Abstract
The circadian clock is an evolutionarily conserved timekeeper that adapts body physiology to diurnal cycles of around 24 h by influencing a wide variety of processes such as sleep‐to‐wake transitions, feeding and fasting patterns, body temperature, and hormone regulation. The molecular clock machinery comprises a pathway that is driven by rhythmic docking of the transcription factors BMAL1 and CLOCK on clock‐controlled output genes, which results in tissue‐specific oscillatory gene expression programs. Genetic as well as environmental perturbation of the circadian clock has been implicated in various diseases ranging from sleep to metabolic disorders and cancer development. Here, we review the origination of circadian rhythms in stem cells and their function in differentiated cells and organs. We describe how clocks influence stem cell maintenance and organ physiology, as well as how rhythmicity affects lineage commitment, tissue regeneration, and aging.
Keywords: aging, circadian rhythms, clock, regeneration, stem cells
Subject Categories: Development & Differentiation, Stem Cells, Transcription
Glossary
- Adpn
adiponutrin
- Arnt
aryl hydrocarbon receptor nuclear translocator
- Arntl
aryl hydrocarbon receptor nuclear translocator‐like
- BAT
brown adipose tissue
- Bmal1
brain and muscle ARNT‐like 1
- bNGF
β‐nerve growth factor
- Cav1
caveolin 1
- CCG
clock‐controlled gene
- CCM
cardiomyocyte‐specific CLOCK mutant
- Cdk1
cyclin‐dependent kinase 1
- CKIδ
casein kinase 1, delta
- CKIε
casein kinase 1, epsilon
- CLOCK
Circadian Locomoter Output Cycles Protein Kaput
- Cry1
cryptochrome 1
- Cry2
cryptochrome 2
- Dbp
D site albumin promoter binding protein
- Dgat2
diacylglycerol O‐acyltransferase 2
- E4bp4
E4 promoter binding protein
- E‐box
enhancer box
- ES
embryonic stem
- GLUT1/8
glucose transporter 1/8
- HDAC3
histone deacetylase 3
- Hlf
hepatic leukemia factor
- HNF6
hepatocyte nuclear factor 6
- KChIP2
Kv channel interacting protein 1
- LIF
leukemia inhibitor factor
- Lipe
lipase, hormone sensitive
- NCoR
nuclear receptor co‐repressor
- Npas2
neuronal PAS domain protein 2
- Nr1d1/2
nuclear receptor subfamily 1, group D, member1/2
- Per1
period circadian clock 1
- Per2
period circadian clock 2
- PGIF‐1
human placental growth factor
- Pnpla2
patatin‐like phospholipase domain containing 2
- Ppp1cc
protein phosphatase 1, catalytic subunit, gamma isoform
- REV‐ERBα/β
reverse ERB
- RGS2
regulator of G‐protein signaling 2
- Rorα/β
RAR‐related orphan receptor alpha/beta
- RRE
Rev‐ErbA/ROR response elements
- SCA1
stem cell antigen 1
- SCF
stem cell factor
- SCN
suprachiasmatic nucleus
- Slc2a1
solute carrier family 2 member 1
- Slc2a8
solute carrier family 2 member 8
- Tcap
titin‐cap
- Tef
thyrotroph embryonic factor
- TGFβ
transforming growth factor beta
- TSPO
translocator protein
- VEGF‐A
vascular endothelial growth factor A
- WAT
white adipose tissue
- ZT
Zeitgeber
Introduction
The fact that planet Earth spins around its axis every 24 h results in day and night cycles that influence organismal functioning, which is illustrated by circadian phenotypes in almost every form of life. The circadian clock, an evolutionarily conserved time‐keeping mechanism, allows organisms to anticipate the fluctuating environment, as illustrated by, for instance, the opening and closing of plant leaves, and to turn it into their advantage.
In mammals, the central or “master clock” consists of around 20,000 neurons that are located in the suprachiasmatic nucleus (SCN) of the hypothalamus. Light that is sensed by the retina is transmitted to the SCN, and via neural and humoral factors such as glucocorticoids and melatonin, “peripheral clocks” in all organs are synchronized to entrain the whole body to diurnal cycles. This rhythm persists in the absence of physiological clues (e.g., in complete darkness), has a “free‐running” period of around 24 h, and is even apparent in cells cultured in vitro 1, 2.
The molecular machinery that underlies the circadian clock consists of a transcriptional/translational feedback loop that is coordinated by the rhythmic binding of a heterodimer of two core clock transcription factors, BMAL1 and CLOCK, to enhancer boxes (E‐boxes) in the promoters of the negative regulators Period (Per) and Cryptochrome (Cry). Subsequent expression of Per and Cry suppresses the transcription of Bmal1 and Clock, thereby establishing an oscillating negative feedback loop (Fig 1). While this core pathway is shared among tissues, the resulting rhythmic transcription of clock‐controlled output genes (CCGs) is highly tissue‐specific. This is necessary to meet the physiological needs of every organ, and it has been shown that 43% of all protein‐coding genes display circadian expression in at least one organ 3. The liver, for instance, has up to 15% of its transcripts expressed in a diurnal manner 3, 4.
Figure 1. The molecular clock pathway.
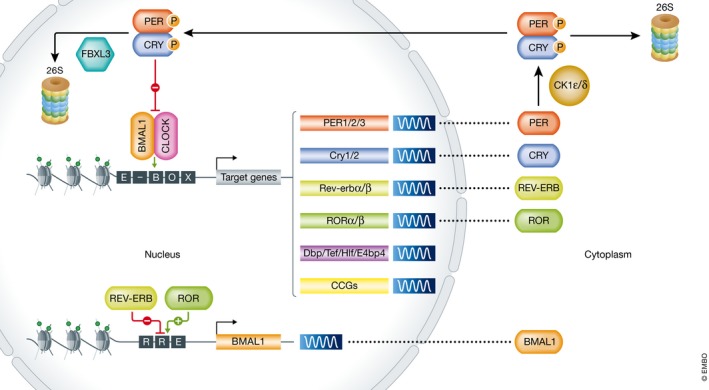
Schematic of the transcriptional/translational feedback loop. The BMAL1:CLOCK heterodimer binds enhancer (E)‐Box elements within regulatory sequences of its target genes, such as Per, Cry, Rev‐erb, Ror, Dbp, Tef, Hlf, E4bp4, and clock‐controlled genes (CCGs). Upon transcriptional induction of Per and Cry, PER and CRY proteins accumulate and dimerize in the cytosol, where they either get degraded by the 26S proteasome upon CK1ε/δ‐mediated phosphorylation or from where they migrate to the nucleus to inhibit BMAL1:CLOCK transcriptional activity and therefore their own transcription. Upon gradual phosphorylation and ubiquitination by FBXL3, they are degraded in the nucleus, completing the first feedback loop. The second BMAL1:CLOCK‐dependent feedback loop is driven by rhythmic Ror and Rev‐erb transcription. Upon accumulation of their respective proteins in the cytosol, ROR and REV‐ERB shuttle to the nucleus where they activate/repress Bmal1 transcription via competitive binding to the REV‐ERB/ROR response (RRE) element in its regulatory sequences. Additional post‐transcriptional/translational/epigenetic modifications mediate robustness of the pathway, thereby establishing cycles of around 24 h of rhythmic BMAL1:CLOCK‐mediated transcriptional activation of CCGs.
The importance of maintaining proper clock function is illustrated by the fact that its disturbance is implicated in multiple pathological conditions, such as impaired metabolism, cardiovascular diseases, sleep disorders, cancer, and even hampered regenerative capacities 5. Therefore, the circadian clock is under intense investigation in differentiated cells, adult stem cells, and even embryonic stem cells.
Embryonic stem (ES) cells are pluripotent cells, derived from the inner cell mass of the blastocyst and can form all cells of the embryo proper 6. In vitro, these cells proliferate indefinitely and can give rise to derivatives of all three germ layers (ecto‐, meso‐, and endoderm). Indeed, directed differentiation toward clinically relevant cell types such as neurons 7, hepatocytes 8, and cardiac cells 9 is common practice nowadays. Therefore, these cells hold great promise for disease modeling, drug development as well as cell‐based regenerative therapies.
In contrast to ES cells, adult stem cells are multipotent cells that can proliferate and differentiate into specific lineages only. This type of stem cells is now known to be present in several organs, such as the bone marrow (hematopoietic stem cells), the intestine (intestinal stem cells), the skin (epidermal stem cells), the heart (cardiac stem cells), and many more organs 10. These cells provide a pool for cell renewal, thereby ensuring tissue homeostasis, but can also be activated specifically upon damage.
In this review, we describe how circadian rhythms evolve during embryonic stem cell differentiation, elaborate on the role of rhythmicity in adult stem cell maintenance, and discuss how the clock controls tissue physiology, regeneration, and aging by driving tissue‐specific gene expression programs.
The molecular clock comprises a transcriptional/translational feedback loop
Practically, all cells of the human body possess a functional circadian clock. The underlying molecular machinery involves a mechanism of transcriptional and translational feedback loops (Fig 1). The central players in this pathway are BMAL1 (brain and muscle ARNT‐like1) and CLOCK (Circadian Locomotor Output Cycles Kaput) that are encoded by the genes Arntl and Clock, respectively. Upon heterodimerization, these bHLH‐PAS (basic‐helix‐loop‐helix Per‐Arnt‐Single‐minded) proteins activate the transcription of other clock genes, such as Period (Per1‐2‐3) and Cryptochrome (Cry1‐2), by binding to E‐boxes near their respective promoters. Protein levels of PER and CRY are tightly regulated by stabilization and proteasome‐based degradation upon (de)phosphorylation 11, 12. After accumulation and dimerization of PER and CRY in the cytoplasm, they translocate to the nucleus in which they inhibit BMAL1:CLOCK‐mediated transcription 13 and therefore also their own transcription 14, 15, 16. Disturbing this molecular negative feedback loop results in an affected period or amplitude of circadian rhythmicity 5, 17.
A second feedback loop consists of two sets of nuclear receptors: the transcriptional activator ROR (RAR‐related orphan receptor, RORα/β) and transcriptional repressor REV‐ERB (REV‐ERBα/β, encoded by Nr1d1/2) that are all activated by the BMAL1:CLOCK heterodimer 18, 19. RORs and REV‐ERBs compete for Rev‐ErbA/ROR response elements (RRE) within the regulatory sequence of core clock genes such as Bmal1, Cry1, E4bp4, and Npas2 to fine‐tune their transcription 20, 21. In addition to transcriptional‐based circadian rhythms, non‐transcriptional oscillatory patterns in post‐transcriptional/translational modulation 22, chromatin modifications 23, binding of RNA binding factors 24, redox 25, and metabolic 26 fluxes also occur. They mainly stabilize the precise regulation of the well‐conserved clock pathway and contribute to its robustness (summarized in detail in 5).
Establishment of the clock through tissue‐specific transcription factors
The core pathway, present in every organ, ultimately results in a set of tissue‐specific clock‐controlled genes (CCGs) that are rhythmically expressed. With up to 15% of all mRNAs in a given tissue oscillating in a diurnal manner, these output genes reflect the specific temporal control of cellular physiology that is unique to each tissue 3. Intriguingly, different groups of genes peak at different times during the day (Fig 2). This is partially established by rhythmic binding of the BMAL1:CLOCK heterodimer onto E‐boxes in proximal and distal cis‐regulatory elements of CCGs. BMAL1:CLOCK abundance, and therefore, binding occurs within a specific and short time window (ZT (Zeitgeber) 6‐ZT9), but the transcription of other genes can peak at other time points in the 24‐h cycle, which suggests that additional regulatory mechanisms regulate the expression of different subsets of CCGs within one organ 27. Among the BMAL1:CLOCK‐controlled transcripts are a number of PAR‐bZIP genes, such as DBP, TEF, HLF, and E4BP4 that on their turn recognize D‐box motifs in the regulatory sequences of other CCGs. Circadian enhancers phasing in ZT9‐ZT12 were found to be enriched for this D‐box motif, while REV‐DR2/ROR motifs were found enriched in regulatory sequences of a distinct set of CCGs that peak around ZT18‐ZT24 27. The rhythmic binding of these respective binding factors (BMAL1/CLOCK, E4BP4, REV‐ERB/ROR) hints toward a molecular mechanism in which phase‐specific oscillators rhythmically influence circadian enhancers 27, 28.
Figure 2. Organ‐specific clock‐controlled genes peak at different times during the circadian cycle.
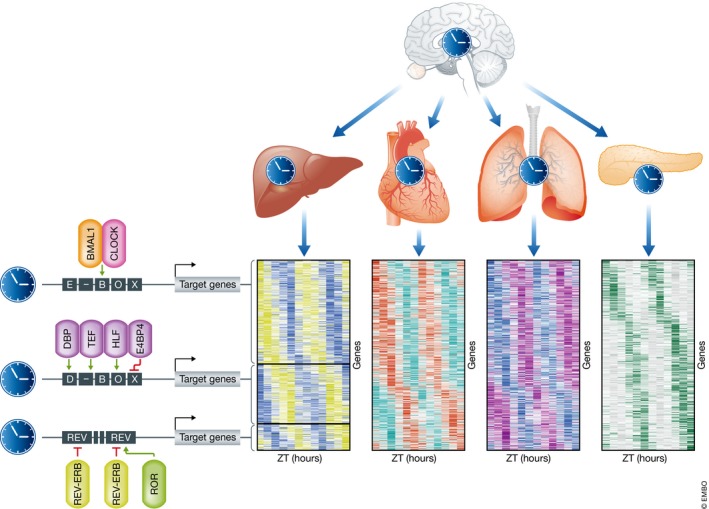
The central clock, located in the suprachiasmatic nucleus in the brain, synchronizes the clocks of peripheral clocks, which on their turn drive rhythmic expression of clock‐controlled genes (CCGs) that are often tissue‐specific (depicted as differentially colored heatmaps). This is mediated by tissue‐specific transcription factors that bind regulatory elements of CCGs, which results in peaks/phases of transcription at different ZTs (zeitgeber times).
Core clock factors are expressed in most organs, but CCGs are often organ‐specific. This can be explained by tissue‐specific chromatin conformations that are established by pioneering factors during development 29. These are able to open up nucleosome‐bound DNA, thereby opening up certain genomic regions to co‐factors that influence gene transcription. Once their binding motifs are accessible, core clock transcription factors can bind and drive rhythmic expression of CCGs by the recruitment of other regulatory complexes. In addition to this, rather than by direct binding, core clock factors can also be tethered to regulatory sequences of CCGs by cell type‐specific transcription factors. For instance, the liver‐specific transcription factor HNF6 (hepatocyte nuclear factor 6) recruits REV‐ERBα to HNF6 binding motifs in the regulatory sequences of liver metabolism genes. Subsequent recruitment of NCoR/HDAC3 (nuclear receptor co‐repressor‐histone deacetylase 3) by REV‐ERBα leads to rhythmic transcriptional repression, which results in oscillatory expression of liver metabolism genes 21, 30. For the heart, Jain and colleagues showed that the transcription factor KLF15 is responsible for REV‐ERBα‐mediated NCoR/HDAC3 facilitated oscillatory target gene expression 31. While this shows how tissue specificity is established, the tethering co‐factors that direct rhythmic target gene expression remain to be determined for many organs.
Tissue‐specific circadian clocks influence organ physiology
In a recent study, Zhang and colleagues profiled CCGs in 12 murine organs 3. These data, combined with other circadian experiments on tissues and cell lines, have been implemented in an online database (http://circadb.hogeneschlab.org) that facilitates a straightforward search for oscillators in any organ of interest. Mining this database can help to understand or explain tissue‐specific circadian physiology. For instance, CCGs in the murine heart are involved in cardiac glucose and fatty acid metabolism (e.g., Dgat2, Adpn, Ppp1cc 32), but also electrophysiology which results in oscillatory contractile properties 33 (e.g., Tcap 34 , Kv4.2, and KChIP2 35). Surprisingly, the cardiac‐specific clock not only drives rhythmic output under normal physiological conditions, but also under pathophysiological conditions as noted by its oscillating responsiveness to myocardial infarction mediated by oscillating phosphorylated Akt and GSK‐3 levels 36. In adipose tissue, the clock drives rhythmicity of lipogenesis and lipolysis, and even lipid release 37, via output genes such as Pnpla2 and Lipe 38. In the pancreas, the clock mainly regulates insulin secretion, the importance of which is indicated by the development of β‐cell failure and diabetes mellitus upon genetic clock ablation 39. In brown adipose tissue (BAT), REV‐ERBα modulates the response to cold tolerance via transcriptional repression of Ucp1 40, while the lung clock steers a phasic response to pro‐inflammatory stimuli 41, highlighting how the circadian clock can drive anticipation to stress.
The skin consists of numerous cell types, such as keratinocytes, melanocytes, and dermal fibroblasts, most of which have been shown to possess circadian clocks (expertly reviewed by Plikus et al 42). These compartmented dermal clocks mediate several circadian responses, such as cycles of DNA replication and repair in keratinocytes 42, 43, 44, 45, 46 as well as oscillatory waves of hair follicle regeneration via hair growth cycles 47. Indeed, BMAL−/− mice show accelerated skin aging 48, suggesting an underlying oscillating pattern of CCGs driving skin homeostasis.
In conclusion, organ‐specific peripheral clocks influence tissue physiology in many ways by driving rhythmic and phased CCG mRNA expression, which entrains organs to deal with diurnal fluctuations of the environment.
The circadian clock in stem cell‐derived cells
In‐depth studies of the molecular clock and its CCGs in different murine organs have significantly increased our understanding of circadian rhythmicity. Nonetheless, the time resolution as well as a necessity of multiple replicates that are needed for these types of in vivo studies results in the requirement of large number of animals. This, in combination with limited options to study transcriptional rhythmicity in humans, has driven the investigation of use of in vitro stem cell‐derived cell types to investigate the circadian clock. This has led to the understanding that pluripotent embryonic stem (ES) cells do not possess a functional clock system (further discussed in the next section), but that a clock emerges in a spontaneous manner upon in vitro differentiation (Fig 3).
Figure 3. The circadian clock during in vitro (de)differentiation.
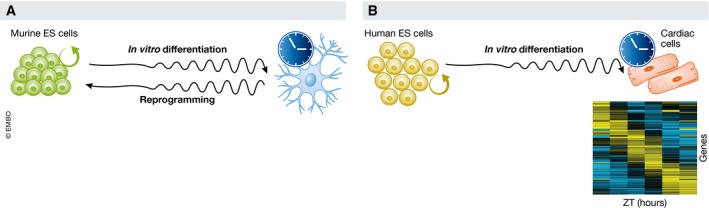
(A) Random differentiation of mouse embryonic stem (ES) cells leads to gradual activation of the molecular circadian clock, while reprogramming decreases rhythmicity of the expression of clock genes. (B) Directed differentiation of human ES cells toward the cardiac lineage leads to activation of the circadian clock that drives oscillatory gene expression of a set of clock‐controlled genes.
In murine pluripotent stem cells, circadian rhythms were shown to be established when differentiation is induced upon withdrawal of leukemia inhibitor factor (LIF) (passive) or by the addition of retinoic acid (active) 49, 50, 51. When reversing differentiation through reprogramming 52, the clock is switched off again 49 (Fig 3A), which indicates that the (in)activation of the diurnal clock is a reversible process that is intensively linked with the differentiation state of a cell.
We have recently shown that human ES cells also lack a circadian clock 53 (Fig 3B) and that a functional circadian clock emerges after directed differentiation toward cardiac cells by the use of growth factors (BMP4 and activin A) 53. In these human ES cell‐derived cardiac cultures, ~7.5% of the transcriptome was expressed with significant diurnal rhythmicity, 20% of which had been shown to oscillate in the murine heart 3. This suggests conserved mechanisms between both species, but still the overlap is modest. Differences might, however, be explained by factors such as cellular heterogeneity. Most circadian transcriptomic experiments are done on whole murine hearts, which include atrial, ventricular and nodal cardiomyocytes, smooth muscle cells, endothelial cells, epicardium cells, pacemaker cells, Purkinje cells, and cardiac fibroblasts 54. Our human ES cell‐derived cardiac cultures are mixed cultures as well, but with a different constitution of cells compared to the murine heart. In addition, in vitro differentiated cardiac cells are known to remain embryonic‐like. Importantly, however, in vitro‐derived cardiac cells are the best proxy for transcriptional oscillations in human hearts. We uncovered several genes, known for their role in human heart physiology, that were expressed in an oscillatory manner. For instance, factors such as TSPO (translocator protein), a therapeutic target, CAV1 (caveolin‐1), an ion channel regulator, PLN (phospholamban), a cardiac contractility regulator, RGS2 (regulator of G‐protein signaling 2), an inhibitor of cardiac hypertrophy, showed rhythmic transcription.
This highlights that unraveling the oscillating transcriptome or proteome of in vitro‐derived cells can lead to the discovery of previously unidentified oscillators that might influence organ physiology as well as therapeutic efficacy. We thus believe that studying phenotypical oscillations of in vitro differentiated human ES cells can serve as a proxy for circadian organ parameters in vivo.
Absence of a functional circadian clock in embryonic stem cells
Conclusions on the absence of a functional circadian clock in murine and human pluripotent ES cells were based on qRT–PCR experiments as well as reporter assays, and while no rhythmicity of clock genes could be observed, most of the core clock genes were found to be expressed in these cells 49, 53, 55. It is evident that the stoichiometry of their transcription is different compared to differentiated cells. Most of the core clock factors are expressed at lower levels in pluripotent stem cells compared to differentiated cells, but Cry1 is an exception as expression levels are higher in stem cells 49, 53, 55 (Fig 4). Since expression ratio of the core genes and availability of clock proteins are the main mechanism by which the diurnal oscillatory network is established and maintained, it is to be expected that such changes result in the absence of a functional clock.
Figure 4. Altered clock factor stoichiometry might shift the functional role of core clock genes toward modulation of proliferation.
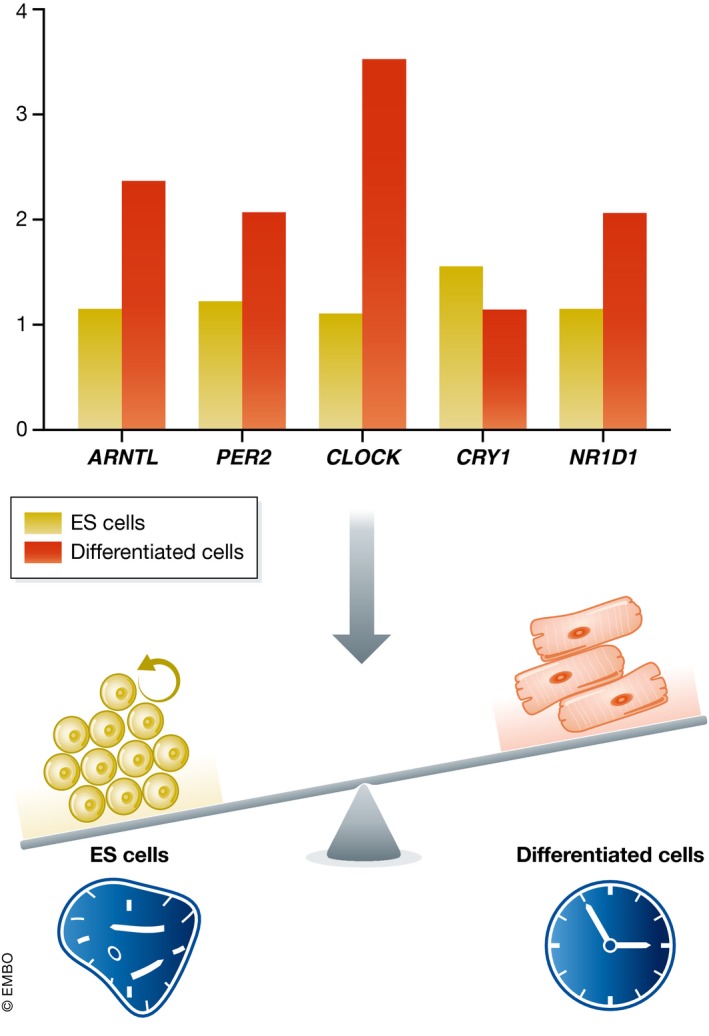
ES cells express core clock factors (ARNTL, PER2, CLOCK, CRY1, and NR1D1) at a different level than differentiated cells. These factors may exert non‐circadian roles in proliferative ES cells that is distinct from their circadian and cell division clock‐related role in differentiated cells.
Aberrant localization of core clock proteins might also contribute to a non‐functional clock in ES cells. It has been proposed that importin α2, encoded by Kpna2, plays a role in this. Importin α2 is a nuclear transporter that shuttles specific pluripotency factors, such as OCT3/4 and differentiation‐related factors like OCT6, out of the nucleus in mouse ES cells in order to retain a pluripotent state 56. This nuclear transporter regulates the cellular differentiation to maintain an undifferentiated state, which induces the retention of core clock factors PER1 and PER2 out of the nucleus, thereby preventing a functional clock pathway through the disturbance of the PER‐based negative feedback loop 57. It is not known yet whether the same holds true for human ES cells, but importin α2 levels gradually decreased upon differentiation toward cardiomyocytes 53, which suggests that similar regulatory mechanisms are involved.
Both stoichiometry and localization differences may contribute to the absence of a clock in ES cells. It would be interesting to study the (transcriptional) effect of overexpression of certain (sets of) core clock factors to see whether this will speed up transcriptional circadian clock formation during differentiation or even whether a functional clock could be established in pluripotent ES cells without them differentiating.
Why are clock genes expressed in embryonic stem cells?
As they are expressed, the question remains whether clock factors do play a role in ES cells even though they do not participate in the functional clock wheelwork that drives the well‐known circadian rhythmicity of CCG expression in differentiated cells. It is known that clock factors control essential cell cycle genes. PER2, for example, can activate p16 (Ink4a), which inhibits the G1‐S transition, in murine fibroblasts 58. Per2 levels in ES cells are lower compared to differentiated cells 49, 53, which might possibly allow stem cells to proliferate faster than differentiated cells. The finding that mice devoid of Per2 are cancer prone is in line with this 59. CRY1 levels on the other hand are upregulated in colorectal cancer cells, and overexpression of Cry1 accelerates the proliferation of HCT116 cells 60. Increased Cry1 levels in ES cells compared to differentiated cells 49, 53 might therefore also influence the difference in proliferation rates between these cell types. CLOCK−/− mouse embryonic fibroblasts display hampered proliferation with concomitant overexpression of p21 and p27, two inhibitors of cellular proliferation 61. In line with this, a recent report demonstrated that CLOCK KO mouse ES cells exhibit decreased proliferation (associated with lower mRNA levels of c‐Myc, Cdk1, and cyclin D1) and increased apoptosis, while pluripotency was unaffected 62. This suggests a strong interaction between cell cycle regulation and clock factors and indicates that altered stoichiometry of clock factors in pluripotent stem cells might shift the role of these factors from regulators of diurnal rhythmicity to key regulators of the cell cycle (Fig 4).
While this hints toward similar consequences of clock factors in proliferation in stem, cancer as well as differentiated cells, other findings report that the regulation of clock genes in stem cells is different or even opposite from the one in differentiated cells. For example, overexpression of a dominant‐negative (DN) form of Bmal1, a positive regulator of Per, Cry, and Dbp in NIH3T3 cells, leads to broad suppression of their mRNA levels. In contrast, overexpression of DN‐Bmal1 in mouse ES cells had no effect on Cry and Dbp expression and induced (rather than reduced) Per2 mRNA levels 49. Remarkably, no attempts to knockout Bmal1 or Per2 have been reported for in vitro‐cultured ES cells. Other (combinatorial) knockout ES cells have been generated for Cry1, Cry2, CKIδ, CKIε, and while these cells show no apparent phenotype when pluripotent, defects in circadian rhythmicity become apparent upon in vitro differentiation 63.
The latter is in line with the observation of impairments later in life rather than at embryonic stages in clock gene knockout mice. Bmal1, Clock, and Per2 knockout mice are not embryonic lethal 48, 64, 65, but a phenotype of premature aging and age‐related pathologies were noted in Bmal1 knockouts 48. That the basis for this is possibly laid during development is indicated by the fact that deletion of both Bmal1 alleles in adult mice (deleted at 3 months of age) does not result in the reduced lifespan, defects in hair regrowth, arthropathy, atherogenesis, and reduced body weight as seen in Bmal1 knockout mice 66. This likely means that BMAL1 exerts a non‐clock‐related role during embryonic development that is important for proper organismal functioning at later stages in life. One could speculate that embryonic BMAL1 depletion leads to epigenetically modified regulatory regions, the result of which only becomes prevalent upon adulthood. Therefore, it would be commendable to study the BMAL1 cistrome (and that of other clock factors) during development and in ES cells, to analyze the putative transcriptional program that is established and which may explain dominant phenotypes later in life.
The role of the clock in adult stem cells
Adult stem cells have the capacity to proliferate as well as to differentiate and, in contrast to embryonic stem cells, contain a functional circadian clock (reviewed by Weger et al 67). Rhythmicity is confirmed to contribute to the properties of these multipotent cells that assure a constant renewal of cells in (some) human organs and can become activated upon injury.
The intestinal epithelium is a good example of this as its stem cells constantly renew the epithelial layer that lines the gut and facilitate regeneration via proliferation and differentiation upon damage 68. The latter has been shown to depend on rhythmic Per and Cycle expression which influences transcription of more than 100 CCGs that are involved in stress response and regeneration 69. Similarly, intestinal stem cell proliferation exhibits a diurnal rhythm in mice that suffer from gastrointestinal syndrome. Here, BMAL1 controls the inflammatory cytokine TNF that drives both p21 and a jnk expression‐mediated stress response, thereby coordinating intestinal regeneration 70.
Similar observations were made in the skin. A robust clock is present in stem and progenitor cells of the basal epidermis, and circadian rhythmicity establishes diurnal cycles of DNA replication and regeneration 43, 44, 45, 46, 71, 72. Interestingly, patchy expression of clock genes creates heterogeneity in the dormant hair follicle stem cells, which results in a subset of cells being more prone to activation. BMAL1 (clock) high subpopulations show more WNT activity and less TGFβ signaling in comparison with clock low cells. Clock ablation leads to the disruption of this equilibrium and results in accumulation of dormant stem cells in Bmal1 knockouts and depletion of the stem cell population upon loss of Per2, which results in premature epidermal aging and increased tumor formation 71. These different phenotypes hint toward a factor‐specific, clock‐unrelated role for these proteins in aging. Another two recent studies show that epidermal as well as skeletal muscle stem cells retain robust circadian rhythms upon aging, but that the set of oscillatory output gene changes from genes involved in homeostasis to tissue‐specific stress‐associated genes involved in DNA damage and inefficient autophagy 73, 74. The authors could prevent age‐associated rewiring of the circadian transcriptome by long‐term caloric restriction in aged mice, which is line with an earlier found correlation between low‐fat diet and reduced aging 73, 75. This means that stem cells can adapt their circadian transcriptome to different conditions of metabolic states and organ‐specific stresses. The question remains whether additional adult stem cell types show this transcriptomic rewiring. Understanding the true mechanism behind this age‐associated switch could lead to therapies ensuring healthy aging.
The circadian clock in (heart) disease and regeneration
The clock has not only been shown to influence healthy (and/or aged) stem cells, but also cancerous cells. Leukemic stem cells have the capacity to self‐renew and are able to propagate disease upon serial transplantation. They display circadian rhythms, and disruption of the clock genes BMAL1 and CLOCK hampers their proliferation, induces myeloid differentiation, and depletes the leukemic stem cell pool 76. This suggests that core clock genes affect disease and that this system could possibly be targeted for (cancer) therapy.
Circadian rhythms have also been shown to play a major role in heart pathophysiology. This is illustrated by increased incidence of myocardial infarction in the morning compared to the evening 77, 78, 79, and a correlation between infarct size and the time of the day at which it occurs is noted 80, 81, 82. Mice that overexpress a dominant‐negative form of the CLOCK protein in cardiomyocytes specifically (CCM) show abolished rhythmicity in cardiac CCG expression, which results in an abrogated circadian response in infarct size after ischemia/reperfusion 36. This shows that the cardiac‐specific clock is important for the heart's response to external stress and that it contributes to the organ its regenerative potential. This is most likely driven by the oscillatory expression of a set of transcripts, but the identity of these CCGs is extremely difficult to uncover in human hearts. Nonetheless, a circadian network of stress‐associated genes was discovered in human ES cell‐derived cardiomyocytes 53. This was found to result in a rhythmic response to doxorubicin, a widely used anti‐cancer drug that causes severe cardiotoxic side effects 83.
We now know that this is not restricted to in vitro ES cell‐derived cardiac cultures as neonatal rat cardiomyocytes 84 and SCA1+ stem cells derived from human hearts 85 also show an oscillatory response to external stressors. Next to that, human SCA1+ cardiac stem cells showed circadian rhythmicity in proliferation and the secretion of a number of paracrine factors, such as β‐nerve growth factor (bNGF), stem cell factor (SCF), human placental growth factor (PGIF‐1), and vascular endothelial growth factor A (VEGF‐A) 85, was found to be rhythmic as well. Strikingly, VEGF‐A, a well‐known growth factor involved in angiogenesis, also oscillated in human ES cell‐derived cardiomyocytes 53 and murine hearts 3. This suggests that the rhythmic nature of this pro‐angiogenic factor may contribute to the oscillatory reaction of the heart to stress. In light of cell‐based cardiac regeneration, one could speculate whether the contribution of human ES cell‐derived cardiomyocytes, or human cardiac stem cells to repair, could be increased by timed administration after cardiac injury.
Future perspectives
In the mammalian body, nearly all cells possess a functional clock to anticipate diurnal variations. Over the last decade, the tissue specificity of the clock has become apparent, which is now known to be established by the interplay between transcription factors and regulatory elements within or close to CCGs. These insights were gained from bulk tissue samples that consist of a heterogeneous population of cells. While the circadian clock is principally organized to synchronize cells, differences in levels of clock genes (and more importantly output genes) have been described. The presence and consequences of this remain to be determined for many (single) cell types within one organ.
Focusing on defined cell types may also help to unravel the mechanisms behind the birth of the clock. There is no functional clock at the earliest steps of development (the oocyte and early zygote), but clock factor levels increase after fertilization, which allows for the gradual development of diurnal clock gene rhythmicity during and after gestation 86, 87, 88. This is in line with the emergence of circadian rhythmicity during differentiation of embryonic stem cells that do not possess a functional circadian clock 49, 53. Clock genes are expressed in ES cells, however, and the emergence of a functional clock system is closely linked to differentiation 49, 50, 51, 53, but these factors may have additional functions in those cells. The intimate link between clock proteins and the cell cycle was discussed above, and stem cell proliferation may be regulated by these factors. Future studies using inducible knockout systems could possibly shed light on the clock‐unrelated roles of clock proteins in pluripotent ES cells (see also Box 1).
Box 1: In need of answers.
-
Do clock factors exert non‐circadian roles in embryonic stem cells?
Although ES cells do not possess a classical transcriptional/translational feedback loop‐based circadian clock, they do express most of the clock factors. Whether they exert specific roles in stem cell maintenance is unknown so far, but knockout during development can lead to premature aging later in life.
-
Which machinery drives metabolic rhythms in embryonic stem cells?
ES cells do not possess a functional circadian core clock machinery, but they do show rhythmic glucose uptake. Metabolic oscillations have also been observed in other cell types such as red blood cells that show oxidation–reduction cycles of peroxiredoxins. Understanding how metabolic ES cell rhythms are established could help to uncover additional non‐traditional oscillatory systems.
-
Do organ‐specific clocks consist of cell type‐specific clocks?
Clock‐controlled genes are organ‐specific and mostly identified in bulk tissue. Deconvoluting this into cell type‐specific output genes may help to understand how subclocks are synchronized to retain proper organ physiology.
-
Can we apply knowledge of circadian rhythms in human embryonic stem cell‐derived cells for regenerative purposes?
Differentiating human ES cells toward specific lineages provides an unlimited source of cells that could be used for therapy. In addition, they can be used to identify clock‐controlled genes of (specific) cell types that cannot be investigated for humans otherwise. Uncovering oscillatory networks involved in cell survival as well as factor secretion could ultimately be used to ameliorate cell‐based therapy after injury.
-
Are small‐molecule modulators of the circadian clock specific and safe?
The generation of synthetic clock modulators has gained attention over the last years. While some have been shown to have beneficial effects in multiple organs/diseases, the specificity and safety of these compounds are underexplored. Thorough investigation will be needed before taking these modulators of the circadian clock to the clinic.
A functional clock is present in adult stem cells, in which circadian oscillations have been shown to drive proliferation and differentiation, thereby facilitating tissue homeostasis and regeneration 89. Targeting the clock in adult stem cells in vivo by the use of drugs might enhance regeneration after damage. Another route for treatment could be ex vivo culturing of stem cells to synchronize their clocks and administering them in the time window in which the patient is most receptive and in which the cells are most likely to engraft. Additional knowledge on the circadian clock in adult stem cells could thus allow for or improve stem cell‐based therapies.
A set of novel synthetic ligands that target components of the clock pathway are currently being investigated for different purposes 90, 91. Compounds, such as SR9009 and SR9011, have been shown to activate the clock factors REV‐ERBα/β, the role of which has been studied extensively in oscillatory metabolic processes of the liver. While these REV‐ERB agonists have been shown to be (beneficial) for energy expenditure and to reverse diet‐induced obesity 92, 93, the effects of these drugs should be closely examined for ramifications on other cellular processes. For instance, SR9009 and SR9011 were shown to induce an anti‐proliferative response in leukemic stem cells 76, therefore providing potential new therapeutic avenues, but their consequences in healthy adult stem cells are unknown. In‐depth characterization of small‐molecule modulators of circadian factors is needed, as it may provide powerful tools for tissue regeneration as well as anti‐cancer therapies.
In conclusion, the circadian clock plays a major role in facilitating tissue homeostasis and regeneration via a number of processes. Circadian rhythms drive stem cell metabolism, self‐renewal, and differentiation, and can even create stem cell heterogeneity in one tissue to protect the organism from stem cell depletion upon activation. Finding methods to preserve the circadian rhythm in stem cells will unequivocally lead to proper tissue homeostasis and healthy aging.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgements
We would like to thank Marit W. Vermunt for carefully and critically reading the manuscript.
See the Glossary for abbreviations used in this article.
References
- 1. Ebihara S, Tsuji K, Kondo K (1978) Strain differences of the mouse's free‐running circadian rhythm in continuous darkness. Physiol Behav 20: 795–799 [DOI] [PubMed] [Google Scholar]
- 2. Balsalobre A, Damiola F, Schibler U (1998) A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell 93: 929–937 [DOI] [PubMed] [Google Scholar]
- 3. Zhang R, Lahens NF, Ballance HI, Hughes ME, Hogenesch JB (2014) A circadian gene expression atlas in mammals: implications for biology and medicine. Proc Natl Acad Sci USA 111: 16219–16224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vollmers C, Gill S, DiTacchio L, Pulivarthy SR, Le HD, Panda S (2009) Time of feeding and the intrinsic circadian clock drive rhythms in hepatic gene expression. Proc Natl Acad Sci USA 106: 21453–21458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dierickx P, Du Pré B, Feyen DAM, Geijsen N, van Veen T, Doevendans PA, van Laake LW (2015) Circadian rhythms in stem cell biology and function In Stem Cells and Cardiac Regeneration, Madonna R, (ed.), pp 57–78. Cham: Springer International Publishing; [Google Scholar]
- 6. Evans MJ, Kaufman MH (1981) Establishment in culture of pluripotential cells from mouse embryos. Nature 292: 154–156 [DOI] [PubMed] [Google Scholar]
- 7. Mertens J, Marchetto MC, Bardy C, Gage FH (2016) Evaluating cell reprogramming, differentiation and conversion technologies in neuroscience. Nat Rev Neurosci 17: 424–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mathapati S, Siller R, Impellizzeri AAR, Lycke M, Vegheim K, Almaas R, Sullivan GJ (2016) Small‐molecule‐directed hepatocyte‐like cell differentiation of human pluripotent stem cells. Curr Protoc Stem Cell Biol 38: 1G.6.1–1G.6.18 [DOI] [PubMed] [Google Scholar]
- 9. Dierickx P, Doevendans PA, Geijsen N, van Laake LW (2012) Embryonic template‐based generation and purification of pluripotent stem cell‐derived cardiomyocytes for heart repair. J Cardiovasc Transl Res 5: 566–580 [DOI] [PubMed] [Google Scholar]
- 10. Avgustinova A, Benitah SA (2016) Epigenetic control of adult stem cell function. Nat Rev Mol Cell Biol 17: 643–658 [DOI] [PubMed] [Google Scholar]
- 11. Keesler GA, Camacho F, Guo Y, Virshup D, Mondadori C, Yao Z (2000) Phosphorylation and destabilization of human period I clock protein by human casein kinase I epsilon. NeuroReport 11: 951–955 [DOI] [PubMed] [Google Scholar]
- 12. Lowrey PL, Shimomura K, Antoch MP, Yamazaki S, Zemenides PD, Ralph MR, Menaker M, Takahashi JS (2000) Positional syntenic cloning and functional characterization of the mammalian circadian mutation tau. Science 288: 483–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yagita K, Yamaguchi S, Tamanini F, van Der Horst GT, Hoeijmakers JH, Yasui A, Loros JJ, Dunlap JC, Okamura H (2000) Dimerization and nuclear entry of mPER proteins in mammalian cells. Genes Dev 14: 1353–1363 [PMC free article] [PubMed] [Google Scholar]
- 14. Kume K, Zylka MJ, Sriram S, Shearman LP, Weaver DR, Jin X, Maywood ES, Hastings MH, Reppert SM (1999) mCRY1 and mCRY2 are essential components of the negative limb of the circadian clock feedback loop. Cell 98: 193–205 [DOI] [PubMed] [Google Scholar]
- 15. Griffin EA, Staknis D, Weitz CJ (1999) Light‐independent role of CRY1 and CRY2 in the mammalian circadian clock. Science 286: 768–771 [DOI] [PubMed] [Google Scholar]
- 16. Brown SA, Kowalska E, Dallmann R (2012) (Re)inventing the circadian feedback loop. Dev Cell 22: 477–487 [DOI] [PubMed] [Google Scholar]
- 17. Siepka SM, Yoo S‐H, Park J, Song W, Kumar V, Hu Y, Lee C, Takahashi JS (2007) Circadian mutant Overtime reveals F‐box protein FBXL3 regulation of cryptochrome and period gene expression. Cell 129: 1011–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Preitner N, Damiola F, Lopez‐Molina L, Zakany J, Duboule D, Albrecht U, Schibler U (2002) The orphan nuclear receptor REV‐ERBalpha controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell 110: 251–260 [DOI] [PubMed] [Google Scholar]
- 19. Sato TK, Panda S, Miraglia LJ, Reyes TM, Rudic RD, McNamara P, Naik KA, FitzGerald GA, Kay SA, Hogenesch JB (2004) A functional genomics strategy reveals Rora as a component of the mammalian circadian clock. Neuron 43: 527–537 [DOI] [PubMed] [Google Scholar]
- 20. Harding HP, Lazar MA (1993) The orphan receptor Rev‐ErbA alpha activates transcription via a novel response element. Mol Cell Biol 13: 3113–3121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang Y, Fang B, Emmett MJ, Damle M, Sun Z, Feng D, Armour SM, Remsberg JR, Jager J, Soccio RE et al (2015) Gene regulation. Discrete functions of nuclear receptor Rev‐erbα couple metabolism to the clock. Science 348: 1488–1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Green CB (2017) Circadian posttranscriptional regulatory mechanisms in mammals. Cold Spring Harb Perspect Biol a030692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Koike N, Yoo S‐H, Huang H‐C, Kumar V, Lee C, Kim T‐K, Takahashi JS (2012) Transcriptional architecture and chromatin landscape of the core circadian clock in mammals. Science 338: 349–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu Y, Hu W, Murakawa Y, Yin J, Wang G, Landthaler M, Yan J (2013) Cold‐induced RNA‐binding proteins regulate circadian gene expression by controlling alternative polyadenylation. Sci Rep 3: 2054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. O'Neill JS, Reddy AB (2011) Circadian clocks in human red blood cells. Nature 469: 498–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Feeney KA, Hansen LL, Putker M, Olivares‐Yañez C, Day J, Eades LJ, Larrondo LF, Hoyle NP, O'Neill JS, van Ooijen G (2016) Daily magnesium fluxes regulate cellular timekeeping and energy balance. Nature 532: 375–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fang B, Everett LJ, Jager J, Briggs E, Armour SM, Feng D, Roy A, Gerhart‐Hines Z, Sun Z, Lazar MA (2014) Circadian enhancers coordinate multiple phases of rhythmic gene transcription in vivo . Cell 159: 1140–1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fang B, Lazar MA (2015) Dissecting the rev‐erbα cistrome and the mechanisms controlling circadian transcription in liver. Cold Spring Harb Symp Quant Biol 80: 233–238 [DOI] [PubMed] [Google Scholar]
- 29. Zaret KS, Carroll JS (2011) Pioneer transcription factors: establishing competence for gene expression. Genes Dev 25: 2227–2241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang Y, Fang B, Damle M, Guan D, Li Z, Kim YH, Gannon M, Lazar MA (2016) HNF6 and Rev‐erbα integrate hepatic lipid metabolism by overlapping and distinct transcriptional mechanisms. Genes Dev 30: 1636–1644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang L, Prosdocimo DA, Bai X, Fu C, Zhang R, Campbell F, Liao X, Coller J, Jain MK (2015) KLF15 establishes the landscape of diurnal expression in the heart. Cell Rep 13: 2368–2375 [DOI] [PubMed] [Google Scholar]
- 32. Bray MS, Shaw CA, Moore MWS, Garcia RAP, Zanquetta MM, Durgan DJ, Jeong WJ, Tsai J‐Y, Bugger H, Zhang D et al (2008) Disruption of the circadian clock within the cardiomyocyte influences myocardial contractile function, metabolism, and gene expression. Am J Physiol Heart Circ Physiol 294: H1036–H1047 [DOI] [PubMed] [Google Scholar]
- 33. Young ME (2016) Temporal partitioning of cardiac metabolism by the cardiomyocyte circadian clock. Exp Physiol 101: 1035–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Podobed PS, Alibhai FJ, Chow C‐W, Martino TA (2014) Circadian regulation of myocardial sarcomeric Titin‐cap (Tcap, telethonin): identification of cardiac clock‐controlled genes using open access bioinformatics data. PLoS One 9: e104907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jeyaraj D, Haldar SM, Wan X, McCauley MD, Ripperger JA, Hu K, Lu Y, Eapen BL, Sharma N, Ficker E et al (2012) Circadian rhythms govern cardiac repolarization and arrhythmogenesis. Nature 483: 96–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Durgan DJ, Pulinilkunnil T, Villegas‐Montoya C, Garvey ME, Frangogiannis NG, Michael LH, Chow C‐W, Dyck JRB, Young ME (2010) Short communication: ischemia/reperfusion tolerance is time‐of‐day‐dependent: mediation by the cardiomyocyte circadian clock. Circ Res 106: 546–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wei E, Ben Ali Y, Lyon J, Wang H, Nelson R, Dolinsky VW, Dyck JRB, Mitchell G, Korbutt GS, Lehner R (2010) Loss of TGH/Ces3 in mice decreases blood lipids, improves glucose tolerance, and increases energy expenditure. Cell Metab 11: 183–193 [DOI] [PubMed] [Google Scholar]
- 38. Shostak A, Husse J, Oster H (2013) Circadian regulation of adipose function. Adipocyte 2: 201–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Marcheva B, Ramsey KM, Buhr ED, Kobayashi Y, Su H, Ko CH, Ivanova G, Omura C, Mo S, Vitaterna MH et al (2010) Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature 466: 627–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gerhart‐Hines Z, Feng D, Emmett MJ, Everett LJ, Loro E, Briggs ER, Bugge A, Hou C, Ferrara C, Seale P et al (2013) The nuclear receptor Rev‐erbα controls circadian thermogenic plasticity. Nature 503: 410–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sundar IK, Yao H, Sellix MT, Rahman I (2015) Circadian molecular clock in lung pathophysiology. Am J Physiol Lung Cell Mol Physiol 309: L1056–L1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Plikus MV, Van Spyk EN, Pham K, Geyfman M, Kumar V, Takahashi JS, Andersen B (2015) The circadian clock in skin: implications for adult stem cells, tissue regeneration, cancer, aging, and immunity. J Biol Rhythms 30: 163–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lin KK, Kumar V, Geyfman M, Chudova D, Ihler AT, Smyth P, Paus R, Takahashi JS, Andersen B (2009) Circadian clock genes contribute to the regulation of hair follicle cycling. PLoS Genet 5: e1000573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gaddameedhi S, Selby CP, Kaufmann WK, Smart RC, Sancar A (2011) Control of skin cancer by the circadian rhythm. Proc Natl Acad Sci USA 108: 18790–18795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Geyfman M, Kumar V, Liu Q, Ruiz R, Gordon W, Espitia F, Cam E, Millar SE, Smyth P, Ihler A et al (2012) Brain and muscle Arnt‐like protein‐1 (BMAL1) controls circadian cell proliferation and susceptibility to UVB‐induced DNA damage in the epidermis. Proc Natl Acad Sci USA 109: 11758–11763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Janich P, Toufighi K, Solanas G, Luis NM, Minkwitz S, Serrano L, Lehner B, Benitah SA (2013) Human epidermal stem cell function is regulated by circadian oscillations. Cell Stem Cell 13: 745–753 [DOI] [PubMed] [Google Scholar]
- 47. Plikus MV, Chuong C‐M (2008) Complex hair cycle domain patterns and regenerative hair waves in living rodents. J Invest Dermatol 128: 1071–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kondratov RV, Kondratova AA, Gorbacheva VY, Vykhovanets OV, Antoch MP (2006) Early aging and age‐related pathologies in mice deficient in BMAL1, the core component of the circadian clock. Genes Dev 20: 1868–1873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yagita K, Horie K, Koinuma S, Nakamura W, Yamanaka I, Urasaki A, Shigeyoshi Y, Kawakami K, Shimada S, Takeda J et al (2010) Development of the circadian oscillator during differentiation of mouse embryonic stem cells in vitro . Proc Natl Acad Sci USA 107: 3846–3851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Paulose JK, Rucker EB, Cassone VM (2012) Toward the beginning of time: circadian rhythms in metabolism precede rhythms in clock gene expression in mouse embryonic stem cells. PLoS One 7: e49555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kowalska E, Moriggi E, Bauer C, Dibner C, Brown SA (2010) The circadian clock starts ticking at a developmentally early stage. J Biol Rhythms 25: 442–449 [DOI] [PubMed] [Google Scholar]
- 52. Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126: 663–676 [DOI] [PubMed] [Google Scholar]
- 53. Dierickx P, Vermunt MW, Muraro MJ, Creyghton MP, Doevendans PA, van Oudenaarden A, Geijsen N, van Laake LW (2017) Circadian networks in human embryonic stem cell‐derived cardiomyocytes. EMBO Rep 18: 1199–1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Xin M, Olson EN, Bassel‐Duby R (2013) Mending broken hearts: cardiac development as a basis for adult heart regeneration and repair. Nat Rev Mol Cell Biol 14: 529–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Umemura Y, Koike N, Ohashi M, Tsuchiya Y, Meng Q‐J, Minami Y, Hara M, Hisatomi M, Yagita K (2017) Involvement of posttranscriptional regulation of Clock in the emergence of circadian clock oscillation during mouse development. Proc Natl Acad Sci USA 114: E7479–E7488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yasuhara N, Yamagishi R, Arai Y, Mehmood R, Kimoto C, Fujita T, Touma K, Kaneko A, Kamikawa Y, Moriyama T et al (2013) Importin alpha subtypes determine differential transcription factor localization in embryonic stem cells maintenance. Dev Cell 26: 123–135 [DOI] [PubMed] [Google Scholar]
- 57. Umemura Y, Koike N, Matsumoto T, Yoo S‐H, Chen Z, Yasuhara N, Takahashi JS, Yagita K (2014) Transcriptional program of Kpna2/Importin‐α2 regulates cellular differentiation‐coupled circadian clock development in mammalian cells. Proc Natl Acad Sci USA 111: E5039–E5048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kowalska E, Ripperger JA, Hoegger DC, Bruegger P, Buch T, Birchler T, Mueller A, Albrecht U, Contaldo C, Brown SA (2013) NONO couples the circadian clock to the cell cycle. Proc Natl Acad Sci USA 110: 1592–1599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Fu L, Pelicano H, Liu J, Huang P, Lee C (2002) The circadian gene Period2 plays an important role in tumor suppression and DNA damage response in vivo . Cell 111: 41–50 [DOI] [PubMed] [Google Scholar]
- 60. Yu H, Meng X, Wu J, Pan C, Ying X, Zhou Y, Liu R, Huang W (2013) Cryptochrome 1 overexpression correlates with tumor progression and poor prognosis in patients with colorectal cancer. PLoS One 8: e61679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Miller BH, McDearmon EL, Panda S, Hayes KR, Zhang J, Andrews JL, Antoch MP, Walker JR, Esser KA, Hogenesch JB et al (2007) Circadian and CLOCK‐controlled regulation of the mouse transcriptome and cell proliferation. Proc Natl Acad Sci USA 104: 3342–3347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lu C, Yang Y, Zhao R, Hua B, Xu C, Yan Z, Sun N, Qian R (2016) Role of circadian gene Clock during differentiation of mouse pluripotent stem cells. Protein Cell 7: 820–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tsuchiya Y, Umemura Y, Minami Y, Koike N, Hosokawa T, Hara M, Ito H, Inokawa H, Yagita K (2015) Effect of multiple clock gene ablations on the circadian period length and temperature compensation in mammalian cells. J Biol Rhythms 31: 48–56 [DOI] [PubMed] [Google Scholar]
- 64. Zheng B, Larkin DW, Albrecht U, Sun ZS, Sage M, Eichele G, Lee CC, Bradley A (1999) The mPer2 gene encodes a functional component of the mammalian circadian clock. Nature 400: 169–173 [DOI] [PubMed] [Google Scholar]
- 65. Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, Laposky A, Losee‐Olson S, Easton A, Jensen DR et al (2005) Obesity and metabolic syndrome in circadian Clock mutant mice. Science 308: 1043–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Yang G, Chen L, Grant GR, Paschos G, Song W‐L, Musiek ES, Lee V, McLoughlin SC, Grosser T, Cotsarelis G et al (2016) Timing of expression of the core clock gene Bmal1 influences its effects on aging and survival. Sci Transl Med 8: 324ra16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Weger M, Diotel N, Dorsemans A‐C, Dickmeis T, Weger BD (2017) Stem cells and the circadian clock. Dev Biol 431: 111–123 [DOI] [PubMed] [Google Scholar]
- 68. Beumer J, Clevers H (2016) Regulation and plasticity of intestinal stem cells during homeostasis and regeneration. Development 143: 3639–3649 [DOI] [PubMed] [Google Scholar]
- 69. Karpowicz P, Zhang Y, Hogenesch JB, Emery P, Perrimon N (2013) The circadian clock gates the intestinal stem cell regenerative state. Cell Rep 3: 996–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Stokes K, Cooke A, Chang H, Weaver DR, Breault DT, Karpowicz P (2017) The circadian clock gene BMAL1 coordinates intestinal regeneration. Cell Mol Gastroenterol Hepatol 4: 95–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Janich P, Pascual G, Merlos‐Suárez A, Batlle E, Ripperger J, Albrecht U, Cheng H‐YM, Obrietan K, Di Croce L, Benitah SA (2011) The circadian molecular clock creates epidermal stem cell heterogeneity. Nature 480: 209–214 [DOI] [PubMed] [Google Scholar]
- 72. Plikus MV, Vollmers C, la Cruzde D, Chaix A, Ramos R, Panda S, Chuong C‐M (2013) Local circadian clock gates cell cycle progression of transient amplifying cells during regenerative hair cycling. Proc Natl Acad Sci USA 110: E2106–E2115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Solanas G, Peixoto FO, Perdiguero E, Jardí M, Ruiz‐Bonilla V, Datta D, Symeonidi A, Castellanos A, Welz P‐S, Caballero JM et al (2017) Aged stem cells reprogram their daily rhythmic functions to adapt to stress. Cell 170: 678–692 [DOI] [PubMed] [Google Scholar]
- 74. Sato S, Solanas G, Peixoto FO, Bee L, Symeonidi A, Schmidt MS, Brenner C, Masri S, Benitah SA, Sassone‐Corsi P (2017) Circadian reprogramming in the liver identifies metabolic pathways of aging. Cell 170: 664–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Brandhorst S, Choi IY, Wei M, Cheng CW, Sedrakyan S, Navarrete G, Dubeau L, Yap LP, Park R, Vinciguerra M et al (2015) A periodic diet that mimics fasting promotes multi‐system regeneration, enhanced cognitive performance, and healthspan. Cell Metab 22: 86–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Puram RV, Kowalczyk MS, de Boer CG, Schneider RK, Miller PG, McConkey M, Tothova Z, Tejero H, Heckl D, Järås M et al (2016) Core circadian clock genes regulate leukemia stem cells in AML. Cell 165: 303–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Muller JE, Stone PH, Turi ZG, Rutherford JD, Czeisler CA, Parker C, Poole WK, Passamani E, Roberts R, Robertson T (1985) Circadian variation in the frequency of onset of acute myocardial infarction. N Engl J Med 313: 1315–1322 [DOI] [PubMed] [Google Scholar]
- 78. Muller JE, Tofler GH, Stone PH (1989) Circadian variation and triggers of onset of acute cardiovascular disease. Circulation 79: 733–743 [DOI] [PubMed] [Google Scholar]
- 79. Peckova M, Fahrenbruch CE, Cobb LA, Hallstrom AP (1998) Circadian variations in the occurrence of cardiac arrests: initial and repeat episodes. Circulation 98: 31–39 [DOI] [PubMed] [Google Scholar]
- 80. Reiter R, Swingen C, Moore L, Henry TD, Traverse JH (2012) Circadian dependence of infarct size and left ventricular function after ST elevation myocardial infarction. Circ Res 110: 105–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Seneviratna A, Lim GH, Devi A, Carvalho LP, Chua T, Koh T‐H, Tan H‐C, Foo D, Tong K‐L, Ong H‐Y et al (2015) Circadian dependence of infarct size and acute heart failure in ST elevation myocardial infarction. PLoS One 10: e0128526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Suarez‐Barrientos A, Lopez‐Romero P, Vivas D, Castro‐Ferreira F, Nunez‐Gil I, Franco E, Ruiz‐Mateos B, Garcia‐Rubira JC, Fernandez‐Ortiz A, Macaya C et al (2011) Circadian variations of infarct size in acute myocardial infarction. Heart 97: 970–976 [DOI] [PubMed] [Google Scholar]
- 83. Lefrak EA, Pitha J, Rosenheim S, Gottlieb JA (1973) A clinicopathologic analysis of adriamycin cardiotoxicity. Cancer 32: 302–314 [DOI] [PubMed] [Google Scholar]
- 84. Du Pré BC, Dierickx P, Crnko S, Doevendans PA, Vos MA, Geijsen N, Neutel D, van Veen TAB, van Laake LW (2017) Neonatal rat cardiomyocytes as an in vitro model for circadian rhythms in the heart. J Mol Cell Cardiol 112: 58–63 [DOI] [PubMed] [Google Scholar]
- 85. Du Pré BC, Demkes EJ, Feyen DAM, Dierickx P, Crnko S, Kok BJM, Sluijter JPG, Doevendans PA, Vos MA, van Veen TAB et al (2017) SCA1(+) cells from the heart possess a molecular circadian clock and display circadian oscillations in cellular functions. Stem Cell Reports 9: 762–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Seron‐Ferre M, Mendez N, Abarzua‐Catalan L, Vilches N, Valenzuela FJ, Reynolds HE, Llanos AJ, Rojas A, Valenzuela GJ, Torres‐Farfan C (2012) Circadian rhythms in the fetus. Mol Cell Endocrinol 349: 68–75 [DOI] [PubMed] [Google Scholar]
- 87. Sládek M, Sumová A, Kováciková Z, Bendová Z, Laurinová K, Illnerová H (2004) Insight into molecular core clock mechanism of embryonic and early postnatal rat suprachiasmatic nucleus. Proc Natl Acad Sci USA 101: 6231–6236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ansari N, Agathagelidis M, Lee C, Korf H‐W, von Gall C (2009) Differential maturation of circadian rhythms in clock gene proteins in the suprachiasmatic nucleus and the pars tuberalis during mouse ontogeny. Eur J Neurosci 29: 477–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Brown SA (2014) Circadian clock‐mediated control of stem cell division and differentiation: beyond night and day. Development 141: 3105–3111 [DOI] [PubMed] [Google Scholar]
- 90. Gloston GF, Yoo S‐H, Chen ZJ (2017) Clock‐enhancing small molecules and potential applications in chronic diseases and aging. Front Neurol 8: 100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Wallach T, Kramer A (2015) Chemical chronobiology: toward drugs manipulating time. FEBS Lett 589: 1530–1538 [DOI] [PubMed] [Google Scholar]
- 92. Kojetin DJ, Burris TP (2014) REV‐ERB and ROR nuclear receptors as drug targets. Nat Rev Drug Discov 13: 197–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Chen Z, Yoo S‐H, Takahashi JS (2012) Small molecule modifiers of circadian clocks. Cell Mol Life Sci 70: 2985–2998 [DOI] [PMC free article] [PubMed] [Google Scholar]


