019) | 0.030 | 2.261 (1.235-4.139) | 0.080 |
| pT4a/b | 0.818 (0.618-1.725) | 0.013 | 1.214 (0.784-1.974) | 0.001 |
| N stage | ||||
| N0 | Reference group | - | ||
| N1 | 1.134 (0.594-2.167) | 0.703 | ||
| N2 | 1.553 (0.861-2.801) | 0.144 | ||
| TNM stage | ||||
| IIB + IIC | Reference group | - | Reference group | - |
| IIIB | 1.034 (0.471-2.269) | 0.933 | 0.884 (0.393-1.989) | 0.765 |
| IIIC | 2.284 (1.202-4.337) | 0.012 | 1.831 (0.935-3.584) | 0.018 |
| Lymph node status | 0.710 (0.411-1.229) | 0.221 | ||
| No. of lymph nodes resected | 0.661 (0.411-1.061) | 0.087 | ||
| Histological subtype | 0.456 (0.243-0.854) | 0.014 | 0.469 (0.225-0.974) | 0.042 |
| Differentiation | 0.439 (0.266-0.725) | 0.001 | 0.662 (0.374-1.170) | 0.156 |
| CA19-9 | 2.458 (1.526-3.960) | 0.000 | 3.372 (1.968-5.778) | 0.000 |
| CEA | 1.268 (0.790-2.036) | 0.326 | 0.608 (0.356-1.038) | 0.072 |
| Chemotherapy | 2.157 (1.323-3.514) | 0.002 | 3.817 (2.194-6.639) | 0.000 |
CRC: Colorectal cancer; LAP: Laparoscopic surgery; OPEN: Open surgery; p-Pathological; CA19-9: Carbohydrate antigen 19-9; CEA: Carcinoembryonic antigen; HR: Hazard ratio.
DISCUSSION
Since the first report of laparoscopic colorectal resection in 1991, some prospective clinical studies of laparoscopic resection for colorectal cancer have confirmed that laparoscopic techniques not only achieve minimally invasive and cosmetic effects but also achieve faster recovery and similar oncologic outcomes compared with open surgery[10-12]. However, due to the large tumor size of T4 colorectal cancer and more frequent invasion of peripheral tissues or nearby organs, laparoscopic complete resection is difficult and has high risks; the majority of clinical studies have fewer cases of T4 colorectal cancer[13,14], and some studies do not enroll any such cases[15,16]. Therefore, the evidence-based data that support the laparoscopic resection in T4 colorectal cancer are limited. Laparoscopic resection of T4 colorectal cancer is regarded a technique that demands precision, and its efficacy remains controversial. The relevant guidelines do not recommend laparoscopy in T4 colorectal cancer[8]. However, due to the maturity and progress of the laparoscopic platform, coupled with the popularity of and improvements in laparoscopic techniques, some surgeons in certain experienced centers have tried to use laparoscopic techniques in T4 colorectal cancer, achieving similar short- and long-term outcomes to open surgery[17-20].
This study decided whether laparoscopic or open surgery should be performed based on the results of preoperative imaging examination and the patient’s condition; the main referenced indicators included the following: tumor location, tumor size, and the scope of invaded organ[21]. We found a statistically significant difference in the postoperative pT stage between the two groups (P = 0.021), with 21 cases of pT4b in the OPEN group. An examination of postoperative surgical outcomes (Table 2) revealed 21 cases of combined organ resection in the OPEN group, with the most commonly invaded organs including the small intestine, gynecological organs, and duodenum; in contrast, the LAP group had only five cases, and the number of cases of combined organ resection was thus significantly different for the two groups (P = 0.001), which is consistent with the results of previous studies[22,23]. These data also demonstrate that T4b stage may be an important consideration for surgeons to select laparoscopic or open surgery because it is very difficult to achieve the goal of complete resection using the “no touch” principle; therefore, guidelines do not recommend laparoscopic resection in T4 colorectal cancer[8]. However, such considerations lead to selective bias in the study, which is one of the limitations in both this study and a retrospective study.
Because of the larger tumor, a wide scope of invasion, combined with resection of other organs, especially due to the lack of laparoscopic experience in some centers, may lead to a high conversion rate. Previous studies of laparoscopic surgery in colorectal cancer (stage II-III)[11,15,24] reported that the conversion rate was 25% in the CLASSIC trial (for colon cancer), 17% in the COLOR trial, and 21% in the COSTSG trial, whereas a retrospective study of pT4 colorectal cancer showed that the conversion rate was 5.6%-24.7%[17,19,22,25-29]; this study showed a conversion rate of 12.9%, which is consistent with reports in the literature. The conversion rates reported by some studies of pT4 colorectal cancer in South Korea and Singapore were 5.6%, 7.7% and 8.6%[22,29,30], which are significantly lower than those from the US and Europe[17,18,25,27] (Table 7). We explain these differences as follows: (1) Laparoscopic technology and experience may differ between Asian and Western countries; (2) the conversion standard used was different; (3) there was a lack of preoperative imaging assessments to select appropriate laparoscopic surgery cases in Western countries; and (4) European populations had a higher BMI. All these factors increase the difficulty of surgery[17-25]. Thus, surgeons should choose the appropriate pT4 colorectal cases to perform laparoscopic surgery in order to reduce the conversion rate and ensure operation safety. We recommend laparoscopic surgery as an option in experienced centers and for T4a cases with tumor sizes < 5 cm and when only a single organ has been invaded by T4 colorectal cancer.
Table 7.
Studies about laparoscopic surgery for pathological T4 colorectal cancer
| Ref. | n (LAP:OPEN) | Study period | Study design | Country | Single/Multi center | Conversion rate | Location | Complication rate (LAP:OPEN) | Tumor stage | Combined resection (LAP:OPEN) | RI rate (LAP:OPEN) | 5 years DFS (LAP:OPEN) | 5 years OS (LAP:OPEN) |
| Park et al[22], 2016 | 93:18 | 2000-2010 | RS | South Korea | Single | 5.6% | Colon | 14.1%:31.5% | - | 9.9%:32.5% | 5.5%:4.5% | 81.8%:73.9% | 95.3%:86.5% |
| rectum | |||||||||||||
| Shukla et al[20], 2015 | 61:22 | 2003-2011 | RS | United States | Single | 21% | Colon | 28%:36% | II:35 | 23%:41% | 0%:4% | 75%:65% | 82%:81% |
| III:48 | (3 yr) | (3 yr) | |||||||||||
| Kang et al[29], 2016 | 52:57 | 2003-2013 | RS | South Korea | Single | 7.7% | Colon | 13.5%:36.8% | II:41 | 13.5%:36.8% | - | 53.6%:62.6% | 60.7%:61.9% |
| III:68 | |||||||||||||
| de’Angelis et al[18], 2016 | 106:106 | 2005-2014 | RPSM | France | Multi | 12.2% | Colon | 29.1%:35.3% | II:85 | 14.2%:18.9% | 5.7%:6.6% | 58.6%:59.9% | 57.6%:50.2% |
| Switzerland | III:127 | ||||||||||||
| de’Angelis et al[17], 2016 | 52:52 | 2005-2015 | RPSM | France | Multi | 21.2% | Rectum | 30.8%:48.1% | II:42 | 26.9%:30.8% | 19.2%:17.3% | 66.7%:64.1% | 55.4%:53.3% |
| Switzerland | III:33 | ||||||||||||
| Spain | IV:29 | ||||||||||||
| Chan et al[30], 2016 | 93:59 | 2008-2014 | RS | Singapore | Single | 8.6% | Colon | - | - | 0%:3.4% | 0%:0.7% | - | 75%:80% |
| Leon et al[27], 2017 | 68:79 | 2008-2015 | RS | Italy | Single | 19% | Colon | 7.4%:16.5% | II:69 | - | 11.8%:11.5% | 40.3%:38.9% | 44.6%:39.4% |
| III:78 | |||||||||||||
| Ahmad et al[25], 2015 | 455:406 | 2011-2012 | RS | Canada | ACSNS | 24.7% | Colon | - | - | - | 26.2%:24.3% | - | - |
| QIP |
LAP: Laparoscopy; RS: Retrospective study; RPSM: Retrospective propensity score matching; ACSNS: American College of Surgeons National Surgical Quality Improvement Program; OS: Overall survival; DFS: Disease-free survival.
In this study, the LAP group had longer operative time (210.8 ± 88.9 min vs 173.5 ± 72.7 min, P = 0.028), which may be related to the lack of experience and the difficulty of this surgery[19]. The resection length (19.5 ± 10.4 cm) obtained in the OPEN group was significantly better (P = 0.046). The literature shows that the incidence of postoperative complications associated with laparoscopic surgery was clearly lower than that associated with open surgery[31]. In this study, the rate of postoperative complication within 30 d in the LAP group was 12.9% (12/101), which was lower than 31.8% (35/110) in the OPEN group, and the most common complications in the OPEN group were infection (incision and abdomen) and disruption of incision, similar to a previous report in the literature[32]. Therefore, attention should be paid to intraoperative sterile principles and the suture of incision.
With regard to postoperative recovery outcomes, the LAP group had clear advantages in time to flatus (P = 0.037), diet (P = 0.003), and ambulation (P = 0.027) and hospital stays (P = 0.004) compared with the OPEN group (Table 4). Laparoscopy has the advantages of minimal invasion and fast recovery, which is in agreement with many earlier clinical studies[12,15,16].
In colorectal cancer surgery, lymph node dissection and R0 resection are important factors affecting long-term survival[33]. We performed D3 lymphadenectomy (parenteral lymph node -middle lymph node-central lymph node)[34] according to the guidelines recommended by the JSCCR. Previous studies have shown that laparoscopic treatment of colorectal cancer achieved an R0 resection rate between 80.8% and 98%[11,16,18]. In this study, the percentage of cases with the number of lymph nodes dissected greater than 12 was 75.2% (76/101) in the LAP group and 65.5% (72/110) in the OPEN group, and the difference was not significantly different (P = 0.134). Concurrently, the R0 resection rate in the LAP group was 98% (99/101), whereas in the OPEN group, it was 97.3% (107/110), which was not significantly different (P = 0.779). Thus, we believe that laparoscopic treatment in pT4 colorectal cancer can achieve similar oncological outcomes to open surgery. Finally, no differences in the 3- and 5-year OS rates (P = 0.060) and in 3- and 5-year DFS rates (P = 0.053) were observed when comparing the two groups, suggesting that laparoscopy may be a valid and effective tool to treat pT4 colorectal cancer without jeopardizing oncologic results, in accordance with the previously reported series. Multivariate analysis in our series detected IIIB/IIIC stage, lymph node status, and CA19-9 as independent predictors of OS, and pT4a/b, IIIC stage, CA19-9, and adjuvant chemotherapy as independent predictors of DFS.
In conclusion, laparoscopic surgery may be safe and acceptable in the treatment of pathologic T4 colorectal cancer patients with fast recovery outcomes and oncologic outcomes compared with open surgery. Thus, laparoscopy should not be regarded as an absolute contraindication in the management of pT4 colorectal cancer. Finally, as this study is only a retrospective study in a single center with a small sample size, the results need to be confirmed by prospective, multi-center and large sample clinical studies.
ARTICLE HIGHLIGHTS
Research background
Laparoscopy has been widely used in the treatment of colorectal cancer and it has achieved a good radical effect in oncology. However, for the current clinical guidelines, laparoscopic surgery is not recommended in T4 colorectal cancer.
Research motivation
Due to the characteristics of T4 colorectal cancer, laparoscopic complete resection is difficult for the resection of this kind of tumor. The current colorectal studies about laparoscopy have fewer cases of T4 colorectal cancer, and some studies do not enroll any such cases. We tried to collect and analyze the data about laparoscopy in T4 colorectal cancer in order to add evidence-based clinical evidence.
Research objectives
We aimed to analyze the short- and long-term outcomes of proven pathological T4 colorectal cancer patients who underwent complete resection by laparoscopic or open surgery.
Research methods
We collected and analyzed the data of pT4 colorectal cancer cases at Guangdong General Hospital from 2006 to 2015. All patients were staged according to the AJCC 7th edition manual for colorectal cancer. We compared the laparoscopy (LAP) group and open (OPEN) group in clinical information, surgical and pathological outcomes, postoperative recovery outcomes, and survival.
Research results
There were 101 cases in the LAP group and 110 cases in the OPEN group [including15 (12.9%) cases of conversion to open surgery]. Clinical information did not differ between the two groups. In terms of blood loss, postoperative complications, and rate of recovery, the LAP group performed significantly more favorably (P < 0.05). With regard to pT4a/b and combined organ resection, there were significantly more cases in the OPEN group (P < 0.05). The 3- and 5-year overall survival rates were 74.9% and 60.5%, respectively, for the LAP group and 62.4% and 46.5%, respectively, for the OPEN group (P = 0.060). The 3- and 5-year disease-free survival rates were 68.0% and 57.3%, respectively, for the LAP group and 55.8% and 39.8%, respectively, for the OPEN group (P = 0.053). Multivariate analysis showed that IIIB/IIIC stage, lymph node status, and CA19-9 were significant predictors of overall survival. PT4a/b, IIIC stage, histological subtype, CA19-9, and adjuvant chemotherapy were independent factors affecting disease-free survival.
Research conclusions
Laparoscopic surgery may be safe and acceptable in the treatment of pathologic T4 colorectal cancer patients with fast recovery outcomes and oncologic outcomes compared with open surgery. We recommend that it can be carried out in experienced centers. It is required to screen the appropriate cases for laparoscopic surgery, optimize the preoperative diagnosis process, and reduce the conversion rate.
Research perspectives
Although our study shows that laparoscopy is able to achieve good clinicopathological and oncologic outcomes similar to those of open surgery, this study is only a retrospective study in a single center with a small sample, and the results need to be confirmed by prospective, multi-center and large sample clinical studies.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): E
Supported by Natural Science Foundation of Guangdong Province, No. 2016A030310328 and No. 2016A030313762.
Institutional review board statement: This study was evaluated and approved by the ethics committee at our institution.
Informed consent statement: All study participants, or their legal guardian, provided informed written consent prior to study enrollment.
Conflict-of-interest statement: All authors declare no conflict of interest.
Data sharing statement: No additional data are available.
Peer-review started: October 27, 2017
First decision: November 8, 2017
Article in press: November 21, 2017
P- Reviewer: Kim SM, Sterpetti AV, Yokoyama S S- Editor: Chen K L- Editor: Wang TQ E- Editor: Huang Y
Contributor Information
Zi-Feng Yang, Department of General Surgery, Guangdong General Hospital, Guangdong Academy of Medical Sciences, Guangzhou 510080, Guangdong Province, China.
De-Qing Wu, Department of General Surgery, Guangdong General Hospital, Guangdong Academy of Medical Sciences, Guangzhou 510080, Guangdong Province, China.
Jun-Jiang Wang, Department of General Surgery, Guangdong General Hospital, Guangdong Academy of Medical Sciences, Guangzhou 510080, Guangdong Province, China.
Ze-Jian Lv, Department of General Surgery, Guangdong General Hospital, Guangdong Academy of Medical Sciences, Guangzhou 510080, Guangdong Province, China.
Yong Li, Department of General Surgery, Guangdong General Hospital, Guangdong Academy of Medical Sciences, Guangzhou 510080, Guangdong Province, China. s_09zfyang@163.com.
References
- 1.Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66:683–691. doi: 10.1136/gutjnl-2015-310912. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.Jacobs M, Verdeja JC, Goldstein HS. Minimally invasive colon resection (laparoscopic colectomy) Surg Laparosc Endosc. 1991;1:144–150. [PubMed] [Google Scholar]
- 4.Veldkamp R, Kuhry E, Hop WC, Jeekel J, Kazemier G, Bonjer HJ, Haglind E, Påhlman L, Cuesta MA, Msika S, Morino M, Lacy AM; COlon cancer Laparoscopic or Open Resection Study Group (COLOR) Laparoscopic surgery vs open surgery for colon cancer: short-term outcomes of a randomised trial. Lancet Oncol. 2005;6:477–484. doi: 10.1016/S1470-2045(05)70221-7. [DOI] [PubMed] [Google Scholar]
- 5.Colon Cancer Laparoscopic or Open Resection Study Group, Buunen M, Veldkamp R, Hop WC, Kuhry E, Jeekel J, Haglind E, Påhlman L, Cuesta MA, Msika S, Morino M, Lacy A, Bonjer HJ. Survival after laparoscopic surgery vs open surgery for colon cancer: long-term outcome of a randomised clinical trial. Lancet Oncol. 2009;10:44–52. doi: 10.1016/S1470-2045(08)70310-3. [DOI] [PubMed] [Google Scholar]
- 6.Di B, Li Y, Wei K, Xiao X, Shi J, Zhang Y, Yang X, Gao P, Zhang K, Yuan Y, et al. Laparoscopic vs open surgery for colon cancer: a meta-analysis of 5-year follow-up outcomes. Surg Oncol. 2013;22:e39–e43. doi: 10.1016/j.suronc.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Deijen CL, Vasmel JE, de Lange-de Klerk ESM, Cuesta MA, Coene PLO, Lange JF, Meijerink WJHJ, Jakimowicz JJ, Jeekel J, Kazemier G, Janssen IMC, Påhlman L, Haglind E, Bonjer HJ; COLOR (COlon cancer Laparoscopic or Open Resection) study group. Ten-year outcomes of a randomised trial of laparoscopic vs open surgery for colon cancer. Surg Endosc. 2017;31:2607–2615. doi: 10.1007/s00464-016-5270-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mathis KL, Nelson H. Controversies in laparoscopy for colon and rectal cancer. Surg Oncol Clin N Am. 2014;23:35–47. doi: 10.1016/j.soc.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 9.Muro K. Systemic chemotherapy for metastatic colorectal cancer -Japanese Society for Cancer of the Colon and Rectum (JSCCR) Guidelines 2016 for treatment of colorectal cancer. Nihon Shokakibyo Gakkai Zasshi. 2017;114:1217–1223. doi: 10.11405/nisshoshi.114.1217. [DOI] [PubMed] [Google Scholar]
- 10.Kim RH, Kavanaugh MM, Caldito GC. Laparoscopic colectomy for cancer: Improved compliance with guidelines for chemotherapy and survival. Surgery. 2017;161:1633–1641. doi: 10.1016/j.surg.2016.11.024. [DOI] [PubMed] [Google Scholar]
- 11.Bonjer HJ, Deijen CL, Abis GA, Cuesta MA, van der Pas MH, de Lange-de Klerk ES, Lacy AM, Bemelman WA, Andersson J, Angenete E, et al. A randomized trial of laparoscopic vs open surgery for rectal cancer. N Engl J Med. 2015;372:1324–1332. doi: 10.1056/NEJMoa1414882. [DOI] [PubMed] [Google Scholar]
- 12.Kang SB, Park JW, Jeong SY, Nam BH, Choi HS, Kim DW, Lim SB, Lee TG, Kim DY, Kim JS, et al. Open vs laparoscopic surgery for mid or low rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): short-term outcomes of an open-label randomised controlled trial. Lancet Oncol. 2010;11:637–645. doi: 10.1016/S1470-2045(10)70131-5. [DOI] [PubMed] [Google Scholar]
- 13.Stevenson AR, Solomon MJ, Lumley JW, Hewett P, Clouston AD, Gebski VJ, Davies L, Wilson K, Hague W, Simes J; ALaCaRT Investigators. Effect of Laparoscopic-Assisted Resection vs Open Resection on Pathological Outcomes in Rectal Cancer: The ALaCaRT Randomized Clinical Trial. JAMA. 2015;314:1356–1363. doi: 10.1001/jama.2015.12009. [DOI] [PubMed] [Google Scholar]
- 14.Jeong SY, Park JW, Nam BH, Kim S, Kang SB, Lim SB, Choi HS, Kim DW, Chang HJ, Kim DY, et al. Open vs laparoscopic surgery for mid-rectal or low-rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): survival outcomes of an open-label, non-inferiority, randomised controlled trial. Lancet Oncol. 2014;15:767–774. doi: 10.1016/S1470-2045(14)70205-0. [DOI] [PubMed] [Google Scholar]
- 15.Guillou PJ, Quirke P, Thorpe H, Walker J, Jayne DG, Smith AM, Heath RM, Brown JM; MRC CLASICC trial group. Short-term endpoints of conventional vs laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet. 2005;365:1718–1726. doi: 10.1016/S0140-6736(05)66545-2. [DOI] [PubMed] [Google Scholar]
- 16.Fleshman J, Branda M, Sargent DJ, Boller AM, George V, Abbas M, Peters WR Jr, Maun D, Chang G, Herline A, Fichera A, Mutch M, Wexner S, Whiteford M, Marks J, Birnbaum E, Margolin D, Larson D, Marcello P, Posner M, Read T, Monson J, Wren SM, Pisters PW, Nelson H. Effect of Laparoscopic-Assisted Resection vs Open Resection of Stage II or III Rectal Cancer on Pathologic Outcomes: The ACOSOG Z6051 Randomized Clinical Trial. JAMA. 2015;314:1346–1355. doi: 10.1001/jama.2015.10529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de’Angelis N, Landi F, Vitali GC, Memeo R, Martínez-Pérez A, Solis A, Assalino M, Vallribera F, Mercoli HA, Marescaux J, et al. Multicentre propensity score-matched analysis of laparoscopic vs open surgery for T4 rectal cancer. Surg Endosc. 2017;31:3106–3121. doi: 10.1007/s00464-016-5332-9. [DOI] [PubMed] [Google Scholar]
- 18.de’Angelis N, Vitali GC, Brunetti F, Wassmer CH, Gagniere C, Puppa G, Tournigand C, Ris F. Laparoscopic vs. open surgery for T4 colon cancer: A propensity score analysis. Int J Colorectal Dis. 2016;31:1785–1797. doi: 10.1007/s00384-016-2646-y. [DOI] [PubMed] [Google Scholar]
- 19.Ng DC, Co CS, Cheung HY, Chung CC, Li MK. The outcome of laparoscopic colorectal resection in T4 cancer. Colorectal Dis. 2011;13:e349–e352. doi: 10.1111/j.1463-1318.2011.02698.x. [DOI] [PubMed] [Google Scholar]
- 20.Shukla PJ, Trencheva K, Merchant C, Maggiori L, Michelassi F, Sonoda T, Lee SW, Milsom JW. Laparoscopic resection of t4 colon cancers: is it feasible? Dis Colon Rectum. 2015;58:25–31. doi: 10.1097/DCR.0000000000000220. [DOI] [PubMed] [Google Scholar]
- 21.Vignali A, Ghirardelli L, Di Palo S, Orsenigo E, Staudacher C. Laparoscopic treatment of advanced colonic cancer: a case-matched control with open surgery. Colorectal Dis. 2013;15:944–948. doi: 10.1111/codi.12170. [DOI] [PubMed] [Google Scholar]
- 22.Park JS, Huh JW, Park YA, Cho YB, Yun SH, Kim HC, Lee WY, Chun HK. Clinically suspected T4 colorectal cancer may be resected using a laparoscopic approach. BMC Cancer. 2016;16:714. doi: 10.1186/s12885-016-2753-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huh JW, Kim HR. The feasibility of laparoscopic resection compared to open surgery in clinically suspected T4 colorectal cancer. J Laparoendosc Adv Surg Tech A. 2012;22:463–467. doi: 10.1089/lap.2011.0425. [DOI] [PubMed] [Google Scholar]
- 24.Clinical Outcomes of Surgical Therapy Study Group, Nelson H, Sargent DJ, Wieand HS, Fleshman J, Anvari M, Stryker SJ, Beart RW Jr, Hellinger M, Flanagan R Jr, Peters W, Ota D. A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med. 2004;350:2050–2059. doi: 10.1056/NEJMoa032651. [DOI] [PubMed] [Google Scholar]
- 25.Elnahas A, Sunil S, Jackson TD, Okrainec A, Quereshy FA. Laparoscopic vs open surgery for T4 colon cancer: evaluation of margin status. Surg Endosc. 2016;30:1491–1496. doi: 10.1007/s00464-015-4360-1. [DOI] [PubMed] [Google Scholar]
- 26.Kim IY, Kim BR, Kim YW. The short-term and oncologic outcomes of laparoscopic vs open surgery for T4 colon cancer. Surg Endosc. 2016;30:1508–1518. doi: 10.1007/s00464-015-4364-x. [DOI] [PubMed] [Google Scholar]
- 27.Leon P, Iovino MG, Giudici F, Sciuto A, de Manzini N, Cuccurullo D, Corcione F. Oncologic outcomes following laparoscopic colon cancer resection for T4 lesions: a case-control analysis of 7-years’ experience. Surg Endosc. 2017 doi: 10.1007/s00464-017-5784-6. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 28.Kim KY, Hwang DW, Park YK, Lee HS. A single surgeon’s experience with 54 consecutive cases of multivisceral resection for locally advanced primary colorectal cancer: can the laparoscopic approach be performed safely? Surg Endosc. 2012;26:493–500. doi: 10.1007/s00464-011-1907-7. [DOI] [PubMed] [Google Scholar]
- 29.Kang J, Baik SH, Lee KY, Sohn SK. Outcomes of laparoscopic surgery in pathologic T4 colon cancers compared to those of open surgery. Int J Colorectal Dis. 2017;32:531–538. doi: 10.1007/s00384-016-2720-5. [DOI] [PubMed] [Google Scholar]
- 30.Chan DK, Tan KK. Laparoscopic surgery should be considered in T4 colon cancer. Int J Colorectal Dis. 2017;32:517–520. doi: 10.1007/s00384-016-2702-7. [DOI] [PubMed] [Google Scholar]
- 31.Ohtani H, Tamamori Y, Arimoto Y, Nishiguchi Y, Maeda K, Hirakawa K. A meta-analysis of the short- and long-term results of randomized controlled trials that compared laparoscopy-assisted and open colectomy for colon cancer. J Cancer. 2012;3:49–57. doi: 10.7150/jca.3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liao CH, Tan EC, Chen CC, Yang MC. Real-world cost-effectiveness of laparoscopy vs open colectomy for colon cancer: a nationwide population-based study. Surg Endosc. 2017;31:1796–1805. doi: 10.1007/s00464-016-5176-3. [DOI] [PubMed] [Google Scholar]
- 33.Yamamoto S, Inomata M, Katayama H, Mizusawa J, Etoh T, Konishi F, Sugihara K, Watanabe M, Moriya Y, Kitano S; Japan Clinical Oncology Group Colorectal Cancer Study Group. Short-term surgical outcomes from a randomized controlled trial to evaluate laparoscopic and open D3 dissection for stage II/III colon cancer: Japan Clinical Oncology Group Study JCOG 0404. Ann Surg. 2014;260:23–30. doi: 10.1097/SLA.0000000000000499. [DOI] [PubMed] [Google Scholar]
- 34.Watanabe T, Muro K, Ajioka Y, Hashiguchi Y, Ito Y, Saito Y, Hamaguchi T, Ishida H, Ishiguro M, Ishihara S, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2016 for the treatment of colorectal cancer. Int J Clin Oncol. 2017 doi: 10.1007/s10147-017-1101-6. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]


