Abstract
Novel vaccines against substances of abuse generate a strong antibody response that prevents the drug from entering the brain. These could become a safe and efficient tool to combat a growing crisis of opioid abuse.
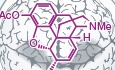
Subject Categories: Chemical Biology, S&S: Health & Disease
In August 2017, US President Donald Trump declared the abuse of opioids by millions of Americans a national state of emergency and highlighted the staggering statistics associated with substance use disorder (SUD), which are at record highs. The NIH reported that in 2015, 2.6 million Americans suffered from opioid use disorder (OUD), which caused US$78.5 billion in healthcare, law enforcement, and lost productivity costs for prescription opioids alone 1. A 2016 National Survey on Drug Use and Health reported that approximately 13 million Americans had misused opioids, including heroin, oxycodone, hydrocodone, and fentanyl, one or more times in the preceding year 2. Most strikingly, the incidence of opioid‐related overdose deaths has quadrupled since 1999, with no signs of slowing. Currently, more than 90 Americans die each day from opioid overdose 3. While the statistics are grim, an increasing commitment and more resources to develop new and efficient therapies for OUD gives hope for effectively combating the drug crisis. Earlier this year, the NIH launched a scientific initiative to combat OUD emphasizing three research areas: improvement of overdose‐reversal interventions; new treatments for opioid addiction; and alternative strategies to safely manage chronic pain 1.
A promising approach for treating addiction is immunopharmacotherapy: vaccination against a drug of abuse to induce a tailored antibody response, which prevents the drug from entering the brain and eliciting its psychoactive effects. Vaccines against heroin, oxycodone, hydrocodone, and fentanyl addiction have already shown great success in preclinical evaluation, but have yet to enter clinical trials. This commentary discusses the clinical potential for opioid vaccines along with the experimental and societal aspects that have so far prevented the development of this approach. Ultimately, the field is on the verge of delivering a vaccine with clinical utility.
Vaccines against opioids
Currently, only four FDA‐approved medications are available for treating OUD—methadone, buprenorphine, naloxone, and naltrexone, which compete directly with opioids at the μ‐opioid receptor in the brain (MOR). Methadone and buprenorphine treat opioid dependence, while naltrexone and naloxone reverse the effects of opioid overdose. While these therapies are extremely beneficial in dire situations, they exhibit significant side effects including dysphoria, abuse, and/or overdose of the therapeutic itself. Most importantly, these therapeutics have failed to thwart the continued growth of OUD in the USA.
Currently, more than 90 Americans die each day from opioid overdose
A complementary strategy for treating OUD is active vaccination using an opioid‐like small‐molecule protein immunoconjugate to stimulate high‐affinity, anti‐opioid IgG antibodies. These antibodies bind circulating drug and thereby prevent the opioid from crossing the blood–brain barrier (BBB) where the MORs are located. Conjugate vaccines are a particularly promising approach because the antibodies thus generated have long half‐lives, which allows for treatment on the timescale of months, far outlasting the metabolism and elimination of small‐molecule therapeutics. Moreover, because immunopharmacotherapy primes the body's own immune system to combat opioid effects, there are limited side effects. Taken together, conjugate vaccines against opioids offer an unconventional strategy that overcomes many of the liabilities associated with traditional pharmaceuticals.
Designing vaccines for drugs of abuse uses a three‐component recipe: the hapten, conjugate protein, and adjuvant. Opioids themselves are not immunogenic, so an opioid analog must be chemically linked, that is, conjugated to an immunogenic carrier protein to create the antigenic motif. The immunoconjugate is then combined with adjuvants, or immunostimulatory agents, to form the vaccine (Fig 1).
Figure 1. Mode of action of vaccines against substances of abuse.

The conjugate vaccine instills a strong antibody response against heroin or other substances of abuse, which prevents the drug from entering the brain and latching on its target receptor.
The concept of anti‐opioid vaccination was first demonstrated in 1974, when self‐administration of heroin by a rhesus monkey was mildly attenuated with a morphine‐bovine serum albumin (BSA) conjugate vaccine 4. However, it was not until the 1990s, when vaccines for cocaine and nicotine were advanced to clinical trials, that the field took on a reinvigorated interest. While unsuccessful in the clinic, these early studies highlighted crucial features of vaccines against drugs of abuse that became the focus of subsequent research efforts. Namely, four fundamental concepts are key to the development of a successful conjugate vaccine: The vaccine's protective effects must not be surmounted by higher drug doses; antibodies must exhibit high binding affinities for the drug; sufficient antibody concentrations must be achieved; and the metabolic profile of the drug must be considered. Recent advancements in vaccine design, pioneered by our laboratory, have sought to address these key considerations.
Hapten design
The most important component in vaccine design is the hapten, which must maximize structural congruence to the native drug to increase the likelihood of producing antibodies with high affinity for the opioid. That being said, the drug mimetic must be chemically modified to install a linker, which covalently attaches the hapten to the carrier protein but must not disturb key functional groups for biological interaction, such as hydrogen bonding and chiral functionality. As a general principle, extending linkers off inherent alkyl chains or aromatic rings typically improves hapten design.
Understanding the pharmacokinetics of opioid metabolism is also essential for designing the vaccine hapten, because metabolism not only alters the chemical structure of the drug, but metabolites often exhibit their own unique psychoactive properties. Heroin itself is a prodrug and is rapidly deacetylated into the metabolites 6‐acetylmorphine (6‐AM) and morphine 5. Importantly, 6‐AM, not heroin, is the principal psychoactive compound. Consequently, hapten design based on the structure of 6‐AM is a successful approach to ensure efficacious vaccination against heroin. In fact, heroin vaccines with haptens that cannot be hydrolyzed to their 6‐AM and/or morphine counterparts fail to effectively block heroin psychoactivity.
The chemical identity of the linker and the method of bioconjugation also determine the efficacy of the vaccine. Two strategies of bioconjugation have been thoroughly investigated: thiol‐maleimide and amide coupling. In short, amide coupling involving a linker with a terminal carboxylic acid, subsequently activated to an N‐hydroxysuccinimide ester prior to coupling, accomplishes an improved anti‐opioid immune response over thiol‐maleimide linkers. As with linker placement, the linker itself should not act as a chemical epitope for antibody generation. As such, the linker should be linear in makeup, avoiding bulky cyclic functionalities. Recent results have suggested that peptidic linkers based on natural amino acids can improve hapten immunogenicity.
Opioid vaccines require the generation of high antibody concentrations with strong affinity for their target to effectively sequester the molar quantities of drug consumed. To achieve these antibody concentrations, vaccines must stimulate a robust T‐helper (Th2)‐type immune response, which requires the right choice of immunogenic carrier protein, adjuvant, and dosing schedule. As stated, a conjugate protein is essential for opioid vaccine efficacy because it converts the hapten from a T‐cell‐independent antigen to a T‐cell‐dependent antigen. Our laboratory has identified the following trend in carrier protein immunogenicity for heroin vaccines: ovalbumin (OVA) ~ BSA < keyhole limpet hemocyanin (KLH) < diphtheria toxoid (DT) < tetanus toxoid (TT). In addition to being the most effective carrier proteins, DT and TT have also been approved for clinical use in carbohydrate conjugate vaccines, validating their safety profile and clinical utility.
… conjugate vaccines against opioids offer an unconventional strategy that overcomes many of the liabilities associated with traditional pharmaceuticals.
Equally essential for conjugate vaccines are the adjuvant formulations, without which the immunoconjugate can only stimulate a weak immune response. In our experience, the most successful adjuvant formulation for opioid vaccines involves two components: colloidal aluminum salts (alum) and cytosine‐phosphodiester‐guanine oligonucleotide (CpG ODN). Alum functions as a depot, lumping the immunoconjugates into larger size particles near the injection site, which is required for uptake by antigen‐presenting cells and successful downstream immune activation. Other adjuvants that directly stimulate Toll‐like receptors (TLRs) of the innate immune system, such as CpG ODN, are now frequently used in combination with alum to enhance the immune response. Compared with alum alone, alum plus CpG ODN has improved the efficacy of our heroin vaccine in both rodent and primate models in attenuating drug potency.
A final consideration for developing efficacious opioid vaccines is the immunization schedule. Opioid vaccination requires the continued maintenance of antibody concentrations with high affinity for the drug. Thus, a vaccination schedule of at least three injections about 4 weeks apart is recommended. Peak antibody concentrations are observed 2–4 weeks after injection. Owing to the rapid decay of antibody titers within 2–4 months of the last injection, vaccines for drugs of abuse will require more frequent booster injections compared to vaccines for infectious diseases.
Safety and efficacy
A key question in the development of opioid vaccines is how to accurately evaluate vaccine efficacy both in vitro and in vivo. Though many vaccines have shown great promise in the developmental stages, the few vaccines that have entered clinical trials have so far failed; understanding these failures helps to improve translation. From an immunological standpoint, antibodies generated upon vaccination must possess high concentrations with tight binding affinity for the drug. The quick‐pass analysis for evaluating antibody titers is enzyme‐linked immunosorbent assay (ELISA). However, ELISAs are limited as a result of consistency between repeat assays, an inability to compare titer results run at different times or by different researchers, and the fact that titers are determined for the structure of the hapten and not the drug itself. Despite these limitations, ELISA is a rapid method to gauge the relative potency of an antigen‐specific immune response, to prompt more quantitative secondary assays, if promising results are observed.
Such secondary assays, namely surface plasmon resonance in the case of opioid vaccines, are necessary to accurately quantify antibody affinities for free drug and cross‐reactivities with other opioids. In this assay, increasing concentrations of free drug and a predetermined dilution of antiserum are added to immobilized hapten. The free drug and hapten compete for antibody binding, and a response signal is measured as a function of free drug concentration, from which antiserum affinities can be calculated. The use of immobilized hapten remains a limitation in this design, and methodologies, such as radioimmunoassay (RIA), are more accurate alternatives. However, RIA requires the use of isotopically labeled drugs as radioactive tracers. Unfortunately, due to the rapid metabolism of heroin, the instability of its radio‐labeled equivalent is not compatible with the required assay times and RIA cannot be employed as a quantitative measure for the characterization of heroin vaccines. Importantly, despite various in vitro immunological assays for characterizing opioid vaccines, the specific levels of antibody titers, concentrations, and affinities required for translation to the clinic are unknown.
A key question in the development of opioid vaccines is how to accurately evaluate vaccine efficacy both in vitro and in vivo.
In vivo behavioral experiments in animal models still represent the best approach for evaluating vaccine efficacy. For opioids, nociception assays such as tail‐flick and hot‐plate tests, which measure acute pain, are the standard for measuring a drug's analgesic effects. In these experiments, an animal's reaction time to nociception stimuli, such as heat, is measured against increasing concentrations of opioid to form a dose–response curve. Successful vaccination yields a right‐shifting of the dose–response curve, which equates to higher opioid doses required to achieve the normal analgesic effect. Degrees of curve shifting offer an excellent quantitative measure for vaccine efficacy. Promising results in antinociception testing prompt more sophisticated in vivo studies such as conditioned‐place preference and self‐administration. These studies evaluate the vaccine's ability to reduce the motivational effects of the opioid and an animal's self‐administration of the drug, respectively.
Ready for clinical testing
Employing these strategies and techniques, recent efforts by our laboratory and others have generated state‐of‐the‐art vaccines against opioids, with promising results in primate models. The current optimal design for a heroin vaccine incorporates a heroin mimic conjugated to TT and formulated with alum and CpG ODN. We installed a peptidic linker off the bridging N‐methyl functionality in heroin with both terminal thiol (HerSH) and carboxylic acid (HerCOOH) moieties for bioconjugation. Our first‐generation HerSH‐TT vaccine reduced heroin antinociception at doses above 5 mg/kg in mice, outperforming the best vaccines in the field, which demonstrated the same effect at 1 mg/kg 6. In rats, this vaccine effectively blocked the effects of 10 times the amount of heroin compared to their unvaccinated counterparts. Our second‐generation HerCOOH‐TT vaccine reduced heroin antinociception at doses higher than 10 mg/kg in rhesus monkeys 7. Remarkably, the vaccine blocked the effects of heroin for more than 8 months with 3‐month boosters. Moreover, the vaccine protected against lethal heroin overdoses, which is critical in a clinical setting.
Vaccines against fentanyl are still in earlier developmental stages, mainly owing to the drug's recent appearance in illicit drug use. Fentanyl and its analogs are commonly used as adulterants in heroin and account for a recent spike in overdose deaths. Our laboratory has developed the first vaccine against fentanyl, a TT conjugate with alum and CpG ODN adjuvants, which generates antibodies with single‐digit nanomolar affinities, shifted the fentanyl dose–response curve 30‐fold in antinociception testing in mice, and protected not only against fentanyl, but commonly observed fentanyl ester analogs 8. Most importantly, the vaccine was able to protect against lethal doses of 2.2 and 4.4 mg/kg; unvaccinated mice experienced 18 and 55% overdose fatalities at these doses.
We are truly at a point where current vaccine developments hover on the brink of translation to the clinic.
Finally, work in our laboratory and the Pravetoni group has pioneered the first vaccines for oxycodone and hydrocodone abuse. Pravetoni's first vaccines used a tetraglycine linker at the 6‐position of oxycodone via an oxime. The second‐generation vaccine, which was conjugated to TT and adjuvanted with alum, was effective against both oxycodone and hydrocodone in mice 9. We investigated oxy‐ and hydrocodone haptens conjugated through the bridging nitrogen, analogously to the aforementioned HerCOOH‐TT vaccine. This accomplished fivefold to 10‐fold shifts in the dose–response curve in mouse antinociception testing, reduced opioid biodistribution, and increased resistance to opioid overdose mortality 10.
Based on the recent results of our heroin vaccine in primate studies, this vaccine is positioned to enter clinical trials upon garnering the necessary financial backing. While vaccines for fentanyl, oxy‐ and hydrocodone are in earlier stages, the wealth of knowledge gained from developing the best‐in‐class heroin vaccine, with regard to hapten design, bioconjugation methodology, and carrier protein and adjuvant choice, has quickly propelled these vaccines through the preclinical pipeline. Current next steps include evaluation in more sophisticated behavioral testing and primate models.
Challenges for trials in humans
Despite the failure of early conjugate vaccines for cocaine and nicotine in clinical trials, we remain optimistic that current opioid vaccines will meet clinical endpoints. Profound scientific advances in the design and development of opioid vaccines, including the optimization of hapten design, bioconjugation methodology, carrier proteins, and adjuvants, have been accomplished. We are truly at a point where current vaccine developments hover on the brink of translation to the clinic.
Yet, there is work to be done. Before initiating a clinical trial, it will be fundamentally important to define the patient population. The ideal vaccine recipient will be patients who desire opioid abstinence, and it is crucial that these individuals are cooperative in their treatment. Because opioid vaccines sequester the drug away from the brain and the MORs, they could induce symptoms of withdrawal and drug cravings. Consequently, combination treatments of vaccination and opioid replacement therapy, such as methadone or buprenorphine, that mitigate these symptoms may offer the optimal treatment strategy for addiction. However, this hypothesis is in early stages, and experiments that evaluate the simultaneous use of opioid vaccination with opioid replacement therapy are required to demonstrate its feasibility.
Moreover, OUD is typically characterized by the consumption of more than one opioid based on user access. As a result, simultaneous immunization with multiple opioid haptens may prove to be the most advantageous strategy. In other words, vaccinating against one opioid may not be enough as individuals can turn toward other opioids for a high. A combination vaccine of 6‐AM and oxycodone conjugates has shown to successfully protect against both opioids as effectively as their individual vaccines. Oxycodone and 6‐AM are structurally similar, however, and it remains unknown whether this vaccination strategy would be effective against structurally unique opioids, such as heroin and fentanyl. A combination vaccine with these components would be particularly useful as heroin is often laced with fentanyl on the street.
… clinically available vaccines will offer individuals suffering OUD another tool to combat their addiction.
Finally, as discussed, the required thresholds for antibody concentrations, affinities, and fold shifts in dose–response curves required for successful translation of vaccines to the clinic is unknown. Unfortunately, until a successful trial is completed, it is not possible to define benchmarks for preclinical vaccine evaluation. With that being stated, failed first‐generation vaccines for cocaine and nicotine were not subjected to the rigorous preclinical testing that is conducted today.
A matter of support and funding
Despite these advancements in preclinical development of opioid vaccines, there are societal influences that play a role in slowing translation to the clinic. The primary bottleneck is lack of financial investment from the pharmaceutical industry. Financial backing is limited for a few reasons: the previous clinical failures of cocaine and nicotine vaccines, but more importantly; the stigma associated with addiction and the hesitancy to treat the condition as any other disease; the fact that a large percentage of opioid users are marginalized in society, without financial resources for meaningful therapy; and concerns about vaccination interfering with necessary pain treatment.
Unfortunately, yet realistically, the pharmaceutical industry is for profit, so a company that does not project a clear return on their investment will hesitate to provide financial backing. Investment from big pharma could also present a conflict of interest, particularly in the case of vaccines for oxycodone and hydrocodone, as these prescription opioids are one of the industry's biggest moneymakers. Despite the increased allocation of resources from the NIH, the dedicated funds from the government are not sufficient to support human clinical trials.
Sidebar A: Recommended reading.
Comprehensive review on conjugate vaccines
Bremer PT, Janda KD (2017) Conjugate vaccine immunotherapy for substance use disorder. Pharmacol Rev 69: 298–315
Key references in opioid vaccine research
Bremer PT, Schlosburg JE, Lively JM, Janda KD (2014) Injection route and TLR9 agonist addition significantly impact heroin vaccine efficacy. Mol Pharm 11: 1075–1080
Bremer PT, Schlosburg JE, Banks ML, Steele FF, Zhou B, Poklis JL, Janda KD (2017) Development of a clinically‐viable heroin vaccine. J Am Chem Soc 139: 8601–8611
Bremer PT, Kimishima A, Schlosburg JE, Zhou B, Collins KC, Janda KD (2016) Combating synthetic designer opioids: a conjugate vaccine ablates lethal doses of fentanyl class drugs. Angew Chem Int Ed Engl 55: 3772–3775
Pravetoni M, Vervacke JS, Distefano MD, Tucker AM, Laudenbach M, Pentel PR (2014) Effects of currently approved carriers and adjuvants on the pre‐clinical efficacy of a conjugate vaccine against oxycodone in mice and rats. PLoS One 8: e96547
Kimishima A, Wenthur CJ, Zhou B, Janda KD (2017) An advance in prescription opioid vaccines: overdose mortality reduction and extraordinary alteration of drug half‐life. ACS Chem Biol 12: 36–40
Stowe GN et al (2011) A vaccine strategy that induces protective immunity against heroin. J Med Chem 54: 5195–5204
Schlosburg JE et al (2013) Dynamic vaccine blocks relapse to compulsive intake of heroin. Proc Natl Sci Acad USA 110: 9036–9041
A common criticism of the development of opioid vaccines is that this approach only counteracts the pharmacology of the drug, and fails to address the underlying causes of addiction. In other words, addiction is often associated with or rooted in psychiatric ailments, with patients commonly suffering anxiety, depression, anger, or post‐traumatic stress, to name a few. The field of opioid vaccine research makes no claims that vaccines offer some sort of magic bullet cure; however, clinically available vaccines will offer individuals suffering OUD another tool to combat their addiction. As is the case with many of the most debilitating diseases, such as cancer and HIV‐1, a multi‐pronged cocktail or holistic approach is required for the best outcomes. We envision a similar strategy for treating addiction, whereby opioid vaccines would be coupled with replacement therapy, as described, and psychosocial support, including counseling, behavior modification, and management of other underlying psychiatric disorders.
In theory, opioid vaccines could even achieve great success prophylactically in at risk populations; however, the stigma associated with vaccinating against opioids may be too much to bear at this stage. Unfortunately, until opioid vaccines “fit the portfolio” of big pharma and insurance companies consider addiction to be just another neurological disease, the translation of vaccines to the human patient population may come too late. Yet, there is hope as the field is much better positioned to deliver a vaccine for OUD addiction than ever before.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1. Volkow ND, Collins FS (2017) The role of science in addressing the opioid crisis. N Engl J Med 377: 391–394 [DOI] [PubMed] [Google Scholar]
- 2. U.S. Department of Health and Human Services (HHS), Office of the Surgeon General (2016) Facing addiction in America: The Surgeon General's report on alcohol, drugs, and health. Washington, DC: HHS; [PubMed] [Google Scholar]
- 3. Rudd RA, Seth P, David F, Scholl L (2016) Increases in drug and opioid‐involved overdose deaths – United States, 2010–2015. MMWR Morb Mortal Wkly Rep 65: 1445–1452 [DOI] [PubMed] [Google Scholar]
- 4. Bonese KF, Wainer BH, Fitch FW, Rothberg RM, Schuster CR (1974) Changes in heroin self‐administration by a rhesus monkey after morphine immunization. Nature 252: 708–710 [DOI] [PubMed] [Google Scholar]
- 5. Selley DE, Cao CC, Sexton T, Schwegel JA, Martin TJ, Childers SR (2001) mu Opioid receptor‐mediated G‐protein activation by heroin metabolites: evidence for greater efficacy of 6‐monoacetylmorphine compared with morphine. Biochem Pharmacol 62: 447–455 [DOI] [PubMed] [Google Scholar]
- 6. Bremer PT, Schlosburg JE, Lively JM, Janda KD (2014) Injection route and TLR9 agonist addition significantly impact heroin vaccine efficacy. Mol Pharm 11: 1075–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bremer PT, Schlosburg JE, Banks ML, Steele FF, Zhou B, Poklis JL, Janda KD (2017) Development of a clinically‐viable heroin vaccine. J Am Chem Soc 139: 8601–8611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bremer PT, Kimishima A, Schlosburg JE, Zhou B, Collins KC, Janda KD (2016) Combating synthetic designer opioids: a conjugate vaccine ablates lethal doses of fentanyl class drugs. Angew Chem Int Ed Engl 55: 3772–3775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pravetoni M, Vervacke JS, Distefano MD, Tucker AM, Laudenbach M, Pentel PR (2014) Effect of currently approved carriers and adjuvants on the pre‐clinical efficacy of a conjugate vaccine against oxycodone in mice and rats. PLoS One 8: e96547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kimishima A, Wenthur CJ, Zhou B, Janda KD (2017) An advance in prescription opioid vaccines: overdose mortality reduction and extraordinary alteration of drug half‐life. ACS Chem Biol 12: 36–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
Acknowledgements
The authors thank Dr. Lisa Eubanks, Dr. Candy Hwang, and Dr. Cody Wenthur for critical discussions. This work was supported by the NIH grants UH3 DA041146 to KDJ and F32 DA044692 to MEO.


