Abstract
Background
Although silent myocardial infarction (SMI) accounts for about half of the total number of MIs, the risk of HF among patients with SMI is not well established.
Objectives
To examine the association between SMI and clinically manifested MI (CMI), compared to no MI, with HF.
Methods
This analysis included 9,243 participants from the Atherosclerosis Risk in Communities (ARIC) study who were free of cardiovascular disease at baseline (ARIC visit-1, 1987–1989). SMI was defined as electrocardiographic evidence of MI without CMI after the baseline until ARIC visit-4 (1996–1998). HF events were ascertained starting from ARIC visit-4 until 2010 in individuals free of HF before that visit.
Results
Between ARIC visit-1 and visit-4, 305 SMI and 331 CMI occurred. After ARIC visit-4 and during a median follow-up of 13.0 years, 976 HF events occurred. The incidence rate of HF was higher in both CMI and SMI than those without MI (incidence rate per 1000 person-years were 30.4, 16.2 and 7.8, respectively; p-value <.001). In a model adjusted for demographics and HF risk factors, both SMI (HR (95% CI): 1.35 (1.02–1.78) and CMI (HR (95% CI): 2.85 (2.31–3.51), compared to no MI, were associated with increased risk of HF. These associations were consistent in subgroups of participants stratified by several HF risk predictors. However, the risk of HF associated with SMI was stronger in those younger than vs. those at or older the median age (53 years) (HR (95%CI) 1.66 (1.00–2.75) vs. 1.19 (0.85–1.66), respectively; interaction p-value <.001).
Conclusions
SMI is associated with increased risk of HF. Future research is needed to examine the cost-effectiveness of screening for SMI as part of HF risk assessment, and to identify preventive therapies to improve the risk of HF among patients with SMI.
Keywords: Silent myocardial infarction, Electrocardiogram, Heart failure
Introduction
Heart failure (HF) is the final outcome of up to 15% of the patients who suffer from acute myocardial infarction (MI) (1–4). The proportion of this segment of the population is likely to increase as the survival of post-MI patients has significantly improved over the last decade (5). Up to one third of the million patients who are hospitalized for HF each year in the United States have a history of MI (6). Several factors, such as recurrent MI, ventricular remodeling, mechanical MI complications, and stunned or hibernating myocardium, lead to HF post-MI (7–8). These conditions might be clinically silent and go unnoticed for a long time.
Silent MI (SMI), defined as evidence of MI on the electrocardiogram (ECG) in the absence of history of MI, accounts for about half of the total number of MIs (9). Previous reports from different populations have shown that both clinical MI (CMI) and SMI are associated with poor prognosis (9–10). However, whether SMI is associated with HF similar to CMI is currently unclear. Furthermore, HF prevalence varies by sex and race, and hence it is possible that sex and race modify the relationship between SMI and HF (11–12). Therefore, the aims of this study were to examine and compare the associations between SMI and CMI, versus no MI, with HF, and to examine the consistency of these associations in subgroups stratified by sex and race as well as HF risk factors.
Methods
Study Design and Population
The Atherosclerosis Risk in Communities (ARIC) study is a community-based, predominantly biracial prospective cohort study that was designed to study atherosclerosis and its clinical outcomes, and variation in cardiovascular risk factors, medical care, and disease by race, gender, location, and date. Details of the ARIC study have been previously published (13). Briefly, from 1987 to 1989 (ARIC visit-1, baseline), 15,792 adults (age 45–64 years) from 4 US communities (Washington County, Maryland; suburbs of Minneapolis, MN; Jackson, MS; and Forsyth County, North Carolina) were prospectively enrolled in the ARIC study. They underwent a phone interview and subsequent clinic visit. Additional examinations were performed in 1990 to 1992 (visit-2), 1993 to 1995 (visit-3), 1996 to 1998 (visit-4), and 2011 to 2013 (visit-5). Participants were mostly white in the Washington County and Minneapolis sites, exclusively black in Jackson, and a mix of both in Forsyth County. The study was approved by the institutional review board at each study site. All participants provided written informed consent.
For the purpose of this analysis, all ARIC participants with good quality and complete ECG data at visit-1 through visit-4 as well as outcome events after visit-4 were considred. The following partcipants were excluded: 47 with reported race neither African-American nor white, 565 participants with ECG data that were not interpretable for the diagnosis of MI due to poor quality or suppression codes by the Minnesota ECG classification, 3,775 with missing ECG in any of the ARIC first four visits including those who died during this period, 201 with missing baseline cardiovascular disease (CVD) risk factors utilized in the models, and 119 missing HF follow-up data. We also excluded 1,706 participants with history of prevalent CVD at baseline which was defined as the presence of ECG evidence of MI, or a self-reported history of physician-diagnosed MI, coronary artery bypass surgery, coronary angioplasty, HF, or stroke. Finally, we excluded 136 cases with HF occurring between ARIC visit-1 and visit-4. After all exclusions (n=6,549), a total of 9,243 participants remained and were included in the analysis.
Baseline Covariates
Baseline (visit-1) age, sex, race, and smoking status were determined by self-report. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Blood samples were obtained after a12-hour fast and were examined in a central laboratory. Diabetes mellitus was defined as a fasting glucose level ≥126 mg/dL (or non-fasting glucose ≥200 mg/dL), a self-reported physician diagnosis of diabetes mellitus, or the use of anti-diabetes medications. Hypertension was defined as systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or the use of blood pressure lowering medications. Medication use was obtained by self-report of medication intake during last 2 weeks and by a review of medications brought by the participants to their visit. Each medication was coded by trained and certified interviewers with the use of a computerized medication classification system. Heart rate data were obtained from the baseline ECG.
Silent MI and Clinical MI
SMI was defined as ECG-evidence of new MI at ARIC visit-2, -3, or -4 that was not present at the baseline visit (visit-1) in the absence of documented CMI. CMI was adjudicated by physician review based on chest pain, cardiac biomarkers/enzymes from hospitalizations, ECG evidence including a new pathological Q wave, coronary heart disease history, and other associated information. All hospitalized events were classified into definite, probable, suspect and no MI. Details of classification and specific criteria for adjudication have been described previously (14). Definite and probable MIs were combined to define CMI in this analysis. Definite hospitalized CMI met ≥1 of the following criteria: evolving diagnostic ECG pattern, diagnostic ECG pattern and abnormal enzymes, or cardiac pain and abnormal enzymes plus evolving ST-T pattern or equivocal ECG pattern. Probable hospitalized MI met ≥1 of the following criteria in the absence of sufficient evidence for definite hospitalized MI: cardiac pain and abnormal enzymes, cardiac pain and equivocal enzymes and either evolving ST-T pattern or diagnostic ECG pattern, or abnormal enzymes and evolving ST-T pattern (10). Participants with both SMI and CMI between ARIC visits 1 and 4 were considered to have CMI.
Resting 10-second standard simultaneous 12-lead ECGs were performed in all participants using identical electrocardiograph (MAC PC, Marquette Electronics Inc, Milwaukee, WI) machines at all clinical sites by trained personnel. These ECGs were processed in a central ECG laboratory (initially at Dalhousie University, Halifax, NS, Canada, and later at the Epidemiological Cardiology Research Center (EPICARE), Wake Forest School of Medicine, Winston-Salem, NC), where all ECGs were visually inspected for quality and technical errors. ECG-evidence of MI was defined using the Minnesota Code (MC) ECG classifications as new appearance of a major Q/QS wave abnormality (MC 1.1 or MC 1.2) or minor Q/QS wave abnormality (MC 1.3) plus major ST-T abnormality (MC 4.1, MC 4.2, MC 5.1, or MC 5.2) (15,16).
Ascertainment of Heart Failure
Incident HF was defined as the first occurrence of HF hospitalization according to the International Classification of Diseases, 9th Revision (ICD-9), code 428 (428.0–428.9) as a diagnosis in any position. These diagnostic codes were obtained during retrospective surveillance of hospital discharges or a death certificate with death from HF in any position or death certificate with an ICD-9 code of 428 or an ICD-10 code of I-50 among any of the diagnoses listed or underlying causes of death on the death certificate (17).
Statistical Analysis
Baseline characteristics were compared by MI status (CMI, SMI, and no MI). Statistical significance for categorical variables was tested using the χ2-method and the analysis of variance or t-test for continuous variables.
Cumulative incidence rates of HF per 1000 person-years occurring after visit-4 were calculated among ARIC participants who had SMI and CMI (versus no MI) that occurred between visit-1 and visit-4. Kaplan-Meier estimates were used to compute the cumulative incidence of HF stratified by the MI status and the difference in estimates was compared using the log-rank procedure. Follow-up time was defined as the time from visit-4 to the diagnosis of HF, death, loss to follow-up, or end of follow-up (December 31, 2010).
After testing for proportional hazard assumptions, Cox proportional hazard analysis was used to examine the association between CMI and SMI (versus no MI) with HF, in models adjusted as follows: Model 1 adjusted for demographics (age, sex, race); Model 2 adjusted for variables in model 1 plus the clinical components of the ARIC study HF risk score (BMI, smoking status, heart rate, systolic blood pressure, use of blood pressure lowering medications, and diabetes mellitus) (18). Since we excluded participants with coronary heart disease, we did not adjust for coronary heart disease although it is a component of the ARIC HF risk score (18). Individuals were censored at the time of HF, death or December 31, 2010, whichever occurred earlier.
Subgroup analysis in the study participants stratified by the clinical components of the ARIC study HF risk score were also examined in models adjusted in a similar fashion to model 2, and p-value for interactions were calculated in each subgroup. For the purpose of subgroup analysis, we used hypertension to replace systolic blood pressure and use of blood pressure lowering medications. We also used the median age (53 years), instead of 65 years which is suggested by the ARIC HF risk score because of the small number of participants with SMI in those above the age of 65 years. On the other hand, we used the BMI of 25 kg/m2 and heart rate of 60 beats/minutes as cut-off points as suggested by ARIC HF risk score (18). All analyses were performed with SAS version 9.3 (SAS Institute Inc, Cary, NC). A 2-sided p-value of<0.05 was considered significant.
RESULTS
Overall, 9,243 (mean age 53.7± 5.7 years, 57.2% women, 20.4% black) participants were included in the analysis. Table 1 shows the baseline characteristics stratified by MI status. Compared with the CMI group, the SMI group had more women, blacks and non-smokers. CMI and SMI had expectedly higher prevalence of coronary heart disease risk factors than the no MI group.
Table 1.
Baseline (ARIC visit-1, 1987–89) Participant Characteristics Stratified by Myocardial Infarction Status
| Characteristics* | No MI (n=8607) | Silent MI (n=305) | Clinical MI (n=331) | p-value† |
|---|---|---|---|---|
| Age (years) | 54 ±5.6 | 55 ±5.9 | 55 ±5.6 | <.001 |
| Women | 5,063 (59%) | 133 (44%) | 87 (26%) | <.001 |
| African-American | 1,771 (21%) | 69 (23%) | 48 (15%) | .017 |
| Heart rate (beats/minute) | 65.6 ±9.6 | 66.9 ±10.5 | 65.3 ±9.6 | .045 |
| Smoking Status | <.001 | |||
| Current | 1,770 (21%) | 75 (25%) | 95 (29%) | |
| Past | 2,800 (32%) | 96 (31%) | 124 (37%) | |
| Never | 4,030 (47%) | 134 (44%) | 111 (34%) | |
| Body mass index (kg/m2) | 27 ±5.0 | 29 ±5.6 | 28 ±4.2 | <.001 |
| Systolic blood pressure (mmHg) | 118 ±17 | 125 ±19 | 125±19 | <.001 |
| Hypertension | 2,280 (27%) | 121 (40%) | 130 (39%) | <.001 |
| BP lowering medication use | 1,910 (22%) | 102 (33%) | 99 (30%) | <.001 |
| Diabetes | 620 (7.3%) | 51 (17%) | 48 (15%) | <.001 |
MI= myocardial infarction; BP= blood pressure
Values expresses as mean± SD or n (%)
p-value for comparison among the three groups using analysis of variance and χ2for continuous and categorical variables, respectively
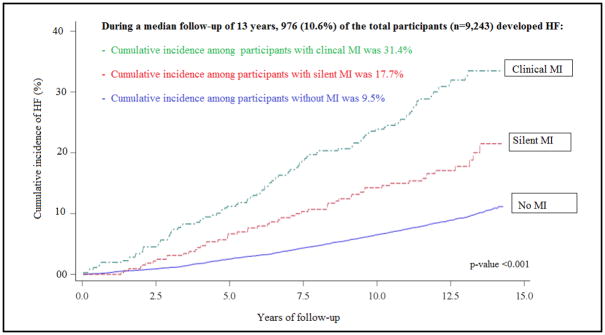
During a median follow- up of 13.0 years (IQR: 12.2, 13.9 years), there were 976 cases of HF; 104 HF cases among CMI group, 54 among SMI group and 818 among the no MI group. The incidence rate of HF was higher in both CMI and SMI than those without MI (incidence rate per 1,000 person year were 30.4, 16.2 and 7.8, respectively; p-value <0.001). The Central Illustration shows the cumulative incidence of HF stratified by MI status.
Central Illustration. Risk of HF Associated with Different Patterns of MI.
Cumulative Incidence of Heart Failure Stratified by Myocardial Infarction Status. MI= Myocardial Infraction; HF= Heart Failure.
In multivariable adjusted Cox proportional hazard models, both CMI and SMI, compared with no MI, were significantly associated with HF, independent of demographics and clinical risk factors (Table 2). However, the magnitude of risk of HF associated with CMI was larger than the risk associated with SMI.
Table 2.
Associations between Type of Myocardial Infarction and Incident Heart Failure
| Participants | Events | Incidence Rate | Model 1* | Model2 † | |||
|---|---|---|---|---|---|---|---|
| n | n | 1000-person- year | HR (95% CI) | p-value | HR (95% CI) | p-value | |
| No MI | 8,607 | 818 | 7.8 | 1 (ref.) | 1 (ref.) | ---- | |
| Silent MI | 305 | 54 | 16.2 | 1.85 (1.40–2.44) | <.001 | 1.35 (1.02–1.78) | .035 |
| Clinical MI | 331 | 104 | 30.4 | 3.49 (2.83–4.30) | <.001 | 2.85 (2.31–3.51) | <.001 |
HR=hazard ratio; CI=confidence interval; MI= myocardial infarction
Model 1 adjusted for age, sex, and race.
Model 2 adjusted for variables in model 1 plus body mass index, smoking status, heart rate, systolic blood pressure, use of blood pressure lowering medications, and diabetes mellitus
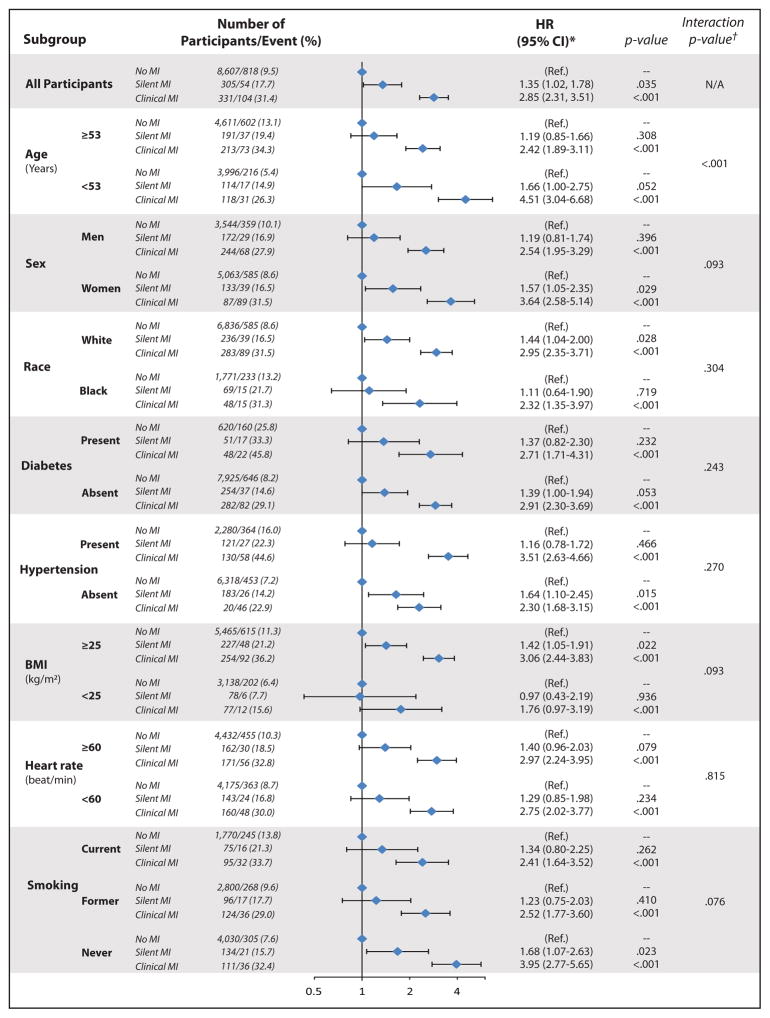
Figure 1 and Supplemental Table 1 show subgroups analyses stratified by demographics and HF risk factors. As shown, the pattern of associations between MI status and HF was consistent among these subgroups i.e. no effect modification by race, diabetes, hypertension, heart rate or heart rate on the association between MI by type and HF. However, there was effect modification by age; the risk of HF associated with SMI was stronger in those younger than vs. those at or older the median age (interaction p-value <0.001). Also the risk of HF associated with SMI was slightly different stronger for women compared with men (interaction p-value = 0.093), and in overweight compared to normal weight (interaction p-value =0.093), and in never smokers (interaction p-value = 0.076).
Figure 1. Associations between Type of Myocardial Infarction and Incident Heart Failure in Subgroups.
HR=hazard ratio; CI=confidence interval; MI= myocardial infarction. Models adjusted for age, sex, race, plus body mass index, smoking status, heart rate, systolic blood pressure, blood pressure lowering medications, and diabetes mellitus (subgroup used in stratification is not included in the model).
DISCUSSION
In this analysis from the ARIC study, we showed that SMI is associated with increased risk of HF independent of HF risk factors (Central Illustration). CMI also was associated with HF, and the association was stronger than that that of SMI. HF has been defined as global pandemic, since it affects around 26 million people worldwide (19). Currently 5.7 million people in the United States have HF, and it is expected that by 2030 >8 million people will have this condition (20). Therefore, identifying a new potential mechanism contributing to this pandemic is of enormous importance. While future research is needed to examine the cost-effectiveness of screening for SMI as part of HF risk assessment, we believe that our report provides novel insights into overlooked and potentially addressable contributor to the HF pandemic.
SMI was first described in 1949 and further characterized in the Framingham Heart Study in 1959 (21,22). Its prevalence in the general population ranges from 0.3–4.8% (23–29). Certain subgroups, such as the elderly, persons with diabetes and women are known to have higher prevalence of up to 15% (25,30,31). These subgroups are also uniquely at higher risk of adverse events. Among individuals with MI, SMI constitutes up to half of the total number of MIs, (30,32) and is associated with increased risk of re-infarction, (30) other coronary heart disease, sudden cardiac death and all-cause mortality (24). To our knowledge, this study is the first to provide evidence that SMI is associated with increased risk of HF as well. Our subgroup analysis shows that the risk of HF associated with SMI is stronger with young age; although they may be less exposed to SMI. Other subgroups of interest that showed borderline effect modification include overweight; stronger association of SMI with HF in overweight than those with normal weight. This could probably explained by the added risk of obesity to HF. On the other hand, there was there was borderline effect modification by smoking where the association was stronger in never smoker than current smoker and was in-between in former smokers. Whether this is due to survival bias or a by-chance finding requires further investigation.
Even though men have higher risk of HF than women, the association between different types of MI and HF did not significantly differ by sex in our study. As a matter of fact, there was tendency for more HF risk associated with SMI in women than men (interaction p-value = 0.093). In the Prevention of REnal and Vascular END-stage Disease (PREVEND) Study, men were more likely to develop HF than women, but women had higher risk of HF with preserved ejection fraction (HFpEF) (33). This finding and the notion that coronary heart disease (of which SMI is part) is an established risk factor for HFpEF (34,35) suggest that examining HF types along with MI types may shed more light on whether effect modification by sex on the association between SMI and HF exists or not. Similarly, Hebert et al. showed that black patients with HFpEF tend to have a lower prevalence of ECG-based MI than whites, although blacks are at a higher risk of HF overall (36). This may partially explain the non-significant slightly stronger association between SMI and HF in whites than blacks in our analysis. Further studies examining sex and race differences in the association between MI (clinical and silent) with different types of HF (HFpEF and HFrEF) might be in a better position to examine the effect modification of race and sex on the associations between SMI and HF.
Not surprisingly, CMI is associated with a stronger association with HF than SMI. ECG changes reflecting ischemic cascade in the myocardium could precede clinical symptoms (37). Therefore, it is possible that SMI represents milder or earlier changes prior to the development of CMI or HF. Also, it is possible that CMI patients might have a larger infarct size than SMI, and thus SMI led to a smaller degree of insult to the myocardium. That is to say, SMI probably remained silent because of being small in size or subclinical, and hence did not significantly impact the myocardium as CMI did, which could explain the stronger risk of HF with CMI than SMI.
Recently, increased life expectancy and better care of post-MI patients in the United States resulted in an upsurge of HF (38). Early detection of HF prior to overt physiological and structural changes may lead to better outcomes (39). Hence, early detection of risk factors has the potential to minimize the burden of HF-related mortality, morbidity and health care costs. In this regard, our study provides evidence for a new risk factor that may be otherwise missed in routine care. Since ECG is a readily available tool with high inter-rater reliability, SMI, as a subclinical risk factor, could be identified with ease. Although guideline-directed therapy has a clear role in preventing future HF among CMI patients and therefore is a part of quality of care core measure (40, 41), it is not known whether such benefit exists for persons with SMI. Thus, SMI could be considered as a condition for American College of Cardiology/American Heart Association Stage-A HF (i.e., at risk for HF) (42). Future studies are needed to study the beneficial effects of screening for SMI and whether guideline-directed therapy in SMI patients have improved outcomes in the same way as among CMI patients.
Our results should be considered in the context of certain limitations. As in other studies with similar design, residual confounding despite adjustment of several confounders remains a possibility. The inability to detect significant interactions in the subgroups analyses could be related to lack of enough power due to the small sample size within the subgroups. Also, including only whites and blacks limits the generalizability of our study to other races/ethnicities. Since the diagnosis of CMI is not reliant on high sensitivity troponin assays, it is possible that the prevalence of CMI was underestimated in ARIC. However, troponin was not available until 1998, the date our ascertainment of SMI ended. Finally, HF was based on hospitalized cases using ICD-9 and ICD-10 codes and not validated by physician review for diagnosis of HF, which might have led to misclassification and underestimation of the true incidence of HF; however, use of inpatient HF events has a high diagnostic specificity in ARIC, as shown previously (17).
Despite these limitations, this is the first report from a large community-based study showing a link between SMI and HF, which provides an opportunity to identify a new risk factor contributing to a strongly emerging pandemic. Strengths of the study include a biracial population with good representation of women, long-term follow up, and well-ascertained variables and outcomes including ECG data evaluated at a central reading center.
Supplementary Material
CLINICAL PERSPECTIVES.
Heart failure (HF) is a frequent complication of myocardial infarction (MI). Although silent MI (SMI) accounts for about half of the total number of MIs, the risk of HF among patients with SMI is not well established. In this analysis from the ARIC study, we showed that SMI is associated with increased risk of HF independent of HF predictors.
TRANSLATIONAL OUTLOOK.
This study provides evidence that SMI is a risk factor for HF. Future studies are needed to examine the cost-effectiveness of screening for SMI as part of HF risk assessment, and to study preventive therapies to improve the risk of HF among patients with ECG-evidence of SMI.
Acknowledgments
Source of Funding: The ARIC Study is carried out as a collaborative study supported by the National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C).
The authors thank the staff and participants of the ARIC study for their important contributions.
Abbreviations
- SMI
Silent Myocardial Infarction
- CMI
Clinically Manifested MI
- HF
Heart failure
- ARIC
Atherosclerosis Risk in Communities study
- LVH
Left Ventricular Hypertrophy
- BMI
Body Mass Index
- HFpEF
Heart Failure with Preserved Ejection Fraction
- HFrEF
Heart Failure with Reduced Ejection Fraction
- ECG
Electrocardiogram
Footnotes
Relationship with industry: None
Disclosures: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shah RV, Holmes D, Anderson M, et al. Risk of heart failure complication during hospitalization for acute myocardial infarction in a contemporary population: Insights from the National Cardiovascular Data ACTION registry. Circ Heart Fail. 2012;5:693–702. doi: 10.1161/CIRCHEARTFAILURE.112.968180. [DOI] [PubMed] [Google Scholar]
- 2.Mocan T, Agoston-Coldea L, Gatfosse M, Rosenstingl S, Mocan LC. Risk factors for heart failure in patients with one prior myocardial infarction episode. Rom J Intern Med. 2008;46:213–221. [PubMed] [Google Scholar]
- 3.Zornoff LA, Skali H, Pfeffer MA, et al. Right ventricular dysfunction and risk of heart failure and mortality after myocardial infarction. J Am Coll Cardiol. 2002;39:1450–1455. doi: 10.1016/s0735-1097(02)01804-1. [DOI] [PubMed] [Google Scholar]
- 4.Minicucci MF, Azevedo PS, Polegato BF, Paiva SA, Zornoff LA. Heart failure after myocardial infarction: Clinical implications and treatment. Clin Cardiol. 2011;34:410–414. doi: 10.1002/clc.20922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cowie MR, Lacey L, Tabberer M. Heart failure after myocardial infarction: A neglected problem. Br J Cardiol. 2005;12:205–208. [Google Scholar]
- 6.Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and Stroke statistics-2017 update: A report from the American Heart Association. Circulation. 2017 doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation. 1990;81:1161–1172. doi: 10.1161/01.cir.81.4.1161. [DOI] [PubMed] [Google Scholar]
- 8.Cleland JG, Torabi A, Khan NK. Epidemiology and management of heart failure and left ventricular systolic dysfunction in the aftermath of a myocardial infarction. Heart. 2005;91(Suppl 2):ii7–13. doi: 10.1136/hrt.2005.062026. discussion ii31, ii43–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pride YB, Piccirillo BJ, Gibson CM. Prevalence, consequences, and implications for clinical trials of unrecognized myocardial infarction. Am J Cardiol. 2013;111:914–918. doi: 10.1016/j.amjcard.2012.11.042. [DOI] [PubMed] [Google Scholar]
- 10.Zhang ZM, Rautaharju PM, Prineas RJ, et al. Race and sex differences in the incidence and prognostic significance of silent myocardial infarction in the atherosclerosis risk in communities (ARIC) study. Circulation. 2016;133:2141–2148. doi: 10.1161/CIRCULATIONAHA.115.021177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roger VL, Weston SA, Redfield MM, et al. Trends in heart failure incidence and survival in a community-based population. JAMA. 2004;292:344–350. doi: 10.1001/jama.292.3.344. [DOI] [PubMed] [Google Scholar]
- 12.Bhatia S, Qazi M, Erande A, Shah K, Amin A, Patel P, Malik S. Racial differences in the prevalence and outcomes of atrial fibrillation in patients hospitalized with heart failure. Am J Cardiol. 2016;117:1468–1473. doi: 10.1016/j.amjcard.2016.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) study: Design and objectves. American Journal of Epidemiology. 1989;129:687–702. [PubMed] [Google Scholar]
- 14.White AD, Folsom AR, Chambless LE, et al. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) study: Methods and initial two years’ experience. J Clin Epidemiol. 1996;49:223–233. doi: 10.1016/0895-4356(95)00041-0. [DOI] [PubMed] [Google Scholar]
- 15.Prineas RJ, Crow RS, Zhang Z-M. The Minnesota Code Manual of Electrocardiographic findings. Springer Science & Business Media; 2009. [Google Scholar]
- 16.Prineas RJ, Crow RS, Blackburn H. The Minnesota Code manual of electrocardiographic findings. John Wright–PSG; Littleton, MA: 1982. [Google Scholar]
- 17.Loehr LR, Rosamond WD, Chang PP, Folsom AR, Chambless LE. Heart failure incidence and survival (from the Atherosclerosis Risk in Communities Study) Am J Cardiol. 2008;101:1016–1022. doi: 10.1016/j.amjcard.2007.11.061. [DOI] [PubMed] [Google Scholar]
- 18. [Accessed September 28, 2017];ARIC Heart Failure Risk Calculator using Clinical Factors. http://aricnews.net/HFCalcs/RiskCalcHFClinFac.html.
- 19.Ponikowski P, Anker SD, AlHabib KF, et al. Heart failure: preventing disease and death worldwide. ESC Heart Failure. 2014;1:4–25. doi: 10.1002/ehf2.12005. [DOI] [PubMed] [Google Scholar]
- 20.Mozaffarian D, Benjamin EJ, Go AS, et al. American Heart Association Statistics Committee; Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2016 Update: A report from the American Heart Association. Circulation. 2016;133:e38–e360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 21.Hipp HR, Behrman JM, Heyer HE. The so-called silent myocardial infarction. Am Pract Dig Treat. 1949;4:64–66. [PubMed] [Google Scholar]
- 22.Stokes J, 3rd, Dawber TR. The silent coronary: The frequency and clinical characteristics of unrecognized myocardial infarction in the framingham study. Ann Int Med. 1959;50:1359–1369. doi: 10.7326/0003-4819-50-6-1359. [DOI] [PubMed] [Google Scholar]
- 23.Ikram MA, van Oijen M, de Jong FJ, Kors JA, Koudstaal PJ, Hofman A, Witteman JC, Breteler MM. Unrecognized myocardial infarction in relation to risk of dementia and cerebral small vessel disease. Stroke. 2008;39:1421–1426. doi: 10.1161/STROKEAHA.107.501106. [DOI] [PubMed] [Google Scholar]
- 24.Maradit-Kremers H, Crowson CS, Nicola PJ, Ballman KV, Roger VL, Jacobsen SJ, Gabriel SE. Increased unrecognized coronary heart disease and sudden deaths in rheumatoid arthritis: A population-based cohort study. Arthritis Rheum. 2005;52:402–411. doi: 10.1002/art.20853. [DOI] [PubMed] [Google Scholar]
- 25.Lundblad D, Eliasson M. Silent myocardial infarction in women with impaired glucose tolerance: The northern sweden monica study. Cardiovasc Diabetol. 2003;2:9. doi: 10.1186/1475-2840-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jonsdottir LS, Sigfusson N, Sigvaldason H, Thorgeirsson G. Incidence and prevalence of recognised and unrecognised myocardial infarction in women. The Reykjavik Study. Eur Heart J. 1998;19:1011–1018. doi: 10.1053/euhj.1998.0980. [DOI] [PubMed] [Google Scholar]
- 27.Sheifer SE, Gersh BJ, Yanez ND, 3rd, Ades PA, Burke GL, Manolio TA. Prevalence, predisposing factors, and prognosis of clinically unrecognized myocardial infarction in the elderly. J Am Coll Cardiol. 2000;35:119–126. doi: 10.1016/s0735-1097(99)00524-0. [DOI] [PubMed] [Google Scholar]
- 28.Nadelmann J, Frishman WH, Ooi WL, et al. Prevalence, incidence and prognosis of recognized and unrecognized myocardial infarction in persons aged 75 years or older: The bronx aging study. Am J Cardiol. 1990;66:533–537. doi: 10.1016/0002-9149(90)90477-i. [DOI] [PubMed] [Google Scholar]
- 29.Boland LL, Folsom AR, Sorlie PD, et al. Occurrence of unrecognized myocardial infarction in subjects aged 45 to 65 years (the ARIC study) Am J Cardiol. 2002;90:927–931. doi: 10.1016/s0002-9149(02)02655-3. [DOI] [PubMed] [Google Scholar]
- 30.Kannel WB, Abbott RD. Incidence and prognosis of unrecognized myocardial infarction. An update on the framingham study. N Eng J Med. 1984;311:1144–1147. doi: 10.1056/NEJM198411013111802. [DOI] [PubMed] [Google Scholar]
- 31.Vacek J. Silent myocardial infarction in the diabetic population. Am J Med. 1984;76:A59, 68. doi: 10.1016/0002-9343(84)90277-8. [DOI] [PubMed] [Google Scholar]
- 32.Kannel WB. Silent myocardial ischemia and infarction: Insights from the Framingham Study. Cardiol Clin. 1986;4:583–591. [PubMed] [Google Scholar]
- 33.Meyer S, Brouwers FP, Voors AA, et al. Sex differences in new-onset heart failure. Clinical Res Cardiol. 2015;104:342–350. doi: 10.1007/s00392-014-0788-x. [DOI] [PubMed] [Google Scholar]
- 34.Hwang SJ, Melenovsky V, Borlaug BA. Implications of coronary artery disease in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2014;63:2817–2827. doi: 10.1016/j.jacc.2014.03.034. [DOI] [PubMed] [Google Scholar]
- 35.Choudhury L, Gheorghiade M, Bonow RO. Coronary artery disease in patients with heart failure and preserved systolic function. Am J Cardiol. 2002;89:719–722. doi: 10.1016/s0002-9149(01)02345-1. [DOI] [PubMed] [Google Scholar]
- 36.Hebert K, Lopez B, Dias A, et al. Prevalence of electrocardiographic abnormalities in a systolic heart failure disease management population by race, ethnicity, and sex. Congest Heart Fail. 2010;16:21–26. doi: 10.1111/j.1751-7133.2009.00126.x. [DOI] [PubMed] [Google Scholar]
- 37.Nesto RW, Kowalchuk GJ. The ischemic cascade: Temporal sequence of hemodynamic, electrocardiographic and symptomatic expressions of ischemia. Am J Cardiol. 1987;59:23C–30C. doi: 10.1016/0002-9149(87)90192-5. [DOI] [PubMed] [Google Scholar]
- 38.Benjamin EJ, Blaha MJ, Chiuve SE, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017;135:e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Couto G, Ouzounian M, Liu PP. Early detection of myocardial dysfunction and heart failure. Nat Rev Cardiol. 2010;7:334–344. doi: 10.1038/nrcardio.2010.51. [DOI] [PubMed] [Google Scholar]
- 40.Borghi C, Ambrosioni E. Double-blind comparison between zofenopril and lisinopril in patients with acute myocardial infarction: Results of the survival of myocardial infarction long-term evaluation-2 (SMILE-2) study. Am Heart J. 2003;145:80–87. doi: 10.1067/mhj.2003.24. [DOI] [PubMed] [Google Scholar]
- 41.Zuanetti G, Latini R, Maggioni AP, Franzosi M, Santoro L, Tognoni G. Effect of the ACE inhibitor lisinopril on mortality in diabetic patients with acute myocardial infarction: Data from the GISSI-3 study. Circulation. 1997;96:4239–4245. doi: 10.1161/01.cir.96.12.4239. [DOI] [PubMed] [Google Scholar]
- 42.Hunt SA, Abraham WT, Chin MH, et al. American College of Cardiology.; American Heart Association Task Force on Practice Guidelines.; American College of Chest Physicians.; International Society for Heart and Lung Transplantation.; Heart Rhythm Society. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. J Am Coll Cardiol. 2005;46(6):e1–82. doi: 10.1016/j.jacc.2005.08.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.